Introduction
Malignant pleural mesothelioma (MPM) is a
biologically aggressive malignancy arising from the pleural
mesothelium and pathogenically linked to occupational or
residential exposure to asbestos fibers (1,2). MPM
has poor outcomes (median overall survival from 6 to 12 months)
with late diagnosis and low treatment response (1,2).
Currently, the main clinicopathological features
with prognostic relevance in patients with MPM include pathological
stage, histological subtype, sex and age (3). Immunohistochemistry plays a leading
role in the diagnosis of this neoplasm and Wilms' Tumor-1 (WT-1),
calretinin, Cytokeratin 5/6 (CK5/6), podoplanin, mesothelin and
osteopontin are immunomarkers currently available for MPM. However,
in the last decade, clinicians have reported a potential prognostic
and predictive role of proteins expressed in MPM, including
caspase-3 (4), autophagy-related
protein 7 (5) and serine and
Arginine-rich splicing factor 1 (6); to the best of our knowledge, however,
reliable prognostic and predictive biomarkers capable of improving
MPM treatment and outcome have not yet been characterized.
In addition to asbestos, other pathogenic agents,
including asbestos-like fibers such as erionite and fluoroedenite
(FE) fibers, induce MPM (7–9).
Several cases of MPM have been reported by previous epidemiological
studies in Biancavilla, a small town near Mt. Etna (Sicily, Italy)
(10–12); FE fibers were isolated in the lava
rocks excavated from a local stone quarry that had been used to
extract building materials for >50 years (10–12).
A strong morphological and size overlap was demonstrated between
the extracted building materials and tremolite amphibolic asbestos
fibers and the International Agency for Research on Cancer declared
FE fibers as environmental carcinogens (12).
Cyclic adenosine monophosphate (cAMP) was the first
secondary messenger discovered and plays crucial roles in cell
targeting and signaling, regulating physiological and pathological
mechanisms (13,14). The transcription of numerous genes
may be regulated by cAMP via the classically kinase A (PKA) and
cAMP-responsive element binding protein (CREB) pathway (13). PKA also serves as a phosphorylating
agent of multiple kinases, including Raf, glycogen synthase kinase
3 (GSK3) and focal adhesion kinase (FAK) (13). The aberrant activation of the
cAMP/PKA pathway has been shown to be involved in many human
neoplasms including liver, brain, prostate, breast and lung cancer,
regulating tumor cell proliferation, invasion and metastatic
potential (13).
To the best of our knowledge, there is few data
about the role of cAMP in MPM. Therefore, the present study
investigated the immunohistochemical expression of cAMP and its
correlation with the clinicopathological parameters in patients
with MPM exposed to FE fibers.
Materials and methods
Sample collection
The present research complied with the Helsinki
Declaration and was approved by the Catania 1 Ethics Committee,
‘Policlinico-Vittorio Emanuele’, Catania, Italy (approval no.
1/2014/PO; 28/05/2014). Written informed consent was obtained from
all patients enrolled in the study. All patients were recruited
from the University-Hospital Policlinico-Vittorio Emanuele.
Clinicopathological features from 49 surgically
treated cases with a histological diagnosis of MPM diagnosed
between January 1996 and December 2014 were collected from patients
(28 males and 21 females; age range: 47–93 years) who were
residents in Biancavilla and exhibited evidence of environmental
exposure to FE. Adequate thoracoscopic biopsy tissue and follow-up
data were available only for 10 of the 49 patients. Inclusion
criteria for histological samples were as previously reported
(5,6): i) Tumor tissue from the
paraffin-embedded blocks had to be sufficient to cut slides for
immunohistochemical analyses and ii) representative tumor tissue
had to be present in paraffin-embedded blocks. Cases with extensive
necrosis were excluded to preserve the immunoreactivity of the
tumor tissue.
Immunohistochemistry
Surgical specimens were fixed in 10% formalin for
12–24 h, embedded in paraffin at 46–68°C, cut to 4–5 µm and stained
with 5% hematoxylin for 4 min and with 1–5% eosin for two min at
room temperature. Each sample was incubated overnight at 4°C with a
rabbit monoclonal anti-cAMP protein kinase catalytic subunit
antibody (clone EP2102Y; cat. no. ab76238; Abcam), diluted at 1:100
in PBS). The presence of brown chromogen within the tumor cytoplasm
was assessed as positive cAMP staining; unaffected human testis
tissue was used as a positive control, while negative control
sections were obtained by omitting the primary antibody. cAMP
immunoreactivity was evaluated within areas of vital tumor tissue,
while necrotic areas were excluded from the analysis.
Immunohistochemical slides were semi-quantified as
previously described (15,16). Immunoreactivity score (IRS) was
obtained by multiplying the intensity of staining (IS; 0–3) and the
percentage of positive cells (extent score, ES; 0–4). IRS ≤6 or
>6 indicated low and high cAMP expression, respectively.
Statistical analysis
The mean and median values of cAMP expression,
expressed as IRS, in FE-induced MPM were non-parametrically
compared by χ2 test. Hazard ratio (HR) was calculated
using the Mantel-Haenszel test. Cancer-specific survival analysis
and comparison of survival curves were performed using the
Kaplan-Meier method and Mantel-Cox log-rank test, respectively.
Spearman's correlation was performed to evaluate the correlation
between clinicopathological and immunohistochemical data. P<0.05
was considered to indicate a statistically significant difference.
Statistical analysis was performed using GraphPad Prism version 7
(GraphPad Software, Inc.).
Results
Clinicopathological features of the
FE-induced MPM cases
The clinicopathological features were as previously
described (6). Briefly, six males
and four females affected by FE-induced MPM with an age range of
50–93 years (mean age, 68.4 years), were part of the study.
According to the World Health Organization (WHO) criteria (17), histopathology showed an epithelioid
morphology in six cases, a sarcomatoid morphology in one case and a
biphasic morphology in the remaining three cases. A mild
predominance of the sarcomatoid component (60 vs. 40% epithelioid)
was observed in two biphasic MPMs, while an almost pure spindle
cell morphology with only scattered glandular structures was seen
in the remaining biphasic case. No signs of apoptosis were found.
The primary clinicopathological and immunohistochemical features
are summarized in Table I.
 | Table I.Clinico-pathological and
immunohistochemical features of the cases from our series. |
Table I.
Clinico-pathological and
immunohistochemical features of the cases from our series.
| Case | Age, years | Sex | Pathological
subtype | Survival time,
months | cAMP IS | cAMP ES | cAMP IRS |
|---|
| 1 | 69 | M | Epithelioid | 2 | 2 | 4 | 8 |
| 2 | 50 | M | Biphasic (20%
epithelioid, 80% sarcomatoid) | 16 | 2 | 2 | 4 |
| 3 | 69 | F | Sarcomatoid | 5 | 3 | 3 | 9 |
| 4 | 74 | F | Epithelioid | 13 | 2 | 2 | 4 |
| 5 | 85 | M | Epithelioid | 23 | 2 | 4 | 8 |
| 6 | 93 | F | Biphasic (40%
epithelioid, 60% sarcomatoid) | 8 | 3 | 4 | 12 |
| 7 | 58 | F | Epithelioid | 18 | 2 | 2 | 4 |
| 8 | 55 | M | Epithelioid | 37 | 1 | 2 | 2 |
| 9 | 75 | M | Biphasic (40%
epithelioid, 60% sarcomatoid) | 60 | 2 | 2 | 4 |
| 10 | 56 | M | Epithelioid | 12 | 2 | 4 | 8 |
Immunohistochemical expression of cAMP
and correlation with clinicopathological parameters
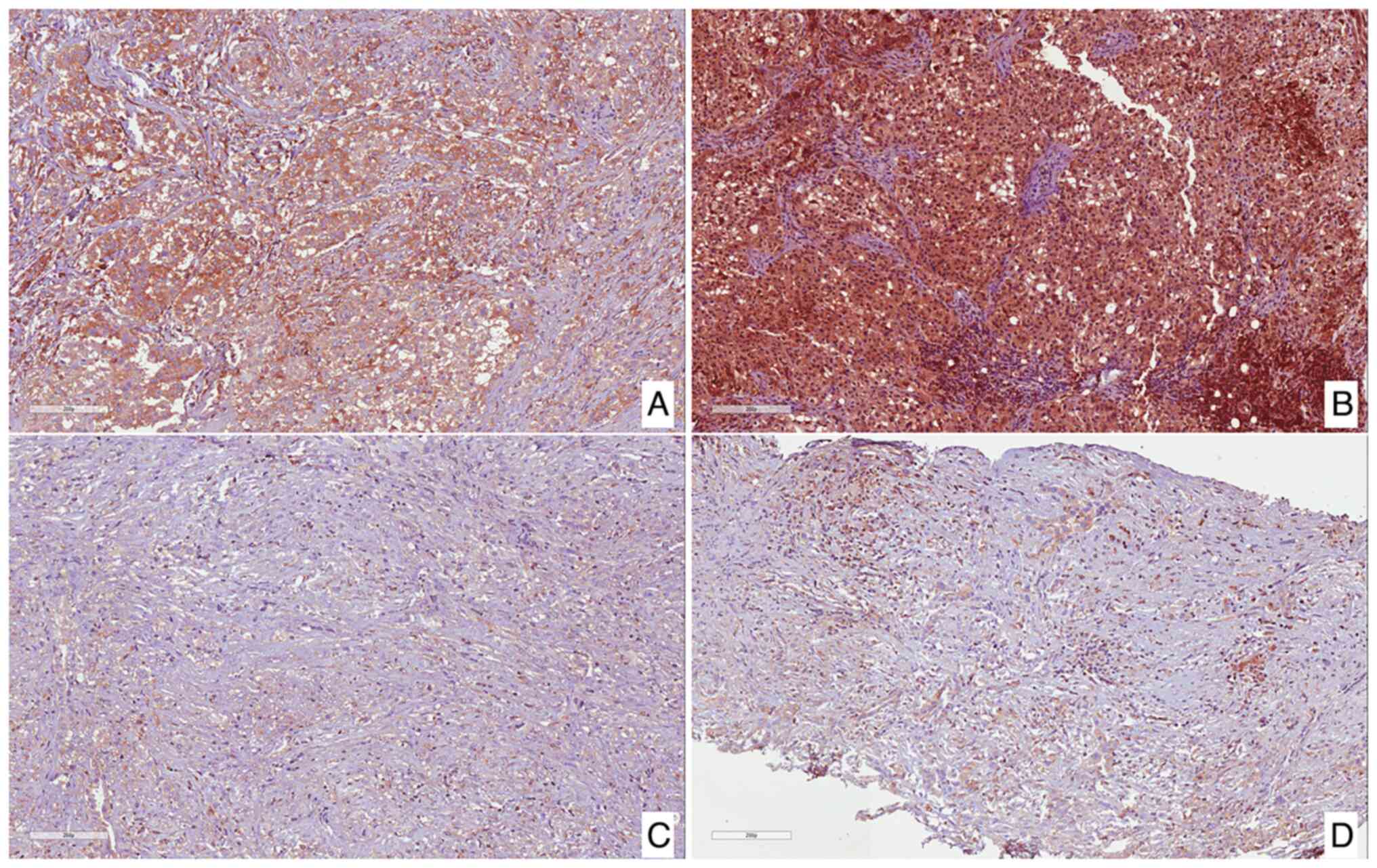
High immunohistochemical expression of cAMP was
found in 5 tumors (50%; Fig. 1A and
B), while the remaining 5 cases (50%) exhibited low
immunoexpression (Fig. 1C and
D).
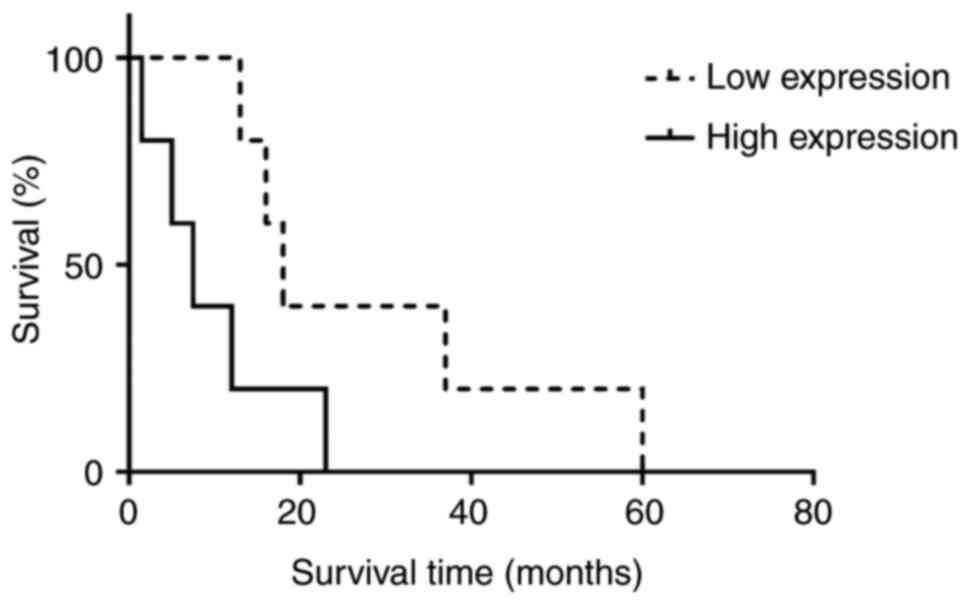
Considering the median overall survival (OS) time
between high (8 months) and low (18 months) cAMP expression, there
was no significant association between cAMP expression and
increased OS and HR was 0.226 (95% CI, 0.049-1.042; Fig. 2). No significant association
between cAMP expression and other clinicopathological variables
(age, sex and MPM pathological subtype) was observed (data not
shown). Moreover, the better prognosis was observed in cases that
exhibited a low immunoexpression of cAMP. By contrast, shorter OS
was found in patients with FE-induced MPM with high cAMP
expression. A correlation between cAMP overexpression and decreased
survival time was found (mean OS, 7.5 for patients with high
expression vs. 18.0 months for patients with low cAMP expression;
Fig. 2).
Discussion
The present study investigated the
immunohistochemical expression of cAMP in patients with MPM
characterized by exposure to FE fibers. High cAMP immunoexpression
in five MPM cases (50%), while the remaining 5 cases (50%)
exhibited low immunoreactivity. In addition, a trend of shorter OS
was found in patients with FE-induced MPM and high cAMP expression
(mean OS time of 7.5 months for patients with high expression vs.
mean OS of 18 months for patients with low cAMP expression).
cAMP acts as an intracellular secondary messenger,
by regulating several processes such as cellular metabolism, ion
channel activation, gene transcription, cell proliferation,
differentiation, cell death and apoptosis (13,14).
cAMP may play both a tumor-suppressive and tumor-promoting role by
interacting with CREB and PKA, which induces the phosphorylation of
multiple kinases, including Raf, GSK3 and FAK (13). In hepatocellular carcinoma, the
role of cAMP in tumorigenesis is controversial because some authors
suggested that increasing cAMP levels inhibits cell proliferation
(18,19), while some clinicians demonstrated
that PKA promotes tumor invasion and metastasis via phosphorylation
of Cdc42-interacting protein 4 (CIP4) (20). In brain tumors, cAMP has been found
to inhibit glioblastoma and medulloblastoma cell proliferation by
increasing p21/p27 and PKA/exchange protein activated by cAMP
(Epac1)/Rap1 signaling (21,22)
and decreasing Hedgehog pathway signaling (23,24),
respectively. It has also been suggested that PKA overexpression
may negatively influence the androgen receptor signaling status of
prostate cancer, leading to the development of androgen resistance
and tumor growth (25,26). Similarly, the cAMP/PKA axis has
been found to stimulate tamoxifen resistance and tumor progression
in estrogen receptor-positive breast cancer (27) and trastuzumab resistance in HER-2
positive breast cancer (28).
Although the involvement of cAMP in tumorigenesis
and tumor growth has been reported in various human neoplasms
(13), little is known about its
role in MPM.
In the past few decades, some clinicians have found
that asbestos fibers rapidly induced CREB1 phosphorylation and
upregulation of CREB target genes, including c-Fos, Early Growth
Response-1 (EGR-1), Mitogen-activated protein kinase phosphatase 1
(MKP1), BCL2 and MMP13, on human mesothelial cell lines (29). Levels of phosphorylated CREB1 and
mRNA of BCL2, c-FOS, MMP9 and MMP13 were found to be increased also
in malignant mesothelioma cell lines (29). Therefore it is hypothesized that
cAMP is upregulated in human mesothelium exposed to asbestos via
the CREB pathway and may represent a novel potential therapeutic
target for MPM treatment (29).
Aromatase may be involved in MPM tumorigenesis via
modulation of cAMP (30,31), Nuvoli et al (32) found that exemestane, an inhibitor
of aromatase, has beneficial effects in a preclinical model of MPM;
specifically, exemestane negatively modulates expression of CD44 by
downregulating cAMP and CREB and inhibits MPM cell proliferation
(32).
Given the controversial role of cAMP in
tumorigenesis, it is likely that its impact is associated with
specific tissue type and other factors on which patient prognosis
typically depends, including tumor stage and microenvironment
(tumor-associated inflammation and fibroblast levels and changes in
tumor extracellular matrix composition) (33).
The present results are in line with the
aforementioned data that attribute a tumor-promoting role to cAMP
in MPM; the present study found a trend of shorter OS in patients
with FE-induced MPM with high immunoexpression of cAMP. However,
the present study is limited by the relatively small cohort of
patients and the lack of statistical significance between cAMP
expression and OS. Larger sample size is required to determine if
there is a statistically significant association between cAMP and
shorter OS. Although very few targeted therapies have been
discovered and introduced in MPM treatment guidelines, patients
with coactivation of AXL and Mesenchymal-epithelial transition
factor (MET) tyrosine kinase receptors benefit from a targeted
treatment with tyrosine kinase inhibitors (34). Future studies should assess the
correlation between cAMP expression and co-activation of AXL and
MET tyrosine kinase receptors and validate the present findings in
animal models and/or MPM cell lines by in vitro
experiments.
Acknowledgements
The authors would like to thank Professor Anthony
Bridgewood (Scientific Bureau of the University of Catania) for
language support.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GB and VF performed immunohistochemical experiments
and statistical analysis. GB, GM, BM, RG and RC performed the
histological examination. GB wrote the manuscript. CLom, VR, CLor
and CLed analyzed and interpreted data. CLom, VR, CLor and CLed
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study complied with the Helsinki
Declaration and was approved by the Catania 1 Ethics Committee,
‘Policlinico-Vittorio Emanuele’, Catania, Italy (approval no.
1/2014/PO; 28/05/2014). Written informed consent was obtained from
the patients enrolled in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perera ND and Mansfield AS: The evolving
therapeutic landscape for malignant pleural mesothelioma. Curr
Oncol Rep. 24:1413–1423. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sinn K, Mosleh B and Hoda MA: Malignant
pleural mesothelioma: Recent developments. Curr Opin Oncol.
33:80–86. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delgermaa V, Takahashi K, Park EK, Le GV,
Hara T and Sorahan T: Global mesothelioma deaths reported to the
world health organization between 1994 and 2008. Bull World Health
Organ. 89:716–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rapisarda V, Ledda C, Migliore M, Salemi
R, Musumeci A, Bracci M, Marconi A, Loreto C and Libra M: FBLN-3 as
a biomarker of pleural plaques in workers occupationally exposed to
carcinogenic fibers: A pilot study. Future Oncol. 11:35–37. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rapisarda V, Broggi G, Caltabiano R,
Lombardo C, Castorina S, Trovato A, Ledda C, Filetti V and Loreto
C: ATG7 immunohistochemical expression in malignant pleural
mesothelioma. A preliminary report. Histol Histopathol.
36:1301–1308. 2021.PubMed/NCBI
|
|
6
|
Broggi G, Angelico G, Filetti V, Ledda C,
Lombardo C, Vitale E, Rapisarda V, Loreto C and Caltabiano R:
Immunohistochemical expression of serine and arginine-rich splicing
factor 1 (SRSF1) in fluoro-edenite-induced malignant mesothelioma:
A preliminary study. Int J Environ Res Public Health. 18:62492021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Filetti V, Vitale E, Broggi G, Hagnäs MP,
Candido S, Spina A and Lombardo C: Update of in vitro, in vivo and
ex vivo fluoro-edenite effects on malignant mesothelioma: A
systematic review (Review). Biomed Rep. 13:602020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ledda C, Loreto C, Pomara C, Rapisarda G,
Fiore M, Ferrante M, Bracci M, Santarelli L, Fenga C and Rapisarda
V: Sheep lymph-nodes as a biological indicator of environmental
exposure to fluoro-edenite. Environ Res. 147:97–101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Filetti V, Loreto C, Falzone L, Lombardo
C, Cannizzaro E, Castorina S, Ledda C and Rapisarda V: Diagnostic
and prognostic value of three microRNAs in environmental
asbestiform fibers-associated malignant mesothelioma. J Pers Med.
11:12052021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paoletti L, Batisti D, Bruno C, Di Paola
M, Gianfagna A, Mastrantonio M, Nesti M and Comba P: Unusually high
incidence of malignant pleural mesothelioma in a town of eastern
Sicily: An epidemiological and environmental study. Arch Environ
Health. 55:392–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Comba P, Gianfagna A and Paoletti L:
Pleural mesothelioma cases in Biancavilla are related to a new
fluoro-edenite fibrous amphibole. Arch Environ Health. 58:229–232.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grosse Y, Loomis D, Guyton KZ,
Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L,
Guha N, Scoccianti C, Mattock H, et al: Carcinogenicity of
fluoro-edenite, silicon carbide fibres and whiskers, and carbon
nanotubes. Lancet Oncol. 15:1427–1428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Kong Q, Wang J, Jiang Y and Hua
H: Complex roles of cAMP-PKA-CREB signaling in cancer. Exp Hematol
Oncol. 9:322020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jackson EK: The 2′,3′-cAMP-adenosine
pathway. Am J Physiol Renal Physiol. 301:F1160–F1167. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Broggi G, Lo Giudice A, Di Mauro M,
Pricoco E, Piombino E, Ferro M, Caltabiano R, Morgia G and Russo
GI: Insulin signaling, androgen receptor and PSMA
immunohistochemical analysis by semi-automated tissue microarray in
prostate cancer with diabetes (DIAMOND study). Transl Res.
238:25–35. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Broggi G, Lo Giudice A, Di Mauro M,
Asmundo MG, Pricoco E, Piombino E, Caltabiano R, Morgia G and Russo
GI: SRSF-1 and microvessel density immunohistochemical analysis by
semi-automated tissue microarray in prostate cancer patients with
diabetes (DIAMOND study). Prostate. 81:882–892. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galateau-Salle F, Churg A, Roggli V and
Travis WD; World Health Organization Committee For Tumors Of The
Pleura, : The 2015 world health organization classification of
tumors of the pleura: Advances since the 2004 classification. J
Thorac Oncol. 11:142–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Massimi M, Ragusa F, Cardarelli S and
Giorgi M: Targeting cyclic AMP signalling in hepatocellular
carcinoma. Cells. 8:15112019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Massimi M, Cardarelli S, Galli F, Giardi
MF, Ragusa F, Panera N, Cinque B, Cifone MG, Biagioni S and Giorgi
M: Increase of intracellular cyclic AMP by PDE4 inhibitors affects
HepG2 cell cycle progression and survival. J Cell Biochem.
118:1401–1411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tonucci FM, Almada E, Borini-Etichetti C,
Pariani A, Hidalgo F, Rico MJ, Girardini J, Favre C, Goldenring JR,
Menacho-Marquez M and Larocca MC: Identification of a CIP4 PKA
phosphorylation site involved in the regulation of cancer cell
invasiveness and metastasis. Cancer Lett. 461:65–77. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen TC, Hinton DR, Zidovetzki R and
Hofman FM: Up-regulation of the cAMP/PKA pathway inhibits
proliferation, induces differentiation, and leads to apoptosis in
malignant gliomas. Lab Invest. 78:165–174. 1998.PubMed/NCBI
|
|
22
|
Moon EY, Lee GH, Lee MS, Kim HM and Lee
JW: Phosphodiesterase inhibitors control A172 human glioblastoma
cell death through cAMP-mediated activation of protein kinase A and
Epac1/Rap1 pathways. Life Sci. 90:373–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen JR, Resnick DZ, Niewiadomski P, Dong
H, Liau LM and Waschek JA: Pituitary adenylyl cyclase activating
polypeptide inhibits gli1 gene expression and proliferation in
primary medulloblastoma derived tumor sphere cultures. BMC Cancer.
10:6762010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge X, Milenkovic L, Suyama K, Hartl T,
Purzner T, Winans A, Meyer T and Scott MP: Phosphodiesterase 4D
acts downstream of Neuropilin to control Hedgehog signal
transduction and the growth of medulloblastoma. Elife.
4:e070682015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neary CL, Nesterova M, Cho YS, Cheadle C,
Becker KG and Cho-Chung YS: Protein kinase A isozyme switching:
Eliciting differential cAMP signaling and tumor reversion.
Oncogene. 23:8847–8856. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pollack A, Bae K, Khor LY, Al-Saleem T,
Hammond ME, Venkatesan V, Byhardt RW, Asbell SO, Shipley WU and
Sandler HM: The importance of protein kinase A in prostate cancer:
Relationship to patient outcome in radiation therapy oncology group
trial 92-02. Clin Cancer Res. 15:5478–5484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kok M, Zwart W, Holm C, Fles R, Hauptmann
M, Veer LJ, Wessels LF, Neefjes J, Stål O, Linn SC, et al:
PKA-induced phosphorylation of ERα at serine 305 and high PAK1
levels is associated with sensitivity to tamoxifen in ER-positive
breast cancer. Breast Cancer Res Treat. 125:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moody SE, Schinzel AC, Singh S, Izzo F,
Strickland MR, Luo L, Thomas SR, Boehm JS, Kim SY, Wang ZC and Hahn
WC: PRKACA mediates resistance to HER2-targeted therapy in breast
cancer cells and restores anti-apoptotic signaling. Oncogene.
34:2061–2071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shukla A, Bosenberg MW, MacPherson MB,
Butnor KJ, Heintz NH, Pass HI, Carbone M, Testa JR and Mossman BT:
Activated cAMP response element binding protein is overexpressed in
human mesotheliomas and inhibits apoptosis. Am J Pathol.
175:2197–2206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stoppoloni D, Salvatori L, Biroccio A,
D'Angelo C, Muti P, Verdina A, Sacchi A, Vincenzi B, Baldi A and
Galati R: Aromatase inhibitor exemestane has antiproliferative
effects on human mesothelioma cells. J Thorac Oncol. 6:583–591.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nuvoli B and Galati R: Cyclooxygenase-2,
epidermal growth factor receptor, and aromatase signaling in
inflammation and mesothelioma. Mol Cancer Ther. 12:844–852. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nuvoli B, Germoni S, Morosetti C, Santoro
R, Cortese G, Masi S, Cordone I and Galati R: Exemestane blocks
mesothelioma growth through downregulation of cAMP, pCREB and CD44
implicating new treatment option in patients affected by this
disease. Mol Cancer. 13:692014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Wang W, Yamada T, Matsumoto K, Sakai
K, Bando Y, Uehara H, Nishioka Y, Sone S, Iwakiri S, et al: Pleural
mesothelioma instigates tumor-associated fibroblasts to promote
progression via a malignant cytokine network. Am J Pathol.
179:1483–1493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marino FZ, Corte CM, Ciaramella V, Erra S,
Ronchi A, Fiorelli A, Vicidomini G, Santini M, Scognamiglio G,
Morgillo F, et al: AXL and MET tyrosine kinase receptors
co-expression as a potential therapeutic target in malignant
pleural mesothelioma. J Pers Med. 12:19932022. View Article : Google Scholar
|