Introduction
The world is experiencing aging populations
regardless of development level (1). Projections suggest twice as a number
of individuals >60 years by 2050, fueled by annual increases to
this population (2). Population
aging is a major issue with extensive consequences for society as a
whole (1). As the elderly
population increases, sarcopenia, the gradual reduction of muscle
mass, strength and function with age (3–5),
arises as a major risk factor (6).
The root of Panax ginseng (Ginseng
Radix, Ginseng) is a promising herb with a number of active
ingredients. Ginsenosides, or saponins, are the main active
ingredients of Ginseng. These gensenosides are further categorized
as protopanaxadiols and protopanaxatriols, represented by
ginsenoside Rb1 and ginsenoside Rg1, respectively. Along with these
ginsenosides, P. ginseng also contains alkaloids,
polysaccharides, essential oil components and phenol and nitrogen
compounds (7).
In traditional Korean medicine, it is believed that
the herb wild ginseng can help restore energy to the elderly
(8). Cultivated ginseng can also
be used but wild ginseng is considered an improved option for
several reasons. It has both higher ginsenoside Rb1 and Rg1 content
(9) and, according to proteomic
analysis, higher amino acid, amino acid-related enzyme and protein
and derivative content (10).
Reports also indicate that wild ginseng may be an antioxidant, an
anti-inflammatory and an anticancer agent while also having
antidiabetic properties (11,12). Due to its diverse biological
properties, it may play a key role in treating diseases that
develop later in life such as sarcopenia with muscle atrophy.
There are several different methods for
administering ginseng in TKM. P. Ginseng has traditionally
been made into a beverage by boiling its decoction in water for
several hours (13), but
solvents, such as water and alcohol, have recently been employed to
concentrate the ginseng extract (14). These oral intake methods for
ginseng have been proven to be clinically effective and safe
(15,16). A new method for administering
ginseng in TKM is through intramuscular or intravenous injection at
acupoints or non-acupoints using pharmacopuncture, a combination of
acupuncture and herbal medicine. This acupuncture technique uses
filtered and sterilized injections of herbal extracts obtained
through various herbal medicine-dependent extraction methods, such
as distillation, alcohol immersion and compression (17).
Wild ginseng pharmacopuncture (WGP) is a common
clinical practice in TKM to cure fatigue and poor functionality of
organs associated with disease (17). There are American wild ginseng
pharmacopuncture (AWGP) and Korean cultivated wild ginseng
pharmacopuncture (KCWGP) types. There have been reports confirming
the effectiveness and safety of pharmacopuncture with KCWG. A
review of an animal model injected with hydrocortisone acetate
reported that ginseng pharmacopuncture may help prevent disease and
enhance immune responses (18).
In addition, there have been several studies on P. ginseng
pharmacopuncture toxicity and animal safety (19,20). In a review of P. ginseng
pharmacopuncture (7), it has been
reported to affect the cardiovascular system and protein synthesis
in humans, to be non-toxic in animals and humans in 25 case
reports, to significantly improve the clinical outcomes of patients
with serious conditions, including cancer and amyotrophic lateral
sclerosis, to minor conditions, such as skin wrinkles and allergic
rhinitis. Previous studies have also reported that muscle injuries
and inflammation are affected by Panax ginseng extract (21,22). In a previous case study involving
two elderly individuals, a combination acupuncture containing wild
ginseng increased muscle-related metrics, such as muscle/fat ratio
and body metabolic rate (23).
However, studies on the muscle-related uses of WGP are rare.
Moreover, the evidence for AWGP is lacking.
Therefore, to further evaluate how AWGP and KCWGP
may be beneficial to muscle function, the present study
investigated the regulation of myogenic and mitochondrial biogenic
factors in C2C12 mouse skeletal muscle cells via activation of the
AMPK and PI3K/Akt/mTOR signaling pathways post-AWGP and KCWGP
treatment.
Materials and methods
Preparation of AWGP and KCWGP
Wild ginseng extract was prepared by external herbal
dispensaries (EHDs) adhering to Korean Good Manufacturing Practice
(K-GMP) standards (24). The AWGP
extract was prepared from American wild ginseng (AWG;
Woominnongsan, Chungbuk, Korea) at Namsangcheon EHD, and the KCWGP
extract was prepared from EHD (Yongin, Korea) with Korean
cultivated wild ginseng (KCWG; Cheonbangnongsan, Seocheon, Korea).
A small piece of ~100 g (for AWG) or 120 g (for KCWG) of wild
ginsengs was extracted in a distillation extractor with 1,000 ml of
distilled water, filtered through a two-layer mesh and dissolved by
stirring with 0.9% NaCl, titrated to pH 7.4. Each extract was
re-filtered through a 0.45 µm syringe filters, sterilized and
sealed in a vial (1 mg/ml).
AWGP and KCWGP extracts in vials were stored at 4°C
until use in the study.
Cell culture and treatments
Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS)
and a penicillin/streptomycin mix (Invitrogen; Thermo Fisher
Scientific, Inc.) as supplements were used to maintain mouse C2C12
myoblasts (CRL-1772; ATCC) in 5% CO2 incubator at 37°C.
Upon becoming confluent, the cells were transferred to a
differentiation medium (DMEM supplemented with 2% horse serum;
Invitrogen; Thermo Fisher Scientific, Inc.) for 5 days to induce
myotube differentiation. The C2C12 myotubes underwent several
possible treatments. They were either treated with AWGP extract or
KCWGP extract at concentrations of 0.5, 1, or 2 mg/ml, with no
treatment at all, or with 2.5 mM of metformin as a reference
drug.
Cell viability assay
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) colorimetric assay was used to verify
cell viability. C2C12 myoblasts were cultured in 96-well plates
(1×104 cells/well) overnight in 5% CO2
incubator at 37°C. Then over a period of 24 h in 5% CO2
incubator at 37°C myoblasts received varying concentrations of
either AWGP or KCWGP extract. The medium of each well was discarded
after treatment and replaced with a 0.5 mg/ml MTT containing medium
followed by a 4 h incubation at 37°C. The formazan crystals were
dissolved by replacing the culture medium with DMSO in equal
volumes and shaking at room temperature (RT) for 15 min. Finally, a
microplate reader (Asys) was used to determine well absorbance at
570 nm.
Western blot
Ice-cold lysis buffer (0.1 ml of 50 mM Tris-HCl (pH
7.2) containing 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate
(SDS), 0.15 M NaCl and 1% NP-40) was used to lyse the cells, which
were then centrifuged at 12,000 × g for 20 min at 4°C. A
bicinchoninic acid (BCA) assay was used to determine the protein
content. Electrophoresis through 10% SDS-acrylamide gels was used
to separate equal amounts of protein (20 µg/ml). An electrical
transfer system was then used to relocate the proteins to
nitrocellulose membranes. The membranes received a treatment of 3%
skimmed milk in TBST buffer (5 mM Tris-HCl, pH 7.6, 136 mM NaCl and
0.1% Tween-20) for 1 h at RT to block non-specific binding.
The membranes underwent incubation with primary
antibodies anti-peroxisome proliferator-activated receptor-γ
coactivator-1-α (PGC-1α, 1:1,000; cat. no. NBP1-04676; Bioss
Antibodies), anti-mitochondrial transcription factor A (TFAM,
1:1,000; cat. no. PA5-68789; Thermo Fisher Scientific, Inc.),
anti-nuclear respiratory factor-1 (NRF-1, 1:1,000; cat. no. 69432s;
Cell Signaling Technology, Inc.), anti-phospho-AMPK (1:500; cat.
no. 44-11509; Thermo Fisher Scientific, Inc.), anti-AMPK (1:500;
cat. no. AHO1332; Thermo Fisher Scientific, Inc.), anti-Sirtuin 1
(SIRT1, 1:1,000; cat. no. 69432s; Cell Signaling Technology, Inc.),
anti-myosin heavy chain (MyHC, 1:1,000; cat. no. sc-376157; Santa
Cruz Biotechnology, Inc.), anti-myoblast determination protein 1
(1:1,000; MyoD; cat. no. sc-377460; Santa Cruz Biotechnology,
Inc.), anti-Myostatin (1:1,000; cat. no. PA5-11936, Invitrogen),
anti-Myogenin (1:1,000; cat. no. sc-377460; Santa Cruz
Biotechnology, Inc.), anti-phospho-AKT (1:500; cat. no. AF887;
R&D Systems, Inc.), anti-AKT (1:500; cat. no. 9272s; Cell
Signaling Technology, Inc.), anti-mTOR (1:500; cat. no. 29725; Cell
Signaling Technology, Inc.) and anti-β-Actin (1:1,000;
MilliporeSigma) overnight at 4°C. The membranes then underwent
incubation at RT for 1 h with horseradish peroxidase-labeled
anti-mouse immunoglobulin G (IgG, 1:1,000; cat no. sc-2005; Santa
Cruz Biotechnology, Inc.). Following incubation, the blots were
washed in 1X TBST three times. Then ECL western detection reagents
(cat no. 1705061; Bio-Rad Laboratories, Inc.) were used for
development and densitometry was used to compare the protein bands
in ImageJ software (1.47J, National Institutes of Health).
Immunocytochemistry
C2C12 myoblasts were cultured in DMEM with 10% FBS
in 5% CO2 at 37°C and was performed to differentiate the
C2C12 myoblasts for 5 days by first seeding them onto Thermanox
plastic coverslips (Nunc; Thermo Fisher Scientific, Inc.).
Following drug treatment, 1X PBS was used to wash the samples on
coverslips. Then 10 min treatment of 4% paraformaldehyde was used
to fix them and a 20 min treatment of 0.1% Triton X-100
(MilliporeSigma) was used to permeabilize them at RT. Another rinse
with 1X PBS was performed and a 30 min treatment at RT with 5%
bovine serum albumin (BSA) was performed to block the coverslips
followed by an overnight incubation with anti-MyHC antibody (1:200;
cat. no. sc-376157; Santa Cruz Biotechnology, Inc.) at 4°C. Next, a
1 h treatment with Alexa Fluor 488-conjugated goat anti-rabbit
antibody (1:500; cat. No. A11011; Thermo Fisher Scientific. Inc.)
at RT was used to label the coverslips and then a 5 min DAPI
treatment was used to counterstain at RT. Lastly, observation of
MyHC expression was performed 24 h after drug treatment with a
fluorescence microscope (Leica DM2500; Leica Microsystems
GmbH).
High-performance liquid chromatography
(HPLC)
The AWGP and KCWGP constituents were identified via
HPLC with standard compounds, rg1, rb1, rg3 and rh2. HPLC analysis
was performed at a wavelength of 203 nm and a flow rate of 0.6
ml/min using Waters HPLC (Waters Corporation) as an instrument and
YMC 3 µm, 4.6×150 mm (AQ; YMC Korea Co., Ltd.) as a column.
An acetonitrile (B) and water (A) gradient solvent
system was used for chromatographic separation using the following
gradient solvent system procedure at RT: 0 min, 20% B; 4 min, 22%
B; 20 min, 33% B; 26 min, 38% B; 40 min, 38% B; 58 min, 50% B; 68
min, 50% B; 70 min, 60% B; 75 min, 60% B; 80 min, 20% B and 90 min,
20% B. The standards mixture (rg1, rb1, rg3 and rh2) was injected
at a volume of 10 µl and a concentration of 0.5 mg/ml and the AWGP
and KCWGP medicinal solutions were injected at a volume of 100
µl.
Data analysis
For consistency, each experiment was repeated three
times with results given as mean ± standard error of mean. One-way
ANOVA and a Tukey's test were performed with GraphPad Prism
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxic effect of AWGP and KCWGP in
C2C12 cells
A cell viability assay was performed to determine
the cytotoxicity of AWGP and KCWGP. The results showed that
treatment of C2C12 cells stimulated with AWGP and KCWGP at a
concentration of 10 mg/ml did not affect cell viability (Fig. 1). In the present study, AWGP and
KCWGP were used at concentrations of 0.5, 1 and 2 mg/ml in C2C12
myotubes.
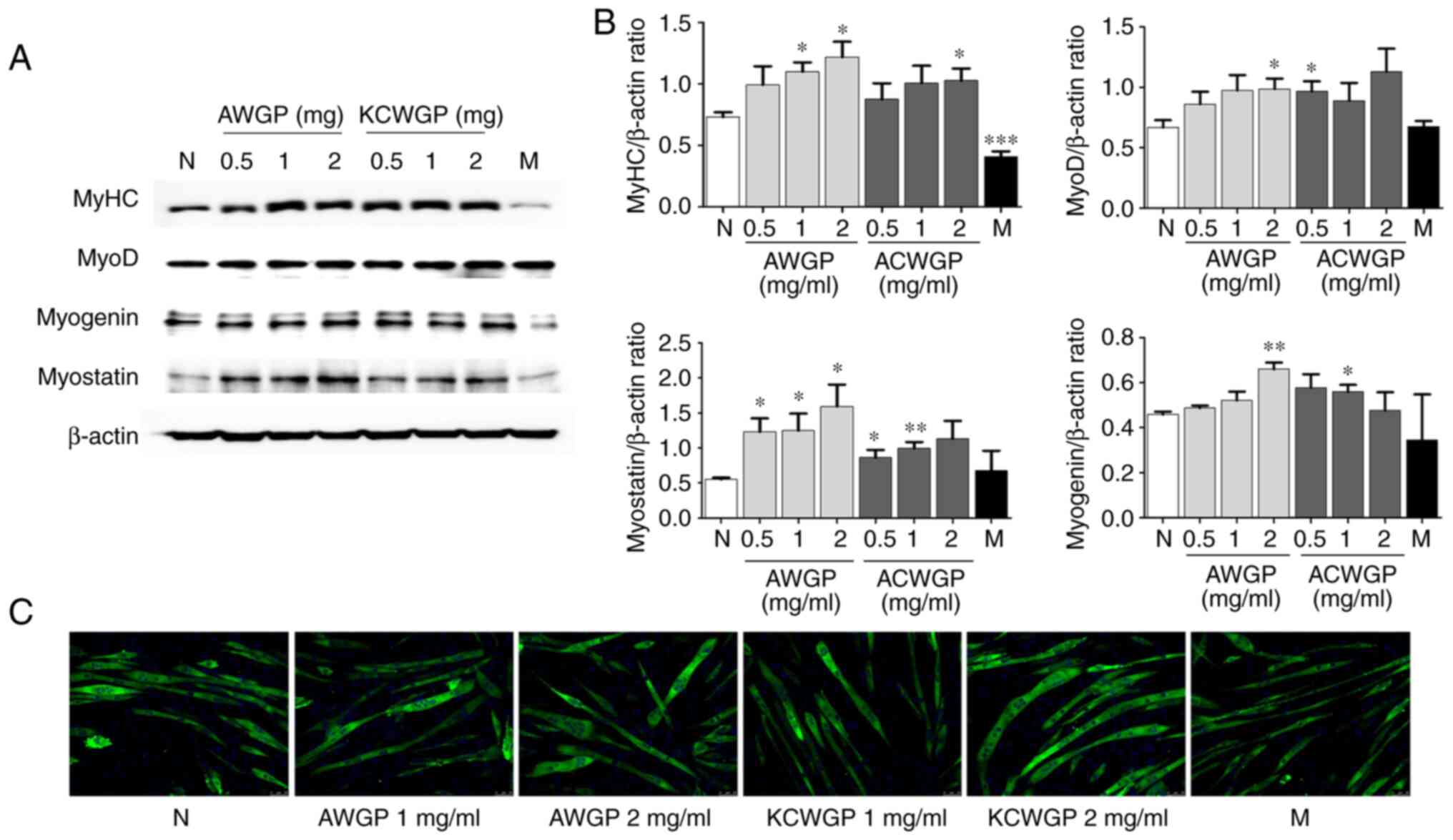
Effects of AWGP and KCWGP on
myogenesis-regulating protein expression in C2C12 myotubes
AWGP and KCWGP's influence on myoblast
differentiation into myotubes was observed using western blotting
to detect myogenesis-regulating proteins, such as MyHC, myostatin,
MyoD and myogenin, in C2C12 myotubes.
The C2C12 myotubes treated with AWGP had higher
MyHC, myostatin, MyoD and myogenin protein expression compared with
the untreated cells. Furthermore, the increases were proportional
to the concentrations of AWGP. The C2C12 myotubes treated with
KCWGP also showed significant increases in MyHC, myostatin, MyoD
and myogenin protein expression compared with the untreated cells,
but only the increases in MyHC and myostatin protein expression
were dose-dependent.
MyHC expression increased significantly in samples
treated with 1 and 2 mg/ml AWGP (P<0.05, respectively), 2 mg/ml
KCWGP (P<0.05) and metformin (P<0.001). MyoD expression
increased significantly in samples treated with 2 mg/ml AWGP
(P<0.05), 0.5 mg/ml AWGP (P<0.01) and 0.5 mg/ml KCWGP
(P<0.05) (Fig. 2A). Myostatin
expression increased significantly in samples treated with all
concentrations of AWGP (P<0.05) and 1 and 2 mg/ml KCWGP
(P<0.05 and P<0.01, respectively). Lastly, myogenin
expression increased significantly in the samples treated with 2
mg/ml AWGP (P<0.01) and 1 mg/ml KCWGP (P<0.05) (Fig. 2B).
The immunocytochemical staining (Fig. 2C) showed that C2C12 myotubes
differentiated into long, wide cylinders with multiple nuclei due
to an increase in MyHC expression following treatment with AWGP or
KCWGP. This change occurred in a dose-dependent manner.
Metformin-treated cells showed an increase in MyHC expression, but
less than in the treatment of AWGP and KCWGP. Therefore, it appears
that muscle differentiation stimulation may be aided by AWGP and
KCWGP (Fig. 2C).
Effects of AWGP and KCWGP on
mitochondrial biogenesis-regulating protein expression in C2C12
myotubes
The influence of AWGP and KCWGP on muscle cell
biogenesis in C2C12 myotubes was confirmed using western blotting
to observe the expression of the mitochondrial biogenesis
regulators, PGC-1α, NRF-1, TFAM and Sirt-1. C2C12 myotubes samples
that were treated with either AWGP or KCWGP had higher expression
of all four regulatory proteins compared with the untreated cells.
PGC-1α expression increased significantly in samples treated with
every experimental dosage of both AWGP (P<0.05) and KCWGP
(P<0.05). Sirt-1 expression increased significantly in samples
treated with 2 mg/ml AWGP (P<0.05) and 1 mg/ml KCWGP
(P<0.05). NRF-1 expression increased significantly only in
samples treated with 0.5 and 1 mg/ml KCWGP (P<0.05). Finally,
TFAM expression increased significantly in samples treated with 1
mg/ml AWGP (P<0.05) and all experimental dosages of KCWGP
(P<0.05). As these four regulatory proteins all showed increased
expression with AWGP and KCWGP treatments, these treatments may be
involved in upregulating transcription factors to improve
mitochondrial biogenesis (Fig.
3).
 | Figure 3.Effect of AWGP and KCWGP on
mitochondrial biogenesis in C2C12 myotubes. C2C12 myoblasts were
differentiated into myotubes for 5 days and then treated with AWGP
or KCWGP (0.1, 1 or 2 mg/ml) or with metformin (2.5 mM) for 45 min.
(A) The expressions of PGC1α, Sirt-1, NRF-1 and TFAM proteins were
analyzed by western blotting. (B) The density of each target was
calculated with the expression of β-actin. The values from the
triplicate of experiments are given as the mean ± standard error of
mean. *P<0.05 vs. untreated cells (N). AWGP, American wild
ginseng pharmacopuncture; KCWGP, Korean cultivated wild ginseng
pharmacopuncture; PGC1α, peroxisome proliferator-activated
receptor-γ coactivator-1-α; Sirt-1, Sirtuin 1; NRF-1, nuclear
respiratory factor-1; TFAM, mitochondrial transcription factor A;
M, metformin-treated cells; N, untreated cells. |
Effects of AWGP and KCWGP on the AMPK
and PI3K/AKT/mTOR pathways in C2C12 myotubes
The influence of AWGP and KCWGP on the AMPK and
PI3K/Akt/mTOR signaling pathways, which activate mitochondrial
biogenesis in C2C12 myotubes, was investigated.
Myotubes treated with AWGP increased the expression
of PI3K (Fig. 4B) and
phosphorylation of AMPK (Fig.
4A), Akt (Fig. 4B) and mTOR
(Fig. 4C) compared with the
untreated cells in a dose-dependent manner. The treatment of KCWGP
treatment increased the expression of PI3K (Fig. 4B) and phosphorylation of AMPK
(Fig. 4A), Akt (Fig. 4B) and mTOR (Fig. 4C) compared with the untreated
cells and there was little difference between the KCWGP
concentrations.
AMPK phosphorylation increased significantly in
samples treated with 1 mg/ml AWGP (P<0.01), 2 mg/ml AWGP
(P<0.001), 0.5 mg/ml KCWGP (P<0.01), 1 mg/ml KCWGP
(P<0.05), 2 mg/ml KCWGP (P<0.01) and metformin (P<0.01).
Akt phosphorylation increased significantly when samples were
treated with 0.5 mg/ml AWGP (P<0.05), 1 mg/ml AWGP (P<0.01),
2 mg/ml AWGP (P<0.01), all concentrations of KCWGP (P<0.05)
and metformin (P<0.01). PI3K expression increased significantly
when samples were treated with all experimental concentrated of
both AWGP and KCWGP (P<0.05) as well as when treated with
metformin (P<0.01). mTOR phosphorylation increased significantly
when samples were treated with all experimental concentrations of
AWGP (P<0.01) and KCWGP (P<0.05). These increases in
phosphorylation imply AWGP and KCWGP may aid in activating the AMPK
and PI3K/AKT/mTOR pathways and enhancing mitochondrial biogenesis
in C2C12 myotubes.
HPLC analysis of AWGP and KCWGP
To identify the constituents of AWGP and KCWGP, HPLC
analysis was performed with standard compounds, rg1, rb1, rg3 and
rh2 (Fig. 5A).
In AWGP (Fig. 5B)
and KCWGP (Fig. 5C), the expected
peaks from rb1, rg1 and rg3 were found, but not the expected peak
from rh2. In the case of KCWGP, a small peak, which was considered
to be an rg3 component, was observed and the rg3 component is
expected to be contained in a trace amount.
Discussion
The regulation of carbohydrate metabolism and the
balancing of energy in the body given proper nutrition is achieved
in skeletal muscles (25). Within
these muscles are mitochondria, which not only supply ATP as
cellular energy (26), but also
can aid in the metabolism of amino acid and the homeostasis of ions
(27). As mitochondria have
diverse roles in moving the myoblasts from the glycolytic state in
muscles (28), the upregulation
of mitochondrial functions appear to promote myoblast
differentiation and muscle functions (29).
The C2C12 myoblast cell line is often used for
investigating skeletal muscle atrophy through in vitro
modeling (30,31). This cell line can easily
differentiate into myotubes in low-serum medium through the
regulation of the myogenic differentiation gene (MyoD)
transcription factor family (32), which includes the myogenic
regulatory factors (MRFs), myogenic factor 5 (Myf5), MyoD and
myogenin (32). MRF activation
causes myoblasts to fuse into myotubes (32). Below the basal membrane of muscle
tissue are the inactive skeletal muscle-derived stem cells required
for myogenesis (33). Upon
activation, they begin myogenic differentiation and proceed through
a cascade of MRFs and structural muscle proteins, including MyHC,
which is a muscle thick filament motor protein that can be used to
indicate maturation (34,35). Thus, MRFs activation causes
myoblasts to fuse into myotubes. First, Myf5 is initially expressed
after satellite cells are activated and then MyoD and myogenin are
sequentially expressed in the newly formed fibers (33). However, in the present study,
treatments of AWGP and KCWGP extracts to C2C12 myotubes
significantly increased MyHC, myostatin, MyoD and myogenin
expression resulting in changes in myotube morphology. This
increased expression implies that myoblast differentiation into
myotubes in muscles can be stimulated with AWGP and KCWGP. Since
the expression of MyoD, myogenin, MyHC and myostatin increased
after treatment with AWGP and KCWGP, these treatments may help
stimulate myoblast differentiation into myotubes in muscle
cells.
Metformin, one of the biguanide drugs, is a
well-known medicine for the treatment of type 2 diabetes as an
anti-hyperglycemic and insulin sensitizing agent. It has been
reported that metformin treatment in C2C12 cells increases the
myotube diameter at 48 h during differentiation (36). In the present study, metformin
decreased MyHC expression in C2C12 myotubes and showed
differentially altered morphology with a longer and thinner myotube
compared with AWGP and KCWGP-treated cells. However, further study
is needed on the differences between AWGP or KCWGP and metformin to
myoblast differentiation.
The PGC1α/SIRT1/AMPK pathways are integral to
mitochondrial biogenesis and the metabolism of glucose in skeletal
muscle (37–39). A number of natural compounds have
been reported to increase mitochondrial biosynthesis and oxidative
phosphorylation through activation of PGC1α, AMPK and SIRT1,
thereby increasing energy expenditure in skeletal muscle (40,41). PGC1α increases the rate of
skeletal muscle respiration and mitochondrial biogenesis, thereby
increasing the consumption of energy (42) and it also interacts with NRF-1, a
transcription factor for a number of mitochondrial genes, including
TFAM, a direct regulator of mitochondrial DNA replication and
transcription (43). In the
present study, PGC1α, NRF-1 and TFAM increased according to the
AWGP and KCWGP treatment concentrations in C2C12 myotubes, but the
change of mitochondrial quantity such as mitochondrial DNA levels
by treatment of AWGP and KCWGP should be further identified to
advance the understanding of the role of drugs on energy metabolism
in muscle.
By providing energy as ATP to cells, mitochondrial
biogenesis ultimately promotes AMPK activation (44). It has been reported that in
skeletal muscle, AMPK, p38 MAPK and AKT activate PGC-1α by changing
the phosphorylation status of residues at distinct sites in the
protein (45). AMPK is
excessively involved in the metabolic condition of skeletal muscle
through its regulation of a number of downstream targets such as
the PI3K/AKT pathway (46). Due
to their effects on anabolic and catabolic cellular processes, AMPK
serves a major role in controlling skeletal muscle development and
growth (46) and in regulating
muscle mass and regeneration (47). AKT has been known to be an
important regulator of both glucose transport (48,49) and activation of glycogen synthesis
in skeletal muscle (50). mTOR is
the main regulator in maintaining muscle mass through protein
synthesis as it controls the balance between metabolic and
catabolic processes. Therefore, the PI3K/Akt/mTOR pathway is
important for muscle maintenance in aged muscles (51). In aging-associated conditions, it
has been reported that ginseng and its bioactive compounds,
ginsenoside Rb1 and Rg3, have anti-aging effects with the molecular
mechanisms such as PPAR-α, GLUTs, FOXO1, caspase-3 and Bcl-2 along
with SIRT1/AMPK, PI3K/Akt, NF-κB and insulin/insulin-like growth
factor-1 pathways as preferential targets (52). Since the AWGP and KCWGP treatments
activated the AMPK and PI3K/Akt/mTOR signaling pathways, they may
increase mitochondrial biogenesis in skeletal muscle cells, thereby
aiding in muscle maintenance in aged muscles.
In the present study, peaks predicted to be rb1, rg1
and rg3 were found in AWGP and KCWGP, but peaks predicted to be rh2
were not found. KCWGP was reported to contain both ginsenoside Rg1
and ginsenoside Rb2 as well as phenolic compounds while having
extraction-dependent variance in the amounts of each substance
(53). American ginseng (P.
quinquefolium) is reported to have the most ginsenoside Rb1
overall, followed by Rg1 and Re (54). Tanaka (55) found that the difference in
ginsenoside content in wild and cultivated Asian ginseng was
insignificant, but Mizuno et al (56) reported that wild ginseng had
higher ginsenoside Rg1, Re and Rd content and lower ginsenoside Rc,
Rb2 and Rb1 content compared with the cultivated roots of Asian
ginseng (P. ginseng). Other studies report positive effects
of rg1, rb1, rg3 and/or rh2 on myogenic differentiation and
myotubes formation by activating the Akt signaling pathway that has
a protective function in muscle weakness and atrophy with chronic
diseases and cancers (57–60).
The HPLC analysis identified rg1, rb1 and rg3 in AWGP and KCWGP.
However, because there is a large diversity of ginsenosides in
AWGP, analysis of various compounds will need to be further
studied.
In the present study, AWGP and KCWGP were
investigated for their regulation of muscle differentiation and
mitochondrial biogenesis via the AMPK and PI3K/AKT/mTOR signaling
pathways in C2C12 myotubes. AWGP and KCWGP significantly increased
the expression of MyoD, myogenin, MyHC and myostatin and increased
the expression of the PGC-1α, NRF-1, TFAM and SIRT1 in the myotubes
through activation of the AMPK and PI3K/Akt/mTOR signaling pathway.
The results suggested that AWGP and KCWGP may aid muscle function
through muscle differentiation and energy metabolism enhancement.
However, to clearly understand the mechanisms of AWGP and KCWGP for
the regulation of muscle differentiation and mitochondrial
biogenesis, further studies on the pathological conditions such as
energy metabolism imbalance will be needed.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Research Foundation
of Korea (NRF; grant nos. 2020R1A6A3A01099936 and
2021R1A2C1012267).
Availability of data and materials
The data generated and analyzed during the study are
available from the corresponding author upon reasonable request.
All materials used in this study are properly included in the
Methods section.
Authors' contributions
HWJ and JHH designed all of the experiments
together. HWJ, SYK and JHH performed the experiments, the
statistical analysis and interpreted the experimental results. SYK
and JHH wrote the manuscript. HWJ revised the manuscript. All
authors have read and approved the final manuscript. HWJ, SYK and
JHH confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
United Nations Population Fund (UNFPA) and
HelpAge International, . Ageing in the twenty-first century: A
celebration and a challenge. https://www.unfpa.org/publications/ageing-twentyfirst-centuryJanuary
1–2012
|
|
2
|
United Nations, . World Population
Prospects: The 2002 revision highlights. United Nations; New York,
NY: 2003, https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2002_world_population_prospects-2002_revision_volume-ii.pdfFebruary
26–2003
|
|
3
|
Kalyani RR, Corriere M and Ferrucci L:
Age-related and disease-related muscle loss: The effect of
diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol.
2:819–829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dodds R and Avan AA: Sarcopenia and
frailty: New challenges for clinical practice. Clin Med (Lond).
16:455–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cruz-Jentoft AJ, Baeyens JP, Bauer JM,
Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y,
Schneider SM, et al: Sarcopenia: European consensus on definition
and diagnosis: Report of the European working group on sarcopenia
in older people. Age Ageing. 39:412–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamel HK: Sarcopenia and aging. Nutr Rev.
61:157–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee IS, Kang KS and Kim SY: Panax
ginseng pharmacopuncture: Current status of the research and
future challenges. Biomolecules. 10:332019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SY, Park JH, Kim HS, Lee CY, Lee H.J,
Kang KS and Kim CE: Systems-level mechanisms of action of Panax
ginseng: A network pharmacological approach. J Ginseng Res.
42:98–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeong HS, Lim CS, Cha BC, Choi SH and Kwon
KR: Component analysis of cultivated ginseng, cultivated wild
ginseng, and wild ginseng and the change of ginsenoside components
in the process of red ginseng. J Pharmacopuncture. 13:63–77. 2010.
View Article : Google Scholar
|
|
10
|
Sun H, Liu F, Sun L, Liu J, Wang M, Chen
X, Xu X, Ma R, Feng K and Jiang R: Proteomic analysis of amino acid
metabolism differences between wild and cultivated Panax
ginseng. J Ginseng Res. 40:113–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Yang WS, Yu T, Sung GH, Park KW,
Yoon K, Son YJ, Hwang H, Kwak YS, Lee CM, et al:
ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red
ginseng water extract. J Ethnopharmacol. 154:218–228. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CH and Kim JH: A review on the
medicinal potentials of ginseng and ginsenosides on cardiovascular
diseases. J Ginseng Res. 38:161–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sung IJ, Ghimeray AK, Chang KJ and Park
CH: Changes in contents of Ginsenoside due to boiling process of
Panax ginseng C.A. Mayer. Korea J Plant Res. 26:726–730.
2013. View Article : Google Scholar
|
|
14
|
Lee SM, Bae BS, Park HW, Ahn NG, Cho BG,
Cho YL and Kwak YS: Characterization of Korean Red Ginseng
(Panax ginseng Meyer): History, preparation method, and
chemical composition. J Ginseng Res. 39:384–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin X, Che DB, Zhang ZH, Yan HM, Jia ZY
and Jia XB: Ginseng consumption and risk of cancer: A
meta-analysis. J Ginseng Res. 40:269–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hernández-García D, Granado-Serrano AB,
Martín-Gari M, Naudí A and Serrano JC: Efficacy of Panax
ginseng supplementation on blood lipid profile. A meta-analysis
and systematic review of clinical randomized trials. J
Ethnopharmacol. 243:1120902019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung C, Jung JH and Lee MS: A clinical
study of immune pharmacopuncturology. Kyungrak Medical Publishing
Co.; pp. 127–133. Chungnam: 2011, (In Korean).
|
|
18
|
Kang SK, Lee HJ and Park YB: Experimental
studies on the effect of Ginseng radix aqua-acupuncture. Int
Symp East-West Med. 1989:61–83. 1989.
|
|
19
|
Lim C, Kwon K and Lee K: Plexiform
neurofibroma treated with pharmacopuncture. J Pharmacopuncture.
17:74–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee K, Yu J, Sun S, Kwon K and Lim C: A
4-week, repeated, intravenous dose, toxicity test of mountain
ginseng pharmacopuncture in sprague-dawley rats. J
Pharmacopuncture. 17:27–35. 2014. View Article : Google Scholar
|
|
21
|
Jung HL, Kwak HE, Kim SS, Kim YC, Lee CD,
Byurn HK and Kang HY: Effects of Panax ginseng
supplementation on muscle damage and inflammation after uphill
treadmill running in humans. Am J Chin Med. 39:441–450. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alvarez AI, De Oliveira ACC, Perez AC,
Vila L, Ferrando A and Prieto JG: The effect of ginseng on muscle
injury and inflammation. J Ginseng Res. 28:18–26. 2004. View Article : Google Scholar
|
|
23
|
Hwang JH and Jung HW: Effects of
pharmacopuncture with wild ginseng complex in 2 elderly patients
with obesity: Case report. Medicine (Baltimore). 97:e115342018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sung SH, Shin BC, Park MJ, Kim KH, Kim JW,
Ryu JY and Park JK: Current status of management on
pharmacopuncture in Korea through introduction of an accreditation
system. J Pharmacopuncture. 22:75–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Papa EV, Dong X and Hassan M: Skeletal
muscle function deficits in the elderly: Current perspectives on
resistance training. J Nat Sci. 3:e2722017.PubMed/NCBI
|
|
26
|
Kelly DP and Scarpulla RC: Transcriptional
regulatory circuits controlling mitochondrial biogenesis and
function. Genes Dev. 18:357–368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hock MB and Kralli A: Transcriptional
control of mitochondrial biogenesis and function. Annu Rev Physiol.
71:177–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sin J, Andres AM, Taylor DJ, Weston T,
Hiraumi Y, Stotland A, Kim BJ, Hwang C, Doran KS and Gottlieb RA:
Mitophagy is required for mitochondrial biogenesis and myogenic
differentiation of C2C12 myoblasts. Autophagy. 12:369–380. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oh SS, Kim S, Moon S, Park DH and Kang JH:
Lactate overload inhibits myogenic activity in C2C12 myotubes. Open
Life Sci. 14:29–37. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen H, Liu T, Fu L, Zhao S, Fan B, Cao J
and Li X: Identification of microRNAs involved in
dexamethasone-induced muscle atrophy. Mol Cell Biochem.
381:105–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang DT, Yin Y, Yang YJ, Lv PJ, Shi Y, Lu
L and Wei LB: Resveratrol prevents TNF-α-induced muscle atrophy via
regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int
Immunopharmacol. 19:206–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langley B, Thomas M, Bishop A, Sharma M,
Gilmour S and Kambadur R: Myostatin inhibits myoblast
differentiation by down-regulating MyoD expression. J Biol Chem.
277:49831–49840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsukamoto S, Shibasaki A, Naka A, Saito H
and Iida K: Lactate promotes myoblast differentiation and myotube
hypertrophy via a pathway involving MyoD in vitro and enhances
muscle regeneration in vivo. Int J Mol Sci. 19:36492018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cole NJ, Hall TE, Martin CI, Chapman MA,
Kobiyama A, Nihei Y, Watabe S and Johnston IA: Temperature and the
expression of myogenic regulatory factors (MRFs) and myosin heavy
chain isoforms during embryogenesis in the common carp Cyprinus
carpio L. J Exp Biol. 207:4239–4248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tajbakhsh S: Skeletal muscle stem cells in
developmental versus regenerative myogenesis. J Intern Med.
266:372–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Crocker CL, Baumgarner BL and Kinsey ST:
β-guanidinopropionic acid and metformin differentially impact
autophagy, mitochondria and cellular morphology in developing C2C12
muscle cells. J Muscle Res Cell Motil. 41:221–237. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cantó C and Auwerx J: PGC-1alpha, SIRT1
and AMPK, an energy sensing network that controls energy
expenditure. Curr Opin Lipidol. 20:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cantó C, Gerhart-Hines Z, Feige JN,
Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P and Auwerx
J: AMPK regulates energy expenditure by modulating NAD+ metabolism
and SIRT1 activity. Nature. 458:1056–1060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ruderman NB, Xu XJ, Nelson L, Cacicedo JM,
Saha AK, Lan F and Ido Y: AMPK and SIRT1: A long-standing
partnership? Am J Physiol Endocrinol Metab. 298:E751–E760. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suwa M, Egashira T, Nakano H, Sasaki H and
Kumagai S: Metformin increases the PGC-1alpha protein and oxidative
enzyme activities possibly via AMPK phosphorylation in skeletal
muscle in vivo. J Appl Physiol (1985). 101:1685–1692. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, et al: Resveratrol improves mitochondrial function and
protects against metabolic disease by activating SIRT1 and
PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang H and Ward WF: PGC-1alpha: A key
regulator of energy metabolism. Adv Physiol Educ. 30:145–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scarpulla RC: Transcriptional paradigms in
mammalian mitochondrial biogenesis and function. Physiol Rev.
88:611–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Banerjee J, Bruckbauer A and Zemel MB:
Activation of the AMPK/Sirt1 pathway by a leucine-metformin
combination increases insulin sensitivity in skeletal muscle, and
stimulates glucose and lipid metabolism and increases life span in
Caenorhabditis elegans. Metabolism. 65:1679–1691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fernandez-Marcos PJ and Auwerx J:
Regulation of PGC-1α, a nodal regulator of mitochondrial
biogenesis. Am J Clin Nutr. 93:884S–890S. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Thomson DM: The role of AMPK in the
regulation of skeletal muscle size, hypertrophy, and regeneration.
Int J Mol Sci. 19:31252018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tao R, Gong J, Luo X, Zang M, Guo W, Wen R
and Luo Z: AMPK exerts dual regulatory effects on the PI3K pathway.
J Mol Signal. 5:12010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu
Q, Crenshaw EB III, Kaestner KH, Bartolomei MS, Shulman GI and
Birnbaum MJ: Insulin resistance and a diabetes mellitus-like
syndrome in mice lacking the protein kinase Akt2 (PKB beta).
Science. 292:1728–1731. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
McCurdy CE and Cartee GD: Akt2 is
essential for the full effect of calorie restriction on
insulin-stimulated glucose uptake in skeletal muscle. Diabetes.
54:1349–1356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Friedrichsen M, Birk JB, Richter EA,
Ribel-Madsen R, Pehmøller C, Hansen BF, Beck-Nielsen H, Hirshman
MF, Goodyear LJ, Vaag A, et al: Akt2 influences glycogen synthase
activity in human skeletal muscle through regulation of
NH2-terminal (sites 2 + 2a) phosphorylation. Am J Physiol
Endocrinol Metab. 304:E631–E639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yoon MS: mTOR as a key regulator in
maintaining skeletal muscle mass. Front Physiol. 8:7882017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bai L, Gao J, Wei F, Zhao J, Wang D and
Wei J: Therapeutic potential of ginsenosides as an adjuvant
treatment for diabetes. Front Pharmacol. 9:4232018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee DY, Choi BS, Lee IH, Kim JH and Gwon
PS: Comparison of index compounds content and antioxidative
activity of wild ginseng pharmacopuncture by extraction method. J
Intern Korean Med. 39:313–322. 2018. View Article : Google Scholar
|
|
54
|
Lim W, Mudge KW and Vermeylen F: Effects
of population, age, and cultivation methods on ginsenoside content
of wild American ginseng (Panax quinquefolium). J Agric Food Chem.
53:8498–8505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tanaka O: Solubilizing properties of
ginseng saponins. Soc Korean Ginseng. 67–74. 1987.
|
|
56
|
Mizuno M, Yamada J, Terai H, Kozukue N,
Lee YS and Tsuchida H: Differences in immunomodulating effects
between wild and cultured Panax ginseng. Biochem Biophys Res
Commun. 200:1672–1678. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Go GY, Jo A, Seo DW, Kim WY, Kim YK, So
EY, Chen Q, Kang JS, Bae GU and Lee SJ: Ginsenoside Rb1 and Rb2
upregulate Akt/mTOR signaling-mediated muscular hypertrophy and
myoblast differentiation. J Ginseng Res. 44:435–441. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Go GY, Lee SJ, Jo A, Lee J, Seo DW, Kang
JS, Kim SK, Kim SN, Kim YK and Bae GU: Ginsenoside Rg1 from Panax
ginseng enhances myoblast differentiation and myotube growth. J
Ginseng Res. 41:608–614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee SJ, Bae JH, Lee H, Lee H, Park J, Kang
JS and Bae GU: Ginsenoside Rg3 upregulates myotube formation and
mitochondrial function, thereby protecting myotube atrophy induced
by tumor necrosis factor-alpha. J Ethnopharmacol. 242:1120542019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee SJ, Im M, Park SK, Kim JY, So EY,
Liang OD, Kang JS and Bae GU: BST204, a Rg3 and Rh2 enriched
ginseng extract, upregulates myotube formation and mitochondrial
function in TNF-α-induced atrophic myotubes. Am J Chin Med.
48:631–650. 2020. View Article : Google Scholar : PubMed/NCBI
|