Introduction
Inflammatory bowel disease (IBD) is a group of
chronic diseases in the digestive system that includes ulcerative
colitis (UC) and Crohn's disease (CD) (1). It is empirically diagnosed based on
endoscopic, pathological, clinical and radiological characteristics
(2), but the pathogenesis of IBD
remains unclear. UC is characterized by pathological damage in the
mucosal layer and colonic ulceration (3), and CD is characterized by
intraabdominal abscesses, fistulas and perianal disease (4). Colorectal cancer (CRC) is one of the
most serious complications of long-term IBD (5). It has the third highest incidence of
all malignant tumors (6). IBD is
frequently diagnosed in 20–30-year-olds followed by another peak in
60–70-year-olds (7), where
patients with IBD are at ~ three times higher risk of developing
CRC compared with that in the general population (8,9).
Colonic inflammation is caused by an imbalance in
the levels of proinflammatory and anti-inflammatory factors and is
associated with the aberrant levels of interleukins (ILs) (10). Chronic inflammation of the mucosa
is one of the main characteristics of IBD, the long-term presence
of which can lead to carcinogenesis (11). CRC associated with colitis
gradually develops from a negative test result for dysplasia, to an
indefinite test result for dysplasia, followed by the development
of low-grade dysplasia, high-grade dysplasia and finally CRC
(12). During the progression
from inflammation to atypical hyperplasia and then CRC, the
expression of NF-κB (13) and S11
calcium binding protein A9 (S100a9), which is a member of the S100
family known to serve roles in the innate immune system (14).
Traditional Chinese medicine and naturally-occurring
compounds have been used to treat tumors because of their low risk
of adverse effects and their therapeutic efficacy against multiple
targets (15). Evodia
rutaecarpa is a type of traditional Chinese medicine that has
been used for the long-term treatment of gastrointestinal
disorders, headaches and postpartum hemorrhage (16). In total, 131 compounds have been
isolated and identified from the extract of Evodia
rutaecarpa, with the majority consisting of alkaloids, terpenes
and phenols (17). Evodiamine
(Evo) is an alkaloid (Fig. 1A)
that can be extracted from Evodia rutaecarpa (18). It can inhibit inflammation by
inhibiting NF-κB (19), where it
has been reported to exert anti-tumor activities against lung
cancer, osteosarcoma, gastric cancer and breast cancer through the
promotion of mitochondrial apoptosis (16). In addition, Evo has been
demonstrated to inhibit inflammation by inhibiting the NLR family
pyrin domain containing 3 inflammasome in mice with dextran sulfate
sodium (DSS)-induced UC (19).
However, the effects of Evo on CRC, including any possible
underlying mechanisms, remain poorly understood.
In the present study, the potential effects of Evo
in a mouse model of DSS-induced UC was examined. In addition, the
possible effects of Evo on CRC were investigated in the
C57BL/6-adenomatous polyposis coli (Apc)MinC/Gpt strain
of mice, which harbours a point-mutation in the Apc gene,
which influences WNT-β-catenin signaling. Based on proteomics
screening, the effects of Evo on inflammation, with emphasis on
NF-κB signaling and S100a9 expression (which plays roles in the
innate immune system), were studied. It is hoped that the data
generated can provide experimental evidence for the potential
clinical value of applying Evo for the treatment of UC or even
CRC.
Materials and methods
Animal experiments
Establishment of the UC mouse model and agent
administration procedure
In total, 75 male wild-type C57BL/6 mice (8 weeks
old; mean weight ± standard error of mean, 23.1±0.3 g; weight
range, 20–25 g) were supplied by Liaoning Changsheng Biotechnology
Co., Ltd. [license no. SCXK (LIAO)-2015-0001; Liaoning, China]. The
animals were housed in a specific pathogen-free (SPF) animal
laboratory where they were allowed unrestricted access to food and
water under a temperature of 22±2°C and 40–60% humidity, with a
12-h light/dark cycle. Experimental protocols were approved by the
Experimental Animal Ethics Committee of Jilin University (approval
no. SY201905008).
Negative control mice received sterile water alone
throughout the experimental period, whilst mice in the UC group
were allowed to freely drink water containing 3% DSS (cat. no.
S14048; Mw, 50,000 Da; Shanghai Yuanye Biological Technology Co.,
Ltd.) for 6 days. From the days 7 to 27, the mice with UC were fed
water containing 3% DSS every 3 days and water without DSS the rest
of the time. On day 7, mice with UC were randomly divided into four
groups and were orally administered with either ddH2O
(model group; n=15), 0.6 g/kg sulfasalazine (SASP; cat. no. BP779;
Mw, 398.39 Da; Sigma-Aldrich, Merck KGaA; positive control group,
n=15), 10 mg/kg evodiamine (Evo; cat. no. B21315; Mw, 303.363 Da;
Shanghai Yuanye Biological Technology Co., Ltd.; n=15) or 30 mg/kg
Evo (n=15) once daily for the following 3 weeks. The dosage of Evo
in this study was identified based on a previous study (19).
During the experimental period, body weight, fecal
consistency and occult blood were evaluated daily. At the end of
the experimental period, the mice were humanely euthanized and
blood, colon, liver, spleen and kidney tissues were collected. The
length of the colon was then measured. The euthanasia was performed
according to the AVMA Guidelines for the Euthanasia of Animals
(20), and the specific method
was as follows: The mice were put into a CO2-free
euthanasia box, before the CO2 was perfused at a rate of
replacing 10–30% of the volume of the euthanasia box per min. After
5 min, if the mice were confirmed to be motionless, not breathing
and with dilated pupils, the CO2 would be turned off the
mice would be observed for 2–3 min. The organ index values were
calculated using the following formula: Organ index (%)=organ
weight (g)/body weight (g) ×100%.
The disease activity index (DAI) is a comprehensive
score of weight loss, stool consistency and rectal bleeding that is
used extensively to evaluate the clinical progress of patients with
colitis (21,22). DAI values were calculated
according to a previously described method (23). Scores were calculated as follows:
Weight loss, 0 (no weight loss), 1 (1–5%), 2 (5–10%), 3 (10–15%), 4
(15–20%) and 5 (>20%); stool consistency, 0 (Normal stool), 1
(Mildly), 2 (Soft stool), 3 (Very soft stool), 4 (Watery stool) and
5 (Completely watery stool); rectal bleeding, 0 (Normal colored
stool), 1 (Brown stool), 2 (Reddish stool), 3 (Mildly bloody
stool), 4 (Bloody stool) and 5 (Very bloody stool). The DAI was
calculated using the following formula: DAI=(weight loss + stool
consistency + rectal bleeding)/3.
Establishment of the CRC mouse model and agent
administration procedure
In total, 24 male C57BL/6-ApcMinC/Gpt
mice (8 weeks old; mean weight ± standard error of mean, 21.7±0.7
g; weight range, 20–25 g) were obtained from GemPharmatech Co.,
Ltd. [licence no. SCXK(SU)2018-0008; Nanjing, China]. Genetic
testing and pathological sections were used to confirm the the
successful establishment of the model. The animals were housed in a
specific-pathogen-free animal laboratory (Jilin University,
Changchun, China) and allowed unrestricted access to sufficient
food and water under a temperature of 22±2°C and 40–60% humidity,
with a 12-h light/dark cycle. The experimental protocols were
approved by the Experimental Animal Ethics Committee of Jilin
University (approval no. SY201905003).
The C57BL/6-ApcMinC/Gpt mice were
randomly divided into two groups (n=12) and were orally
administered with either ddH2O (control group) or 10
mg/kg Evo every other day for 8 weeks. The body weight of the mice
was recorded once a week. At the end of the experimental period,
the mice were humanely euthanized, before blood, colon, liver,
spleen and kidney tissues were collected. The organ index values
were then calculated as aforementioned.
Label-free quantification of proteins in the
colons of UC mice
Colon tissue samples (n=3) from mice in the control,
model and 10 mg/kg Evo-treated groups were fully lysed (25 mM
Tris•HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 1%
SDS). Protein concentrations were determined using a BCA assay kit
(cat. no. 23227; Thermo Fisher Scientific, Inc.) as previously
described (24). A total of 100
µg total protein of each group was diluted to 1 mg/ml and mixed
with 4 volumes of cold acetone. The mixture was thoroughly shaken
at a low temperature (−20°C; 30 min) and the proteins were
precipitated by centrifugation at 4°C at 10,000 × g for 10 min. The
sediment was collected. The protein precipitate was re-dissolved in
ammonium bicarbonate (100 mM ammonium bicarbonate, 1% sodium
deoxycholate, pH 8.5), reduced (5 mM TCEP at 55°C for 10 min),
alkylated (10 mM iodoacetamide at room temperature for 15 min) and
enzymatically hydrolyzed (2 µg trypsin solution at room temperature
for 5 min; Promega Corporation). After removing sodium deoxycholate
(the sodium deoxycholate was precipitated with 2% trifluoroacetic
acid, centrifuged at 10,000 × g for 10 min at room temperature, and
the supernatant was collected) from the peptide samples, they were
desalted using a desalting column (cat. no. DC18150; Biocomma
Limited) at room temperature.
The peptide samples prepared as aforementioned were
analyzed using liquid chromatography-mass spectrometry (LC-MS)/MS
(25). The details of the
reaction were as follows: Nano-UPLC liquid phase system
EASY-nLC1200 (Thermo Fisher Scientific, Inc.); positive ion
detection mode; precursor scan range, 350-1,600 m/z; nitrogen gas
temperature, 20°C; spray voltage, 1.5 kv; flow rate, 300 nl/min.
The results were processed using MaxQuant (1.5.6.0; Max Planck
Institute of Biochemistry). The protein database is from the
UNIPROT database (Uniprot_mouse_2016_09; http://www.uniprot.org/). The protein sequences and
their inverted decoy sequences were used in the MaxQuant searches.
Label-free quantification (LFQ) was used because it matches the
samples between runs. The samples were normalized to keep the
median total protein concentration in each group consistent before
performing MaxQuant analysis and LFQ. Fold differences in protein
concentrations were defined to be significant if the A/B ratio was
>1.5 or <0.67. Subsequently, Gene Ontology (GO)
(clusterProfiler_3.12.0; http://guangchuangyu.github.io/software/clusterProfiler/),
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
(clusterProfiler_3.12.0; http://guangchuangyu.github.io/software/clusterProfiler/)
and protein interaction analyses (STRINGdb_1.20.0; http://cn.string-db.org/cgi/input.pl)
were performed. GO and KEGG used Fisher's precision probability
test (P<0.05; n=3) and protein interaction analyses used random
background model (no threshold filtering).
Detection of cytokines in serum and colon tissues
of mice with UC and CRC
IL-1β (cat. no. KT2040-A), IL-2 (cat. no. KT2698-A),
IL-6 (cat. no. KT2163-A), IL-8 (cat. no. KT2123-A), IL-15 (cat. no.
KT2172-A), IL-17 (cat. no. KT2170-A), IL-22 (cat. no. KT9441-A),
TNF-α (cat. no. KT2132-A), IFN-γ (cat. no. KT2182-A) concentrations
in the colon tissues, which were lysed using Radio
Immunoprecipitation Assay (cat. no. R0010; Beijing Solarbio Science
& Technology Co., Ltd.) containing 1% protease inhibitor
cocktail (cat. no. P8340; Sigma-Aldrich; Merck KGaA) and 2%
phenylmethanesulfonyl fluoride (cat. no. P7626; Sigma-Aldrich;
Merck KGaA), and/or serum of mice with UC and CRC were measured
using commercialized ELISA kits (Jiangsu Kete Bio-Technology Co.,
Ltd.) according to the manufacturer's protocols.
Histological analysis
Colon tissues were fixed with 4% paraformaldehyde
for 48 h at room temperature and dehydrated using an ascending
ethanol gradient from 50 to 100%. The tissues were cleared with
xylene and embedded in paraffin wax blocks. Before staining, 5-µm
thick colon tissue sections were dewaxed in xylene, rehydrated
through a descending ethanol gradient from 100–70% and washed in
PBS. This was followed by staining with hematoxylin for 6 min and
eosin for 1 min at 20–25°C in sequence and examined under a light
microscope (magnification, ×40 and ×400; Olympus Corporation) as
previously described (26).
Detection of apoptosis in colon tissues of mice
with CRC
The extent of apoptosis in colon tissues of mice
with CRC was detected using a Terminal Deoxynucleotidyl
Transferase-Mediated dUTP In Situ Nick End Labelling Assay
kit (cat. no. G1501; Wuhan Servicebio Technology Co., Ltd.)
according to the manufacturer's protocols. Briefly, the sections
prepared from colon tissues were retrieved with proteinase K for 22
min at 37°C. Terminal deoxynucleotidyl transferase (TdT) enzyme and
dUTP were added after breaking the membrane, and incubated at 37°C
for 2 h. Subsequently, the sections were washed with PBS three
times for 5 min. The sections were then stained with DAPI (2 µg/ml)
(cat. no. G1012; Wuhan Servicebio Technology Co., Ltd.) at room
temperature and incubated without strong and direct light for 10
min. The sections were finally mounted with anti-fluorescence
quenching mounting medium (cat. no. G1401; Wuhan Servicebio
Technology Co., Ltd.). The fluorescent images were photographed
using a fluorescence microscope (magnification, ×200; Nikon
Corporation).
Western blotting
Colon tissues of mice with UC and CRC were collected
and washed immediately in D-Hank's buffer (8 g/l NaCl, 0.4 g/l KCl,
1 g/l glucose, 60 mg/l KH2PO4, 47.5 mg/l
Na2HPO4, pH 7.2). The colon tissues were then
lysed using Radio Immunoprecipitation Assay (cat. no. R0010;
Beijing Solarbio Science & Technology Co., Ltd.) containing 1%
protease inhibitor cocktail (cat. no. P8340; Sigma-Aldrich; Merck
KGaA) and 2% phenylmethanesulfonyl fluoride (cat. no. P7626;
Sigma-Aldrich; Merck KGaA), and protein concentration in the
lysates was determined using a BCA protein assay kit, as previously
described (24). The protein
lysates (40 µg/lane) were resolved by 12% SDS-PAGE and transferred
onto PVDF membranes. The membranes were blocked with 5% bovine
serum albumin (cat. no. A8010; Beijing Solarbio Science &
Technology Co., Ltd.) in Tris-buffered saline at 4°C for 4 h and
then incubated with primary antibodies against phosphorylated
(p)-NF-κB (cat. no. ab86299; 1:4,000; Abcam), p-inhibitor NF-κB
kinase α+β (IKKα+β; cat. no. ab195907; 1:500; Abcam), total
(T)-NF-κB (cat. no. ab7970; 1:1,000), T-IKKα+β (cat. no. ab178870;
1:1,000; Abcam), p-IκBα (cat. no. ab12135; 1:500; Abcam), T-IκBα
(cat. no. ab32518; 1:2,000; Abcam), GAPDH (cat. no. ab8245;
1:2,000; Abcam), S100a9 (cat. no. A9842; 1:1,000; ABclonal Biotech
Co., Ltd.), Toll-like receptor 4 (TLR4; cat. no. bs-20594R,
1:2,000), myeloid differentiation factor 88 (MyD88; cat. no.
bs-1047R, dilution: 1:300; BIOSS) overnight at 4°C. They were then
incubated with goat anti-rabbit IgG-HRP (cat. no. E-AB-1003;
1:5,000; Elabscience Biotechnology, Inc.) or goat anti-mouse
IgG-HRP (cat. no. E-AB-1001; 1:5,000; Elabscience Biotechnology,
Inc.) for 4 h at 4°C. The bands were detected using an
Ultrasensitive ECL Chemiluminescence Kit (cat. no. P10200; Suzhou
New Saimei Biotechnology Co., Ltd.) and imaging system (BioSpectrum
600; BIOSS), and then quantified using Image J software (v1.8.0;
National Institutes of Health).
Cell Culture
SW480 cells, a human colon adenocarcinoma cell line
(The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences), were cultured at 37°C in a 5% CO2 incubator
with DMEM (cat. no. CM-0223B; Procell Life Science & Technology
Co., Ltd.) containing 10% FBS (cat. no. 164210; Procell Life
Science & Technology Co., Ltd.), 100 µg/ml streptomycin and 100
U/ml penicillin (Thermo Fisher Scientific, Inc.).
Cell viability assay
SW480 cells were seeded into 96-well plates at the
density of 8×103 cells/well and treated with Evo at
doses of 20–200 µM for 24 h at 37°C. Cell viability was detected
using MTT assay (26). Briefly,
MTT was added (5 mg/ml) to 96-well plates and incubated for 4 h at
37°C. Subsequently, the liquid was aspirated, dissolved in DMSO and
the optical density value at 490 nm was measured.
Mitochondrial membrane potential (MMP)
detection
SW480 cells were seeded into six-well plates at a
density of 2×105 cells/well and treated with 100 and 200
µM Evo for 12 h at 37°C. The collected cells were incubated with
the JC-1 dye (Final concentration, 5 µg/ml; cat. no. BB-4105;
BestBio) at 37°C for 20 min in the dark and then washed with PBS
for three times. A fluorescence microscope (magnification, ×100;
Nikon Corporation) was used to observe the fluorescence intensity
of the cells (brighter red fluorophores represent higher
mitochondrial membrane potential).
Cell cycle detection
SW480 cells were seeded into six-well plates at a
density of 2×105 cells/well and treated with 100 and 200
µM Evo for 12 h at 37°C. Collected cells were washed with ice-cold
PBS and incubated with 70% pre-cooled ethanol for >3 h at −20°C.
The cells were then exposed to the Muse® Cell Cycle
Reagent (cat. no. MCH100106; MilliporeSigma) at room temperature
for 30 min in the dark. Muse® Cell Analyzer (cat. no.
0500-3115; MilliporeSigma) was used to detect the conditions of the
cell cycle.
Immunofluorescence of p-NF-κB
Immunofluorescence of p-NF-κB was performed using a
NF-κB Activation, Nuclear Translocation Assay Kit (cat. no. SN368;
Beyotime Institute of Biotechnology). Briefly, SW480 cells were
seeded into glass-bottom cell culture dishes at a seeding density
of 2×105 cells/well and treated with 100 and 200 µM Evo
for 12 h at 37°C. Collected cells were washed with ice-cold PBS and
then incubated with the primary NF-κB p65 antibody (undiluted) at
4°C overnight after fixing (cold 100% methanol at −20°C) for 10 min
and blocking (5% BSA) for 60 min at 37°C. The cells were then
incubation with a Cy3-conjugated secondary antibody (undiluted) for
1 h at room temperature. Subsequently, the cells were stained with
DAPI (undiluted) for 5 min at room temperature and then visualized
by a confocal microscope (magnification, ×400; LSM710; Carl Zeiss
AG).
Statistical analysis
All data are presented as the mean ± standard error
of the mean (SEM). In vitro, all experiments were repeated
six times. Kruskal-Wallis test followed by Dunn's test was used to
compare differences in the DAI among each group. One-way analysis
of variance and Tukey's post hoc multiple comparisons test were
performed using the SPSS 16.0 software (SPSS, Inc.) in other data.
P<0.05 was considered to indicate a statistically significant
difference.
Theoretical analysis
The three-dimensional structure of NF-κB (NCBI no.
NP_033071.1) was built using the online tool SWISS-MODEL (release
date, 2017-03-08; http://swissmodel.expasy.org/). The template and the
structure of the complex between the kinase-inducible
domain-interacting domain of CREB binding protein (CBP) (PDB ID,
5U4K) and the transactivation domain 1 of p65 showed 93.33%
sequence identify (27). The
ligand, Evo, which was downloaded from chemspider (http://www.chemspider.com/), was docked onto T-NF-κB
and p-NF-κB (S536P) using AutoDock 4.2 (Olson Lab at the Scripps
Institute; http://autodock.scripps.edu/) (28,29). The size of the docking box was set
to 20×20×25 and the length of each grid was 0. 0375 nm. The
molecular docking was calculated by the Lamarckian genetic
algorithm. Amber 16 software (Amber is developed in an active
collaboration of David Case at Rutgers University; http://ambermd.org/) was used to analyze the four
systems, T-NF-κB, p-NF-κB, T-NF-κB-Evo and p-NF-κB-Evo (30) for 50 nsec molecular dynamics (MD)
simulations. The amber ff99SB forcefield, which could give a force
to an atom before MD, was applied to the proteins and ligands
(31).
Results
Effects of Evo on DSS-induced UC
DAI and weight loss are important parameters used to
evaluate the degree of inflammation in patients with UC (32,33). Compared with that in healthy mice,
significant weight loss (Table
SI), significant reductions in colon length (Fig. 1B and C) and increased DAI scores
on day 27 (Fig. 1D) were observed
in mice with DSS-induced UC. These symptoms were ameliorated after
Evo administration (Fig. 1B-D and
Table SI).
Furthermore, mice with DSS-induced UC showed a
damaged intestinal epithelium, fewer numbers of goblet cells and
dense exfoliated lymphocyte infiltration into the submucosa
(Fig. 1E). By contrast, both SASP
and Evo treatment markedly improved these pathologic changes in the
colon tissues (Fig. 1E).
Anti-UC effects of Evo is associated
with NF-κB signaling
In the colon tissue samples analyzed, 23,519
peptides, 3,375 groups of proteins, 3,316 quantifiable proteins and
838 differentially expressed proteins were identified. The
administration of Evo at a dose of 10 mg/kg significantly increased
the levels of 129 proteins and decreased the levels of 129 proteins
in the colons of mice with DSS-induced UC (Fig. 2A and Table SII). Through STRINGdb analysis of
protein interactions between the groups, potent interactions were
found among 258 molecules in the colons between the UC model and
Evo-treated UC mice (Figs. 2B and
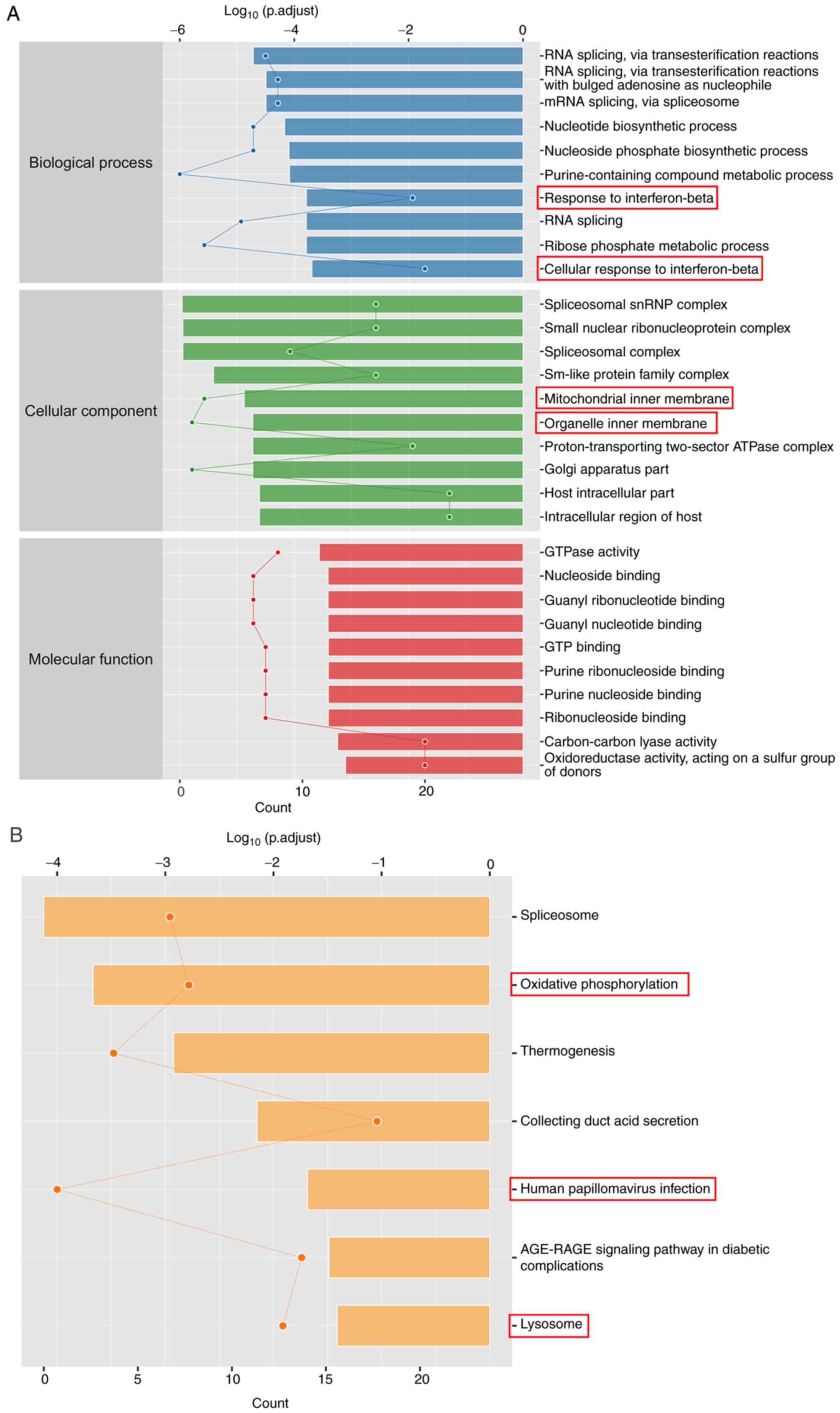
S1). GO enrichment analysis
showed that the 258 proteins with significantly changed expression
levels were associated with ‘response to IFN-β’, ‘cellular response
to IFN-β’, ‘mitochondrial inner membrane’ and ‘organelle inner
membrane’ (Fig. 3A). According to
KEGG enrichment analysis, Evo altered processes in ‘oxidative
phosphorylation’, ‘human papillomavirus infection’ and ‘lysosome’
(Fig. 3B). These results suggest
that Evo may reduce the inflammatory response in UC.
Compared with healthy mice, significantly increased
levels of IL-1β, IL-2, IL-6, IL-8 and TNF-α were observed in the
colon tissues of mice with UC (Fig.
4A-F). However, 3 weeks of Evo administration resulted in
significant reductions in the colonic levels of IL-1β, IL-2, IL-6,
IL-8, TNF-α and IFN-γ (Fig.
4A-F). However, ad libitum drinking of DSS only caused a
significant increase in the concentration of IL-1β in the serum
(Table SIII). In addition, Evo
treatment significantly reduced the serum concentrations of IL-1β,
IL-6 and IL-8 in mice with DSS-induced UC (Table SIII).
 | Figure 4.Evo ameliorates inflammation in the
colonic tissues of mice in UC. Evo reduced the levels of (A) IL-1β,
(B) IL-2, (C) IL-6, (D) IL-8, (E) TNF-α and (F) IFN-γ in the
colonic tissues of mice with UC (n=6). (G) Evo exerted
anti-inflammatory effects by inhibiting NF-κB signaling. Compared
with their levels in vehicle-treated mice with UC, Evo reduced the
phosphorylation levels of NF-κB, IKKα/β and IκBα and the expression
levels of S100a9, TLR4 and MyD88 in the colons of mice with UC
(n=3). The levels of each protein were normalized to those of
GAPDH. Data are presented as the mean ± SEM and were analyzed using
one-way ANOVA followed by Tukey's test. #P<0.05,
##P<0.01 and ###P<0.001 vs. CTRL;
*P<0.05, **P<0.01 and ***P<0.001 vs. 3.0% DSS-only. Evo,
evodiamine; DSS, dextran sodium sulfate; CTRL, control; UC,
ulcerative colitis; SASP, sulfasalazine; p-, phosphorylated; t-,
total; TLR4, Toll-like receptor 4; MyD88, myeloid differentiation
primary response 8; S100a9, S100 calcium binding protein A9; IκBα,
inhibitor of NF-κBα; IKK, IκB kinase. |
NF-κB is considered to be a key regulator of
inflammation (11). Evo
significantly reduced the phosphorylation of NF-κB, IKKα/β and IκBα
and the expression levels of S100a9, TLR4 and MyD88 in the colon
tissues of mice with UC compared with those in vehicle-treated mice
with UC (Fig. 4G).
Anti-CRC effects of Evo in
ApcMinC/Gpt mice through NF-кB signaling
Evo significantly reduced the viability of SW480
cells (Fig. S2A) and caused cell
cycle arrest at the G2/M phase (Fig. S2B). In addition, Evo inhibited
mitochondrial membrane potential (Fig. S2C), reduced the expression of
p-NF-кB and suppressed the translocation of p-NF-кB from the
cytoplasm to the nucleus (Fig.
S2D) in SW480 cells.
ApcMinC/Gpt mice, which spontaneously
develop colorectal tumors, were used in the present study to
examine the effects of Evo on CRC (34). After 8 weeks of Evo
administration, the numbers and sizes of the colonic tumors were
markedly reduced (Fig. 5A and F),
whereas the degree of weight loss in the ApcMinC/Gpt
mice was significantly lower (Fig.
5B). Evo also significantly reduced the liver (Fig. 5C) and kidney index values
(Fig. 5D). In addition, Evo
significantly enhanced the spleen index values (Fig. 5E) of mice with CRC, suggesting
that the administration of Evo reduced inflammation. Compared with
that in the control mice, an increase in green fluorescence
representing apoptotic cells in tumor tissues was observed,
indicating that Evo administration promoted tumor tissue apoptosis
(Fig. S3).
Compared with vehicle-treated ApcMinC/Gpt
mice with CRC, 8 weeks of Evo administration significantly
suppressed the serum concentrations of IL-1β, IL-6, IL-22 and TNF-α
whilst significantly increasing the concentration of IL-15
(Fig. 6A-G). In colonic tissues,
Evo administration significantly reduced the levels of IL-1β, IL-2,
IL-6, IL-17, IL-22 and TNF-α, but significantly increased the
expression levels of IL-15 (Fig.
6A-G).
 | Figure 6.Evo exerts anti-inflammatory effects
in ApcMinC/Gpt mice by inhibiting NF-κB signaling. Evo
reduced the levels of (A) IL-1β, (B) IL-2, (C) IL-6, (D) IL-17, (E)
IL-22 and (F) TNF-α, whilst increasing the levels of (G) IL-15 in
the serum and colon samples of ApcMinC/Gpt mice (n=6).
(H) Evo suppressed the phosphorylation of NF-κB, IKKα/β and IκBα
and the expression levels of S100a9 in colonic tissues (n=3). Data
are presented as the mean ± SEM and were analyzed by one-way ANOVA
followed by Tukey's test. ^P<0.05,
^^P<0.01 and ^^^P<0.001 vs. CTRL. Evo,
evodiamine; CTRL, control; S100a9, S100 calcium binding protein A9;
p-, phosphorylated; t-, total; IκBα, inhibitor of NF-κBα; IKK, IκB
kinase. |
The NF-κB pathway has been reported to regulate the
expression of oncogenes and proinflammatory genes (11). IL-6-activated NF-κB has been
previously found to promote the development of CRC by acting on
intestinal epithelial cells (35). During the development of CRC,
constitutive activation of this pathway promotes the malignant
transformation and proliferation of colonic epithelial cells
(36). Following 8 weeks of Evo
administration, the phosphorylation levels of NF-κB, IKKα/β and
IκBα were significantly reduced and the expression levels of S100a9
were also significantly reduced, in the colon compared with those
in vehicle-treated ApcMinC/Gpt mice with CRC (Fig. 6H).
MD simulations
Theoretical models were built to explain the
inhibition of NF-κB activation by Evo. Sequence alignment between
the templates, XP_004627287.1 and XP_020020483.1 (the closest
protein number which compared in Blast) and NF-κB is shown in
Fig. 7A, whereas the docking of
Evo onto NF-κB is shown in Fig.
7B. Asp533, Ile537, Phe534 and Ser536 (phosphorylated residue)
were found to be important residues for Evo binding. The effects of
the protein chains and the solvent environment on the secondary
structure of the protein were subsequently determined (Fig. 7C and D). Evo binding to either
T-NF-κB or p-NF-κB may increase the probability of α helix
formation. To confirm whether the conformational changes were
continuous and stable, principal components analysis and
cross-correlation analyses were further performed. The
cross-correlation analyses of the four systems indicated that the
significant movement mainly occurred between the regions of the α
helix (Fig. 7E). According to
free energy landscape model, the structures of the two most stable
conformations of Evo binding to T-NF-κB and p-NF-κB revealed that
the conformational changes in the α helix existed during MD
simulations (Fig. 7F).
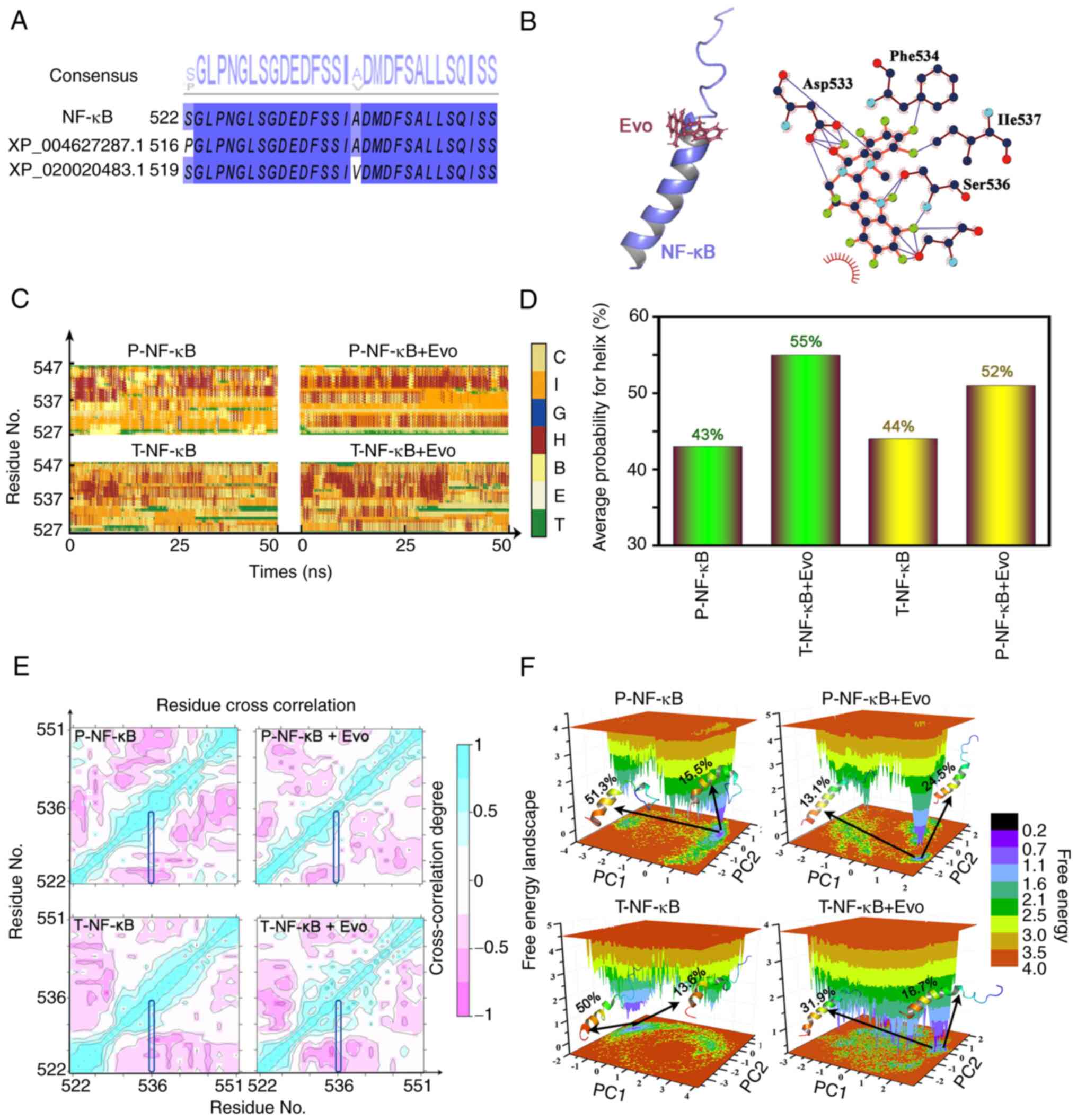 | Figure 7.Docking data of Evo onto NF-κB. (A)
Sequence alignment of NF-κB, XP_004627287.1 and XP_020020483.1 (the
closest protein number which compared in Blast). (B) The structure
of the complex and the potential interaction between NF-κB and Evo.
(C) Changes in the secondary structure of T-NF-κB, T-NF-κB + Evo,
p-NF-κB and p-NF-κB + Evo. (D) The average probability of a helix
which might influence the NF-κB in T-NF-κB, T-NF-κB + Evo, p-NF-κB,
and p-NF-κB + Evo. (E) Cross-correlation maps for T-NF-κB, T-NF-κB
+ Evo, p-NF-κB, and p-NF-κB + Evo. (F) Free energy landscape for
T-NF-κB, T-NF-κB + Evo, p-NF-κB, and p-NF-κB +Evo. Evo, evodiamine;
T-total; p-, phosphorylated; C, coil; I, 5-helix; G, 3-helix; H,
α-helix; B, β-bridge; E, β-sheet; T, turn. |
Discussion
The present study systemically investigated effects
of Evo on inflammation, UC and CRC, with focus on NF-κB signaling.
Inflammation has been reported to regulate every stage of tumor
development, from initiation to and metastasis (37). A number of pathways, including
NF-κB, are activated during chronic inflammation, which can promote
tumor development by promoting epithelial cell proliferation and
angiogenesis (11). During the
early events of the development of colitis-associated CRC, the
TP53 gene is mutated, leading to the constitutive activation
of NF-κB and increased inflammation (35). This inflammatory environment can
potentiate DNA damage and ultimately lead to mutations in the
APC gene and tumor initiation (35). APC is a frequently mutated
gene in human sporadic CRC and is almost always mutated in familial
APC (38). The loss of
APC function is frequently an early event in the
pathogenesis of CRC.
Epithelial cells and immune cells in the intestines
of mice with colitis express a variety of proinflammatory mediators
(39). As an effective NF-κB
activator, IL-1β appears during the early stages of intestinal
inflammation and sustains the inflammatory environment in colonic
tissues (40). High expression
levels of IL-1β have been observed in the tumors from mice with
DSS/azoxymethane-induced colitis-associated CRC and in non-colitic
APCΔ468 mice (41).
IL-1β promotes the production of IL-6 (41), which contributes to the
development of IBD and the tumorigenesis of CRC through its
receptor, IL-6R (42). Both IL-1β
and IL-6 levels were found to be suppressed by Evo administration
in mice with UC and CRC in the present study.
TNF-α is an effective proinflammatory cytokine that
serves important roles in immune regulation, the inflammatory
response, proliferation and death of all cell types (43). The levels of TNF-α are frequently
found to be elevated in the blood, stool samples and mucous
membranes of patients with UC (44). The production and release of TNF-α
is stimulated by IFN-γ and IL-1 (43), where TNF-α activates NF-κB through
TNF receptor-related factor 2 (45). TNF-α is also an effective
activator of intestinal epithelial cells where it stimulates the
production of the proinflammatory cytokine IL-8, which is a key
mediator of inflammation in the C-X-C chemokine family (46). An association between the severity
of inflammation and the levels of IL-8 in the colonic mucosa has
been reported, where elevated IL-8 levels have been found in the
colonic mucosa of patients with UC (47). However, upon stimulation, the
production of a large amounts of IL-8 is induced, which
chemotactically attracts polymorphonuclear leukocytes, monocytes
and macrophages to the site of inflammation, where IL-8 then
aggravates the inflammation (48). The promoter region of IL-8 has
binding sites for transcription factors, such as activator protein
1 and NF-κB (48). Once
activated, NF-κB may cause the overexpression of proinflammatory
cytokines, thereby promoting a Th1-dependent lymphocyte immune
response (49). IL-6 promotes the
chemotaxis of neutrophils, promotes colonic necrosis and ultimately
tissue destruction (50). In the
present study, the inhibitory activity of Evo on NF-κB was noted in
the DSS-induced mouse UC model. Evo improved the symptoms of UC by
inhibiting the DSS-induced activation of NF-κB, downregulating the
levels of proinflammatory genes IL-1β, IL-6, IL-8 and TNF-α whilst
preventing the infiltration of inflammatory cells.
In ApcMinC/Gpt mice with CRC, Evo further
promoted the levels of IL-15 and inhibited IL-17 and −22 in the
serum and colonic tissues according to the results of ELISA. IL-15
enhances the proliferation and activation of natural killer cells
and CD8+ T cells, which in turn promote humoral and cell-mediated
immune responses, thereby inhibiting tumor growth (41). The loss of or reduction in IL-15
expression in human CRC results in higher risks of recurrence
(41). In sporadic CRC, mutations
in the APC gene cause the loss of cell polarity and tight
junctions, leading to bacterial invasion and production of IL-17
(35). In IBD, high levels of
IL-17 have been found in the inflamed intestinal mucosa (51). Accordingly, the simultaneous
neutralization of IL-17 and TNF-α may switch off NF-κB signaling,
which may negate the mitogenic effects of factors secreted by CRC
cells (52). Furthermore, Evo
strongly suppressed the levels of IL-22, which is found at high
levels in the serum and intestines of patients with IBD (52). IL-22 expression has also been
found to be upregulated in leukocytes infiltrating the tumor mass
in patients with colitis-related CRC (11). The activation of IL-22R in turn
leads to an increase in IL-8 and TNF-α levels (51). These results suggest that the
suppression of NF-κB signaling serves an important role in
mediating the anti-CRC effects of Evo in ApcMinC/Gpt
mice.
Furthermore, the dysregulation of S100a9, a
Ca2+-binding protein of the S100 family that controls
acute and chronic inflammation, has been widely observed in IBD
(3). S100a8/a9 functions as an
‘alarm’ protein at the site of the inflammation by activating TLR4
(53), which subsequently
activates NF-κB (54). In colonic
tissues of mice with UC or CRC, Evo markedly regulated the levels
of S100a9, p-NF-κB and its upstream proteins. TLR4, a
lipopolysaccharide receptor, is expressed at high levels in the
colonic tissues of patients with UC and mice with DSS-induced
colitis (55). The activation of
TLR4 promotes the signaling cascade mediated by MyD88, which leads
to the activation of NF-κB and the release of IL-6 (56). NF-κB expression and activation are
markedly increased in the inflamed intestines of patients with IBD,
in addition to those animals of experimental colitis models
(11). Once NF-κB is activated,
the NF-κB inhibitor, IκB, which binds to NF-κB, is also
phosphorylated by the IKK complex. p-IκB causes the translocation
of NF-κB to the nucleus, where it activates the transcription of
related target genes encoding inflammatory factors, especially IL-6
(57). In SW480 cells, Evo
exposure potently suppressed the translocation of p-NF-κB from the
cytoplasm into the nucleus. Experimental and theoretical data in
the present study suggest that Evo may directly inhibit the
phosphorylation of NF-κB. It has been reported that phosphorylated
serine can promote electron transfer, since they facilitate the
formation or destruction of various non-bonding interactions
(58). Evo binding to p-NF-κB may
induce new interactions with NF-κB, which facilitate the formation
of an α helix in p-NF-κB, as indicated by MD simulations.
There are a number of limitations in the present
study. It only investigated the effects of Evo on UC and CRC in
relation to NF-κB signaling from the perspective of inflammation.
However, because of the selection of animal models, an in-depth
study of the role of Evo in the transition from UC to
colitis-related CRC was not possible. In addition, the effects of
mitochondrial function on the anti-inflammatory effects of Evo
would require further study.
Altogether, data from the present study suggest that
Evo can reduce the inflammatory response in UC and CRC by
preventing damage of the intestinal mucosal barrier and by
regulating the secretion of inflammatory cytokines. The suppression
on the activation of NF-κB serve central roles in mediating these
effects. The findings provide experimental evidence that Evo may be
promising as an effective treatment option in clinics for colitis
and CRC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology
Development Project of Jilin Province in China (grant nos.
20200708037YY, 20200708068YY and 20200708091YY) and the Natural
Sciences Foundation of Jilin Province in China (grant nos.
20200201030JC and 20200201122JC).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The datasets generated and/or analyzed during the current
study are available in the ProteomeXchange Consortium via the PRIDE
partner repository (dataset identifier PXD027745), (Username,
reviewer_pxd027745@ebi.ac.uk;
Password, pZmVuoRu; http://www.ebi.ac.uk/pride/archive).
Authors' contributions
CL and YQ contributed to the conceptual design of
the research. YonZ, YaqZ, YanZ, WW, WM and YuZ performed the
experiments. YonZ and YaqZ processed the data. YonZ and YaqZ wrote
the manuscript. WM and YuZ helped perform the analysis with
constructive discussions. YonZ and YaqZ confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All the animal experiments have been approved by the
Experimental Animal Ethics Committee of Jilin University (approval
nos. SY201905008 and SY201905003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APC
|
adenomatous polyposis coli
|
|
CRC
|
colorectal cancer
|
|
DAI
|
disease activity index
|
|
DSS
|
dextran sodium sulfate
|
|
Evo
|
Evodiamine
|
|
GO
|
Gene Ontology
|
|
IBD
|
Inflammatory bowel disease
|
|
IKKα+β
|
inhibitor of NF-κB kinase α+β
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
MD
|
molecular dynamics
|
|
MMP
|
mitochondrial membrane potential
|
|
MyD88
|
myeloid differentiation factor 88
|
|
SASP
|
Sulfasalazine
|
|
TLR4
|
Toll-like receptor 4
|
|
UC
|
ulcerative colitis
|
References
|
1
|
Yue B, Ren YJ, Zhang JJ, Luo XP, Yu ZL,
Ren GY, Sun AN, Deng C, Wang ZT and Dou W: Anti-inflammatory
effects of fargesin on chemically induced inflammatory bowel
disease in mice. Molecules. 23:13802018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Wei L, Wang J, Qin Z, Wang J, Lu
Y, Zheng X, Peng Q, Ye Q, Ai F, et al: Suppression colitis and
colitis-associated colon cancer by anti-S100a9 antibody in mice.
Front Immunol. 8:17742017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernstein CN, Fried M, Krabshuis JH, Cohen
H, Eliakim R, Fedail S, Gearry R, Goh KL, Hamid S, Khan AG, et al:
World gastroenterology organization practice guidelines for the
diagnosis and management of IBD in 2010. Inflamm Bowel Dis.
16:112–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao Y, Guo Q, Zhao K, Zhou Y, Li W, Pan
C, Qiang L, Li Z and Lu N: Small molecule GL-V9 protects against
colitis-associated colorectal cancer by limiting NLRP3 inflammasome
through autophagy. Oncoimmunology. 7:e13756402017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doubeni CA, Laiyemo AO, Major JM,
Schootman M, Lian M, Park Y, Graubard BI, Hollenbeck AR and Sinha
R: Socioeconomic status and the risk of colorectal cancer: An
analysis of more than a half million adults in the national
institutes of health-AARP diet and health study. Cancer.
18:3636–3644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Assadsangabi A and Lobo AJ: Diagnosing and
managing inflammatory bowel disease. Practitioner. 257:13–18.
22013.PubMed/NCBI
|
|
8
|
Porta C, Ippolito A, Consonni FM, Carraro
L, Celesti G, Correale C, Grizzi F, Pasqualini F, Tartari S,
Rinaldi M, et al: Protumor steering of cancer inflammation by p50
NF-κB enhances colorectal cancer progression. Cancer Immunol Res.
6:578–593. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vilarrasa Rull E and González Lama Y:
Clinical features of hidradenitis suppurativa and Crohn disease:
What do these two entities have in common? Actas Dermosifiliogr.
107 (Suppl 2):S21–S26. 2016.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pichai MV and Ferguson LR: Potential
prospects of nanomedicine for targeted therapeutics in inflammatory
bowel diseases. World J Gastroenterol. 18:2895–2901. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo CX and Zhang H: The role of
proinflammatory pathways in the pathogenesis of colitis-associated
colorectal cancer. Mediators Inflamm. 2017:51260482017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bressenot A, Cahn V, Danese S and
Peyrin-Biroulet L: Microscopic features of colorectal neoplasia in
inflammatory bowel diseases. World J Gastroenterol. 20:3164–3172.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho HT, Kim JH, Heo W, Lee HS, Lee JJ,
Park TS, Lee JH and Kim YJ: Explosively puffed ginseng ameliorates
ionizing radiation-induced injury of colon by decreasing oxidative
stress-related apoptotic cell execution in mice. J Med Food.
22:490–498. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan L, Wu R, Ye L, Wang H, Yang X, Zhang
Y, Chen X, Zuo G, Zhang Y, Weng Y, et al: S100A8 and S100A9 are
associated with colorectal carcinoma progression and contribute to
colorectal carcinoma cell survival and migration via Wnt/β-catenin
pathway. PLoS One. 8:e620922013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Y, Li CS and Dong Q: Chinese herb
related molecules of cancer-cell-apoptosis: A minireview of
progress between Kanglaite injection and related genes. J Exp Clin
Cancer Res. 27:312008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang J and Hu C: Evodiamine: A novel
anti-cancer alkaloid from Evodia rutaecarpa. Molecules.
14:1852–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Z, He X, Han W, Chen X, Liu P, Zhao
X, Wang X, Zhang L, Wu S and Zheng X: Genus tetradium L.: A
comprehensive review on traditional uses, phytochemistry, and
pharmacological activities. J Ethnopharmacol. 231:337–354. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu H, Jin H, Gong W, Wang Z and Liang H:
Pharmacological actions of multi-target-directed evodiamine.
Molecules. 18:1826–1843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen P, Zhang Z, Zhu K, Cao H, Liu J, Lu
X, Li Y, Jing Y, Yuan X, Fu Y, et al: Evodiamine prevents dextran
sulfate sodium-induced murine experimental colitis via the
regulation of NF-κB and NLRP3 inflammasome. Biomed Pharmacother.
110:786–795. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cima G: AVMA guidelines for the euthanasia
of animal. (2013 Edition). JAVMA J Am Vet Med Assoc. 242:715–716.
2013.PubMed/NCBI
|
|
21
|
Kim JJ, Shajib MS, Manocha MM and Khan WI:
Investigating intestinal inflammation in DSS-induced model of IBD.
J Vis Exp. 60:36782012.
|
|
22
|
Teng S, Hao J, Bi H, Li C, Zhang Y, Zhang
Y, Han W and Wang D: The protection of crocin against ulcerative
colitis and colorectal cancer via suppression of NF-κB-mediated
inflammation. Front Pharmacol. 12:6394582021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin HS, Satsu H, Bae MJ, Zhao Z, Ogiwara
H, Totsuka M and Shimizu M: Anti-inflammatory effect of chlorogenic
acid on the IL-8 production in Caco-2 cells and the dextran
sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food
Chem. 168:167–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dou B, Hu W, Song M, Lee RJ, Zhang X and
Wang D: Anti-inflammation of Erianin in dextran sulphate
sodium-induced ulcerative colitis mice model via collaborative
regulation of TLR4 and STAT3. Chem Biol Interact. 324:1090892020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ficarro SB, McCleland ML, Stukenberg PT,
Burke DJ, Ross MM, Shabanowitz J, Hunt DF and White FM:
Phosphoproteome analysis by mass spectrometry and its application
to saccharomyces cerevisiae. Nat Biotechnol. 20:301–305. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Wang J, Wang C, Li Z, Liu X,
Zhang J, Lu J and Wang D: Pharmacological Basis for the Use of
Evodiamine in Alzheimer's Disease: Antioxidation and Antiapoptosis.
Int J Mol Sci. 19:15272018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lecoq L, Raiola L, Chabot PR, Cyr N,
Arseneault G, Legault P and Omichinski JG: Structural
characterization of interactions between transactivation domain 1
of the p65 subunit of NF-κB and transcription regulatory factors.
Nucleic Acids Res. 45:5564–5576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Norgan AP, Coffman PK, Kocher JP, Katzmann
DJ and Sosa CP: Multilevel parallelization of AutoDock 4.2. J
Cheminform. 3:122011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu J, Li X, Zhang S, Ye H, Zhao H, Jin H
and Han W: Exploring stereochemical specificity of
phosphotriesterase by MM-PBSA and MM-GBSA calculation and steered
molecular dynamics simulation. J Biomol Struct Dyn. 35:3140–3151.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee TS, Hu Y, Sherborne B, Guo Z and York
DM: Toward fast and accurate binding affinity prediction with
pmemdGTI: An efficient implementation of GPU-accelerated
thermodynamic integration. J Chem Theory Comput. 13:3077–3084.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hermosilla L, Prampolini G, Calle P,
García de la Vega JM, Brancato G and Barone V: Extension of the
AMBER force field for nitroxide radicals and combined QM/MM/PCM
approach to the accurate determination of EPR parameters of DMPO-H
in solution. J Chem Theory Comput. 9:3626–3636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Li Y, Shen P, Li S, Lu X, Liu J,
Cao Y, Liu B, Fu Y and Zhang N: Administration of geniposide
ameliorates dextran sulfate sodium-induced colitis in mice via
inhibition of inflammation and mucosal damage. Int Immunopharmacol.
49:168–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
34
|
He J, Yang A, Zhao X, Liu Y, Liu S and
Wang D: Anti-colon cancer activity of water-soluble polysaccharides
extracted from Gloeostereum incarnatum via Wnt/β-catenin signaling
pathway. Food Sci Hum Wellness. 10:460–470. 2021. View Article : Google Scholar
|
|
35
|
Lasry A, Zinger A and Ben-Neriah Y:
Inflammatory networks underlying colorectal cancer. Nat Immunol.
17:230–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Uttarawichien T, Khumsri W, Suwannalert P,
Sibmooh N and Payuhakrit W: Onion peel extract inhibits cancer cell
growth and progression through the roles of L1CAM, NF-κB, and
angiogenesis in HT-29 colorectal cancer cells. Prev Nutr Food Sci.
26:330–337. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu YH, Gu L, Li YJ, Lin X, Shen H, Cui K,
Chen L, Zhou F, Zhao Q, Zhang J, et al: miR-148a inhibits colitis
and colitis-associated tumorigenesis in mice. Cell Death Differ.
24:2199–2209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim HY, Jeon H, Bae CH, Lee Y, Kim H and
Kim S: Rumex japonicus Houtt. Alleviates dextran sulfate
sodium-induced colitis by protecting tight junctions in mice.
Integr Med Res. 9:1003982020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng F, Zhang Y, Li Q, Zeng F and Wang K:
Inhibition of dextran sodium sulfate-induced experimental colitis
in mice by angelica sinensis polysaccharide. J Med Food.
23:584–592. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
West NR, McCuaig S, Franchini F and Powrie
F: Emerging cytokine networks in colorectal cancer. Nat Rev
Immunol. 15:615–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L and Karin M: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nikolaus S and Schreiber S: Treatment of
inflammatory bowel disease. Dtsch Med Wochenschr. 138:205–208.
2013.PubMed/NCBI
|
|
44
|
Mitselou A, Grammeniatis V, Varouktsi A,
Papadatos SS, Katsanos K and Galani V: Proinflammatory cytokines in
irritable bowel syndrome: A comparison with inflammatory bowel
disease. Intest Res. 18:115–120. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han S, Yoon K, Lee K, Kim K, Jang H, Lee
NK, Hwang K and Young Lee S: TNF-related weak inducer of apoptosis
receptor, a TNF receptor superfamily member, activates NF-kappa B
through TNF receptor-associated factors. Biochem Biophys Res
Commun. 305:789–796. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Khan MR, Uwada J, Yazawa T, Islam MT, Krug
SM, Fromm M, Karaki S, Suzuki Y, Kuwahara A, Yoshiki H, et al:
Activation of muscarinic cholinoceptor ameliorates tumor necrosis
factor-α-induced barrier dysfunction in intestinal epithelial
cells. FEBS Lett. 589:3640–3647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bruno ME, Rogier EW, Arsenescu RI,
Flomenhoft DR, Kurkjian CJ, Ellis GI and Kaetzel CS: Correlation of
biomarker expression in colonic mucosa with disease phenotype in
crohn's disease and ulcerative colitis. Dig Dis Sci. 60:2976–2984.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Park SY, Ku SK, Lee ES and Kim JA:
1,3-Diphenylpropenone ameliorates TNBS-induced rat colitis through
suppression of NF-κB activation and IL-8 induction. Chem Biol
Interact. 196:39–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Durko Ł, Stasikowska-Kanicka O,
Wagrowska-Danilewicz M, Danilewicz M and Malecka-Panas E:
Expression of epithelial growth factor receptor, tumor necrosis
factor-α and nuclear factor κB in inflammatory bowel diseases. Prz
Gastroenterol. 8:262–267. 2013.PubMed/NCBI
|
|
50
|
Tesoriere L, Attanzio A, Allegra M,
Gentile C and Livrea MA: Indicaxanthin inhibits NADPH oxidase
(NOX)-1 activation and NF-κB-dependent release of inflammatory
mediators and prevents the increase of epithelial permeability in
IL-1β-exposed Caco-2 cells. Br J Nutr. 111:415–423. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hundorfean G, Neurath MF and Mudter J:
Functional relevance of T helper 17 (Th17) cells and the IL-17
cytokine family in inflammatory bowel disease. Inflamm Bowel Dis.
18:180–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
De Simone V, Franzè E, Ronchetti G,
Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald
TT, Pallone F, et al: Th17-type cytokines, IL-6 and TNF-α
synergistically activate STAT3 and NF-kB to promote colorectal
cancer cell growth. Oncogene. 34:3493–3503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aranda CJ, Ocón B, Arredondo-Amador M,
Suárez MD, Zarzuelo A, Chazin WJ, Martínez-Augustin O and Sánchez
de Medina F: Calprotectin protects against experimental colonic
inflammation in mice. Br J Pharmacol. 175:3797–3812. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kwon CH, Moon HJ, Park HJ, Choi JH and
Park DY: S100A8 and S100A9 promotes invasion and migration through
p38 mitogen-activated protein kinase-dependent NF-κB activation in
gastric cancer cells. Mol Cells. 35:226–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luo X, Yue B, Yu Z, Ren Y, Zhang J, Ren J,
Wang Z and Dou W: Obacunone protects against ulcerative colitis in
mice by modulating gut microbiota, attenuating TLR4/NF-κB signaling
cascades, and improving disrupted epithelial barriers. Front
Microbiol. 11:4972020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shi YJ, Hu SJ, Zhao QQ, Liu XS, Liu C and
Wang H: Toll-like receptor 4 (TLR4) deficiency aggravates dextran
sulfate sodium (DSS)-induced intestinal injury by down-regulating
IL6, CCL2 and CSF3. Ann Transl Med. 7:7132019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Han W, Zhu J, Wang S and Xu D:
Understanding the phosphorylation mechanism by using quantum
chemical calculations and molecular dynamics simulations. J Phys
Chem B. 121:3565–3573. 2017. View Article : Google Scholar : PubMed/NCBI
|