Introduction
Ischemic stroke is a refractory disease that can
seriously harm human health and life. At present, the main
treatment of stroke is to restore the blood supply to ischemic area
as soon as possible to rescue dying neurons, glial cells and
vascular endothelial cells (1). For
acute ischemic stroke, the only effective way to restore blood
supply is to use thrombolysis drugs and endovascular therapy within
3–4.5 h of the onset. This narrow treatment window and various
complications, such as aneurysmal perforations induced by the
microcatheter and thromboembolic events, also limit the use of
endovascular therapy (2). How to
effectively restore the blood supply in the ischemic brain tissue
has become a key focus for stroke research (3). Stem cell-based therapy has been
intensively applied to ischemic diseases. A number of previous
studies have confirmed that the transplantation of stem cells can
reduce tissue damage after ischemia and can promote the functional
recovery of injured tissues (4–6).
Endothelial progenitor cells (EPCs) have been shown to promote
angiogenesis in vitro and in vivo (7,8).
However, there are always risks involved in stem cell
transplantation, such as vascular embolism caused by transplanted
cells, genetic variation of cells cultured repeatedly in
vitro, and the possibility of tumorigenesis and teratogenesis.
Recently, the transport function and mechanism of extracellular
microbubbles have attracted increased attention in various
disciplines (9). In a broad sense,
there are two extracellular vesicles: Exosomes and microvesicles.
Microvesicles are ectosomes, or microparticles, a type of
extracellular vesicle released from the cell membrane and are often
uneven in size (diameter, ~1,000 nm). Exosomes are relatively
uniform in size and form from the membrane of polyvesicles in the
cell (10). Exosomes contain
proteins, lipids, coding or non-coding RNAs and other bioactive
substances similar to the source cells, such as cytokines and
growth factors, and serve an important role in regulating the
physiological functions of cells (11). A number of previous studies have
investigated the repair of tissue damage by stem cell-derived
exosomes. For example, the direct transplantation of exosomes
secreted by stem cells into damaged tissues was reported to have a
similar role in repairing tissue damage as that of transplanted
stem cells (12). In addition,
mesenchymal stem cell (MSC)-derived exosomes can promote the
regeneration of nerval blood vessels to enhance the recovery of
nerve function (13). In animal
models of stoke and brain injury, MSC-derived exosomes were shown
to enhance the coordination ability of movement by a horizontal
transfer of mRNA, improving post-stroke neuroregeneration and
rescuing cognitive impairments (14–16).
In addition, EPC-derived exosomes have exhibited anti-apoptosis
activity that promotes the proliferation and angiogenesis of
endothelial cells (14) and the
proliferation and differentiation of vascular endothelial cells
(17). Sahoo et al (18) reported that the exosomes secreted by
CD34+ stem cells promote proliferation, migration and
angiogenesis of endothelial cells in vitro. Therefore, stem
cell-derived exosomes may also serve a role in promoting cell
regeneration and repair, and they may be used to replace stem cells
for therapy, thus avoiding the immune rejection that may result
from stem cell transplantation.
In the present study, stem cell-derived exosomes
were isolated and purified, and the effect and mechanism of repair
on ischemia-reperfusion (IR) brain injury were investigated in
model rats. The findings may provide a new insight on alleviating
ischemic brain injury by EPCs and may facilitate the clinical
translation of stem cell regenerative medicine.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (n=35; weight, 300 g;
age, 9–10 weeks), were purchased from Slykingda Experimental Animal
[Hunan, China; permit no. scxk (Xiang) 2016–0002]. Pregnant SD rats
(n=3; weight 300 g; age, 9–10 weeks), were purchased from Tianqin
Biotech [permit no. scxk (Xiang) 2016–0217]. All animal experiments
and animal care were conducted in accordance with the criteria of
the Laboratory Animals Welfare Act, the Guide for the Care and Use
of Laboratory Animals provided by the Institutional Animal Care and
Use Committee of Nanchang University. All experimental protocols
for the use of animals were approved by the Animal Care and Use
Committee of Nanchang University (Nanchang, China). All rats were
housed under pathogen-free conditions at 30–70% humidity and 26°C
and had access to standard rodent food and water ad libitum
and maintained under a 12-h light/dark cycle. Experiments were
performed on rats between 7 and 10 weeks of age. Animals were
euthanized after completion of the experiments and prior to tissue
collection by CO2 asphyxiation at a flow rate of 20%
cage volume displacement/minute (5 l/min). Death after exposure to
CO2 was confirmed based on careful assessment of the
rats for cardiac arrest.
Reagents and instruments
TUNEL assay kit (cat. no. C1088) was purchased from
Beyotime Institute of Biotechnology. Rabbit antibodies against CD31
(cat. no. bs-20321R; 1:1,000) and GSK-3β (cat. no. bs-0028R;
1:1,000) were obtained from BIOSS; rabbit antibody against VEGF
(cat. no. AF5109; 1:1,000) was obtained from Affinity; mouse
monoclonal antibodies against β-actin (cat. no. TA-09; 1:2,000);
HRP-conjugated goat anti-mouse IgG (H + L; cat. no. ZB-2305;
1:2,000) and HRP-conjugated goat anti-rabbit IgG (H + L; cat. no.
ZB-2301; 1:2,000) were purchased from Zhongshan Golden Bridge
Biotechnology Co., Ltd (OriGene Technologies, Inc.); rabbit
polyclonal anti-phosphorylated (p)-GSK-3β (cat. no. AF2016; 1:500)
was purchased from Affinity Biosciences, Ltd.; PVDF membrane (cat.
no. IPVH00010) was purchased from MilliporeSigma; SuperSignal West
Pico Chemiluminescent Substrate (cat. no. 34077) was obtained from
Thermo Fisher Scientific, Inc.; Ultrasensitive Chemiluminescence
Imaging system (ChemiDoc XRS+) and CFX Connect Real-Time
PCR Detection system were purchased from Bio-Rad Laboratories, Inc.
Ultrapure RNA Extraction kit (cat. no. CW0581M) was purchased from
CWBIO; HiScript II Q RT SuperMix for qPCR (cat. no. R223-01) was
obtained from Vazyme; and Universal SYBR Green qPCR Master Mix was
purchased from Applied Biosystems; Thermo Fisher Scientific,
Inc.
EPC isolation
EPC isolation was performed as reported previously
(19). Briefly, 3-day-old neonatal
SD rats (n=3) from the pregnant females were sacrificed by
decapitation and sterilized by soaking in 75% ethanol for 5 min.
The tibia and femur were isolated, and the attached muscles were
removed. The tibia and femur were washed with PBS and the bone
marrow was washed into a Petri dish. The bone marrow was repeatedly
pipetted to form a single cell suspension, which was then carefully
added to the surface of 4 ml mixture of Ficoll, hydroxyethyl starch
550 and meglumine diatrizoate (20)
and centrifuged at 500 × g for 20 min at 25–26°C. The cells in the
buffy coat fractions were collected, diluted with EBM-2 medium
(cat. no. CC-3156; Lonza Group, Ltd.) and pelleted at 500 × g at
25–26°C for 5 min. Cells were resuspended in EBM-2 medium and
cultured in 2% CO2 at 37°C. The cells were then cultured
in serum-free EBM-2 medium in a 2% CO2 incubator at 37°C
for 48 h, collected and stored at −80°C until exosome extraction.
All operations were performed in laminar hoods to avoid microbial
contamination.
Immunofluorescence assay
EPCs (104 cells/ml) were inoculated onto
a microscope cover glass and cultured in EBM-2 medium in 2%
CO2 at 37°C until cells reached 90% confluency. The
slides were washed with PBS three times (3 min each), fixed at
25–26°C with 4% paraformaldehyde for 15 min and permeated with 0.5%
Triton X-100 (prepared in PBS) at room temperature for 20 min. The
slides were then soaked in PBS for 5 min for three times (3 min
each) at 25–26°C. Cells were blocked with 5% BSA (CoWin
Biosciences) at 37°C for 30 min. Diluted primary rabbit
anti-coagulation factor VIII antibody (cat. no. bs-2974R; BIOSS;
1:200) was added and the slides were incubated at 4°C overnight.
The slides were subsequently incubated with Cy3-conjugated goat
anti-rabbit IgG secondary antibody (1:200; cat no. S0011; Affinity
Biosciences) at 37°C for 30 min. The nuclei were stained with DAPI
at 25–26°C for 1 h and the slides were examined under a
fluorescence microscope.
Exosome extraction
EPCs were rapidly thawed at 37°C and the supernatant
was centrifuged at 2,000 × g for 30 min at 4°C. The supernatant was
centrifuged again at 12,000 × g for 45 min at 4°C to remove larger
vesicles. The supernatant was then filtered through a membrane
(0.45 µm pore size) and pelleted by centrifuging at 11,0000 × g for
70 min at 4°C. The pellet was resuspended with 10 ml precooled 1X
PBS. The exosome suspension was injected into a NanoFCM N30E
nanoflow detector (Malvern Instruments, Ltd.) to determine the size
distribution (diameter and number).
Transmission electron microscopy
(TEM)
EPCs were fixed in 2.5% glutaraldehyde at 25–26°C
for 1 h. After washing in pre-cooled PBS, the EPCs were dehydrated
using ethanol and acetone, soaked in embedding solution overnight
at room temperature and embedded in epoxy resin. Embedded cell
blocks were cut into ultrathin sections (50-nm) and stained with 2%
uranyl acetate for 30 min at 25–26°C, and washed with water five
times (10 sec each time). Then, the sections were stained with 1%
lead citrate for 15 min at 25–26°C and washed five times (10 sec
each time) before TEM at 80 kV.
IR model and treatment
A classical suture method was used to establish IR
models (21). Briefly, rats were
anesthetized by intraperitoneal injection of ketamine 100 mg/kg
(Shanghai Hengrui Pharmaceutical Co., Ltd.) and xylazine 10 mg/kg
(Hubei Xinmingtai Chemical Co., Ltd.). A smooth incision was made
along the middle line of the neck to separate bluntly the left
sternocleidomastoid muscles and cervical muscles and to expose the
right common carotid artery. The carotid artery was separated at
the trident point to expose internal and external carotid arteries.
The proximal ends of the right common carotid artery and external
carotid artery were ligated, and the internal carotid artery was
clamped with a vascular clip. A small incision was made on the
right common carotid artery 1 cm away from the trigeminal nerve. A
monofilament was introduced along the carotid artery into the brain
to block the blood flow of the middle cerebral artery. After 2 h
embolization, the thread was withdrawn. Nerve function defect was
evaluated 24 h after reperfusion and scored as follows: i) 0, no
symptoms of nerve injury; ii) 1, incomplete extension of the left
front paw; iii) 2, circling left; iv) 3, falling to the left; and
v) 4, loss of consciousness and unable to walk autonomously
(22).
The 35 male SD rats were randomly divided into five
groups (n=7 rats/group): i) untreated rats (control); ii) sham
operation without plus inserted (sham); iii) IR model rats injected
with 50 µl PBS (model); iv) model rats injected with 50 µl EPC cell
suspensions at 6×106 cells/ml (model + EPC); v) and
model rats injected with 50 µl exosome suspension at 0.6 µg/µl
(model + exosome) once a day for 3 days. The dose of exosomes used
was selected based on a previous study (23).
Three days before modelling, rats were anesthetized,
fixed on a brain stereotaxic apparatus and a 1.5 cm longitudinal
incision was made in the middle of the skin of the head. A skull
drill was used to make a hole 0.22 mm posterior to and 10 mm to the
right side of the bregma; care was taken to avoid damaging the
dura. A total of 50 µl suspension (aforementioned) was administered
using a microsyringe injector into the lateral ventricle below the
surface of the skull. The needle was withdrawn slowly 5 min
following injection, and the injection site was sterilized twice
with iodophor and sutured. The mice were then placed on a thermal
pad and reared in the cage when the animals were awake from the
anesthesia.
2,3,5-triphenyltetrazolium chloride
(TTC) staining
Rats were anesthetized as aforementioned and
perfused with 20 ml PBS into the brain. The brain was dissected to
isolate the cerebellum, brain stem and olfactory bulb. The brain
tissues were frozen at −20°C for 30 min and the cerebellum and
olfactory bulb were removed. The remaining brain tissue was
sectioned (2 mm thick) and stained in 2% TTC dye solution in the
dark at 37°C for 15 min; during this period, the sections were
turned over every 5 min. The infarcted area was gray-white, and the
non-infarcted area was dark red.
Hematoxylin and eosin (H&E)
staining
H&E staining was conducted to examine the tissue
damage as previously described (24). Briefly, the brain tissue was
dehydrated in an ascending series of ethanol (70, 80, 90 and 100%)
at 25–26°C for 5 min at each concentration and cleared with xylene.
Dehydrated tissue was embedded in paraffin, sectioned (4-µm thick),
dewaxed with xylene and rehydrated in a series of ethanol (100, 90,
80, 70, 50, 30 and 0%) at 25–26°C for 5 min at each concentration.
The sections were stained with an aqueous hematoxylin solution at
25–26°C for 3 min, differentiated with hydrochloric acid for 15
sec, briefly washed with tap water at 25–26°C for 60 sec, and
counterstained with eosin at 25–26°C for 3 min. Sections were
washed in distilled water, dehydrated and cleared as previously
described, then the sections were sealed and examined under a CX41
light microscope (Olympus Corporation) at ×200 magnification to
observe pathological changes, including the number of nerve cells
and glial cells.
TUNEL assay
A TUNEL assay was used to detect apoptotic cells in
the brain tissues as described previously (25). Briefly, brain tissue sections (4-µm)
were baked at 65°C for 2 h, rehydrated in a descending ethanol
series and treated with proteinase K (50 µg/ml) for 30 min at 37°C.
The sections were rinsed with PBS three times (5 min each) and
incubated with TUNEL detection solution at 37°C in the dark for 1
h, according to the supplier's protocols. The slide was then
incubated with DAPI at room temperature in the dark for 3 min; the
excess DAPI was rinsed away with PBS and the slide was blotted dry
with absorbent paper. The slides were sealed with anti-fluorescence
quenching solution and observed under a CX41 fluorescence
microscope (Olympus Corporation) in 10 fields of view.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the brain tissues using
an RNA Extraction kit (Takara Bio, Inc.) according to the
manufacturer's instructions. RNA concentrations were quantified
using a Nanodrop spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.) and subsequently reverse transcribed into
cDNA using the High-Capacity cDNA Transcriptase Reverse kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol. qPCR was conducted using the Universal
SYBR Green qPCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on a CFX96 Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.) using the primers listed in Table I. Relative mRNA expression levels
were determined using the 2−ΔΔCq method after
normalization with β-actin as an internal reference (26). qPCR was carried out in a total
volume of 15 µl containing 1 µl of diluted and pre-amplified cDNA,
10 µl Universal SYBR Green qPCR Master Mix and 1.5 µl of each
forward and reverse primer. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 10 min; followed by 40
cycles of 95°C for 15 sec and 57°C for 60 sec.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′-3′) | Primer length,
nt | Amplicon size,
bp | Annealing
temperature,°C |
|---|
| VEGF | F:
AATTGAGACCCTGGTGGACA | 20 | 246 | 58.47 |
|
| R:
CTATCTTTCTTTGGTCTGCATTCAC | 25 |
|
|
| CD31 | F:
AGGTGACAGAAGGTGGGATT | 20 | 299 | 56.85 |
|
| R:
CTGGATTTGAAACTTGGGTG | 20 |
|
|
| β-actin | F:
GCCATGTACGTAGCCATCCA | 20 | 375 | 59.53 |
|
| R:
GAACCGCTCATTGCCGATAG | 20 |
|
|
Immunofluorescence assays
Brain sections (4-µm) were fixed at 25–26°C in 4%
paraformaldehyde for 10–15 min and rinsed three times with PBS (3
min each). The cells were cleared with 0.5% Triton X-100 (in PBS)
at room temperature for 20 min and washed with PBS three times (5
min each). After blocking with 5% BSA at 37°C for 30 min, anti-CD31
antibody (1:1,000) was added and the plates were incubated
overnight at 4°C. The plates were then immersed in PBS three times
(3 min each) and incubated with Cy3-conjugated goat anti-rabbit IgG
(1:200; cat. no. CW0159S; CoWin Biosciences) at 25–26°C for 1 h.
Subsequently, the slides were incubated with an anti-VEGF antibody
(1:1,000) at 25–26°C for 30 min and then incubated with diluted
Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:200; cat. no.
ZF-0511; Zhongshan Golden Bridge Biotechnology Co., Ltd; OriGene
Technologies) at 37°C for 45 min and counterstained with DAPI at
25–26°C in the dark for 5 min. Images were captured using a
fluorescence microscope (Olympus Corporation).
Western blotting
Brain tissues (0.2 g) were lysed with RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd.) containing
protease inhibitors cocktail and quantitated using a BCA kit (CoWin
Biosciences) according to the manufacturer's instructions. After
denaturing by boiling at 100°C for 5 min, 50 µg protein was
separated by 10% SDS-PAGE, transferred to PVDF membranes, blocked
with 5% non-fat milk in 1X TBS-0.1% Tween-20 buffer for 4 h at room
temperature and then detected by incubation with the following
primary antibodies (at the aformentioned dilutions) at 4°C
overnight: Mouse monoclonal anti-β-actin, rabbit polyclonal
anti-Wnt3α, rabbit polyclonal anti-Gsk-3β and rabbit polyclonal
anti-p-Gsk-3β. Subsequently, the membranes were incubated with
HRP-conjugated goat anti-mouse IgG or HRP-conjugated goat
anti-rabbit IgG secondary antibodies (at the aformentioned
dilutions) at 25–26°C for 1 h. Protein bands were visualized using
the SuperSignal West Pico Chemiluminescent Substrate (cat. no.
34077; Thermo Fisher Scientific, USA). Densitometric analysis was
conducted using Quantity One software (version v4.6.6; Bio-Rad
Laboratories, Inc.) using β-actin as the internal control.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean obtained from at least three independent experiments.
Statistical comparisons between groups were assessed using one-way
ANOVA with Tukey's post hoc tests. Ordinal data obtained for nerve
defect scoring were analyzed using the Kruskal-Wallis test followed
by Dunn's post hoc tests. Statistical analysis was performed using
SPSS 21.0 software (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Characterization of EPCs and
exosomes
Immunofluorescence results demonstrated that
isolated EPCs had red fluorescence with a wavelength 640 nm emitted
from factor VIII (Fig. 1A). TEM and
nanoflow measurements confirmed that the isolated exosomes
exhibited the cup-like shape with double membranes (Fig. 1B) and were in the expected size
range (30–299 nm), with the majority of exosomes being 60–80 nm in
diameter (Fig. 2).
EPC and exosome treatment reduce
infarcted area and nerve defects
Results from TTC staining revealed that the
infarcted area increased significantly after IR modeling compared
with the control and sham groups (Fig.
3A). EPC and exosome treatments significantly reduced the
infarcted area compared with the untreated model group (Fig. 3A). Similarly, IR modelling
significantly increased nerve defects compared with the control
(Fig. 3B); the EPC and exosome
treatments significantly reduced the defect score (Fig. 3B), and the improvement was more
notable with exosome than with EPC (P<0.05; Fig. 3B).
EPC and exosome treatment reduce
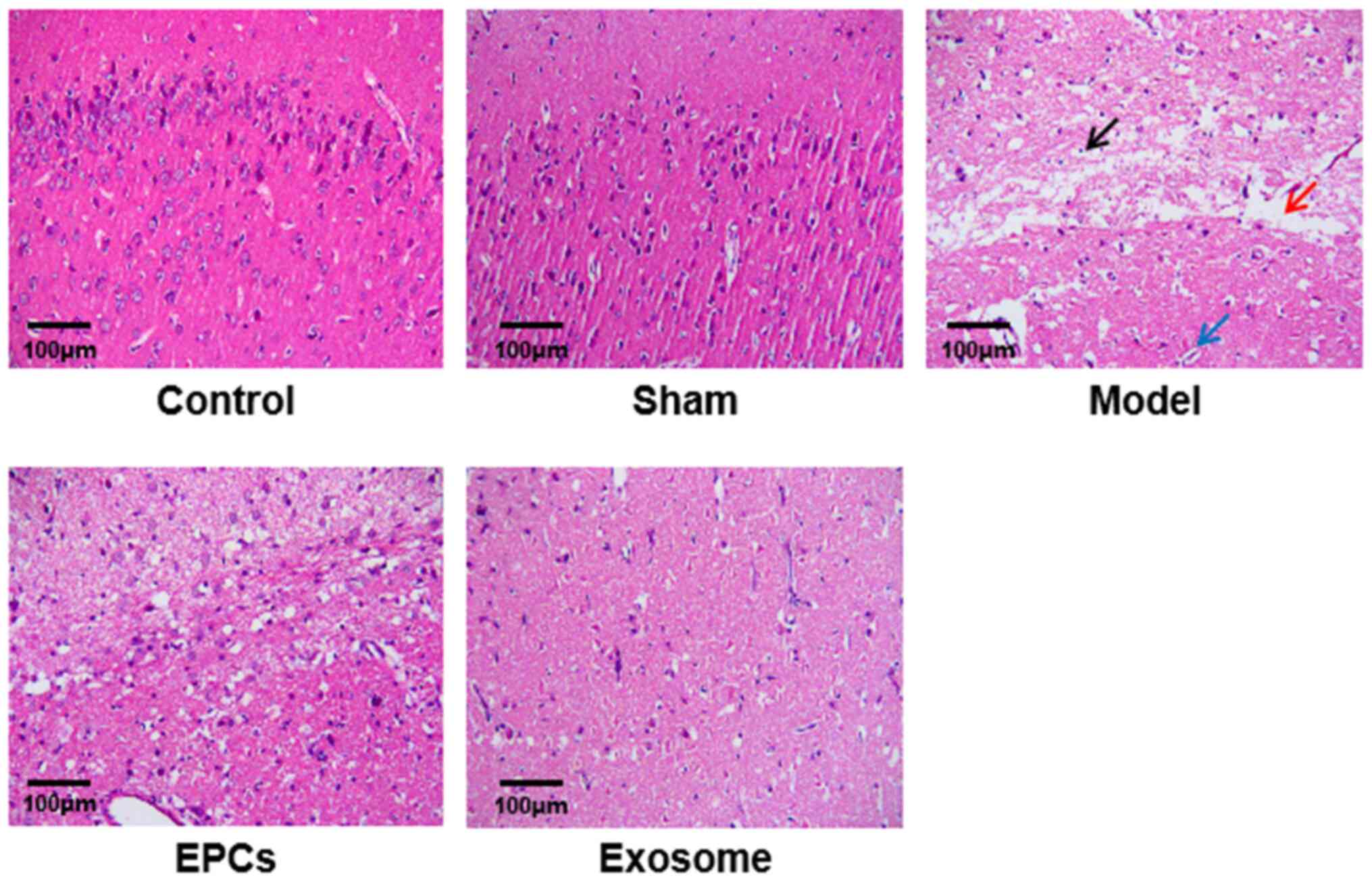
IR-induced degeneration and necrosis of nerve cells
H&E staining revealed that in control and sham
rats, the boundary of the cortex and gray matter of the brain was
clear without edema and necrosis; the nerve cells were arranged
orderly and evenly, the cell membrane was intact and there was a
clear nucleus and nucleolus (Fig.
4). In model group, the nerve fibers were slightly necrotic and
swollen, the number of nerve cells was reduced and glial cells were
proliferated. In the EPC-treated rats, the brain tissue showed mild
liquefaction and degeneration, mild edema in the stroma and
decreased number of nerve cells which were distributed less evenly;
glial cells were proliferated. In the exosome-treated rats, the
distribution of nerve cells was more uniform, the degeneration and
necrosis of cells were less intensive, and the proliferation of
glial cells was remarkable (Fig.
4).
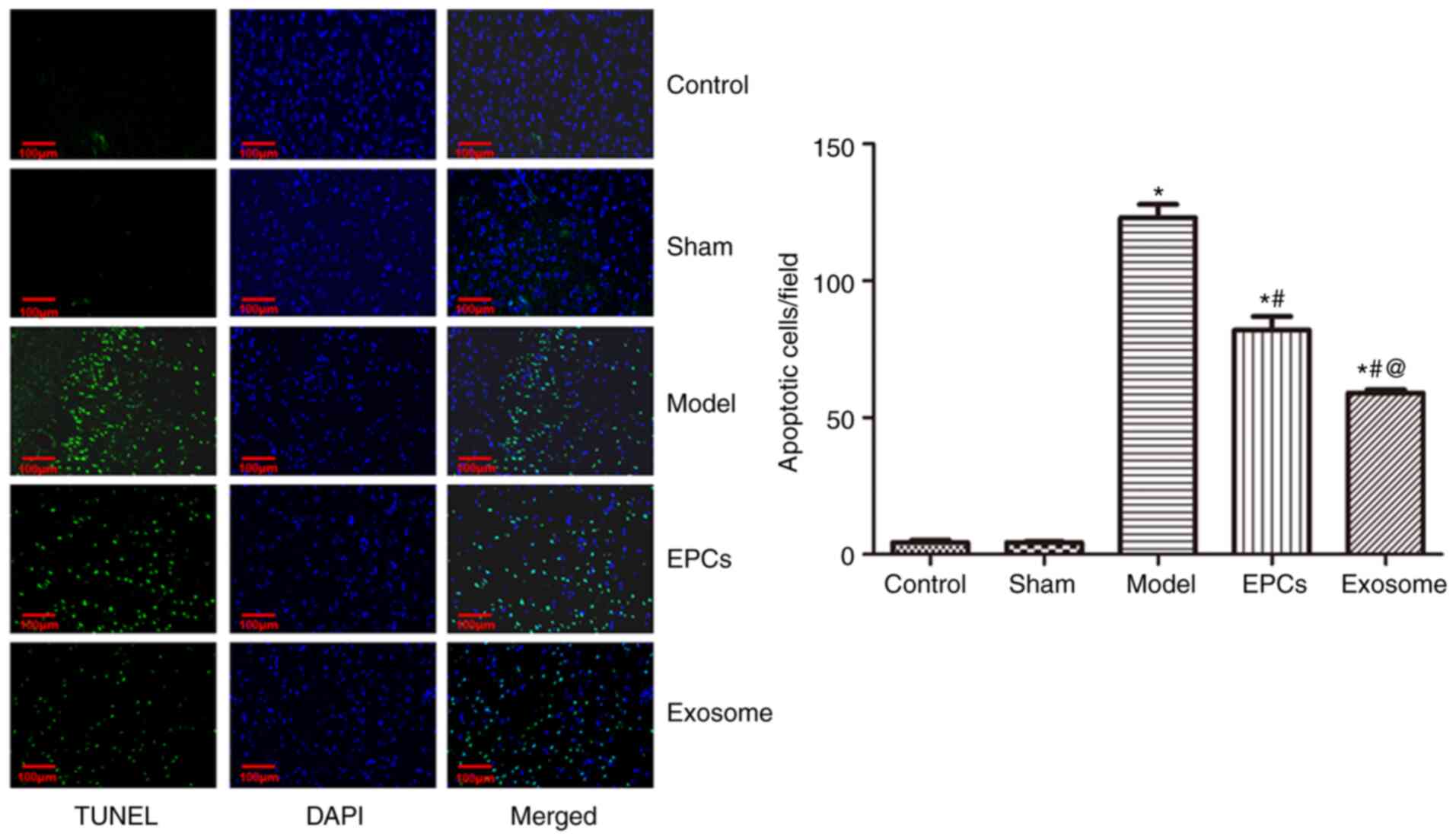
EPC and exosome treatments reduce
IR-induced apoptosis in nerve cells
The TUNEL assay results revealed that, compared with
the control, the number of apoptotic cells was significantly
increased after IR modelling (P<0.05; Fig. 5). Compared with the untreated model
group, apoptosis was significantly decreased following EPC and
exosome treatments (both P<0.05). Compared with EPC treatment,
model rats treated with exosomes exhibited a significant reduction
in apoptosis (P<0.05).
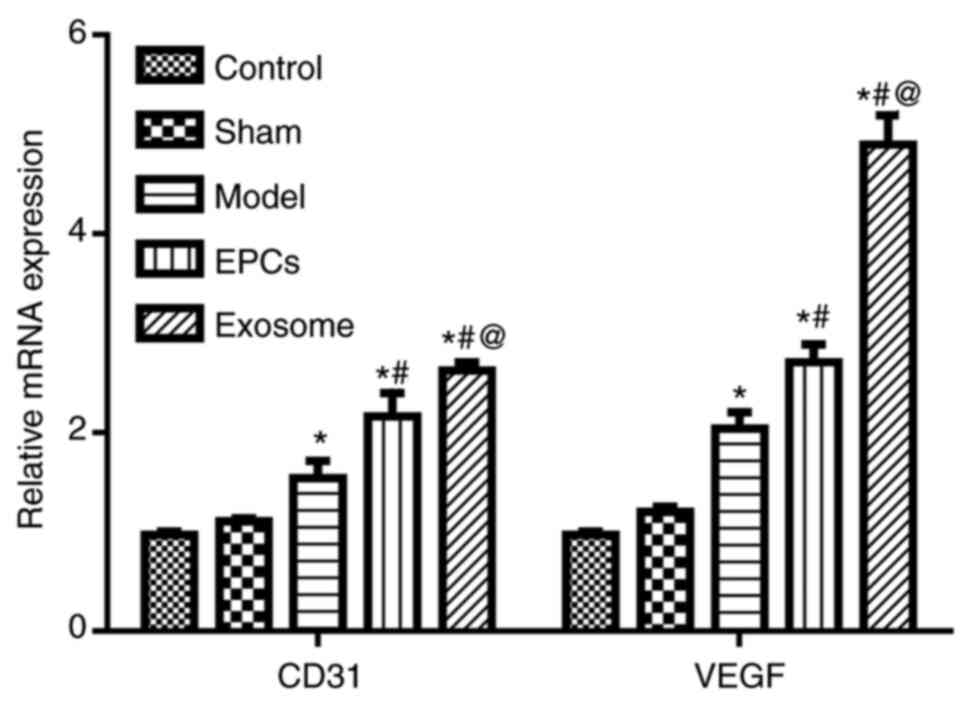
EPC and exosome treatments upregulate
CD31 and VEGF expression
qPCR results revealed that the mRNA expression
levels of CD31 and VEGF were significantly increased after IR
modelling compared with the control group (P<0.05; Fig. 6); the expressions were further
upregulated following EPC and exosome treatments compared with the
untreated model group (both P<0.05). Similarly,
immunofluorescence assay results demonstrated that the protein
expression levels of CD31 and VEGF were significantly increased
after IR modelling (P<0.05) compared with the control and sham
groups, and they were further upregulated following EPC and exosome
treatments (P<0.05), particularly with exosomes (P<0.05)
(Fig. 7) compared with the model
group.
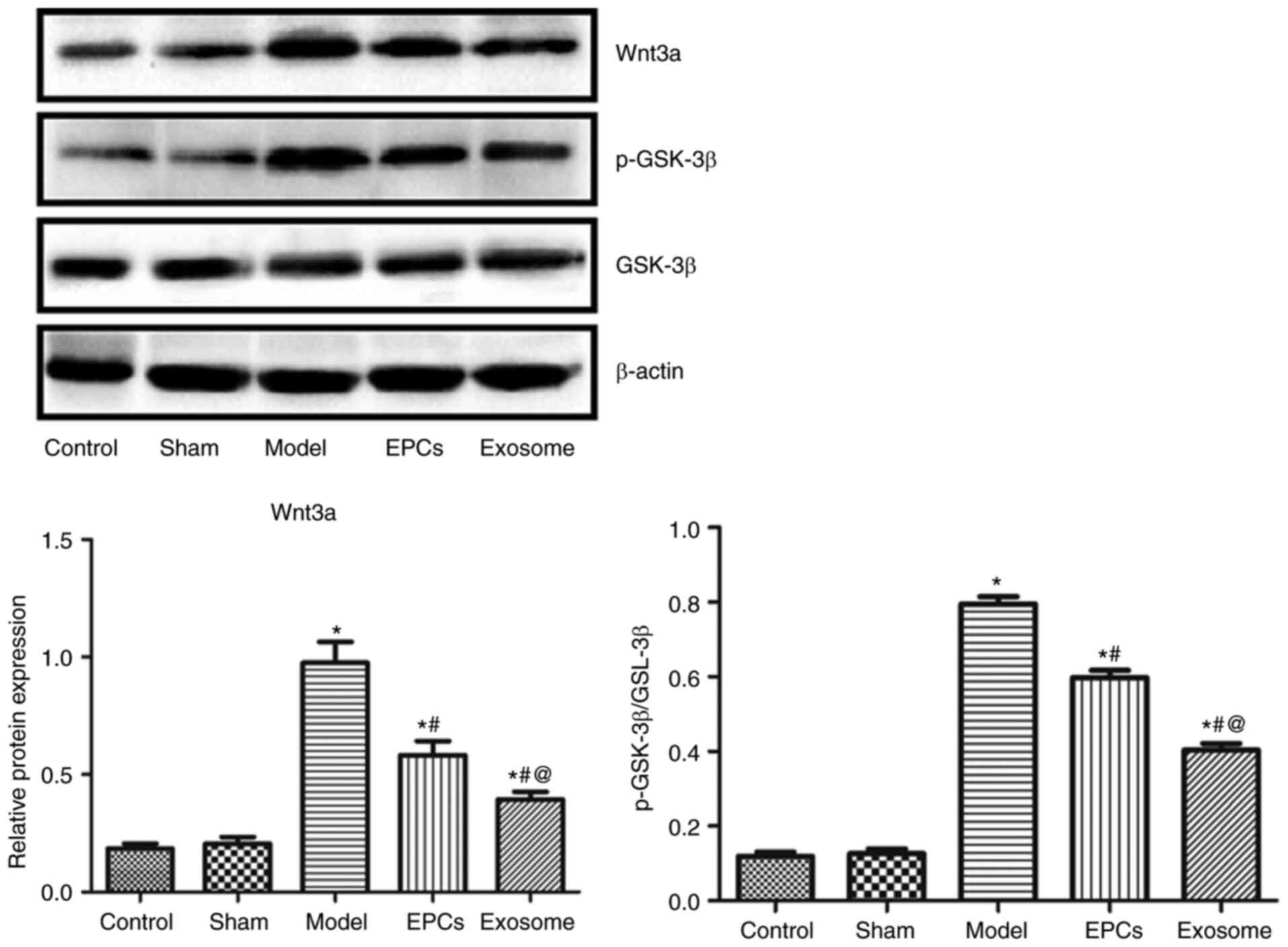
EPC and exosome treatment
downregulated Wnt3a and p-GSK-3 expression
Western blot analysis revealed that the protein
expression levels of Wnt3a, GSK-3β and p-GSK-3β were significantly
increased after IR modelling (P<0.05) compared with the control
and sham groups, and were significantly downregulated in model rats
treated with EPCs or exosomes compared with the control and sham
groups; no significant difference in the ratio of p-GSK-3β to
GSK-3β expression levels were detected (P>0.05) compared with
the control and sham groups (Fig.
8). Exosome treatment resulted in a more marked downregulation
compared with EPC treatment (P<0.05).
Discussion
Stroke is a common disease with high morbidity. It
can be divided into hemorrhagic stroke and ischemic stroke, which
is more common (27). To better
understand ischemic cerebrovascular disease, it is very important
to establish a relevant animal model for experimental
investigation. In the present study, the classical suture method
was used to occlude the middle cerebral artery to generate an IR
model rat. The success of modelling was confirmed by the presence
of infarcted area and reduced nerve defect score. Exosomes are
extracellular vesicles with a diameter of 30–100 nm and a double
layered membrane; they contain a variety of bioactive substances
such as proteins, lipids and nucleic acids (28). Exosomes could stably exist in
extracellular spaces and deliver proteins and RNA to the targeted
cells to reprogram the recipient cells (29) and could play an important role in
various physiological and pathological processes (30). In the present study, EPCs were
isolated, and exosomes were extracted from the supernatant of EPCs.
TEM revealed that the extraction of exosomes was successful, as the
extracted exosomes exhibited the expected double membrane structure
and size.
A previous study reported that MSC-derived exosomes
could improve the recovery of neural function by promoting
neurovascular regeneration (13).
Therapeutic effects of MSC-derived exosomes have been confirmed in
animal models of stroke and brain injury, resulting in significant
improvements in motor coordination and space learning ability
(14–16). In addition, previous studies have
demonstrated that EPC-secreted exosomes promote the proliferation
and vessel formation of endothelial cells (14), as well as the proliferation and
differentiation of vascular endothelial cells through
anti-apoptotic effects (17). Bian
et al (31) found that the
exosomes from bone marrow MSCs promote angiogenesis in ischemic
myocardium, reduce myocardial infarction area and improve cardiac
function. Sahoo et al (18)
also demonstrated that the exosomes secreted by CD34+
stem cells promote the proliferation, migration and vessel
formation of endothelial cells in vitro.
CD31 is present on the surface of platelets,
neutrophils, monocytes and certain types of T cells, as well as in
the junctions between endothelial cells. It may be involved in
leukocyte migration, angiogenesis and integrin activation (32,33).
The expression of CD31 is significantly upregulated after cerebral
ischemia and is further increased after treatment with exosomes
(19). The angiogenesis of
endothelial cells is regulated by a number of angiogenic genes,
including VEGF (17,34). VEGF is an important angiogenic
factor that mediates the proliferation and migration of endothelial
cells and maintains the survival of vascular endothelium after
binding with kinase insert domain receptor in vascular endothelial
cells (35). EPCs usually exist in
bone marrow. When peripheral tissues are damaged by ischemia and
hypoxia, VEGF and other substances are produced in the injured
tissues to mobilize and recruit EPCs to the ischemic and hypoxic
tissues, where the EPCs are integrated into the blood vessels to
promote the extension of the original blood vessels and to provide
materials for angiogenesis by secreting a variety of
angiogenesis-related substances, such as VEGF (36,37).
As a consequence, angiogenesis and functional recovery of ischemic
tissues are facilitated (38,39).
Data from the present study demonstrated that the expression of
CD31 and VEGF increased significantly after cerebral ischemia and
further increased after EPC or exosome treatment, suggesting that
one of the mechanisms underlying exosome-mediated angiogenesis is
to upregulate the expression of angiogenesis related-genes and
proteins in the endothelial cells, thus promoting angiogenesis.
The Wnt signaling pathway is a well-known
intracellular signaling pathway, which is highly conserved
evolutionally. This pathway is involved in the proliferation,
differentiation and axon formation of neural stem cells, and serves
an important role in the formation and maintenance of the
blood-brain barrier, cerebral vascular regeneration and remodeling
(40). A previous study
demonstrated that Wnt signaling pathway serves an important role in
injury repair and neurovascular remodeling following ischemic
stroke (41). It has been reported
that treatment with the GSK-3β inhibitor TWS119 reduces
neurological deficit score and increases brain edema, infarct
volume and blood-brain barrier damage as a result of Wnt signaling
pathway activation (42). A
previous study also found that the Wnt signaling pathway in rats is
activated when ischemic stroke occurs (35). Activated Wnt signaling pathway is
accompanied with increased GSK-β phosphorylation (43,44).
The present study also revealed that the levels of Wnt3 and p-GSK-β
were increased after cerebral ischemia. The levels of Wnt3 and
p-GSK-β were downregulated in model rats treated with EPCs or
exosomes, suggesting that EPC derived-exosomes may be
neuroprotective by inhibiting the expression of Wnt3 and
phosphorylation of GSK-β.
There are limitations to the present study. First,
the therapeutic effects of EPC exosomes on ischemic stroke were
investigated in vivo, but not in vitro. The dose of
EPC exosomes has not been optimized since only a single dose was
used in this study. Although EPC-derived exosomes serve
neuroprotective effects by inhibiting Wnt3 expression and GSK-β
phosphorylation, as well as promote angiogenesis by upregulating
the expression of angiogenesis-related genes and proteins in
endothelial cells (42), there is a
lack of investigations on the mechanisms underlying its regulation.
Moreover, microRNAs (miRNAs/miRs) in exosomes serve an important
role in injury repair. For example, M1 macrophages are known to
promote inflammation; on other hand, exosomes secreted from
adipose-derived stem cells rich in miR-30d-5p inhibited
autophagy-mediated polarization of microglia to M1, thereby
preventing brain damage caused by inflammation (45). Therefore, it is worthy to screen
miRNAs in EPC-derived exosomes and to investigate their possible
mechanisms. Since this study was focused on the Wnt/GSK-β pathway,
the downstream effectors have not been investigated. Therefore, as
these genes and proteins, such as downstream effectors, are likely
to play role in the observed therapeutic effect, they should be
investigated to further elucidate the therapeutic mechanisms and
potential in attenuating IR-induced damage. For example, Petherick
et al (46) found that the
accumulation of β-catenin inhibited the p62/SQSTM1 promoter,
leading to autophagy inhibition, and Chen et al (47) reported that TNFα inhibits osteogenic
differentiation by inhibiting the Wnt/β-catenin pathway, and
subsequently inhibits autophagy. The use of autophagy inducers
restores the TNFα-mediated differentiation process and positively
regulates the Wnt/β-catenin pathway.
In conclusion, the present study demonstrated that
EPC-derived exosomes reduced apoptosis and promoted angiogenesis,
and may serve a protective role to nerve cells with IR-induced
damage. Therefore, exosomes may be considered as a potential
therapeutic agent for stroke, although clinical studies in humans
are required to validate these findings. Compared with other
therapeutics, such as intravenous thrombolysis and endovascular
mechanical thrombectomy, exosomes may target the recipient cells
selectively due to expression of tissue-specific antigens on the
surface of exosome.
Acknowledgements
Not applicable.
Funding
This work was funded by Jiangxi Provincial Science and
Technology Department (grant no. 20161BBH80075).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RH, TC and XL designed the study. RH and TC
collected the data and performed the analyses. RH, TC and XL
drafted the manuscript. RH and XL confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments and animal care were
conducted in accordance with the criteria of the Laboratory Animals
Welfare Act, the Guide for the Care and Use of Laboratory Animals
provided by the Institutional Animal Care and Use Committee
Nanchang University (Nanchang, China). All experimental protocols
for the use of animals were approved by the Animal Care and Use
Committee Nanchang University (Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EPC
|
endothelial progenitor cell
|
|
IR
|
ischemia-reperfusion
|
|
SD
|
Sprague-Dawley rats
|
|
TEM
|
transmission electron microscopy
|
|
TTC
|
2,3,5-triphenyltetrazolium
chloride
|
References
|
1
|
Jena I, Nayak SR, Behera S, Singh B, Ray
S, Jena D, Singh S and Sahoo SK: Evaluation of ischemia-modified
albumin, oxidative stress, and antioxidant status in acute ischemic
stroke patients. J Nat Sci Biol Med. 8:110–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshimura S, Sakai N, Uchida K, Yamagami
H, Ezura M, Okada Y, Kitagawa K, Kimura K, Sasaki M, Tanahashi N,
et al: Endovascular therapy in ischemic stroke with acute
large-vessel occlusion: Recovery by endovascular salvage for
cerebral ultra-acute embolism Japan Registry 2. J Am Heart Assoc.
7:e0087962018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moussouttas M and Papamitsakis NIH:
Critique on the use of early short-term dual antiplatelet therapy
following minor acute cerebral ischemic events. Cerebrovasc Dis.
49:237–243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdelwahid E, Siminiak T, Guarita-Souza
LC, Teixeira de Carvalho KA, Gallo P, Shim W and Condorelli G: Stem
cell therapy in heart diseases: A review of selected new
perspectives, practical considerations and clinical applications.
Curr Cardiol Rev. 7:201–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gutierrez-Fernandez M, Rodriguez-Frutos B,
Ramos-Cejudo J, Otero-Ortega L, Fuentes B and Diez-Tejedor E: Stem
cells for brain repair and recovery after stroke. Expert Opin Biol
Ther. 13:1479–1483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee EJ, Park HW, Jeon HJ, Kim HS and Chang
MS: Potentiated therapeutic angiogenesis by primed human
mesenchymal stem cells in a mouse model of hindlimb ischemia. Regen
Med. 8:283–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Y, Ip JE, Huang J, Zhang L, Matsushita
K, Liew CC, Pratt RE and Dzau VJ: Essential role of ICAM-1/CD18 in
mediating EPC recruitment, angiogenesis, and repair to the
infarcted myocardium. Circ Res. 99:315–322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong XH, Liu H, Wang SJ, Liang SW and Wang
GG: Exosomes derived from SDF1-overexpressing mesenchymal stem
cells inhibit ischemic myocardial cell apoptosis and promote
cardiac endothelial microvascular regeneration in mice with
myocardial infarction. J Cell Physiol. 234:13878–13893. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue M, Chen W, Xiang A, Wang R, Chen H,
Pan J, Pang H, An H, Wang X, Hou H and Li X: Hypoxic exosomes
facilitate bladder tumor growth and development through
transferring long non-coding RNA-UCA1. Mol Cancer. 16:1432017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi
C, Huang NP, Xiao ZD, Lu ZH, Tannous BA and Gao J: Surface
functionalized exosomes as targeted drug delivery vehicles for
cerebral ischemia therapy. Biomaterials. 150:137–149. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M and
Lu L: Exosomes derived from miR-181-5p-modified adipose-derived
mesenchymal stem cells prevent liver fibrosis via autophagy
activation. J Cell Mol Med. 21:2491–2502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG and
Chopp M: Systemic administration of exosomes released from
mesenchymal stromal cells promote functional recovery and
neurovascular plasticity after stroke in rats. J Cereb Blood Flow
Metab. 33:1711–1715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deregibus MC, Cantaluppi V, Calogero R, Lo
Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B and Camussi G:
Endothelial progenitor cell derived microvesicles activate an
angiogenic program in endothelial cells by a horizontal transfer of
mRNA. Blood. 110:2440–2448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim DK, Nishida H, An SY, Shetty AK,
Bartosh TJ and Prockop DJ: Chromatographically isolated CD63+CD81+
extracellular vesicles from mesenchymal stromal cells rescue
cognitive impairments after TBI. Proc Natl Acad Sci USA.
113:170–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doeppner TR, Herz J, Gorgens A, Schlechter
J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B and
Hermann DM: Extracellular vesicles improve post-stroke
neuroregeneration and prevent postischemic immunosuppression. Stem
Cells Transl Med. 4:1131–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cantaluppi V, Biancone L, Figliolini F,
Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R,
Salizzoni M, Tetta C, et al: Microvesicles derived from endothelial
progenitor cells enhance neoangiogenesis of human pancreatic
islets. Cell Transplant. 21:1305–1320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sahoo S, Klychko E, Thorne T, Misener S,
Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, et al:
Exosomes from human CD34(+) stem cells mediate their proangiogenic
paracrine activity. Circ Res. 109:724–728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roy J: Primary microglia isolation from
mixed cell cultures of neonatal mouse brain tissue. Brain Res.
1689:21–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu L, Li X, Zhang E, Liang H, Li W, Wang
S, Song S and Ji A: The effect of leech extracts on endothelial
cell coagulation-related factors and endothelial dysfuction-related
molecules. Clin Exp Hypertens. 41:220–230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao J, Zhao Y, Zheng W, Lu Y, Feng G and
Yu S: Neuroprotective effect of curcumin on transient focal
cerebral ischemia in rats. Brain Res. 1229:224–232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei EQ, Zhu CY, Xu QQ, Yu YP, Zhu YF and
Zheng MZ: An improved quantitative method for evaluating
neurological deficits in mice with focal cerebral ischemia. Sheng
Li Xue Bao. 55:742–747. 2003.(In Chinese). PubMed/NCBI
|
|
23
|
AbuBakr N, Haggag T, Sabry D and Salem ZA:
Functional and histological evaluation of bone marrow stem
cell-derived exosomes therapy on the submandibular salivary gland
of diabetic Albino rats through TGFβ/Smad3 signaling pathway.
Heliyon. 6:e037892020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008.pdb prot4986. 2008.
|
|
25
|
Kyrylkova K, Kyryachenko S, Leid M and
Kioussi C: Detection of apoptosis by TUNEL assay. Methods Mol Biol.
887:41–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esenwa C and Gutierrez J: Secondary stroke
prevention: Challenges and solutions. Vasc Health Risk Manag.
11:437–450. 2015.PubMed/NCBI
|
|
28
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Guo P, He Y, Chen Z, Chen L, Luo
Y, Qi L, Liu Y, Wu Q, Cui Y, et al: HCC-derived exosomes elicit HCC
progression and recurrence by epithelial-mesenchymal transition
through MAPK/ERK signalling pathway. Cell Death Dis. 9:5132018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miranda AM, Lasiecka ZM, Xu Y, Neufeld J,
Shahriar S, Simoes S, Chan RB, Oliveira TG, Small SA and Di Paolo
G: Neuronal lysosomal dysfunction releases exosomes harboring APP
C-terminal fragments and unique lipid signatures. Nat Commun.
9:2912018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bian S, Zhang L, Duan L, Wang X, Min Y and
Yu H: Extracellular vesicles derived from human bone marrow
mesenchymal stem cells promote angiogenesis in a rat myocardial
infarction model. J Mol Med (Berl). 92:387–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Figueiredo CC, Pereira NB, Pereira LX,
Oliveira LAM, Campos PP, Andrade SP and Moro L: Double
immunofluorescence labeling for CD31 and CD105 as a marker for
polyether polyurethane-induced angiogenesis in mice. Histol
Histopathol. 34:257–264. 2019.PubMed/NCBI
|
|
33
|
Shih YT, Wang MC, Yang TL, Zhou J, Lee DY,
Lee PL, Yet SF and Chiu JJ: β(2)-Integrin and Notch-1
differentially regulate CD34(+)CD31(+) cell plasticity in vascular
niches. Cardiovasc Res. 96:296–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shima DT, Gougos A, Miller JW, Tolentino
M, Robinson G, Adamis AP and D'Amore PA: Cloning and mRNA
expression of vascular endothelial growth factor in ischemic
retinas of Macaca fascicularis. Invest Ophthalmol Vis Sci.
37:1334–1340. 1996.PubMed/NCBI
|
|
35
|
Melincovici CS, Bosca AB, Susman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|
|
36
|
Kamel NM, Abd El Fattah MA, El-Abhar HS
and Abdallah DM: Novel repair mechanisms in a renal
ischaemia/reperfusion model: Subsequent saxagliptin treatment
modulates the pro-angiogenic GLP-1/cAMP/VEGF, ANP/eNOS/NO,
SDF-1α/CXCR4, and Kim-1/STAT3/HIF-1α/VEGF/eNOS pathways. Eur J
Pharmacol. 861:1726202019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Liu H, Xu C, Deng M, Song M, Yu X,
Xu S and Zhao X: VEGF promotes endothelial progenitor cell
differentiation and vascular repair through connexin 43. Stem Cell
Res Ther. 8:2372017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kutikhin AG, Sinitsky MY, Yuzhalin AE and
Velikanova EA: Shear stress: An essential driver of endothelial
progenitor cells. J Mol Cell Cardiol. 118:46–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumar VV, Heller M, Gotz H, Schiegnitz E,
Al-Nawas B and Kammerer PW: Comparison of growth & function of
endothelial progenitor cells cultured on deproteinized bovine bone
modified with covalently bound fibronectin and bound vascular
endothelial growth factor. Clin Oral Implants Res. 28:543–550.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kahn M: Wnt signaling in stem cells and
cancer stem cells: A tale of two coactivators. Prog Mol Biol Transl
Sci. 153:209–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oh SH, Kim HN, Park HJ, Shin JY and Lee
PH: Mesenchymal stem cells increase hippocampal neurogenesis and
neuronal differentiation by enhancing the Wnt signaling pathway in
an Alzheimer's disease model. Cell Transplant. 24:1097–1109. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang W, Li M, Wang Y, Li Q, Deng G, Wan J,
Yang Q, Chen Q and Wang J: GSK-3β inhibitor TWS119 attenuates
rtPA-induced hemorrhagic transformation and activates the
Wnt/β-catenin signaling pathway after acute ischemic stroke in
rats. Mol Neurobiol. 53:7028–7036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen S, Sun YY, Zhang ZX, Li YH, Xu ZM and
Fu WN: Transcriptional suppression of microRNA-27a contributes to
laryngeal cancer differentiation via GSK-3β-involved Wnt/β-catenin
pathway. Oncotarget. 8:14708–14718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Liu Y, Shao R and Li W:
Cdc42-interacting protein 4 silencing relieves pulmonary fibrosis
in STZ-induced diabetic mice via the Wnt/GSK-3β/β-catenin pathway.
Exp Cell Res. 359:284–290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang M, Wang H, Jin M, Yang X, Ji H,
Jiang Y, Zhang H, Wu F, Wu G, Lai X, et al: Exosomes from
MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced,
autophagy-mediated brain injury by promoting M2
microglial/macrophage polarization. Cell Physiol Biochem.
47:864–878. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Petherick KJ, Williams AC, Lane JD,
Ordóñez-Morán P, Huelsken J, Collard TJ, Smartt HJ, Batson J, Malik
K, Paraskeva C and Greenhough A: Autolysosomal β-catenin
degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J.
32:1903–1916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen L, Yang Y, Bao J, Wang Z, Xia M, Dai
A, Tan J, Zhou L, Wu Y and Sun W: Autophagy negative-regulating Wnt
signaling enhanced inflammatory osteoclastogenesis from Pre-OCs in
vitro. Biomed Pharmacother. 126:1100932020. View Article : Google Scholar : PubMed/NCBI
|