Introduction
One-lung ventilation (OLV) has been commonly applied
in clinical settings (1); however,
it can result in inhibition of hypoxemic pulmonary
vasoconstriction, the imbalance of ventilation/perfusion (V/Q), the
production of oxygen free radical products, inflammation and
ischemia/reperfusion (I/R) injury, mainly in the surgical lung,
which can lead to severe pulmonary injury and is an issue for
anesthesiologists (2,3). Current methods of OLV do not
satisfactorily improve the prognosis of patients. Previous studies
have reported that the core problem of OLV-associated pulmonary
injury is mitochondrial damage (4,5).
According to previous studies, nicorandil, a US Food and Drug
Administration-approved mitochondrial ATP-sensitive potassium
channel (mitoKATP)-specific opener, has beneficial effects on the
heart by opening the mitoKATP (6–8). To
the best of our knowledge, only one study has previously reported
its effect on pulmonary injury (9).
It is important to protect pulmonary function during
thoracic surgery anesthesia, particularly that of the collapsed
lung. This is very important for the perioperative safety of
patients and guarantees the rapid recovery of patients after
surgery, which is the core intention of enhanced recovery after
surgery (ERAS) protocols (10).
How to ensure the stability and clearance of the surgical field
while maintaining the stability of vital signs during OLV, as well
as the early recovery of postoperative pulmonary function, is an
unsolved clinical issue.
Acute lung injury can cause changes in mitochondrial
respiratory function and enzyme activity, the production of
mitochondrial oxygen free radicals, mitochondrial calcium overload
and mitochondrial permeability transition. The activation of PI3K
can initiate the phosphorylation of various phosphatidylinositol
intermediates and the generated phosphatidylinositol (3,4,5)-trisphosphate can be combined with Akt.
After binding, Akt is transferred from the cytoplasm to the cell
membrane with the help of 3-phosphoinositide-dependent protein
kinase 1. The threonine phosphorylation site (Thr308) and serine
phosphorylation site (Ser473) on Akt are phosphorylated to promote
its activation. Phosphorylated (p)-Akt regulates cell functions,
such as anti-apoptotic functions, by phosphorylating numerous
enzymes and kinases (11).
Moreover, p-Akt can phosphorylate Bcl-2 family member BAD so that
it can bind to the effector protein instead of Bcl-XL, thereby
inhibiting apoptosis. Furthermore, mitochondrial regulation of
apoptosis is closely associated with the Bcl-2 protein.
Pro-apoptotic members of this family, such as Bax, trigger the
release of mitochondrial apoptotic factors into the cytoplasm by
acting on the mitochondrial membrane permeability transition pore
(mPTP), which leads to the activation of cysteine proteases. The
anti-apoptotic protein Bcl-2 serves a role in preventing cell
apoptosis. Oxidative stress products are closely associated with
mitochondrial dysfunction and apoptosis (12). The mPTP is the main target of
reactive oxygen species (ROS) (12). After the mPTP is opened, cytochrome
c is released into the cytoplasm, which causes cell
dysfunction.
Nicorandil can open the KATP channel on the vascular
smooth muscle cell membrane, causing potassium ion outflow and
leading to hyperpolarization of the cell membrane, which will
inhibit the opening of voltage-dependent calcium ion channels, thus
leading to reduced calcium ion influx (13). Whether nicorandil can act on
mitoKATP to serve a protective role in the lungs requires
elucidation.
Our previous study in rabbits demonstrated that
nicorandil protected against lung injury in the collapsed lung
during surgery (13). The present
study assessed the beneficial effect of nicorandil on OLV-induced
pulmonary injury in clinical thoracic surgery and evaluated its
mechanisms in combination with the results of the rabbit OLV model
(13).
Materials and methods
Patient information
The present study was approved by the Affiliated
Hospital of Nantong University ethics committee (approval no.
2017-K031-D01; Nantong, China). A total of 60 American Society of
Anesthesiologists class I–II (14)
patients undergoing thoracoscopic radical resection for lung cancer
at the Affiliated Hospital of Nantong University, Yangzhou Hongquan
Hospital (Yangzhou, China) and Shuyang Hospital Affiliated of
Xuzhou Medical University (Suqian, China) between January 2018 and
December 2020 were enrolled in the present study. The patients
volunteered and were randomly divided into the following groups: i)
The sham group; ii) the control group; and iii) the nicorandil
group [n=20 cases (10 male and 10 female cases)/group]. The 30 male
and 30 female patients were 55–75 years old and weighed 50–70 kg.
Their lung, brain, liver and kidney functions, and biochemical
tests were normal prior to surgery. An electrocardiogram (EKG) was
used to assess chronic myocardial ischemia, and changes to the
ST-segment and T-wave. Nicorandil and nitroglycerin can be applied
for myocardial protection. Patients with a history of digestive
ulcers and the use of sulfa drugs were excluded. Subjects
participated voluntarily and were able to withdraw; therefore, a
potential exit rate was considered in the calculation of the sample
size. After initial screening according to inclusion and exclusion
criteria, informed consent was signed to collect basic information,
disease history and anthropometric information of the patients in a
face-to-face manner. The four enrolled cases in the control group
and five enrolled cases in the nicorandil group that were benign
were withdrawn from the study. Patients who were enrolled in the
control and nicorandil groups underwent thoracoscopic radical
resection of lung cancer after pathological biopsy. Patients who
were enrolled in the sham group did not undergo thoracoscopic
radical resection of lung cancer due to the mass being benign;
their tissue samples were obtained from a pathological biopsy.
Instruments
The following instruments were used in the present
study: Single double-sided clean bench (Suzhou Purification
Engineering Installation Co., Ltd.), transmission electron
microscope (Hitachi, Ltd.), ELISA plate reader (BioTek Instruments,
Inc.), fluorescence quantitative (q)PCR instrument (Eppendorf),
fluorescence microscope (Olympus Corporation), gel imaging analysis
system (Beijing Yuanpinghao Biotech Co., Ltd.), SK-30 high-speed
refrigerated centrifuge (Sigma-Aldrich; Merck KGaA), Cryostat
CM1900-UV (Leica Microsystems GmbH), multi-function monitor
(Spacelabs Healthcare; OSI Systems, Inc.), DNA thermal cycler
PTC-200 (Eppendorf) and paraffin microtome RM2245 (Leica
Microsystems GmbH).
Experimental methods
Anesthesia method
Atropine (0.5 mg) was injected intramuscularly
before surgery. In the operation room, Ringer's solution was
infused at 10 ml/kg/h. The signal lines of the EKG, the probe of
pulse oxygen saturation (SpO2) and the artery catheter
were placed to continuously monitor the vital signs [heart rate
(HR), SpO2 and mean arterial pressure (MAP)] using a
Spacelabs monitor. Midazolam (50 µg/kg), sufentanil (0.6 µg/kg),
propofol (2 mg/kg) and vecuronium (0.2 mg/kg) were used for
induction. A double lumen bronchial catheter was inserted ~3 min
after induction and mechanical ventilation was performed with a
tidal volume (VT) of 8 ml/kg and a respiratory rate (RR) of 12
beats/min. During OLV, the VT was adjusted to 5 ml/kg and the RR
was adjusted to 16 beats/min. Anesthesia maintenance was performed
by infusion of propofol (200 mg/h, remifentanil 0.3 (µg/kg/min) and
atracurium (50 mg/h). OLV was performed when the double lumen
bronchial catheter was inserted and two-lung ventilation (TLV) was
restored before the incision was closed. Anesthesia was terminated
after the operation. The patients were admitted to the
post-anesthesia care unit and were then connected to a
patient-controlled intravenous analgesia pump [sufentanil (4.5
µg/kg) and azasetron (20 mg); total volume, 150 ml]. Neostigmine (2
mg) and atropine (1 mg) antagonized residual muscle relaxant before
consciousness, reflexes and breathing were completely restored. The
patients were returned to the ward after extubation.
Patient treatment
Patients in the nicorandil group were treated
according to the manufacturer's protocol for nicorandil (SHKB
pharmaceutical), venous access was opened 1 h before induction and
nicorandil (2 mg/h) was infused for 2 h until 1 h after OLV. OLV
and TLV were performed and thoracoscopic radical resection of lung
cancer was successfully completed. Patients in the control group
were treated according to the same method as The nicorandil group,
with the exception that nitroglycerin (0.3 µg/kg/min) was used
instead of nicorandil. Patients in the sham group were treated with
nitroglycerin (0.3 µg/kg/min) as was used in the control group
without an OLV phase. Immediately after the operation, the target
lung tissue that was removed by wedge resection was rapidly frozen
and underwent pathological examination and was confirmed to be
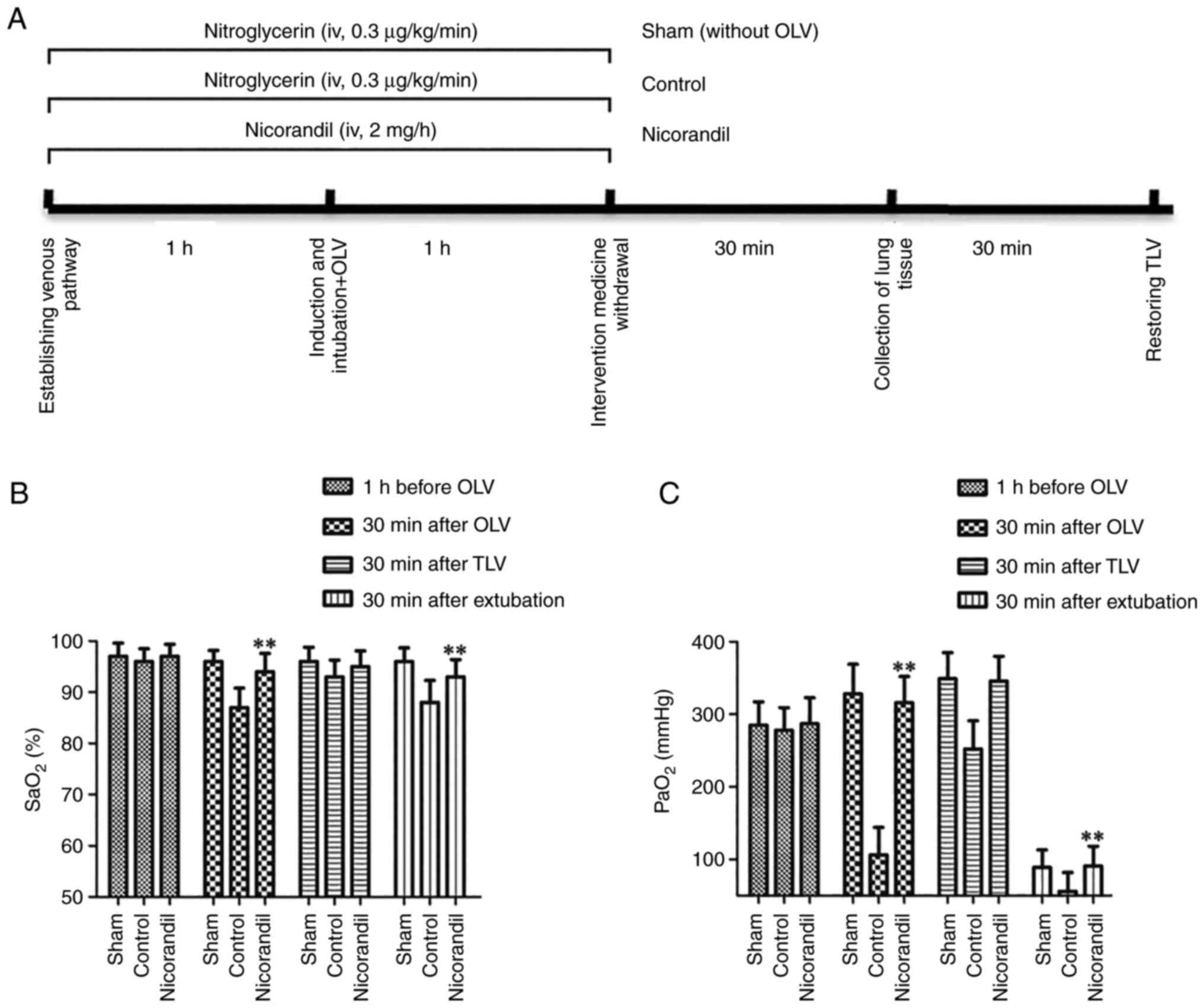
benign in the sham group. A schematic diagram of the experimental
design was presented in the Fig.
1A.
Observation items
MAP, HR and SpO2 were recorded at four
time points, including before administration of nicorandil or
nitroglycerin, 30 min after OLV, 30 min after TLV and 30 min after
extubation. Arterial blood gas analysis was performed for arterial
partial pressure for oxygen (PaO2) and arterial oxygen
desaturation (SaO2) at the above four time points. Light
microscopy and electron microscopy were used to evaluate the
microstructure of the lung. A TUNEL assay was utilized to evaluate
apoptosis and assess the proportion of apoptotic cells to total
observed cells (AI). The levels of malondialdehyde (MDA) and TNF-α,
and the activity of superoxide dismutase (SOD) were assessed using
ELISA. The MDA (cat. no. S0131S), SOD (cat. no. S0103) and TNF-α
(cat. no. PT518) ELISA kits were all obtained from Beyotime
Institute of Biotechnology and were performed according to the
manufacturer's protocols. The protein expression levels of PI3K,
Akt, p-Akt, NF-κB and hypoxia-inducible factor 1α (HIF-1α) were
assessed using western blotting. Reverse transcription (RT)-qPCR
was used to assess the mRNA expression level of HIF-1α. The protein
expression levels of apoptosis-related proteins, including Bax,
Bcl-2 and caspase-3, were also semi-quantified using western
blotting.
Specimen collection
A total of 1 ml arterial blood was collected at the
four aforementioned time points for blood gas analysis, immediate
assessment and ELISA. When the surgical specimen was removed, three
pieces (~1 cm3) of lung tissue around the mass were
collected. One piece was fixed with 4% paraformaldehyde (PFA) at
4°C overnight for use in subsequent H&E staining and TUNEL
staining. Another piece was frozen in liquid nitrogen and then
placed in an ultra-low temperature (−80°C) experimental freezer for
RT-qPCR and western blotting. The remaining piece of lung tissue
was fixed using 2.5% glutaraldehyde and stored at 4°C overnight for
assessment using scanning electron microscopy.
Detection methods
All subsequent detection methods were previously
reported for use in animal experiments (11).
Measurement of the pulmonary injury
score (H&E staining)
For histopathological analysis, lung tissue samples
were fixed in 4% PFA at 4°C overnight, and embedded in paraffin.
Subsequently, sections (5 µm) were prepared, stained with
hematoxylin at room temperature for 5 min and with eosin at room
temperature for 3 min for pathological observation. Finally, the
samples were examined using an OLYMPUS-CX23 light microscope
(magnification, ×400; Olympus Corp.). Each slide was assessed
according to the following criteria: i) Alveolar septal congestion;
ii) alveolar hemorrhage; iii) intra-alveolar cell infiltrates; and
iv) intra-alveolar fibrin deposition (15).
Transmission electron microscopy
Lung tissues (1 mm3) were fixed using
2.5% glutaraldehyde at 4°C, overnight, followed by 1% osmium
tetroxide and embedded in epoxy resin 618 at room temperature
overnight. Subsequently, these samples were cut into ultrathin
sections (0.1 µm) and examined under a Hitachi HT-7700 transmission
electron microscope (magnification, ×3000; HT-7700; Hitach,
Ltd.).
TUNEL assay
Sections (10 µm) were dewaxed at room temperature
for 30 min, rehydrated using ethyl alcohol according to kit
instructions, protein was removed using proteinase K, the sample
was immersed in equilibration buffer and incubated with terminal
deoxynucleotidyl transferase buffer containing FITC-12-dUTP
labeling mix at 37°C for 1 h. The nuclei were stained with Hoechst
(1 µg/ml) at room temperature for 5 min and slides were assessed
using a fluorescence microscope (magnification, ×200) after being
covered with mounting media (Beyotime Institute of Biotechnology).
Three fields of view were randomly selected for assessment. The
apoptosis index (AI) was calculated as the percentage of
TUNEL-positive nuclei among the total number of nuclei in a
randomly selected area.
Western blotting
Lung tissues were homogenized using a RIPA lysis
buffer (Beyotime Institute of Biotechnology Co., Ltd.), which
contains protease, phosphatase and phosphorylase inhibitors. The
total protein concentration in the supernatant was assessed using a
BCA protein assay. Subsequently, an equal amount (30 µg) of
proteins were separated by SDS-PAGE on 5 or 10% gels and then
transferred to a PVDF membrane. After blocking with 5% fat-free
milk at room temperature for 30 min, the membranes were incubated
with primary antibodies overnight at 4°C. After washing three times
with TBS-Tween (0.1%), the membranes were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibody
(1:5,000; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature. Protein bands were visualized using the ECL
chemiluminescence detection kit (Vazyme Biotech Co., Ltd.) and
analyzed using a EDAS120 gel imaging system (Kodak). The antibodies
used were as follows: NF-κB (1:500; cat. no. MAB3026;
MilliporeSigma), β-actin (1:1,000; cat. no. 3700; Cell Signaling
Technology, Inc.), PI3K (1:1,000; cat. no. AF1966; Beyotime
Institute of Biotechnology), Akt (1:1,000; cat. no. AF0045;
Beyotime Institute of Biotechnology), p-Akt (1:1,000; cat. no.
AF1546; Beyotime Institute of Biotechnology), HIF-1α (1:500; cat.
no. NB100-105; Novus Biologicals, LLC), Bax (1:1,000; cat. no.
AB026; Beyotime Institute of Biotechnology), Bcl-2 (1:1,000; cat.
no. AF0060; Beyotime Institute of Biotechnology) and caspase-3
(1:1,000; cat. no. 9665; Cell Signaling Technology, Inc.),
HRP-conjugated secondary goat anti-rabbit antibody (1:5,000; cat.
no. AP156P; MilliporeSigma), and HRP-conjugated secondary goat
anti-mouse antibody (1:5,000; cat. no. AP124P; MilliporeSigma).
RT-qPCR
Total RNA was extracted from lung tissue specimens
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Subsequently, total RNA was reverse transcribed to cDNA at 42°C for
60 min by using BeyoRT™ (cat. D7166; Beyotime Institute
of Biotechnology) according to manufacturer's protocol. qPCR was
performed on cDNA. Primer sequences used were as follows: HIF-1α
(NM_001082782) forward (F), 5′-CAACATCACCACCATACA-3′ and reverse
(R), 5′-TCAGGAGCAGTAGTTCTTT-3′; and GAPDH (NM_001082253) F,
5′-AGAGCACCAGAGGAGGACG-3′ and R, 5′-CTGGGATGGAAACTGTGAAGAG-3′. The
lengths of amplified products were 148 and 105 bp for HIF-1α and
GAPDH, respectively. Amplification reaction conditions were as
follows: HIF-1α, 94°C pre-denaturation for 3 min, followed by 32
cycles of 94°C denaturation for 30 sec, 56°C annealing for 30 sec
and 72°C extension for 45 sec; and GAPDH, 94°C pre-denaturation for
3 min, followed by 32 cycles of 94°C denaturation for 30 sec, 57°C
annealing for 30 sec and 72°C extension for 45 sec. The SYBR Green
PCR Master Mix kit [Roche Diagnostics (Shanghai) Co., Ltd.] was
used for qPCR. Fluorescence intensity was assessed using a
fluorescence qPCR instrument and the results were generated
compared with the sham group. The relative expression of mRNA
(normalized against GAPDH) was calculated using the
2−∆∆Cq method (16).
Statistical analysis
All data are presented as the mean ± standard
deviation and each experiment was repeated at least three times.
Statistical analysis was performed using GraphPad Prism (version 5;
GraphPad Software, Inc.). Multiple comparisons were analyzed using
one-way ANOVA followed by Holm-Sidak post hoc correction. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of nicorandil on patient
oxygenation in OLV-induced pulmonary injury
The present study evaluated the oxygenation of
patients by assessing their SaO2 and PaO2.
For SaO2 and PaO2 (blood gas analysis), the
levels in the nicorandil group were significantly improved compared
with those in the control group at the 30 min after OLV and 30 min
after TLV (P<0.01; Fig. 1B and
C). The changes in the trend of SpO2 (data not
shown) were the same as those of SaO2. These data
suggested that nicorandil had beneficial effects on the pulmonary
oxygenation of patients.
Effect of nicorandil on the
microstructure of OLV-induced injured lungs
The microstructure, as assessed using H&E
staining and electron microscopy, was markedly improved in the
nicorandil group compared with that in the control group. The
results of H&E staining demonstrated that the alveolar
structure in the control group was severely damaged, partially
collapsed and disappeared. A large amount of hyperemia was observed
in the lung tissue and there were numerous red blood cells and
inflammatory cells. In the control group, the alveolar wall was
thick and edematous, whereas this was less observed in the
nicorandil group, which appeared similar to the sham group with
normal alveolar structure, including a thin alveolar wall, less
infiltration of red blood cells and inflammatory cells, and no
obvious exudation (Fig. 2). The
results of electron microscopy demonstrated that the nuclear
fractions of the three types of cells in the control group
exhibited pyknosis and lobulation, and the perinuclear space was
enlarged. The lamellar bodies of type II epithelial cells were
emptying, and the microvilli on the cell membrane became thinner
and smaller. The nicorandil group was similar to the sham group,
which was close to the ultrastructure of normal lung cells,
including full cell nucleus without pyknosis, lobulation and
extension of the perinuclear space. The lamellar body of type II
epithelial cells, which had more microvilli, was not empty
(Fig. 3).
Effect of nicorandil on apoptosis in
OLV-induced injured lungs
The results of the TUNEL assay demonstrated that the
control group had a markedly increased proportion of apoptotic
cells and a high AI, compared with in the sham and nicorandil
groups. The nicorandil and sham groups demonstrated minimal
apoptosis. There was a significant difference in AI (P<0.01)
between nicorandil and control groups (Fig. 4).
Effect of nicorandil on OLV-induced
pulmonary injury via downregulation of oxidative stress
Oxidative stress status was evaluated via assessment
of MDA and TNF-α levels, and SOD activity. The MDA and TNF-α levels
in the operated lung in the nicorandil group were significantly
lower than those in the control group (P=0.0008 and P<0.01,
respectively; Fig. 5A and C). The
activity of SOD in the nicorandil group was significantly higher
compared with that in the control group (P<0.01; Fig. 5B). These results suggested that
nicorandil may have beneficial effects on the operated lung by
decreasing oxidative stress.
Signaling pathways involved in the
effect of nicorandil on OLV-induced pulmonary injury
The protein expression levels of p-Akt and PI3K in
The nicorandil group were significantly higher compared with those
in the control group (P<0.01; Fig.
6). The protein expression levels of NF-κB in the nicorandil
group were significantly lower compared with those in the control
group (P<0.01; Fig. 7A and C).
Furthermore, HIF-1α mRNA and protein expression levels in the
nicorandil group were significantly higher compared with those in
the control group (P<0.01; Figs.
5D, 7A and C). Bax and Bcl-2
are apoptosis-related genes and caspase-3 is the main execution
protein of apoptosis in pulmonary damage. The protein expression
levels of Bax and caspase-3 in The nicorandil group were
significantly decreased compared with those in the control group
(P<0.01; Fig. 7A, D and F). By
contrast, the protein expression levels of Bcl-2 in the nicorandil
group were significantly increased compared with those in the
control group (P<0.01; Fig. 7A and
E). These results suggested that nicorandil acted on mitoKATP
via the PI3K/Akt signaling pathway, which downregulated NF-κB
expression and upregulated HIF-1α expression in nicorandil group in
the process of inhibiting apoptosis.
Discussion
ERAS requires close multidisciplinary collaboration
with the aim of minimizing perioperative injury, improving the
prognosis of patients, accelerating recovery and reducing medical
expenses. OLV is a basic requirement of ERAS in clinical settings.
OLV technology, which can completely separate the operated lung
from the contralateral lung and avoid secretions or exuded blood
flowing to the healthy side, has become the favored core technology
for anesthesiologists (1).
In thoracic surgery, an anesthesiologist must
simultaneously address the violent fluctuations in the
pathophysiology and mechanical stress in the lung tissue. These
problems are caused by complete collapse of the surgical side and
partial collapse of the contralateral side, resulting in hypoxemia
due to the uncoordinated V/Q ratio. I/R injury, inflammatory
reaction and mechanical stress can result in pulmonary injury,
seriously affecting the recovery and prognosis of patients
(2,3). Significant hypoxemia can occur in
6–20% of patients with OLV due to increased atelectasis (17–19).
According to a previous report, an increase in airway pressure
during OLV may result in acute pulmonary injury (20,21).
Surgical patients should receive low VT, low to moderate positive
end-expiratory pressure and a high fraction of inspired oxygen
(6). Furthermore, Nieman et
al (7) reported that the
damage caused by OLV after I/R, accompanied by collapse and
re-expansion of the surgical side of the lung, should be reduced as
much as possible.
Szegedi et al (8) hypothesized that the oxidative stress
in the surgical side was greater because gravity affects V/Q
matching. The body has numerous different signaling pathways for
producing ROS, including the xanthine oxidase signaling pathway
(9,22). Molecular oxygen can become a
superoxide radical (O2−) in mitochondria (23). The presence of oxygen during
reperfusion promotes the metabolism of hypoxanthine by xanthine
oxidase, which forms ROS. Hydrogen peroxide produces a highly toxic
hydroxyl via the Haber-Weiss reaction, which is promoted by the
increase in free iron during ischemia. The aforementioned forms of
ROS lead to major lung tissue damage. ROS, TNF-α and IL-6 are
involved in pulmonary injury, which occurs during I/R, as they
alter cellular proteins, lipids and ribonucleic acids, which leads
to cell dysfunction, apoptosis or cell death (24).
In the present study, pulmonary injury, which
occurred in OLV, was decreased by nicorandil and this effect has
been previously demonstrated in a rabbit model of OLV (13). The protective ventilation strategy
(low VT, high frequency and occasional expansion of the lung)
should be considered first in thoracic anesthesia; however, it
cannot affect all the factors that cause pulmonary injury. A
traditional drug (nicorandil, a mitoKATP opener), which has
previously been used clinically for myocardial infarction, has been
reported to be effective in prevention of pulmonary injury in an
animal experiment. Based on the mechanism of action, nicorandil may
serve protective roles in myocardial and pulmonary injury.
Nicorandil may become another major method of treating pulmonary
injury associated with OLV (25–27).
In the present study, nicorandil and low-dose
nitroglycerin had different effects on certain lung indicators.
Nicorandil was used at a conventional dose (close to the high dose
for rabbits) for myocardial protection, based on animal experiments
and the manufacturer's protocols. Nicorandil (2 mg/h) was infused
before anesthetic induction and continued to be administered for 2
h during the operation. Nicorandil treatment was associated with
significantly improved SaO2 and PaO2 after
OLV and after extubation. It demonstrated a beneficial effect on
lung function due to an improvement in oxygenation. According to
the literatures, nicorandil has little effect on hemodynamics and
airway pressure (28,29). Low-dose nitroglycerin also
demonstrated a negligible effect in the normal physiological range
on hemodynamics and lung indicators.
During OLV in thoracic surgery, nicorandil markedly
influenced oxygenation and this was associated with a statistically
significant increase in the SaO2 and PaO2
levels of patients, which was similar to those results previously
reported in the rabbit OLV model (13). Therefore, the present study
evaluated the material basis of the effect, which can be considered
in terms of microstructure, inflammatory response and functional
molecules. The present study demonstrated that the microstructure
of the operated lung, which was improved due to nicorandil, was
seriously damaged in the control group, including the cell nucleus,
cell membrane and alveoli. H&E staining demonstrated that
nicorandil, could serve protective roles in the alveoli, which
exhibited little congestion and secretion. The alveolar wall
exhibited minimal edema, thickening and exudation. Furthermore,
electron microscopy demonstrated that nicorandil group had no
pyknosis and lobulation of the nucleus, no marked emptying of
lamellar bodies and no decrease in microvilli in type II epithelial
cells. These results all demonstrated that nicorandil had a
beneficial effect on the lung in the clinical setting.
In the present study, the selection of molecular
indicators was based on the previously reported rabbit OLV
experiment (13), and the
consistency between the two experiments was evaluated. The present
study demonstrated that the nicorandil group exhibited lower MDA
and TNF-α levels compared with those in the control group, which
demonstrated that nicorandil reduced oxidative stress and
inflammation. SOD, which can scavenge ROS, serves a vital role in
I/R injury (30). Furthermore, the
present study demonstrated that nicorandil reduced I/R injury to
protect the lung via SOD, the activity of which was significantly
increased in nicorandil group. Induction of apoptosis can occur in
two ways. The intrinsic pathway is dependent on mitochondria and
activated by ROS, whereas the extrinsic pathway is dependent on
inflammatory molecules, such as TNF-α. Features of apoptosis
include chromatin compression, cytoplasmic shrinkage, the
appearance of apoptotic bodies and interruption of DNA (31). Apoptosis depends on energy.
Forgiarini et al (32)
reported that an increase in caspase-3 activity can result in the
formation of more apoptotic cells after 45 min of ischemia.
Therefore, the present study considered the mechanism of nicorandil
and evaluated how the apoptosis of lung cells was regulated via a
certain signaling pathway after the inflammatory response.
In the present study, the effect of nicorandil was
regulated by apoptosis-related genes with opposite effects,
including Bax and Bcl-2. Caspase-3, regulated by Bax/Bcl-2, is a
vital terminal enzyme in apoptosis (15,33,34).
Our previously reported rabbit study revealed that the nicorandil
group had lower Bax expression and higher Bcl-2 expression, the
ratio of which resulted in the decreased protein expression level
of caspase-3 (13). Therefore,
nicorandil treatment was demonstrated to be associated with
decreased apoptosis. The results of the present study indicated
important mechanisms, such as decreased protein expression levels
of caspase-3 and Bax, and the upregulation of the Bcl-2, which
resulted in less hypoxemia and oxidative damage, and improved the
microstructure of the operated lung by decreasing apoptosis in the
nicorandil group. The results were consistent with the observed
TUNEL results. The results of the TUNEL assay indicated that the AI
of nicorandil group was significantly less than that of the control
group, whereas the control group is similar to the positive control
group in the previous animal experiment (13). However, how nicorandil acts on
apoptotic genes and the underlying mechanism require further
investigation.
Nicorandil can activate the opening of mitoKATP on
alveolar cells and a number of studies have demonstrated that
nicorandil relies on PI3K/Akt, which is a signaling pathway
involved in cell proliferation and apoptosis (27,35).
In the present study, the protein expression levels of PI3K and the
p-Akt/Akt ratio in the nicorandil group were significantly
increased, which was similar to the previously reported rabbit
experiment (13). These results
indicated that nicorandil acted on mitoKATP via PI3K/Akt to reduce
apoptosis in the operated lung. Due to its inhibitory effect on
inflammation, the present study examined two transcription factors
that were closely associated with nicorandil. NF-κB exists in
almost all cells, and has an important physiological role in
inflammation, stress, apoptosis and organ ischemia/reperfusion
damage. In ventilatory lung injury, the NF-κB pathway is activated,
and can produce a large number of chemokines and cytokines,
subsequently triggering the aggregation of neutrophils, monocytes,
macrophages and other inflammatory cells (36). The protein expression levels of
NF-κB, which is related to inflammation, were significantly
decreased in the nicorandil group, whereas the protein expression
levels of HIF-1α, another nuclear transcription factor, were
significantly increased. Zhao et al (37) reported that HIF-1α was one of the
vital mechanisms for I/R pulmonary injury after the application of
dimethyloxalylglycine.
In short, compared with previously reported animal
experiments, consistent effects and mechanisms have been
demonstrated in the clinical setting. Nicorandil had a beneficial
effect by reducing apoptosis in the operated lung in clinical
thoracic surgery. It may serve a beneficial role by inhibiting the
overloading of calcium in mitochondria, shutting off mPTP (38), reducing the release of
apoptosis-inducing factors and cytochrome c, simultaneously
triggering activation of the PI3K/Akt signaling pathway around the
cell membrane, downregulating NF-κB expression, upregulating HIF-1α
expression and then reducing expression of Bax/Bcl-2, caspase-3 and
apoptosis (32).
In conclusion, nicorandil demonstrated beneficial
effects on the operated lung in clinical thoracic surgery via
reduction of apoptosis. However, further research regarding the
effects of nicorandil on cytochrome c and mPTP activity is
required in the future.
Acknowledgements
The authors would like to thank Professor Maorong
Jiang (Nantong University, Nantong, Jiangsu, China) for assistance
with the design of experiments.
Funding
The present study was supported by the Science and Technology
Plan Projects of Nantong City, Jiangsu Province, P.R. China (grant
no. MSZ18094) and the General Topic Projects of Nantong Municipal
Health Committee, Jiangsu, China (grant no. MA2021007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and ZWu designed the study. CW, ZWu, ZL, ZWa, HK
and XH performed the research. CW, ZWu, ZL and ZWa analyzed the
data and confirmed the authenticity of all the raw data. CW and ZWu
wrote the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experiments were reviewed and approved by the
Ethics Committee for Clinical Experimentation of The Affiliated
Hospital of Nantong University (approval no. 2017-K031-D01). The
enrolled patients provided written informed consent for
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ERAS
|
enhanced recovery after surgery
|
|
OLV
|
one-lung ventilation
|
|
TLV
|
two-lung ventilation
|
|
HR
|
heart rate
|
|
I/R
|
ischemia/reperfusion
|
|
mitoKATP
|
mitochondrial ATP-sensitive potassium
channel
|
|
SaO2
|
arterial oxygen desaturation
|
|
SpO2
|
pulse oxygen saturation
|
|
PaO2
|
arterial partial pressure for
oxygen
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
V/Q
|
ventilation/perfusion
|
|
mPTP
|
mitochondrial membrane permeability
transition pore
|
References
|
1
|
Bernasconi F and Piccioni F: One-lung
ventilation for thoracic surgery: Current perspectives. Tumori.
103:495–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dolch ME, Choukèr A, Hornuss C, Frey L,
Irlbeck M, Praun S, Leidlmair C, Villinger J and Schelling G:
Quantification of propionaldehyde in breath of patients after lung
transplantation. Free Radic Biol Med. 85:157–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ju NY, Gao H, Huang W, Niu FF, Lan WX, Li
F and Gao W: Therapeutic effect of inhaled budesonide
(Pulmicort® Turbuhaler) on the inflammatory response to
one-lung ventilation. Anaesthesia. 69:14–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shiva S, Sack MN, Greer JJ, Duranski M,
Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari
N, et al: Nitrite augments tolerance to ischemia/reperfusion injury
via the modulation of mitochondrial electron transfer. J Exp Med.
204:2089–2102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu B, Tewari AK, Zhang L, Green-Church
KB, Zweier JL, Chen YR and He G: Proteomic analysis of protein
tyrosine nitration after ischemia reperfusion injury: Mitochondria
as the major target. Biochim Biophys Acta. 1794:476–485. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serpa Neto A, Filho RR, Rocha LL and
Schultz MJ: Recent advances in mechanical ventilation in patients
without acute respiratory distress syndrome. F1000Prime Rep.
6:1152014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nieman GF, Satalin J, Andrews P, Aiash H,
Habashi NM and Gatto LA: Personalizing mechanical ventilation
according to physiologic parameters to stabilize alveoli and
minimize ventilator induced lung injury (VILI). Intensive Care Med
Exp. 5:82017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szegedi LL, D'Hollander AA, Vermassen FE,
Deryck F and Wouters PF: Gravity is an important determinant of
oxygenation during one-lung ventilation. Acta Anaesthesiol Scand.
54:744–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jerome SN, Doré M, Paulson JC, Smith CW
and Korthuis RJ: P-selectin and ICAM-1-dependent adherence
reactions: Role in the genesis of postischemic no-reflow. Am J
Physiol. 266:H1316–H1321. 1994.PubMed/NCBI
|
|
10
|
Huang H, Ma H and Chen S: Enhanced
recovery after surgery using uniportal video-assisted thoracic
surgery for lung cancer: A preliminary study. Thorac Cancer.
9:83–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie Y, Shi X, Sheng K, Han G, Li W, Zhao
Q, Jiang B, Feng J, Li J and Gu Y: PI3K/Akt signaling transduction
pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol Med
Rep. 19:783–791. 2019.PubMed/NCBI
|
|
12
|
Kerkhofs M, La Rovere R, Welkenhuysen K,
Janssens A, Vandenberghe P, Madesh M, Parys JB and Bultynck G:
BIRD-2, a BH4-domain-targeting peptide of Bcl-2, provokes
Bax/Bak-independent cell death in B-cell cancers through
mitochondrial Ca2+-dependent mPTP opening. Cell Calcium.
94:1023332021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Ke H, Xu X, Chen J, Sun D and Ji
F: Protective effect of nicorandil on collapse-induced lung injury
in rabbits by inhibiting apoptosis. Int J Mol Med. 44:725–736.
2019.PubMed/NCBI
|
|
14
|
Schupper AJ, Shuman WH, Baron RB, Neifert
SN, Chapman EK, Gilligan J, Gal JS and Caridi JM: Utilization of
the American society of anesthesiologists (ASA) classification
system in evaluating outcomes and costs following deformity spine
procedures. Spine Deform. 9:185–190. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin L, Zhang L, Yu L, Han L, Ji W, Shen H
and Hu Z: Time-dependent changes of autophagy and apoptosis in
lipopolysaccharide-induced rat acute lung injury. Iran J Basic Med
Sci. 19:632–637. 2016.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karzai W and Schwarzkopf K: Hypoxemia
during one-lung ventilation: Prediction, prevention, and treatment.
Anesthesiology. 110:1402–1411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rozé H, Lafargue M and Ouattara A: Case
scenario: Management of intraoperative hypoxemia during one-lung
ventilation. Anesthesiology. 114:167–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SM, Kim WH, Ahn HJ, Kim JA, Yang MK,
Lee CH, Lee JH, Kim YR and Choi JW: The effects of prolonged
inspiratory time during one-lung ventilation: A randomised
controlled trial. Anaesthesia. 68:908–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szegedi LL, Bardoczky GI, Engelman EE and
d'Hollander AA: Airway pressure changes during one-lung
ventilation. Anesth Analg. 84:1034–1037. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kilpatrick B and Slinger P: Lung
protective strategies in anaesthesia. Br J Anaesth. 105 (Suppl
1):i108–i116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cuzzocrea S, Riley DP, Caputi AP and
Salvemini D: Antioxidant therapy: A new pharmacological approach in
shock, inflammation, and ischemia/reperfusion injury. Pharmacol
Rev. 53:135–159. 2001.PubMed/NCBI
|
|
23
|
Dalton TP, Shertzer HG and Puga A:
Regulation of gene expression by reactive oxygen. Annu Rev
Pharmacol Toxicol. 39:67–101. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ambros JT, Herrero-Fresneda I, Borau OG
and Boira JM: Ischemic preconditioning in solid organ
transplantation: From experimental to clinics. Transpl Int.
20:219–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Zhang J, Cui W, Liu F, Xie R, Yang
X, Gu G, Zheng H, Lu J, Yang X, et al: Cardioprotective effects of
single oral dose of nicorandil before selective percutaneous
coronary intervention. Anatol J Cardiol. 15:125–131. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saha KK, Kumar A, Deval MM, Saha KK, Jacob
RV, Jagdale L and Kaul SK: Nicorandil infusion during off-pump
coronary artery bypass grafting reduces incidence of intra-aortic
balloon pump insertion. Innovations (Phila). 11:123–127. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su Q, Li L, Zhao J, Sun Y and Yang H:
Effects of nicorandil on PI3K/Akt signaling pathway and its
anti-apoptotic mechanisms in coronary microembolization in rats.
Oncotarget. 8:99347–99358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanaka K, Kato K, Takano T, Katagiri T,
Asanoi H, Nejima J, Nakashima M, Kamijo T and Sakanashi M: Acute
effects of intravenous nicorandil on hemodynamics in patients
hospitalized with acute decompensated heart failure. J Cardiol.
56:291–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Minami Y, Nagashima M, Kajimoto K, Shiga T
and Hagiwara N: Acute efficacy and safety of intravenous
administration of nicorandil in patients with acute heart failure
syndromes: Usefulness of noninvasive echocardiographic hemodynamic
evaluation. J Cardiovasc Pharmacol. 54:335–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen C, Lu W, Wu G, Lv L, Chen W, Huang L,
Wu X, Xu N and Wu Y: Cardioprotective effects of combined therapy
with diltiazem and superoxide dismutase on myocardial
ischemia-reperfusion injury in rats. Life Sci. 183:50–59. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van de Schepop HA, de Jong JS, van Diest
PJ and Baak JP: Counting of apoptotic cells: A methodological study
in invasive breast cancer. Clin Mol Pathol. 49:M214–M217. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Forgiarini LA Jr, Grün G, Kretzmann NA, de
Muñoz GA, de Almeida A, Forgiarini LF and Andrade CF: When is
injury potentially reversible in a lung ischemia-reperfusion model?
J Surg Res. 179:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu G, Zhang J, Chen H, Wang C, Qiu Y, Liu
Y, Wan J and Guo H: Effects and mechanisms of alveolar type II
epithelial cell apoptosis in severe pancreatitis-induced acute lung
injury. Exp Ther Med. 7:565–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Z, Dai F, Ren W, Liu H, Li B and Chang
J: Angiotensin II induces apoptosis of human pulmonary
microvascular endothelial cells in acute aortic dissection
complicated with lung injury patients through modulating the
expression of monocyte chemoattractant protein-1. Am J Transl Res.
8:28–36. 2016.PubMed/NCBI
|
|
35
|
Yu D, Fan C, Zhang W, Wen Z, Hu L, Yang L,
Feng Y, Yin KJ and Mo X: Neuroprotective effect of nicorandil
through inhibition of apoptosis by the PI3K/Akt1 pathway in a mouse
model of deep hypothermic low flow. J Neurol Sci. 357:119–125.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suzuki T, Yamashita K, Jomen W, Ueki S,
Aoyagi T, Fukai M, Furukawa H, Umezawa K, Ozaki M and Todo S: The
novel NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin, prevents
local and remote organ injury following intestinal
ischemia/reperfusion in rats. J Surg Res. 149:69–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao X, Jin Y, Li H, Wang Z, Zhang W and
Feng C: Hypoxia-inducible factor 1 alpha contributes to pulmonary
vascular dysfunction in lung ischemia-reperfusion injury. Int J
Clin Exp Pathol. 7:3081–3088. 2014.PubMed/NCBI
|
|
38
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|