Introduction
Lung cancer is a leading cause of cancer-associated
mortality worldwide, and is frequently associated with a poor
prognosis due to being diagnosed at an advanced stage (1). The most prevalent histological
subtype of lung cancer is lung adenocarcinoma (LUAD), which
accounts for ~60% of cases globally (2). In recent years, notable progress has
been made in LUAD treatment with the use of molecular targeted
therapies (3). Notably, small
molecule-based targeted therapy has been shown to significantly
improve the treatment outcomes of patients with carrying targetable
molecular alterations, such as epidermal growth factor receptor
(EGFR)-activating mutations, and anaplastic lymphoma kinase (ALK)
and ROS1 translocations (4–6).
Despite recent therapeutic advances, the worldwide 5-year survival
rate for patients diagnosed with LUAD remains <20% (7). Consequently, chemotherapy remains the
standard of care for these patients in routine clinical practice.
To improve patient survival rates and quality of life, personalized
molecular-targeted therapies are urgently required.
In order to identify potential therapeutic targets
for LUAD, Gene Expression Profiling Interactive Analysis (GEPIA)
was employed to investigate genes associated with LUAD prognosis.
As a result, anillin (ANLN), an actin-binding protein with
ubiquitous expression, was identified as a novel therapeutic target
for LUAD (8). ANLN possesses
multiple domains, including a myosin- and actin-binding domain, a
RhoA-binding domain and a C-terminal pleckstrin homology domain
(9,10). It has been demonstrated that ANLN
serves a critical role in promoting tumor growth, migration and
cytokinesis (11). Of particular
note, ANLN has been suggested by several studies to be potentially
involved in the pathogenesis of LUAD (12). Elevated levels of ANLN have been
reported in LUAD cells, and it has been proposed that ANLN may
serve as a crucial determinant in distinguishing between low- and
high-risk LUAD cases with unfavorable prognoses (12–15).
Despite these findings, the precise biological functions and
mechanisms underlying the involvement of ANLN in LUAD pathogenesis
remain unknown, highlighting the need for further research in order
to develop effective therapeutic strategies.
Pyroptosis is an inflammatory form of programmed
cell death that features cell swelling, cell membrane rupture and
the release of cytoplasmic contents, including pro-inflammatory
molecules such as mature interleukin-1β and −18 (16). Studies have demonstrated that
pyroptosis has a significant role in the pathogenesis of LUAD
(17–20). Meanwhile, ANLN is known to have a
role in regulating cellular behavior. However, the extent of ANLN's
potential involvement in the pathogenesis of LUAD via the
regulation of pyroptosis remains uncertain (21,22).
Therefore, the present study aimed to explore the relationship
between ANLN and pyroptosis in the progression of LUAD.
Materials and methods
Gene expression analysis using
publicly available datasets
Gene expression levels of ANLN in tumors and
adjacent normal samples were obtained from TCGA-LUAD dataset
(accession no: 202208, 535 LUAD tissue samples and 59 adjacent
normal samples). GEPIA (http://gepia.cancer-pku.cn/) was used for gene
expression analysis. Kaplan-Meier analysis (http://kmplot.com/analysis/) was performed with
TCGA-LUAD dataset (accession no: 202208). The log-rank test was
used to assess the significance of between-group differences.
Cell lines and culture
The human LUAD cell lines A549 and H1299 were
procured from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences, and were cultured in RPMI-1640 medium
(cat. no. A1049101; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; cat. no. 10100147;
Gibco; Thermo Fisher Scientific, Inc.) and penicillin (100
U/ml)/streptomycin (100 µg/ml) for <15 passages. The cells were
authenticated by Shanghai Biowing Applied Biotechnology Co., Ltd.
and were maintained under standard cell culture conditions at 37°C
in a humidified incubator with 5% CO2.
Small interfering RNA (siRNA)
transfection
In order to suppress ANLN expression, siRNA
sequences were employed. Three distinct siRNA sequences were
synthesized by Guangzhou Anernor Biotechnology Co., Ltd., as
follows: si-ANLN #1 sense, 5′-GAGAGAAUCUUCAGAGAAAAA-3′ and
antisense, 5′-UUUUUCUCUGAAGAUUCUCUC-3′; si-ANLN #2 sense,
5′-GUGAAGAGAAAUCUUGUACAA-3′ and antisense,
5′-UUGUACAAGAUUUCUCUUCAC-3′; si-ANLN #3 sense,
5′-CACUGAAGUAGAAGUUUCUAA-3′ and antisense,
5′-UUAGAAACUUCUACUUCAGUG-3′; non-targeting negative control (NC)
sense, 5′-UUCUCCGAACGAGUCACGU-3′ and antisense,
5′-ACGUGACUCGUUCGGAGAA-3′. A549 and H1299 cells (1×105)
were seeded in each well of a six-well plate. After 12 h, cells
were transfected with 80 nM of control siRNA and ANLN siRNA.
Lipofectamine® 2000 reagent (cat. no. 11668019;
Invitrogen; Thermo Fisher Scientific, Inc.) was used for the
transfection experiments (5 µl/well). After culture at 37°C for 24
h, the transfection medium was replaced with fresh RPMI-1640
culture medium and the cells were further incubated at 37°C for 48
h.
Western blot analysis
Proteins were extracted from A549 and H1299 cells
using RIPA lysis buffer (Beyotime Institute of Biotechnology)
containing 0.1 M PMSF and protease inhibitors (Roche Diagnostics).
The protein concentration was determined using a BCA Protein Assay
Kit (Shanghai Yeasen Biotechnology Co., Ltd.). Subsequently, the
proteins (20 µg/lane) were separated by 10% SDS-PAGE, transferred
onto PVDF membranes (MilliporeSigma) and blocked with 3% bovine
serum albumin (cat. no. AR1006; Boster Biological Technology) for 2
h at room temperature. Primary antibodies against ANLN (cat. no.
ab211872; Abcam; 1:1,000 dilution), GAPDH (cat. no. GB15002; Wuhan
Servicebio Technology Co., Ltd.; 1:5,000 dilution), interleukin
(IL)-18 (cat. no. ab243091; Abcam; 1:1,000 dilution),
apoptosis-associated speck-like protein containing a CARD domain
(ASC; cat. no. ab283684; Abcam; 1:1,000 dilution), pro-caspase-1
(cat. no. ab179515; Abcam; 1:1,000 dilution), caspase-1 (cat. no.
ab207802; Abcam; 1:1,000 dilution), NLR pyrin domain-containing
(NLRP)3 (cat. no. ab263899; Abcam; 1:1,000 dilution),
cleaved-gasdermin D (GSDMD; cat. no. ab215203; Abcam; 1:1,000
dilution) and IL-1β (cat. no. ab254360; Abcam; 1:1,000 dilution)
were incubated with the membranes overnight at 4°C. After washing
with TBST (1X) containing 0.3% Tween-20, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (cat. no. 074-1506 and 074-1807; KPL; 1:5,000 dilution)
for 2 h at room temperature. Immunoreactive bands were visualized
using an ECL kit (Beyotime Institute of Biotechnology) and were
semi-quantified using Image J software (Image J 1.8.0; National
Institutes of Health).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from A549 and H1299 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and reverse transcribed into cDNA with the
PrimeScript™ RT reagent Kit (Takara Biotechnology Co., Ltd.),
according to the manufacturer's instructions. qPCR was performed on
an ABI 7900HT PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using TB Green® Premix Ex Taq™ II
(Takara Biotechnology Co., Ltd.). The primer sequences for qPCR
were as follows: ANLN forward, 5′-CGCCTCAGACTCCTGGTTTT-3′ and
reverse, 5′-GCTCCAGCAGTTTCTCCGTA-3′; GAPDH forward,
5′-AATGGGCAGCCGTTAGGAAA-3′ and reverse, 5′-GCGCCCAATACGACCAAATC-3′.
Amplifications were performed following the procedure of a two-step
method (95°C for 30 sec, 1 cycle; followed by 40 cycles of 95°C for
10 sec and 60°C for 30 sec; and melting curve stage). The fold
change in mRNA expression levels was calculated using the
2−ΔΔCq method and normalized to GAPDH (23).
Colony formation assay
A549 and H1299 cells with ANLN knockdown were seeded
at a density of 500 cells/well in 12-well plates and were incubated
for 14 days at 37°C with 5% CO2 in a humidified
incubator. Subsequently, the culture medium was discarded and the
cells were washed with PBS. Following fixation with 4%
paraformaldehyde at room temperature for 30 min, the cells were
stained using Giemsa stain solution at room temperature for 60 min.
Finally, the colonies were manually counted. A cluster with >50
cells was considered a colony.
TUNEL staining
Cell death was evaluated using TUNEL staining, which
was performed with an In Situ Cell Death Detection kit
(Roche Diagnostics) according to the manufacturer's protocol. A549
and H1299 cells (1×105) were seeded in each well of a
six-well plate and cultured at 37°C for 48 h. Then the cells were
fixed on coverslips with 4% paraformaldehyde at room temperature
for 30 min and subsequently treated with 0.1% Triton X-100 for 10
min at room temperature. The cells were then washed with PBS and
incubated with 50 µl TUNEL reaction mixture at 37°C for 1 h,
followed by counterstaining with DAPI (1:100) for 10 min at room
temperature. Finally, the cells were mounted with ProLong Gold
antifade reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Visualization of the cells was achieved using an inverted
fluorescence microscope (Nikon Corporation) and three random fields
of view were observed.
Transwell assay
The migration of A549 and H1299 cells was assessed
using a Transwell kit (cat. no. 354480, Corning, Inc.).
Specifically, 5×104 cells were suspended in 300 µl
serum-free medium and seeded into the upper chamber of the
Transwell with 8-µm pore chambers inserted into 24-well plates,
whereas 500 µl medium containing 30% FBS was added to the lower
chamber. Following a 48-h incubation period at 37°C, the migrated
cells on the underside of the membrane were fixed with 0.5% crystal
violet at room temperature for 30 min, washed with PBS and
air-dried. Images of six randomly selected fields were captured
under a light microscope and the number of migrated cells was
determined.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism software (version 9.0; GraphPad Software, Inc.). All
experiments were repeated three times and data are expressed as the
mean ± standard deviation. Two-group comparisons were analyzed
using the unpaired Student's t-test, whereas one-way ANOVA followed
by Tukey's post-hoc test was use for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
High expression of ANLN is associated
with a poor prognosis in LUAD
Using The Cancer Genome Atlas (TCGA) database
(https://www.cancer.gov/ccg/research/genome-sequencing/tcga),
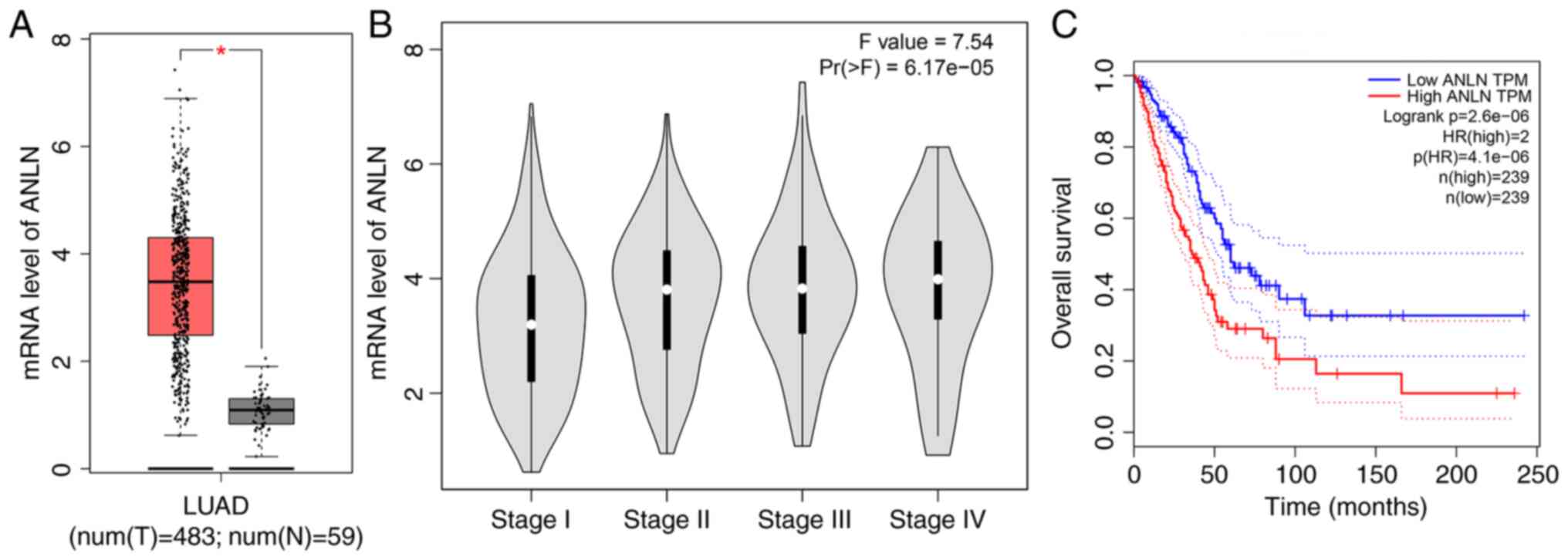
a significant increase in the mRNA expression levels of ANLN was
detected in LUAD tissues compared with adjacent normal samples
(P<0.05; Fig. 1A).
Subsequently, by analyzing TCGA and Genotype-Tissue Expression
databases on the GEPIA website, it was revealed that the mRNA
expression levels of ANLN were high in LUAD and were gradually
increased with advancing clinical stage (Fig. 1B). Moreover, Kaplan-Meier Plotter
database analysis revealed that patients with high ANLN expression
had significantly poorer overall survival (OS) than those
expressing low levels of ANLN (HR=2, log-rank
P=2.6×10−6; Fig. 1C).
Collectively, these findings suggested that ANLN may serve as a
promoter gene in LUAD.
Knockdown of ANLN promotes cell death,
and suppresses the colony formation and migration of A549 and H1299
cells
To investigate the potential role of ANLN in LUAD,
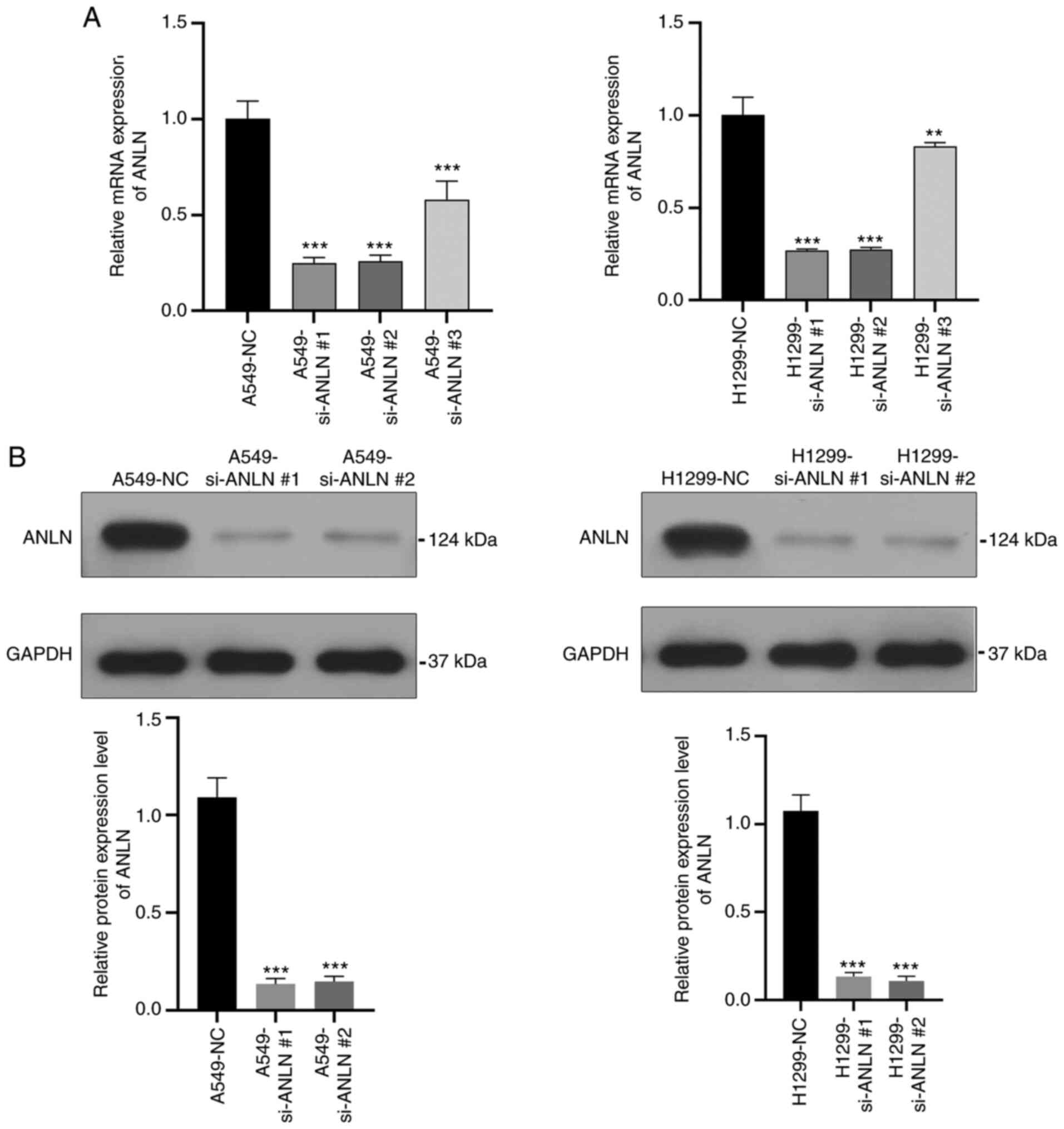
the present study transfected A549 and H1299 cells with an ANLN
siRNA. As demonstrated by qPCR and western blot analysis, the mRNA
and protein expression levels of ANLN were significantly decreased
in the ANLN knockdown groups compared with those in the NC groups
(Fig. 2A and B), indicating that
ANLN was effectively downregulated in these cells. Moreover,
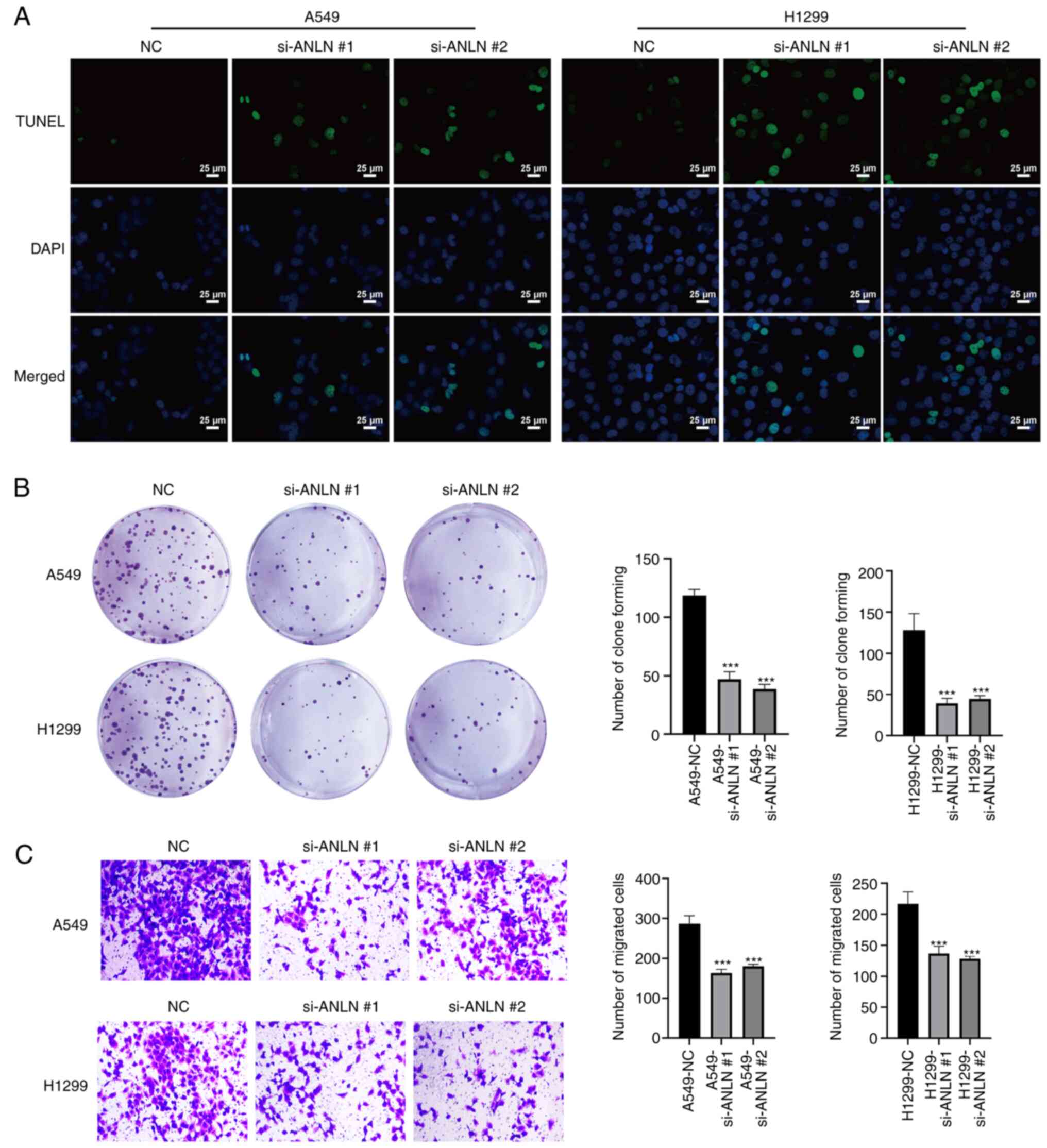
TUNEL/DAPI double staining revealed a marked increase in the number
of TUNEL-positive dead cells in the ANLN knockdown groups compared
with in the NC groups (Fig. 3A).
In addition, the colony-forming ability of A549 and H1299 cells was
significantly reduced upon ANLN silencing compared with that in the
NC groups (Fig. 3B). Furthermore,
knockdown of ANLN in A549 and H1299 cells significantly attenuated
cell migration compared with that in the NC groups, as confirmed by
Transwell assays (Fig. 3C).
Knockdown of ANLN potentiates
pyroptosis in A549 and H1299 cells
The present study analyzed the expression of
pyroptosis-associated proteins to investigate the potential effects
of ANLN on cell viability through a pyroptosis-dependent mechanism.
The results indicated that ANLN knockdown led to an increase in the
expression levels of inflammasome/pyroptosis-related proteins, such
as caspase-1, NLRP3, cleaved-GSDMD, IL-1β, ASC and IL-18, thus
suggesting that the knockdown of ANLN may trigger pyroptosis in
A549 and H1299 cells (Fig. 4A and
B).
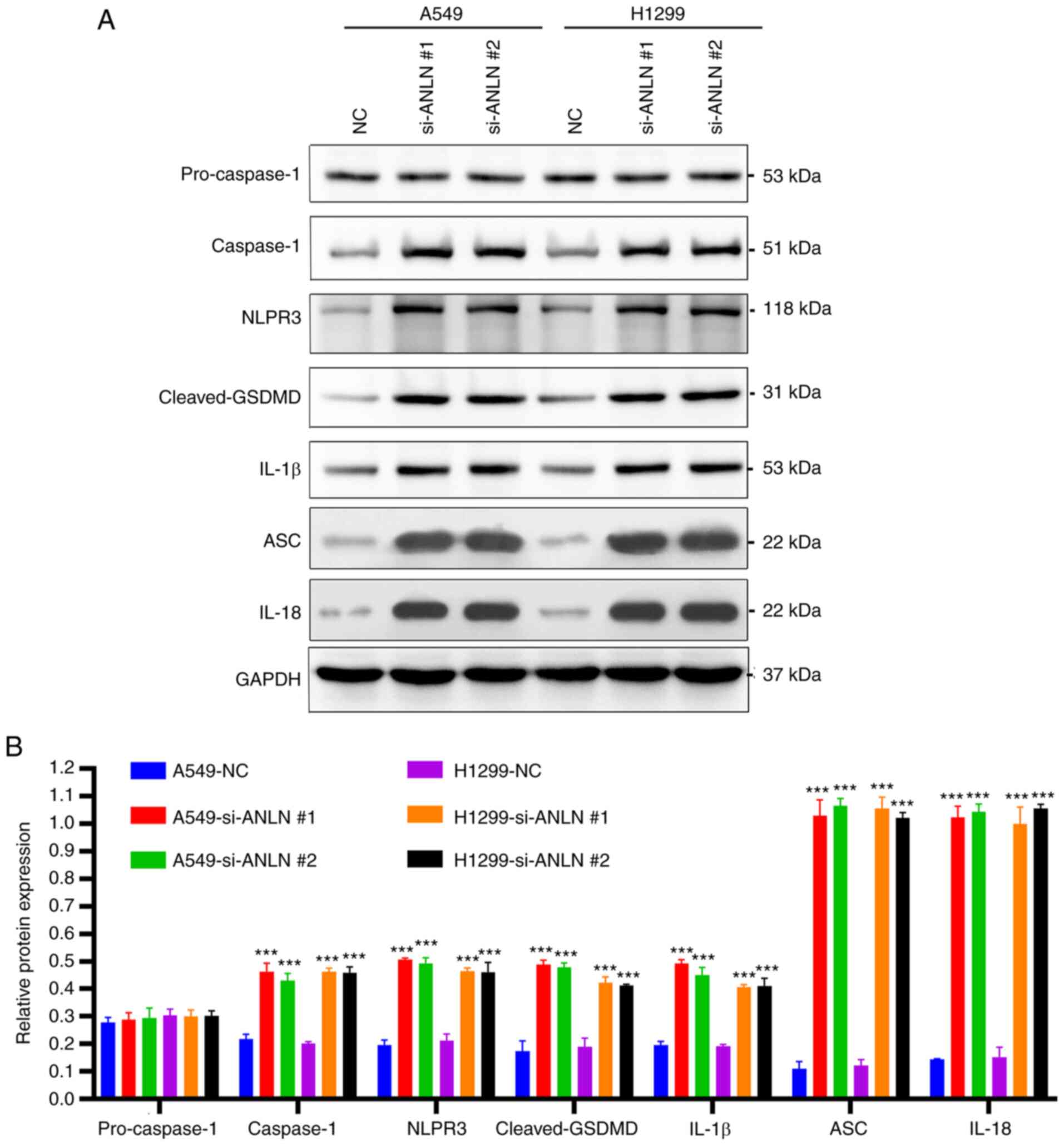 | Figure 4.ANLN knockdown induces pyroptosis in
A549 and H1299 cells. (A) Protein expression levels of
pro-caspase-1, caspase-1, NLRP3, cleaved-GSDMD, IL-1β, ASC and
IL-18 protein level in A549 and H1299 cells were detected by
western blot analysis. (B) Grayscale analysis was performed by
ImageJ software. Data are presented as the mean ± SD (n=3).
***P<0.001 vs. NC. ANLN, anillin; ASC, apoptosis-associated
speck-like protein containing a CARD domain; GSDMD, gasdermin D;
IL, interleukin; NC, negative control; NRP3, NLR family pyrin
domain-containing 3; si, small interfering. |
Discussion
The aim of the present study was to identify a
potential therapeutic target for patients with LUAD, a type of lung
cancer that accounts for ~60% of all cases and has a 5-year
survival rate of <20% (2,7).
Although significant progress has been made in the molecular
targeted therapy of LUAD, particularly for those with specific
mutations in EGFR, ALK, RET and ROS1 (3,24–27),
the high heterogeneity and complex molecular patterns of LUAD limit
the applicability of these targeted therapies, thus leaving a
number of patients without effective treatment options (28). Therefore, it is critical to
discover new targets for the treatment of LUAD.
ANLN, an actin-binding protein involved in cell
division, serves a crucial role in cell proliferation and migration
(29). Notably, localization of
ANLN varies during different phases of the cell cycle. During
interphase, the ANLN protein is localized exclusively in the
nucleus, whereas it becomes cytoplasmic during mitosis (30). Furthermore, ANLN has been
identified as a poor prognostic marker associated with aggressive
cancer phenotypes in multiple types of cancer, including bladder
cancer (31), hepatocellular
carcinoma (32), colorectal cancer
(33), head and neck squamous cell
carcinoma (34) and stage I LUAD
(14). The present study
demonstrated that high ANLN expression was significantly associated
with clinical stage progression in patients with LUAD and that
those exhibiting higher expression levels of ANLN had poorer
prognoses. These findings are consistent with previously reported
results, which suggest that ANLN could serve as a novel prognostic
indicator for patients with LUAD.
As an actin-binding protein, ANLN has a crucial role
in regulating various cellular processes, such as cell
proliferation, apoptosis and invasion. Silencing ANLN in colorectal
carcinoma cell lines has been reported to result in reduced
proliferation in vitro and in vivo, along with
induction of G0/G1 cell cycle arrest
(35). Similarly, knockdown of
ANLN in breast cancer cells was shown to markedly inhibit cell
proliferation, migration and invasion, leading to cell cycle arrest
in the G2/M phase and the induction of apoptosis
(36). Exosomal ANLN-210 has also
been found to promote macrophage polarization via the PTEN/PI3K/Akt
signaling pathway, thereby stimulating tumor growth in head and
neck squamous cell carcinoma (34). The present study confirmed that
silencing ANLN markedly increased cell death, and suppressed the
colony formation and migration of A549 and H1299 cells,
highlighting the potential therapeutic significance of targeting
ANLN in LUAD.
Pyroptosis is a unique form of programmed cell death
that is highly dependent on inflammation, which is distinct from
apoptosis (37). Pyroptosis can be
induced via caspase-1 (mammals), human caspase-4 and caspase-5, or
mouse caspase-11 (38).
Inflammasomes, such as the NLRP3 inflammasome, initiate caspase-1
activation (39,40), and the adaptor protein ASC
facilitates the interaction between NLRP3 and caspase-1 (41,42).
Upon activation, caspase-1 cleaves GSDMD to generate the N-terminal
fragment of GSDMD (GSDMD-N), which is crucial for pyroptosis
(43). GSDMD-N interacts with the
lipids of the inner membrane, leading to pore formation in the
plasma membrane, ultimately causing cellular swelling and rupture,
and the release of pro-inflammatory cytokines, resulting in
pyroptotic cell death (44,45).
Previous studies have reported the induction of pyroptosis in
endothelial and bronchial epithelial cells through caspase-1
activation. For example, caspase-1 inflammasome activation has been
shown to mediate homocysteine-induced pyroptosis in endothelial
cells (46). Similarly, cigarette
smoke extract can induce pyroptosis in human bronchial epithelial
cells via the ROS/NLRP3/caspase-1 pathway (47). WSPM2.5 also induces pyroptosis
through caspase-1/IL-1β/IL-18- and ATP/P2Y-dependent mechanisms in
human bronchial epithelial cells (48). The present study revealed that ANLN
knockdown increased the protein expression levels of caspase-1,
which suggests that caspase-1 activation may be involved in ANLN
knockdown-induced pyroptosis in A549 and H1299 cells. Human
caspase-4 and caspase-5, or mouse caspase-11, can directly cleave
GSDMD to induce pyroptosis (38).
In the context of Caspase-1-independent pyroptosis, caspase-4,
caspase-5 and caspase-11 serve as initiating activators and
directly recognize LPS from gram-negative bacteria in the host
cytoplasm through their CARD domains (49). Although caspase-4 has been
suggested to cleave pro-IL-1β and pro-IL-18 in epithelial cells,
further confirmation of this finding is required (50).
Analysis of prognosis has revealed a lower survival
rate in patients with LUAD who exhibit low expression levels of
NLRP7, NLRP1, NLRP2 and nucleotide binding oligomerization domain
containing 1, as well as high expression levels of caspase-6
(17). Additionally, activation of
the NLRP3 inflammasome has been shown to accelerate the progression
of LUAD by promoting the proliferation and metastasis of lung
cancer cells (18). Conversely,
downregulation of GSDMD has been demonstrated to mitigate tumor
proliferation via the intrinsic mitochondrial apoptotic pathway, as
well as through inhibition of EGFR/Akt signaling, and is associated
with favorable prognostic outcomes in LUAD (19,20).
In the current study, it was revealed that knockdown of ANLN
significantly increased the expression levels of caspase-1, NLRP3,
cleaved-GSDMD, IL-1β, ASC and IL-18 in A549 and H1299 cells. These
findings suggested that silencing ANLN may have a critical role in
promoting pyroptotic cell death in LUAD. Therefore, these data
demonstrated that ANLN knockdown could lead to pyroptosis in A549
and H1299 cells, which may affect the progression of LUAD.
In conclusion, knockdown of ANLN can significantly
disrupt the progression of LUAD by inducing pyroptosis, and
suppressing the colony formation and migration of A549 and H1299
cells. These findings indicated that ANLN may serve as a promising
therapeutic target for the treatment of LUAD.
Acknowledgements
Not applicable.
Funding
This work was supported by the Key Research and Development
Program of Hainan Province, China (grant no. ZDYF2019189).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS, YK and DC conceived and designed this study. LS,
YK and LYS carried out the analyses and also participated in the
study design. LS wrote the manuscript. YK and LS confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Szczepanski AP, Tsuboyama N, Watanabe J,
Hashizume R, Zhao Z and Wang L: POU2AF2/C11orf53 functions as a
coactivator of POU2F3 by maintaining chromatin accessibility and
enhancer activity. Sci Adv. 8:eabq24032022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Ren F, Sun D, Liu J, Liu B, He Y,
Pang S, Shi B, Zhou F, Yao L, et al: CircKEAP1 suppresses the
progression of lung adenocarcinoma via the miR-141-3p/KEAP1/NRF2
axis. Front Oncol. 11:6725862021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan J, Yuan B, Zeng L, Liu B, Chen Y,
Meng X, Sun R, Lv X, Wang W and Yang S: Identification and
validation of tumor microenvironment-related genes of prognostic
value in lung adenocarcinoma. Oncol Lett. 20:1772–1780. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazières J, Zalcman G, Crinò L, Biondani
P, Barlesi F, Filleron T, Dingemans AM, Léna H, Monnet I,
Rothschild SI, et al: Crizotinib therapy for advanced lung
adenocarcinoma and a ROS1 rearrangement: Results from the EUROS1
cohort. J Clin Oncol. 33:992–999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller KD, Nogueira L, Devasia T, Mariotto
AB, Yabroff KR, Jemal A, Kramer J and Siegel RL: Cancer treatment
and survivorship statistics, 2022. CA Cancer J Clin. 72:409–436.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia H, Gao Z, Yu F, Guo H and Li B:
Actin-binding protein anillin promotes the progression of
hepatocellular carcinoma in vitro and in mice. Exp Ther Med.
21:4542021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strausberg RL, Feingold EA, Grouse LH,
Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler
GD, Altschul SF, et al: Generation and initial analysis of more
than 15,000 full-length human and mouse cDNA sequences. Proc Natl
Acad Sci USA. 99:16899–16903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ota T, Suzuki Y, Nishikawa T, Otsuki T,
Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, et al:
Complete sequencing and characterization of 21,243 full-length
human cDNAs. Nat Genet. 36:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lian YF, Huang YL, Wang JL, Deng MH, Xia
TL, Zeng MS, Chen MS, Wang HB and Huang YH: Anillin is required for
tumor growth and regulated by miR-15a/miR-16-1 in HBV-related
hepatocellular carcinoma. Aging (Albany NY). 10:1884–1901. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng F, Xu Z, Zhou J, Zhang R and Gong X:
ANLN Regulated by miR-30a-5p mediates malignant progression of lung
adenocarcinoma. Comput Math Methods Med. 2021:95492872021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo C, Lei M and Zhang Y, Zhang Q, Li L,
Lian J, Liu S, Wang L, Pi G and Zhang Y: Systematic construction
and validation of an immune prognostic model for lung
adenocarcinoma. J Cell Mol Med. 24:1233–1244. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng Y, Chen X, Huang C, Song J, Feng S,
Chen X and Zhou R: Screening and validation of significant genes
with poor prognosis in pathologic stage-I lung adenocarcinoma. J
Oncol. 2022:37940212022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Luo Y, Cheng T, Chen J, Yang H,
Wen X, Jiang Z, Li H and Pan C: Development and validation of a
prognostic N6-methyladenosine-related immune gene signature for
lung adenocarcinoma. Pharmgenomics Pers Med. 14:1549–1563.
2021.PubMed/NCBI
|
|
16
|
Broz P, Pelegrin P and Shao F: The
gasdermins, a protein family executing cell death and inflammation.
Nat Rev Immunol. 20:143–157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin W, Chen Y, Wu B, Chen Y and Li Z:
Identification of the pyroptosis-related prognostic gene signature
and the associated regulation axis in lung adenocarcinoma. Cell
Death Discov. 7:1612021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu H, Deng W, Zhang Y, Chang Y, Shelat VG,
Tsuchida K, Lino-Silva LS and Wang Z: NLRP3 activation in
tumor-associated macrophages enhances lung metastasis of pancreatic
ductal adenocarcinoma. Transl Lung Cancer Res. 11:858–868. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Qiu X, Xi G, Liu H, Zhang F, Lv T
and Song Y: Downregulation of GSDMD attenuates tumor proliferation
via the intrinsic mitochondrial apoptotic pathway and inhibition of
EGFR/Akt signaling and predicts a good prognosis in non-small cell
lung cancer. Oncol Rep. 40:1971–1984. 2018.PubMed/NCBI
|
|
20
|
Wang Y, Kong H, Zeng X, Liu W, Wang Z, Yan
X, Wang H and Xie W: Activation of NLRP3 inflammasome enhances the
proliferation and migration of A549 lung cancer cells. Oncol Rep.
35:2053–2064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai X, Chen X, Hakizimana O and Mei Y:
Genetic interactions between ANLN and KDR are prognostic for breast
cancer survival. Oncol Rep. 42:2255–2266. 2019.PubMed/NCBI
|
|
22
|
Wang Y, Zhang JW, Wang JW, Wang JL, Zhang
SC, Ma RY, Zhang J, Li Y, Liu PJ, Xue WJ, et al: BMSCs
overexpressed ISL1 reduces the apoptosis of islet cells through
ANLN carrying exosome, INHBA, and caffeine. Cell Mol Life Sci.
79:5382022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harrison PT, Vyse S and Huang PH: Rare
epidermal growth factor receptor (EGFR) mutations in non-small cell
lung cancer. Semin Cancer Biol. 61:167–179. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muller IB, de Langen AJ, Giovannetti E and
Peters GJ: Anaplastic lymphoma kinase inhibition in metastatic
non-small cell lung cancer: Clinical impact of alectinib. Onco
Targets Ther. 10:4535–4541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Drilon A, Wang L, Hasanovic A, Suehara Y,
Lipson D, Stephens P, Ross J, Miller V, Ginsberg M, Zakowski MF, et
al: Response to Cabozantinib in patients with RET fusion-positive
lung adenocarcinomas. Cancer Discov. 3:630–635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bergethon K, Shaw AT, Ou SH, Katayama R,
Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang
R, et al: ROS1 rearrangements define a unique molecular class of
lung cancers. J Clin Oncol. 30:863–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan AC and Tan DSW: Targeted therapies for
lung cancer patients with oncogenic driver molecular alterations. J
Clin Oncol. 40:611–625. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hall G, Lane BM, Khan K, Pediaditakis I,
Xiao J, Wu G, Wang L, Kovalik ME, Chryst-Stangl M, Davis EE, et al:
The human FSGS-causing ANLN R431C mutation induces dysregulated
PI3K/AKT/mTOR/Rac1 signaling in podocytes. J Am Soc Nephrol.
29:2110–2122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi Y, Ma X, Wang M, Lan S, Jian H, Wang
Y, Wei Q and Zhong F: Comprehensive analyses reveal the
carcinogenic and immunological roles of ANLN in human cancers.
Cancer Cell Int. 22:1882022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Chen L, Ju L, Qian K, Liu X, Wang
X and Xiao Y: Novel biomarkers associated with progression and
prognosis of bladder cancer identified by co-expression analysis.
Front Oncol. 9:10302019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang F, Cai J, Hu K, Liu W, Lu S, Tang B,
Li M, Wu W, Ren Z and Yin X: An immune-related gene signature
predicting prognosis and immunotherapy response in hepatocellular
carcinoma. Comb Chem High Throughput Screen. 25:2203–2216. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang G, Shen W, Cui L, Chen W, Hu X and Fu
J: Overexpression of Anillin (ANLN) is correlated with colorectal
cancer progression and poor prognosis. Cancer Biomark. 16:459–465.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo E, Mao X, Wang X, Guo L, An C, Zhang
C, Song K, Wang G, Duan C, Zhang X, et al: Alternatively spliced
ANLN isoforms synergistically contribute to the progression of head
and neck squamous cell carcinoma. Cell Death Dis. 12:7642021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Cao P, Cao F, Wang S, He Y, Xu Y
and Wang Y: ANLN, regulated by SP2, promotes colorectal carcinoma
cell proliferation via PI3K/AKT and MAPK signaling pathway. J
Invest Surg. 35:268–277. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Z, Hu S, Li X, Liu Z, Han D, Wang Y,
Wei L, Zhang G and Wang X: MiR-16-5p suppresses breast cancer
proliferation by targeting ANLN. BMC Cancer. 21:11882021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu P, Zhang X, Liu N, Tang L, Peng C and
Chen X: Pyroptosis: Mechanisms and diseases. Signal Transduct
Target Ther. 6:1282021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Man SM and Kanneganti TD: Converging roles
of caspases in inflammasome activation, cell death and innate
immunity. Nat Rev Immunol. 16:7–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brocker CN, Kim D, Melia T, Karri K,
Velenosi TJ, Takahashi S, Aibara D, Bonzo JA, Levi M, Waxman DJ and
Gonzalez FJ: Long non-coding RNA Gm15441 attenuates hepatic
inflammasome activation in response to PPARA agonism and fasting.
Nat Commun. 11:58472020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsuchiya K, Nakajima S, Hosojima S, Thi
Nguyen D, Hattori T, Manh Le T, Hori O, Mahib MR, Yamaguchi Y,
Miura M, et al: Caspase-1 initiates apoptosis in the absence of
gasdermin D. Nat Commun. 10:20912019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Paik S, Kim JK, Silwal P, Sasakawa C and
Jo EK: An update on the regulatory mechanisms of NLRP3 inflammasome
activation. Cell Mol Immunol. 18:1141–1160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Looi CK, Hii LW, Chung FF, Mai CW, Lim WM
and Leong CO: Roles of inflammasomes in Epstein-Barr
virus-associated nasopharyngeal cancer. Cancers (Basel).
13:17862021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Evavold CL, Hafner-Bratkovič I, Devant P,
D'Andrea JM, Ngwa EM, Boršić E, Doench JG, LaFleur MW, Sharpe AH,
Thiagarajah JR and Kagan JC: Control of gasdermin D oligomerization
and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell.
184:4495–4511.e19. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Vasconcelos NM, Van Opdenbosch N, Van
Gorp H, Parthoens E and Lamkanfi M: Single-cell analysis of
pyroptosis dynamics reveals conserved GSDMD-mediated subcellular
events that precede plasma membrane rupture. Cell Death Differ.
26:146–161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha
X, Cheng X, Wang J, Qin X, Yu J, et al: Caspase-1 inflammasome
activation mediates homocysteine-induced pyrop-apoptosis in
endothelial cells. Circ Res. 118:1525–1539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang MY, Jiang YX, Yang YC, Liu JY, Huo
C, Ji XL and Qu YQ: Cigarette smoke extract induces pyroptosis in
human bronchial epithelial cells through the ROS/NLRP3/caspase-1
pathway. Life Sci. 269:1190902021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fu X, Hong W, Li S, Chen Z, Zhou W, Dai J,
Deng X, Zhou H, Li B and Ran P: Wood smoke particulate matter
(WSPM2.5) induces pyroptosis through both caspase-1/IL-1β/IL-18 and
ATP/P2Y-dependent mechanisms in human bronchial epithelial cells.
Chemosphere. 307:1357262022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kayagaki N, Wong MT, Stowe IB, Ramani SR,
Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP,
Muszyński A, et al: Noncanonical inflammasome activation by
intracellular LPS independent of TLR4. Science. 341:1246–1249.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Knodler LA, Crowley SM, Sham HP, Yang H,
Wrande M, Ma C, Ernst RK, Steele-Mortimer O, Celli J and Vallance
BA: Noncanonical inflammasome activation of caspase-4/caspase-11
mediates epithelial defenses against enteric bacterial pathogens.
Cell Host Microbe. 16:249–256. 2014. View Article : Google Scholar : PubMed/NCBI
|