Introduction
Vascular disease is the core of diabetes mellitus
(DM), which endangers life and health. Severe vascular disease can
cause insufficient blood supply and function loss in the heart,
brain, kidney, eyes, lower limbs and other important tissues and
organs, which seriously threatens human life and health (1). Endothelial injury induced by
hyperglycemia is the most critical initial step in the development
of diabetic vasculopathy (2).
Damaged endothelial cells can secrete a large number of
proinflammatory and procoagulant cytokines, which can induce
platelet aggregation, thrombosis, adhesion and infiltration of
mononuclear macrophages and release of inflammatory factors.
Long-term chronic vascular wall inflammation can stimulate the
proliferation and migration of vascular smooth muscle cells and the
deposition of extracellular matrix, eventually leading to vascular
stenosis and occlusion (3).
Therefore, it is of great clinical value to explore the prevention
and treatment strategies and elucidate the specific mechanism of
diabetes-induced vascular endothelial injury.
Melatonin, a hormone secreted by the pineal gland to
regulate biological rhythm, serves an important role in maintaining
human physiological function (4).
Studies have found that melatonin has anti-inflammatory and
antioxidant protective effects in diabetes-induced vascular
endothelial cell injury (5,6), but
the specific mechanism remains to be elucidated.
Pyroptosis, a type of newly discovered inflammatory
cell death, depends on the activation of caspase-1 (7). The NOD-like receptor family pyrin
domain containing 3 (NLRP3) inflammasome, one of the most
extensively studied pyroptotic inflammasomes, is composed of
NOD-like receptor family, pyrin domain-containing 3 (NLRP3), the
adaptor ASC and pro-caspase-1 (8).
Activation of the NLRP3 inflammasome involves two sequential steps
(priming and assembly) triggered by two signals respectively. The
priming step triggered by the first signal (e.g.,
pathogen-associated molecular pattern molecules such as
lipopolysaccharide) activates NF-κB signaling and induces the
transcription of pre-IL-1β and NLRP3. The second signal (e.g.,
damage-associated molecular patterns such as ATP) triggers several
signaling pathways, including potassium efflux, reactive oxygen
species (ROS) production, and lysosomal damage, all of which
stimulate the assembly of the NLRP3 inflammasome. Activation of the
NLRP3 inflammasome induces the transformation of caspase-1 into its
activated form, cleaved-caspase-1. For one thing, this enzyme
cleaves gasdermin D (GSDMD) into gasdermin-N domain (GSDMD-N),
which oligomerizes on the cell membrane to form cell membrane
pores. For another, cleaved-caspase-1 promotes the maturation of
the pro-inflammatory cytokines IL-1β and IL-18, which are released
into the extracellular space through GSDMD cell membrane pores, to
induce a strong inflammatory response in cells and finally
pyroptosis (9). Previous studies
have found that melatonin can inhibit NLRP3 inflammasome activation
and pyroptosis (10,11). However, whether melatonin can
inhibit endothelial pyroptosis and serve a protective role in
diabetes-induced vascular endothelial injury is rarely
reported.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is an activator of antioxidant response elements (ARE) and a key
target when reducing oxidative stress. Nrf2 binds to kelch-like
ECH-associated protein 1 (Keap1) in the cytoplasm under
physiological conditions (12).
When exposed to oxidative stress, extracellular signal-regulated
protein kinases, protein kinase C and phosphatidylinositol 3-kinase
signaling pathways promote the dissociation of Keap1-Nrf2 and the
activation of Nrf2 through phosphorylation. Activated
phosphorylated-Nrf2 is translocated into the nucleus and binds to
the cis-acting AREs, initiating the transcription of a series of
antioxidant enzyme genes, including heme oxygenase-1 (HO-1), NADPH:
Quinone Oxidoreductase 1 (NQO1), superoxide dismutase (SOD) and
glutathione peroxidase (GSH-PX), to maintain the stability of the
intracellular environment (13).
Previous studies have found that melatonin serves an antioxidant
role by activating the Nrf2 pathway (14,15).
ROS is a key upstream mechanism of NLRP3 inflammasome activation
and pyroptosis (16). Therefore,
the present study hypothesized that melatonin could inhibit ROS
production by activating Nrf2 pathway and further inhibit NLRP3
inflammasome activation and pyroptosis in diabetes-induced vascular
endothelial cell injury.
MT1 and MT2 melatonin membrane receptors are G
protein-coupled receptors widely expressed in the cardiovascular
system. Previous studies have shown that melatonin can bind to the
melatonin receptor (MT1/MT2) on the cell membrane surface, activate
the phosphorylated kinase pathway in the cytoplasm, and then
participate in the regulation of normal physiological functions
(17). The present study
determined whether melatonin attenuates high glucose (HG)-induced
pyroptosis of vascular endothelial cells through the Nrf2-ROS-NLRP3
pathway. Meanwhile, whether melatonin protects vascular endothelial
cells from HG-induced damage through MT1/MT2-mediated Nrf2
signaling was also investigated.
Materials and methods
Cell culture and experimental
design
Primary human umbilical vein endothelial cells
(HUVECs; cat. no. 8000) were purchased from ScienCell Research
Laboratories (Carlsbad, CA, USA) and cultured in endothelial cell
medium (ScienCell Research Laboratories, Inc.) supplemented with 5%
fetal bovine serum under 5% CO2 at 37°C. All experiments
were performed between cell passages three and five. Melatonin (100
µM; MilliporeSigma), a specific NLRP3 inhibitor MCC950 (50 µM;
Selleck Chemicals), and a melatonin receptor antagonist luzindole
(5 µM; MilliporeSigma) were added to the cell cultures 3 h in
advance before being co-incubated with HG (30 mM) for 72 h. All
experiments were performed at least three times and representative
results are presented in the present study.
Small interfering RNA (siRNA)
treatment
The predesigned siRNA duplexes for Nrf2 and the
negative control (NC) were purchased from HippoBio Co., Ltd.
Table I lists the sequences of all
Nrf2 siRNAs and the non-targeting NC-siRNA used. Cells were
incubated in 6-cm dishes until they were 80% confluent and the
original medium was replaced with fresh basal medium 2 h before
siRNA transfection. Two sterile 1.5-ml centrifuge tubes were
prepared and 100 µl Opti-MEM (Invitrogen; Thermo Fisher Scientific,
Inc.) was added to each. siRNA (2 µl, 20 µM) was added to one of
the centrifuge tubes and was thoroughly mixed to dilute the RNA.
LipoRNAi MAX (2 µl; Invitrogen; Thermo Fisher Scientific, Inc.) was
added to the other centrifuge tube and was mixed to dilute the
LipoRNAi MAX. Both centrifuge tubes were maintained at room
temperature for 5 min. Subsequently, siRNA and LipoRNAi MAX were
transferred to the same centrifuge tube and were thoroughly mixed;
the transfection complex was then maintained for 25 min at room
temperature. The HUVECs to be transfected were removed from the
incubator, the transfection complex was added to the culture dish
in drops, and the cells were gently shaken. The transfection
complex was incubated with cells at 37°C for 48 h. Finally, the
transfected cells were harvested for subsequent verification and
experiments.
 | Table I.The sequences of all Nrf2 siRNAs and
the siRNA-NC used. |
Table I.
The sequences of all Nrf2 siRNAs and
the siRNA-NC used.
| Name | Sequence |
|---|
| hNRF2 siRNA-1
sense |
GGUUGAGACUACCAUGGUUTT |
| hNRF2 siRNA-1
antisense |
AACCAUGGUAGUCUCAACCAG |
| hNRF2 siRNA-2
sense |
GCCCAUUGAUGUUUCUGAUTT |
| hNRF2 siRNA-2
antisense |
AUCAGAAACAUCAAUGGGCCC |
| hNRF2 siRNA-3
sense |
GCAGUUCAAUGAAGCUCAATT |
| hNRF2 siRNA-3
antisense |
UUGAGCUUCAUUGAACUGCTC |
| si-NC sense |
GUCUACUGCUAUGUCUGUATT |
| si-NC
antisense |
AAUACAGACAUAGCAGUAGAC |
Enzyme-linked immunosorbent assay
(ELISA)
The HUVEC culture supernatants were collected
following an intervention for 72 h. The concentrations of IL-1β
(cat. no. SEA563Hu) and IL-18 (cat. no. SEA064Hu) in the cell
culture supernatants were determined using ELISA kits (both from
Cloud-Clone Corp.) according to the manufacturer's
instructions.
Detection of ROS production
ROS levels in the HUVECs were measured using an ROS
assay kit (Beijing Solarbio Science & Technology Co., Ltd.) in
accordance with the manufacturer's instructions.
Cell viability assay
A Cell Counting Kit (CCK-8) assay (Nanjing Jiancheng
Bioengineering Institute) was used to measure the level of cell
viability.
Cell death assay
Pyroptotic cell death was assessed using a lactate
dehydrogenase (LDH) release assay and Hoechst 33342/propidium
iodide (PI) staining. For LDH release, the LDH activity of the cell
culture supernatants was measured using an LDH assay kit (Nanjing
Jiancheng Bioengineering Institute). For Hoechst 33342/PI double
staining, the collected cells were washed with PBS and subsequently
incubated with PI (5 µl) for 10 min at 37°C in the dark. After
three washes with PBS, the nuclei were stained with Hoechst 33342
(10 µl) for 30 min at 37°C in the dark. Images were captured under
a fluorescence microscope. The proportion of PI-positive cells was
calculated using Image J software (version 1.46; National
Institutes of Health).
Reverse transcription-quantitative
(RT-q) PCR
The experimental method, which had been described in
our previous study (4), was as
follows: Total RNA was extracted from cells (1×106)
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.) and quantified with a NanoDrop 2000 Spectrophotometer (Thermo
Fisher Scientific, Inc.). Complementary DNA (cDNA) was synthesized
with 1 µg total RNA using a High-Capacity cDNA Reverse
Transcription kit (Takara Bio, Inc.); the temperature protocol was:
42°C for 60 min, 70°C for 15 min, and then 4°C for 5 min. qPCR was
then performed in a CFX96 Touch Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.) using SYBR Premix (Shanghai Yeasen
Biotechnology Co., Ltd.). The thermocycling conditions were as
follows: Predenaturation step at 95°C for 3 min, followed by 40
cycles of 95°C for 10 sec and 60°C for 40 sec. Each sample was
analyzed in triplicate. Table II
lists the primer sequences used. The relative mRNA expression
levels were normalized to β-actin expression using the
2−ΔΔCq method (18).
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| β-actin |
TCCTCCTGAGCGCAAGTACTCC |
CATACTCCTGCTTGCTGATCCAC |
| Nrf2 |
TCAGCGACGGAAAGAGTATGA |
CCACTGGTTTCTGACTGGATGT |
| NLRP3 |
AAGGAAGTGGACTGCGAGAA |
AACGTTCGTCCTTCCTTCCT |
| Caspase-1 |
GGCATGACAATGCTGCTACA |
TCTGGGACTTGCTCAGAGTG |
Western blotting
Total protein or nuclear protein (nucleoprotein
extraction kit, Beijing Solarbio Science & Technology Co.,
Ltd.) was extracted from the collected HUVECs, and protein
concentration was determined using a BCA Protein Assay Kit (cat.
no. P0012A; Beyotime Institute of Biotechnology). Subsequently,
equal amounts of protein lysates (30 µg/lane) were separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene fluoride (PVDF) membranes
(MilliporeSigma). After transfer, the PVDF membranes was incubated
in 25 ml blocking buffer (1X Tris-buffered saline and 0.1% Tween-20
with 5% non-fat dry milk) for 1 h at room temperature and incubated
overnight at 4°C with the following primary antibodies: β-actin
(1:1,000; MDL; cat. no. MD6553), Histone H3 (1:1,000; Abcam; cat.
no. ab1791), Nrf2 (1:1,000; CST; cat. no. 12721T), heme oxygenase-1
(HO-1; 1:1,000; Affinity; cat. no. AF5393), NQO1 (1:1,000; Novus;
cat. no. NB200-209SS), superoxide dismutase 2 (SOD2; 1:1,000;
Novus; cat. no. NB100-1992SS), NLRP3 (1:1,000; Abcam; cat. no.
ab214185), ASC (1:1,000; ABclonal Biotech Co., Ltd.; cat. no.
A1170), cleaved-caspase-1 (1:1,000; CST; cat. no. 4199T), GSDMD
(1:1,000; Abcam; cat. no. ab210070), and cleaved N-terminal GSDMD
(1:1,000; Abcam; cat. no. ab215203). The membranes were then
incubated with HRP-linked anti-mouse IgG (cat. no. 7076; 1:3,000;
Cell Signaling Technology, Inc.) or HRP-linked anti-rabbit IgG
(cat. no. 7074; 1:3,000; Cell Signaling Technology, Inc.) for 1 h
at room temperature. The antibody-antigen complexes were detected
using enhanced electrochemiluminescence reagents (Santa Cruz
Biotechnology, Inc.) and densitometric analysis was conducted using
Image J software (version 1.46; National Institutes of Health).
Statistical analysis
All data were analyzed using GraphPad Prism version
9.0 (Dotmatics). All experimental data are represented as the means
± standard deviation. The Shapiro-Wilk test was used to evaluate
Gaussian distributions, while the Brown-Forsythe test was used to
evaluate homogeneity of variance. All data fitted the Gaussian
distribution and the variances were equal. One-way ANOVA followed
by Šídák's multiple comparisons test was used to compare
differences between three or more groups. P<0.05 was considered
to indicate a statistically significant difference.
Discussion
The present study found that melatonin inhibited
HG-induced pyroptosis of vascular endothelial cells by activating
the Nrf2 pathway to inhibit NLRP3 inflammasome activation. It was
further found that melatonin protects vascular endothelial cells
from HG-induced injury through the activation of Nrf2 pathway,
which is mediated by MT1/MT2.
Previous studies have found that melatonin is
beneficial for a variety of cardiovascular diseases, such as
ischemia-reperfusion injury (21),
hypertension (22), and
atherosclerosis (23). However,
some clinical studies have come to different conclusions. For
example, several randomized controlled trials have found benefits
of melatonin in heart failure (24), ST segment elevation myocardial
infarction (STEMI) (25), coronary
artery bypass grafting (26), and
percutaneous coronary intervention (27). However, some randomized controlled
trials have found no significant positive effect of melatonin in
STEMI (28), acute coronary
syndromes (29) and macrovascular
surgery (30) despite its safety.
Therefore, more experiments are needed to further evaluate the
underlying molecular mechanisms of melatonin activity.
The current study, found through CCK-8 experiment
under the condition of high glucose of 30 mM that the therapeutic
concentration of melatonin of 100 µM could minimize the adverse
effects of high glucose on the vitality of HUVECs. However, higher
concentrations of melatonin showed similar or even reduced
protective effects. In fact, one of our previous studies found that
a therapeutic concentration of 100 µM of melatonin was optimal for
alleviating the adverse effects of cigarette smoke extractants on
the vitality of HUVECs (11). In
addition, in a number of studies on the protection of vascular
endothelial cells with melatonin, 100 µM is used as the therapeutic
concentration of melatonin and it showed an ideal cellular
protective effect (31,32). Therefore, the present study set the
concentration gradient of melatonin treatment at around 100 µM and
found the aforementioned results. As for why melatonin showed
similar or even reduced protective effect with higher concentration
under the experimental conditions of the present study, it was
hypothesized that it might be related to the pharmacological
properties of melatonin itself, which still needs to be verified in
further studies.
Endothelial cell pyroptosis is involved in the
pathogenesis of atherosclerosis (19,33).
Studies have found that melatonin reduces endothelial cell
pyroptosis. For example, a recent study revealed that melatonin
prevents endothelial cell pyroptosis by upregulating TET2 to
inhibit the methylation of UQCRC1 and improving mitochondrial
function (34). Zhang et al
(20) reported that melatonin
prevents endothelial cell pyroptosis via MEG3/miR-223/NLRP3 axis in
atherosclerosis. Our previous study has also demonstrated that
melatonin alleviates cigarette smoke-induced endothelial cell
pyroptosis through inhibiting ROS/NLRP3 axis (11). However, the role of melatonin in
diabetes-induced pyroptosis of vascular endothelial cells is rarely
reported. The current study has clarified the protective role of
melatonin in HG-induced pyroptosis of vascular endothelial
cells.
Nrf2 is a key nuclear transcription factor that
regulates antioxidant gene expression. Studies (35,36)
have reported that during the early stages of diabetes, the
expression of endothelial nitric oxide synthase (eNOS) and
antioxidant genes may be upregulated as a compensatory mechanism in
response to ROS/reactive nitrogen species, however, as diabetes
progresses, NADPH oxidase continues to be activated and the
continued overexpression of ROS will lead to eNOS uncoupling,
mitochondrial dysfunction and decreased expression of antioxidant
genes mediated by Nrf2. Finally, the cells enter a vicious circle
of antioxidant imbalance. Consistent with the results of previous
studies, the present study found that HUVEC initially showed an
increased compensatory expression of Nrf2 under the intervention of
high glucose, but with the extension of high glucose injury time,
Nrf2 expression also gradually decreased.
ROS is a key regulator of inflammasome activation
(37,38). Our previous study also confirmed
that cigarette smoke causes endothelial pyroptosis through the
ROS/NLRP3 axis (11). Therefore,
it is particularly important to find suitable ROS inhibitors to
reduce diabetes-induced vascular injury associated with NLRP3
inflammasome. Increasing evidence has shown that the Nrf2 signaling
pathway interacts with inflammasomes at multiple points. A recent
study revealed that in renal ischemia/reperfusion injury, Nrf2
regulates the inhibition of NLRP3 inflammasome activity (39). In addition, Hou et al
(40) reported that Nrf2 inhibits
NLRP3 inflammasome activity through regulating Trx1/TXNIP complex
in cerebral ischemia reperfusion injury. However, only a few
studies have evaluated the interaction between Nrf2 and NLRP3
inflammasomes in diabetes-induced vascular injury. Previous studies
have shown that melatonin may serve a therapeutic role in different
diseases, such as ischemia-reperfusion injury (41), oxidative stress injury (42) and neurotoxicity (43), through activation of Nrf2 signaling
pathway. Previous studies have found that melatonin can exert
anti-inflammatory effects through Nrf2-NLRP3 pathway. One study
found that melatonin attenuates LPS-induced acute depression-like
behavior and NLRP3 inflammasome activity in microglia via the
SIRT1/Nrf2 pathway (44). Another
study found that melatonin alleviates sepsis-induced cardiac injury
by activating Nrf2 pathway and inhibiting NLRP3 inflammasome
(45). In addition, our previous
study also found that melatonin may effectively protect
smoking-caused vascular injury and atherosclerosis through
Nrf2/ROS/NLRP3 signaling pathway (46). However, it remains to be clarified
whether melatonin serves a protective role through Nrf2/NLRP3
pathway in vascular endothelial injury induced by HG. Considering
different disease states, the effect and mechanism of drugs may
change. The present study confirmed that melatonin alleviated
HG-induced endothelial cell pyroptosis by activating the Nrf2
pathway to inhibit NLRP3 inflammasome activation.
MT1/MT2 is a G-protein-coupled receptor existing in
cell membrane and widely exists in the nervous and cardiovascular
systems (49,50). Cui et al confirmed the
normal expression of MT1/MT2 in HUVEC by western blotting (51). Therefore, the current study did not
conduct experiments to verify it again. However, the lack of
verification of MT1/MT2 expression in HUVECs remains a potential
limitation of the present study. As for the model of vascular
endothelial cells with high glucose injury, the glucose
concentrations in most previous studies were controlled within the
range of 20–40 mM, among which 25 and 30 mM are the most used at
present (52–55) because they are closer to the blood
glucose values of severe hyperglycemia in humans. In addition, in
order to observe pyroptosis and the protective effect of melatonin
more markedly, the present study selected a glucose concentration
of 30 mM. However, the lack of an addition concentration to examine
any dose-dependent effect is also a potential limitation of the
present study.
In conclusion, the current study showed that
melatonin may serve a protective role in HG-induced vascular
endothelial cell pyroptosis by activating the Nrf2 pathway to
inhibit NLRP3 inflammasome activation. In addition, it was further
found that melatonin attenuates HG-induced vascular endothelial
cell injury by interacting with its receptors (MT1/MT2) to promote
activation of Nrf2 pathway (Fig.
5). These findings provide a new theoretical basis and a
promising therapeutic strategy for the early prevention and
treatment of diabetic vasculopathy.
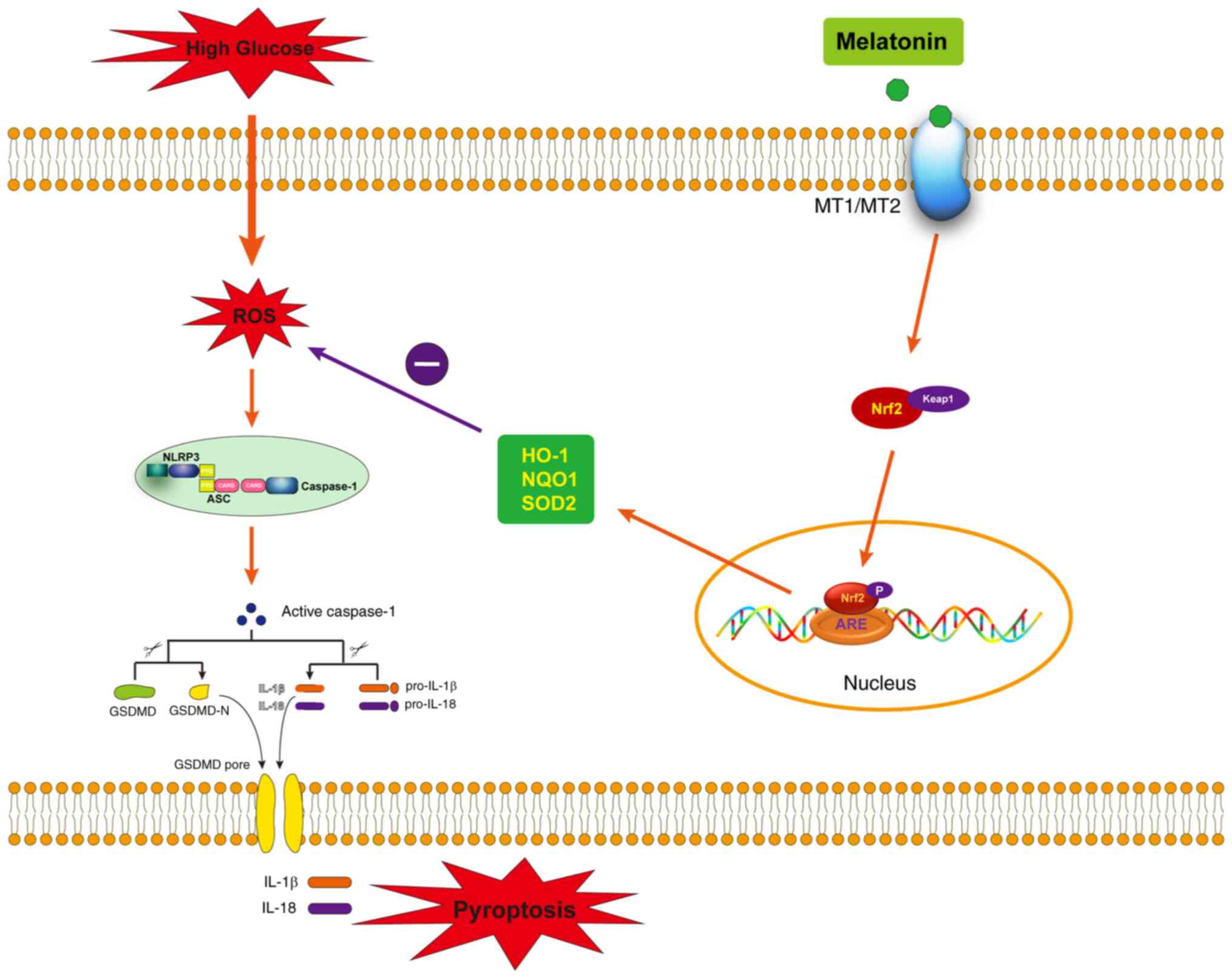 | Figure 5.A schematic hypothetical model
showing the specific signaling mechanisms by which melatonin
attenuates HG-induced endothelial cell pyroptosis through
MT1/MT2-Nrf2-ROS-NLRP3 signaling pathway. HG, high glucose; Nrf2,
nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen
species; NLRP3, NOD-like receptor family, pyrin domain-containing
3; HO-1, heme oxygenase-1; NQO1, oxygenase-1 (HO-1), NADPH: Quinone
Oxidoreductase 1; SOD2, superoxide dismutase 2; Keap1, kelch-like
ECH-associated protein 1. |
Results
Melatonin activates the Nrf2 pathway
in HUVECs under HG conditions
In this part of the experiment, an optimal melatonin
protective concentration was first determined through CCK-8 by
setting control group, HG (30 mM) + different melatonin
concentrations (0 µM-1 M) treatment group. It was found that a
melatonin concentration of 100 µM was the most effective in
alleviating the loss of cell viability caused by HG and 100 µM was
selected as the treatment concentration for subsequent experiments
(Fig. 1A). Next, in order to
select an appropriate time for HG intervention, HUVECs were
incubated with 30 mM HG medium and the expression of Nrf2 protein
in the nucleus of HUVECs was observed by western blotting with
prolonged HG intervention time. It was found that the expression of
n-Nrf2 increased to the highest level after 6 h of HG incubation
and then gradually decreased as HG incubation time was prolonged
(Fig. 1B and C). In order to
observe more marked therapeutic effect of melatonin in the
follow-up experiments, 72 h was chosen as the intervention time for
HG. Finally, it was confirmed by western blotting that melatonin
treatment could activate Nrf2 signaling pathway in HUVECs under HG
condition. The result showed that melatonin promoted the expression
of n-Nrf2, HO-1 and SOD2 (Fig.
1D-H).
Melatonin activates the Nrf2 pathway
in HUVECs mainly through the MT1/MT2 pathway under HG
conditions
Luzindole is an effective competitive MT1/MT2
membrane receptor antagonist and is widely used in melatonin
research. In this part of the experiment, it was found that the
protective effect of melatonin in promoting n-Nrf2 expression was
significantly reduced when MT1/MT2 was blocked by Luzindole under
HG conditions (Fig. 2A and B).
Similarly, the effect of melatonin on promoting the expression of
HO-1, NQO1 and SOD2 proteins under HG conditions was also
significantly reduced when MT1/MT2 was blocked by Luzindole
(Fig. 2C-F).
 | Figure 2.Mel activates the Nrf2 pathway in
HUVECs mainly through the MT1/MT2 pathway under HG conditions. (A)
Western blot analysis of n-Nrf2 expression in HUVECs of each group.
(B) Semi-quantitative analysis of n-Nrf2 relative expression. (C)
Western blot analysis of HO-1, NQO1 and SOD2 expression in HUVECs
of each group. (D-F). Semi-quantitative analysis of HO-1, NQO1 and
SOD2 relative expression. Results are presented as the mean ±
standard deviation. *P<0.05, **P<0.01 and ***P<0.001. Mel,
melatonin; Nrf2, nuclear factor erythroid 2-related factor 2;
HUVECs, human umbilical vein endothelial cells; HG, high glucose;
HO-1, heme oxygenase-1; NQO1, oxygenase-1 (HO-1), NADPH: Quinone
Oxidoreductase 1; SOD2, superoxide dismutase 2; Luz, Luzindole. |
Melatonin or MCC950 inhibits
HG-induced endothelial cell pyroptosis
Hoechst 33342/PI staining and LDH release assay are
often used to observe pyroptotic cell death (19,20).
In this part of the experiment, it was found that the proportion of
PI-positive cells and LDH release of HUVEC in the HG group were
significantly increased compared with the control group. However,
following treatment with the NLRP3 specific inhibitor MCC950 and
melatonin, HG-mediated pyroptosis was significantly reduced
(Fig. 3A-C). Then the protein
expression levels of Gasdermin D (GSDMD), a key executioner of the
pyroptotic pathway and the N-terminal GSDMD cleavage product
(GSDMD-N), which can induce pyroptosis was examined. Western
blotting results showed that GSDMD and GSDMD-N were significantly
increased in HG-incubated HUVEC compared with the control group.
However, following treatment with MCC950 and melatonin, HG-mediated
increases in GSDMD and GSDMD-N were significantly reduced (Fig. 3D-F).
 | Figure 3.Mel or MCC950 inhibits HG-induced
endothelial cell pyroptosis. (A) Representative pyroptotic cell
death images of each group obtained using Hoechst33342/PI staining
(original magnification, ×100; scale bar, 100 µm. (B) The
proportion of PI-positive cells in each group. (C) LDH release
activity in each group. (D) Western blot analysis of GSDMD and
GSDMD-N expression in HUVECs of each group. Semi-quantitative
analysis of (E) GSDMD and (F) GSDMD-N relative expression. Results
are presented as the mean ± standard deviation. *P<0.05,
**P<0.01 and ***P<0.001. Mel, melatonin; HG, high glucose;
PI, propidium iodide; LDH, lactate dehydrogenase; GSDMD, gasdermin
D; GSDMD-N, gasdermin D-N; HUVECs, human umbilical vein endothelial
cells; NC, negative control. |
Melatonin inhibits ROS/NLRP3
inflammasome pathway in HUVECs under HG conditions by activating
the Nrf2 pathway
In this part of the experiment, in order to confirm
that melatonin inhibits the activation of ROS/NLRP3 inflammasome
pathway in vascular endothelial cells induced by HG mainly through
or at least partly through Nrf2 signaling pathway, Nrf2 expression
was knocked down by siRNA interference, which was verified by
RT-qPCR. hNrf2 siRNA-2 was selected as the interference sequence
(Fig. 4A). Next, western blotting
results showed that melatonin treatment could significantly reduce
the overexpression of NLRP3, ASC and cleaved-caspase-1 protein
induced by HG, and the protective effect of melatonin was also
significantly decreased after knockdown of Nrf2 expression
(Fig. 4B-E). In addition,
melatonin treatment could significantly attenuate the increase of
NLRP3 mRNA and caspase-1 mRNA expression induced by HG, while the
protective effect of melatonin was significantly reduced after Nrf2
knockdown (Fig. 4F and G). It was
also found by ELISA that melatonin treatment significantly
attenuated the increased secretion of IL-1β and IL-18 in culture
supernatants induced by HG, and this protective effect of melatonin
was also significantly decreased after knockdown of Nrf2 expression
(Fig. 4H and I). Finally, ROS, a
key activator upstream of the NLRP3 inflammasome was measured.
Results showed that melatonin treatment significantly attenuated
the increase of ROS production induced by HG, and this protective
effect of melatonin was also significantly decreased after
knockdown of Nrf2 expression (Fig.
4J).
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant number 81970417), the Capital Medical
University School Cultivation Fund (Natural Category; grant number
PYZ21047), the Beijing Friendship Hospital Seed Plan Talent Project
(grant number YYZZ202017) and the Capital Health Research and
Development of Special Grant (grant number 2020-2-1102).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, HF and CL conceived and designed the present
study. XW, RZ and BM performed the experiments. XW, WW and RZ
interpreted the results, analyzed the data and wrote the paper. XW,
WW and LN analyzed the data and designed the figures and table. HF
and CL reviewed and edited the manuscript. XW, HF and CL confirmed
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vijan S: Type 2 diabetes: Ann Intern Med.
171:ITC65-ITC80. 2019.
|
|
2
|
Kaur R, Kaur M and Singh J: Endothelial
dysfunction and platelet hyperactivity in type 2 diabetes mellitus:
Molecular insights and therapeutic strategies. Cardiovasc Diabetol.
17:1212018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gimbrone MA Jr and Garcia-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma B, Wang X, Zhang R, Niu S, Rong Z, Ni
L, Di X, Han Q and Liu C: Cigarette smoke extract stimulates PCSK9
production in HepG2 cells via ROS/NF-κB signaling. Mol Med Rep.
23:3312021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salmanoglu DS, Gurpinar T, Vural K,
Ekerbicer N, Dariverenli E and Var A: Melatonin and L-carnitin
improves endothelial disfunction and oxidative stress in type 2
diabetic rats. Redox Biol. 8:199–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tiong YL, Ng KY, Koh RY, Ponnudurai G and
Chye SM: Melatonin inhibits high glucose-induced ox-LDL/LDL
expression and apoptosis in human umbilical endothelial cells. Horm
Mol Biol Clin Investig. 41:2020.PubMed/NCBI
|
|
7
|
Zhaolin Z, Guohua L, Shiyuan W and Zuo W:
Role of pyroptosis in cardiovascular disease. Cell Prolif.
52:e125632019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komada T and Muruve DA: The role of
inflammasomes in kidney disease. Nat Rev Nephrol. 15:501–520. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Gan L, Xu Y, Luo D, Ren Q, Wu S and
Sun C: Melatonin alleviates inflammasome-induced pyroptosis through
inhibiting NF-κB/GSDMD signal in mice adipose tissue. J Pineal Res.
63:2017. View Article : Google Scholar
|
|
11
|
Wang X, Bian Y, Zhang R, Liu X, Ni L, Ma
B, Zeng R, Zhao Z, Song X and Liu C: Melatonin alleviates cigarette
smoke-induced endothelial cell pyroptosis through inhibiting
ROS/NLRP3 axis. Biochem Biophys Res Commun. 519:402–408. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li C, Cheng L, Wu H, He P, Zhang Y, Yang
Y, Chen J and Chen M: Activation of the KEAP1-NRF2-ARE signaling
pathway reduces oxidative stress in Hep2 cells. Mol Med Rep.
18:2541–2550. 2018.PubMed/NCBI
|
|
13
|
Yamamoto M, Kensler TW and Motohashi H:
The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for
maintaining redox homeostasis. Physiol Rev. 98:1169–1203. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Qiao B, Gao F, Wang H, Miao S and
Zhao H: Melatonin protects H9c2 cells against
ischemia/reperfusion-induced apoptosis and oxidative stress via
activation of the Nrf2 signaling pathway. Mol Med Rep.
18:3497–3505. 2018.PubMed/NCBI
|
|
15
|
Li Y, Yu H, Xu Z, Shi S, Wang D, Shi X,
Wang Y, Zeng B, Deng H, Deng X, et al: Melatonin ameliorates
ANIT-induced cholestasis by activating Nrf2 through a
PI3K/Akt-dependent pathway in rats. Mol Med Rep. 19:1185–1193.
2019.PubMed/NCBI
|
|
16
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20:33282019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cecon E, Oishi A and Jockers R: Melatonin
receptors: Molecular pharmacology and signalling in the context of
system bias. Br J Pharmacol. 175:3263–3280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z,
Lin Y, Bai X, Liu X, Chen X, et al: Nicotine promotes
atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis.
Cell Death Dis. 9:1712018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J,
Li M, Zhao T, Yang H, Xu R, et al: Melatonin prevents endothelial
cell pyroptosis via regulation of long noncoding RNA
MEG3/miR-223/NLRP3 axis. J Pineal Res. 64:2018. View Article : Google Scholar
|
|
21
|
Zhang Y, Wang Y, Xu J, Tian F, Hu S, Chen
Y and Fu Z: Melatonin attenuates myocardial ischemia-reperfusion
injury via improving mitochondrial fusion/mitophagy and activating
the AMPK-OPA1 signaling pathways. J Pineal Res. 66:e125422019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scheer FAJL, Van Montfrans GA, van Someren
EJ, Mairuhu G and Buijs RM: Daily nighttime melatonin reduces blood
pressure in male patients with essential hypertension.
Hypertension. 43:192–197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng X, Wan Y, Xu Y, Zhou Q, Wang Y and
Zhu H: Melatonin alleviates myosin light chain kinase expression
and activity via the mitogen-activated protein kinase pathway
during atherosclerosis in rabbits. Mol Med Rep. 11:99–104. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sadeghi M, Khosrawi S,
Heshmat-Ghahdarijani K, Gheisari Y, Roohafza H, Mansoorian M and
Hoseini SG: Effect of melatonin on heart failure: Design for a
double-blinded randomized clinical trial. ESC Heart Fail.
7:3142–3150. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghaeli P, Solduzian M, Vejdani S and
Talasaz AH: Comparison of the effects of melatonin and oxazepam on
anxiety levels and sleep quality in patients with
ST-segment-elevation myocardial Infarction following primary
percutaneous coronary intervention: A randomized clinical trial.
Ann Pharmacother. 52:949–955. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nasseh N, Khezri MB, Farzam S, Shiravandi
S and Shafikhani AA: The effect of melatonin on cardiac biomarkers
after coronary artery bypass graft surgery: A double-blind,
randomized pilot study. J Cardiothorac Vasc Anesth. 36:3800–3805.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dominguez-Rodriguez A, Abreu-Gonzalez P,
de la Torre-Hernandez JM, Consuegra-Sanchez L, Piccolo R,
Gonzalez-Gonzalez J, Garcia-Camarero T, Del Mar Garcia-Saiz M,
Aldea-Perona A, Reiter RJ, et al: Usefulness of early treatment
with melatonin to reduce infarct size in patients with ST-segment
elevation myocardial infarction receiving percutaneous coronary
intervention (from the melatonin adjunct in the acute myocardial
infarction treated with angioplasty trial). Am J Cardiol.
120:522–526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ekeloef S, Halladin N, Fonnes S, Jensen
SE, Zaremba T, Rosenberg J, Jonsson G, Aarøe J, Gasbjerg LS,
Rosenkilde MM and Gögenur I: Effect of intracoronary and
intravenous melatonin on myocardial salvage index in patients with
ST-elevation myocardial infarction: A randomized placebo controlled
trial. J Cardiovasc Transl Res. 10:470–479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zahid JA, Isbrand A, Kleif J,
Schou-Pedersen AV, Lykkesfeldt J, Madsen MT and Gögenur I: The
effect of melatonin on endothelial dysfunction in patients after
acute coronary syndrome: The MEFACS randomized clinical trial. J
Pineal Res. 67:e126002019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kücükakin B, Wilhelmsen M, Lykkesfeldt J,
Reiter RJ, Rosenberg J and Gögenur I: No effect of melatonin to
modify surgical-stress response after major vascular surgery: A
randomised placebo-controlled trial. Eur J Vasc Endovasc Surg.
40:461–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao S, Wang Y, Zhang X, Zheng L, Zhu B,
Yao S, Yang L and Du J: Melatonin protects against
hypoxia/reoxygenation-induced dysfunction of human umbilical vein
endothelial cells through inhibiting reactive oxygen species
generation. Acta Cardiol Sin. 34:424–431. 2018.PubMed/NCBI
|
|
32
|
Dayoub JC, Ortiz F, López LC, Venegas C,
Del Pino-Zumaquero A, Roda O, Sanchez-Montesinos I,
Acuña-Castroviejo D and Escames G: Synergism between melatonin and
atorvastatin against endothelial cell damage induced by
lipopolysaccharide. J Pineal Res. 51:324–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qian Z, Zhao Y, Wan C, Deng Y, Zhuang Y,
Xu Y, Zhu Y, Lu S and Bao Z: Pyroptosis in the initiation and
progression of atherosclerosis. Front Pharmacol. 12:6529632021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zeng J, Tao J, Xia L, Zeng Z, Chen J, Wang
Z, Meng J and Liu L: Melatonin inhibits vascular endothelial cell
pyroptosis by improving mitochondrial function via up-regulation
and demethylation of UQCRC1. Biochem Cell Biol. 99:339–347. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Afzal-Ahmed I, Mann GE, Shennan AH, Poston
L and Naftalin RJ: Preeclampsia inactivates glucose-6-phosphate
dehydrogenase and impairs the redox status of erythrocytes and
fetal endothelial cells. Free Radic Biol Med. 42:1781–1790. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paravicini TM and Touyz RM: NADPH
oxidases, reactive oxygen species, and hypertension: Clinical
implications and therapeutic possibilities. Diabetes Care. 31
(Suppl 2):S170–S180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Minutoli L, Puzzolo D, Rinaldi M, Irrera
N, Marini H, Arcoraci V, Bitto A, Crea G, Pisani A, Squadrito F, et
al: ROS-mediated NLRP3 inflammasome activation in brain, heart,
kidney, and testis ischemia/reperfusion injury. Oxid Med Cell
Longev. 2016:21830262016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fan Y, Zhang X, Yang L, Wang J, Hu Y, Bian
A, Liu J and Ma J: Zinc inhibits high glucose-induced NLRP3
inflammasome activation in human peritoneal mesothelial cells. Mol
Med Rep. 16:5195–5202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su Y, Wang Y, Liu M and Chen H: Hydrogen
sulfide attenuates renal I/R-induced activation of the inflammatory
response and apoptosis via regulating Nrf2-mediated NLRP3 signaling
pathway inhibition. Mol Med Rep. 24:5182021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hou Y, Wang Y, He Q, Li L, Xie H, Zhao Y
and Zhao J: Nrf2 inhibits NLRP3 inflammasome activation through
regulating Trx1/TXNIP complex in cerebral ischemia reperfusion
injury. Behav Brain Res. 336:32–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi S, Lei S, Tang C, Wang K and Xia Z:
Melatonin attenuates acute kidney ischemia/reperfusion injury in
diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling
pathway. Biosci Rep. 39:BSR201816142019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shah SA, Khan M, Jo MH, Jo MG, Amin FU and
Kim MO: Melatonin stimulates the SIRT1/Nrf2 signaling pathway
counteracting lipopolysaccharide (LPS)-induced oxidative stress to
rescue postnatal rat brain. CNS Neurosci Ther. 23:33–44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sadek KM, Lebda MA and Abouzed TK: The
possible neuroprotective effects of melatonin in aluminum
chloride-induced neurotoxicity via antioxidant pathway and Nrf2
signaling apart from metal chelation. Environ Sci Pollut Res Int.
26:9174–9183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Arioz BI, Tastan B, Tarakcioglu E, Tufekci
KU, Olcum M, Ersoy N, Bagriyanik A, Genc K and Genc S: Melatonin
attenuates LPS-induced acute depressive-like behaviors and
microglial NLRP3 inflammasome activation through the SIRT1/Nrf2
pathway. Front Immunol. 10:15112019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rahim I, Sayed RK, Fernández-Ortiz M,
Aranda-Martínez P, Guerra-Librero A, Fernández-Martínez J, Rusanova
I, Escames G, Djerdjouri B and Acuña-Castroviejo D: Melatonin
alleviates sepsis-induced heart injury through activating the Nrf2
pathway and inhibiting the NLRP3 inflammasome. Naunyn Schmiedebergs
Arch Pharmacol. 394:261–277. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao Z, Wang X, Zhang R, Ma B, Niu S, Di
X, Ni L and Liu C: Melatonin attenuates smoking-induced
atherosclerosis by activating the Nrf2 pathway via NLRP3
inflammasomes in endothelial cells. Aging (Albany NY).
13:11363–11380. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen X, Xi Z, Liang H, Sun Y, Zhong Z,
Wang B, Bian L and Sun Q: Melatonin prevents mice cortical
astrocytes from hemin-induced toxicity through activating
PKCα/Nrf2/HO-1 signaling in vitro. Front Neurosci. 13:7602019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jumnongprakhon P, Govitrapong P, Tocharus
C and Tocharus J: Melatonin promotes blood-brain barrier integrity
in methamphetamine-induced inflammation in primary rat brain
microvascular endothelial cells. Brain Res. 1646:182–192. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pandi-Perumal SR, Trakht I, Srinivasan V,
Spence DW, Maestroni GJ, Zisapel N and Cardinali DP: Physiological
effects of melatonin: Role of melatonin receptors and signal
transduction pathways. Prog Neurobiol. 85:335–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gobbi G and Comai S: Sleep well.
Untangling the role of melatonin MT1 and MT2 receptors in sleep. J
Pineal Res. 66:e125442019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cui P, Yu M, Luo Z, Dai M, Han J, Xiu R
and Yang Z: Intracellular signaling pathways involved in cell
growth inhibition of human umbilical vein endothelial cells by
melatonin. J Pineal Res. 44:107–114. 2008.PubMed/NCBI
|
|
52
|
Duan MX, Zhou H, Wu QQ, Liu C, Xiao Y,
Deng W and Tang QZ: Andrographolide protects against HG-induced
inflammation, apoptosis, migration, and impairment of angiogenesis
via PI3K/AKT-eNOS SIgnalling in HUVECs. Mediators Inflamm.
2019:61683402019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Leng B, Zhang Y, Liu X, Zhang Z, Liu Y,
Wang H and Lu M: Astragaloside IV suppresses high glucose-induced
NLRP3 inflammasome activation by inhibiting TLR4/NF-κ B and CaSR.
Mediators Inflamm. 2019:10824972019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang J, Shen X, Liu J, Chen W, Wu F, Wu W,
Meng Z, Zhu M and Miao C: High glucose mediates NLRP3 inflammasome
activation via upregulation of ELF3 expression. Cell Death Dis.
11:3832020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nie J: UNC0321 inhibits high glucose
induced apoptosis in HUVEC by targeting Rab4. Biomed Pharmacother.
131:1106622020. View Article : Google Scholar : PubMed/NCBI
|



















