Introduction
Preeclampsia (PE) is a significant pregnancy-related
disorder affecting 3 to 5% of pregnant women globally, marked by
hypertension and proteinuria, appearing after 20 weeks of gestation
(1). PE has been the third leading
cause of maternal and neonatal morbidity and mortality with
>60,000 mortalities in pregnant women with PE annually across
the world (2,3). Currently, there are still no
effective therapies for PE beyond delivery of the placenta
(4,5). Despite ongoing research, the etiology
of PE is not fully understood. However, studies have linked
inappropriate remodeling of spiral arteries, immune dysregulation,
inadequate trophoblastic invasion and endothelial damage to this
condition (3,6,7).
MSCs display a capacity of potential self-renewal,
broad differentiation potential, low immunogenicity and readily
accessible properties, which offer MSCs advantages over other
cell-based therapies (8,9). Moreover, factors secreted by MSCs
have been correlated with angiogenesis and trophoblast formation,
and can maintain successful pregnancy (10–12).
However, the molecular mechanisms are still unclear. MSCs perform
their therapeutic roles through paracrine mechanism. Exosomes are
small extracellular vesicles that range from 30–100 nm in size and
contain various biomolecules including nucleic acids, lipids,
proteins, mRNA, miRNA and other non-coding RNAs (13). Exosomes derived from MSCs (MSC-Ex)
can alleviate liver fibrosis, acute and chronic kidney injury,
myocardial ischemia/reperfusion damage and acute tubular injury
(14–16).
Exosomes have gained a lot of attention as a
potential method for delivering therapeutics due to their
non-immunogenicity and non-toxicity (17). Exosomes can be genetically
engineered or their surface chemically modified to specifically
target cells or tissues, allowing them to accumulate in tumor
tissues (18). This enables more
precise and targeted delivery of therapeutics. The tumor-targeting
capability of exosomes was conferred by linking lysosome-associated
membrane glycoprotein 2b (LAMP-2B), a well-characterized exosomal
membrane protein (19), to
internalizing arginine-glycine-aspartic acid (CRGDKGPDC), which is
widely recognized as an efficient cell membrane penetration peptide
targeting αvβ3 integrins and neuropilin-1 (NRP-1) receptors
(20). The CCG ligand (CCGKRK) has
also shown selectivity towards placental tissue in mice (21). To the best of our knowledge, there
is currently no study that has attempted to conjugate CCGKRK to
LAMP-2B in order to enable exosomes target the placenta.
It is hypothesized that uteroplacental
ischemia/hypoxia caused by impaired trophoblast invasion and
uterine spiral arteriole remodeling is one of the leading causes of
PE (6). During the first trimester
of pregnancy, trophoblast cells are known to proliferate and
survive in a hypoxic environment, which is beneficial for mural
trophectoderm proliferation and spiral artery remodeling (22). Thus, during the initial phase of
embryonic development, hypoxia-inducible factor-1α (HIF1α), an
important transcription factor in placental development, is highly
expressed in in trophoblast subpopulations. However, sustained
hypoxia or HIF1α expression after 9 weeks of gestation will lead
trophoblast cells to fail to differentiate from a proliferative to
an invasive phenotype, shallow invasion of the trophoblasts and
insufficient myometrial spiral artery transformation, which is
strongly associated with early-onset PE (23,24).
Therefore, HIF1α deletion might facilitate trophoblast
invasion.
The present research is dedicated to exploring
whether short hairpin RNA (shRNA)-HIF1α (sh-HIF1α) carried by
MSC-Ex can effectively enhance the invasion ability of placental
cells by conjugating sh-HIF1α-CCGKRK to LAMP-2B, thus providing a
potential new therapeutic option for the prevention or treatment of
PE (25). MSCs exosomes were used
as a cell-based carrier for sh-HIF1α in the present study.
Materials and methods
JEG-3 cells culture
JEG-3 cells (ATCC; cat. no. HTB-36) were cultured in
DMEM (cat. no. 10313039) adding 10% FBS (cat. no. 16140071) as well
as 1% penicillin/streptomycin (cat. no. 15140122) (Gibco; Thermo
Fisher Scientific, Inc.) under atmospheric oxygen tension (~21%)
and 5% CO2 at 37°C.
Vector construction and lentiviral
infection
HIF1α-overexpressing lentivirus (GV492-HIF1α) was
constructed based on human HIF1α sequences from the Ensembl
database (www.ensembl.org, Ensembl gene:
ENSG00000100644) and synthesized by Shanghai GeneChem Co., Ltd. A
3rd generation system was used to package the lentivirus. The
vectors (100 nM) and packaging plasmids (vector:packaging
vector:envelope ratio, 10:3:1) were co-transfected into
1×106 293T cells (The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 8 h. The lentivirus-HIF1α and
negative control lentivirus (empty vector) were collected and
filtered through a 0.45 µM filter 3 days after transfection. JEG-3
cells were then seeded in six-well plates at a density of
1×105 cells/well and cultured at 37°C in 5%
CO2. The JEG-3 cells were transduced with lentiviral
vectors at a multiplicity of infection (MOI) of 10 at 37°C for 8 h,
followed by replacement with fresh medium. JEG-3 cells were grown
for 48 h and subsequently treated with puromycin (1 µg/ml) for 48 h
to select stably transduced cells and 0.5 µg/ml puromycin was used
for maintenance. Subsequently, transduction efficiency was
determined using immunofluorescence microscopy, and the expression
of HIF1α was detected using quantitative PCR and western
blotting.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA-iSo PluS (cat. no. 9109; Takara Bio, Inc.) was
used to isolate total RNA from JEG-3 cells and MSC-Ex. cDNA was
produced by cDNA Synthesis SuperMix (cat. no. 11119ES60; Shanghai
Yeasen Biotechnology Co., Ltd.). The reverse transcription
procedure was as follows: 25°C for 5 min; 42°C for 30 min; and 85°C
for 5 min. The cDNA was subjected to qPCR using SYBR Green qPCR Mix
(cat. no. HY-K0501A; MedChemExpress) using the ABI 7500 Real-Time
PCR system (Thermo Fisher Scientific, Inc.). The following
ingredients were used to a total of 20 µl: cDNA (1 µl), SYBR Premix
ex Taq (2X; 10 µl), reverse primer (10 µM; 0.4 µl), forward primer
(10 µΜ; 0.4 µl) and double-distilled water (to 20 µl).
Amplifications were performed following the procedure of a two-step
method (95°C for 30 sec; 1 cycle at 95°C for 10 sec followed by
95°C for 10 sec; 60°C for 30 sec and 40 cycles; and melting curve
stage). Relative HIF1α expression was normalized to GAPDH mRNA
level and calculated via the 2−ΔΔCq method (26). The sequences of primers used were
as follows: GAPDH forward 5′-GGGAGCCAAAAGGGTCAT-3′, and reverse
5′-GAGTCCTTCCACGATACCAA-3′; HIF1α forward,
5′-GGCGCGAACGACAAGAAAAA-3′, and reverse
5′-GGCTGTGTCGACTGAGGAAA-3′.
Western blotting
Proteins were extracted using RIPA lysis buffer
(cat. no. HY-K1001; MedChemExpress) from JEG-3 cells and MSCs-Ex.
The concentration of the isolated protein was quantified via BCA
Protein Assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.).
Isolated proteins (5 µg) were mixed with 5X SDS-PAGE protein
loading buffer (cat. no. 20315ES05; Shanghai Yeasen Biotechnology
Co., Ltd.). Proteins (20 µg) were separated on 12% SDS-acrylamide
gels, followed by transferring onto PVDF membranes, which were
incubated with 5% non-fat milk for 1 h at room temperature, and
with rabbit anti-HIF-1α (1:1,000; cat. no. ab179483; Abcam), rabbit
anti-CD9 (1:1,000; cat. no. ab236630; Abcam), rabbit anti-CD81
(1:1,000; cat. no. ab79559; Abcam), rabbit anti-LAMP-2B (1:1,000;
cat. no. ab18529; Abcam), rabbit anti-TSG101 (1:1,000; cat. no.
ab125011; Abcam) and rabbit anti-GAPDH (1:10,000; cat. no.
10494-1-AP; ProteinTech Group, Inc.) overnight at 4°C.
Subsequently, membranes were incubated with horseradish
peroxidase-conjugated mouse anti-rabbit IgG (1:5,000; cat. no.
BM2006; Boster Biological Technology) for 1 h at room temperature.
Protein bands were determined via ECL western blot detection
reagents (Thermo Fisher Scientific, Inc.). The protein gray value
was calculated using ImageJ (Version 1.5.3; National Institutes of
Health).
Glucose uptake and lactate
production
Transfected JEG-3 cells were seeded in six-well
plates at the concentration of 3×105/well. After
culturing at 37°C for 48 h, the cells were collected and lysed with
Cell and Tissue Lysis Buffer for Glucose Assay (cat. no. S3062;
Beyotime Institute of Biotechnology). The samples were then
centrifuged at 1,200 × g at 4°C for 10 min to obtain the
supernatant of cells. The glucose concentration in the supernatant
was determined using the glucose oxidase method (Amplex Red
Glucose/Glucose Oxidase Assay kit; cat. no. MP 22189; Invitrogen;
Thermo Fisher Scientific, Inc.). An appropriate amount of 1X
reaction buffer was added to dilute 400 mM glucose to produce a
glucose concentration of 0–200 µM to make the standard curve.
Subsequently, 50 µl of each standard, controls and samples were
added to the individual wells of the microplate in duplicate. The
absorbance at OD 590 nm was measured using a microplate reader
(LT-4000; Labtech International Ltd,).
The lactate in the supernatant was measured
according to the instructions of the Lactate Detection kit (cat.
no. K607-100; BioVision; Abcam). A total of 10 µl samples, 90 µl
distilled water, 40 µl reagent 2 and 60 µl chromogen solution were
added to the well and mixed thoroughly. Then the wells were
incubated at 37°C in dark for 30 min followed by measuring the
absorbance at 530 nm.
Cell viability assay using Cell
Counting Kit-8 (CCK-8)
Transfected JEG-3 cells were passed onto the 96-well
plates (1×103 cells/well) and cultured in an incubator
for 24, 48, 72, 96 and 120 h at 37°C. Next, CCK-8 regent (10
µl/well; cat. no. A311-01; Vazyme Biotech Co., Ltd.) was added to
the cells and kept at 37°C for 3 h. Eppendorf
BioPhotometer® D30 (Eppendorf) was used to acquire the
values of absorbance at 450 nm. The cell viability was calculated
from the absorbance values for five consecutive days. The cell
viability percentage was calculated as follows: Cell viability
(%)=(sample absorbance/control absorbance) ×100.
Cell invasion assay
Transwell assay was conducted to measure the changes
in JEG-3 cells invasion after HIF1α lentivirus transfection.
Transwell chambers (cat. no. 3402; Corning Life Sciences) were
pre-coated with Matrigel (cat. no. 356234; Corning Life Sciences)
for 6 h at 37°C. A 150-µl JEG-3 cell suspension in serum-free DMEM
was seeded in the upper layer of the six-well Transwell chamber
(7×103 cells/well). In addition, 600 µl DMEM containing
10% FBS was added to the lower chamber. After 48 h incubation at
37°C, JEG-3 cells on the basolateral chamber were washed twice
using PBS, and stained with 1% crystal violet for 30 min at room
temperature. JEG-3 cells were visualized and images were captured
under the light microscope (magnification, ×200; Olympus cX2;
Olympus Corporation) after washing using PBS twice.
Mesenchymal stem cells (MSCs)
identification
MSCs were obtained from Procell Life Science &
Technology Co., Ltd. (cat. no. CP-H166), which were primary human
MSCs that have not been immortalized. The cells were kept in DMEM
medium adding 10% FBS and 100 U/ml penicillin and streptomycin.
Then the surface markers of the cultured MSCs were identified using
CD90 and CD44 by immunofluorescence assays. MSCs were plated in a
35-mm confocal dish and cultured for 48 h followed by fixing with
4% paraformaldehyde for 20 min at room temperature. After rinsing
with PBS, cells were permeabilized with 0.5% Triton X100 in PBS and
blocked with 3% BSA for 1 h at room temperature. Cells were then
incubated overnight with anti-CD44 (1:50; cat. no. 15675-1-AP;
ProteinTech Group, Inc.), anti-CD90 (1:50; cat. no. 66766-1-Ig;
ProteinTech Group, Inc.), anti-CD34 (1:50; cat. no. 60287-1-Ig;
ProteinTech Group, Inc.) and anti-CD45 (1:50; cat. no. 14486-1-AP;
ProteinTech Group, Inc.) antibodies at 4°C followed by 2 h
incubation with goat anti-mouse IgG (H+L) Cy3-conjugated and goat
anti-rabbit IgG (H+L) FITC-conjugated secondary antibodies (1:100;
cat. no. BA1031 and BA1105, respectively; Boster Biological
Technology). After three TBS-0.05% Tween-20 washing steps, cells
were incubated with DAPI (cat. no. D1306; Thermo Fisher Scientific,
Inc.) for 10 min at 37°C. Finally, cells were observed using
Olympus fluorescence microscope BX53 (Olympus Corporation). The
positive rate was calculated using the following formula: Positive
rate=(number of positive cells/number of total cells) ×100%. The
cell number were analyzed using ImageJ software (1.4; National
Institutes of Health).
Homing peptide, sh-HIF1α peptide
nucleic acid conjugates
The LAMP-2B + 5′-TGTTGTGGTAAACGTAAA-3′ (gene
sequence of CCGKRK) gene was amplified from cDNA template by PCR,
then purified and recovered with a gel recovery kit (Beijing
Solarbio Science & Technology Co., Ltd.). The gene was
subsequently cloned and ligated to the GV493 vector
(hU6-MCS-CBh-MCS-IRES-puromycin; Shanghai GeneChem Co., Ltd.). A
3rd generation system was used to package the lentivirus.
Additionally, an sh-HIF1α sequence (5′-ACGACAAGAAAAAGATAAGTT-3′)
was also ligated into the same GV493 vector using an independent
promoter. Upon transduction into cells, the lentiviral particles
generated in this manner facilitated the overexpression of both
LAMP-2B + CGKRK and HIF1α shRNA. The vectors (100 nM) and packaging
plasmids (vector:packaging vector:envelope ratio, 10:3:1) were
co-transfected into 1×106 293T cells with Lipofectamine
2000 at 37°C for 8 h. The sh-HIF1α-LAMP-2B lentivirus and negative
control (empty vector) were collected and filtered through a 0.45
µM filter after 293T cells were cultured at 37°C for 3 days. MSCs
were then transduced with lentiviral vectors at a MOI of 10 for 8 h
followed by replacement with fresh medium. MSCs were cultured at
37°C for 48 h and subsequently treated with puromycin (1 µg/ml) for
48 h to select stably transduced cells, 0.5 µg/ml puromycin was
used for maintenance.
Isolation of exosomes
MSCs supernatant was collected and centrifugated at
2,000 × g for 30 min at 4°C. Following being filtered through 0.22
µm syringe filter (cat. no. SLGVR13SL; MilliporeSigma), which was
further centrifuged at 120,000 g overnight at 4°C using Optima
XPN-100 high-speed freezing centrifuge (cat. no. CP100MX; Hitachi,
Ltd.). Exosomes were precipitated from the supernatants. The
sediments of exosomes were resuspended by cold PBS, and
ultracentrifuged again at 120,000 × g for 90 min at 4°C. The final
sediments of exosomes were resuspended in cold PBS or SDT lysate
buffer, and immediately stored at −80°C.
Pep-sh-HIF1α and exosomes binding
Exosomes (30 g) were preincubated with
LAMP-2B-CCGKRK-sh-HIF1α peptide (30 g) overnight at 4°C, followed
by washing with PBS five times in 2-ml ultracentrifuge tubes and
filtration with 100-kDa diafiltration tube (MilliporeSigma) to
remove unbound peptides. Subsequently, peptide-exosome complexes
(30 g) were incubated with 4-mm aldehyde/sulfate latex beads
(Invitrogen; Thermo Fisher Scientific, Inc.) for 15 min at room
temperature under rotation followed by washing with PBS for three
times. Then recovered beads were subjected to various tests.
Transmission electron microscopy
Purified exosomes supernatant was resuspended PBS.
The exosome pellets were fixed with 2.5% glutaraldehyde for 1 h at
room temperature, and post-fixed with 1% osmium for 1 h at room
temperature. A total of 20 µl exosomes suspensions were added onto
copper grid carefully, blotted up and stained with 2%
phosphotungstic acid (PTA) for 2 min at room temperature. Sample
was imaged using a transmission electron microscope (cat. no.
HT-7700; Hitachi, Ltd.).
Nanoparticle tracking analysis
Isolated exosome was diluted to 1 ml in TBS with
0.1% Pluronic F-68 and 2 mmol/l EDTA for the next analysis. Size of
exosomes was determined using Nanosight Tracking Analysis by
utilizing nanoparticle tracking analysis (NTA; N30E; NanoFCM, Inc.)
referring to the documented protocol (17).
Flow cytometry
To characterize individual exosomes by flow
cytometry, exosomes were labeled with 1,1′-dioctadecyltetramethyl
indotricarbocyanine iodide (cat. no. DL22065, Duolaimi
Biotechnology Co., Ltd.), which labels the lipid in exosomes, as
per the manufacturer's instruction and subjected to flow cytometry.
To measure the percentage of pep-positive exosomes in total
exosomes, DiR-labeled exosomes (5 g) were incubated with homing
peptide (CCGKRK)-labeled anti-mouse antibody (1:20) generated in
collaboration with NovoPro Bioscience, Inc. in 4% BSA for 30 min at
4°C, followed by 1:10 dilution with PBS, and were analyzed with
flow cytometry (FACSCalibur; BD Biosciences). Uncoated beads were
used as negative controls for gating.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism software (8.0; GraphPad Software, Inc.). All experiments were
repeated thrice and data in the present study are presented as with
mean ± standard deviation. Kolmogorov-Smirnov testing for normality
of distribution was used to determine if variables were parametric
or non-parametric. Unpaired Student's t-test was used for two group
parametric comparisons, and one-way ANOVA followed by Tukey's post
hoc test was used for multiple parametric comparisons. Mann-Whitney
U testing was used for nonparametric variables. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression level of HIF1α in
trophoblast cells
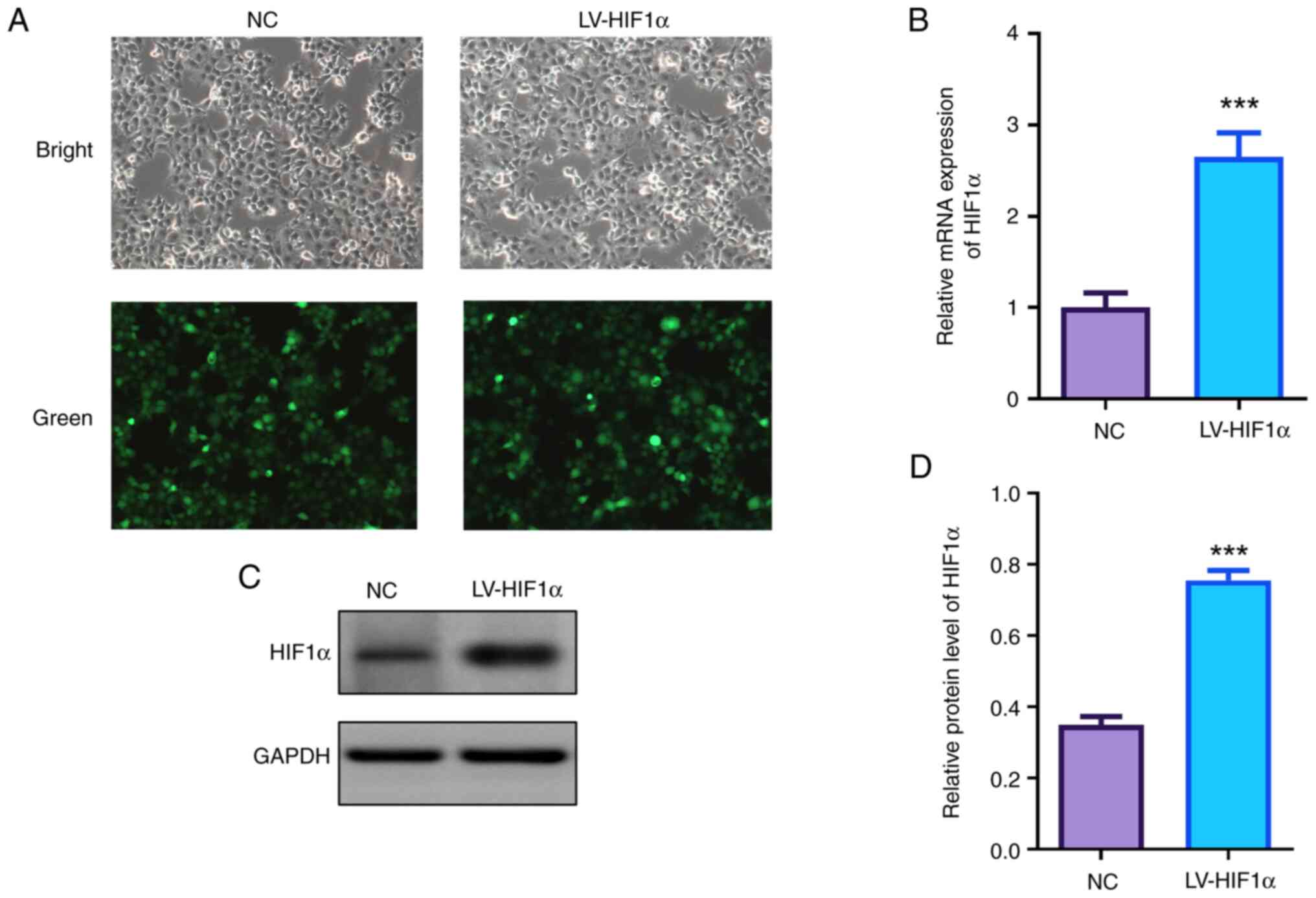
HIF1α was overexpressed in JEG-3 cells via
lentiviral transfection. Fluorescence microscopy confirmed high
efficiencies of lentivirus transfection with a large proportion of
GFP-positive cells (Fig. 1A).
Analysis of both mRNA and protein levels revealed a significant
increase of HIF1α in the transfected cells when compared with the
control group detected by RT-qPCR and western blotting (Fig. 1B-D).
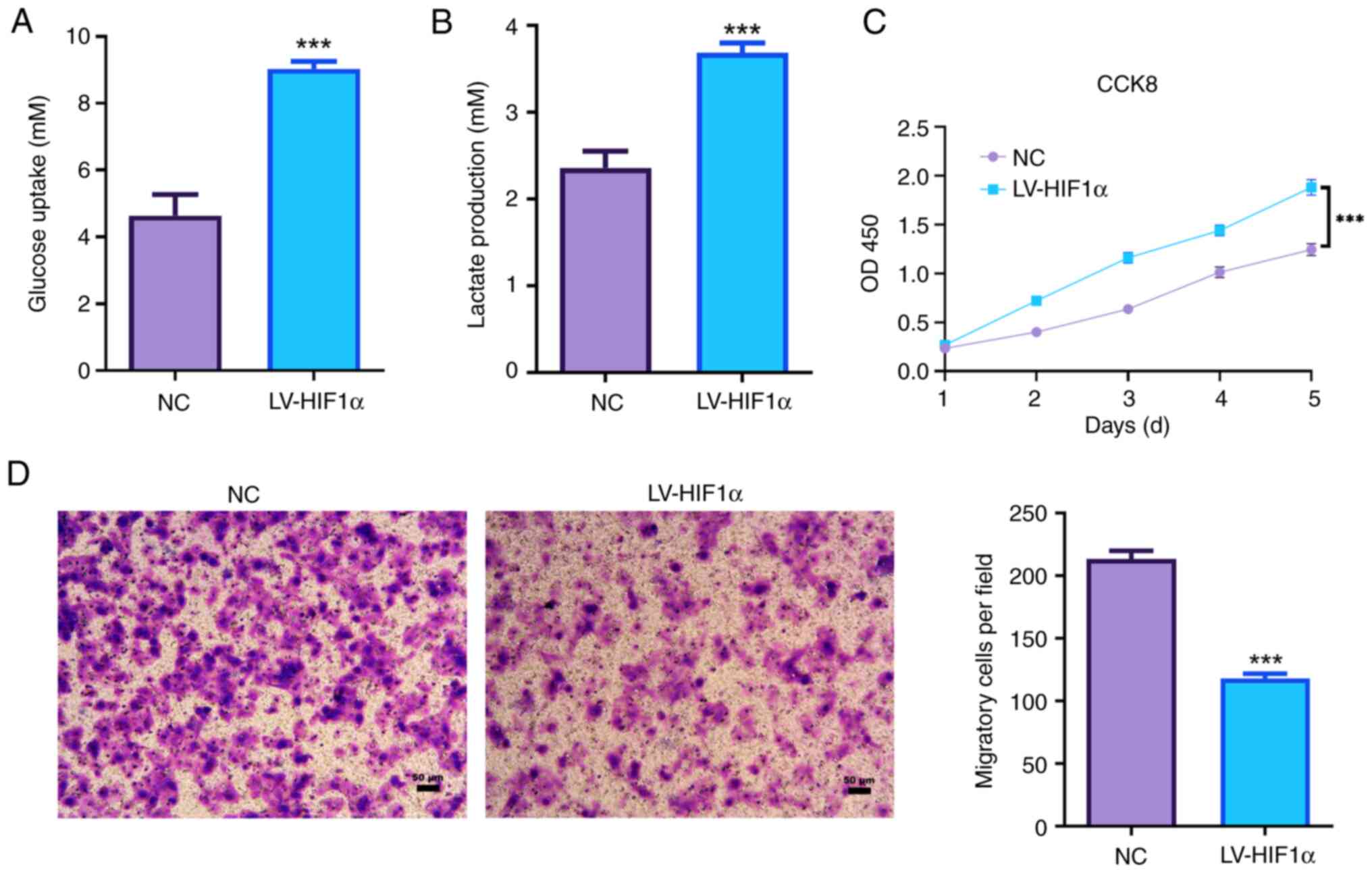
HIF1α promotes aerobic glycolysis and
decreased the invasion capacity of trophoblast cells
Elevated HIF1α in JEG-3 cells led to a significant
increase in glucose uptake compared with the NC group (Fig. 2A). Additionally, there was a
significant elevation in lactate production in HIF1α-overexpressed
JEG-3 cells (Fig. 2B).
Overexpression of HIF1α significantly enhanced the proliferation
ability of JEG-3 cells (Fig. 2C).
Moreover, increased expression of HIF1α significantly suppressed
the invasion ability of JEG-3 cells via Transwell invasion assays
(Fig. 2D). These observations
suggested that HIF1α was associated with the invasion and aerobic
glycolysis of JEG-3 cells.
Observation and identification of
human MSCs
CD44 and CD90 are cell surface markers that are
expressed by human MSCs (27). The
present study demonstrated that the MSCs bought from Procell Life
Science & Technology Co., Ltd. were spindle-shaped
(fibroblast-like cells), which is typical morphology of MSCs.
Results of immunofluorescence assay indicated that both
CD90-positive and CD44-positive rates of MSCs were ~100%, while
lacking CD34 and CD45 human leukocyte markers (Fig. 3A). The results revealed that the
purity of the isolated MSCs was high enough for the subsequent
experiments.
Characterization of isolated
exosomes
The exosomes harvested from the supernatant of MSCs
were characterized using electron microscopy, which demonstrated a
presence of small membrane-bound vesicles (Fig. 4A). This observation is consistent
with the typical morphology of exosomes (28). The majority of the population was
in the size range of 50–130 nm in diameter (Fig. 4A), which is consistent with
previously reported features of exosomes (29). Western blotting was applied to
determine the level of the typical exosomal markers CD9 antigen
(CD9), CD81 antigen (CD81), exosomal protein LAMP-2B and tumor
susceptibility gene 101 (TSG101) (30). As expected, the exosomal markers,
including CD63, CD81, LAMP-2B and TSG101, were enriched in exo-NC
and exo-pep-sh-HIF1α compared with those in cells (Fig. 4B). Approximately 69.94% of exosomes
were labeled by DiR, and ~87.56% of DiR-labeled exosomes were
modified with homing peptide (CCGKRK) demonstrated by flow
cytometry exosomes (Fig. 4C).
These findings support the conclusion that the homing peptide
successfully binds to exosomes LAMP-2B.
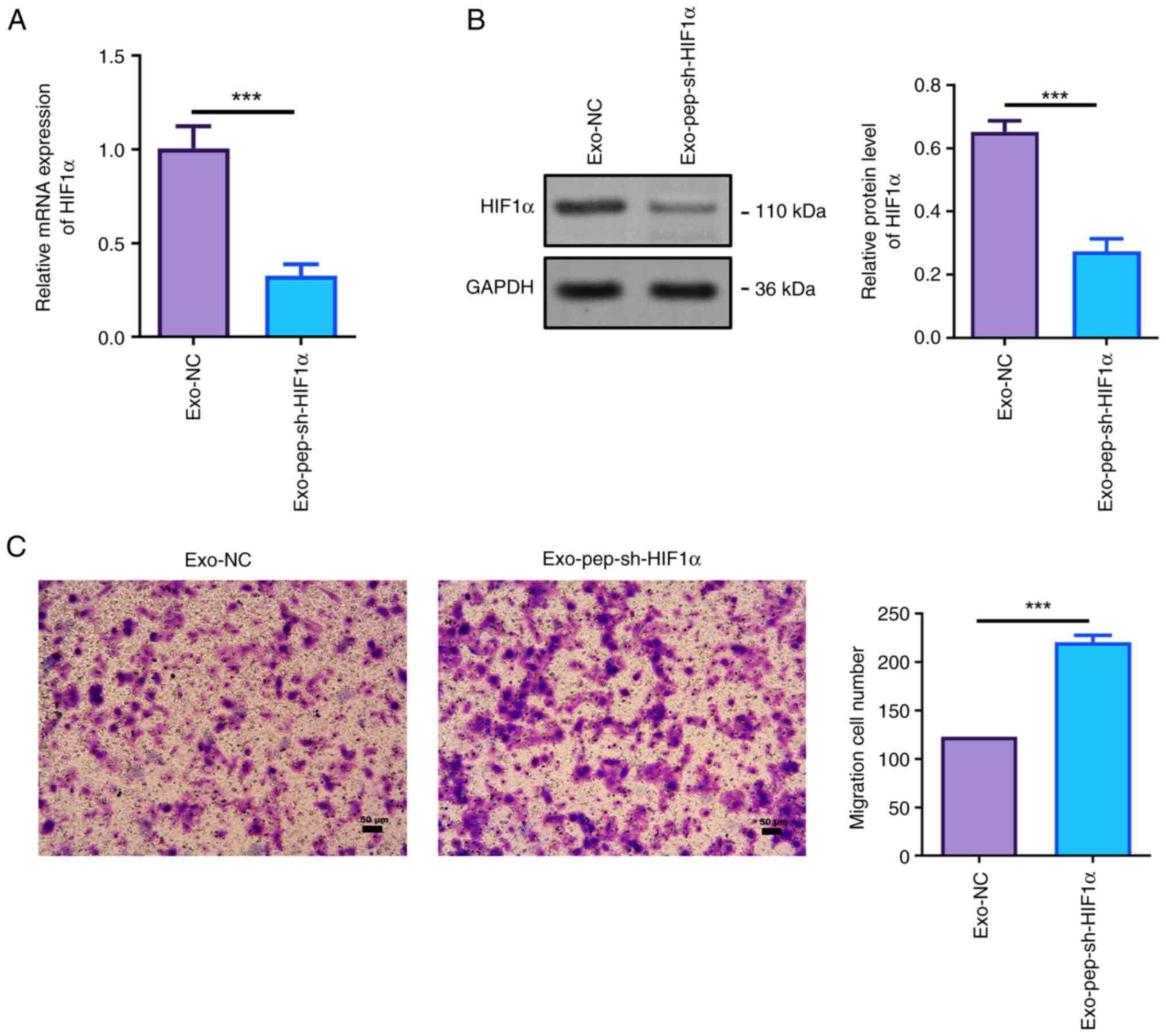
HIF1α knockdown of MSCs-derived
exosomes increases the invasion capacity of trophoblast cells
Next, HIF1α levels in MSC-Ex-treated JEG-3 cells
were tested. The RT-qPCR assay revealed that the HIF1α level in
MSC-Ex-treated JEG-3 cells was significantly decreased compared
with the exo-NC group (Fig. 5A).
In addition, HIF1α protein level in HIF1α-silenced MSC-Ex-treated
JEG-3 cells was also significantly suppressed (Fig. 5B). Knockdown of HIF1α significantly
enhanced the invasion ability of trophoblast JEG-3 cells (Fig. 5C), which plays an important role in
embryo implantation.
Discussion
PE is often linked to reduced invasiveness of fetal
trophoblast cells, leading to inadequate uterine placental
perfusion (31). Sustained hypoxia
leads trophoblasts to fail to differentiate from a proliferative to
an invasive phenotype after 9 weeks of gestation, resulting in
failed implantation (23,32). The current study conjugated an
anchor peptide specific CCG ligand (CCGKRK) to exosomal membrane
protein LAMP-2B to target HIF1α deletion exosomes to the placenta,
which significantly enhanced the invasion ability of trophoblast.
The results unveil new potential therapeutic targets for the
prevention and treatment of PE.
At present, there are no effective treatments for
PE. The few drugs licensed for pregnancy disorders often lead to
serious systemic toxicity as their low molecular weights cross the
placenta from mother to fetus (33,34).
Thus, developing targeted therapies will be a highly effective
therapeutic strategy for PE. HIF1α, a key regulator of
intracellular oxygen metabolism, plays an important role in the
development of the tumor (35).
HIF1α is significantly upregulated to maintain trophoblasts in a
proliferative, non-invasive and immature phenotype, which share
some similarities with tumor cells in the biological processes,
during the initial phase of embryonic development (23,36).
After that, oxygen levels in the intervillous spaces increase from
2% O2 before 9 weeks to 8% O2 at 10 to 12
weeks of pregnancy (37).
Correspondingly, HIF1α are rapidly reduced after 9 weeks of
gestation. However, preeclamptic women have significantly higher
oxidative stress and serum HIF1α levels compared with normal
pregnant women, which leads the trophoblasts development to remain
arrested at an immature stage, causing inadequate trophoblast
invasion (38,39). HIF1α-overexpressed pregnant mice
have significantly elevated blood pressure, decreased placental
weights and histopathological placental abnormalities (40). Serum HIF1α levels have already been
used to predict PE (23). In the
present study, upregulated HIF1α was confirmed to significantly
promote the proliferation while suppressing the invasion of
trophoblast cells. This suggested that HIF1α deletion might be a
potential therapeutic possibility for PE.
Moreover, PE is implicated in abnormal glucose and
lipid metabolism (41–43). Gestational diabetes mellitus is one
of the most common and important complications of pregnancy
(44). Chronic fetal hypoxia
causes deficits in oxidative glucose metabolism and enhancement of
glycolytic metabolism, which causes energy production via glucose
oxidation to be replaced by glycolysis (43). This abnormal aerobic glycolysis is
also called metabolic reprogramming, a phenomenon known as the
Warburg Effect, which is a hallmark of cancer, such as breast
cancer, gastric cancer and colorectal cancer (45–47).
However, a potential role for aerobic glycolysis in PE has not been
elucidated. HIF1α facilitates glucose transporters and glycolytic
enzymes expression that promote glucose uptake and glycolysis in
Th17 cells (48). Thus, elevated
levels of HIF1α in the hypoxic microenvironments at 10 to 12 weeks
of gestation might mean trophoblasts fail to switch to an invasive
phenotype by promoting aerobic glycolysis. The present study
revealed that HIF1α overexpression promoted the uptake of glucose
and the production of lactate in trophoblast cells. However, HIF-1α
overexpression led to increased proliferation of trophoblast cells.
The enhanced glucose and lactate production did not definitively
exclude the possibility of cell proliferation-induced promotion.
Thus, the enhanced glucose and lactate production resulted from
increasing cell number or upregulation of HIF-1α overexpression
needs further investigation.
Disrupting the expression of disease-causing genes
by gene editing is already a well-established technique (49). However, the method to achieving
efficient intervention of HIF1α expression in the placenta needs to
be investigated. In recent years, several research reports have
focused on delivering therapeutic drugs specifically to the
placenta via nanoparticles, which exert high chemical stability,
high drug loading capacity and low toxicity (50,51).
Notably, extensive studies have confirmed the positive effects of
MSCs on PE (11,52–54).
In various PE rat models, MSC transplantation improves PE symptoms
and inhibits inflammatory cytokines such as TNF-α and IL-6,
providing evidence that human umbilical cord-derived MSCs may be a
possible therapy for preeclampsia (53,55,56).
MSCs exert their therapeutic functions via secretion of bioactive
products, namely the exosomes, which have a high biocompatibility,
low toxicity and low immunogenicity that makes them ideal drug
delivery carriers of anti-PE drugs (57). The present study succeeded in
conjugating CCG ligand (CCGKRK) to the exosomal membrane protein
LAMP-2B, which led MSCs-derived exosomes to target the placenta. In
the present study, MSC exosomes were used as a biocompatible drug
carrier to target HIF1α siRNA to the placenta. To the best of our
knowledge, this is the first attempt to make exosomes target
placental tissue by genetic engineering. Notably, silencing HIF1α
encouraged an aggressive phenotype that facilitated trophoblasts
invasion, suggesting that HIF1α deletion is a potent way to
interfere with the progression of PE. Clinically, some factors can
be used to predict PE (58). If it
poses a high risk of PE, some measures can be taken to intervene PE
in advance, such as enhancing the invasion ability of trophoblasts
by carrying sh-HIF1α to placenta by exosomes before PE is
diagnosed.
There were some pitfalls and drawbacks in present
study. Firstly, the experiments in vitro were only conducted
in JEG3 cells, a commonly used choriocarcinoma cell line that
serves as a model of villous trophoblast cells, whereas
immortalized human chorionic trophoblast cells such as HTR-8 cells
are considered to be an improved cell model to study trophoblast
function. Therefore, further studies should be conducted using
HTR-8 cells to confirm the findings presented in this study.
Additionally, due to limited experimental conditions, the
feasibility and effectiveness of targeted delivery of HIF1α
deletion exosomes to the placenta by conjugating CCG ligand
(CCGKRK) to LAMP-2B in animal models or human tissue samples has
not yet been confirmed. In the present study, knockdown of HIF1α
was proposed as a potential treatment to increase trophoblast
invasion. However, it is essential to consider the potential
effects of HIF1α knockdown on early pregnancy processes, such as
implantation and placental development. Therefore, targeted and
precise control of HIF1α knockdown is necessary to avoid any
adverse effects on these critical events. Further research is
required to elucidate the underlying mechanisms and optimize the
therapeutic approach to ensure the safety and efficacy of HIF
knockdown in the context of pregnancy complications.
In summary, the present study facilitated the
targeted delivery of HIF1α deletion exosomes to the placenta by
conjugating CCG ligand (CCGKRK) to LAMP-2B, and provided a novel
platform for the development of placenta-specific therapeutics.
Silencing HIF1α was confirmed to be an effective therapeutic target
for PE by promoting trophoblast invasion.
Acknowledgements
Not applicable.
Funding
This study was supported by Hainan Provincial Natural Science
Foundation of China (no. 821MS128 and 822MS164) and National
Natural Science Fund Cultivating 530 Project of Hainan General
Hospital (grant no. 2021MSXM04). Scientific research project of
health industry in Hainan Province (grant no. 22A200234).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FC and HC conceived and designed this study. FC and
DM carried out the analyses and also participated in the study
design. HC wrote the manuscript. RF participated in the analysis of
data and helped edit the manuscript. FC and HC confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The medical ethics committee of Hainan General
Hospital waives the requirement for authors to obtain ethical
approval for the use of commercially available cells.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Broumand F, Lak SS, Nemati F and Mazidi A:
A study of the diagnostic value of Inhibin A Tests for occurrence
of preeclampsia in pregnant women. Electronic Physician.
10:6186–6192. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duley L: The global impact of
pre-eclampsia and eclampsia. Semin Perinatol. 33:130–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poon LC, Shennan A, Hyett JA, Kapur A,
Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P,
McIntyre HD, et al: The international federation of gynecology and
obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide
for first-trimester screening and prevention. Int J Gynaecol
Obstet. 145 (Suppl 1):S1–S33. 2019. View Article : Google Scholar
|
|
4
|
Rahma H, Indrawan IWA, Nooryanto M,
Rahajeng and Keman K: Effect of a black cumin (Nigella
sativa) ethanol extract on placental angiotensin II type
1-receptor autoantibody (AT1-AA) serum levels and endothelin-1
(ET-1) expression in a preeclampsia mouse model. J Taibah Univ Med
Sci. 12:528–533. 2017.PubMed/NCBI
|
|
5
|
Belay Tolu L, Yigezu E, Urgie T and
Feyissa GT: Maternal and perinatal outcome of preeclampsia without
severe feature among pregnant women managed at a tertiary referral
hospital in urban Ethiopia. PLoS One. 15:e02306382020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller EC, Wilczek A, Bello NA, Tom S,
Wapner R and Suh Y: Pregnancy, preeclampsia and maternal aging:
From epidemiology to functional genomics. Ageing Res Rev.
73:1015352022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spradley FT, Palei AC and Granger JP:
Immune mechanisms linking obesity and preeclampsia. Biomolecules.
5:3142–3176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Song Y, Liu F, Liu D, Miao H, Ren J,
Xu J, Ding L, Hu Y, Wang Z, et al: Long Non-coding RNA MALAT1
promotes proliferation, angiogenesis, and immunosuppressive
properties of mesenchymal stem cells by inducing VEGF and IDO. J
Cell Biochem. 118:2780–2791. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh S, Jang AY, Chae S, Choi S, Moon J, Kim
M, Spiekerkoetter E, Zamanian RT, Yang PC, Hwang D, et al:
Comparative analysis on the anti-inflammatory/immune effect of
mesenchymal stem cell therapy for the treatment of pulmonary
arterial hypertension. Sci Rep. 11:20122021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burlacu A, Grigorescu G, Rosca AM, Preda
MB and Simionescu M: Factors secreted by mesenchymal stem cells and
endothelial progenitor cells have complementary effects on
angiogenesis in vitro. Stem Cells Dev. 22:643–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang D, Na Q, Song GY and Wang L: Human
umbilical cord mesenchymal stem cell-derived exosome-mediated
transfer of microRNA-133b boosts trophoblast cell proliferation,
migration and invasion in preeclampsia by restricting SGK1. Cell
Cycle. 19:1869–1883. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi JH, Jung J, Na KH, Cho KJ, Yoon TK
and Kim GJ: Effect of mesenchymal stem cells and extracts derived
from the placenta on trophoblast invasion and immune responses.
Stem Cells Dev. 23:132–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosseini R, Asef-Kabiri L, Yousefi H,
Sarvnaz H, Salehi M, Akbari ME and Eskandari N: The roles of
tumor-derived exosomes in altered differentiation, maturation and
function of dendritic cells. Mol Cancer. 20:832021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen
L, Wang M, Zhou Y, Zhu W, Li W and Xu W: Exosomes derived from
human umbilical cord mesenchymal stem cells alleviate liver
fibrosis. Stem Cells Dev. 22:845–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bjorge IM, Kim SY, Mano JF, Kalionis B and
Chrzanowski W: Extracellular vesicles, exosomes and shedding
vesicles in regenerative medicine-a new paradigm for tissue repair.
Biomater Sci. 6:60–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gatti S, Bruno S, Deregibus MC, Sordi A,
Cantaluppi V, Tetta C and Camussi G: Microvesicles derived from
human adult mesenchymal stem cells protect against
ischaemia-reperfusion-induced acute and chronic kidney injury.
Nephrol Dial Transplant. 26:1474–1483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Q, Yao J, Wu X, Li J, Li G, Tang W, Liu
J and Wan M: Emodin attenuates severe acute pancreatitis-associated
acute lung injury by suppressing pancreatic exosome-mediated
alveolar macrophage activation. Acta Pharm Sin B. 12:3986–4003.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kojima R, Bojar D, Rizzi G, Hamri GC,
El-Baba MD, Saxena P, Ausländer S, Tan KR and Fussenegger M:
Designer exosomes produced by implanted cells intracerebrally
deliver therapeutic cargo for Parkinson's disease treatment. Nat
Commun. 9:13052018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai J, Duan J, Liu R, Du Y, Luo Q, Cui Y,
Su Z, Xu J, Xie Y and Lu W: Engineered targeting tLyp-1 exosomes as
gene therapy vectors for efficient delivery of siRNA into lung
cancer cells. Asian J Pharm Sci. 15:461–471. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu C, Chen X, Huang Y and Chen Y:
Co-administration of iRGD with peptide HPRP-A1 to improve
anticancer activity and membrane penetrability. Sci Rep.
8:22742018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beards F, Jones LE, Charnock J, Forbes K
and Harris LK: Placental homing Peptide-microRNA inhibitor
conjugates for targeted enhancement of intrinsic placental growth
signaling. Theranostics. 7:2940–2955. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burton GJ, Hempstock J and Jauniaux E:
Nutrition of the human fetus during the first trimester-a review.
Placenta. 22 (Suppl A):S70–S77. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tianthong W and Phupong V: Serum
hypoxia-inducible factor-1alpha and uterine artery Doppler
ultrasound during the first trimester for prediction of
preeclampsia. Sci Rep. 11:66742021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao H, Wong RJ and Stevenson DK: The
impact of hypoxia in early pregnancy on placental cells. Int J Mol
Sci. 22:96752021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mol BWJ, Roberts CT, Thangaratinam S,
Magee LA, de Groot CJM and Hofmeyr GJ: Pre-eclampsia. Lancet.
387:999–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lan T, Luo M and Wei X: Mesenchymal
stem/stromal cells in cancer therapy. J Hematol Oncol. 14:1952021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma L, Wei J, Zeng Y, Liu J, Xiao E and
Kang Y and Kang Y: Mesenchymal stem cell-originated exosomal
circDIDO1 suppresses hepatic stellate cell activation by
miR-141-3p/PTEN/AKT pathway in human liver fibrosis. Drug Deliv.
29:440–453. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang C, Tang S, Shen D, Li X, Liang L,
Ding Y and Xu B: Circulating plasma exosomal miRNA profiles serve
as potential metastasis-related biomarkers for hepatocellular
carcinoma. Oncol Lett. 21:1682021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu YM, Tseng CH, Chen YC, Yu WY, Ho MY,
Ho CY, Lai MMC and Su WC: Exosome-delivered and Y RNA-derived small
RNA suppresses influenza virus replication. J Biomed Sci.
26:582019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang P, Dai A, Alexenko AP, Liu Y,
Stephens AJ, Schulz LC, Schust DJ, Roberts RM and Ezashi T:
Abnormal oxidative stress responses in fibroblasts from
preeclampsia infants. PLoS One. 9:e1031102014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsumoto L, Hirota Y, Saito-Fujita T,
Takeda N, Tanaka T, Hiraoka T, Akaeda S, Fujita H, Shimizu-Hirota
R, Igaue S, et al: HIF2α in the uterine stroma permits embryo
invasion and luminal epithelium detachment. J Clin Invest.
128:3186–3197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim MK, Lee SM, Oh JW, Kim SY, Jeong HG,
Kim SM, Park CW, Jun JK, Hahn SK and Park JS: Efficacy and side
effect of ritodrine and magnesium sulfate in threatened preterm
labor. Obstet Gynecol Sci. 61:63–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Driul L, Londero AP, Adorati-Menegato A,
Vogrig E, Bertozzi S, Fachechi G, Forzano L, Cacciaguerra G, Perin
E, Miceli A and Marchesoni D: Therapy side-effects and predictive
factors for preterm delivery in patients undergoing tocolysis with
atosiban or ritodrine for threatened preterm labour. J Obstet
Gynaecol. 34:684–689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mei T, Wang Z, Wu J, Liu X, Tao W, Wang S
and Chen F: Expression of GLUT3 and HIF-1α in meningiomas of
various grades correlated with peritumoral brain edema. Biomed Res
Int. 2020:16823522020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Wu Y and Liu H: Expression and role
of miR-338-3p in peripheral blood and placenta of patients with
pregnancy-induced hypertension. Exp Ther Med. 20:418–426. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jauniaux E, Watson A and Burton G:
Evaluation of respiratory gases and acid-base gradients in human
fetal fluids and uteroplacental tissue between 7 and 16 weeks'
gestation. Am J Obstet Gynecol. 184:998–1003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iriyama T, Wang W, Parchim NF, Song A,
Blackwell SC, Sibai BM, Kellems RE and Xia Y: Hypoxia-independent
upregulation of placental hypoxia inducible factor-1α gene
expression contributes to the pathogenesis of preeclampsia.
Hypertension. 65:1307–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ali LE, Salih MM, Elhassan EM, Mohmmed AA
and Adam I: Placental growth factor, vascular endothelial growth
factor, and hypoxia-inducible factor-1α in the placentas of women
with pre-eclampsia. J Matern Fetal Neonatal Med. 32:2628–2632.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tal R, Shaish A, Barshack I, Polak-Charcon
S, Afek A, Volkov A, Feldman B, Avivi C and Harats D: Effects of
hypoxia-inducible factor-1alpha overexpression in pregnant mice:
Possible implications for preeclampsia and intrauterine growth
restriction. Am J Pathol. 177:2950–2962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ueki N, Takeda S, Koya D and Kanasaki K:
The relevance of the Renin-Angiotensin system in the development of
drugs to combat preeclampsia. Int J Endocrinol. 2015:5727132015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu M, Li J, Baker PN and Tong C:
Revisiting preeclampsia: A metabolic disorder of the placenta. FEBS
J. 289:336–354. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Illsley NP, Caniggia I and Zamudio S:
Placental metabolic reprogramming: Do changes in the mix of
energy-generating substrates modulate fetal growth? Int J Dev Biol.
54:409–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bosdou JK, Anagnostis P, Goulis DG, Lainas
GT, Tarlatzis BC, Grimbizis GF and Kolibianakis EM: Risk of
gestational diabetes mellitus in women achieving singleton
pregnancy spontaneously or after ART: A systematic review and
meta-analysis. Hum Reprod Update. 26:514–544. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhong D, Li Y, Huang Y, Hong X, Li J and
Jin R: Molecular mechanisms of exercise on cancer: A bibliometrics
study and visualization analysis via CiteSpace. Front Mol Biosci.
8:7979022021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu Y, Lu J, Tang Y, Xie W, Zhang H, Wang
B, Zhang S, Hou W, Zou C, Jiang P and Zhang W: PINK1 deficiency in
gastric cancer compromises mitophagy, promotes the Warburg effect,
and facilitates M2 polarization of macrophages. Cancer Lett.
529:19–36. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jing Z, Liu Q, He X, Jia Z, Xu Z, Yang B
and Liu P: NCAPD3 enhances Warburg effect through c-myc and E2F1
and promotes the occurrence and progression of colorectal cancer. J
Exp Clin Cancer Res. 41:1982022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Q, Wang L, Jiang J, Lin S, Luo A,
Zhao P, Tan W and Zhang M: Critical role of AdipoR1 in regulating
Th17 cell differentiation through modulation of HIF-1α-dependent
glycolysis. Front Immunol. 11:20402020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun J, Carlson-Stevermer J, Das U, Shen M,
Delenclos M, Snead AM, Koo SY, Wang L, Qiao D, Loi J, et al:
CRISPR/Cas9 editing of APP C-terminus attenuates β-cleavage and
promotes α-cleavage. Nat Commun. 10:532019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang B, Tan L, Yu Y, Wang B, Chen Z, Han
J, Li M, Chen J, Xiao T, Ambati BK, et al: Placenta-specific drug
delivery by trophoblast-targeted nanoparticles in mice.
Theranostics. 8:2765–2781. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li L, Yang H, Chen P, Xin T, Zhou Q, Wei
D, Zhang Y and Wang S: Trophoblast-targeted nanomedicine modulates
placental sFLT1 for preeclampsia treatment. Front Bioeng
Biotechnol. 8:642020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chu Y, Chen W, Peng W, Liu Y, Xu L, Zuo J,
Zhou J, Zhang Y, Zhang N, Li J, et al: Amnion-derived mesenchymal
stem cell exosomes-mediated autophagy promotes the survival of
trophoblasts under hypoxia through mTOR pathway by the
downregulation of EZH2. Front Cell Dev Biol. 8:5458522020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang D, Fu L, Wang L, Lin L, Yu L, Zhang
L and Shang T: Therapeutic benefit of mesenchymal stem cells in
pregnant rats with angiotensin receptor agonistic
autoantibody-induced hypertension: Implications for
immunomodulation and cytoprotection. Hypertens Pregnancy.
36:247–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Qu HM, Qu LP, Pan XZ and Mu LS:
Upregulated miR-222 targets BCL2L11 and promotes apoptosis of
mesenchymal stem cells in preeclampsia patients in response to
severe hypoxia. Int J Clin Exp Pathol. 11:110–119. 2018.PubMed/NCBI
|
|
55
|
Wang LL, Yu Y, Guan HB and Qiao C: Effect
of human umbilical cord mesenchymal stem cell transplantation in a
rat model of preeclampsia. Reprod Sci. 23:1058–1070. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fu L, Liu Y, Zhang D, Xie J, Guan H and
Shang T: Beneficial effect of human umbilical cord-derived
mesenchymal stem cells on an endotoxin-induced rat model of
preeclampsia. Exp Ther Med. 10:1851–1856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xiong ZH, Wei J, Lu MQ, Jin MY and Geng
HL: Protective effect of human umbilical cord mesenchymal stem cell
exosomes on preserving the morphology and angiogenesis of placenta
in rats with preeclampsia. Biomed Pharmacother. 105:1240–1247.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stepan H, Galindo A, Hund M, Schlembach D,
Sillman J, Surbek D and Vatish M: Clinical utility of sFlt-1 and
PlGF in screening, prediction, diagnosis and monitoring of
pre-eclampsia and fetal growth restriction. Ultrasound Obstet
Gynecol. 61:168–180. 2023. View Article : Google Scholar : PubMed/NCBI
|