Introduction
Skin damage due to ultraviolet (UV) radiation, known
as photoaging, and due to over time, known as intrinsic aging, are
considered distinct processes. Photoaged skin has various
appearances, such as wrinkles, mottled pigmentation and
telangiectasia. Intrinsic skin aging causes thinning of the
epidermis and fine wrinkles. These processes occur naturally over
time, and they depend on the accumulation of inflammatory
mediators, such as free radicals (1). Intrinsic aging is also caused by
extracellular matrix (ECM) destruction during dermal fibroblast
aging. ECM-like collagen fibers and elastins are produced and
maintained by dermal fibroblasts (2). Therefore, the impairment of
fibroblast function affects the mechanical properties of skin
connective tissue. In addition, inflammatory mediators, including
cytokines, can induce skin aging (3). For example, UVB exposure induces the
production of tumor necrosis factor (TNF)-α (4). TNF-α activates matrix
metalloproteinases (MMPs), increasing inflammation and degradation
of several types of collagen (5–7).
Thus, the regulation of TNFα activity may be a novel target for
treating aged skin.
The regulation of cell proliferation and
differentiation in multicellular organisms involves complex
processes primarily regulated by external growth factors provided
by the microenvironment of proliferating cells. The
mitogen-activated protein kinase (MAPK) pathway regulates the cell
cycle by influencing cell death and survival processes (8). Among various MAPKs, extracellular
signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK) and
p38 are associated with skin aging. The ERK pathway mainly mediates
cellular responses to growth factors, such as epidermal growth
factor and phorbol ester. On the other hand, the JNK and p38
pathways mediate cellular responses to cytokines, such as TNF and
interleukin (IL)-1, and physical stressors, such as UV radiation or
osmotic shock (9,10). ERK activity is reduced, while JNK
activity is increased in aged human skin compared with young skin
in vivo (11). The MAPK
pathway plays a role in modulating cell growth and procollagen
synthesis (12), and it is also
involved in MMP-1-regulated signaling pathways (13).
Polydeoxyribonucleotide (PDRN), a mixture of
deoxyribonucleotides derived from salmon sperm, is commonly used
for dermatological purposes, such as treating chronic wounds and
diabetic foot ulcers (14,15). PDRN activates dermal fibroblasts
and enhances the synthesis of vascular endothelial growth factor.
These biological mechanisms of PDRN suggest that it could exert a
major influence on ameliorating skin cell aging (16–19).
Multiple studies have reported the beneficial effects of PDRN on
skin health (14–19). However, the detailed molecular
mechanisms remain poorly understood. Previously, keratinocytes or
fibroblasts were studied alone or in co-culture in skin aging
studies (14,16,18).
In these studies, no notable differences in experimental PDRN
effects were elucidated between the aforementioned types of skin
cells. In the current study, it was confirmed that PDRN did not
differentially affect the cell viability of fibroblasts and
keratinocytes. However, the effective PDRN concentration and
intracellular signaling transduction differed between epidermal
keratinocytes and dermal fibroblasts. The aim was to elucidate the
molecular mechanism by which PDRN promoted skin healing by
confirming the effect of PDRN treatment on epidermal keratinocytes
and dermal fibroblasts, and by assessing collagen and inflammatory
cytokine levels, including those of TNF-α, IL-1β, IL-6, monocyte
chemotactic protein (MCP)1, inducible nitric oxide synthase (iNOS),
regulated by ERK signaling.
Materials and methods
Cell culture
The present study was approved by the Institutional
Review Board (IRB) of Hallym University Sacred Heart Hospital (IRB
no. HALLYM 2019-07-029; Republic of Korea). Human epidermal
keratinocytes (HEKs) were isolated from foreskins of patients
through circumcisions (20).
Epidermal layers were separated from dermis via enzymatic
digestion. Tissues were incubated with 25 U/ml dispase in Hank's
Balanced Salt Solution for 20 h at 4°C. The following day, the
epidermis was separated from whole tissue, and keratinocytes were
dissociated from the epidermal layer by digesting the layer with
0.05% trypsin-ethylenediaminetetraacetic acid (EDTA) solution for
15 min. Cells were cultured in 0.07 mM Ca2+ 154CF medium
(Thermo Fisher Scientific, Inc.) containing human keratinocyte
growth supplement (Thermo Fisher Scientific, Inc). All cell types
were incubated in a humidified 5% CO2 incubator at 37°C.
Primary cells at passages 2–8 were used, with enzymatic
dissociation using trypsin-EDTA for every passage.
Human dermal fibroblasts (HDFs) were prepared as
previously described (21,22). Briefly, human dermal tissue samples
were sectioned to a thickness of 4 mm. Within the first week,
keratinocytes migrated out of the biopsy tissue. Fibroblasts
appeared 7–10 days after the first outer growth of keratinocytes.
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc.), high glucose media supplemented with 20% fetal bovine serum
(FBS; Cytiva) and antibiotics (100 U/ml penicillin and 100 µg/ml
streptomycin; Thermo Fisher Scientific, Inc.) favored the growth of
fibroblasts over keratinocytes. Homogenous fibroblast cultures were
obtained via keratinocyte dilution after two passages. HDFs were
maintained in DMEM supplemented with 10% FBS.
Cell proliferation and cytotoxicity
assay
HDFs (1×103 cells/well) and HEKs
(5×103 cells/well) were seeded in 96-well culture plates
(SPL Life Sciences). After incubation for 24 h, cells were
serum-starved for 16 h and treated with PDRN
(Placentex®; Mastelli srl Officina Bio-Farmaceutica) at
different concentrations (0, 1, 5, 10, 50 and 100 µg/ml) for an
additional 24, 48, and 72 h. Cell viability was measured by using
the water-soluble tetrazolium-8 (WST-8) Cell Proliferation kit
(Quanti-MAX™; Biomax Co. Ltd.) in order to investigate cytotoxicity
and cell proliferation. Cytotoxicity was measured within 24 h, and
proliferation rate was measured at 24, 48 and 72 h post-treatment.
Briefly, medium-containing plates were incubated with WST-8 reagent
at 37°C for 30 min, and absorbance at a wavelength of 450 nm was
measured using a microplate reader.
Cell migration assay
HDFs (2×105 cells/well) and HEKs
(4×105 cells/well) were plated in six-well culture
plates (SPL Life Sciences). When the cells reached 80–90%
confluence, serum was removed for 16 h. Cells were scratched in the
middle with a 200-µl pipette tip. The starting point was marked
with a marker pen at the bottom of the plate. Medium was replaced
with either serum-depleted medium containing PDRN (1, 5, 10, 50 and
100 µg/ml) or serum-depleted medium without PDRN as a control, and
the cells were incubated for 0, 12 and 24 h. Digital images of the
scratches were acquired using a phase-contrast light microscope
(TS100; Nikon Corporation). Images were randomly divided into three
parts, the number of migrated cells was counted and the experiment
was repeated at least three times.
Enzyme-linked immunosorbent assay
(ELISA) of collagen type I and III
HDFs (2×105 cells/well) and HEKs
(4×105 cells/well) were seeded in six-well culture
plates in complete growth medium. After starving cells for 16 h,
PDRN was added for 24 h. The culture supernatants were then
collected for ELISA to determine the concentrations of specific
proteins. Supernatants were assayed using commercially available
Human Pro-Collagen I α 1 (cat. no. ab210966; Abcam) and collagen
type III (cat. no. MBS761779; MyBioSource, Inc.) ELISA kits
according to the manufacturer's instructions. Measurements were
performed at least three times. The mean values of samples were
used to calculate concentrations on the basis of a standard curve
obtained with standard proteins provided from the kits.
Western blotting
To investigate the molecular mechanism involved in
PDRN induction, HDFs (2×105 cells/well) and HEKs
(4×105 cells/well) were seeded in six-well culture
plates in growth medium for 24 h. Cells were serum-starved for 16 h
before supplementation with PDRN for 0, 5, 15, 30, 60 and 120 min.
Confirming changes in MAPK phosphorylation kinetics under
stimulation within 2 h has been widely used as a method to monitor
MAPK activation in vitro (23,24).
Protein expression levels of phosphorylated ERK1/2, JNK and p38
were determined by immunoblotting. Cells were washed using ice-cold
phosphate-buffered saline. After washing, the cells were lysed in
RIPA buffer containing 150 mM NaCl, 1% Triton X-100, 1% sodium
deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris pH 7.5,
2 mM EDTA, protease inhibitor cocktail solution and phosphatase
inhibitor cocktail solution, producing a 1X final concentration
(GenDEPOT LLC) on ice for 30 min. After centrifugation at 20,000 ×
g at 4°C for 30 min, a BCA protein assay (cat. no. 23227; Pierce;
Thermo Fisher Scientific, Inc.) was used for protein quantitation
and the resulting supernatant was boiled at 95°C for 5 min in
SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer. The
samples (25 µg/lane) were subjected to 10% SDS-PAGE and
electrotransferred to immobilon polyvinylidene fluoride membranes
(Merck KGaA). Non-specific binding was blocked with skimmed milk
(5%) for 1 h at room temperature. Membranes were then stored at 4°C
overnight with specific primary antibodies (1:1,000 dilution)
against phospho-ERK (sc-7383), ERK 1/2 (sc-514302), phospho-p38
(sc-166182), p38α/β MAPK (sc-7972), phospho-JNK (sc-6254), JNK
(sc-7345) and α-tubulin (sc-5286) purchased from Santa Cruz
Biotechnology, Inc. The membranes were washed using Tris-buffered
saline with 0.05% Tween 20 and incubated for 1 h at room
temperature with horseradish peroxidase-conjugated secondary
antibody (1:3,000 dilution; cat. no. sc-516102; Santa Cruz
Biotechnology, Inc.). Protein bands were visualized using an
enhanced chemiluminescence detection system (Amersham imager 600;
GE Healthcare). Phosphorylation was semi-quantified with Image J
software (National Institutes of Health) by comparing the density
of the phosphorylated form and total protein.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated with TRIzol®
reagent (Molecular Research Center, Inc.) according to the
manufacturer's protocol. complementary DNA (cDNA) was synthesized
using Maxime RT PreMix kit (Intron Biotechnology, Inc.) according
to the manufacturer's instructions. qPCR was performed using SYBR
Green dye using the KAPA SYBR® FAST qPCR Master Mix (2X)
kit (Roche Diagnostics GmbH). A comparative Cq method
(2−ΔΔCq) (25) was used
to quantify relative gene expression, and β-actin was used as an
endogenous reference control for all transcripts. A LightCycler 96
Real-Time PCR System (Roche Diagnostics GmbH) was used, with
thermal cycling conditions consisting of 95°C for 3 min, followed
by 45 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 1
sec. Primers were designed according to published cDNA or genomic
sequences (26,27). The sequences of the primers were as
follows: β-actin (forward, GAGACCTTCAACACCCCAGC; reverse,
ATGTCACGCACGATTTCCCT; NG_007992), TNF-α (forward,
CCCAGGGACCTCTCTCTAATC; reverse, ATGGGCTACAGGCTTGTCACT; NG_007462),
interleukin (IL)-1β (forward, GATGAAGTGCTCCTTCCAGG;
reverse, GCATCTTCCTCAGCTTGTCC; NG_008851), IL-6 (forward,
CAATCTGGATTCAATGAGGAGAC; reverse, TTTTTCTGCAGGAACTGGATCAG;
NG_011640), monocyte chemotactic protein 1 (MCP1; forward,
TTCCCCTAGCTTTCCCCAGA; reverse, TCCCAGGGGTAGAACTGTGG; NG_012123) and
inducible nitric oxide synthase (iNOS; forward,
AAAGTTTGACCAGAGGACCC; reverse, TCCTTTGTTACCGCTTCCAC;
NG_011470).
Statistical analysis
All experiments were performed at least three times,
and data are expressed as the mean ± standard error of the mean
(SEM). Statistical comparisons were performed using various
methods. One-way analysis of variance (ANOVA) followed by Dunnett's
post hoc test using Microsoft Excel (Microsoft Corporation) and
GraphPad Prism 9 software (Dotmatics). For the analysis of the
proliferation assay, two-way ANOVA was performed followed by
Dunnett's and Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PDRN increases proliferation and
migration of human skin cells
The proliferation of HDFs and HEKs was investigated
in vitro to examine the effect of PDRN on the bioactivity of
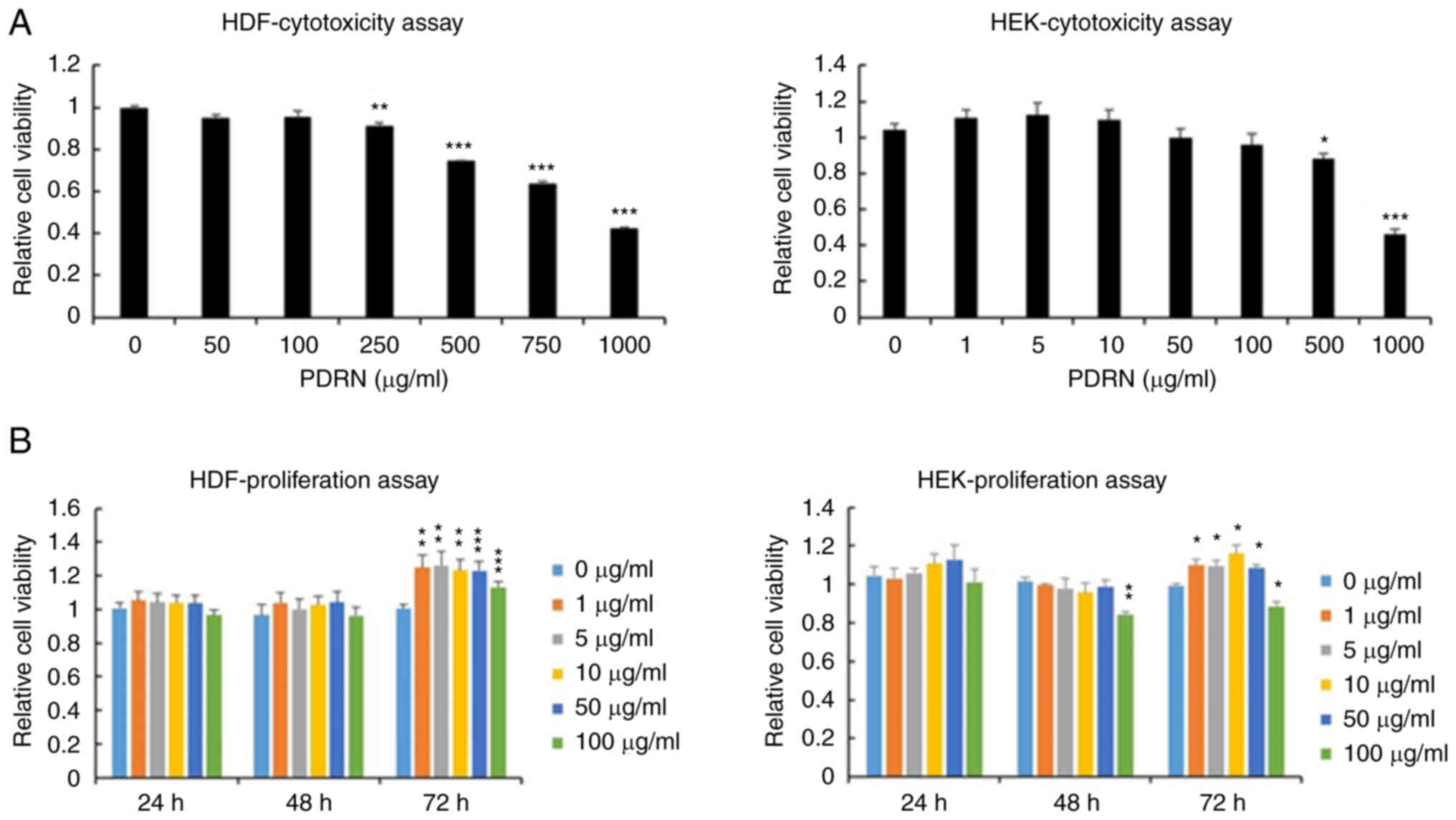
these cells. The cytotoxicity effects of PDRN were demonstrated in
HDFs and HEKs. HDFs survived in medium supplemented with 100 µg/ml
of PDRN; however, cytotoxicity towards HEKs was observed at a PDRN
concentration of >500 µg/ml (Fig.
1A). Next, the optimal concentration of PDRN was determined for
maximum intensity of cell response with minimum cytotoxicity. The
proliferation and migration of HDFs and HEKs was assessed at 24, 48
and 72 h after PDRN treatment at dose of 0, 1, 5, 10, 50 and 100
µg/ml. HDF and HEK proliferation increased with PDRN treatment, but
HEK proliferation decreased upon addition of 100 µg/ml of PDRN at
48 and 72 h (Fig. 1B). These
results show that PDRN increases skin cell proliferation, but a
high concentration of PDRN can inhibit HEK proliferation. HDF
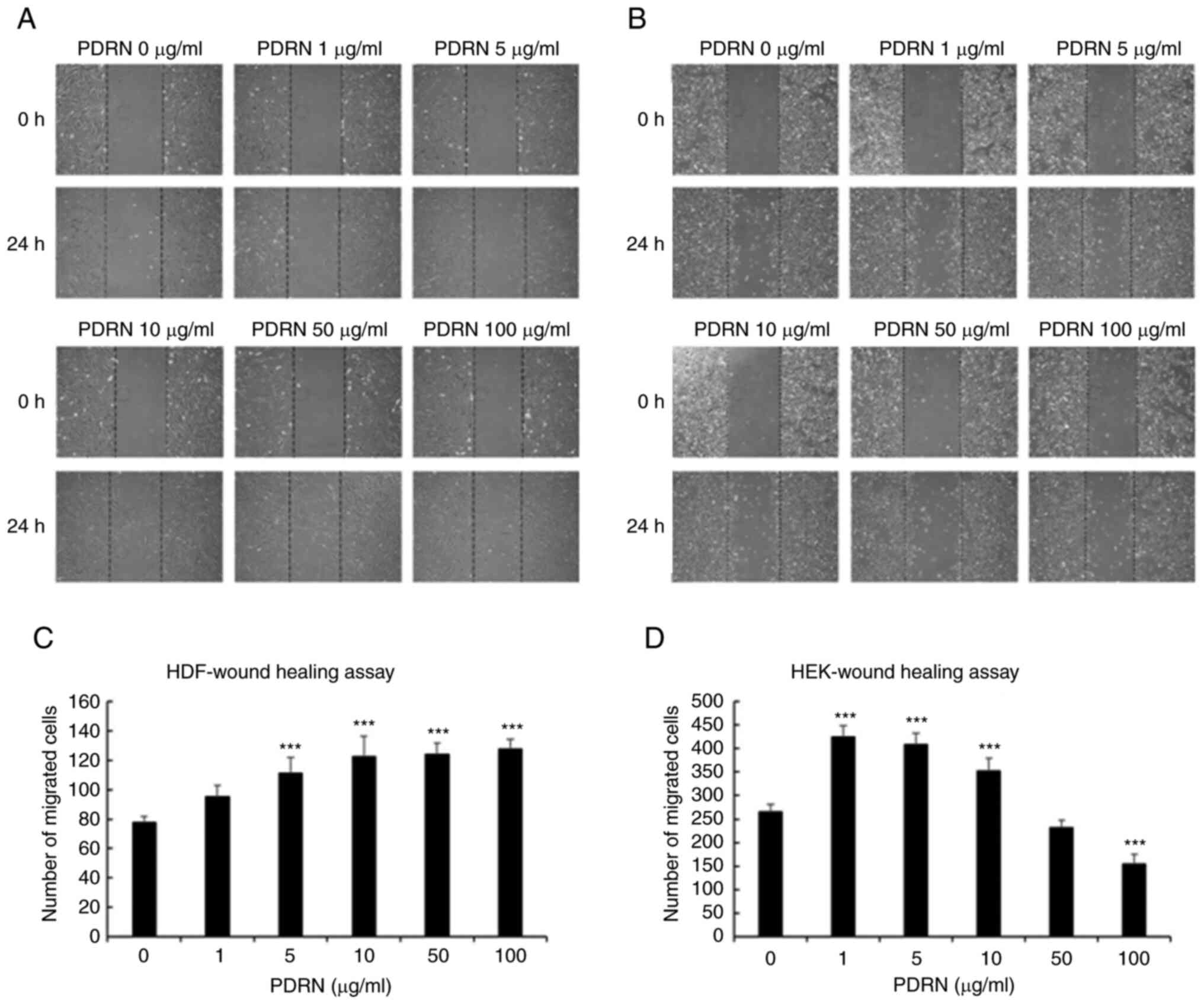
migration increased with increasing PDRN concentration, and there
was no difference in migration upon treatment with >10 µg/ml
PDRN (Fig. 2A and C). HEK
migration was highest upon treatment with 1 µg/ml PDRN but
suppressed at ≥50 µg/ml PDRN (Fig. 2B
and D). Based on these results, it was confirmed that PDRN
increased the proliferation and migration of skin cells. The
optimal PDRN concentration for the treatment of HDFs and HEKs was
determined to be 10 and 1 µg/ml, respectively.
Intracellular signaling cascades in
PDRN-treated human skin cells
Transcription-regulating protein kinases, including
MAPK family members ERK and JNK, have previously been shown to be
phosphorylated after PDRN treatment in fibroblasts or melanocytes
(28,29). The activation of the MAPK family
members was thus investigated using western blotting. HDFs and HEKs
were serum-starved for 16 h, followed by treatment with a
serum-free medium supplemented with 10 µg/ml of PDRN for HDFs, and
1 µg/ml of PDRN for HEKs. The cells were harvested after 0, 5, 15,
30, 60 and 120 min. As shown in Fig.
3A and C, treatment with PDRN significantly increased ERK
phosphorylation without altering total ERK levels in HDFs.
Phosphorylation of the p46 JNK isoform was also increased by PDRN
treatment, but the level of the phosphorylated-p54 JNK isoform did
not change and there was no statistically significant differences
(Fig. S1A). The increase of p38
phosphorylation was not statistically significant. However, ERK
phosphorylation decreased significantly after PDRN treatment in
HEKs, before they adapted to the treatment (Fig. 3B and D). Phosphorylated JNK and p38
levels did not change during PDRN treatment in HEKs (Fig. S1B). Based on these results, it was
concluded that PDRN induces opposite effects on MAPK activation
depending on the skin cell type, and it is necessary to investigate
the underlying intracellular signal transduction mechanisms.
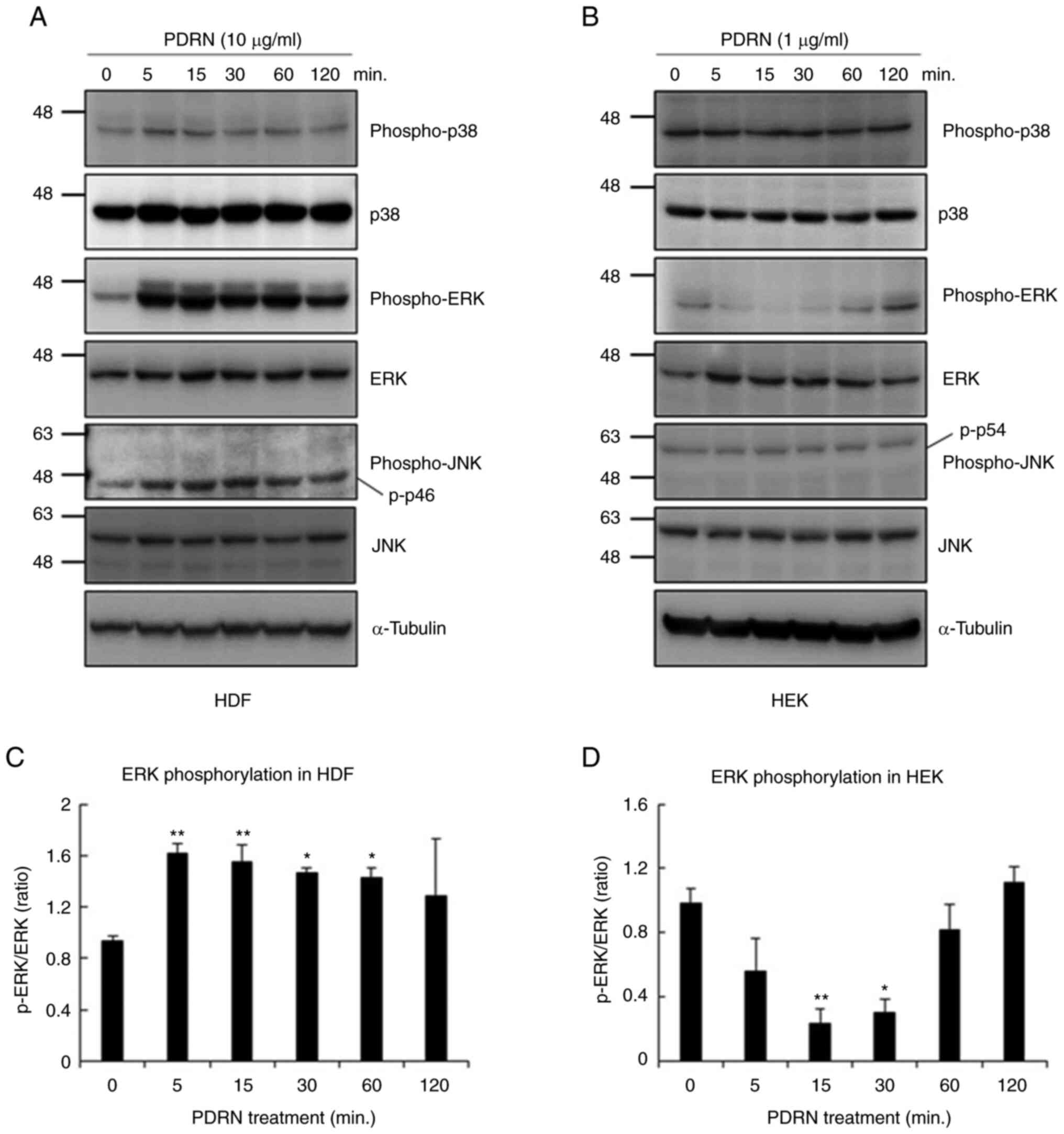 | Figure 3.Effects of PDRN on mitogen-activated
protein kinase pathway activation in human skin cells. Cells were
cultured without serum for 16 h, followed by addition of PDRN to
culture medium for 0, 5, 15, 30, 50 and 120 min. Cell lysates were
analyzed by western blotting using targeted protein antibodies in
(A) HDFs and (B) HEKs. Phosphorylated ERK was quantified with Image
J software by comparing the density of phospho-ERK and ERK in (C)
HDFs and (D) HEKs. Similar results were obtained in three different
experiments. *P<0.05 and **P<0.01 vs. 0 min. PDRN,
polydeoxyribonucleotide; HDF, human dermal fibroblasts; HEK, human
epidermal keratinocytes; ERK, extracellular signal-regulated
kinase; JNK, c jun N terminal kinase. |
PDRN enhances collagen accumulation by
activating the ERK pathways in HDFs
To further understand the mechanism underlying
PDRN-induced ERK activation, the selective ERK inhibitor PD98059
was used in HDFs. A total of 1 µM PD98059 was added to HDFs, and
cells were incubated for 30 min before PDRN treatment. Fig. 4A shows that PD98059 significantly
suppressed PDRN-induced ERK phosphorylation, and the WST-8 assay
showed that the significant increase in cell proliferation induced
by PDRN treatment was reversed by treatment with PD98059. Moreover,
PDRN-mediated HDF migration was inhibited by PD98059 treatment
(Fig. 4B). Next, collagen protein
expression was investigated by ELISA after 24 h of PDRN treatment
of HDFs (Fig. 4C). Collagen type I
and III protein levels were increased after PDRN treatment compared
with those in the untreated control, but PD98059 decreased collagen
secretion in a dose-dependent manner. The regulation of MMP
expression by PDRN treatment was also examined via RT-qPCR
(Fig. 4D). PDRN decreased the
expression of MMP1, 2 and 3 in HDFs, whereas PD98059 rescued MMP
expression in these cells. These results suggest that PDRN
treatment triggers collagen accumulation in HDFs via ERK
phosphorylation.
 | Figure 4.Effect of ERK inhibition on
PDRN-induced collagen accumulation around HDFs. To explore the
underlying mechanism of PDRN-induced ERK activation, the
proliferation and migration of HDFs stimulated with PDRN in the
presence of PD98059 were measured using (A) WST-8 and (B) wound
healing assays. Original magnification, ×40. Cells were cultured
without serum for 16 h, followed by addition of PDRN and PD98059 to
the culture medium for 24 h. (C) hPro-Collagen I α 1 and hCollagen
type III expression levels were determined using ELISA in HDFs. (D)
MMP-1, 2 and 3 expression levels were examined by reverse
transcription-quantitative PCR. Each treatment was performed in
triplicate at least, and the data are presented as mean ± SEM.
*P<0.05, **P<0.01 and ***P<0.005 vs. PDRN or indicated
control. PDRN, polydeoxyribonucleotide; HDF, human dermal
fibroblasts; ERK, extracellular signal-regulated kinase; WST-8,
water-soluble tetrazolium-8; p-, phosphorylated; h, human; MMP,
matrix metalloproteinases. |
PDRN suppresses the pro-inflammatory
effect by inhibiting the ERK pathway in HEKs
As ERK phosphorylation was reduced in HEKs, collagen
levels were also assessed, but these could not be measured due to
low expression levels (data not shown). It was speculated that ERK
inhibition by PDRN would manifest different effects in
keratinocytes compared with fibroblasts. ERK activation is related
to inflammatory responses, and their regulation is a crucial factor
in skin repair. To examine the effects of PDRN on ERK
signaling-mediated inflammatory responses, the expression levels of
proinflammatory cytokines in HEKs were determined after 24 h PDRN
treatment (Fig. 5). The expression
levels of TNF-α and IL-6 decreased with PDRN treatment in a
dose-dependent manner, while those of IL-1β and iNOS were inhibited
within the non-toxic concentration range for HEKs of ≤1 µg/ml. MCP1
expression levels were suppressed when 0.5 µg/ml of PDRN was used,
but expression levels were not suppressed when cells were treated
with >0.5 µg/ml. It is noteworthy that expression levels of
inflammatory cytokines in HDFs could not be determined due to
either low expression or no significant change was observed even
after treatment with PDRN (data not shown).
Discussion
PDRN is a mixture of deoxyribonucleotides with a
molecular weight range of 50–1,500 kDa and derived from human
placenta or salmon sperm (30).
According to previous experiments, the most relevant mechanism of
action of PDRN is the engagement of adenosine A2A receptors. PDRN
binds to the adenosine A2A receptor, and it induces the synthesis
of vascular endothelial growth factor, which improves angiogenesis
and consequently promotes wound healing (30,31).
PDRN inhibits apoptosis and exerts anti-inflammatory effects by
downregulating inflammatory cytokines, including iNOS, IL-1β, IL-6
and TNF-α (32). Also, PDRN
stimulates nucleic acid synthesis through a salvage pathway to
recover bases and nucleosides generated from the breakdown of DNA
and RNA (33). Therefore, PDRN
reactivates normal cell proliferation by generating nucleotides and
nucleosides that contribute to DNA formation. Although it was
confirmed that PDRN exerts opposite effects on ERK activity is HDFs
and HEKs in the present study, PDRN-treated HDFs and HEKs showed
increased proliferation with reduced mRNA expression levels of cell
cycle arrest proteins p21, p27, p57 and p53 (Fig. S2). These results suggest that the
PDRN-induced increase in the proliferation of ERK-inhibited
keratinocytes may be due to the PDRN-induced salvage pathway.
Most studies on the effects of PDRN on skin
regeneration have been conducted using fibroblasts (17,19,29,33).
However, under physiological conditions, the normal wound healing
process is characterized by complex and integrated processes that
require the interactions of inflammatory cells, fibroblasts,
keratinocytes and endothelial cells, and the involvement of growth
factors and enzymes. Normal wound healing proceeds through four
phases: Hemostasis, inflammation, proliferation, maturation and
remodeling (34,35). In the present study, it was
confirmed that PDRN not only improved cell proliferation and
migration, but it also promoted accumulation of collagen. The most
notable histological changes in intrinsic and photo-aged skin occur
within the dermis (36,37), and changes in collagen have been
suggested to be the cause of wrinkling observed in photoaged and
naturally aged skin (38,39). UV irradiation enhances MMP
synthesis in human skin in vivo, and MMP-mediated collagen
destruction constitutes a notable proportion of connective tissue
damage that occurs during photoaging. Collagen destruction in
intrinsic skin aging may be due to elevated MMP expression and an
accompanying reduction in collagen synthesis, similar to photoaging
(39,40). Increased MMP-mediated degradation
of aged and photoaged skin results in decrease in collagen, causing
wrinkles in aged skin. Fibroblasts were treated with PD98059, an
ERK inhibitor, and a decrease in PDRN-mediated collagen
accumulation was observed along with an increase in MMP expression
levels. These results suggest that PDRN activates collagen
synthesis via ERK phosphorylation.
Inflammation during skin aging reduces collagen
activity and increases MMP levels (41,42).
Inflammation also disrupts the rate of cell proliferation in all
skin layers, leading to thinning of the epidermis, flattening of
the dermo-epidermal junction and increased irregular pigment
production, eventually resulting in an increased incidence of skin
cancer (43). UVR can activate
signaling pathways (44,45), and it promotes the downstream
signaling pathway intermediates, such as ERK1/2 and p38 (46–48).
During skin aging, cytokines produced by keratinocytes and dermal
fibroblasts potentiate inflammation by binding to their receptors
on adjacent cells. The increased production of MCP1 induces the
expression of reactive oxygen species, MMPs and other inflammatory
mediators that can damage the dermal matrix (49). Finally, inflammatory cytokines can
upregulate the synthesis of their receptors or nuclear factors,
such as NF-κB, iNOS and JNK, further augmenting the inflammatory
response (50,51) and aggravating the damaging effects
on the skin. In the current study, it was confirmed that
proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, MCP-1 and
iNOS related to skin aging, were reduced after PDRN treatment of
HEKs. By contrast, ERK phosphorylation was found to be reduced by
PDRN in keratinocytes, and the effective concentration of PDRN was
also lower than that required by fibroblasts.
The expression changes of IL-6 and TNF-α in
keratinocytes and fibroblasts were additionally investigated using
ELISA kits. IL-6 was decreased in both cell types, and TNF-α was
decreased only in fibroblasts (data not shown). The reason TNF-α
expression was decreased in keratinocytes is likely due to the
absence of inflammation-inducing factors. Therefore, it can be
expected that TNF-α will be reduced by PDRN treatment in future
experiments when keratinocytes are inflamed (52,53).
In the results of the present study, mRNA expression levels of
inflammatory cytokines in fibroblasts could not be determined due
to either low levels or there was no significant change even after
treatment with PDRN. Transcriptional regulation of inflammatory
cytokines does not appear to be affected by PDRN in fibroblasts,
but the translation and secretion of cytokines seems to have been
affected by PDRN through signaling pathways other than ERK
signaling transmission.
PDRN is commonly used for dermatological purposes.
In the present study, the effects of PDRN on fibroblasts and
keratinocytes were investigated in vitro and the possible
underlying molecular mechanisms were examined by investigating the
bioactivities of skin cells. PDRN affects cell proliferation and
migration, the expression levels of fibrotic proteins such as
collagen type I and III, and inflammatory cytokines via the ERK
pathway. PDRN has a positive effect on skin regeneration, but the
mechanism that regulates it differs depending on cell type.
Therefore, it may exert different effects on skin regeneration in
different cell types. Increased ERK phosphorylation in PDRN-treated
HDFs induced the synthesis of collagen type I and III proteins.
However, the suppression of ERK phosphorylation in PDRN-treated
HEKs resulted in decrease of pro-inflammatory cytokine levels. The
findings of the current study indicate that PDRN has potential for
skin rejuvenation via dermal remodeling and epidermal
anti-inflammation effects, and that signal transductions can exert
opposing effects in different cell types, which needs to be taken
into consideration in drug effect tests in skin research.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Hallym University Research
Fund and the National Research Foundation of Korea grant funded by
the Korea government (grant no. 2019R1G1A100658813).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EJP and SMS designed the project. SMS and EJB
performed the experiments. SMS, EJB, KJK, KHK and EJP evaluated
data. SMS and EJB wrote this article. SMS, EJB, KJK, KHK and EJP
edited the manuscript. EJP supervised the project. SMS and EJP
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This experiment was approved by the IRB of Hallym
University Sacred Hospital (Anyang, Republic of Korea) (approval
no. HALLYM 2019-07-029). All procedures in the present study were
conducted in accordance with the IRB of Hallym University Sacred
Hospital approved protocols. Written informed consent was obtained
for patient participation at the initial time that the samples were
taken.
Patient consent for publication
Written informed consent was obtained from the
patient(s) for their anonymized information to be published in this
article.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
cDNA
|
complementary DNA
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
ECM
|
extracellular matrix
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
FBS
|
fetal bovine serum
|
|
HDFs
|
human dermal fibroblasts
|
|
HEKs
|
human epidermal keratinocytes
|
|
IL
|
interleukin
|
|
INOS
|
inducible nitric oxide synthase
|
|
IRB
|
Institutional Review Board
|
|
JNK
|
cjun Nterminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MCP1
|
monocyte chemotactic protein 1
|
|
MMPs
|
matrix metalloproteinases
|
|
PAGE
|
polyacrylamide gel electrophoresis
|
|
PCR
|
polymerase chain reaction
|
|
PDRN
|
polydeoxyribonucelotide
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
ROS
|
reactive oxygen species
|
|
SDS
|
sodium dodecyl sulfate
|
|
SEM
|
standard error of the mean
|
|
TNF
|
tumor necrosis factor
|
|
UV
|
ultraviolet
|
|
WST-8
|
water-soluble tetrazolium-8
|
References
|
1
|
El-Domyati M, Attia S, Saleh F, Brown D,
Birk DE, Gasparro F, Ahmad H and Uitto J: Intrinsic aging vs.
photoaging: A comparative histopathological, immunohistochemical,
and ultrastructural study of skin. Exp Dermatol. 11:398–405. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quan T and Fisher GJ: Role of
Age-associated alterations of the dermal extracellular matrix
microenvironment in human skin aging: A mini-review. Gerontology.
61:427–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borg M, Brincat S, Camilleri G,
Schembri-Wismayer P, Brincat M and Calleja-Agius J: The role of
cytokines in skin aging. Climacteric. 16:514–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bashir MM, Sharma MR and Werth VP: UVB and
proinflammatory cytokines synergistically activate TNF-alpha
production in keratinocytes through enhanced gene transcription. J
Invest Dermatol. 129:994–1001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agius E, Lacy KE, Vukmanovic-Stejic M,
Jagger AL, Papageorgiou AP, Hall S, Reed JR, Curnow SJ,
Fuentes-Duculan J, Buckley CD, et al: Decreased TNF-alpha synthesis
by macrophages restricts cutaneous immunosurveillance by memory
CD4+ T cells during aging. J Exp Med. 206:1929–1940. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dada LA and Sznajder JI: Mitochondrial
Ca2+ and ROS take center stage to orchestrate
TNF-α-mediated inflammatory responses. J Clin Invest. 121:1683–5.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci. 17:8682016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verheij M, Bose R, Lin XH, Yao B, Jarvis
WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, et al:
Requirement for ceramide-initiated SAPK/JNK signalling in
stress-induced apoptosis. Nature. 380:75–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raingeaud J, Gupta S, Rogers JS, Dickens
M, Han J, Ulevitch RJ and Davis RJ: Pro-inflammatory cytokines and
environmental stress cause p38 mitogen-activated protein kinase
activation by dual phosphorylation on tyrosine and threonine. J
Biol Chem. 270:7420–7426. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin MH, Rhie GE, Kim YK, Park CH, Cho KH,
Kim KH, Eun HC and Chung JH: H2O2 accumulation by catalase
reduction changes MAP kinase signaling in aged human skin in vivo.
J Invest Dermatol. 125:221–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen A and Davis BH: UV irradiation
activates JNK and increases alphaI(I) collagen gene expression in
rat hepatic stellate cells. J Biol Chem. 274:158–164. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brennan M, Bhatti H, Nerusu KC,
Bhagavathula N, Kang S, Fisher GJ, Varani J and Voorhees JJ: Matrix
metalloproteinase-1 is the major collagenolytic enzyme responsible
for collagen damage in UV-irradiated human skin. Photochem
Photobiol. 78:43–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JY, Pak CS, Park JH, Jeong JH and Heo
CY: Effects of polydeoxyribonucleotide in the treatment of pressure
ulcers. J Korean Med Sci. 29 (Suppl 3):S222–S227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Squadrito F, Bitto A, Irrera N, Pizzino G,
Pallio G, Minutoli L and Altavilla D: Pharmacological activity and
clinical use of PDRN. Front Pharmacol. 8:2242017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galeano M, Bitto A, Altavilla D, Minutoli
L, Polito F, Calò M, Lo Cascio P, Stagno d'Alcontres F and
Squadrito F: Polydeoxyribonucleotide stimulates angiogenesis and
wound healing in the genetically diabetic mouse. Wound Repair
Regen. 16:208–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thellung S, Florio T, Maragliano A,
Cattarini G and Schettini G: Polydeoxyribonucleotides enhance the
proliferation of human skin fibroblasts: Involvement of A2
purinergic receptor subtypes. Life Sci. 64:1661–1674. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valdatta L, Thione A, Mortarino C, Buoro M
and Tuinder S: Evaluation of the efficacy of
polydeoxyribonucleotides in the healing process of autologous skin
graft donor sites: A pilot study. Curr Med Res Opin. 20:403–408.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YJ, Kim MJ, Kweon DK, Lim ST and Lee
SJ: Polydeoxyribonucleotide activates mitochondrial biogenesis but
reduces MMP-1 activity and melanin biosynthesis in cultured skin
cells. Appl Biochem Biotechnol. 191:540–554. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin SM, Baek EJ, Oh DY, Kim KH, Kim KJ
and Park EJ: Functional validation of co-culture model of human
keratinocytes and neuronal cell line for sensitive skin by using
transient receptor potential channel vanilloid subfamily member 1
antagonist. Skin Res Technol. 29:e132752023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vangipuram M, Ting D, Kim S, Diaz R and
Schüle B: Skin punch biopsy explant culture for derivation of
primary human fibroblasts. J Vis Exp. 7:e37792013.PubMed/NCBI
|
|
22
|
Cho EB, Park GS, Park SS, Jang YJ, Kim KH,
Kim KJ and Park EJ: Effect of platelet-rich plasma on proliferation
and migration in human dermal fibroblasts. J Cosmet Dermatol.
18:1105–1112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoo DH, Im YS, Oh JY, Gil D and Kim YO:
DUSP6 is a memory retention feedback regulator of ERK signaling for
cellular resilience of human pluripotent stem cells in response to
dissociation. Sci Rep. 13:56832023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwon Y, Mehta S, Clark M, Walters G, Zhong
Y, Lee HN, Sunahara RK and Zhang J: Non-canonical β-adrenergic
activation of ERK at endosomes. Nature. 611:173–179. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alvarado-Vazquez PA, Bernal L, Paige CA,
Grosick RL, Moracho Vilrriales C, Ferreira DW, Ulecia-Morón C and
Romero-Sandoval EA: Macrophage-specific nanotechnology-driven CD163
overexpression in human macrophages results in an M2 phenotype
under inflammatory conditions. Immunobiology. 222:900–912. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shan X, Zhang Y, Chen H, Dong L, Wu B, Xu
T, Hu J, Liu Z, Wang W, Wu L, et al: Inhibition of epidermal growth
factor receptor attenuates LPS-induced inflammation and acute lung
injury in rats. Oncotarget. 8:26648–26661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noh TK, Chung BY, Kim SY, Lee MH, Kim MJ,
Youn CS, Lee MW and Chang SE: Novel Anti-melanogenesis properties
of polydeoxyribonucleotide, a popular wound healing booster. Int J
Mol Sci. 17:14482016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hwang KH, Kim JH, Park EY and Cha SK: An
effective range of polydeoxyribonucleotides is critical for wound
healing quality. Mol Med Rep. 18:5166–5172. 2018.PubMed/NCBI
|
|
30
|
Veronesi F, Dallari D, Sabbioni G, Carubbi
C, Martini L and Fini M: Polydeoxyribonucleotides (PDRNs) from skin
to musculoskeletal tissue regeneration via adenosine A2A
receptor involvement. J Cell Physiol. 232:2299–2307. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Altavilla D, Bitto A, Polito F, Marini H,
Minutoli L, Di Stefano V, Irrera N, Cattarini G and Squadrito F:
Polydeoxyribonucleotide (PDRN): A safe approach to induce
therapeutic angiogenesis in peripheral artery occlusive disease and
in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem.
7:313–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han JH, Jung J, Hwang L, Ko IG, Nam OH,
Kim MS, Lee JW, Choi BJ and Lee DW: Anti-inflammatory effect of
polydeoxyribonucleotide on zoledronic acid-pretreated and
lipopolysaccharide-stimulated RAW 264.7 cells. Exp Ther Med.
16:400–405. 2018.PubMed/NCBI
|
|
33
|
Sini P, Denti A, Cattarini G, Daglio M,
Tira ME and Balduini C: Effect of polydeoxyribonucleotides on human
fibroblasts in primary culture. Cell Biochem Funct. 17:107–114.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lavker RM: Structural alterations in
exposed and unexposed aged skin. J Invest Dermatol. 73:59–66. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gilchrest BA: Skin aging and photoaging:
An overview. J Am Acad Dermatol. 21:610–603. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fisher GJ, Wang ZQ, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–2148. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Varani J, Warner RL, Gharaee-Kermani M,
Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher G and Voorhees
JJ: Vitamin A antagonizes decreased cell growth and elevated
collagen-degrading matrix metalloproteinases and stimulates
collagen accumulation in naturally aged human skin. J Invest
Dermatol. 114:480–486. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chung JH, Seo JY, Choi HR, Lee MK, Youn
CS, Rhie G, Cho KH, Kim KH, Park KC and Eun HC: Modulation of skin
collagen metabolism in aged and photoaged human skin in vivo. J
Invest Dermatol. 117:1218–1224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sárdy M: Role of matrix metalloproteinases
in skin ageing. Connect Tissue Res. 50:132–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fisher GJ, Choi HC, Bata-Csorgo Z, Shao Y,
Datta S, Wang ZQ, Kang S and Voorhees JJ: Ultraviolet irradiation
increases matrix metalloproteinase-8 protein in human skin in vivo.
J Invest Dermatol. 117:219–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Neagu M, Constantin C, Caruntu C, Dumitru
C, Surcel M and Zurac S: Inflammation: A key process in skin
tumorigenesis. Oncol Lett. 17:4068–4084. 2019.PubMed/NCBI
|
|
44
|
López-Camarillo C, Ocampo EA, Casamichana
ML, Pérez-Plasencia C, Alvarez-Sánchez E and Marchat LA: Protein
kinases and transcription factors activation in response to
UV-radiation of skin: Implications for carcinogenesis. Int J Mol
Sci. 13:142–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rosette C and Karin M: Ultraviolet light
and osmotic stress: Activation of the JNK cascade through multiple
growth factor and cytokine receptors. Science. 274:1194–1197. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang RP, Wu JX, Fan Y and Adamson ED: UV
activates growth factor receptors via reactive oxygen
intermediates. J Cell Biol. 133:211–220. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peus D, Meves A, Vasa RA, Beyerle A,
O'Brien T and Pittelkow MR: H2O2 is required for UVB-induced EGF
receptor and downstream signaling pathway activation. Free Radic
Biol Med. 27:1197–1202. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Son Y, Cheong YK, Kim NH, Chung HT, Kang
DG and Pae HO: Mitogen-activated protein kinases and reactive
oxygen species: How can ROS activate MAPK pathways? J Signal
Transduct. 2011:7926392011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kang JS, Kim HN, Jung DJ, Kim JE, Mun GH,
Kim YS, Cho D, Shin DH, Hwang YI and Lee WJ: Regulation of
UVB-induced IL-8 and MCP-1 production in skin keratinocytes by
increasing vitamin C uptake via the redistribution of SVCT-1 from
the cytosol to the membrane. J Invest Dermatol. 127:698–706. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Trefzer U, Brockhaus M, Lötscher H, Parlow
F, Budnik A, Grewe M, Christoph H, Kapp A, Schöpf E, Luger TA, et
al: The 55-kD tumor necrosis factor receptor on human keratinocytes
is regulated by tumor necrosis factor-alpha and by ultraviolet B
radiation. J Clin Invest. 92:462–470. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takii T, Akahoshi T, Kato K, Hayashi H,
Marunouchi T and Onozaki K: Interleukin-1 up-regulates
transcription of its own receptor in a human fibroblast cell line
TIG-1: Role of endogenous PGE2 and cAMP. Eur J Immunol.
22:1221–1227. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bitto A, Polito F, Irrera N, D'Ascola A,
Avenoso A, Nastasi G, Campo GM, Micali A, Bagnato G, Minutoli L, et
al: Polydeoxyribonucleotide reduces cytokine production and the
severity of collagen-induced arthritis by stimulation of adenosine
A(2A) receptor. Arthritis Rheum. 63:3364–3371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Castellini C, Belletti S, Govoni P and
Guizzardi S: Anti Inflammatory property of PDRN-An in vitro study
on cultured macrophages. Adv Bioscience Biotechnol. 8:13–26. 2017.
View Article : Google Scholar
|