Introduction
Primary nephrotic syndrome (PNS) is a crucial factor
leading to chronic kidney disease in children. The most common
pathological types are minimal change disease and focal segmental
glomerular stiffness (1,2). Although the majority of affected
children benefit from hormone therapy and exhibit a good prognosis,
some children do not tolerate hormone therapy. If they are not
treated with other drugs in time, they may eventually develop
glomerulosclerosis and end-stage renal disease. To date, the
mechanisms through which PNS develops into glomerulosclerosis
remain to be elucidated. Therefore, enhancing the basic research of
PNS, searching for PNS-related pathogenic molecules and further
researching associated drug targets may provide early intervention
treatment for PNS with important clinical significance.
The main pathological mechanisms associated with PNS
are podocyte and glomerular injury (3,4).
The most typical feature of podocyte injury is podocyte shedding or
apoptosis from the outside surface of the glomerular basement
membrane (4,5). The rearrangement of the cytoskeleton
leads to phenotypic transformation, which leads to podocytes losing
their epithelial features and acquiring the ability of movement and
migration (6,7). The occurrence and development of
glomerular sclerosis can cause podocyte damage. Proteinuria is a
key clinical feature of PNS and podocyte damage is also a cause of
proteinuria. Therefore, the present study approached the
pathogenesis of PNS from the perspective of podocyte injury.
CXC motif chemokine ligand 16 (CXCL16), as a member
of the CXC chemokine family, is closely associated with kidney
diseases. Studies have demonstrated that CXCL16 expression is
upregulated in several kidney injury models from animals (8,9),
as well as blood and urine samples of patients with kidney disease
(10,11). Blocking the mRNA and protein
expression of CXCL16 has been shown effectively improve the
occurrence and development of kidney disease (12,13).
In addition, when cells are overwhelmed by oxidative
stress, genes involved in cell death signaling are activated to
induce apoptosis or necrosis to remove irreversibly damaged cells
(14). Therefore, an increase in
the level of apoptosis may underline the pathogenesis of kidney
injury. Furthermore, oxidative stress and inflammation, which are
often associated, are characteristic features of kidney disease
(15,16). Extracellular regulated protein
kinase (ERK), as a member of the mitogen-activated protein kinase
(MAPK) family, which is widely involved in regulating cell growth,
cell cycle, apoptosis and differentiation processes, is an
important signal of intracellular signal transduction (17,18). ERK1 and ERK2, two major ERK
subtypes, are proteins encoded by two splicing variants of the same
gene (19), which are 84%
identical at the amino acid level (20). Lakshmanan et al (21) found that ERK1/2 protein expression
was incremental in the renal biopsy tissues of mice with diabetic
nephropathy. However, ERK1/2 expression in PNS renal tissue has
rarely been reported.
The present study aimed to assess the expression and
function of CXCL16 in PNS kidney tissues and cell lines and to
search for target proteins that interact with CXCL16. Through
functional analysis, the role of CXCL16 and its target protein in
podocyte proliferation, apoptosis and migration was confirmed. In
addition, a search was performed for the signaling pathway
regulated by CXCL16, in order to provide a theoretical basis for
the study of PNS.
Materials and methods
Clinical tissue samples
A total of 15 human renal tissue samples and normal
tissue (children who underwent resection of Wilms tumor and the
normal renal tissue 3 cm outside the lesion was taken as control)
were obtained from Shandong Provincial Hospital (Table I). The present study was approved
and conducted under the guidance of the ethics committee of the
Shandong Provincial Hospital. All samples were obtained with the
written informed consent from the patients (or signed by the
child's guardian) with PNS. The present study was approved by the
clinical research ethics committee of Shandong Provincial Hospital
(approval no. SWYX: NO.2020-152).
 | Table I.Baseline demographic and clinical
characteristics. |
Table I.
Baseline demographic and clinical
characteristics.
| Characteristic | PNS | Normal |
|---|
| Sex,
male/female | 9/6 | 7/8 |
| Age, mean ± SD,
years | 8.53±2.47 | 7.07±3.61 |
| Albumin, mean ± SD,
g/l | 25.19±6.84 | 43.27±1.95 |
| Cholesterol, mean ±
SD, mmol/l | 10.33±3.81 | 3.99±0.44 |
| 24-hour urine,
protein, mean ± SD, mg/kg | 118.44±79.8 | - |
Cell lines and culture
Conditionally immortalized human podocytes (HPCs)
were a kind gift from Professor Fan Yi (Shandong University)
(22,23) and maintained in modified RPMI-1640
medium (MilliporeSigma) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and penicillin/streptomycin
(100 µg/ml, Nanjing KeyGen Biotech Co., Ltd.) at 33°C with 5%
CO2 for 2–3 days. In order to induce podocytes to
undergo differentiation, the culture temperature was changed from
33°C to 37°C for 2 weeks; and the other conditions remained
unaltered.
Plasmids
The lentiviral transferring plasmid,
pCDH-CMV-Puro-CXCL16 (termed pCDH-CXCL16) was purchased from Vigene
Biosciences, Inc. The sequences of the CXCL16 shRNA and ERK1/2
shRNA were amplified and inserted into pLKO.1-TRC control (pLKO.1)
to generate recombinant plasmid for lentiviral vector production.
The construction of recombinant plasmid was as previously described
(24). Briefly, the sequences of
shCXCL16 and shERK1/2 were amplified and inserted into pLKO.1 to
generate recombinant pLKO.1-shCXCL16 and pLKO.1-ERK1/2 for
lentivirus production. All shRNA primer sequences are presented in
Table II. Using synthetic
shCXC16 and shERK1/2 sequences as templates for PCR. PCR
amplifications were performed with Phanta Max Super-Fidelity DNA
Polymerase (cat. no. DC505; Vazyme Biotech Co., Ltd.), using the
following thermocycling conditions: 35 cycles at 95°C for 15 sec,
60°C for 30 sec and 72°C for 15 sec; extension at 72°C for 1 min;
and maintained at 4°C. The restriction enzymes were used to perform
a double digest of the plasmid and the PCR gel purification
products. The products were separated by 1% agarose gel
electrophoresis, purified and incubated with T4 ligase overnight at
4°C. The products were transformed into Escherichia coli,
and a single colony was randomly selected. The plasmid was
extracted using a plasmid extraction kit (cat. no. DC201; Vazyme
Biotech Co., Ltd.). The plasmids were subsequently sent to
GenScript Biotech for sequencing.
 | Table II.shRNA sequences. |
Table II.
shRNA sequences.
| shRNA | Sequence
(5′→3′) |
|---|
|
shCXCL16-human-F1 |
CCGGCGTCCCAATGAAACCACCATTCTCGAGAATGGTGGTTTCATTGGGACGTTTTTG |
|
shCXCL16-human-R1 |
AATTCAAAAACGTCCCAATGAAACCACCATTCTCGAGAATGGTGGTTTCATTGGGACG |
|
shCXCL16-human-F2 |
CCGGCCATCGGTTCAGTTCATGAATCTCGAGATTCATGAACTGAACCGATGGTTTTTG |
|
shCXCL16-human-R2 |
AATTCAAAAACCATCGGTTCAGTTCATGAATCTCGAGATTCATGAACTGAACCGATGG |
| shERK2-human-F |
CCGGTGGAATTGGATGACTTGCCTACTCGAGTAGGCAAGTCATCCAATTCCATTTTT |
| shERK2-human-R |
AATTAAAAATGGAATTGGATGACTTGCCTACTCGAGTAGGCAAGTCATCCAATTCCA |
Lentivirus packaging and
transduction
293T cells (Conservation Genetics CAS Kunming Cell
Bank) were cultured in a 10 cm cell culture dish at 37°C with 5%
CO2 overnight. At 80% confluence, 293T cells were
co-transfected with lentiviral plasmids (5.94 µg lentivirus-CXCL16,
5.94 µg lentivirus-CXCL16 shRNA1, 2, 5.94 µg lentivirus-ERK2 shRNA,
4.86 µg helper plasmid psPAX2 and 9.0 µg pMD2.G using 40 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) to produce the recombinant lentivirus. The 4.86
µg empty vectors pCDH and pLKO-1 were also transfected as control.
At 10 h post-transfection, the cell culture medium was replaced
with DMEM medium containing 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin. Following transfection for 48 h, the medium was
collected. The lentiviral titer was approximately 1×107
to 1×108 transduction units (TU)/ml.
Cell proliferation assay
Different groups of HPCs (5×103
cells/well) were cultured in 96-well plates. After the HPCs were
allowed to adhere to the plate (12 h after cell plating), cell
viability was detected at 0, 1, 3 and 5 days. Briefly, the
procedure used for the Cell Counting Kit-8 (CCK-8) reagent kit
(cat. no. C0038, Beyotime, Institute of Biotechnology) involved the
addition of 10% reconstituted CCK-8 reagent in 100 µl of culture
medium volume and returning to an incubator at 37°C for 1 h. The
optical density (OD) value determined using a microplate reader
(BioTek Instruments, Inc.) at 450 nm.
Transwell migration assay
Briefly, 200 µl HPC (2×105 cells)
suspension was seeded into the culture insert, while 500 µl
RPMI-1640 medium containing 10% FBS were added to the lower
chamber. The Transwell chamber was incubated at 37°C for 12 h. The
inner surface of the basement membrane was cleaned. The membrane
was then dried, inverted. Images were captured from six random of
fields (magnification, ×200) using an light microscope (BX54;
Olympus Corporation).
Flow cytometry
Cell apoptosis was measured using an Apoptosis
Detection kit [cat. no. 40302ES20; Yeasen Biotechnology (Shanghai)
Co., Ltd.]. The different groups of HPCs were detached with 0.25%
trypsin (cat. no. T1320, Beijing Solarbio Science & Technology
Co., Ltd.), centrifuged at 200 × g for 5 min at room temperature
and washed three times with cold 4°C PBS. Subsequently, 200 µl
binding buffer were used for re-suspension, followed by the
addition of 5 µl PI reagent and 5 µl Annexin-V-FITC. The mixture
was reacted in an incubator at 25°C for 15 min in the dark and 400
µl binding buffer were then added. Finally, the cells were detected
using a FACScan flow cytometer (BD Biosciences) within 2 h at
wavelengths of 488 and 615 nm. Stained cells were analyzed using
the FACScan flow cytometry system (BD Biosciences). The apoptotic
rate was calculated as the percentage of early + late apoptotic
cells.
Reverse transcription-quantitative PCR
(RT-qPCR)
mRNA was extracted from cells (1×107/ml)
and tissues (20 mg) using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) and was reverse transcribed into cDNA using the
PrimeScript Reverse Transcription kit (Vazyme Biotech Co. Ltd), the
temperature protocol used for reverse transcription was as follows:
50°C for 20 min and 85°C for 2 min. qPCR was performed using the
ABI-7300 System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) in a total volume of 20 µl according to the manufacturer's
protocols. The primers sequences used were as follows: CXCL16
forward, 5′-AAACCACCATTCACACTGCG-3′ and reverse,
5′-GAGTCAGGTGCCACAGGTAT-3′; ERK2, forward,
5′-GATCTGTGACTTTGGCCTGG-3′; and reverse,
5′-TGTGGTTCAGCTGGTCAAGA-3′; β-actin, forward,
5′-GGCATCCTCACCCTGAAGTA-3′; and reverse,
5′-GGGGTGTTGAAGGTCTCAAA-3′. The following thermocycling conditions
were used for qPCR: 95°C for 5 min; 40 cycles of 95°C for 10 sec
and 60°C for 30 sec. Relative gene expression was determined using
the 2−ΔΔCq method (25) using β-actin as an internal
control.
Western blot analysis
The HPCs in the different groups were harvested and
extracted using RIPA lysis buffer (Thermo Fisher Scientific, Inc)
supplemented with protease and phosphatase inhibitors(Thermo Fisher
Scientific, Inc). Total protein was quantified using the BCA
Protein Assay kit (Beyotime Institute of Biotechnology). Total
protein (equal, 30 µg/lane) was separated on 10% SDS-PAGE and
transferred to PVDF membranes (Millipore). The membranes were then
incubated with 5% non-fat milk (Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature for 1 h. Finally, the
membranes were incubated with the following primary antibodies at
4°C overnight: CXCL16 (1:1,000; cat. no. ab101404, Abcam), ERK1/2
(1:1,000; cat. no. ab17942, Abcam), ZO-1 (1:1,000; cat. no.
ab96587, Abcam), P-cadherin (1:1,000; cat. no. ab2420604, Abcam),
vimentin (1:1,000; cat. no. ab137321, Abcam) N-cadherin (1:1,000;
cat. no. ab76011, Abcam) and β-actin (1:1,000; cat. no. ab8226,
Abcam). The following day, the membranes were washed three times
and then incubated with goat anti-rabbit IgG (1:10,000; cat. no.
ab205718, Abcam) or anti-mouse IgG (1:10,000; cat. no. ab6789,
Abcam) at room temperature for 2 h. The membranes were detected
using ECL reagent (Thermo Fisher Scientific, Inc.) and images of
each protein band were obtained using an enhanced chemiluminescence
detection system (4600; Tanon Science and Technology Co.,
Ltd.).
Immunohistochemistry (IHC)
The clinical tissues were provided by Shandong
Provincial Hospital for IHC and were then fixed in 10% formaldehyde
for 24 h, immersed in 75% ethanol for 2 h followed by tissue
dehydration: 80% ethanol 20 min, 90% ethanol 20 min, 95% ethanol 20
min, 100% ethanol I 10 min, 100% ethanol II 10 min, xylene I 10
min, xylene II 10 min, xylene III 10 min, paraffin I 40 min,
paraffin wax II 1 h, Paraffin III 1 h and embedded in paraffin. The
tissue was sectioned at 5 µm and attached to slides. After blocking
endogenous peroxides and non-specific proteins, the tissues
sections were incubated with primary antibodies, CXCL16 (cat. no.
ab119350, Abcam) and ERK1/2 (cat. no. ab17942, Abcam) at 4°C
overnight. The following day, the sections were washed three times
and were incubated with HRP-goat-anti-rabbit antibody (1:10,000;
cat. no. ab205718, Abcam) at 37°C for 30 min. The tissues sections
were then stained with diaminobenzidine for 3 min and the nuclei
were counterstained with hematoxylin for 2 min at room temperature.
The CXCL16 and ERK1/2 antibodies were used at a dilution of 1:200.
IHC images of each tissues were randomly obtained using a light
microscope (BX53M; Olympus Corporation), then were analyzed using
the Image-Pro Plus 6.0 image analysis system (Media Cybernetics,
Inc.).
Statistical analysis
Values were presented as the mean ± standard
deviation. Differences between two groups were analyzed using a
Student's t-test. One-way analysis of variance and Tukey or LSD
post hoc test were used for comparisons between multiple groups.
The correlation between ERK1/2 and CXCL16 was measured by
Spearman's correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
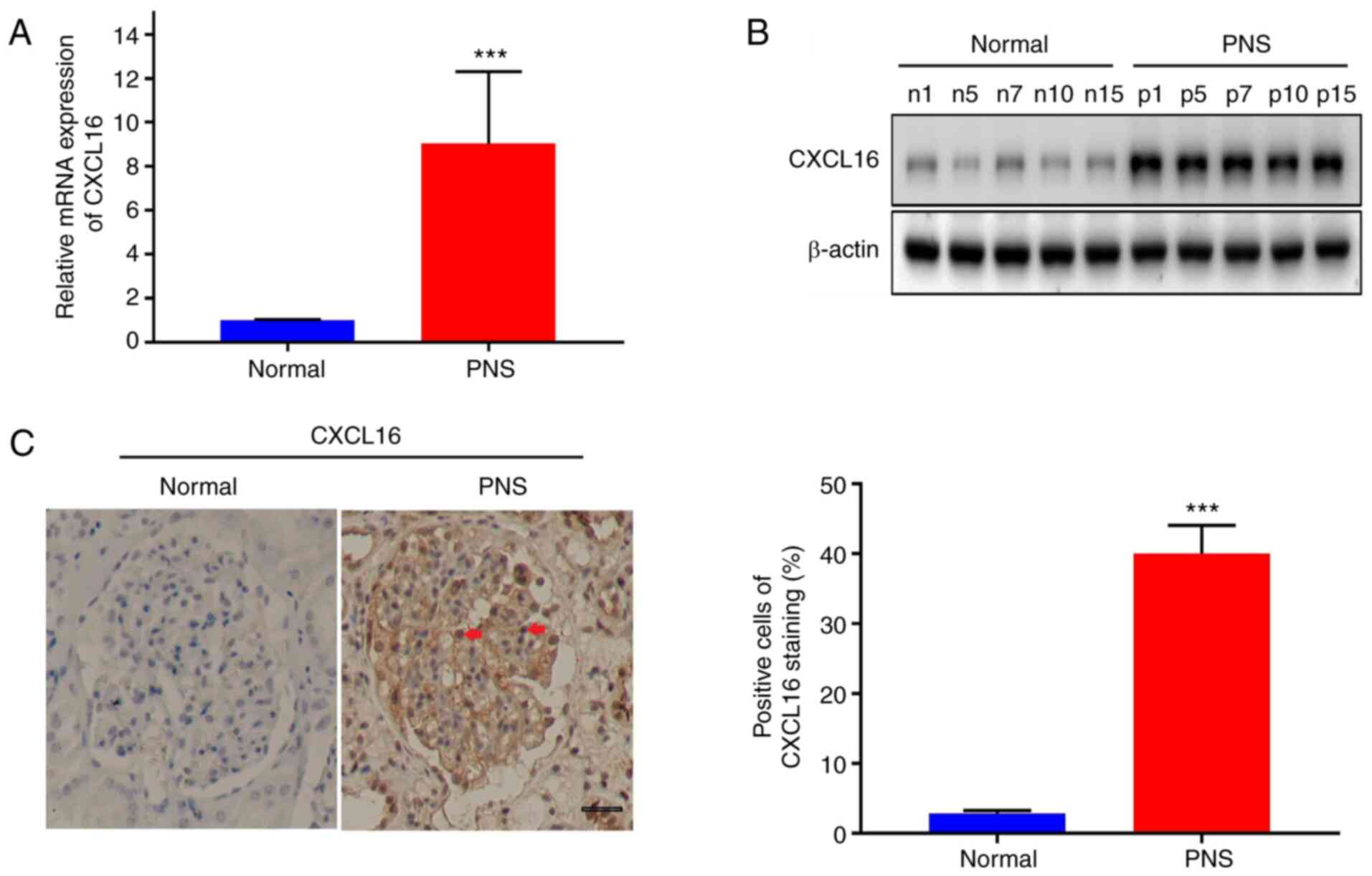
CXCL16 is upregulated in PNS
The present study first evaluated CXCL16 expression
levels in renal tissues from patients from PNS and healthy
individuals. As illustrated in Fig.
1A, CXCL16 expression in podocyte of the renal tissues of the
15 patients with PNS was significantly upregulated compared with
that in the paired normal tissues (P<0.001). Accordingly, five
tissues from each group were randomly selected for western blot
analysis. As illustrated in Fig.
1B, CXCL16 protein expression from the podocyte in PNS renal
tissues was significantly higher than that in normal tissues
(P<0.001). Furthermore, IHC was conducted. As illustrated in
Fig. 1C, CXCL16 expression in
podocyte of the renal tissue of patients with PNS was significantly
higher than that of the normal control (P<0.001).
CXCL16 regulates the growth, migration
and apoptosis of human podocytes
Previous studies have indicated that CXCL16 promotes
mouse podocyte migration and regulates the progression of PNS;
however, there are still areas of uncertainty. To clarify this, the
present study examined the effects of CXCL16 after overexpression
or knockdown on human podocytes. HPCs were transfected with
lentivirus to overexpress or knockdown CXCL16. Western blot
analysis confirmed the overexpression or knockdown of CXCL16
(P<0.01, Fig. 2A and B). The
overexpression of CXCL16 suppressed the growth of human podocytes
and the knockdown of CXCL16 promoted the proliferation of podocytes
(P<0.01, P<0.001; Fig. 2C and
D). Consistent with this finding, CXCL16 overexpression
promoted HPC apoptosis and the knockdown of CXCL16 suppressed the
apoptosis of HPCs (P<0.05; Fig. 2E
and F). In addition, Transwell assays were performed on HPCs
stably transfected with CXCL16 or CXCL16 shRNA. The results
indicated that CXCL16 overexpression promoted HPC migration
(P<0.001; Fig. 2G), whereas
CXCL16 knockdown significantly suppressed the migration of HPCs
(P<0.01; Fig. 2H). Overall,
these findings demonstrated that CXCL16 regulated the progress of
PNS by inhibiting the growth and promoting the apoptosis of
podocytes, which caused podocytes to lose the characteristics of
normal cells and obtain a higher migration and movement
ability.
ERK1/2 expression is upregulated in
PNS
In order to reveal the potential downstream targets
of CXCL16, a literature search was performed. The results
demonstrated that ERK1/2 may be the potential downstream target of
CXCL16 and may be regulated by CXCL16 (26,27). To verify the association between
CXCL16 and ERK1/2, the expression of ERK1/2 in PNS was verified.
The ERK2 mRNA and ERK1/2 protein levels were evaluated in the same
renal tissues from patients with PNS using qPCR (P<0.001,
Fig. 3A), western blot analysis
(P<0.01, Fig. 3B) and IHC
(P<0.001, Fig. 3C). Notably,
compared with normal tissues, the expression of ERK1/2 in PNS
tissues was also upregulated and there was a positive association
between ERK2 and CXCL16 expression (Fig. 3D). The expression of CXCL16 and
ERK1/2 was then verified in HPCs in which was stably overexpressed
or knocked down. As shown in Fig. 3E
and F, ERK1/2 expression increased with the overexpression of
CXCL16 expression and decreased with the knockdown of CXCL16
(P<0.01). The aforementioned results suggested that ERK1/2 may
be a potential downstream target of CXCL16.
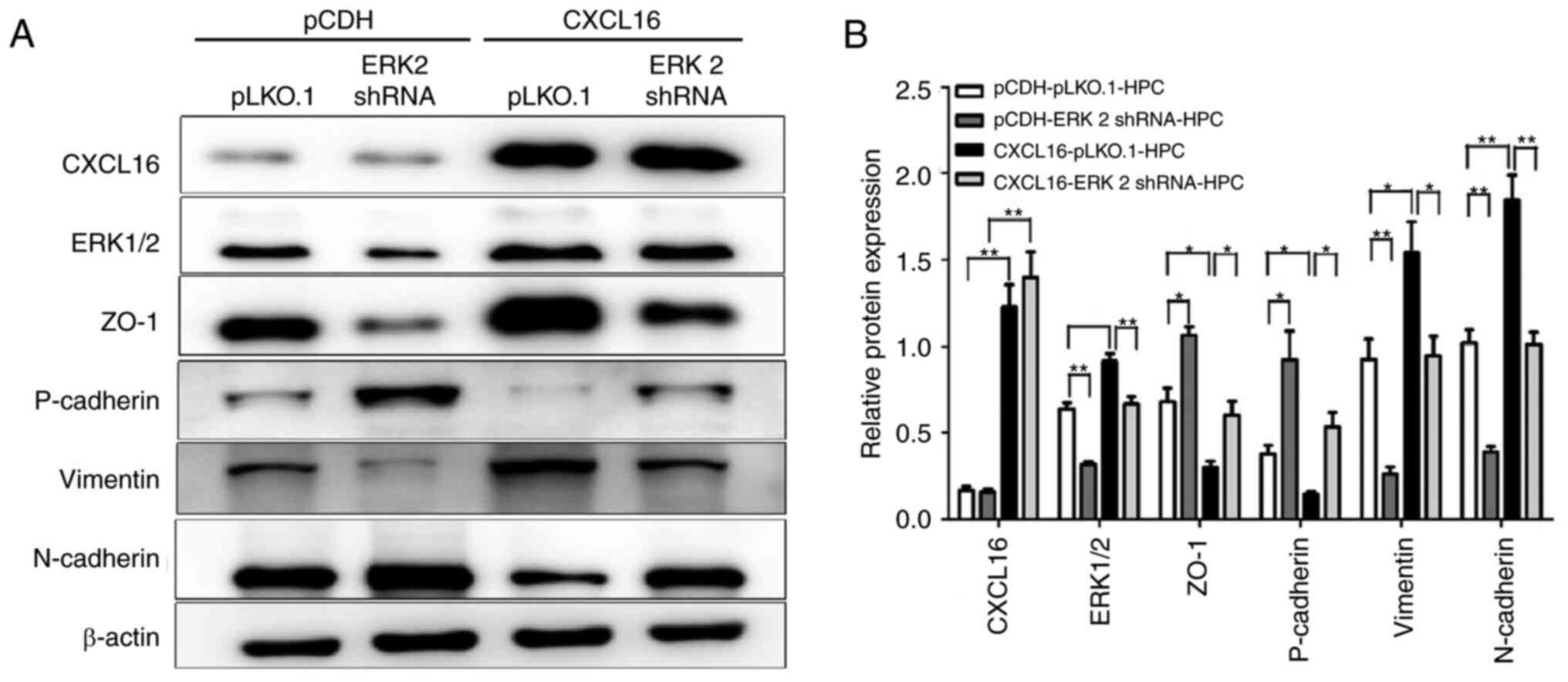
ERK1/2 is a downstream protein of
CXCL16 and is involved in regulating podocyte growth, migration,
apoptosis and EMT
To define the role of ERK1/2 upregulation in PNS,
the recombinant lentivirus plasmid of ERK1/2 shRNA was first
constructed and the expression of ERK2 was knocked down in HPCs
overexpressing CXCL16 (P<0.001, Fig. 4A). The effects of this knockdown
were then evaluated on the ability of cell proliferation and
apoptosis. As indicated in Fig. 4B
and C, the knockdown of ERK1/2 expression reversed the
inhibitory effects of CXCL16 on HPC proliferation and reduced the
apoptosis of HPCs which was induced by CXCL16 (P<0.5, P<0.01
and P<0.001). Subsequently, the effects of ERK2 knockdown on
podocyte migration were evaluated and the results were consistent
with the expectations. The knockdown of ERK2 inhibited the
increased migratory ability of HPCs induced by CXCL16 (P<0.01
and P<0.001; Fig. 4D). EMT of
HPCs is the main pathway leading to HPC dysfunction, proteinuria
and glomerulosclerosis (28,29). Therefore, the present study
detected the expression of N-cadherin, P-cadherin, vimentin and
zonula occludens-1 (ZO-1) proteins related to EMT. Of note, it was
found that the knockdown of ERK1/2 expression in
CXCL16-overexpressing HPCs reversed the CXCL16-induced expression
of protein molecules associated with EMT (P<0.5 and P<0.01;
Fig. 5A and B).
Discussion
PNS is a kidney disease. The clinical features of
PNS are the following: Large amounts of proteinuria,
hypoproteinemia, hyperlipidemia and varying degrees of edema
(4). The pathogenesis of PNS has
not yet been fully elucidated. At present, podocyte and glomerular
injury are considered the main pathological characteristics of PNS
(2,3). Podocytes are terminally
differentiated cells lining the outside surface of the glomerular
basement membrane (30). Podocyte
injury can lead to podocyte fusion, which causes podocyte apoptosis
(31). It also causes
cytoskeleton rearrangement (32),
which causes podocytes to acquire the ability of movement and
migration, resulting in glomerular sclerosis and proteinuria
(5). In the present study, a high
CXCL16 expression was found in human PNS samples and podocytes;
CXCL16 was also found to regulate the proliferation, apoptosis and
migration of podocytes, which is closely associated with podocyte
injury and PNS pathogenesis.
ERK is a member of the mitogen-activated protein
kinase (MAPK) family, which is widely involved in regulating cell
growth, cell cycle, apoptosis and differentiation processes. ERK1/2
expression in PNS renal tissue has rarely been reported. The
activation of ERK1/2 has been shown to be responsible for the
beneficial effects of adrenocorticotropic hormone on the glomerular
filtration barrier in patients with nephrotic syndrome (33). The complement membranous attack
complex can induce the phosphorylation of JNK and ERK1/2 and AS-IV
is able to block the complement- induced phosphorylation of JNK and
ERK1/2 (34). The present study
detected the ERK2 expression in PNS and normal renal tissue
samples. The results revealed that ERK2 expression in renal tissue
samples from children with PNS was upregulated at both mRNA and
protein levels and it was thus hypothesized that ERK 2 may be
involved in the pathogenesis of PNS. CXCL16 has been reported that
to be involved in acute kidney injury by regulating ERK1/2 to
induce the apoptosis and inhibit the proliferation of renal tubular
epithelial cells (35). In the
present study, it was found that the knockdown of ERK2 expression
in podocytes overexpressing CXCL16 significantly prevented the
inhibition of podocyte proliferation, podocyte apoptosis and the
increase in podocyte migratory ability induced by CXCL16; this
suggested that CXCL16 inhibits podocyte proliferation and induces
podocyte apoptosis and migration via ERK2. This study also has some
shortcomings. Due to the poor phosphorylation of ERK1/2, only the
expression of total ERK1/2 protein was detected and the changes in
ERK1/2 phosphorylation should be detected. The next step is to
supplement the changes in ERK1/2 phosphorylation and explore
related signaling pathways.
Recent studies have demonstrated that the EMT of
podocytes is involved in various disease process, such as podocyte
dysfunction, proteinuria and glomerulosclerosis (36,37). Under certain conditions, podocyte
injury leads to phenotypic transition, which causes podocytes to
lose their epithelial characteristics, such as the downregulation
of P-cadherin and ZO-1, so as to obtain the characteristics of
interstitial cells, such as an increase in N-cadherin and vimentin
expression (38); podocytes
acquire the ability of movement and migration, which renders
podocytes unable to maintain the glomerular filtration barrier,
eventually leading to the occurrence of kidney diseases (39,40). For example, Shi et al
(41) report that ginsenoside Rg1
alleviates diabetic nephropathy (DN) caused by podocyte injury by
inhibiting the EMT pathway of podocytes. Xing et al
(42) report that phosphatase and
tensin homolog inhibited the EMT process of podocytes and was an
underlying target for the therapy of DN. In addition, some studies
have confirmed that CXCL16 promotes the occurrence of breast cancer
by regulating ERK1/2 protein and inducing EMT (26,43). In the present study, CXCL16 was
found to induce podocyte EMT by regulating ERK1/2.
In conclusion, the findings of the present study
suggested that the CXCL16/ERK1/2 pathway regulated human podocytes
growth, migration, apoptosis and EMT. In addition, the
CXCL16/ERK1/2 axis was found to be involved in the
pathophysiological mechanisms of PNS. These findings may provide an
underlying target for further and more in-depth research of
PNS.
Acknowledgements
Not applicable.
Funding
Natural Science Foundation of Shandong Province (grant no.
ZR2015HM009) and the Shandong Key Research and Development Program
(grant no. 2017GSF218005) provided the cost of our research
content.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC designed the present study. YC, ZW, QL and MT
performed the experiments. YC and SS analyzed the data. YC and ZW
drafted the manuscript and analyzed data. YC, ZW, QL, MT, YZ, LY
and JW collected and interpreted data, and revised the final
manuscript. YC and SS confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethical approval and consent to
participate
This study was approved by the clinical research
ethics committee of Shandong Provincial Hospital (approval number
SWYX:NO.2020-152). All samples were obtained with the written
informed consent from the patients (or signed by the child's
guardian) with PNS.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PNS
|
primary nephrotic syndrome
|
|
CXCL16
|
CXC motif chemokine ligand 16
|
|
ERK1/2
|
extracellular signal-regulated kinases
1 and 2
|
References
|
1
|
McCaffrey J, Lennon R and Webb NJ: The
non-immunosuppressive management of childhood nephrotic syndrome.
Pediatr Nephrol. 31:1383–1402. 2016. View Article : Google Scholar
|
|
2
|
Zhao X, Hwang DY and Kao HY: The role of
glucocorticoid receptors in podocytes and nephrotic syndrome. Nucl
Receptor Res. 5:1013232018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian X, Kim JJ, Monkley SM, Gotoh N,
Nandez R, Soda K, Inoue K, Balkin DM, Hassan H, Son SH, et al:
Podocyte-associated talin1 is critical for glomerular filtration
barrier maintenance. J Clin Invest. 124:1098–1113. 2014. View Article : Google Scholar
|
|
4
|
Andolino TP and Reid-Adam J: Nephrotic
syndrome. Pediatr Rev. 36:117–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ranganathan S: Pathology of
podocytopathies causing nephrotic syndrome in children. Front
Pediatr. 4:322016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noris M and Remuzzi G: Non-muscle myosins
and the podocyte. Clin Kidney J. 5:94–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reiser J and Altintas MM: Podocytes.
F1000Res. 5:F10002016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu T, Xie C, Bhaskarabhatla M, Yan M,
Leone A, Chen SS, Zhou XJ, Putterman C and Mohanet C: Excreted
urinary mediators in an animal model of experimental immune
nephritis with potential pathogenic significance. Arthritis Rheum.
56:949–959. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teramoto K, Negoro N, Kitamoto K, Iwai T,
Iwao H, Okamura M and Miura K: Microarray analysis of glomerular
gene expression in murine lupus nephritis. J Pharmacol Sci.
106:56–67. 2018. View Article : Google Scholar
|
|
10
|
Lin Z, Gong Q, Zhou Z, Zhang W, Liao S,
Liu Y, Yan XX, Pan X, Lin S and Li X: Increased plasma CXCL16
levels in patients with chronic kidney diseases. Eur J Clin Invest.
41:836–845. 2011. View Article : Google Scholar
|
|
11
|
Gong Q, Wu F, Pan X, Yu J, Li Y, Lu T, Li
X and Lin Z: Soluble C-X-C CXC motif chemokine ligand 16 levels are
increased in gout patients. Clin Biochem. 45:1368–1373. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia GE, Truong LD, Li P, Zhang P,
Johnson RJ, Wilson CB and Feng L: Inhibition of CXCL16 attenuates
inflammatory and progressive phases of anti-glomerular basement
membrane antibody-associated glomerulonephritis. Am J Pathol.
170:1485–1496. 2017. View Article : Google Scholar
|
|
13
|
Riedel JH, Paust HJ, Turner JE, Tittel AP,
Krebs C, Disteldorf E, Wegscheid C, Tiegs G, Velden J, Mittrücker
HW, et al: Immature renal dendritic cells recruit regulatory
CXCR6(+) invariant natural killer T cells to attenuate crescentic
GN. J Am Soc Nephrol. 23:1987–2000. 2012. View Article : Google Scholar
|
|
14
|
Liu B, Zhang H, Tan X, Yang D, Lv Z, Jiang
H, Lu J, Baiyun R and Zhang Z: GSPE reduces lead-induced oxidative
stress by activating the Nrf2 pathway and suppressing miR153 and
GSK-3βn rat kidney. Oncotarget. 8:42226–42237. 2017. View Article : Google Scholar
|
|
15
|
Zheng X, Li S, Li J, Lv Y, Wang X, Wu P,
Yang Q, Tang Y, Liu Y and Zhang Z: Hexavalent chromium induces
renal apoptosis and autophagy via disordering the balance of
mitochondrial dynamics in rats. Ecotoxicol Environ Saf.
204:1110612020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang D, Yang Q, Fu N, Li S, Han B, Liu Y,
Tang Y, Guo X, Lv Z and Zhang Z: Hexavalent chromium induced heart
dysfunction via Sesn2-mediated impairment of mitochondrial function
and energy supply. Chemosphere. 264((Pt 2)): 1285472021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu N and Malemud CJ: Extracellular
signal-regulated kinase: A regulator of cell growth, inflammation,
chondrocyte and bone cell receptor-mediated gene expression. Int J
Mol Sci. 20:37922019. View Article : Google Scholar
|
|
18
|
Kehat I and Molkentin JD: Extracellular
signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac
hypertrophy. Ann N Y Acad Sci. 1188:96–102. 2010. View Article : Google Scholar
|
|
19
|
Liu F, Yang X, Geng M and Huang M:
Targeting ERK, an Achilles' Heel of the MAPK pathway, in cancer
therapy. Acta Pharm Sin B. 8:552–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boulton TG, Nye SH, Robbins DJ, Ip NY,
Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH
and Yancopoulos GD: ERKs: A family of protein-serine/threonine
kinases that are activated and tyrosine phosphorylated in response
to insulin and NGF. Cell. 65:663–675. 1991. View Article : Google Scholar
|
|
21
|
Lakshmanan AP, Thandavarayan RA, Watanabe
K, Sari FR, Meilei H, Giridharan VV, Sukumaran V, Soetikno V,
Arumugam S, Suzuki K and Kodama M: Modulation of AT-1R/MAPK cascade
by an olmesartan treatment attenuates diabetic nephropathy in
streptozotocin-induced diabetic mice. Mol Cell Endocrinol.
348:104–111. 2012. View Article : Google Scholar
|
|
22
|
Zhou D, Zhou M, Wang Z, Fu Y, Jia M, Wang
X, Liu M, Zhang Y, Sun Y, Zhou Y, et al: Progranulin alleviates
podocyte injury via regulating CAMKK/AMPK-mediated autophagy under
diabetic conditions. J Mol Med (Berl). 97:1507–1520. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu M, Liang K, Zhen J, Zhou M, Wang X,
Wang Z, Wei X, Zhang Y, Sun Y, Zhou Z, et al: Sirt6 deficiency
exacerbates podocyte injury and proteinuria through targeting notch
signaling. Nat Commun. 8:4132017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Wang Z, Li Q, Yu L, Zhu Y, Wang J
and Sun S: OxLDL promotes podocyte migration by regulating CXCL16,
ADAM10 and ACTN4. Mol Med Rep. 22:1976–1984. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–8. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao G, Wang X and Wang J, Zu L, Cheng G,
Hao M, Sun X, Xue Y, Lu J and Wang J: CXCL16/CXCR6 chemokine
signaling mediates breast cancer progression by pERK1/2-dependent
mechanisms. Oncotarget. 6:14165–14178. 2015. View Article : Google Scholar
|
|
27
|
Xiao XC, Gu YL, Chen SX, Wang GJ and Yuan
L: Effect of SHIP-1 on invasion, migration and PI3K-AKT signaling
pathway of leukemic cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
26:324–329. 2018.(In Chinese).
|
|
28
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar
|
|
29
|
Kang YS, Li Y, Dai C, Kiss LP, Wu C and
Liu Y: Inhibition of integrin-linked kinase blocks podocyte
epithelial-mesenchymal transition and ameliorates proteinuria.
Kidney Int. 78:363–373. 2010. View Article : Google Scholar
|
|
30
|
Wang L, Sun S, Zhou A, Yao X and Wang Y:
oxLDL-induced lipid accumulation in glomerular podocytes: Role of
IFN-gamma, CXCL16, and ADAM10. Cell Biochem Biophys. 70:529–538.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kwoh C, Shannon MB, Miner JH and Shaw A:
Pathogenesis of nonimmune glomerulopathies. Annu Rev Pathol.
1:349–374. 2006. View Article : Google Scholar
|
|
32
|
Takeda T, McQuistan T, Orlando RA and
Farquhar MG: Loss of glomerular foot processes is associated with
uncoupling of podocalyxin from the actin cytoskeleton. J Clin
Invest. 108:289–301. 2001. View
Article : Google Scholar
|
|
33
|
Bergwall L, Wallentin H, Elvin J, Liu P,
Boi R, Sihlbom C, Hayes K, Wright D, Haraldsoon B, Nystrom J and
Buvall L: Amplification of the melanocortin-1 receptor in nephrotic
syndrome identifies a target for podocyte cytoskeleton
stabilization. Sci Rep. 8:157312018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng R, Deng Y, Chen Y, Fan J, Zhang M,
Zhong Y, Zhu R and Wang L: Astragaloside IV attenuates complement
membranous attack complex induced podocyte injury through the MAPK
pathway. Phytother Res. 26:892–898. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang W, Hua T, Li J, Zheng L, Wang Y, Xu
M and Qi G: CXCL16 is activated by p-JNK and is involved in
H2O2-induced HK-2 cell injury via p-ERK signaling. Am J Transl Res.
10:3723–3732. 2018.PubMed/NCBI
|
|
36
|
Ying Q and Wu G: Molecular mechanisms
involved in podocyte EMT and concomitant diabetic kidney diseases:
An update. Ren Fail. 39:474–483. 2017. View Article : Google Scholar
|
|
37
|
Boini KM, Xia M, Xiong J, Li C, Payne LP
and Li PL: Implication of CD38 gene in podocyte
epithelial-to-mesenchymal transition and glomerular sclerosis. J
Cell Mol Med. 16:1674–1685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patrakka J and Tryggvason K: Molecular
make-up of the glomerular filtration barrier. Biochem Biophys Res
Commun. 396:164–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Welsh GI and Saleem MA: The podocyte
cytoskeleton-key to a functioning glomerulus in health and disease.
Nat Rev Nephrol. 8:14–21. 2011. View Article : Google Scholar
|
|
40
|
Ha TS: Roles of adaptor proteins in
podocyte biology. World J Nephrol. 2:1–10. 2013. View Article : Google Scholar
|
|
41
|
Shi Y, Gao Y, Wang T, Wang X, He J, Xu J,
Wu B and Li Y: Ginsenoside rg1 alleviates podocyte EMT passage by
regulating AKT/GSK3 β/β-Catenin pathway by restoring autophagic
activity. Evid Based Complement Alternat Med. 30:19036272020.
|
|
42
|
Xing L, Liu Q, Fu S, Li S, Yang L, Liu S,
Hao J, Yu L and Duan H: PTEN inhibits high glucose-induced
phenotypic transition in podocytes. J Cell Biochem. 116:1776–1784.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mir H, Kapur N, Gales DN, Sharma PK,
Oprea-Ilies G, Johnson AT, Singh R and Singh S: CXCR6-CXCL16 axis
promotes breast cancer by inducing oncogenic signaling. Cancers
(Basel). 13:35682021. View Article : Google Scholar : PubMed/NCBI
|