Introduction
Leukemia is the sixth most lethal cancer accounting
for 4% of all cancer cases (1)
and arises from the bone marrow and the lymphatic system (2). Based on the rapidity of
proliferation (acute or chronic) and originator cell (myeloid cell
or lymphoid cell), leukemia can be classified into four types:
Acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML),
chronic lymphocytic leukemia (CLL), chronic myeloid leukemia (CML)
and chronic myelomonocytic leukemia (CMML) (3). In 2020, the American Cancer Society
reported that leukemia developed 474,519 new cases globally and
caused 311,594 deaths (4).
Clinical treatment for leukemia includes chemotherapy,
radiotherapy, immunotherapy, bone marrow transplantation and even
traditional Chinese medicine (5,6).
Among them, chemotherapy is the first choice for leukemia therapy
(2,7). Doxorubicin (DOX), an anthracycline
antibiotic that can inhibit topoisomerase and induce oxidative
stress to kill cells, is a well-established chemotherapeutic drug
for leukemia treatment (8–10).
DOX resistance is a major clinical problem in the leukemia
treatment and can lead to rapid deterioration in leukemia (10). It has been demonstrated that the
amplification of the multi-drug resistance gene mdr1 or increased
expression of glyoxalase I contributes to DOX resistance of
leukemia cells (11–13). In addition, epigenetic mechanisms,
such as DNA modification and histone modification, have been
involved in DOX resistance of leukemia cells (14–16).
Ferroptosis is a novel form of cell death, first
defined in 2012 (17).
Intracellular iron ion accumulation and reactive oxygen species
(ROS)-mediated lipid peroxidation are two hallmarks of ferroptosis
(18). It has been documented
that a series of extrinsic or intrinsic pathways can trigger
ferroptosis, such as inhibition of cystine/glutamate transporter,
activation of the iron transporter transferrin, or blockade of
glutathione peroxidase GPX4 (19). Ferroptosis has been shown to link
a number of human diseases including neurodegenerative diseases,
organ injury and cardiovascular diseases (20). In addition, recent evidence
indicates that induction of ferroptosis is a potential strategy to
eliminate cancer cells (21).
Currently, a series of anti-tumor drugs associated with ferroptosis
have been developed, such as nuclear factor erythroid 2-related
factor 2 (Nrf2) inhibitors, GSH inhibitors and iron activators
(21).
Triptolide is a natural diterpenoid epoxide,
extracted from the Chinese traditional herb thunder god vine
(Tripterygium wilfordii) (22). Triptolide has been used to treat
autoimmune disorders, such as rheumatoid arthritis and systemic
lupus erythematosus for a number of years (23). Accumulating evidence indicates
that triptolide also exhibits anti-tumor activities in multiple
types of human cancer, including leukemia, lung cancer, breast
cancer, colon cancer and prostate cancer (24–28). Currently, several triptolide
derivatives are in clinical phase I/II trials for cancer therapy
(29). However, the molecular
mechanisms underlying the anti-cancer activity of triptolide remain
to be elucidated.
The present study showed that Nrf2 served a critical
role in leukemia cell resistance to DOX. Triptolide can induce
leukemia cell ferroptosis via downregulation of Nrf2 to overcome
leukemia cell resistance to DOX. Thus, the present study suggested
that a combination of triptolide and DOX is a potential strategy
for leukemia treatment.
Materials and methods
Collection of patient samples
A total of 30 patients with leukemia (15 men and 15
women; age range, 15–35 years) admitted to Jin'an District Hospital
(Fuzhou, China) between May 1, 2020, and December 1, 2020, were
enrolled in the current study. Leukemia patient blood samples
(n=10; 4 men and 6 women) and DOX-resistant Leukemia patient blood
samples (n=20; 11 men and 14 women) were collected according to
institutional regulation of the Hospital Clinic Ethical Committee
and according to the declaration of Helsinki. Informed written
consent was given by all patients. The blood samples were separated
to obtain peripheral blood mononuclear cells (PBMCs) using a cell
isolator (LTS1077-1; Tianjin Haoyang Biological Manufacture Co.,
Ltd.) (30). Cells were subjected
to western blot analyses or reverse transcription-quantitative
(RT-q) PCR analyses.
Cell culture and drug treatment
Human leukemia chronic myelogenous leukemia K562 and
acute promyelocytic leukemia HL-60 cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS; Hyclone; Cytiva). HEK293T (Human
embryonic kidney) cells were cultured in DMEM medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Hyclone;
Cytiva). All cells were purchased from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences and were
grown in a medium supplemented with 100 µg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin (Gibco;
Thermo Fisher Scientific, Inc.). Cells were maintained in a
humidified 37°C incubator under a 5% CO2 atmosphere.
Cells at 80% confluence were treated with 1 µg/ml DOX (cat. no.
HY-15142A; MedChemExpress) for 48 h, 40 nM berberine (cat. no.
HY-N0716; MedChemExpress) for 24 h, 40 nM metformin (cat. no.
HY-15763; MedChemExpress), 40 nM artemisinin (cat. no. HY-B0094;
MedChemExpress) for 24 h, 40 nM curcumin (cat. no. HY-N0005;
MedChemExpress) for 24 h, 2 µM erastin (cat. no. HY-B0627;
MedChemExpress) for 48 h and 40 nM triptolide (cat. no. HY-32735;
MedChemExpress) for 24 or 48 h at 37°C.
Plasmid transfection and lentiviral
infection
Recombinant lentiviruses were amplified by
transfecting HEK 293T cells at 75% confluence with 6 µg pMD2.G and
6 µg psPAX2 packaging plasmids (the 2nd generation lentiviral
packaging plasmid; Addgene, Inc.) and 6 µg lentivirus-based Nrf2
expression plasmid (pLVX-puro; Addgene, Inc.) or 6 µg
lentiviral-based short hairpin (sh)RNAs (pLKO.1-puro; Addgene,
Inc.) specific for green fluorescent protein (GFP,
CAAATCACAGAATCGTCGTAT; negative control) or Nrf2 (#1,
GCTCCTACTGTGATGTGAAAT; 2#, GGAGTGTCAGTATGTTGAA) using
Lipofectamine® 2000 (cat. no. 11668; Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C. Viruses were collected at 72 h
after transfection. K562 or HL-60 cells at 40% confluence were
infected with a recombinant lentivirus in the presence of 10 µg/ml
polybrene, followed by 12 h incubation at 37°C with 5%
CO2. After 36 h of infection, cells were treated with 2
µg/ml puromycin for 24 h. The viable cells were used to further
experiments. The infection efficiency was ~85%.
Western blot analyses
Cells were collected, washed with cold PBS and
resuspended in EBC250 lysis buffer (250 mM NaCl, 50 mM Tris pH 8.0,
0.5% Nonidet P-40, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 2
µg/ml aprotinin and 2 µg/ml leupeptin) (31). The protein concentration was
assessed using the BCA method. Equal amounts of total protein (20
µg) were loaded, separated by SDS-PAGE (4% stacking gel, 10%
running gel), transferred to PVDF membranes (MilliporeSigma). The
membrane was then blocked with 5% skimmed milk powder for 1 h at
room temperature and hybridized to an appropriate primary antibody
(4°C, overnight) and HRP-conjugated secondary antibody
(anti-rabbit: 1:3,000; cat. no. AB0101; anti-mouse: 1:3,000, cat.
no. AB0102; Shanghai Abways Biotechnology Co., Ltd.; room
temperature, 1 h) for subsequent detection by enhanced
chemiluminescence using an ECL kit (cat. no. P0018S; Beyotime
Institute of Biotechnology). The images was analyzed using ImageJ
software 1.8.0 (National Institutes of Health). Primary antibodies
for GAPDH (cat. no. AF7021; 1:1,000), Nrf2 (cat. no. AF0639;
1:1,000), catalase (cat. no. DF7545; 1:1,000), superoxide dismutase
(SOD)2 (cat. no. AF5144; 1:1,000), glutathione peroxidase (GPX)4
(cat. no. DF6701; 1:1,000) and were purchased from Affinity
Biosciences. Antibody for Lamin B (cat. no. ab32535; 1:1,000) was
purchased from Abcam.
RT-qPCR
Total RNA was extracted from 1×106 cells
using RNA easy Plus Mini kit (Qiagen) according to the
manufacturer's protocol. RNA was reverse-transcribed into cDNAs
using M-MLV First Strand kit (Invitrogen) according to the
manufacturer's protocol. qPCR analyses of Nrf2 (forward:
GGTTTCTTCGGCTACGTTT; reverse: ACTTCTTTTTCCATTGAGGGTATA), catalase
(forward: CTCCGGAACAACAGCCTTCT; reverse: ATAGAATGCCCGCACCTGAG),
SOD2 (forward: TAGCTCTTCAGCCTGCACTG; reverse:
GCTTCCAGCAACTCCCCTTT), GPX4 (forward: TGGACGAGGGGAGGAGC; reverse:
GGGACGCGCACATGGT) and GAPDH (forward: TCAAGAAGGTGGTGAAGCAGG;
reverse: TCAAAGGTGGAGGAGTGGGT) were performed in CFX96 Real-Time
PCR System (Bio-Rad Laboratories, Inc.) using SoFast EvaGreen
Supermix (Bio-Rad Laboratories, Inc.), according to the
manufacturer's protocol. The reactions were carried out in a
96-well plate at 95°C for 5 min, followed by 40 cycles of 95°C for
15 sec and 58°C for 30 sec. GAPDH expression was used as an inner
control to normalize gene expression by the 2−ΔΔCq
method (31). All experiments
were performed three times in triplicate.
Cell viability analyses
Cell viability assay (CCK-8) was performed using a
CCK-8 assay Kit (cat. no. CK04; Dojindo Molecular Technologies,
Inc.) as described in the manufacturer's instruction (32). Briefly, 10 µl CCK-8 reagent was
incubated with the cells at 37°C for 4 h, and absorbance was
quantified at 450 nm using an ELx800 Absorbance Microplate Reader
(BioTek Instruments Inc.).
Measurement of ROS levels and lipid
oxidation by flow cytometry
K562 or HL-60 cells (2×105) were seeded
in 24-well plates in the absence or presence of triptolide. For the
measurement of ROS levels, cells were washed and subjected to the
procedures as described in the Reactive Oxygen Species Assay kit
(cat. no. D6470, Beijing Solarbio Science & Technology Co.,
Ltd.) (33). Measurement of lipid
oxidation was performed using BODIPY 581/591 C11 assay kit (cat.
no. D3861; Invitrogen; Thermo Fisher Scientific, Inc.) as described
in the manufacturer's instruction (33). Then the 2×104 cells
were analyzed using a flow cytometer (Beckman Coulter, Inc.) and
the data were analyzed using FlowJo software V10 (FlowJo, LLC).
Statistical analyses
GraphPad Prism 6.0 (GraphPad Software Inc.) was used
for data recording and calculation. All experiments were performed
at least three times. Data were presented as means ± standard
deviation. Quantitative data were analyzed statistically using
unpaired Student's t-test for two groups (Figs. 1 and 2) and one-way ANOVA followed by Tukey's
post-hoc test for >2 groups (Figs.
3 and 4) to assess the
significance.
 | Figure 1.Nrf2 serves a critical role in
leukemia cell resistance to DOX. K562 or HL-60 cells were grown in
a regular RPMI-1640 medium in the presence or absence of 0.8 µg/ml
DOX for 24 h alternatively. On day 40, viable DOX-resistant K562 or
HL-60 cells (K562-DR or HL-60-DR) were collected for further
experiments. (A) K562 cells, K562/DR, HL-60 cells, or HL-60/DR were
treated with an indicated concentration of DOX (DOX) for 48 h. Cell
viability was determined using CCK8 assays. K562, K562/DR, HL-60,
or HL-60/DR cells were subjected to (B) western blot or (C and D)
qPCR analyses. (E) K562, K562/DR, HL-60, or HL-60/DR cells were
subjected to fractionation of cytoplasm and nucleus, followed by
western blot analyses. (F) K562/DR or HL-60/DR cells stably
expressing shGFP, shNrf2-1, or shNrf2-2 were subjected to western
blot analyses. (G and H) Cells were treated with 1 µg/ml DOX for 48
h. Cell viability was determined using CCK8 assays. Peripheral
blood mononuclear cells (PBMC) from leukemia patients (n=10) or
DOX-resistance leukemia patients (n=20) were examined by (I and J)
western blot analyses or (K) qPCR analyses. Data were derived from
at least three independent experiments and were presented as means
± standard deviation. ***P<0.001; **P<0.01; *P<0.05. Nrf2,
nuclear factor erythroid 2-related factor 2; DOX, doxorubicin;
qPCR, quantitative PCR. |
Results
Nrf2 is essential for leukemia cell
resistance to DOX
Chemotherapy is the first choice for leukemia
treatment. DOX is a well-established chemotherapeutic drug for
leukemia treatment. However, DOX resistance is a major clinical
problem in leukemia treatment. Currently, the molecular
mechanism(s) by which leukemia cells resistance to DOX remains to
be elucidated. To explore this issue, DOX-resistant K562 cells
(K562/DR) and DOX-resistant HL-60 cells (HL-60/DR) were first
established upon a low dose of DOX treatment. As shown in Fig. 1A, compared with normal K562 or
HL-60 cells, K562/DR or HL-60/DR cells exhibited strong resistance
to DOX. Oxidative stress is an important hallmark of cancer cells
(34). It is reported that DOX
can induce the production of ROS to kill cancer cells (9). Next, the present study examined
whether oxidative stress also served a role in leukemia cell
resistance to DOX. As shown in Fig.
1B, K562/DR or HL-60/DR cells had higher Nrf2, a master
regulator of the antioxidant response, protein expression than
normal K562 or HL-60 cells, as evidenced by western blot analyses.
It has been documented that Nrf2 is a transcription factor that
regulates a series of genes expression involved in oxidative stress
response, including catalase, SOD2 and GPX4. Consistent with Nrf2,
K562/DR or HL-60/DR cells also exhibited higher catalase, SOD2 and
GPX4 protein expression than normal K562 or HL-60 cells (Fig. 1B). In addition, qPCR analyses also
showed that compared with normal K562 or HL-60 cells, K562/DR or
HL-60/DR cells had higher Nrf2, catalase SOD2 and GPX4 mRNA levels
(Fig. 1C and D; P<0.001). It
is reported that Nrf2 can both localize in the cytoplasm and
nucleus (35). However, only
nuclear localization of Nrf2 can regulate antioxidant genes
transcription. Therefore, the localization of Nrf2 was also
examined in our system. As shown in Fig. 1E, compared with normal leukemia
cells, nuclear localization of Nrf2 protein expression
significantly increased in K562/DR or HL-60/DR cells. Notably,
silencing of Nrf2 significantly sensitized K562/DR or HL-60/DR
cells to DOX (Fig. 1F-H;
P<0.05).
To further investigate whether the expression of
Nrf2 and its downstream target genes are also upregulated in
clinical DOX-resistant leukemia samples, leukemia patient blood
samples (n=10) and DOX-resistant leukemia patient blood samples
(n=20) were harvested. Levels of Nrf2, catalase, SOD2 and GPX4 in
leukemia PBMC (n=10) and DOX-resistant leukemia PBMC (n=20) were
then examined by western blot and quantitative PCR analyses. As
shown in Fig. 1I-K, DOX-resistant
leukemia PBMC exhibited significantly upregulated Nrf2, catalase,
SOD2 and GPX4 protein and mRNA expression than normal leukemia PBMC
(P<0.05).
Together, these results suggested that Nrf2 may play
a role in leukemia cell resistance to DOX.
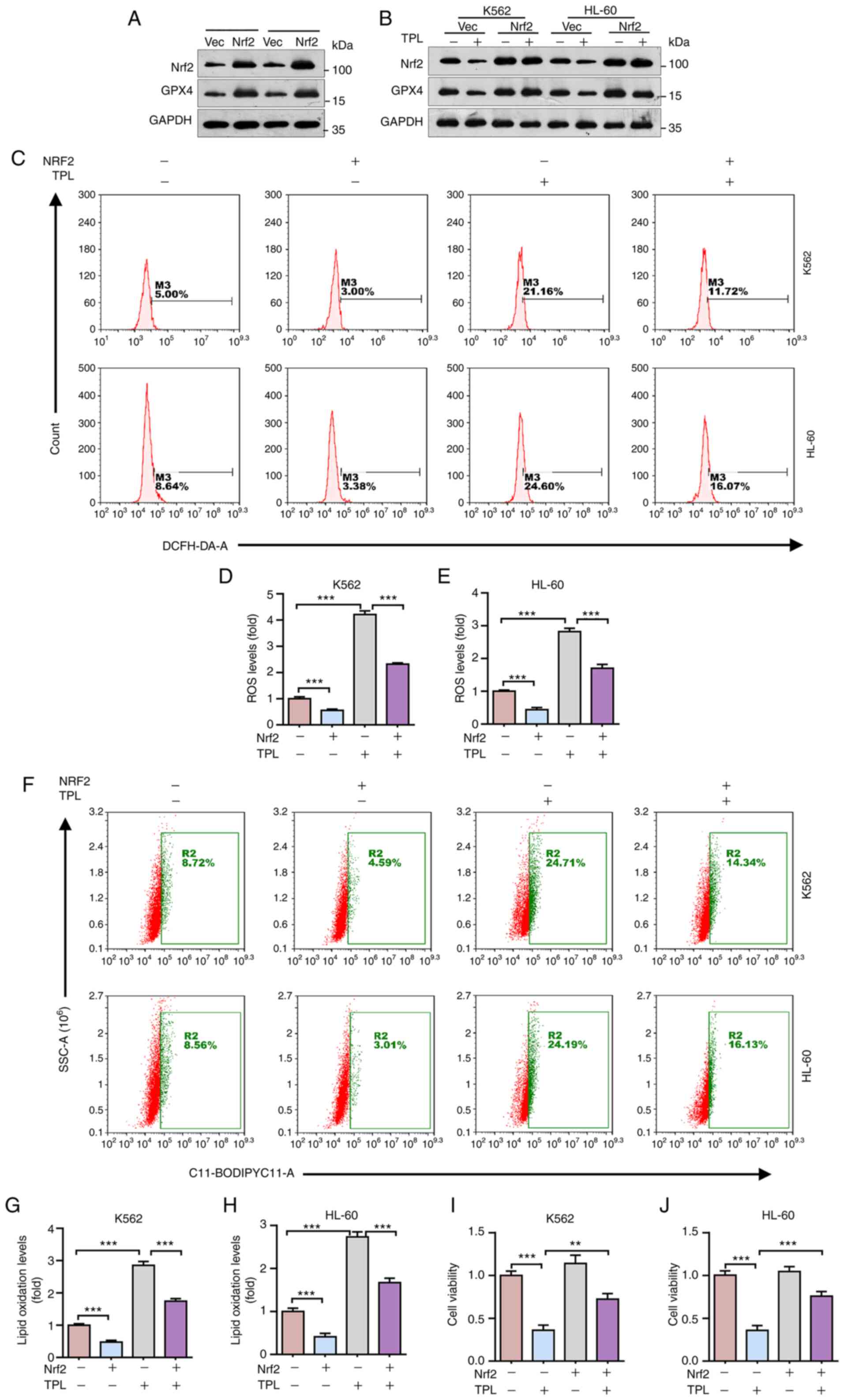
Triptolide inhibits Nrf2 expression
and induces leukemia cells ferroptosis
The aforementioned data indicated that Nrf2 served a
critical role in leukemia cell resistance to DOX. It was thus
hypothesized that inhibition of Nrf2 may be a potential strategy
for overcoming DOX resistance in leukemia cells. To search for
potential small chemical Nrf2 inhibitors, the effects of several
known chemical compounds on Nrf2 expression were examined. As shown
in Fig. 2A, triptolide, a
clinical-approved chemical drug used to treat autoimmune disorders,
remarkedly inhibited Nrf2 protein expression, concomitant with
reduced GPX4 expression. The effects of triptolide on cellular ROS
levels were also examined. As shown in Fig. 2B and C, triptolide markedly
increased ROS levels in K562 and HL-60 cells, as evidenced by
DCFH-DA analyses (P<0.001). Triptolide also significantly
promoted lipid oxidation in K562 and HL-60 cells, as evidenced by
BODIPY 581/591 C11 analyses (Fig. 2D
and E; P<0.001). Since increased ROS and lipid oxidation and
decreased GPX4 expression are hallmarks of ferroptosis, the effects
of triptolide on leukemia cell viability were therefore examined.
As shown in Fig. 2F and G,
triptolide markedly inhibited K562 or HL-60 cell viability,
indicating that triptolide induced leukemia cell ferroptosis.
Together, these results demonstrated that triptolide can inhibit
Nrf2 expression and induce leukemia cell ferroptosis.
Ectopic expression of Nrf2 inhibits
triptolide-induced ferroptosis
Next, the present study investigated the role of
Nrf2 in triptolide-induced leukemia cell ferroptosis. To examine
this issue, K562 or HL-60 cells which stably express Nrf2 were
first established. As shown in Fig.
3A, ectopic expression Nrf2 significantly increased GPX4
protein expression, consistent with the previous report (36). Furthermore, ectopic expression of
Nrf2 restored GPX4 protein expression inhibited by triptolide
(Fig. 3B). In addition, ectopic
expression of Nrf2 also markedly reduced endogenous ROS levels and
triptolide-induced ROS levels in K562 and HL-60 cells (Fig. 3C-E; P<0.001). Next, the role of
Nrf2 on triptolide-induced leukemia cell lipid oxidation was
examined. As shown in Fig. 3F-H,
triptolide significantly induced lipid oxidation in K562 and HL-60
cells, which can be significantly rescued by ectopic expression of
Nrf2 (P<0.001), suggesting that Nrf2 is critical in
triptolide-induced ferroptosis. Indeed, it was found that ectopic
expression of Nrf2 can inhibit triptolide-induced downregulation of
K562 and HL-60 cell viability (Fig.
3I-J; P<0.001). Together, these results indicated that
triptolide promotes leukemia cell ferroptosis via downregulation of
Nrf2 expression.
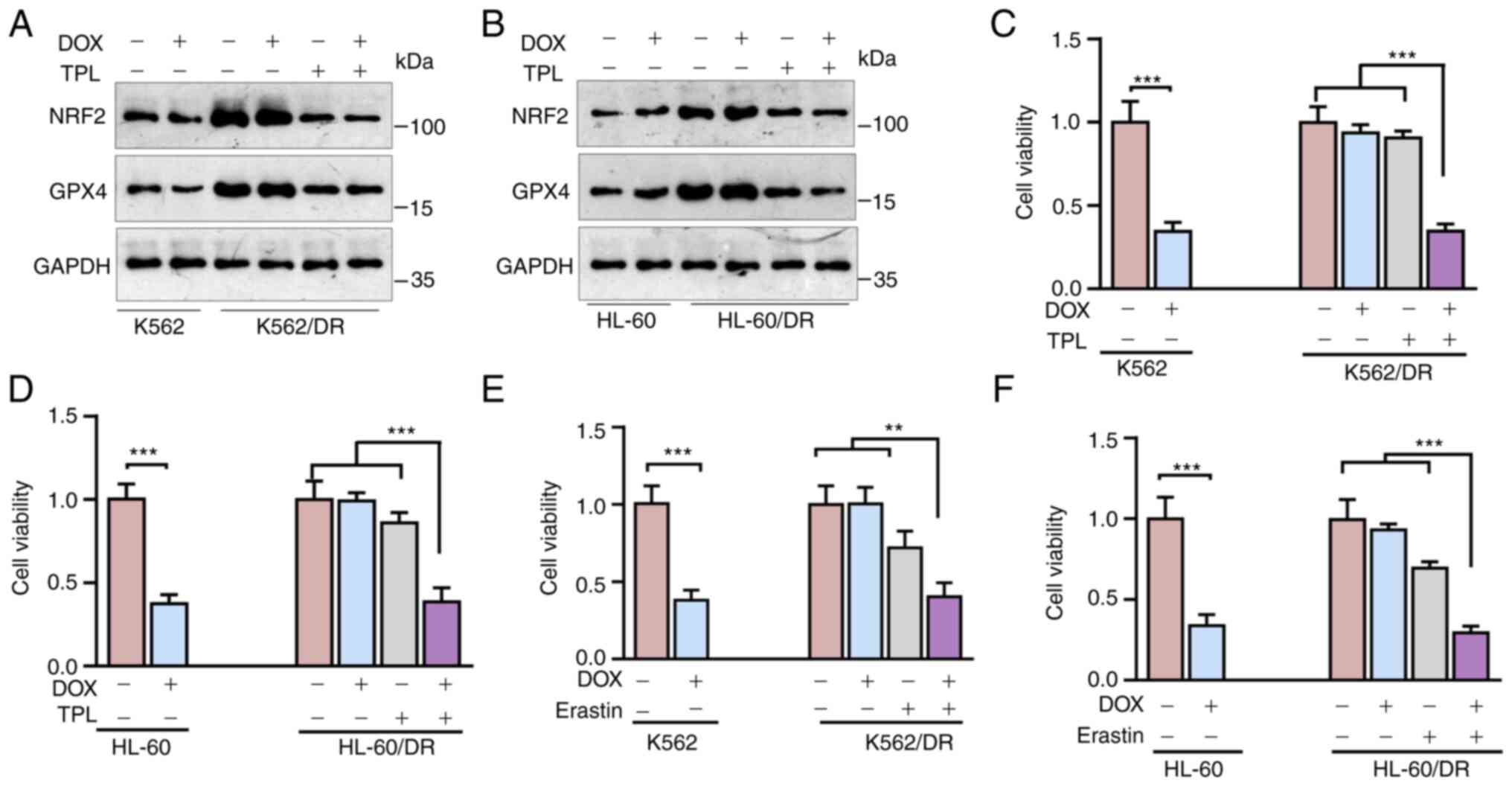
Triptolide sensitizes leukemia cells
to DOX
The aforementioned data indicated that Nrf2 served a
critical role in leukemia cell resistance to DOX (Fig. 1) and triptolide inhibits Nrf2
expression to induce leukemia cell ferroptosis (Figs. 2 and 3). Therefore, it was hypothesized that
treatment with triptolide may overcome leukemia cell resistance to
DOX. To examine this hypothesis, triptolide was used to treat
K562/DR or HL-60 cells. As shown in Fig. 4A and B, triptolide significantly
suppressed Nrf2 and GPX4 expression. Furthermore, DOX significantly
inhibited normal K562 or HL-60 cell viability (P<0.001), but it
had little effect on K562/DR or HL-60 cell viability (Fig. 4C and D). Notably, treatment with
low-dose triptolide did not affect K562/DR or HL-60 cell viability,
but it could re-sensitize K562/DR or HL-60 cells to DOX (Fig. 4C and D). In addition, consistent
with triptolide, erastin, a ferroptosis inducer, could also
re-sensitize K562/DR or HL-60 cells to DOX (Fig. 4E and F). Together, these results
suggested that triptolide promotes ferroptosis via suppressing Nrf2
to sensitize leukemia cells to DOX.
Discussion
The present study showed that the expression of
Nrf2, the master regulator of cellular antioxidation response, is
significantly increased in the clinical DOX-resistant leukemia
sample. Notably, the silencing of Nrf2 markedly sensitized
DOX-resistant leukemia cells to DOX. In addition, the present study
showed that triptolide, a natural diterpenoid epoxide used to treat
autoimmune disorders, can inhibit Nrf2 expression to induce
leukemia cells ferroptosis and sensitize DOX-resistant leukemia
cells to DOX.
DOX is widely used to treat leukemia. However, DOX
resistance is a major clinical problem for leukemia therapy.
Therefore, it is important to investigate the molecular mechanism
by which leukemia cells resistance to DOX and explore new
strategies to overcome DOX resistance. It is reported that
piperlongumine, a ROS inducer, can reverse leukemia cell resistance
to DOX via the PI3K/Akt pathway (37). In addition, indomethacin, a
cyclooxygenase inhibitor, can also overcome DOX resistance by
decreasing glutathione (38). The
present study indicated that Nrf2 served an important role in
leukemia cell resistance to DOX and highlighted a potential
strategy of combination therapy using triptolide and DOX in
leukemia treatment.
Nrf2 mRNA and protein levels were significantly
upregulated in DOX-resistant leukemia cells and clinical
DOX-resistant leukemia samples. An important question is: How Nrf2
is upregulated in DOX-resistant leukemia? It is reported that Nrf2
protein stability is tightly regulated by Kelch-like ECH-associated
protein 1 (Keap1) in the cytoplasm (34). Upon oxidative stress, Keap1
separates from Nrf2, which leads to the stabilization of Nrf2
protein and results in Nrf2 translocation to the nucleus (34). In addition, activation of ERK
signaling also can promote Nrf2 translocation to the nucleus and
stabilize Nrf2 protein (35,39). It has been documented that
oncogenic K-RasG12D can increase the transcription of
Nrf2 via activating ERK signaling (40). Notably, DOX can induce ROS
production and activate ERK signaling (41,42). Therefore, it is plausible that DOX
promotes Nrf2 expression via induction of ROS or activation of ERK,
which needs to be further investigated.
The present study clearly demonstrated that
triptolide can significantly inhibit Nrf2 expression in leukemia
cells. A recent report shows that triptolide can suppress Nrf2
target genes expression via decreasing nuclear localization of Nrf2
in lung cancer cells (43). In
addition, triptolide can also serve as an inhibitor of Nrf2, which
suppresses Nrf2 transcriptional activity in glioma cells (44). Together, these observations
demonstrate that triptolide can inhibit Nrf2 in multiple levels,
including protein expression, nuclear localization and
transcriptional activity.
Notably, the present study indicated that
triptolide-mediated downregulation of Nrf2 led to leukemia cells
ferroptosis. It also showed that Nrf2 serves a critical role in the
DOX resistance of leukemia cells. Therefore, it is plausible that
triptolide can promote leukemia cell sensitivity to DOX via
downregulation of Nrf2 expression. Indeed, the present study
indicated that treatment with triptolide sensitized DOX-resistant
leukemia cells to DOX. Together, the present study suggested that
the combined use of triptolide and DOX may be a promising
therapeutic strategy for leukemia therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jin'an District Hospital
Research Foundation (grant no. JA2021KJ005) to GL.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and GL conceived and designed the experiments.
XW, SC and KH performed the experiments. XW, SC and KH analyzed the
data. XW and GL wrote the manuscript. XW and GL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Fuzhou Jin'an District Hospital (Fuzhou, China;
approval no. JA-KJ2021-011).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Du Y and Chen B: Detection approaches for
multidrug resistance genes of leukemia. Drug Des Devel Ther.
11:1255–1261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yi Y, Gao L, Wu M, Ao J, Zhang C, Wang X,
Lin M, Bergholz J, Zhang Y and Xiao ZJ: Metformin sensitizes
leukemia cells to vincristine via activation of AMP-activated
protein kinase. J Cancer. 8:2636–2642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brunning RD: Classification of acute
leukemias. Semin Diagn Pathol. 20:142–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burnett AK, Kell J and Rowntree C: Acute
myeloid leukemia: Therapeutic indications. Curr Opin Hematol.
7:333–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burnett A, Wetzler M and Löwenberg B:
Therapeutic advances in acute myeloid leukemia. J Clin Oncol.
29:487–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang HW, Ma KL, Liu H and Zhou JY:
Reversal of multidrug resistance in leukemia cells using a
transferrin-modified nanomicelle encapsulating both doxorubicin and
psoralen. Aging (Albany NY). 12:6018–6029. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lothstein L, Israel M and Sweatman TW:
Anthracycline drug targeting: Cytoplasmic versus nuclear-a fork in
the road. Drug Resist Updat. 4:169–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kruk I, Michalska T, Kładny J and
Kubera-Nowakowska L: Luminescence investigations of redox cycling
of adriamycin. Chemosphere. 44:83–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davies GF, Roesler WJ, Juurlink BH and
Harkness TA: Troglitazone overcomes doxorubicin-resistance in
resistant K562 leukemia cells. Leuk Lymphoma. 46:1199–1206. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakamoto H, Mashima T, Kizaki A, Dan S,
Hashimoto Y, Naito M and Tsuruo T: Glyoxalase I is involved in
resistance of human leukemia cells to antitumor agent-induced
apoptosis. Blood. 95:3214–3218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakamoto H, Mashima T, Sato S, Hashimoto
Y, Yamori T and Tsuruo T: Selective activation of apoptosis program
by S-p-bromobenzylglutathione cyclopentyl diester in glyoxalase
I-overexpressing human lung cancer cells. Clin Cancer Res.
7:2513–2518. 2001.PubMed/NCBI
|
|
13
|
Xia CQ and Smith PG: Drug efflux
transporters and multidrug resistance in acute leukemia:
Therapeutic impact and novel approaches to mediation. Mol
Pharmacol. 82:1008–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Altucci L, Clarke N, Nebbioso A,
Scognamiglio A and Gronemeyer H: Acute myeloid leukemia:
Therapeutic impact of epigenetic drugs. Int J Biochem Cell Biol.
37:1752–1762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waldmann T and Schneider R: Targeting
histone modifications-epigenetics in cancer. Curr Opin Cell Biol.
25:184–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu T, Guo Q, Guo H, Hou S, Li J and Wang
H: Quantitative analysis of histone H3 and H4 post-translational
modifications in doxorubicin-resistant leukemia cells. Biomed
Chromatogr. 30:638–644. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bebber CM, Müller F, Prieto Clemente L,
Weber J and von Karstedt S: Ferroptosis in cancer cell biology.
Cancers (Basel). 12:1642020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Z, Zhang L, Zheng J, Sun H and Shao C:
Ferroptosis: Biochemistry and biology in cancers. Front Oncol.
11:5792862021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv
H, AlQudsy LHH and Shang P: Ferroptosis, a novel pharmacological
mechanism of anti-cancer drugs. Cancer Lett. 483:127–136. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai J, Yi M, Tan Y, Li X, Li G, Zeng Z,
Xiong W and Xiang B: Natural product triptolide induces
GSDME-mediated pyroptosis in head and neck cancer through
suppressing mitochondrial hexokinase-II. J Exp Clin Cancer Res.
40:1902021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Q: Triptolide and its expanding
multiple pharmacological functions. Int Immunopharmacol.
11:377–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pigneux A, Mahon FX, Uhalde M, Jeanneteau
M, Lacombe F, Milpied N, Reiffers J and Belloc F: Triptolide
cooperates with chemotherapy to induce apoptosis in acute myeloid
leukemia cells. Exp Hematol. 36:1648–1659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shao H, Ma J, Guo T and Hu R: Triptolide
induces apoptosis of breast cancer cells via a mechanism associated
with the Wnt/β-catenin signaling pathway. Exp Ther Med. 8:505–508.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Isharwal S, Modi S, Arora N, Uhlrich C
III, Giri B, Barlass U, Soubra A, Chugh R, Dehm SM, Dudeja V, et
al: Minnelide inhibits androgen dependent, castration resistant
prostate cancer growth by decreasing expression of androgen
receptor full length and splice variants. Prostate. 77:584–596.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oliveira A, Beyer G, Chugh R, Skube SJ,
Majumder K, Banerjee S, Sangwan V, Li L, Dawra R, Subramanian S, et
al: Triptolide abrogates growth of colon cancer and induces cell
cycle arrest by inhibiting transcriptional activation of E2F. Lab
Invest. 95:648–659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Philips BJ, Kumar A, Burki S, Ryan JP,
Noda K and D'Cunha J: Triptolide-induced apoptosis in non-small
cell lung cancer via a novel miR204-5p/Caveolin-1/Akt-mediated
pathway. Oncotarget. 11:2793–2806. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Noel P, Von Hoff DD, Saluja AK, Velagapudi
M, Borazanci E and Han H: Triptolide and its derivatives as cancer
therapies. Trends Pharmacol Sci. 40:327–341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ni WJ and Leng XM: Down-regulated miR-495
can target programmed cell death 10 in ankylosing spondylitis. Mol
Med. 26:502020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Niu W, Xu L, Li J, Zhai Y, Sun Z, Shi W,
Jiang Y, Ma C, Lin H, Guo Y and Liu Z: Polyphyllin II inhibits
human bladder cancer migration and invasion by regulating
EMT-associated factors and MMPs. Oncol Lett. 20:2928–2936. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang X, Li X, Zhang D and Han W:
Astragaloside-IV alleviates high glucose-induced ferroptosis in
retinal pigment epithelial cells by disrupting the expression of
miR-138-5p/Sirt1/Nrf2. Bioengineered. 13:8240–8254. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deshmukh P, Unni S, Krishnappa G and
Padmanabhan B: The Keap1-Nrf2 pathway: Promising therapeutic target
to counteract ROS-mediated damage in cancers and neurodegenerative
diseases. Biophys Rev. 9:41–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zipper LM and Mulcahy RT: Erk activation
is required for Nrf2 nuclear localization during pyrrolidine
dithiocarbamate induction of glutamate cysteine ligase modulatory
gene expression in HepG2 cells. Toxicol Sci. 73:124–134. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma CS, Lv QM, Zhang KR, Tang YB, Zhang YF,
Shen Y, Lei HM and Zhu L: NRF2-GPX4/SOD2 axis imparts resistance to
EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer
cells. Acta Pharmacol Sin. 42:613–623. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang Q and Yan S: Piperlongumine reverses
doxorubicin resistance through the PI3K/Akt signaling pathway in
K562/A02 human leukemia cells. Exp Ther Med. 9:1345–1350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Asano T, Tsutsuda-Asano A and Fukunaga Y:
Indomethacin overcomes doxorubicin resistance by decreasing
intracellular content of glutathione and its conjugates with
decreasing expression of gamma-glutamylcysteine synthetase via
promoter activity in doxorubicin-resistant leukemia cells. Cancer
Chemother Pharmacol. 64:715–721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nguyen T, Sherratt PJ, Huang HC, Yang CS
and Pickett CB: Increased protein stability as a mechanism that
enhances Nrf2-mediated transcriptional activation of the
antioxidant response element. Degradation of Nrf2 by the 26 S
proteasome. J Biol Chem. 278:4536–4541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
DeNicola GM, Karreth FA, Humpton TJ,
Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES,
et al: Oncogene-induced Nrf2 transcription promotes ROS
detoxification and tumorigenesis. Nature. 475:106–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Asensio-López MC, Soler F, Pascual-Figal
D, Fernández-Belda F and Lax A: Doxorubicin-induced oxidative
stress: The protective effect of nicorandil on HL-1 cardiomyocytes.
PLoS One. 12:e01728032017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu J, Mao W, Ding B and Liang CS:
ERKs/p53 signal transduction pathway is involved in
doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am
J Physiol Heart Circ Physiol. 295:H1956–H1965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nam LB, Choi WJ and Keum YS: Triptolide
downregulates the expression of NRF2 target genes by increasing
cytoplasmic localization of NRF2 in A549 cells. Front Pharmacol.
12:6801672021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu D, Liu Y, Zhou Y, Ruiz-Rodado V, Larion
M, Xu G and Yang C: Triptolide suppresses IDH1-mutated malignancy
via Nrf2-driven glutathione metabolism. Proc Natl Acad Sci USA.
117:9964–9972. 2020. View Article : Google Scholar : PubMed/NCBI
|