Introduction
Myocardial hypertrophy is an independent risk factor
for cardiovascular disease and a key cause of heart failure
(1). The condition may be caused
by valvular disease, hypertension, myocardial infarction and
cardiomyopathy amongst other pathological mechanisms (1). Cardiomyocyte hypertrophy is an
indicator of myocardial remodeling and induces decreased myocardial
contractility, which progresses to heart failure (2). Therefore, it is necessary to
determine factors offering a protective effect against cardiac
hypertrophy to decrease the incidence of heart failure and
subsequent mortality. Resveratrol (Res) is a natural non-flavonoid
polyphenol with anti-inflammatory, cardioprotective,
neuroprotective, antioxidant and anti-aging properties that is
abundant in food such as grapes, mulberries and blueberries
(3,4). Preclinical studies have reported the
potential of Res as a treatment for severe cardiomyopathy (5,6).
However, mechanisms underlying the cardioprotective action of Res
remain unknown.
Network pharmacology is a powerful multi-component,
multi-target tool which has been used to study therapeutic
mechanisms of herbal medicines (7,8).
The approach has been employed in mechanistic studies associated
with numerous types of chronic disease, including obesity,
diabetes, chronic obstructive pulmonary and cardio-cerebral
vascular disease (9,10). Therefore, evaluation of the
mechanistic actions of multi-target drugs in the treatment of
complex diseases draws on abundant datasets to inform preliminary
investigation.
The present study evaluated the pharmacological
action of Res in cardiac hypertrophy using network pharmacology and
animal experiments. Potential Res targets were predicted and
cross-referenced with therapeutic targets associated with
cardiomyopathy. Res targets associated with cardiac hypertrophy
were integrated to establish a multi-target network. Cytoscape and
Metascape were used to analyze significant genes in the Gene
Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes
(KEGG) databases. Significant targets and pathways obtained from
network pharmacology were verified using western blotting. The
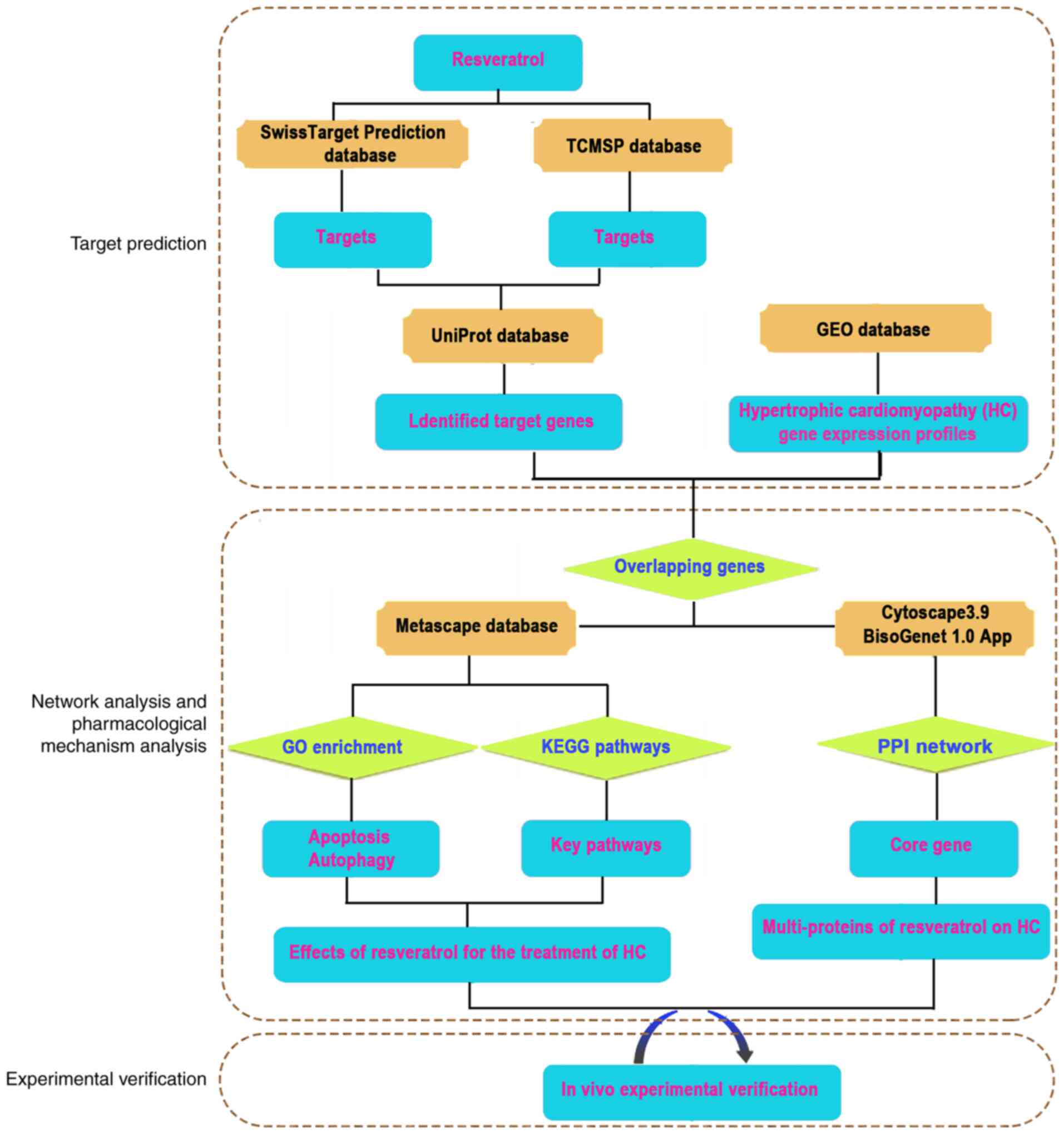
process of network pharmacology and animal experiments is presented
in Fig. 1.
Materials and methods
Construction of Res target
library
The chemical structure of Res was downloaded from
Pubchem (pubchem.ncbi.nlm.nih.gov) and Res targets were predicted
and downloaded from the Traditional Chinese Medicine Systems
(TCMSP; http://tcmsp-e.com/) and Swiss Target
Prediction (swisstargetprediction.ch) databases (the default
parameters of the websites were utilized), using the inclusion
criteria of P≤0.5 (11). Genes
corresponding to targets were identified from UniProt (12).
Acquisition of cardiac hypertrophy
gene dataset
Target genes associated with cardiac hypertrophy
were downloaded from the Gene Expression Omnibus database (GEO;
ncbi.nlm.nih.gov/geo). The gene expression profiles of myocardial
tissue removed surgically from 54 male and 52 female patients with
hypertrophic cardiomyopathy were downloaded from the GSE36961
dataset (P<0.05; log2 fold change >1).
Molecular docking and enrichment
analysis
The structure of the signal transducer and activator
of transcription (STAT3) protein was downloaded from the Protein
Data Bank (rcsb.org/) and molecular docking performed using
AutoDock Vina (version, 1.2.0) (13). The online Database for Annotation,
Visualization and Integrated Discovery (david.ncifcrf.gov/home.jsp)
enrichment analysis service was used to analyze enriched genes
using GO terms and KEGG pathways. Venn diagrams were generated
using Venny 2.1.0 (bioinfogp.cnb.csic.es/tools/venny/) to
demonstrate potential associations between datasets. Functional GO
and KEGG analysis of potential targets of Res for cardiac
hypertrophy treatment was performed using Metascape (version, 3.5)
(metascape.org) and pathways with P≤0.05 were considered to be
statistically significant for cardiac hypertrophy treatment. A
diagram of Res-associated signaling pathways based on GO and KEGG
analyses was generated using the ‘Pathview’ package in the Toolkit
for Biologists software (version, 1.098667) (14).
Protein-protein interaction (PPI)
network
Interactions between potential Res targets
associated with hypertrophic cardiomyopathy in both males and
females were evaluated using a PPI network generated using the
BisoGenet 1.0 plugin (apps.cytoscape.org/apps/bisogenet) for
Cytoscape 3.9 (15). The
Molecular Complex Detection (MCODE) plugin (version 2.0.2,
apps.cytoscape.org/apps/mcode) for Cytoscape 3.9 was used to
visualize protein-protein interactions with topology parameters
evaluated using the Cytoscape network analyzer tool.
Compound-target associations were presented in a pharmacological
network map constructed using Cytoscape 3.9 (the default parameters
were utilized). Core gene-encoding proteins were screened for using
MCODE score >5.
Animal experiments
A total of 20 healthy adult female and 20 male
Sprague Dawley rats (eight weeks of age, 200–250 g) were supplied
by the Animal Center of Qiqihar Medical University. Rats were
housed with temperature and humidity at 22±2°C and 45±15%,
respectively, under a 12 h light-dark cycle, with free access to
food and water. Rats were acclimated for 7 days prior to the
experiment. Rats were randomly divided into four groups (each group
contained 5 male and 5 female rats) as follows: i) Control; ii)
isoprenaline (ISO); iii) Res and iv) Res + 3-Methyladenine (3-MA).
Rats in the ISO group were administered 0.9% saline by gavage (in
equal volume to Res group, about 2 ml) for 4 weeks and subcutaneous
ISO injection (5 mg/kg/day) on days 22–28. The Res group were
administered Res (80 mg/kg/day) by gavage for 4 weeks (16) and subcutaneous ISO injections (5
mg/kg/day) from day 22–28. The Res + 3-MA group was administered
Res (80 mg/kg/day) by gavage, 3-MA (20 mg/kg/day) by
intraperitoneal injection for 4 weeks and subcutaneous ISO
injection (5 mg/kg/day) on days 22–28. Rats in the control group
were administered 0.9% saline (in equal volume to Res group, about
2 ml) by gavage for 4 weeks and subcutaneous 0.9% saline (in equal
volume to ISO, about 0.5 ml) injections on days 22–28. Rats were
euthanized using intraperitoneal injection of 3% sodium
pentobarbitone (240 mg/kg) following echocardiography and the
ventricular myocardia was excised. The present study was approved
by the Committee on the Ethics of Animal Experiments of Qiqihar
Medical University (Qiqihar, heilingjiang, approval no.
QMU-AECC-2019-53).
Echocardiography
Echocardiography was performed 24 h after the final
treatment. Briefly, rats were anaesthetized with intraperitoneal
injection of 3% sodium pentobarbitone (80 mg/kg) and the thoracic
area was shaved. M-mode recording of the short-axis view was
performed on the shaved chest wall. Left ventricular end-systolic
dimension (LVESd), LV end-diastolic dimension (LVEDd), LV
fractional shortening (FS) and ejection fraction (EF) were assessed
by echocardiography using a V6 imaging system [VINNO Technology
(Suzhou) Co., Inc.] with high-frequency ultrasound transducer.
Tissue collection and analysis
The heart and left ventricle of euthanized rats were
isolated. The ratio of left ventricle weight: body weight (LVW/BW)
and heart weight: BW (HW/BW) were calculated to evaluate the degree
of ventricular hypertrophy.
Measurement of malondialdehyde (MDA)
and lactate dehydrogenase (LDH)
MDA and LDH levels in heart homogenate was assessed
using MDA assay kit and LDH assay kit (Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's protocol
and optical density at 530 and 450 nm was quantified using a
microplate reader (Thermo Fisher Scientific, Inc.).
Measurement of atrial natriuretic
peptide (ANP) mRNA expression levels
Total RNA was isolated from left ventricle tissue
with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). According to the manufacturer's protocols, total
RNA was reverse transcribed with mRNA reverse transcription kit
(cat. no. RR037A, Takara Bio, Inc.). The resulting cDNA was used as
template for RT-qPCR using SYBR-Green Master Mix (cat. no. A25742,
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions, the PCR program was as follows: 95°C for 30 sec,
followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec.
Melting curves were used to ensure that only the correct product
was amplified. Primer sequences were as follows: ANP forward (F),
5′-GGGAAGTCAACCCGTCTCA-3′ and reverse (R),
5′-GGGCTCCAATCCTGTCAAT-3′ and GAPDH F, 5′-GAGACAGCCGCATCTTCTTG-3′
and R, 5′-ATACGGCCAAATCCGTTCAC-3′. Data were normalized to
expression of GAPDH mRNA as endogenous control and quantified using
the 2−ΔΔCq method (17).
Western blotting
Myocardial tissue was homogenized with cold RIPA
buffer with 1% phenylmethylsulfonyl fluoride (P0013C, Beyotime
Institute of Biotechnology), Total protein concentrations were
measured by a BCA kit (Beyotime Institute of Biotechnology). Equal
amounts of protein (30 µg) was separated using 8 or 10% SDS-PAGE
and electro-transferred to PVDF membranes (MilliporeSigma).
Membranes were blocked with 5% blocking buffer for 1 h at room
temperature and incubated with primary antibodies as follows:
Anti-ANP (1:1,000; cat. no. sc-515701, Santa Cruz Biotechnology,
Inc.), anti-Bcl-2 (1:1,000; cat. no. sc-7382, Santa Cruz
Biotechnology, Inc.), anti-Bax (1:1,000; cat. no. sc-20067, Santa
Cruz Biotechnology, Inc.), anti-cytochrome C (CytC; 1:1,000; cat.
no. sc-13156, Santa Cruz Biotechnology, Inc.) and
anti-autophagy-related gene 5 (Atg5; 1:1,000; cat. no.sc-133158,
Santa Cruz Biotechnology, Inc.) at 4°C overnight. GAPDH (1:1,000;
sc-166545, Santa Cruz Biotechnology, Inc) was used as the internal
control. Membranes were incubated with Secondary goat antimouse
antibody were used (diluted 1:5,000, cat. no. BA1050; Boster
Biological Technology, Ltd.) for 1.5 h at room temperature. The
membranes were washed three times in Wash Buffer and proteins were
detected using ECL reagent (Beyotime Institute of Biotechnology).
Relative protein expression levels were semi-quantified using
ImageJ software (National Institutes of Health; version 1.48) and
presented as fold-change compared with control.
Statistical analysis
All data are presented as mean ± SEM and represent
at least three independent experiments. Statistical comparisons
were made one-way ANOVA followed by a post hoc analysis (Tukey
test) and GraphPad Prism software (GraphPad Software, Inc.,version
7.0). P<0.05 was considered to indicate a statistically
significant difference.
Results
Res target characteristics for
hypertrophic cardiomyopathy
The molecular structure of Res
(C14H12O3) is presented in
Fig. 2A. A total of 230 potential
Res targets from the TCMSP and Swiss Target Prediction databases
and 444 cardiac hypertrophy-associated targets from the GSE36961
dataset, in the form of differential gene expression profiles for
male and female patients with hypertrophic cardiomyopathy, were
screened to characterizing the potential association between Res
and cardiac hypertrophy (Fig. 2B and
C). A total of 8 proteins overlapped in the intersection of Res
and cardiac hypertrophy-associated targets, of which 3 were
identified in females only (Fig.
2D).
Bioinformatics analysis of target
genes
Molecular docking analysis demonstrated the binding
of Res to STAT3 (Fig. 3).
Enrichment analysis demonstrated a further 10 pathways, including
‘IL-4 and IL-13 signaling’ and ‘TGF-β signaling pathway’, that were
associated with Res treatment of cardiac hypertrophy. Further
analysis demonstrated that Res intervening pathways, including
‘IL-5 signaling pathway’ and ‘lipid metabolism in senescent cells’
were associated with ‘apoptosis’, whereas ‘TGF-β signaling pathway’
and ‘positive regulation of glycolytic process’ were associated
with ‘autophagy’. Therefore, apoptosis and autophagy may be
potential therapeutic mechanistic targets of Res in hypertrophic
cardiomyopathy.
PPI network of target genes
The BisoGenet 1.0 plugin for Cytoscape 3.9 was used
to analyze the 129 Res targets associated with male patients with
hypertrophic cardiomyopathy and a network of PPI associations with
1,206 nodes and 21,105 connections was constructed (Fig. 4A). A total of four modules of the
core gene-encoded proteins had an MCODE score >5 as predicted
using the MCODE plugin (version 2.0.2) in Cytoscape 3.9 (Fig. 4B). Analysis of the 215 Res targets
associated with female patients with hypertrophic cardiomyopathy
generated a PPI network with 3,350 nodes and 63,783 connections
(Fig. 4C). A total of eight
modules of the core gene-encoded proteins had an MCODE score >5,
predicted using the MCODE plugin (version 2.0.2) in Cytoscape 3.9
(Fig. 4D). The female PPI network
was more complex than the male network and featured more core
proteins.
Res reverses ISO-induced myocardial
hypertrophy
The cardiac index and ANP mRNA and protein
expression levels were assessed as cardiac hypertrophy indicators.
HW/BW, LVW/BW, the levels of ANP mRNA and protein expression were
increased in ISO group. Treatment with Res significantly decreased
HW/BW and LVW/BW values and decreased the levels of ANP mRNA and
protein expression compared with the ISO group. Moreover, Res +
3-MA significantly increased the levels of ANP mRNA and protein
expression compared with Res-alone (Fig. 5A-D).
Res reverses ISO-induced cardiac
dysfunction
Echocardiographic parameters were assessed in M-mode
(Fig. 6A); treatment with Res
produced significant increases in EF% and FS% compared with the ISO
group (Fig. 6B and C). These
results demonstrated the importance of Res in cardiac hypertrophy.
Furthermore, both LVESd and LVEDd were significantly decreased in
the Res group compared with the ISO group. No significant
differences were demonstrated for EF%, FS% and LVEDd between the
Res and Res + 3-MA groups; however, LVESd exhibited a significant
increase in the Res + 3-MA group compared with the Res group
(Fig. 6D and E). In summary, ISO
and 3-MA treatment both led to progressive cardiac enlargement,
however, Res improved cardiac function.
Res decreases ISO-induced oxidative
stress in the heart
The effect of Res on oxidative stress was
investigated. Treatment with ISO significantly increased cardiac
MDA and LDH levels compared with the control and these increases
were significantly reversed by treatment with Res (Fig. 7). However, levels of MDA (Fig. 7A) were markedly higher and levels
of LDH (Fig. 7B) significantly
higher in the Res + 3-MA compared with the Res group.
Res downregulates apoptosis and
upregulates autophagy
The protein expression levels of Bcl-2 and Atg5 were
significantly decreased by ISO treatment compared with the control.
Res caused significant upregulation of Bcl-2 and Atg5 expression
compared with the ISO group. Expression levels of pro-apoptosis
proteins Bax and CytC significantly increased in the ISO group
compared with control. However, this effect was significantly
decreased by Res treatment (Fig.
8A-E). However, Res + 3-MA treatment significantly reversed
changes in protein expression levels of CytC, Bcl-2 and Atg5
compared with the Res group (Fig.
8A-E).
Discussion
Cardiac hypertrophy is an adaptive response to
pathological stimuli but prolonged hypertrophy results in cardiac
dysfunction and heart failure. Volume overload or elevated pressure
is a key cause and pathological remodeling is characterized by
hypertrophied cardiomyocytes, interstitial fibrosis, perivascular
fibrosis and decreased cardiac compliance leading to malignant
arrhythmia, heart failure and potential sudden cardiac death
(18–20). A previous study demonstrated
associations between cardiac hypertrophy and apoptosis, oxidative
stress, autophagy and aging (21). These connections highlight the
importance of elucidating the precise mechanism of cardiac
hypertrophy.
Res is a plant-derived polyphenol present in grape
skin, peanut, cranberries and veratrum (22). Numerous biological activities have
been ascribed to Res, including potent anti-inflammatory,
antioxidant, anti-aging, insulin sensitization and cardioprotective
activities, which are reported to protect of nerve and heart tissue
(16). Furthermore, anti-aging
activity has been suggested due to upregulation of hydrogen
peroxide-dependent autophagy (23). Res has also been reported to
inhibit vascular smooth muscle remodeling and growth and
proliferation of cardiac fibroblasts (24). Res (80 mg/kg) via intragastric
administration has been reported to protect against
L-arginine-induced ANP, which may be associated with enhancement of
sirtuin 1-mediated deacetylation of p53 and heat shock
transcription factor 1 (16). To
the best of our knowledge, however, little detailed information
regarding Res protective mechanisms has been reported.
Network pharmacology has allowed previous studies of
the anti-cardiac hypertrophy mechanism of Res to be performed
(25–27) and may facilitate identification of
potential drug treatments for complex disease (28,29). The present study demonstrated the
presence of 230 Res targets in the Swiss Target Prediction and
TCMSP databases and 444 sex-specific targets associated with
cardiac hypertrophy in the GSE36961 dataset. A total of 8 Res
targets were also associated with cardiac hypertrophy, 5 in males
and 8 in females. The intersection of Res and cardiac hypertrophy
targets was visualized using the ‘Res-target-disease’ network and
GO functional and KEGG pathway enrichment analyses were performed.
STAT3, Myc and apoptotic processes were keytargets of Res in
hypertrophic cardiomyopathy. Molecular docking analysis
demonstrated the potential regulatory effect of Res on STAT3, a
protein which is known to inhibit apoptosis and numerous
autophagy-associated proteins (30). STAT3 is reported to be a
suppressor of autophagy, which suggested that the Res therapeutic
mechanism may involve autophagy in both male and female patients.
Moreover, recent evidence suggests that STAT3 may promote
cardiomyocyte metabolism and function by an effect on endothelial
autophagy (31). The effect of
STAT3 on autophagy has also been reported in cardiac fibrosis
(32–34). In summary, effects of Res on
apoptosis and autophagy may underlie the potential mechanisms of
Res in treating cardiac hypertrophy.
PPI networks demonstrated that Res action in female
patients with hypertrophic cardiomyopathy was more complex and
contained more core proteins than the equivalent action in male
patients with hypertrophic cardiomyopathy. In both physiological
and pathological scenarios and pre-menopausal, females exhibit
enhanced protection against cardiac hypertrophy compared with
males. Such protection disappears following the menopause; however
it can be partially restored by estrogen replacement therapy.
Estrogen antagonizes the pro-hypertrophy signaling pathway, whereas
androgens have the opposing effect (35). The network pharmacology approach,
encompassing various pharmacological effects of traditional drugs
and the interaction between chemical molecules and target proteins
at the molecular level, demonstrates interactions which support the
suggestion that Res achieves its cardioprotective role in
inhibiting cardiomyocyte apoptosis by increasing autophagy
(36).
Pathological indicators, such as ANP, MDA and LDH,
cardiac morphological indicators, such as HW/BW and LVW/BW, and
cardiac structural indicators, such as EF%, FS%, LVESd and LVEDd,
were analyzed in vivo to demonstrate the effects of Res. Res is
demonstrated to reverse cardiac hypertrophy via complex mechanisms
which remain to be clarified; however autophagy appears to be a key
factor (37). However, the role
of autophagy in myocardial hypertrophy is controversial. Previous
studies have reported activation of general autophagy as having
both protective and detrimental roles during cardiac hypertrophy
and heart failure (38,39). Both Li et al (40) and Wang et al (41) reported that ISO-induced cardiac
hypertrophy is accompanied by a significant decrease in autophagy
and that enhanced autophagy may attenuate cardiac hypertrophy. The
network pharmacology in the present study identified autophagy as a
potential therapeutic Res target in cardiac hypertrophy.
Furthermore, hypertrophic responses induced by ISO were alleviated
by Res treatment via regulation of autophagy in the present study.
Moreover, the autophagy inhibitor 3-MA stimulated hypertrophic
responses in rats. However, HW/BW and LVW/BW, which demonstrated
the therapeutic effect of Res on cardiac function, were not
affected by 3-MA. However, 3-MA markedly decreased the reversal of
Res-induced cardiac dysfunction, which suggested that the
regulation of autophagy was involved. Apoptosis was also associated
with the therapeutic effect of Res. Autophagy is reported to be
associated with apoptosis, which affects homeostasis of the
intracellular environment, initiated by both exogenous and
endogenous pathways (42,43). The results of the present study
demonstrated that apoptosis significantly increased following ISO
treatment; this effect was significantly reversed by Res treatment.
It could be hypothesized that 3-MA decreased the therapeutic effect
of Res by suppressing autophagy and promoting apoptosis, two forms
of programmed cell death. Autophagy may be regarded as a
double-edged sword (44).
Interactions between apoptosis and autophagy are highly complex and
environmentally dependent and it can be concluded that both are
mechanisms involved in the treatment of cardiac hypertrophy by Ras
(45). Mitochondrial cytochrome C
release and apoptosis are both inhibited by Bcl-2 binding to Bax
(46). Bax is a proapoptotic
member of the Bcl-2 family activated by apoptotic signaling,
leading to cytochrome C release (47). This is stimulated by proapoptotic
Bax, Bad, BH3-interacting domain death agonist and Bcl-2
antagonist/killer and inhibited by anti-apoptotic Bcl-xl and/or
Bcl-2 (48). Res significantly
reversed ISO-induced changes to Bcl-2, Bax and cytochrome C
expression in the present study. 3-MA markedly reversed the
regulation of apoptosis-associated proteins by Res, indicating that
protection against myocardial hypertrophy involves autophagy and
apoptosis. One potential limitation of this study would be that the
mechanism of Res promoting the Atg5 require further study. Further
investigation is therefore needed to understand the association
between autophagy and the cardiac function following treatment with
Res.
In conclusion, the results of the integrated network
pharmacological and animal experiments in the present study
demonstrated that Res ameliorated myocardial hypertrophy via
regulation of autophagy and apoptosis-associated signaling
pathways. These results may inform further studies on the use of
Res in cardiac disease. Res may be an effective multi-target
anti-myocardial hypertrophy drug.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Natural Science
Foundation of China (grant no. 82074148 and 31751004) and the
Postdoctoral Scientific Research Foundation of Heilongjiang
Province (grant no. LBH-Q18128).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SR, LS and YL designed the experiments and wrote and
revised the manuscript. WWJ, SL and WX performed the experiments
and analyzed the data. PMY, YYZ and DX performed the experiments.
SR and LS confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Committee on the Ethics of Animal Experiments of Qiqihar Medical
University (Qiqihar, heilingjiang, approval no.
QMU-AECC-2019-53).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nakamura M and Sadoshima J: Mechanisms of
physiological and pathological cardiac hypertrophy. Nat Rev
Cardiol. 15:387–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ott C, Jung T, Brix S, John C, Betz IR,
Foryst-Ludwig A, Deubel S, Kuebler WM, Grune T, Kintscher U and
Grune J: Hypertrophy-reduced autophagy causes cardiac dysfunction
by directly impacting cardiomyocyte contractility. Cells.
10:8052021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaito A, Posadino AM, Younes N, Hasan H,
Halabi S, Alhababi D, Al-Mohannadi A, Abdel-Rahman WM, Eid AH,
Nasrallah GK and Pintus G: Potential adverse effects of
resveratrol: A literature review. Int J Mol Sci. 21:20842020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian B and Liu J: Resveratrol: A review of
plant sources, synthesis, stability, modification and food
application. J Sci Food Agric. 100:1392–1404. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peñalver P, Belmonte-Reche E, Adán N, Caro
M, Mateos-Martín ML, Delgado M, González-Rey E and Morales JC:
Alkylated resveratrol prodrugs and metabolites as potential
therapeutics for neurodegenerative diseases. Eur J Med Chem.
146:123–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen C, Zou LX, Lin QY, Yan X, Bi HL, Xie
X, Wang S, Wang QS, Zhang YL and Li HH: Resveratrol as a new
inhibitor of immunoproteasome prevents PTEN degradation and
attenuates cardiac hypertrophy after pressure overload. Redox Biol.
20:390–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Yang SH, Zhong K, Jiang T, Zhang
M, Kwan HY and Su T: Network pharmacology-based strategy for the
investigation of the anti-obesity effects of an ethanolic extract
of zanthoxylum bungeanum maxim. Front Pharmacol. 11:5723872020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin X, Shao T, Huang L, Wen X, Wang M, Wen
C and He Z: Simiao decoction alleviates gouty arthritis by
modulating proinflammatory cytokines and the gut ecosystem. Front
Pharmacol. 11:9552020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Liu T, Yang L, Ma Y, Dou F, Shi L,
Wen A and Ding Y: Study on the multi-targets mechanism of triphala
on cardio-cerebral vascular diseases based on network pharmacology.
Biomed Pharmacother. 116:1089942019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Peng L, Jin L, Fu H and Shou Q:
Network pharmacology analysis of the identification of
phytochemicals and therapeutic mechanisms of paeoniae radix alba
for the treatment of asthma. J Immunol Res. 2021:96593042021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daina A, Michielin O and Zoete V:
SwissTargetPrediction: Updated data and new features for efficient
prediction of protein targets of small molecules. Nucleic Acids
Res. 47((W1)): W357–W364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
UniProt Consortium: UniProt: The universal
protein knowledgebase in 2021. Nucleic Acids Res. 49(D1):
D480–D489. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010.PubMed/NCBI
|
|
14
|
Chen C, Chen H, Zhang Y, Thomas HR, Frank
MH, He Y and Xia R: TBtools: An integrative toolkit developed for
interactive analyses of big biological data. Mol Plant.
13:1194–1202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martin A, Ochagavia ME, Rabasa LC, Miranda
J, Fernandez-de-Cossio J and Bringas R: BisoGenet: A new tool for
gene network building, visualization and analysis. BMC
Bioinformatics. 11:912010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang N, Zhang F, Yang L, Zou J, Wang H,
Liu K, Liu M, Zhang H, Xiao X and Wang K: Resveratrol protects
against L-arginine-induced acute necrotizing pancreatitis in mice
by enhancing SIRT1-mediated deacetylation of p53 and heat shock
factor 1. Int J Mol Med. 40:427–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng S, Lu XF, Qi YD, Li J, Xu J, Yuan TY,
Wu XY, Ding Y, Li WH, Zhou GQ, et al: LCZ696 ameliorates oxidative
stress and pressure overload-induced pathological cardiac
remodeling by regulating the Sirt3/MnSOD pathway. Oxid Med Cell
Longev. 2020:98150392020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wolf CM: Hypertrophic cardiomyopathy:
Genetics and clinical perspectives. Cardiovasc Diagn Ther. 9 (Suppl
2):S388–S415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Halliday BP, Cleland JGF, Goldberger JJ
and Prasad SK: Personalizing Risk Stratification for sudden death
in dilated cardiomyopathy: The past, present, and future.
Circulation. 136:215–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiao Y, Zhu B, Tian A and Li Z: PEG-coated
gold nanoparticles attenuate β-adrenergic receptor-mediated cardiac
hypertrophy. Int J Nanomedicine. 12:4709–4719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galiniak S, Aebisher D and
Bartusik-Aebisher D: Health benefits of resveratrol administration.
Acta Biochim Pol. 66:13–21. 2019.PubMed/NCBI
|
|
23
|
Du L, Chen E, Wu T, Ruan Y and Wu S:
Resveratrol attenuates hydrogen peroxide-induced aging through
upregulation of autophagy in human umbilical vein endothelial
cells. Drug Des Devel Ther. 13:747–755. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Lu H, Xie S, Wu C, Guo Y, Xiao Y,
Zheng S, Zhu H, Zhang Y and Bai Y: Resveratrol suppresses the
myofibroblastic phenotype and fibrosis formation in kidneys via
proliferation-related signalling pathways. Br J Pharmacol.
176:4745–4759. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li W, Yuan G, Pan Y, Wang C and Chen H:
Network pharmacology studies on the bioactive compounds and action
mechanisms of natural products for the treatment of diabetes
mellitus: A review. Front Pharmacol. 8:742017.PubMed/NCBI
|
|
26
|
Zhang W, Bai Y, Wang Y and Xiao W:
Polypharmacology in drug discovery: A review from systems
pharmacology perspective. Curr Pharm Des. 22:3171–3181. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu TH, Tu WQ, Tao WC, Liang QE, Xiao Y
and Chen LG: Verification of resveratrol inhibits intestinal aging
by downregulating ATF4/Chop/Bcl-2/Bax signaling pathway. Based on
network pharmacology and animal Front Pharmacol.
11:10642020.PubMed/NCBI
|
|
28
|
Liu B, Palmfeldt J, Lin L, Colaço A,
Clemmensen KKB, Huang J, Xu F, Liu X, Maeda K, Luo Y and Jäättelä
M: STAT3 associates with vacuolar H+-ATPase and
regulates cytosolic and lysosomal pH. Cell Res. 28:996–1012. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Wang S, Liu T, Ma Y, Huang S, Lei
L, Wen A and Ding Y: Resveratrol: Multi-targets mechanism on
neurodegenerative diseases based on network pharmacology. Front
Pharmacol. 11:6942020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
You L, Wang Z, Li H, Shou J, Jing Z, Xie
J, Sui X, Pan H and Han W: The role of STAT3 in autophagy.
Autophagy. 11:729–739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao X, Li B, Han X, Zhang X, Dang M, Wang
H, Du F, Zeng X and Guo C: Soluble receptor for advanced glycation
end-products promotes angiogenesis through activation of STAT3 in
myocardial ischemia/reperfusion injury. Apoptosis. 25:341–353.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu X, Zhu Z, Jiang L, Sun X, Jia Z, Qian
S, Li J and Ma L: Matrine increases NKG2D ligand ULBP2 in K562
cells via inhibiting JAK/STAT3 pathway: A potential mechanism
underlying the immunotherapy of matrine in leukemia. Am J Transl
Res. 7:1838–1849. 2015.PubMed/NCBI
|
|
33
|
Zhang YG, Zhu X, Lu R, Messer JS, Xia Y,
Chang EB and Sun J: Intestinal epithelial HMGB1 inhibits bacterial
infection via STAT3 regulation of autophagy. Autophagy.
15:1935–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang N, Xin H, Xu P, Yu Z and Shou D:
Erxian decoction attenuates TNF-α induced osteoblast apoptosis by
modulating the Akt/Nrf2/HO-1 signaling pathway. Front Pharmacol.
10:9882019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goncalves GK, Scalzo S, Alves AP, Agero U,
Guatimosim S and Reis AM: Neonatal cardiomyocyte hypertrophy
induced by endothelin-1 is blocked by estradiol acting on GPER. Am
J Physiol Cell Physiol. 314:C310–C322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang XQ, Xu ZT, Zhang GP, Hou N, Mo QX,
Wei J, Jiang X, Liu Y and Luo JD: Endophilin A2 attenuates cardiac
hypertrophy induced by isoproterenol through the activation of
autophagy. Am J Transl Res. 11:5065–5075. 2019.PubMed/NCBI
|
|
37
|
Shirakabe A, Zhai P, Ikeda Y, Saito T,
Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B and Sadoshima J:
Drp1-dependent mitochondrial autophagy plays a protective role
against pressure overload-induced mitochondrial dysfunction and
heart failure. Circulation. 133:1249–1263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Ding Y, Li M, Yuan J, Yu Y, Bi X,
Hong H, Ye J and Liu P: MicroRNA-34c-5p provokes
isoprenaline-induced cardiac hypertrophy by modulating autophagy
via targeting ATG4B. Acta Pharm Sin B. 12:2374–2390. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luan Y, Luan Y, Feng Q, Chen X, Ren KD and
Yang Y: Emerging role of mitophagy in the heart: Therapeutic
potentials to modulate mitophagy in cardiac diseases. Oxid Med Cell
Longev. 2021:32599632021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Chen X, Li P, Xiao Q, Hou D and Kong
X: CD47 antibody suppresses isoproterenol-induced cardiac
hypertrophy through activation of autophagy. Am J Transl Res.
12:5908–5923. 2020.PubMed/NCBI
|
|
41
|
Wang SY, Ni X, Hu KQ, Meng FL, Li M, Ma
XL, Meng TT, Wu HH, Ge D, Zhao J, et al: Cilostazol alleviate
nicotine induced cardiomyocytes hypertrophy through modulation of
autophagy by CTSB/ROS/p38MAPK/JNK feedback loop. Int J Biol Sci.
16:2001–2013. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie C, Liu S, Wu B, Zhao Y, Chen B, Guo J,
Qiu S and Cao YM: miR-19 promotes cell proliferation, invasion,
migration, and EMT by inhibiting SPRED2-mediated autophagy in
osteosarcoma cells. Cell Transplant. 29:9636897209624602020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang Z, Takahashi Y, Chen C, Liu Y, He H,
Tsotakos N, Serfass JM, Gebru MT, Chen H, Young MM and Wang HG:
Atg2A/B deficiency switches cytoprotective autophagy to
non-canonical caspase-8 activation and apoptosis. Cell Death
Differ. 24:2127–2138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng Q, Wang H, Pang J, Ji L, Han J, Wang
Y, Qi X, Liu Z and Lu L: Prevention of wogonin on colorectal cancer
tumorigenesis by regulating p53 nuclear translocation. Front
Pharmacol. 9:13562018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Valcourt DM, Dang MN, Scully MA and Day
ES: Nanoparticle-mediated co-delivery of notch-1 antibodies and
ABT-737 as a potent treatment strategy for triple-negative breast
cancer. ACS Nano. 14:3378–3388. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cusack CL, Swahari V, Hampton Henley W,
Michael Ramsey J and Deshmukh M: Distinct pathways mediate axon
degeneration during apoptosis and axon-specific pruning. Nat
Commun. 4:18762013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Acuner Ozbabacan SE, Keskin O, Nussinov R
and Gursoy A: Enriching the human apoptosis pathway by predicting
the structures of protein-protein complexes. J Struct Biol.
179:338–346. 2012. View Article : Google Scholar : PubMed/NCBI
|