Introduction
Hepatitis B virus (HBV) infection is a major public
health problem worldwide (1). In
2016, the 194 member states of the World Health Organization
committed to eliminating viral hepatitis as a public health threat
by 2030, with a particular focus on hepatitis B virus and hepatitis
C virus infection (2). China is
the country with the highest burden of HBV infection in the world
and will be a major contributor to the global elimination of
hepatitis B disease by 2030 (3).
However, there are still large gaps in achieving the
goals of reducing mortality and improving coverage of diagnosis and
treatment, which shows that China still faces enormous challenges
in the elimination of HBV infection by 2030 (3). High coverage of hepatitis B diagnosis
and treatment has become one of the most difficult goals to achieve
globally. A report in 2016 estimated that only 16.1 million people
(19% of the estimated total) with chronic HBV infection were
diagnosed in China (compared to a target of 90% by 2030) (3,4), and
only 2.8 million people (10–11% of those with chronic HBV infection
treated in China in 2016) with chronic HBV infection were currently
receiving the necessary treatment (compared to a target of 80% by
2030) (3,5). Diagnosis and treatment of HBV
infection need to be improved urgently; however, the pathogenesis
of chronic hepatitis B (CHB) is complicated and has not previously
been elucidated. One of the crucial concepts, which is generally
accepted, is that HBV does not directly kill liver cells, and it is
the immune response induced after HBV infection, which is the main
cause of liver cell injury and inflammation (6). To address this knowledge gap, the
present study evaluated the immune mechanism of the pathogenesis of
hepatitis B, especially the mechanism of hepatitis B e antigen
(HBeAg)-induced immune injury, in order to support the development
of better diagnosis and treatment methods, to support the World
Hepatitis 2030 goal.
HBeAg has strong immunogenicity and serves an
important role in the early stage of HBV infection (7). The natural history of chronic HBV
infection still lacks accurate indicators. Given the importance of
HBeAg in the pathogenesis of CHB, recent European Association for
the Study of the Liver guidelines (8) suggested that based on the status of
HBeAg, chronic HBV infection could be divided into 5 stages: HBeAg
(+) chronic infection stage, HBeAg (+) chronic hepatitis stage,
HBeAg (−) chronic infection stage, HBeAg (−) chronic hepatitis
stage and hepatitis B surface antigen (HBsAg) (−) stage. It can
therefore be concluded that HBeAg serves an important role in the
progression of HBV infection.
Our previous studies have reported the
immunopathogenesis of HBV-related antigens in liver disease after
HBV infection. Preliminary results showed that HBeAg, but not
hepatitis B core antigen or HBsAg, serves a key role in the
activation of macrophages, ultimately aggravating the liver injury
by stimulating the production of macrophage inflammatory factors
(9–11). Therefore, HBeAg levels have been
advocated as a useful biomarker for HBeAg positive patients.
Macrophages, one of the most important innate immune
cells, are known for their powerful phagocytosis and high
plasticity. Liver macrophages, also known as Kupffer cells, are
essential for liver tissue homeostasis and promote the progression
of liver disease (12). Certain
previous studies have focused on the inflammatory diseases caused
by macrophages since the discovery of the two subtypes of
macrophages (M1 and M2) (12,13).
M1 macrophages are pro-inflammatory cells that are responsible for
inflammatory signaling, whereas M2 macrophages are
anti-inflammatory cells that participate in the resolution of the
inflammatory process through the production of anti-inflammatory
cytokines that contribute to tissue healing (14). Specific environmental signals
further determine the polarization and function of hepatic
macrophages (14,15). After HBV infection, HBeAg induces
microRNA (miR)-155 expression and promotes liver injury by
increasing inflammatory cytokine production in macrophages
(9,11). Therefore, it can be hypothesized
that HBeAg could improve M1 activation and promote the production
of inflammatory cytokines.
Previous studies have reported that M1-type
inflammatory macrophages are not only conventionally associated
with T helper cell 1 response, but also T helper cell 17 (Th-17)
responses, including promotion of interleukin 17A (IL-17A)
production and Th-17 differentiation (16,17).
IL-17A secreted by Th-17 is a proinflammatory cytokine with dual
effects in immune responses, including the early beneficial
responses against infection and the detrimental effects associated
with autoimmunity and inflammatory diseases. During HBV infection,
Th-17 cells may contribute to disease progression and the
pathogenesis of liver injury (18). Whether HBeAg, as a strong immune
mediator, can promote the differentiation of Th-17, thus leading to
liver inflammation and fibrosis has been rarely reported.
The present study evaluated the relationship between
M1 macrophage and related cytokines, and Th-17 differentiation in
CHB infection and assessed the hypothesis that the HBeAg-M1
macrophage-Th-17-IL-17A axis may be another immune pathway by which
HBeAg positive patients develop inflammation and fibrosis.
Materials and methods
Patients and healthy controls
A total of 42 participants, including 15 healthy
controls, 15 patients who were HBeAg positive and 12 patients who
were HBeAg negative, were recruited. The 27 patients with CHB were
divided into three groups according to their pathological results
as follows: i) light-moderate CHB (n=12), ii) severe CHB (n=8), and
iii) hepatitis B cirrhosis (n=7). All participants were negative
for antibodies to hepatitis A virus, hepatitis C virus (HCV),
hepatitis D virus, hepatitis E virus and human immunodeficiency
virus (HIV), and other causes of chronic liver damage, including
alcoholic hepatitis, nonalcoholic hepatitis, drug-induced liver
injury, autoimmune liver disease or Wilson disease, liver
cirrhosis, decompensated liver disease, hepatocellular carcinoma
and other kinds of malignancies were excluded. The CHB patients
with alanine transaminase (ALT) £2× upper limit of normal (ULN;
ULN, 40 U/l) (8) were hospitalized
or followed up in Yantai Qishan Hospital from January 2019-December
2021 and all underwent liver biopsies. The liver tissues of healthy
volunteers with normal liver functions were obtained during surgery
following liver trauma. None of the patients received anti-HBV
agents or steroids in the 6 months prior to sampling. The
diagnostic criteria for CHB were based on the Guidelines for the
Prevention and Treatment of Chronic Hepatitis B (2019) issued by
The Chinese Society of Infectious Diseases (Chinese Medical
Association) (6) and the degree of
pathological injury of liver tissue (6). There were no significant differences
in age and gender between patients and healthy controls. Clinical
characteristics of the enrolled participants were summarized in
Table I. Peripheral blood samples
were collected from the patients and the healthy control after
obtaining written informed consent. The present study received
ethical approval from Yantai Qishan Hospital Research and Ethics
Committee.
 | Table I.Clinical and laboratory
characteristics of enrolled subjects. |
Table I.
Clinical and laboratory
characteristics of enrolled subjects.
|
Characteristics | Healthy control
(n=15) | HBeAg positive
(n=15) | HBeAg negative
(n=12) |
|---|
| Age, median ± SD
(years) | 49.93±14.25 | 49.87±12.05 | 53.50±13.79 |
| Sex |
|
|
|
| Male,
n | 9 | 5 | 7 |
| Female,
n | 6 | 10 | 5 |
| ALT, median ± SD
(U/l) | 28.20±9.06 | 50.34±20.25 | 34.75±16.47 |
| AST, median ± SD
(U/l) | 31.27±8.71 | 63.35±37.96 | 36.73±20.45 |
| GGT, median ± SD
(U/l) | 28.67±10.40 | 78.06±77.66 | 54.83±42.67 |
| ALP, median ± SD
(U/l) | 56.47±11.08 | 124.00±80.34 | 96.00±38.00 |
| logHBVDNA | - | 4.09±1.85 | 3.17±1.34 |
Clinical parameters
All patients systematically underwent a complete
biochemical examination, ultrasonography and liver biopsy within 3
days. Blood samples for use in the present study were collected
before liver biopsy. Biochemical tests were performed using
commercial assays in the Yantai Qishan hospital laboratory
according to the manufacturer's protocols. The ULN for ALT was set
as 40 U/l (8). HBeAg levels were
assessed using a microparticle-based enzyme immunoassay in a
commercially available HBeAg Regent Kit (cat. no. 07P6474, Abbott
Pharmaceutical Co. Ltd.), in which there was a direct relationship
between the HBeAg level in the sample and the intensity of the
resulting chemiluminescent reaction, measured as relative light
units (RLU). HBeAg levels were based on the ratio of sample RLU to
a control cutoff RLU (S/CO), which thereby produced
semi-quantitative results that were proportional to the HBeAg
level. HBeAg titers ≥1.0 S/CO were scored as positive, whereas
HBeAg titers <1.0 S/CO were scored as negative.
Liver biopsy
Liver tissue was obtained by ultrasound-guided
percutaneous liver biopsy using a 16-guage biopsy needle. These
specimens were fixed using 4% paraformaldehyde at room temperature
for 72 h, paraffin-embedded, cut into 4mm thick sections and
stained using hematoxylin and eosin (H&E; as detailed below)
and Gomori reticular fiber staining (adtailed below). A minimum of
1.5 cm of liver tissue with at least 6 portal tracts was required
for diagnosis. According to Scheuer's classification, the degree of
inflammatory activity and fibrosis were divided into 0–4 grades (G)
and 0–4 stages (S) (19) by two
pathologists blinded to the clinical data. G1-2, S0-2 were defined
as mild-moderate CHB; G3, S1-3 were defined as severe CHB, G4, S2-4
were defined as cirrhosis. When the opinions of the two experts
were inconsistent, the higher G score was used (19).
H&E staining
After heating at 65°C for 30 min, liver tissue
sections were dewaxed at room temperature (soaked in xylene,
anhydrous ethanol and 95% ethanol for 5, 1 and 1 min respectively,
and washed with tap water for 2 min), hydrated (washed with
distilled water for 2 min), stained with hematoxylin for 5 min,
differentiated using hydrochloric acid alcohol (pH 3, 75% alcohol)
for several seconds, soaked in ammonia for 1 min and stained with
eosin for 2 min, all at room temperature. Sections were then washed
with distilled water, dehydrated using an increasing alcohol
gradient (95% alcohol for 1 min and anhydrous alcohol for 1 min),
washed with xylene and sealed with neutral resin. The stained
section were assessed and imaged using a light microscope.
Gomori reticular fiber staining
The staining was performed according to the
manufacturer's protocol. Ammoniacal silver solution was prepared
using 0.4 ml of 10% sodium hydroxide solution and 4 ml of 10%
aqueous silver nitrate solution (Merck KGgA), with the precipitate
dissolved by the gradual addition of with concentrated ammonia
(28%) while shaking the container continuously until the solution
was clear. The working solution was 1 ml ammoniac silver solution
diluted to 10 ml with distilled water. Staining was performed as
previously described (20).
Gomori-stained sections were imaged at ×200 magnification using an
Olympus BX51 light microscope. Reticular fibers were identified by
black staining.
Flow cytometry analysis
Peripheral blood monocytes were prepared using Human
Lymphocyte Separation liquid (cat. no. 7121011; Dakewe Biotech Co.,
Ltd.), according to the manufacturer's protocols. Th-17 cells were
prepared and activated as follows, the monocytes were stimulated
using Cell Activation Cocktail (with Brefeldin A) (cat. no. 423303;
BioLegend, Inc.) stimulating solution and then blocked using Human
TruStain FcX™ Fc receptor blocking solution (cat. no. 422301;
BioLegend, Inc.). CD4+ T cells were gated on the basis
of forward and side light scatter using a FITC-conjugated
anti-human CD3 antibody (1:20; cat. no. 300305; BioLegend, Inc.)
incubated at 4°C for 20 min in the dark and APC-conjugated
antihuman CD4 antibody (1:20; cat. no. 357407; BioLegend, Inc.)
incubated at 4°C for 20 min in the dark. For intracellular
staining, the cells were fixed and permeabilized using fixation
buffer (cat. no. 420801; BioLegend, Inc.) at room temperature for
20 min and intracellular staining permeabilization wash buffer
(cat. no. 421002; BioLegend, Inc.) at room temperature for 10 min,
and then stained using PE-conjugated IL-17A antibodies (1;20; cat.
no. 512305; BioLegend, Inc.) incubated at room temperature in the
dark for 20 min. IL-17A staining was performed to label Th-17
cells. The Th-17 cell subset was defined as CD4IL-17A double
positive.
M1 and M2 macrophages were prepared and activated by
assessing the membrane expression levels of CD14, CD86 and CD163 in
human monocytes. Macrophages were gated on the basis of forward and
side light scatter and using a FITC-conjugated anti-human CD14
antibody (1:20; cat. no. 301803; Dakewe Biotech Co., Ltd.)
incubated at 4°C for 20 min in the dark and Fixable Viability Dye
(cat. no. 65-0865-14, Thermo Fisher Scientific, Inc.) at room
temperature for 10 min. The following antibodies were used for
staining: PE-conjugated CD86 (1:20; cat. no. 305405; Dakewe Biotech
Co., Ltd.) and APC-conjugated antihuman CD163 (1:20; cat. no.
326509; Dakewe Biotech Co., Ltd.), both were incubated at 4°C for
20 min in the dark. All flow cytometry data were acquired using a
BD LSRFortessa Cell Analyzer (BD Biosciences) and analyzed using
FlowJo version 10 software (FlowJo LLC).
Enzyme-linked immunosorbent assay
(ELISA)
Peripheral serum was separated by centrifugation at
400 × g for 40 min at room temperature and the concentration of
interleukin 6 (IL-6) (cat. no. KE0007, Proteintech Group, Inc.),
tumor necrosis factor-α (TNF-α) (cat. no. KE000681, Proteintech
Group, Inc.), interleukin 10 (IL-10) (cat. no. 1111002, Dakewei
Biological), IL-17A (cat. no. ab216167, Abcam) and interleukin 1β
(IL-1β) (cat. no. 1110122, Dakewei Biological) were assessed using
commercially available ELISA kits, which were used according to the
manufacturer's protocols. Optical density values were quantified at
450 nm. Standard curves were established and cytokine levels were
calculated.
Statistical analysis
All numerical data were presented as mean ± standard
deviation or median (interquartile range). Comparison of two groups
was performed using Student's t-test or Mann Whitney U test.
Comparison of ≥3 groups was performed using one-way analysis of
variance (ANOVA), LSD was used as the ANOVA post-hoc pairwise test
when 3 group were compared and Tukey's was used as the ANOVA
post-hoc pairwise test when >3 groups were included in the
comparison. Spearman correlation analysis was performed between the
frequency of circulating Th-17 cells and other parameters. All
analyses were performed using SPSS 17.0 (SPSS, Inc.) and GraphPad
Prism (GraphPad Software; Dotmatics). P<0.05 was considered to
indicate a statistically significant difference.
Results
HBV infection promotes the
accumulation of CD14+ cells in the peripheral blood
To evaluate changes in the macrophage subset in
peripheral blood between patients with HBV infection and healthy
controls, the proportion of CD14+ (a surface localized
indicator of macrophage maturation) cells was first assessed using
flow cytometry. The number of CD14+ macrophages was
significantly increased after HBV infection compared with the
healthy control group (3.26±1.75% vs. 8.75±4.42%; Fig. 1). Furthermore, the HBeAg positive
group had a significantly higher proportion of CD14+
macrophages compared with the healthy control group (10.63±4.40%
vs. 3.26±1.75%). The HBeAg negative group showed an increase;
however, this was not statistically significant compared with the
healthy control group (6.33±3.29% vs. 3.26±1.75%) and was markedly
lower compared with the HBeAg positive group (6.33±3.29% vs.
10.63±4.40%).
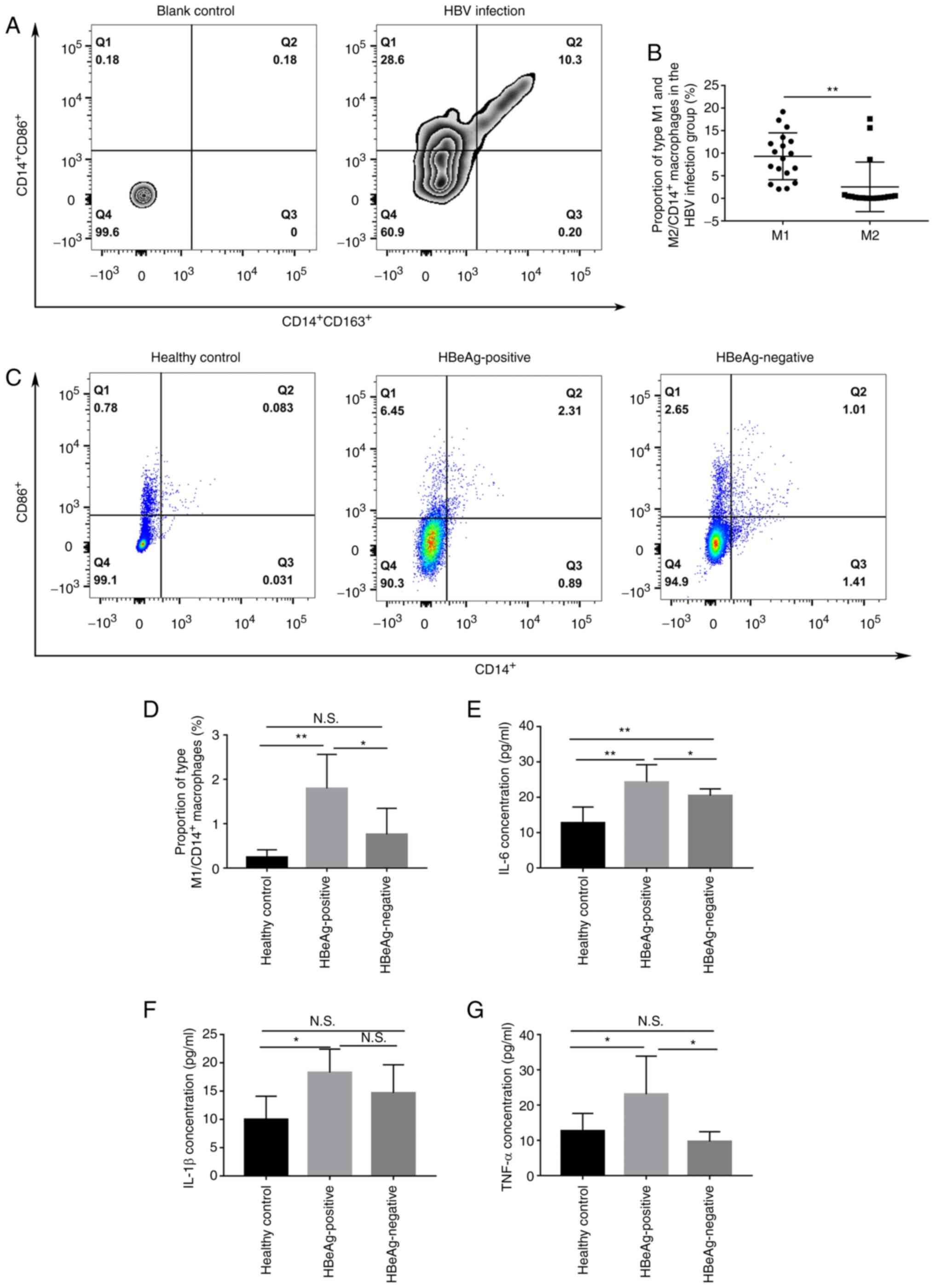
HBeAg promotes the polarization of
macrophages towards the M1 phenotype
As previously reported (14,15,21),
CD14+ CD86+ was used to identify M1
macrophages and CD14+ CD163+ was used to
identify M2 macrophages for the assessment of the polarization of
macrophages using flow cytometry. The results demonstrated that M1
was the main phenotype of macrophages in the peripheral blood of
patients with HBV infection with significantly higher levels of M1
macrophages compared with M2 macrophages (9.32±5.19% vs.
2.54±5.49%; Fig. 2A and B).
Moreover, the proportion of M1 polarization was significantly
higher in the HBeAg positive group compared both with the healthy
control group and HBeAg negative groups (1.79±0.77% vs. 0.24±0.17%
and 1.79±0.77% vs. 0.76±0.59%, respectively; Fig. 2C and D). Furthermore, the protein
expression levels of IL-6, IL-1β and TNF-α in the peripheral serum
was assessed and the protein expression levels of these cytokines
were all significantly higher in HBeAg positive group compared with
the healthy control group (Fig.
2E-G). Moreover, IL-6 and TNF-α levels were significantly
higher in the HBeAg positive group compared with those in the HBeAg
negative group (Fig. 2E and
G).
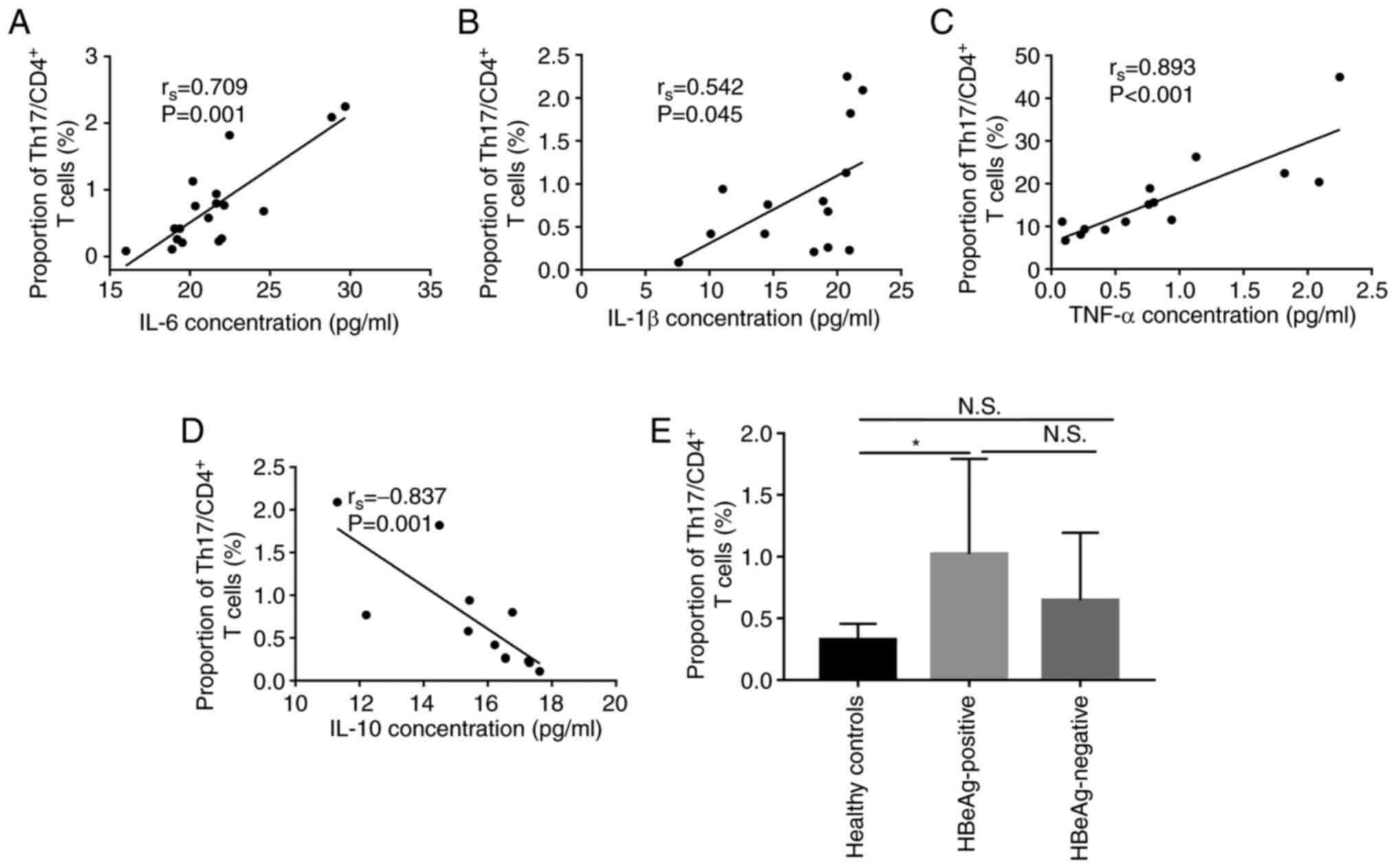
M1 macrophages may promote
CD4+ T lymphocyte differentiation to Th-17
As both macrophage and Th-17 cells serve important
roles in the promotion of HBV-associated immune-mediated liver
injury (9–11), the present study evaluated the
correlation of IL-6, IL-1β, TNF-α and IL-10 protein expression
levels in the serum with the percentage of Th-17 cells in the
CD4+ T subset (Th-17 cells/CD4+T cells), in
peripheral blood samples. These factors are mainly produced by
macrophages and are involved in the differentiation of Th-17 in
numerous cases (22–31). Significant positive correlation
between IL-6 (rs=0.709, P=0.001), IL-1β
(rs=0.542, P=0.045) and TNF-α (rs=0.893,
P=0.000) cytokines and the percentage of Th-17 cells in
CD4+ T subset was demonstrated in patients with CHB
(including HBeAg positive and HBeAg negative patients; Fig. 3A-C. However, the data demonstrated
that the protein expression level of IL-10 was significantly
negatively correlated with the percentage of Th-17cells in
CD4+ T subset (rs=−0.837, P=0.001, Fig. 3D). Furthermore, the percentage of
Th-17 cells was significantly higher in HBeAg-positive group
compared with the healthy control group (1.02±0.77% vs. 0.33±0.13%,
p=0.028, Fig. 3E). These data
demonstrated that the cytokines produced by M1 polarization were
positively correlated with the proportion of Th-17 in
CD4+ cells. Moreover, the differentiation ratio of Th-17
was the highest in the peripheral blood of HBeAg positive patients.
Therefore, it could be hypothesized that M1 polarization might
promote Th-17 differentiation.
IL-17A is associated with disease
severity and liver inflammation/fibrosis
We hypothesized that IL-17A, as a cytokine with
major functions in Th-17 cells, mediated the occurrence of liver
inflammation and fibrosis in patients with CHB. To evaluate this
hypothesis, the relationship between IL-17A and,
inflammatory/fibrosis and the severity of liver disease was
assessed. In peripheral blood, the data demonstrated that, the
IL-17A protein expression level was significantly positively
correlated with serum ALT levels (rs=0.737, P<0.001,
Fig. 4A). To further evaluate
whether IL-17A was histopathogenic, on the relationship of the
level of IL-17A in the serum with inflammation and fibrosis in
liver tissues was assessed. The pathological tissues of patients
with chronic hepatitis B were divided into light-moderate, severe
and cirrhosis groups using the Scheuer scoring system (Fig. 4B). The IL-17A level in the serum
was significantly positively correlated with the Scheuer
inflammation and fibrosis scores (rs=0.523, P=0.009 and
rs=0.434, P=0.034, respectively). IL-17A staining was
used to label Th-17 cells and assess the percentage of IL-17A
positive Th-17 cells in the peripheral blood using flow cytometry.
These results demonstrated that the percentage of IL-17A positive
cells in the peripheral blood reflected the severity of the
disease. The percentage of IL-17A positive cells in the hepatitis B
cirrhosis group was significantly higher compared with that in
severe CHB patients (1.77±0.49% vs. 0.76±0.14%, P<0.01),
light-moderate CHB patients (1.77±0.49% vs. 0.24±0.10%, P<0.01)
and the healthy control group (1.77±0.49% vs. 0.39±0.21%,
P<0.01); however, there was no significant difference between
patients with mild-moderate CHB and the healthy control group
(Fig. 4E and F). Patients with
light-moderate CHB and healthy volunteers both expressed a markedly
lower percentage of IL-17A positive cells compared with severe CHB
patients (0.24±0.10% vs. 0.76±0.14%, P=0.004 and 0.39±0.21% vs.
0.76±0.14%, P=0.050, respectively; Fig. 4E).
Discussion
A recent study reported that people with ‘normal’
ALT levels can suffer from liver histology abnormalities and may
require antiviral treatment (32).
A study from the Netherlands (33)
that included 2991 patients reported that, significant liver
fibrosis occurred in 7.2% of patients with ALT <1ULN and in 25%
of patients whose ALT was 1–2 times ULN. Another study (34) reported that among untreated
patients with ALT <1ULN or 1–2 times ULN, the prevalence of
fibrosis and cirrhosis was 35.9 and 17.9%, respectively. However,
the mechanism of inflammation and fibrosis of liver tissue in
patients with ALT ≤2ULN with chronic hepatitis B infection and
whether there are any detectable factors to reflect the degree of
liver pathological damage in patients with ALT ≤2ULN was not clear.
Based on these clinical issues, the present study evaluated
patients with chronic hepatitis B with ALT ≤2ULN.
Numerous articles have reported the relationship of
the effect and mechanism of HBV-associated antigen and hepatitis B
virus infection (9,11,35–37);
however, the role of HBeAg, with strong immunogenicity, in the
immune injury of liver tissue has still not been thoroughly
elucidated. The present study provides new insights into HBeAgs
role in the disease progression of CHB, and demonstrated a
potential route for HBeAg-induced liver inflammation and fibrosis.
In the present study, peripheral blood mononuclear cells from HBeAg
positive and negative patients with CHB were used to analyze the
frequency, cytokine production and polarization of macrophages. The
results demonstrated that cytokines produced by M1 macrophages were
then presented to naive T cells and promoted the differentiation of
Th cells into Th-17 cells, and then triggered over activation of
Th-17 cells to produce IL-17A, which ultimately damaged the liver
tissues. Circulating IL-17A levels were significantly positively
correlated with the level of inflammation and fibrosis in liver
histopathology, and were markedly positively correlated with the
severity of the disease.
Macrophages are derived from the mononuclear
phagocytic system (38,39). As precursor cells, monocytes in the
blood migrate to tissues and become macrophage, especially during
inflammation (40). Because it is
difficult to isolate large numbers of macrophages from human
organs, the present study isolated monocytes from peripheral blood
instead. Compared with healthy individuals, HBV patients
demonstrated a markedly higher proportion of the CD14+
macrophage subset. The aforementioned results demonstrate that HBV
infection promoted the accumulation of CD14+ cells in
peripheral blood.
Different stimuli activate the generation of M1
(‘destroy’) and M2 (‘heal’) macrophages (21). M1 and M2 macrophages differ in
phenotype and their release of pro-and anti-inflammatory cytokines,
respectively (14). M1 macrophages
undergo classical activation, characterized by up-regulation of
CD86 and secretion of pro-inflammatory cytokines, such as IL-1β,
IL-6 and TNF-α. M2 polarized macrophage activation however is
characterized by up-regulation of CD163 and increased IL-10
production (14). The phenotype of
not only macrophages but also the cytokines were compared between
CHB patients and healthy controls. These results showed markedly
higher expression of CD14+CD86+ (M1
macrophages) in HBV infected patients, which was significantly
higher in the HBeAg positive group. These data indicated the
monocytes from HBV infected patients were predominantly M1
polarized, which was possibly related to HBeAg. Furthermore, the
levels of IL-6, IL-1β and TNF-α were assessed, which were markedly
higher in the HBeAg positive group compared with those in the HBeAg
negative and healthy control groups. These results suggested that
the polarization of M1 and M2 was unbalanced after HBV infection,
and that the polarization of M1 was predominant. Cytokines produced
by polarized M1 resulted in liver inflammatory damage. This
phenomenon was more apparent in samples from patients with HBeAg
positive HBV infection. These results indicated that,
over-differentiated M1 ‘disruptors’ led to the presence of
persistent inflammation in vivo, which served a key role in
the pathogenesis of HBeAg positive patients.
Numerous studies have reported that Th-17 cells
generate a proinflammatory response (41–43).
Th-17 cell subsets are differentiated from naive CD4+ T
cells and mainly secrete IL-17A, IL-17F and granulocyte-macrophage
colony-stimulating factor. IL-6 and IL-1β are the inducers of Th-17
differentiation and Th-17 downstream product IL-17A (43). The present study demonstrated that
cytokines (IL-6, IL-1β and TNF-α) produced after M1 polarization
were significantly positively correlated with the proportion of
Th-17 cells, whereas the cytokine (IL-10) produced after M2
polarization demonstrated a significant negative correlation with
the percentage of Th-17 cells. Th-17 cells are heterogeneous and
able to display both pathogenic and non-pathogenic phenotypes
depending on the microenvironment (44). IL-6 and IL-1β induce pathogenic
Th-17cells to cause immune pathologies in certain tissues (45). The present study also demonstrated
that IL-6, IL-1β and TNF-α concentrations in the HBeAg positive
group were markedly higher compared with those in the HBeAg
negative and control groups. Which indicated that in HBeAg positive
patients, M1-released cytokines promoted the differentiation of
Th-17 cells into a pathogenic phenotype rather than non-pathogenic
phenotype. Furthermore, as the HBeAg positive group had a higher
ratio of Th-17 differentiated cells than the HBeAg negative group
and HBeAg has strong immunogenicity in the protein encoded by HBV,
it could be hypothesized that HBeAg can directly promote the
differentiation of pathogenic Th-17. However, further evaluation
using in vitro cell testing is required. The participation
of macrophages in Th-17 responses has been previously proposed,
though little is known about the specifics of their involvement
(46) and this hypothesis requires
further investigation.
To further assess the role of Th-17 in the
pathogenesis of HBV-induced liver inflammation and fibrosis, the
present study examined the correlation between serum IL-17A levels
and, liver inflammation and fibrosis scores and disease severity.
Gomori reticular fiber staining was used to evaluate the degree of
liver fibrosis (47). Reticular
fibers are generally located between liver cells and liver sinuses,
and degradation or collapse of reticular fibers in the hepatic
sinusoid can be considered a pathological feature during the
initiation and/or progression of hepatic fibrosis (48). The results of the present study
demonstrated that the expression of IL-17A increased with the
severity of the disease. Consistent with these findings, increased
IL-17A-associated tissue damage had also been reported in patients
infected with HIV (49), human
herpesvirus (50), respiratory
syncytial virus (51), influenza
virus (52) and dengue virus
(53). The present study
demonstrated that the level of IL-17A was positively correlated
with inflammation and fibrosis. Data from numerous studies
(54–57) suggest that there is a potential
correlation between IL-17A-producing cells (i.e., Th-17 cells) or
IL-17A levels and the development of liver fibrosis follow HBV or
HCV infection. However, a previous study reported a negative
correlation between the serum IL-17A concentration and the severity
of inflammatory lesions in the liver tissue of patients with active
autoimmune hepatitis (AIH) (58).
This might be due to the different patient inclusion criteria; in
the present study, patients were infected with HBV and in the
aforementioned study, patients had AIH. This indicates that the
mechanism by which IL-17A caused hepatitis differed depending on
the infectious agent and environment. At present, the pathway that
regulates the progression of the macrophage to M1 and stimulates
Th-17 differentiation to produce IL-17A may be one of the
pathogeneses of CHB, particularly for patients with HBeAg positive
CHB.
It is important to emphasize that the relationship
between HBeAg and M1 macrophages and the cytokines they secrete,
such as IL-6, IL-1β and TNF-α, and Th-17 cells requires further
confirmation in a cell model (using co-culture of cells) and the
absence of this is a limitation of the present study. Due to the
updated diagnostic and therapeutic guidelines for chronic hepatitis
B in China, it is difficult to obtain liver biopsy samples from
patients with chronic hepatitis B with ALT ≤2ULN (the number of
liver biopsies decreased dramatically in patients with ALT ≤2ULN).
Therefore, while the sample size and results of the different
experimental groups in this study have reached statistical
significance, the sample size was small. Moreover, the present
study relied on the data from a single center and the sample size
is small; hence, it is essential to confirm the findings from
multiple centers or expand the sample size in future experiments to
support its value in clinical practice.
The results of the present study indicated that
HBeAg might be the initiator of the onset of HBeAg positive CHB by
the promotion of M1 macrophage differentiation. IL-6, IL-1β and
TNF-α secreted by M1-type macrophages contribute to the
differentiation of naive T cells into pathogenic Th-17 cells. A
potential correlation between Th-17 or IL-17A levels and a more
severe disease stage of HBV progression has been suggested and
mechanistic studies have reported a positive link between IL-17A
production and the development of liver fibrosis/inflammation and
the severity of CHB (18,42). The present study provided new
evidence for a different route of pathogenesis in HBeAg positive
and HBeAg negative patients with CHB. Moreover, the HBeAg-M1
macrophage-Th-17-IL-17A axis may be another immune pathway by which
HBeAg positive patients can develop inflammation and fibrosis.
≤2ULN Evaluation of whether control of Th-17 functions and
anti-IL-17A therapy could potentially serve as a targeted strategy
for treatment of HBV infection in future studies would be of
use.
Acknowledgements
The authors would like to thank Professor Qiang Zhu
(Shandong University, China) for their assistance with language
editing.
Funding
This study was funded by The Yantai Science and Technology
Bureau (grant no. 2020YD060) and WBE Liver Fibrosis Foundation
(grant no. CFHPC2021011).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the first and corresponding author on
reasonable request.
Authors' contributions
JQ was responsible for conception and design. LS, NZ
and YW were responsible for collection and assembly of the data. LS
was responsible for the methodology and LS and JY were responsible
for analysis and interpretation. LS, JY and JQ were responsible for
writing the original draft. JQ provided administrative support. LS,
JY and JQ confirm the authenticity of all the raw data. All authors
read and approved the final draft of the manuscript.
Ethics approval and consent to
participate
The research protocol and consent program were
approved by Yantai Qishan Hospital Research and Ethics Committee
(approval no. 201901).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ULN
|
upper limit of normal
|
|
HBV
|
hepatitis B virus
|
|
HCV
|
hepatitis C virus
|
|
HIV
|
human immunodeficiency virus
|
|
AIH
|
autoimmune hepatitis
|
|
CHB
|
chronic hepatitis B
|
|
HBsAg
|
hepatitis B surface antigen
|
|
HBeAg
|
hepatitis B e antigen
|
|
ALT
|
alanine transaminase
|
|
Th17
|
T helper cell 17
|
|
IL-17A
|
interleukin 17A
|
|
IL-6
|
interleukin 6
|
|
IL-10
|
interleukin 10
|
|
IL-1β
|
interleukin 1β
|
|
TNF-α
|
tumor necrosis factor-α
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
CD4
|
cluster of differentiation 4
|
|
CD3
|
cluster of differentiation 3
|
|
CD86
|
cluster of differentiation 86
|
|
CD163
|
cluster of differentiation 163
|
|
CD14
|
cluster of differentiation 14
|
|
OD
|
optical density
|
|
RLU
|
relative light units
|
|
S/CO
|
ratio of sample RLU to control cutoff
RLU
|
|
ANOVA
|
analysis of variance
|
|
AST
|
aspartate transaminase
|
|
ALP
|
alkaline phosphatase
|
|
GGT
|
glutamyltranspeptidase
|
References
|
1
|
Shih C, Yang CC, Choijilsuren G, Chang CH
and Liou AT: Hepatitis B virus. Trends Microbiol. 26:386–387. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen MH, Wong G, Gane E, Kao JH and
Dashiki G: Hepatitis B virus: Advances in prevention, diagnosis,
and therapy. Clin Microbiol Rev. 33:e00046–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu J, Liang W, Jing W and Liu M:
Countdown to 2030: Eliminating hepatitis B disease, China. Bull
World Health Organ. 97:230–238. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polaris Observatory Collaborators: Global
prevalence, treatment, and prevention of hepatitis B virus
infection in 2016: A modelling study. Lancet Gastroenterol Hepatol.
3:383–403. 2018. View Article : Google Scholar
|
|
5
|
Asrani SK, Devarbhavi H, Eaton J and
Kamath PS: Burden of liver diseases in the world. J Hepatol.
70:151–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chinese Society of Infectious Diseases,
Chinese Medical Association, . Chinese Society of Hepatology,
Chinese Medical Association: The guidelines of prevention and
treatment for chronic hepatitis B (2019 version). Zhonghua Gan Zang
Bing Za Zhi. 27:938–961. 2019.(In Chinese). PubMed/NCBI
|
|
7
|
Iannacone M and Guidotti LG: Immunobiology
and pathogenesis of hepatitis B virus infection. Nat Rev Immunol.
22:19–32. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
European Association for the Study of the
Liver. Electronic address, . simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver: EASL 2017 Clinical
practice guidelines on the management of hepatitis B virus
infection. J Hepatol. 67:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Bian H, Li F, Li X, Zhang D, Sun
S, Song S, Zhu Q, Ren W, Qin C and Qi J: HBeAg induces the
expression of macrophage miR-155 to accelerate liver injury via
promoting production of inflammatory cytokines. Cell Mol Life Sci.
75:2627–2641. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen W, Song J, Bian H, Yang X, Xie X, Zhu
Q, Qin C and Qi J: The functions and targets of miR-212 as a
potential biomarker of cancer diagnosis and therapy. J Cell Mol
Med. 24:2392–2401. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie X, Lv H, Liu C, Su X, Yu Z, Song S,
Bian H, Tian M, Qin C, Qi J and Zhu Q: HBeAg mediates inflammatory
functions of macrophages by TLR2 contributing to hepatic fibrosis.
BMC Med. 19:2472021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saqib U, Sarkar S, Suk K, Mohammad O, Baig
MS and Savai R: Phytochemicals as modulators of M1-M2 macrophages
in inflammation. Oncotarget. 9:17937–17950. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chávez-Galán L, Olleros ML, Vesin D and
Garcia I: Much more than M1 and M2 macrophages, there are also
CD169(+) and TCR(+) macrophages. Front Immunol.
6:2632015.PubMed/NCBI
|
|
15
|
Krenkel O and Tacke F: Liver macrophages
in tissue homeostasis and disease. Nat Rev Immunol. 17:306–321.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sommerfeld SD, Cherry C, Schwab RM, Chung
L, Maestas DR Jr, Laffont P, Stein JE, Tam A, Ganguly S, Housseau
F, et al: Interleukin-36γ-producing macrophages drive
IL-17-mediated fibrosis. Sci Immunol. 4:eaax47832019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung L, Maestas DJ Jr, Lebid A, Mageau A,
Rosson GD, Wu X, Wolf MT, Tam AJ, Vanderzee I, Wang X, et al:
Interleukin 17 and senescent cells regulate the foreign body
response to synthetic material implants in mice and humans. Sci
Transl Med. 12:eaax37992020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu W, Li J, Chen F, Zhu H, Peng G and Chen
Z: Circulating Th17 cells frequency is associated with the disease
progression in HBV infected patients. J Gastroenterol Hepatol.
25:750–757. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chinese Society of Hepatology, Chinese
Medical Association; Chinese Society of Gastroenterology: Chinese
Medical Association, Chinese Society of Infectious Diseases;
Chinese Medical Association, . Consensus on the diagnosis and
treatment of hepatic fibrosis (2019). J Dig Dis. 21:127–138. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwint OA, Labraga M, Cervino CO, Haffar
M, Sequeiros PH and Marcos HJ: A modification of the staining
technique of reticular fibres for image analysis of the cardiac
collagen network. Cardiovasc Pathol. 13:213–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Xie L, Wang S, Lin J, Liang J and
Xu J: Azithromycin promotes alternatively activated macrophage
phenotype in systematic lupus erythematosus via PI3K/Akt signaling
pathway. Cell Death Dis. 9:10802018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei Y, Jing J, Peng Z, Liu X and Wang X:
Acacetin ameliorates insulin resistance in obesity mice through
regulating Treg/Th17 balance via MiR-23b-3p/NEU1 axis. BMC Endocr
Disord. 21:572021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi QZ, Yu HM, Chen HM, Liu M and Cheng X:
Exosomes derived from mesenchymal stem cells regulate Treg/Th17
balance in aplastic anemia by transferring miR-23a-3p. Clin Exp
Med. 21:429–437. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu D, Ma J, Gong Q, Senouthai S, Wang J,
You Y and Pinhu L: Fractalkine mediates lymphocyte inflammation and
tubulointerstitial lesions by modifying the Treg/Th17 balance in
lupus-prone MRL/lpr mice. Am J Transl Res. 12:6170–6186.
2020.PubMed/NCBI
|
|
25
|
Du YY, Chen ZX, Liu MY, Liu QP, Lin CS,
Chu CQ and Xu Q: Leonurine regulates Treg/Th17 balance to attenuate
rheumatoid arthritis through inhibition of TAZ expression. Front
Immunol. 11:5565262020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei S, Sun J, Li Y, Xu K, Wang M and Zhang
Y: Losartan attenuates atherosclerosis in uremic mice by regulating
Treg/Th17 balance via mediating PTEN/PI3K/Akt pathway. Nephron.
146:528–538. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi X, Huang H, Zhou M, Liu Y, Wu H and
Dai M: Paeonol attenuated vascular fibrosis through regulating
Treg/Th17 balance in a gut microbiota-dependent manner. Front
Pharmacol. 12:7654822021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu YJ, Tang B, Wang FC, Tang L, Lei YY,
Luo Y, Huang SJ, Yang M, Wu LY, Wang W, et al: Parthenolide
ameliorates colon inflammation through regulating Treg/Th17 balance
in a gut microbiota-dependent manner. Theranostics. 10:5225–5241.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen T, Gao J, Xiang P, Chen Y, Ji J, Xie
P, Wu H, Xiao W, Wei Y, Wang S, et al: Protective effect of
platycodin D on liver injury in alloxan-induced diabetic mice via
regulation of Treg/Th17 balance. Int Immunopharmacol. 26:338–348.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei C, Huang L, Zheng Y and Cai X:
Selective activation of cannabinoid receptor 2 regulates Treg/Th17
balance to ameliorate neutrophilic asthma in mice. Ann Transl Med.
9:10152021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo J, Wang LY, Wu J, Xu LF and Sun M: The
JAK2 inhibitor AG490 regulates the Treg/Th17 balance and alleviates
DSS-induced intestinal damage in IBD rats. Clin Exp Pharmacol
Physiol. 47:1374–1381. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nguyen MH, Garcia RT, Trinh HN, Lam KD,
Weiss G, Nguyen HA, Nguyen KK and Keeffe EB: Histological disease
in Asian-Americans with chronic hepatitis B, high hepatitis B virus
DNA, and normal alanine aminotransferase levels. Am J
Gastroenterol. 104:2206–2213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sonneveld MJ, Brouwer WP, Hansen BE, Chan
HL, Piratvisuth T, Jia JD, Zeuzem S, Chien RN, Choi H, de Knegt RJ,
et al: Very low probability of significant liver inflammation in
chronic hepatitis B patients with low ALT levels in the absence of
liver fibrosis. Aliment Pharmacol Ther. 52:1399–13406.
2020.PubMed/NCBI
|
|
34
|
Göbel T, Erhardt A, Herwig M, Poremba C,
Baldus SE, Sagir A, Heinzel-Pleines U and Häussinger D: High
prevalence of significant liver fibrosis and cirrhosis in chronic
hepatitis B patients with normal ALT in central Europe. J Med
Virol. 83:968–973. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang YZ, Yan F, Pan KC, Zhu JS, Chen HZ,
Zhu M, Lin X, Zhao HH and Xiao M: Hepatitis B e antigen perturbs
the LPS-stimulated production of inflammatory cytokines by
mononuclear-derived dendritic cells. Zhonghua Gan Zang Bing Za Zhi.
21:590–593. 2013.(In Chinese). PubMed/NCBI
|
|
36
|
Lin C, Zou H and Wang S: Hepatitis B e
antigen seroconversion is related with the function of dendritic
cells in chronic hepatitis B virus infection. Gastroenterol Res
Pract. 2014:4139522014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen W, Bian H, Xie X, Yang X, Bi B, Li C,
Zhang Y, Zhu Q, Song J, Qin C and Qi J: Negative feedback loop of
ERK/CREB/miR-212-3p inhibits HBeAg-induced macrophage activation. J
Cell Mol Med. 24:10935–10945. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nelson PJ, Rees AJ, Griffin MD, Hughes J,
Kurts C and Duffield J: The renal mononuclear phagocytic system. J
Am Soc Nephrol. 23:194–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao Q, Wang Y, Wang XM, Lu J, Lee VW, Ye
Q, Nguyen H, Zheng G, Zhao Y, Alexander SI and Harris DC: Renal
F4/80+ CD11c+ mononuclear phagocytes display phenotypic and
functional characteristics of macrophages in health and in
adriamycin nephropathy. J Am Soc Nephrol. 26:349–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guiteras R, Flaquer M and Cruzado JM:
Macrophage in chronic kidney disease. Clin Kidney J. 9:765–771.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Beringer A and Miossec P: IL-17 and
IL-17-producing cells and liver diseases, with focus on autoimmune
liver diseases. Autoimmun Rev. 17:1176–1185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Chen S and Xu K: IL-17 expression
is correlated with hepatitis B-related liver diseases and fibrosis.
Int J Mol Med. 27:385–392. 2011.PubMed/NCBI
|
|
43
|
Lafdil F, Miller AM, Ki SH and Gao B: Th17
cells and their associated cytokines in liver diseases. Cell Mol
Immunol. 7:250–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Omenetti S, Bussi C, Metidji A, Iseppon A,
Lee S, Tolaini M, Li Y, Kelly G, Chakravarty P, Shoaie S, et al:
The intestine harbors functionally distinct homeostatic
tissue-resident and inflammatory Th17 cells. Immunity. 51:77–89.
e62019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu B and Wan Y: Molecular control of
pathogenic Th17 cells in autoimmune diseases. Int Immunopharmacol.
80:1061872020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zubair A, Jamal S and Mubarik A:
Morphometric analysis of hepatic steatosis in chronic hepatitis C
infection. Saudi J Gastroenterol. 15:11–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wen SL, Feng S, Tang SH, Gao JH, Zhang LH,
Tong H, Yan ZP and Fang DZ: Collapsed reticular network and its
possible mechanism during the initiation and/or progression of
hepatic fibrosis. Sci Rep. 6:354262016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Campillo-Gimenez L, Casulli S, Dudoit Y,
Seang S, Carcelain G, Lambert-Niclot S, Appay V, Autran B, Tubiana
R and Elbim C: Neutrophils in antiretroviral therapy-controlled HIV
demonstrate hyperactivation associated with a specific IL-17/IL-22
environment. J Allergy Clin Immunol. 134:1142–1152.e5. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Stout-Delgado HW, Du W, Shirali AC, Booth
CJ and Goldstein DR: Aging promotes neutrophil-induced mortality by
augmenting IL-17 production during viral infection. Cell Host
Microbe. 6:446–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Reed M, Morris SH, Owczarczyk AB and
Lukacs NW: Deficiency of autophagy protein Map1-LC3b mediates
IL-17-dependent lung pathology during respiratory viral infection
via ER stress-associated IL-1. Mucosal Immunol. 8:1118–1130. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li C, Yang P, Sun Y, Li T, Wang C, Wang Z,
Zou Z, Yan Y, Wang W, Wang C, et al: IL-17 response mediates acute
lung injury induced by the 2009 pandemic influenza A (H1N1) virus.
Cell Res. 22:528–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jain A, Pandey N, Garg RK and Kumar R:
IL-17 level in patients with dengue virus infection & its
association with severity of illness. J Clin Immunol. 33:613–618.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang GL, Zhang T, Zhao QY, Xie C, Lin CS
and Gao ZL: Increased IL-17-producing CD8+ T cell
frequency predicts short-term mortality in patients with hepatitis
B virus-related acute-on-chronic liver failure. Ther Clin Risk
Manag. 14:2127–2136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cho HJ, Kim SS, Nam JS, Oh MJ, Kang DR,
Kim JK, Lee JH, Kim B, Yang MJ, Hwang JC, et al: Higher serum
interleukin-17A levels as a potential biomarker for predicting
early disease progression in patients with hepatitis B
virus-associated advanced hepatocellular carcinoma treated with
sorafenib. Cytokine. 95:118–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jhun J, Lee S, Kim H, Her YM, Byun JK, Kim
EK, Lee SK, Cho ML and Choi JY: HMGB1/RAGE induces IL-17 expression
to exaggerate inflammation in peripheral blood cells of hepatitis B
patients. J Transl Med. 13:3102015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ma WT, Yao XT, Peng Q and Chen DK: The
protective and pathogenic roles of IL-17 in viral infections:
Friend or foe? Open Biol. 9:1901092019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gutkowski K, Gutkowska D, Kiszka J,
Partyka M, Kacperek-Hartleb T, Kajor M and Hartleb M: Serum
interleukin-17 levels predict inflammatory activity in patients
with autoimmune hepatitis. Pol Arch Intern Med. 128:150–156.
2018.PubMed/NCBI
|