Introduction
Retinal pathological neovascularization is a primary
characteristic of neovascular age-related macular degeneration,
retinopathy of prematurity, retinal vein occlusion and diabetic
retinopathy and it is the main cause of refractory blindness
worldwide (1,2). Typical choices for treatment of
retinal neovascularization involve vitrectomy, retinal laser
photocoagulation and cryotherapy; however, they are invasive and
the functional results are typically suboptimal (3–6).
Vascular endothelial growth factor (VEGF) and its receptors have
been shown to be pivotal for the production and advancement of
neovascular eye diseases; they have therefore become the ideal
targets for anti-angiogenesis therapy (7). However, anti-VEGF agents can also
induce local and systemic side effects (8). Therefore, to create novel
therapeutic targets, it is necessary to obtain fuller knowledge of
the mechanisms of ocular neovascularization (6).
The pathogenesis for ocular vascular diseases is
associated with hypoxia, chronic inflammation and high level of
angiogenic factors such as VEGF, platelet-derived growth factor-B
(PDGF-B) and stromal cell-derived factor-1 (SDF-1)α (9). VEGF is pivotal in retinal
neovascularization prompted by hypoxia-induced retinal injury.
Hypoxia in the retina causes compensatory alterations in blood
flow, the overexpression of cytokines and angiogenesis (10,11). Currently, the lack of efficacious
anti-VEGF treatments could be due to the impacts of this treatment
on the HIF pathway-mediated expression of other pro-angiogenic
factors, including PDGF-B, insulin-like growth factor 1,
erythropoietin and SDF-1 (12,13).
SDF is part of the CXC subfamily of chemokines and
it was first cloned from murine bone marrow and described as a
pre-B cell growth stimulating factor (14). The chemokine receptor, chemokine
(C-X-C motif) receptor 4 (CXCR4), was first cloned as an orphan
chemokine receptor and it was determined to be expressed on
numerous cell types, including monocytes, lymphocytes and
hematopoietic and endothelial progenitor cells (15–18). CXCR4 regulates numerous
activities, including chemotaxis, adhesion, proliferation and
survival (19). Moreover, CXCR4
is also detected in endothelial cells, which suggests a possible
role for SDF-1α/CXCR4 cell signaling in angiogenesis (20). Considerable evidence suggests that
SDF-1α/CXCR4 signaling is involved in the process of pathological
neovascularization (21–29). Jin et al (21) report SDF-1α is implicated in
revascularization of ischemic hind limbs through recruitment of
CXCR4+ hemangiocytes. Our previous studies found that
CXCR4 expressed on leukocytes, such as monocytes, stimulates
monocytes chemotaxis, resulting in recruitment of leukocytes to
inflammatory sites; SDF-1α-treated mice exhibited enhanced
alkali-induced corneal neovascularization through enhanced
intracorneal progenitor cells infiltration and increased macrophage
VEGF expression (22,23).
CXCR4/SDF-1α signaling is pivotal in the progression
of a few types of ocular neovascularization, such as corneal
neovascularization, diabetic retinopathy and oxygen-induced
ischemic retinopathy (22–29),
but the precise mechanism of its effects in ocular
neovascularization still needs further exploration. In the present
study, HREC bio-functions were examined in SDF-1α recombinant
protein or CXCR4 antagonist treated groups and was compared with
control group in vitro. The expression of angiogenic factors
and transcription factors in HRECs were detected and compared. The
present study provided the definitive evidence of critical role of
SDF-1α/CXCR4 signaling in HREC behavior of tube formation,
proliferation and migration.
Materials and methods
Reagents and antibodies
CXCR4 antagonist (AMD3100 octahydrochloride, cat.
no. 3299/50) was purchased from Tocris Bioscience. Recombinant
human SDF-1α (CXCL12) protein (cat. no. 350-NS-050) was purchased
from R&D Systems. CCK-8 kit was purchased from Dojindo
Laboratories, Inc. Trypsin-EDTA was purchased from MilliporeSigma.
Rabbit anti-bFGF, VEGF, IL-8 and ICAM-1 antibodies were purchased
from Santa Cruz Biotechnology, Inc. Primers were synthesized by
GeneScript. Total RNA extraction kit and reverse transcription kit
were purchased from Qiagen Sciences, Inc. D2000 DNA Ladder (cat.
no. M1060) was purchased from Solarbio. Gelred nucleic acid stain
(cat. no. SCT123) was purchased from Sigma-Aldrich. Matrigel was
purchased from Becton, Dickinson and Company. Dulbecco's modified
Eagle medium (DMEM) was purchased from HyClone (Cytiva). Fetal
bovine serum (FBS) was purchased from PAA laboratories (Cytiva).
Mouse anti-human GAPDH antibody (cat. no. AF0006, 1:1,000),
HRP-labeled goat anti-mouse IgG(H+L) (cat. no. A0216, 1:1,000) and
HRP-labeled goat anti-rabbit IgG(H+L) (cat. no. A0208, 1:1,000) was
purchased from Beyotime Institute of Biotechnology. APC-conjugated
mouse anti-human CD106 antibody (cat. no. ab103173) and Alexa Fluor
700-conjugated mouse anti-human CD54 antibody (cat. no. ab275944)
were purchased from Abcam. Rabbit anti-Erk 1,2 monoclonal antibody
(cat. no. orb178404; Clone B20-U; 1:5,000), Rabbit
anti-phosphorylated (p-) ERK 1,2 monoclonal antibody (cat. no.
orb178405; Clone G15-B; 1:5,000), rabbit anti-PI3K P85
(phospho-Tyr467) polyclonal antibody (cat. no. orb14998, 1:5,000)
and rabbit anti PI3K polyclonal antibody (cat. no. orb1089274,
1:1,000) were purchased from Biorbyt Ltd. Human retinal vascular
endothelial cells (HRECs; cat. no. YS0884) were purchased from Yaji
Biological Technology Co., Ltd.
Cell culture and treatment of
HRECs
The HRECs were cultivated with DMEM (HyClone;
Cytiva) containing 10% (v/v) FBS, 100 µg/ml streptomycin and 100
U/ml penicillin (HyClone; Cytiva) and incubated in an incubator
under humid, 5% CO2 and 37°C conditions (30). The HRECs were exposed to PBS
treated control group, SDF-1α groups, in which 10, 50, 100 and 200
ng/ml of recombinant human SDF-1α protein were added and CXCR4
antagonist groups, to which SDF-1α protein (200 ng/ml) combined
with CXCR4 antagonist were added (1 nmol/ml). The HRECs were
passaged by trypsinization at ~90% confluence and subcultured in
either 6-well or 96-well plates with the SDF-1α protein and/or the
CXCR4 antagonist for either 12 h or 24 h depending on the assay
conditions. The cells cultured were all at 37°C.
Cell migration assay
Cell horizontal migration ability was detected by
wound healing assay for assessment of the effects of SDF-1α/CXCR4
signaling on the migration of HRECs, as described in detail
previously (31). The cells were
seeded in a 6-well plate and scratched with a 100 µl pipette tip to
obtain scratches of a constant width when cells reached ~80%
confluence. After scratching, the well was gently washed twice with
PBS to remove the detached cells. Fresh serum-free medium (DMEM)
was then added into each well. The cells were then treated with
human recombinant SDF-1α protein or SDF-1α protein plus CXCR4
antagonist in the experimental wells, whereas the control wells
were treated with PBS. Images were captured of the cells invading
the wound line at 0, 12 and 24 h with Olympus TMS inverted phase
contrast microscope (Olympus Corporation) and measured distances
traveled by the cells from the wound edge to the cell-free space to
calculate the migration rate.
Cell proliferation assay
HREC proliferation was analyzed using Cell Counting
Kit-8 (Dojindo Molecular Technologies, Inc.) (32). The cells were diluted and seeded
in a 96-well plate at a density of 8,000 cells/well in 100 µl of
DMEM with 10% FBS and different concentrations of SDF-1α protein or
SDF-1α protein plus CXCR4 antagonist. After incubation at 37°C for
24 h, 10 µl CCK-8 was added to each well and incubated at 37°C for
2 h in an incubator. Subsequently, absorbance was measured at a
wavelength of 450 nm using a microplate reader (Thermo Multiskan EX
plate reader; Thermo Fisher Scientific, Inc.).
Tube formation assay
A tube formation assay was performed to assess the
effect of SDF-1α/CXCR4 signaling on HRECs. In brief, Matrigel (50
µl/well; Becton, Dickinson and Company) was applied to a 96-well
plate and plate was put into a 37°C incubator for 30 min. Then,
HRECs were seeded onto the gel and kept for 6 h at 37°C condition.
Image-Pro Plus 6.0 (Media Cybernetics, Inc.) was used for imaging,
followed by statistical analyses on tube number. Each experiment
was conducted in triplicate.
Reverse transcription-quantitative
(RT-q)PCR
RT-qPCR was used to analyze the transcript levels of
CXCR4, VEGF, bFGF, IL-1β, IL-6, IL-8, ICAM-1, IL-18, TNF-α,
monocyte chemotactic protein 1, zonula occludens-1 and VE-cadherin
(33). The total RNA was
extracted from HRECs (2×105 cells) using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The RNA
concentration and the absorbance values on A260 and A280 nm were
measured by Nanodrop Nd-1000 spectrophotometer (Invitrogen; Thermo
Fisher Scientific, Inc.) and the OD260/OD280 ratio of RNA was
between 1.8 and 2.0, which could be used as a template for reverse
transcription. Thereafter, complementary (c)DNA was generated via
RT reaction by using a PrimeScript first strand cDNA synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Subsequent qPCR was performed using SYBR
green reagent (Takara Biotechnology Co., Ltd.) on an ABI 7000 PCR
instrument (Thermo Fisher Scientific, Inc.). The 20 µl PCR reaction
mixture consisted of 10 µl SYBR Premix Ex Taq (Takara Biotechnology
Co., Ltd.), 2 µl cDNA template, 0.8 µl Primer (0.4 µl each forward
and reverse) and 7.2 µl dH2O. The primer pairs used are
listed in Table I. All primers
used were purchased from Genescript. PCR was performed by initial
denaturation at 95°C for 10 min, followed by 40 cycles of 10 sec at
95°C and 20 sec at 50°C and a final extension of 25 sec at 72°C.
The relative mRNA levels were measured by quantification cycle
values using the 2−ΔΔCq method (34). Data display the average of
triplicate experiments.
 | Table I.Sequences of primers used for reverse
transcription PCR. |
Table I.
Sequences of primers used for reverse
transcription PCR.
| Primers | Sequence
(5′→3′) | Product Size
(bp) | Annealing
temperature (°C) | PCR Cycle
(sec) |
|---|
| CXCR4 | F:
TGTCCATTCCTTTGCCTCTTTTG | 1,020 | 57 | 37 |
|
| R:
GTCCACCTCGCTTTCCTTTG |
|
|
|
| VEGF | F:
CTTGCTGCTCTACCTCCACC | 118 | 60 | 40 |
|
| R:
GCAGTAGCTGCGCTGATAGA |
|
|
|
| bFGF | F:
CAAGCGGCTGTACTGCAAAA | 100 | 60 | 40 |
|
| R:
TAGCTTGATGTGAGGGTCGC |
|
|
|
| IL-1β | F:
GCAGAAGTACCTGAGCTCGC | 109 | 60 | 40 |
|
| R:
CCTGGAAGGAGCACTTCATCT |
|
|
|
| IL-6 | F:
CAATAACCACCCCTGACCCA | 106 | 60 | 40 |
|
| R:
AAGCTGCGCAGAATGAGATG |
|
|
|
| IL-8 | F:
GGTGCAGTTTTGCCAAGGAG | 117 | 60 | 40 |
|
| R:
GTGTGGTCCACTCTCAATCACT |
|
|
|
| ICAM-1 | F:
CCAGGAGACACTGCAGACAG | 100 | 60 | 40 |
|
| R:
CTTCACTGTCACCTCGGTCC |
|
|
|
| IL-18 | F:
TGACCAAGGAAATCGGCCTC | 117 | 60 | 40 |
|
| R:
GCCATACCTCTAGGCTGGCT |
|
|
|
| TNF-α | F:
GCTGCACTTTGGAGTGATCG | 119 | 60 | 40 |
|
| R:
CTACAGGCTTGTCACTCGGG |
|
|
|
| MCP-1 | F:
GATCTCAGTGCAGAGGCTCG | 105 | 60 | 40 |
|
| R:
TCAGCACAGATCTCCTTGGC |
|
|
|
| ZO-1 | F:
TCAAAGGGAAAGCCTCCTGA | 108 | 60 | 40 |
|
| R:
ATACTGCGAGGGCAATGGAG |
|
|
|
|
VE-cadherin | F:
CTTCACCCAGACCAAGTACACA | 113 | 60 | 40 |
|
| R:
ACTTGGTCATCCGGTTCTGG |
|
|
|
| GAPDH | F:
CAAATTCCATGGCACCGTCA | 108 | 60 | 40 |
|
| R:
GCATCGCCCCACTTGATTTT |
|
|
|
Western blot analysis
Immunoblotting analysis was adopted for the
detection of HRECs expression levels of VEGF, bFGF, IL-8, ICAM and
ERK1/2 as well as associated phosphorylated signaling proteins of
ERK1/2 and PI3K. 6-well plates were used to culture the HRECs
(2.5×105 cells) in DMEM containing 10% FBS. The cell
medium was replaced with the media without serum for another 24 h
once 95% cell confluence was achieved. Then, the starved cells were
incubated for another 24 h in serum-free DMEM with human
recombinant SDF-1α protein or SDF-1α protein plus CXCR4 antagonist.
The treated cells were washed twice using chilled PBS. Then,
protein lysate (Beyotime Institute of Biotechnology) was added to
each well, prior to collecting the proteins on ice. Lysate protein
concentrations were evaluated using the BCA method (Beyotime
Institute of Biotechnology). SDS-PAGE was performed using in-house
produced 10% gels. Equal amounts of protein (50 µg) were loaded per
lane. The separated proteins were transferred onto PVDF membranes
(0.45 µm) purchased from MilliporeSigma and were blocked with 5%
skimmed milk dissolved in 1X TBS containing 0.3% Tween-20 for 1.5 h
at room temperature to inhibit endogenous reactions. The membranes
were then incubated with the blocking buffer-diluted primary
antibodies overnight at 4°C. After rinsed the following day using a
Tris-HCl (pH 7.4) buffer (20 mM) as well as Tween-20 (0.1%),
membranes were incubated again at room temperature with the
corresponding secondary antibodies bound to horseradish peroxidase
for 1 h. The protein blots were promptly visualized using a 1
Tanon-5200 Multi-imaging System after treatment using an enhanced
chemiluminescence (ECL) kit obtained from Tanon Science and
Technology. The relative protein levels were quantified by ImageJ
(version 1.5, National Institutes of Health). The experiments were
performed in triplicate.
Flow cytometry
Flow cytometry was performed to identify the feature
of HRECs. The HRECs were seeded in 60 mm wells (1×106
cells) cultured with DMEM containing 10% FBS. When 95% cell
confluence was achieved, the cells were harvested by trypsinization
for staining. After washing twice using chilled PBS, the cells
(1×106 per 100 µl) were co-stained with 10 µl
APC-conjugated mouse anti-human CD106 and Alexa Fluor
700-conjugated mouse anti-human CD54 antibodies or IgG isotype as
control for 30 min at 4°C. After washing with PBS, the cells were
analyzed with Beckman coulter FC500 Flow Cytometer. Data were
analyzed using FlowJo 7.6 (Tree Star). The experiments were
performed in triplicate.
Statistical analysis
SPSS 20.0 (IBM Corp.) was employed to perform all
data analyses. All data were depicted as mean ± standard error
(number of observations). Comparisons between variables were
carried out by a two-tailed unpaired Student's t test. Comparisons
among multiple datasets were performed by one-way analysis of
variance (ANOVA) and Bonferroni correction was used to adjust
P-values for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
The expression of CXCR4 in HRECs
First, the expression of CXCR4 in HRECs was
examined. CXCR4 mRNA and protein expression were detected in HRECs.
The expression of CXCR4 in HRECs suggested the possible involvement
of the SDF-1α/CXCR4 interactions in the biological function of
HRECs. Additionally, the expression of CD106 and CD54 in HRECs was
examined using flow cytometry to identify the characteristic of
HRECs (Fig. 1).
Effects of SDF-1α/CXCR4 signaling on
cell proliferation
To determine the effects of SDF-1α/CXCR4 signaling
on vascular endothelial cell bio-function, the effect of
SDF-1α/CXCR4 signaling in cell proliferation of HRECs in
vitro was assessed. After incubation with SDF-1α or SDF-1α plus
CXCR4 antagonist for 24 h, cell viability was evaluated. HRECs
incubated with SDF-1α showed a significant increasing in cell
proliferation compared with the control, while HRECs incubated with
CXCR4 antagonist after precondition with SDF-1α showed a
significant reduction in cell proliferation compared with 100 ng/ml
or 200 ng/ml SDF-1α groups (Fig.
2). Optical density (OD) value quantification demonstrated that
SDF-1α/CXCR4 signaling was capable of promoting cell proliferation.
These data indicated that an enhancement in proliferation of HRECs
after SDF-1α stimulation was responsible for the promotion effect
of SDF-1α/CXCR4 signaling on tube formation of HRECs in
vitro. In addition, the elevating of cell proliferation of
HRECs peaked in the 200 ng/ml SDF-1α group when compared with other
groups including 10, 50 and 100 ng/ml SDF-1α groups as well as 500
ng/ml SDF-1α group or other higher concentration groups, so the
dose of 200 ng/ml SDF-1α was chosen as the intervention
concentration to treat HRECs in subsequent protein examining
experiment and three-dimensional Matrigel vascular tube formation
assay.
Effects of SDF-1α/CXCR4 signaling on
cell migration
The effects of SDF-1α/CXCR4 signaling on HREC
migration have yet to be reported, to the best of the authors'
knowledge. To evaluate whether SDF-1α/CXCR4 signaling affects the
process of the migration of HRECs, a scratch wound assay was
performed in vitro to measure the migration property of
HRECs in different concentration of SDF-1α or SDF-1α combined with
CXCR4 antagonist. As shown in Fig.
3, compared with control group, a significantly accelerate
wound closure was shown in the group treated with SDF-1α and the
wound almost closed at 24 h after injury. However, the wound area
was still wide in HRECs with CXCR4 antagonist treatment after
precondition with SDF-1α at 24 h. The quantitative data of the
migration distance were shown in Fig.
3B and C. Those data showed that SDF-1α/CXCR4 signaling had the
potential of promoting the migration property of HRECs.
Effects of SDF-1α/CXCR4 signaling on
tube formation of HRECs
To determine whether SDF-1α/CXCR4 signaling plays a
role in the process of tube formation of HRECs, HREC cell line was
seeded in Matrigel-coated 96-well plates. After incubation for 12
h, the cells were able to form tubes. HRECs incubated with SDF-1α
showed a significant increasing in tube formation compared with
control cells (Fig. 4). After
incubated with SDF-1α and CXCR4 antagonist, the number of tubes
formed was significantly reduced. Tube formation quantification and
statistical analysis demonstrated SDF-1α/CXCR4 signaling was able
to promote HREC tube formation.
Enhanced angiogenic factors, p-ERK1/2
and p-PI3K expression in SDF-1α treated HRECs
The balance between angiogenic and anti-angiogenic
elements establishes the results of angiogenesis operations in
different situations (35).
Therefore, the mRNA and protein expression of angiogenic factors in
HRECs were next determined. Among the angiogenic associated
factors, such as VEGF, bFGF, IL-8 and ICAM-1, which were detected,
the mRNA expression of VEGF, bFGF, IL-8 and ICAM-1
were increased in SDF-1α treated cells compared with control groups
(Fig. 5). VEGF, bFGF, IL-8 and
ICAM-1 protein expression also revealed that VEGF, bFGF, IL-8 and
ICAM-1were increased by the treatment with SDF-1α compared with
PBS-treatment (Fig. 6). These
analyses indicated that the SDF-1α treatment elevated the
expression of the angiogenic factors, VEGF, bFGF, IL-8 and ICAM-1
and as a result, overturned the balance to encourage
angiogenesis.
 | Figure 6.Effect of SDF-1α/CXCR4 signaling on
VEGF, bFGF, IL-8 and ICAM-1 expression of HRECs. (A) Protein
extracts were obtained and subjected to western blotting.
Representative results of VEGF, bFGF, IL-8 and ICAM-1 expression of
HRECs were determined. (B) Statistical analysis of ratios of VEGF,
bFGF, IL-8 and ICAM-1 to GAPDH protein. All values represent mean ±
standard error of the mean (n=6-8 animals). *P<0.05 vs. control.
SDF, stromal cell-derived factor; CXCR, chemokine (C-X-C motif)
receptor; HRECs, human retinal endothelial cells; bFGF, basic
fibroblast growth factor; ICAM, intercellular cell adhesion
molecule. |
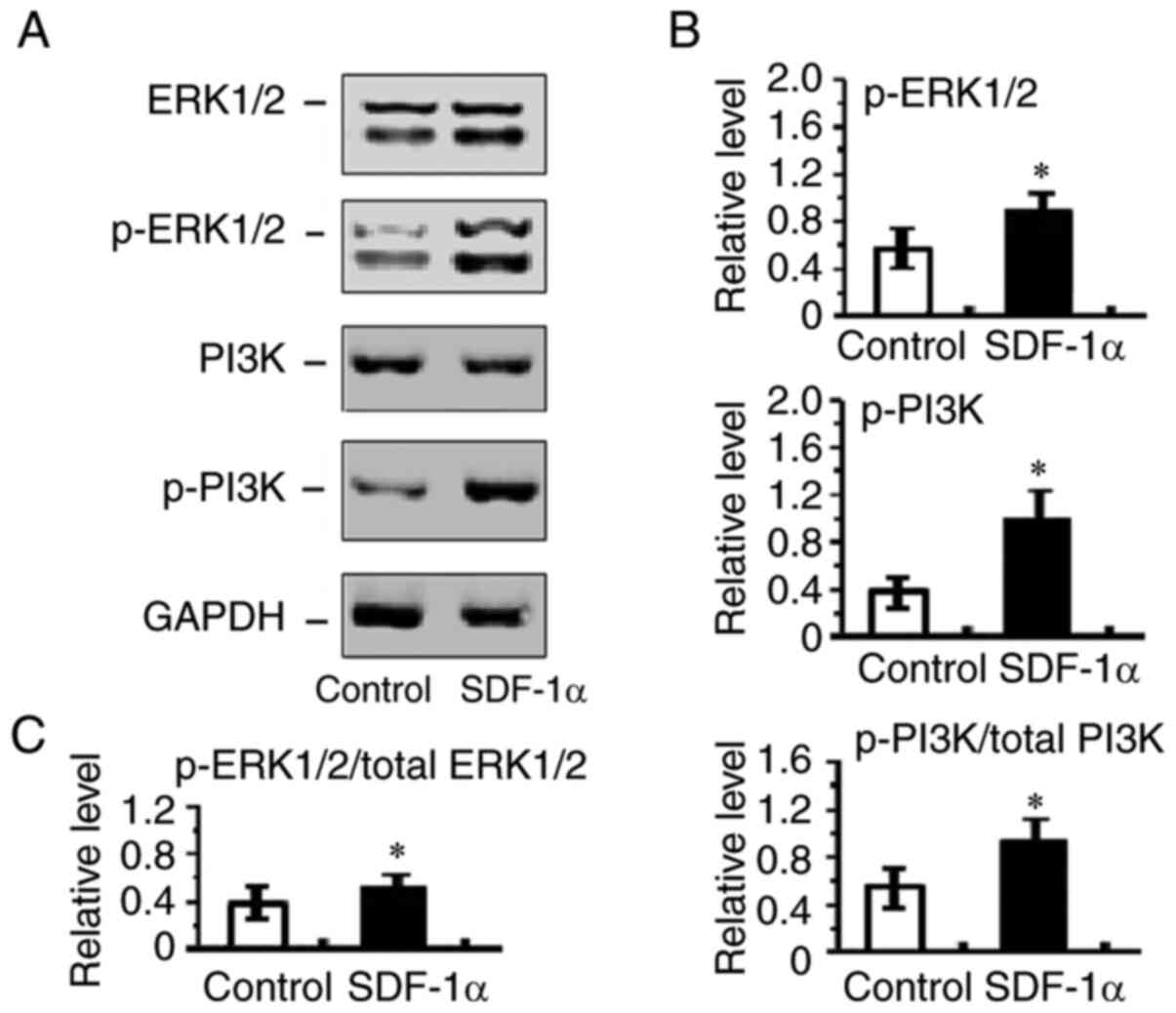
The present study also examined p-ERK1/2 and p-PI3K
expression in HRECs. PI3K and ERK1/2 activation are integral
components of pro-angiogenic signaling pathway and promotes
endothelial migration and proliferation. The present study sought
to determine whether SDF-1α/CXCR4 signaling had effects on cell
migration and proliferation through activation of ERK1/2 and PI3K
in HRECs. It was found that p-ERK1/2 and p-PI3K expression were
markedly increased in SDF-1α treated HRECs (Fig. 7). These results suggested that
SDF-1α induces ERK1/2 and PI3K activation and therefore promoted
angiogenesis.
Discussion
The authors have previously documented that
SDF-1α-treated mice showed improved alkali-induced corneal
neovascularization via amplified intracorneal progenitor cell
infiltration and elevated VEGF expression by macrophages, while
SDF-1α neutralizing antibody- or CXCR4 antagonist-treated mice
demonstrated impeded experimental alkali-induced corneal
neovascularization via downregulated VEGF and C-Kit expression
(22,23). The results provided evidence that
SDF-1α/CXCR4 signaling is implicated in corneal neovascularization
and its potential of pro-angiogenesis may be through indirect
effects of promoting VEGF secreting by intracorneal macrophages and
C-Kit positive progenitor cell migration. In addition, various
evidence indicate that SDF-1α/CXCR4 signaling may have direct
effects on vascular endothelial biofunction (36–39). However, further exploration on the
mechanism of these direct effects is required. In order to
delineate the direct effects of SDF-1α/CXCR4 signaling on vascular
endothelial function of proliferation, migration and tube
formation, the present study performed an in vitro study
using HRECs to evaluate SDF-1α/CXCR4 signaling directed
pro-angiogenesis efficacy.
The present study showed that SDF-1α/CXCR4 signaling
has the ability to increase tube formation of HRECs by promoting
HREC proliferation and migration and VEGF, bFGF, IL-8 and ICAM-1
production of HRECs. These results indicated that SDF-1α/CXCR4
signaling has pro-angiogenesis property not only through activating
cell types of monocytes/macrophages but also through activating
vascular endothelial migration, proliferation and pro-angiogenic
cytokine secretion. Thus, the data verified the hypothesis that
SDF-1α/CXCR4 signaling has an important role in angiogenesis
through indirect and direct pathways, which had not been confirmed
in our previous study (22,23).
Endothelial migration and proliferation are initial
steps for angiogenesis (40). Any
effects on these two steps may subsequently have an impact on
vascular tube formation (40).
Various studies indicate that VEGF, bFGF, IL-8 and ICAM-1 are
involved in vascular endothelial migration and proliferation. VEGF
and bFGF as well as other pro-angiogenic cytokines promote the
process of vascular endothelial migration and proliferation while
ADAMTS-1 and TSP-1 inhibit these processes (41,42). SDF-1/CXCR4 signaling promotes
angiogenesis through multiple pathways, including recruiting
macrophages, c-Kit positive cells and stroma cells and elevating
expression level of pro-angiogenic factors by macrophages and
stroma cells (43). The present
study also examined the effects of SDF-1/CXCR4 signaling on HREC
migration and proliferation (22,23). Consistent with the hypothesis of
the present study, in SDF-1α stimulating groups, both HRECs
migration width and proliferation rate were greater compared with
those in the PBS treated group, while in the CXCR4
antagonist-treated groups, HRECs migration width and proliferation
rate were reduced more compared with the SDF-1α treated groups.
This indicated that directly promoting endothelial migration and
proliferation would be another crucial pathway for SDF-1/CXCR4
signaling implicated in the process of angiogenesis.
The process of angiogenesis is precisely modulated
by a series of pro- and anti-angiogenic molecules under physiologic
condition, while under pathologic condition, the expression balance
upset, serious consequences, such as neovascularization, may occur
(44). Angiogenic factors, such
as VEGF and bFGF have strong efficacy in stimulating blood vessel
formation (45). These factors
are expressed by various cells, including fibroblasts, macrophages,
neutrophils and also by vascular endothelial cells themselves
(46). The present study detected
the mRNA and protein expression of VEGF, bFGF, IL-8, ICAM-1 and
other cytokines in HRECs and the results showed that the expression
of VEGF, bFGF, IL-8 and ICAM-1 in SDF-1α treated cells were
significantly higher compared with control cells. It indicated that
SDF-1/CXCR4 signaling is implicated in the process of angiogenesis
by altering pro-angiogenic milieu and thereby causing
neovascularization (47,48). These results are consistent with
other reports (49–51), which report that SDF-1α promotes
pro-angiogenic cytokine expression in endothelial cells. The
previous reports and the results of the present study imply that
SDF-1/CXCR4 signaling would be a candidate for treating
vascularization diseased by blocking or silencing the
signaling.
To explore the mechanisms of how SDF-1/CXCR4
signaling mediated HREC capillary tube formation, the present study
also evaluated the influence of SDF-1/CXCR4 signaling on signal
expression of PI3K/Akt and ERK1/2. Several signaling pathways are
involved in the process of angiogenesis. Activation of PI3K/Akt and
ERK1/2 in endothelial cells is a crucial intracellular signaling
step for angiogenesis (52,53). Barbero et al (54) report that SDF-1/CXCR4 axis are
capable of activating various signaling pathways, including
PI3K/Akt and ERK1/2, in the process of tumor development and
promote tumor vascular growth through these activated signaling
pathways (55). Lin et al
(56) report that SDF-1/CXCR4
signaling can promote tumor cell proliferation and migration by
activating PI3K/Akt signaling. Based on these studies, the present
study examined whether SDF-1/CXCR4 signaling promoted HRECs
proliferation, migration or capillary tube formation through
PI3K/Akt and ERK1/2. The present study found that SDF-1/CXCR4
signaling promoted the expression of active p-PI3K and p-ERK1/2,
suggesting that SDF-1/CXCR4 signaling had pro-angiogenesis property
via activating PI3K/Akt and ERK1/2 signaling.
In conclusion, the findings of the present study
illustrated a novel mechanism of SDF-1/CXCR4 signaling effects on
the process of neovascularization. It promoted HRECs capillary tube
formation by promoting cell proliferation and cell migration. The
effects may work by enhancing cytokine expression, such as VEGF and
bFGF, and promoting these functions of HRECs via activating
PI3K/Akt and ERK1/2 signaling. These results could provide a
theoretical basis for the possibility of suppressing ocular
neovascularization by inhibiting SDF-1/CXCR4 signaling using
anti-SDF-1 antibody or anti-CXCR4 antagonist or other blocking
agents.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation in China (grant no. 81970830), Suzhou Municipal Natural
Science Foundation (grant no. SKJY2021056) and the Soochow Scholar
Project of Soochow University (grant no. R5122001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GL, HW, XL and XY designed the study, led the
experiments, prepared figures and wrote the manuscript. XY, XL, HW,
YX, LC and ZC analyzed the data and prepared the figures. GL and PL
conceived, designed and coordinated the study as well as drafted
the manuscript. GL and PL confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Campochiaro PA: Ocular neovascularization.
J Mol Med (Berl). 91:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee YM, Lee YR, Kim CS, Jo K, Sohn E, Kim
JS and Kim J: Cnidium officinale extract and butylidenephthalide
inhibits retinal neovascularization in vitro and in vivo. BMC
Complement Altern Med. 16:2312016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Multicenter trial of cryotherapy for
retinopathy of prematurity, . 3 1/2-Year outcome-structure and
function. Cryotherapy for retinopathy of prematurity cooperative
group. Arch Ophthalmol. 111:339–344. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Phelps DL: Retinopathy of prematurity.
Pediatr Rev. 16:50–56. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsilimbaris MK, Kontadakis GA, Tsika C,
Papageorgiou D and Charoniti M: Effect of panretinal
photocoagulation treatment on vision-related quality of life of
patients with proliferative diabetic retinopathy. Retina.
33:756–761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ameri H, Liu H, Liu R, Ha Y,
Paulucci-Holthauzen AA, Hu S, Motamedi M, Godley BF, Tilton RG and
Zhang W: TWEAK/Fn14 pathway is a novel mediator of retinal
neovascularization. Invest Ophthalmol Vis Sci. 55:801–813. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Witmer AN, Vrensen GF, Van Noorden CJ and
Schlingemann RO: Vascular endothelial growth factors and
angiogenesis in eye disease. Prog Retin Eye Res. 22:1–29. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu W, Bai Y, Han N, Wang F, Zhao M, Huang
L and Li X: Inhibition of pathological retinal neovascularization
by semaphorin 3A. Mol Vis. 19:1397–1405. 2013.PubMed/NCBI
|
|
9
|
Praidou A, Androudi S, Brazitikos P,
Karakiulakis G, Papakonstantinou E and Dimitrakos S: Angiogenic
growth factors and their inhibitors in diabetic retinopathy. Curr
Diabetes Rev. 6:304–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gariano RF and Gardner TW: Retinal
angiogenesis in development and disease. Nature. 438:960–966. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang SX and Ma JX: Ocular
neovascularization: Implication of endogenous angiogenic inhibitors
and potential therapy. Prog Retin Eye Res. 26:1–37. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fong GH: Mechanisms of adaptive
angiogenesis to tissue hypoxia. Angiogenesis. 11:121–140. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liekens S, Schols D and Hatse S:
CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell
mobilization. Curr Pharm Des. 16:3903–3920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Federsppiel B, Melhado IG, Duncan AM,
Delaney A, Schappert K, Clark-Lewis I and Jirik FR: Molecular
cloning of the cDNA and chromosomal localization of the gene for a
putative seven-transmembrane segment (7-TMS) receptor isolated from
human spleen. Genomics. 16:707–712. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nomura H, Nielsen BW and Matsushima K:
Molecular cloning of cDNAs encoding a LD78 receptor and putative
leukocyte chemotactic peptide receptors. Int Immunol. 5:1239–1249.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aiuti A, Webb IJ, Bleul C, Springer T and
Gutierrez-Ramos JC: The chemokine SDF-1 is a chemoattractant for
human CD34+ hematopoietic progenitor cells and provides a new
mechanism to explain the mobilization of CD34+ progenitors to
peripheral blood. J Exp Med. 185:111–120. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jo DY, Rafii S, Hamada T and Moore MA:
Chemotaxis of primitive hematopoietic cells in response to stromal
cell-derived factor-1. J Clin Invest. 105:101–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bleul CC, Fuhlbrigge RC, Casasnovas JM,
Aiuti A and Springer TA: A highly efficacious lymphocyte
chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med.
184:1101–1109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pablos JL, Amara A, Bouloc A, Santiago B,
Caruz A, Galindo M, Delaunay T, Virelizier JL and
Arenzana-Seisdedos F: Stromal-cell derived factor is expressed by
dendritic cells and endothelium in human skin. Am J Pathol.
155:1577–1586. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin DK, Shido K, Kopp HG, Petit I,
Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B,
et al: Cytokine-mediated deployment of SDF-1 induces
revascularization through recruitment of CXCR4+ hemangiocytes. Nat
Med. 12:557–567. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu G, Lu P, Li L, Jin H, He X, Mukaida N
and Zhang X: Critical role of SDF-1α-induced progenitor cell
recruitment and macrophage VEGF production in the experimental
corneal neovascularization. Mol Vis. 17:2129–2138. 2011.PubMed/NCBI
|
|
23
|
Liu GQ, Lu PR, Li LB and Zhang XG:
Inhibited experimental corneal neovascularization by neutralizing
anti-SDF-1α antibody. Int J Ophthalmol. 5:7–12. 2012.PubMed/NCBI
|
|
24
|
Sonmez K, Drenser KA, Capone A Jr and
Trese MT: Vitreous levels of stromal cell-derived factor 1 and
vascular endothelial growth factor in patients with retinopathy of
prematurity. Ophthalmology. 115:1065–1070.e1. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Butler JM, Guthrie SM, Koc M, Afzal A,
Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB and Scott EW:
SDF-1 is both necessary and sufficient to promote proliferative
retinopathy. J Clin Invest. 115:86–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhutto IA, McLeod DS, Merges C, Hasegawa T
and Lutty GA: Localisation of SDF-1 and its receptor CXCR4 in
retina and choroid of aged human eyes and in eyes with age related
macular degeneration. Br J Ophthalmol. 90:906–910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lima e Silva R, Shen J, Hackett SF, Kachi
S, Akiyama H, Kiuchi K, Yokoi K, Hatara MC, Lauer T, Aslam S, et
al: The SDF-1/CXCR4 ligand/receptor pair is an important
contributor to several types of ocular neovascularization. FASEB J.
21:3219–3230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sengupta N, Afzal A, Caballero S, Chang
KH, Shaw LC, Pang JJ, Bond VC, Bhutto I, Baba T, Lutty GA and Grant
MB: Paracrine modulation of CXCR4 by IGF-1 and VEGF: Implications
for choroidal neovascularization. Invest Ophthalmol Vis Sci.
51:2697–2704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee E and Rewolinski D: Evaluation of
CXCR4 inhibition in the prevention and intervention model of
laser-induced choroidal neovascularization. Invest Ophthalmol Vis
Sci. 51:3666–3672. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Z, Liu G, Xiao Y and Lu P:
Adrenomedullin22-52 suppresses high-glucose-induced migration,
proliferation, and tube formation of human retinal endothelial
cells. Mol Vis. 20:259–269. 2014.PubMed/NCBI
|
|
31
|
Liu G, Zhang W, Xiao Y and Lu P: Critical
Role of IP-10 on reducing experimental corneal neovascularization.
Curr Eye Res. 40:891–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chao TI, Xiang S, Chen CS, Chin WC, Nelson
AJ, Wang C and Lu J: Carbon nanotubes promote neuron
differentiation from human embryonic stem cells. Biochem Biophys
Res Commun. 384:426–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu P, Li L, Liu G, van Rooijen N, Mukaida
N and Zhang X: Opposite roles of CCR2 and CX3CR1 macrophages in
alkali-induced corneal neovascularization. Cornea. 28:562–569.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Farnoodian M, Wang S, Dietz J, Nickells
RW, Sorenson CM and Sheibani N: Negative regulators of
angiogenesis: Important targets for treatment of exudative AMD.
Clin Sci (Lond). 131:1763–1780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mao L, Huang M, Chen SC, Li YN, Xia YP, He
QW, Wang MD, Huang Y, Zheng L and Hu B: Endogenous endothelial
progenitor cells participate in neovascularization via CXCR4/SDF-1
axis and improve outcome after stroke. CNS Neurosci Ther.
20:460–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li B, Bai W, Sun P, Zhou B, Hu B and Ying
J: The effect of CXCL12 on endothelial progenitor cells: Potential
target for angiogenesis in intracerebral hemorrhage. J Interferon
Cytokine Res. 35:23–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tu TC, Nagano M, Yamashita T, Hamada H,
Ohneda K, Kimura K and Ohneda O: A chemokine receptor, CXCR4, which
is regulated by hypoxia-inducible factor 2α, is crucial for
functional endothelial progenitor cells migration to ischemic
tissue and wound repair. Stem Cells Dev. 25:266–276. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang YB, Liu YF, Lu XT, Yan FF, Wang B,
Bai WW and Zhao YX: Rehmannia glutinosa extract activates
endothelial progenitor cells in a rat model of myocardial
infarction through a SDF-1 α/CXCR4 cascade. PLoS One. 8:e543032013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Griffioen AW and Molema G: Angiogenesis:
potentials for pharmacologic intervention in the treatment of
cancer, cardiovascular diseases, and chronic inflammation.
Pharmacol Rev. 52:237–268. 2000.PubMed/NCBI
|
|
41
|
Hsu YP, Staton CA, Cross N and Buttle DJ:
Anti-angiogenic properties of ADAMTS-4 in vitro. Int J Exp Pathol.
93:70–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lawler PR and Lawler J: Molecular basis
for the regulation of angiogenesis by thrombospondin-1 and −2. Cold
Spring Harb Perspect Med. 2:a0066272012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kryczek I, Wei S, Keller E, Liu R and Zou
W: Stroma-derived factor (SDF-1/CXCL12) and human tumor
pathogenesis. Am J Physiol Cell Physiol. 292:C987–C995. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martínez A: A new family of angiogenic
factors. Cancer Lett. 236:157–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Uno K, Hayashi H, Kuroki M, Uchida H,
Yamauchi Y, Kuroki M and Oshima K: Thrombospondin-1 accelerates
wound healing of corneal epithelia. Biochem Biophys Res Commun.
315:928–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sakaguchi I, Ikeda N, Nakayama M, Kato Y,
Yano I and Kaneda K: Trehalose 6,6′-dimycolate (Cord factor)
enhances neovascularization through vascular endothelial growth
factor production by neutrophils and macrophages. Infect Immun.
68:2043–2052. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Edelman JL, Castro MR and Wen Y:
Correlation of VEGF expression by leukocytes with the growth and
regression of blood vessels in the rat cornea. Invest Ophthalmol
Vis Sci. 40:1112–1123. 1999.PubMed/NCBI
|
|
48
|
Lai CM, Spilsbury K, Brankov M, Zaknich T
and Rakoczy PE: Inhibition of corneal neovascularization by
recombinant adenovirus mediated antisense VEGF RNA. Exp Eye Res.
75:625–634. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Newey SE, Tsaknakis G, Khoo CP,
Athanassopoulos T, Camicia R, Zhang Y, Grabowska R, Harris AL,
Roubelakis MG and Watt SM: The hematopoietic chemokine CXCL12
promotes integration of human endothelial colony forming
cell-derived cells into immature vessel networks. Stem Cells Dev.
23:2730–2743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Smadja DM, Bièche I, Uzan G, Bompais H,
Muller L, Boisson-Vidal C, Vidaud M, Aiach M and Gaussem P: PAR-1
activation on human late endothelial progenitor cells enhances
angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system.
Arterioscler Thromb Vasc Biol. 25:2321–2327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hamed S, Egozi D, Dawood H, Keren A,
Kruchevsky D, Ben-Nun O, Gilhar A, Brenner B and Ullmann Y: The
chemokine stromal cell-derived factor-1α promotes endothelial
progenitor cell-mediated neovascularization of human transplanted
fat tissue in diabetic immunocompromised mice. Plast Reconstr Surg.
132:239e–250e. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fernández JG, Rodríguez DA, Valenzuela M,
Calderon C, Urzúa U, Munroe D, Rosas C, Lemus D, Díaz N, Wright MC,
et al: Survivin expression promotes VEGF-induced tumor angiogenesis
via PI3K/Akt enhanced β-catenin/Tcf-Lef dependent transcription.
Mol Cancer. 13:2092014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Barbero S, Bonavia R, Bajetto A, Porcile
C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T and
Schettini G: Stromal cell-derived factor 1alpha stimulates human
glioblastoma cell growth through the activation of both
extracellular signal-regulated kinases 1/2 and Akt. Cancer Res.
63:1969–1974. 2003.PubMed/NCBI
|
|
55
|
Wu D, Guo X, Su J, Chen R, Berenzon D,
Guthold M, Bonin K, Zhao W and Zhou X: CD138-negative myeloma cells
regulate mechanical properties of bone marrow stromal cells through
SDF-1/CXCR4/AKT signaling pathway. Biochim Biophys Acta.
1853:338–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin ML, Lu YC, Chen HY, Lee CC, Chung JG
and Chen SS: Suppressing the formation of lipid raft-associated
Rac1/PI3K/Akt signaling complexes by curcumin inhibits
SDF-1α-induced invasion of human esophageal carcinoma cells. Mol
Carcinog. 53:360–379. 2014. View Article : Google Scholar : PubMed/NCBI
|