Introduction
Diabetes is a metabolic disease characterized by
chronic hyperglycemia caused by multiple etiologies, which is
caused by defects in insulin secretion and/or utilization (1). Insulin secretion and its regulation
play an important role in glucose metabolism and homeostasis
(2). The abnormal and
incontrollable insulin secretion in β cells is closely related to
the occurrence of diabetes, but its molecular mechanism has not
been fully understood and remains need to be further clarified. In
recent years, the role of VEGFB in regulating lipid and glucose
metabolism has attracted extensive attention. The present study
found that VEGFB is related to total cholesterol (TC), triglyceride
(TG), and glycosylated hemoglobin (GHb) in T2DM patients (3). In type 2 diabetes mellitus (T2DM)
mice, systemic inhibition of VEGFB improves glucose tolerance and
insulin sensitivity (4). Specific
VEGFB overexpression in rats can ameliorate diabetes by improving
insulin action (5). Targeted
overexpression of the VEGFB signal can improve some key factors
that promote the development of T2DM, including glucose tolerance,
abnormal lipid metabolism and β cell function. Therefore, VEGFB may
become an important regulatory approach in the development of
T2DM.
Insulin secretion is a complex process, and calcium
channels on β cell membrane play an important regulatory role
during this process (6). The
change in intracellular Ca2+ concentration is closely
related to insulin secretion (7).
Previous studies have shown that abnormal insulin secretion in
patients with diabetes may be related to the dysfunction of the
intracellular calcium signaling pathway (8). At present and to the best of the
authors' knowledge, the pathophysiological mechanism of abnormal
insulin secretion remains unclear. Although it has been revealed
that VEGFB can regulate insulin secretion by affecting fatty acid
content, its specific regulatory mechanism also needs to be studied
in the future. VEGFB transduces signals through the protein kinase
C pathway (9). Therefore, can
VEGFB stimulate the release of Ca2+ through
phosphatidylinositol 3-kinase, phospholipase C-1, GTPase activating
protein, and other signal proteins after it combines with VEGFR1.
The answer to these scientific questions will help to further
analyze the pathogenesis of diabetes and provide a theoretical
basis for the precise treatment for it.
The potential molecular mechanism of abnormal
insulin secretion in β cells of T2DM mice were examined. VEGFB
knockout (KO) or overexpression can inhibit or activate
phospholipase C gamma (PLCγ) and inositol 1,4,5-triphosphate
receptor IP3R signaling pathway in a VEGFR1-dependent way. Then,
the change of PLCγ/IP3R caused by VEGFB/VEGFR1 will alter the
expression of key factors on the calcium/calmodulin signaling
pathways such as PPP3CA. KO or overexpression of VEGFB can cause
altered insulin secretion by changing the calcium concentration in
β cells and affect the glucose tolerance and insulin sensitivity of
T2DM mice. The present study demonstrated that VEGFB can regulate
insulin secretion via PLCγ and the IP3R-evoked
Ca2+/CaMK2 signaling pathway.
Materials and methods
Experimental animals
The experiments on mice were approved (IACUC
approval no: 2022-210) by the animal ethics committee of Binzhou
Medical University (Yantai, China). All mice were raised at 24°C,
12/12-h light/dark, 50% humidity). C57BL/6 male mice (n=6) (age, 4
weeks-old; weight, 16–18 g), were selected into 5 experimental
groups: wild-type (WT), streptozocin (STZ)-WT, STZ-KO,
adeno-associated virus (AAV)-control, and AAV–VEGFB186
group. VEGFB+/+ mice fed standard diet (SD, Rodent Diet
with 10% kcal% fat, Jiangsu Xietong Pharmaceutical Bio-engineering
Co., Ltd.) were named the WT group (n=6). A total of four groups of
mice induced by STZ and high-fat diet (HFD, Rodent Diet with 60%
kcal% fat, Research Diets, Inc.) were T2DM models. WT group was not
induced with STZ. VEGFB+/+ T2DM mice were named STZ-WT
(n=6), and VEGFB−/− T2DM mice were named STZ-KO (n=6).
VEGFB+/+ T2DM mice injected with AAV targeting
VEGFB186 were named the AAV–VEGFB186 group
(n=6), and mice in the AAV-control group were injected with
non-targeting VEGFB186 and were regarded as the negative
control (n=6). WT, STZ-WT and STZ-KO groups of mice were euthanized
and measured in the 24th week. Some mice developed complications of
T2DM after 32 weeks due to the longer course of T2DM, therefore the
latter two groups were in the 32nd week (10–13).
In the animal experiment, all mice were administered 3% isoflurane,
and sacrificed by cervical dislocation after blood collection from
the eyeball. Pancreatic tissues of mice were removed and fixed with
4% paraformaldehyde or 2.5% glutaraldehyde.
Cell culture and treatment
Mouse islet β cell line Min6 was purchased from the
Procell Life Science&Technology Company and was cultured in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) medium containing
10% fetal bovine serum, and 1% Penicillin/streptomycin (P/S). The
temperature inside the incubator was 37°C and the gas proportion
was 5% CO2. Min6 cells were adhered in the six-well
plate to the confluence of 50% and grouped into negative control
(NC) and silencing (SI) groups. Cells in the NC group were treated
with non-targeting VEGFB sequence (5′UUCUCCGAACGUGUCACGUTT3′, 3′
ACGUGACACGUUCGGAGAATT 5′) while SI groups were treated with VEGFB
KO sequence [VEGFB small interfering RNA (siRNA):
5′GAACACAGCCAATGTGAAT 3′] in the SI group. JetPRIME and Jet buffer
(Polyplus-transfection® Inc., United States) were used
to transfect the sequence within 48 h at room temperature and then
the cells in two groups were detected the efficiency of
transfection by reverse transcription-quantitative (RT-q) PCR and
western blot analysis.
VEGFB KO mouse
VEGFB KO mouse model was constructed by CRISPR/Cas 9
technology. The work was undertaken by the Saiye (Guangzhou)
Biotechnology Co., Ltd. The gRNA sequences were as follows: gRNA-1,
5′-AAGGGCTCCGTCCTTGAGTCAGG-3′; and gRNA-2,
5′-CAGGGGATGACTTATGGGCCAGG-3′. The wild-type mice were not
transfected with any control construct. A total of two pairs of
primers were used for the PCR cycle and the sequences were as
follows: primer 1 forward, 5′-TCTCAAGGTTGGCGGAAGTGG-3′ and reverse,
5′-CAAACTCACCATGTCACCAAGGAG-3′; and primer 2 forward,
5′-TCTCAAGGTTGGCGGAAGTGG-3′ and reverse,
5′-TTGGGATCACGCAAGATAAGGG-3′. Mice genotypes were identified by 12%
agarose gel electrophoresis and visualized by ethidium bromide,
VEGFB+/+ and VEGFB−/− mice were screened for
the T2DM model. In the present study, the protein and mRNA levels
of VEGFB expression in VEGFB+/+ and VEGFB−/−
mice were detected in the pancreas (Fig. 1A-D).
 | Figure 1.Construction of the experimental
animal model and the effect of VEGFB on food and body weight. (A)
Gene identification of VEGFB knockout mice. (B and C) The protein
expression of VEGFB in mice (n=6). (D) The mRNA expression of VEGFB
in mice (n=3). (E) The flow diagram of animal experiment design.
(F) Fluorescent expression of AAV-control and
AAV–VEGFB186 in T2DM mice. (G and H) The protein
expression of VEGFB in T2DM mice (n=6). (I) mRNA expression of
VEGFB in mice (n=3). (J and K) Body weight and food intake of WT,
STZ-WT, and STZ-KO mice (n=9). (L and M) TC and TG content of WT,
STZ-WT, and STZ-KO mice (n=10). *P<0.05 vs. WT;
#P<0.05 vs. STZ-WT. (N and O) Body weight and food
intake of WT, AAV-control and AAV–VEGFB186 mice (n=9).
(P and Q) TC and TG content of WT, AAV-control and
AAV–VEGFB186 mice (n=10). *P<0.05 vs. WT;
#P<0.05 vs. AAV-control. VEGFB, vascular endothelial
growth factor B; AAV, adeno-associated virus; T2DM, type 2 diabetes
mellitus; WT, wild-type; STZ, streptozocin; KO, knockout; TC, total
cholesterol; TG, triglyceride. |
T2DM mouse model
The mice were fed with HFD from the 8th week. STZ
was intraperitoneally injected twice at a dose of 30 mg/kg within
the 15–16th weeks, with an interval of 1 day between the two
injections (14,15). The blood glucose of mice was
measured at 0, 3, 10 and 30 days after injection of STZ. Four weeks
after STZ injection, the mice with fasting blood glucose (FBG)
≥11.1 mmol/l were defined as the T2DM model (Fig. 1E).
Overexpression of VEGFB in T2DM
mouse
The AAV vector was purchased from the OBiO
Technology (Shanghai) Corp., Ltd.
AAV-CAG-VEGFB186-P2A-EGFP-3×FLAG-WPRE was regarded as an
overexpression vector and AAV-CAG-EGFP-3×FLAG-WPRE was a control
vector. A total of eight weeks after injection with STZ, the mice
whose FBG was ≥16.7 mmol/l were prepared for intraperitoneal AAV
infection into the pancreas. The virus titer was controlled
>1.0×1012 on each side (Fig. 1F-I).
Measurement of weight, FBG and
postprandial blood glucose (PBG)
From the 8th week, the three indicators were
measured at a fixed time every week. The mice were not fed within
12 h and FBG was examined. After the mice were administered a
resumption of diet for 2 h, the PBG was measured. The blood was
drawn from the tail vein by using a Roche blood glucose meter
(Roche Diabetes Care, Inc.) for FBG and PBG measurement.
Isolation of islet cells
A total of three mice in each group were used to
isolate islets, and 100–150 islet cell clusters could be collected
from each mouse. The pancreas was removed, and the peripheral
adipose tissue was isolated and placed in Hank's buffer after the
mice's death. Collagenase P (0.5 mg/ml; Roche) was injected through
the pancreatic duct and digested for 10 min after complete
expansion of the pancreas. Hank's buffer was pre-cooled at 4°C to
stop digestion, and cell mass was selected under the stereoscopic
microscope (Olympus Corporation).
Western blot analysis
Lysates consisting of 1% cocktail RIPA (cat. no.
R0010; Beijing Solarbio Science & Technology Co., Ltd.) and
PMSF (cat. no. 36978; Gibco; Thermo Fisher Scientific, Inc.) were
added to the cells from islet cell clusters on ice for 30 min. The
loading buffer (cat. no. D1020-5; Beijing Solarbio Science &
Technology Co., Ltd.) was added to the supernatant and heated after
centrifugation. Protein samples (20 µg protein /lane) were
transferred onto PVDF membranes after separating in 10% SDS-PAGE
gel. After blocking with 5% skimmed milk at room temperature for 1
h, membranes were incubated with primary antibodies at 4°C
(Table I). After 12 h, membranes
were incubated with secondary antibodies (1:5,000, cat. no. S0001,
Affinity, HRP) at room temperature for 2 h. Optical density was
detected after samples were treated with enhanced chemiluminescence
reaction (Tanon 5200; Tanon Science & Technology Co., Ltd.).
The blots were performed densitometric analysis by ImageJ software
(version 1.52a, National Institutes of Health)
 | Table I.List of primary antibodies used. |
Table I.
List of primary antibodies used.
| Primary
antibody | Dilution ratio | Source | Cat. no. | Supplier |
|---|
| PPP3CA | 1:1,000 | Rabbit | DF6208 | Affinity |
| PLCγ | 1:1,000 | Rabbit | AF6210 | Affinity |
| IP3R | 1:1,000 | Rabbit | DF3000 | Affinity |
| CAMK2 | 1:1,000 | Rabbit | AF6493 | Affinity |
| VEGFR1 | 1:1,000 | Rabbit | AF6204 | Affinity |
| VEGFB | 1:1,000 | Rabbit | AF7019 | Affinity |
| β-actin | 1:1,000 | Mouse |
T0022 | Affinity |
RT-qPCR
Total RNA was collected from islet cell clusters
with TriQuick Reagent (cat. no. R100; Beijing Solarbio Science
& Technology Co., Ltd.). RNA-easy Isolation Reagent (Vazyme
Biotech Co., Ltd.) reverse transcription and real-time detection
were accomplished with TB Green Premix Ex Taq II (Takara Bio, Inc.)
fluorescence quantitative kit on PCR QuantStudio 3 (Thermo Fisher
Scientific, Inc.). The thermo cycling conditions of RT-qPCR were as
follows: Initial denaturation at 95°C for 30 sec; then 40 cycles
were conducted at 95°C for 5 sec and 60°C for 34 sec; the
dissolution process was performed at 95°C, 60°C and 95°C for 15
sec, 1 min and 15 sec, respectively in the end. The primer
sequences were as follows: VEGFB forward, 5′-GCTGGGCACTAGTTGTTTG-3′
and reverse, 5′-AGCCACCAGAAGAAAGTGG-3′; and β-actin forward,
5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and reverse,
5′-ATGGAGCCACCGATCCACA-3′. The 2−ΔΔCq method was used to
quantify the expression of mRNA by using β-actin as an internal
reference gene (16).
ELISA and colorimetry
Serums from five mice were collected for measurement
of blood glucose (cat. no. ml016964), GHb (cat. no. ml063816) and
insulin (cat. no. ml001983) content with a microplate reader
(BioTek Corp.) by ELISA. A standard curve was established according
to the measured value of the standard and the sample content was
calculated. TG (cat. no. A110-1-1), and TC (cat. no. A111-1-1; both
from Nanjing Jiancheng Bioengineering Institute) were detected by
the colorimetry method according to the manufacturer's
instructions.
Hematoxylin and eosin (H&E)
Staining
Pancreatic tissues of 3 mice in each group were
fixed with 4% paraformaldehyde at 4°C for 12 h. After dehydration
by an automatic dehydrator, tissue was embedded with paraffin, and
sliced into 5-µm sections. The section was dewaxed to water with
xylene and stained with hematoxylin for 5 min and eosin for 1 min
at room temperature. After sealing with neutral glue, the images of
the samples were acquired by the optical microscope (OLYMPUS-DP27;
Olympus Corporation).
Transmission electron microscopy
The pancreas tissues of 3 mice in each group were
fixed with 2.5% glutaraldehyde solution and 1% osmic acid at 4°C
for 12 h. Tissues were subjected to mixed treatment with entrapment
agent and acetone (v/v=1/2) after dehydration with gradient
alcohol, pure entrapment agent-permeated and embedded.
Subsequently, they were sliced with Reichert ultra-thin microtome
(70 nm). Lead citrate solution and uranyl acetate 50% ethanol
saturated solution were used for staining at room temperature for
10 min, respectively. A transmission electron microscope (JEM-1400;
JEOL, Ltd.) was used to observe and capture images of the
sections.
Immunofluorescence
After paraffin removal with xylene, gradient
hydration with ethanol and antigen repair with sodium citrate and
3% H2O2 solution-eliminated endogenous
peroxidase activity, 5% goat serum (cat. no. SL038, Solarbio) was
used for blocking at 37°C for 30 min. Then the antibody mixture of
insulin (1:200; cat. no. 66198-1-ig; ProteinTech Group, Inc.) and
glucagon (1:100; cat. no. ab92517; Abcam) was added dropwise and
incubated for 12 h in a 4°C wet box. The next day, the mixture of
fluorescent goat anti-rabbit IgG/TRITC (1:100; cat. no. ZF-0317;
OriGene Technologies, Inc.) and goat anti-mouse IgG/FITC (1:100;
cat. no. ZF-0314; OriGene Technologies, Inc.) was added and
incubated for 1 h. Afterwards, it was stained with 10 µg/ml DAPI,
rinsed, blocked, and stored at 4°C without light after washing with
PBS. Images were captured by a confocal laser scanning microscope
(LSM880; Zeiss AG).
Oral glucose tolerance test (OGTT) and
intraperitoneal insulin tolerance test (IPITT)
During the OGTT, mice were not fed within 12 h and
then ravaged with 40% glucose at the dose of 2 mg/kg. During the
IPITT, the mice were injected intraperitoneally with 0.5 UI/kg
insulin after fasting for 6 h. Blood glucose at 0, 15, 30, 60, 90
and 120 min was detected.
Islet secretion function index
FBG, fasting insulin (FINS), insulin increment
(ΔI30), and glucose increment (ΔG30) at 30 min in OGTT, 1 and 2 h
of PBG of five mice in each group were detected. Insulin secretion
index of the steady-state model (HOMA-β)=FINS ×20/(FBG-3.5); Islet
β cells secretion index (ΔI30/ΔG30)=the ratio of insulin increment
to glucose increment in OGTT at 30 min; modified β cells function
index=(FINS × FBG)/(PBG 1 h + PBG 2h-2FBG).
Glucose stimulation
Min6 cell line and islet cells from 3 mice in each
group were used for the detection. Fresh islets and Min6 cells were
cultured overnight in the sugar-free medium at 37°C. After washing,
they were cultured with 2.8 mmol/l low-sugar medium at 37°C for 2
h, and incubation medium was collected to detect insulin (Shanghai
Enzyme-linked Biotechnology Co., Ltd.; cat. no. ml001983) and
intracellular Ca2+ content (Shanghai Enzyme-linked
Biotechnology Co., Ltd.; cat. no. ml058009). And then 16.7 mmol/l
high-sugar medium was replaced for the incubation.
Calcium content analyses
Intracellular calcium content was detected according
to the manufacturer's instructions (cat. no. ml058009). Diluted
standard and samples were added to the 96-well plate with 50 µl,
and then the antibodies were added with 50 µl. The membrane plate
was covered, gently shaken and mixed, and incubated at 37°C for 1
h. The enzyme HRP was added after washing with buffer three times
and incubated at 37°C for 30 min. A total of 50 µl of substrates A
and B was added to each well, gently shaken and mixed, and
incubated at 37°C for 10 min without light. The OD value was
measured at a wavelength of 450 nm after adding 50 µl of
termination solution.
Proteomic analysis
Islet cells of five VEGFB+/+ and
VEGFB−/− mice were isolated. Meanwhile, islet cells of
five VEGFB+/+STZ and VEGFB−/−STZ mice were
isolated for proteomic analysis. PBS containing protease inhibitors
and phosphatase inhibitors were used to treat cells. And then
homogenated in a denatured buffer containing urea, HEPES. The
Bradford assay (Bio-Rad Laboratories, Inc.) was used to examine the
protein content. DL-Dithiothreitol solution and Iodoacetic amide
solution were added. Trypsin/Lys-C (FUJIFILM Wako Pure Chemical
Corporation) was added so that the final concentration of the
sample digestion buffer was 5% (w/w) trypsin/protein.
Trifluoroacetic acid and acetonitrile (ACN) were used for column
washing. The peptide elution fractions were labeled with 6-plex TMT
reagent and then the labeled peptide was acidified with formic acid
(pH 2.5), and the sample was filtered and desalted through C18
Stage-tips, and completely dried in a vacuum centrifuge. Peptides
were dissolved and separated by RPLC-MS using the EASY-nLC 1000
system (Thermo Fisher Scientific, Inc.). The peptide was washed at
250 l/min with ACN concentrated from 4–100%. All results of data
were analyzed by using a QExactive plus Orbitrap mass spectrometer
(Thermo Fisher Scientific, Inc.). The mass spectrometer was
operated in the positive ion module to obtain the investigation
mass spectrum with 7000 resolution and the successive high
collision dissociation fragmentation spectrum. The bioinformatics
tools used to analyze the heatmap were HIPLOT
(hiplot.com.cn/home/index.html) and Gene Set Analysis Toolkit
(https://www.webgestalt.org).
Statistical analysis
SPSS 22.0 statistical software (IBM Corp.) was used
to analyze all data. The results were shown as the mean ± SD.
One-way ANOVA followed by Dunnett's post hoc test was used, while
comparisons between two groups were assessed using paired Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
VEGFB regulates glucolipid metabolism
and insulin sensitivity in T2DM mice
From the 14th week, the weight of mice fed HFD was
higher than those of mice fed SD, and the weight of STZ-KO mice was
higher in comparison with STZ-WT mice in the 18th week (Fig. 1J). There was no significant
difference between SD and HFD feeding except in the 22nd and 24th
week (Fig. 1K). In the 24th week,
the TC and TG of STZ-KO mice were significantly higher than those
of STZ-WT mice (Fig. 1L and M).
When the T2DM mice were administered AAV injection, the weight and
food intake of AAV–VEGFB186 mice were decreased from the
30th week (Fig. 1N and O). And the
TC and TG contents were decreased when compared with STZ mice
(Fig. 1P and Q).
From the 18th week, the FBG and PBG of T2DM mice
were increased. In STZ-KO mice, FBG was increased from the 22nd
week and PBG was increased from the 18th week compared with STZ-WT
mice (Fig. 2A and B). Under the
HFD condition, the serum glucose and GHb of STZ-KO mice were higher
than those of STZ-WT mice in the 24th week (Fig. 2C and D). OGTT and IPITT revealed
that the ability to regulate blood glucose in T2DM mice and the
efficiency of glucose uptake and utilization promoted by insulin
decreased. Blood glucose of STZ-KO mice was higher than that of
STZ-WT mice with the stimulation of glucose and insulin. At the
same time, the area under the curve also increased (Fig. 2E-H).
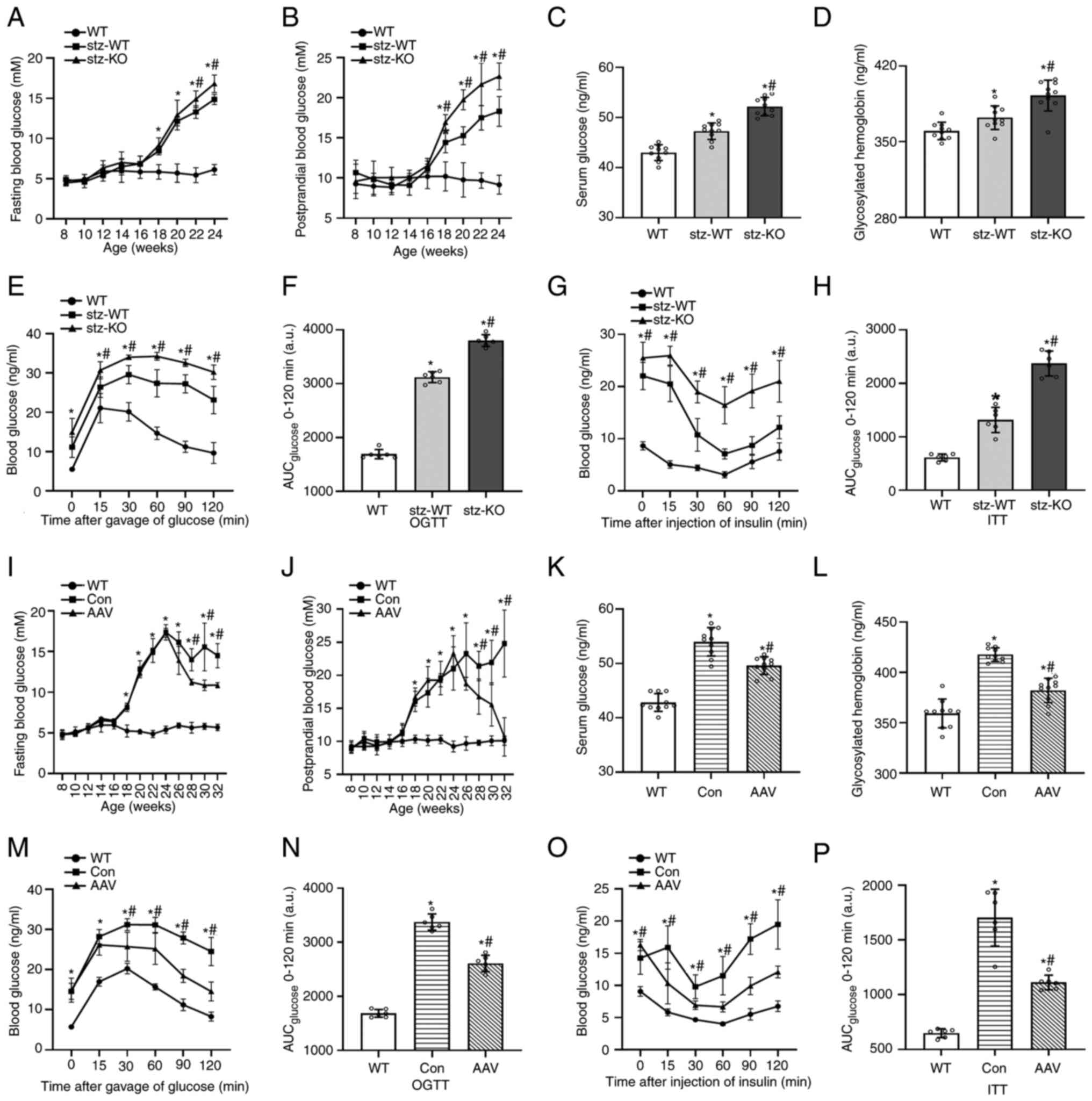 | Figure 2.Effect on glucolipid metabolism in
T2DM mice after VEGFB knockout and overexpression. (A and B) FBG
and PBG curves of WT, STZ-WT and STZ-KO mice from the 8th to 24th
weeks (n=9). (C and D) Serum glucose and GHb contents of WT, STZ-WT
and STZ-KO mice (n=10). (E-H) OGTT, AUC of OGTT, IPITT, AUC of
IPITT of WT, STZ-WT and STZ-KO mice (n=6). *P<0.05 vs. WT;
#P<0.05 vs. STZ-WT. (I and J) FBG and PBG curves of
WT, AAV-control and AAV–VEGFB186 mice from the 8th to
32nd weeks (n=9). (K and L) Serum glucose and GHb contents of WT,
AAV-control and AAV–VEGFB186 mice (n=10). (M-P) OGTT,
AUC of OGTT, IPITT, AUC of IPITT of WT, AAV-control and
AAV–VEGFB186 mice (n=6). *P<0.05 vs. WT;
#P<0.05 vs. AAV-control. T2DM, type 2 diabetes
mellitus; VEGFB, vascular endothelial growth factor B; FBG, fasting
blood glucose; PBG postprandial blood glucose; STZ, streptozocin;
WT, wild-type; KO, knockout; AAV, adeno-associated virus; GHb,
glycosylated hemoglobin; OGTT, oral glucose tolerance test; IPITT,
intraperitoneal insulin tolerance test; AUC, area under the
curve. |
Compared with the Con group, the body weight and
blood glucose of mice in the AAV group decreased significantly from
the 24th week, especially the decrease in PBG, indicating that
VEGFB has a therapeutic effect on blood glucose in T2DM mice
(Fig. 2I and J). In addition,
serum glucose and GHb were significantly lower in the 32nd week
(Fig. 2K and L). The OGTT and ITT
results demonstrated that the glucose tolerance and insulin
sensitivity of AAV–VEGFB186 mice increased compared with
the WT group but decreased compared with control group (Fig. 2M-P).
VEGFB affects insulin secretion of
islet β cells in T2DM mice
Compared with WT, the serum insulin and insulin
secretion function of T2DM mice was lower, while the serum insulin
and insulin secretion function of the STZ-KO group was
significantly lower than those of the STZ-WT group (Fig. 3A-D). After AAV injection, the serum
insulin and insulin secretion function of AAV–VEGFB186
mice were higher than AAV-control mice (Fig. 3E-H).
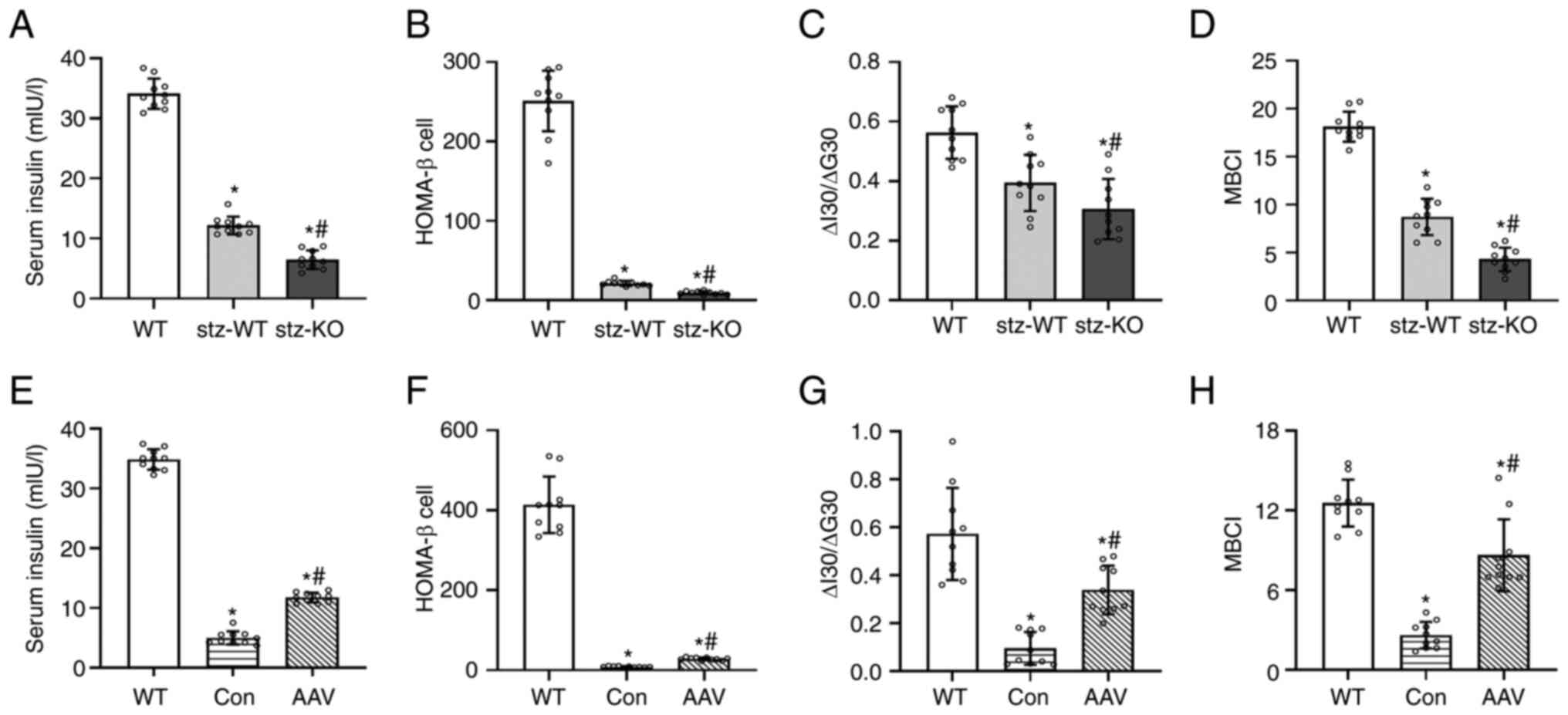 | Figure 3.Effect of up- and downregulated VEGFB
on serum insulin and β cell function index. (A) Serum insulin
contents of WT, STZ-WT and STZ-KO mice (n=10). (B-D) HOMA-β,
ΔI30/ΔG30, and MBCI of WT, STZ-WT and STZ-KO mice (n=10).
*P<0.05 vs. WT; #P<0.05 vs. STZ-WT. (E) Serum
insulin contents of WT, AAV-control and AAV–VEGFB186
mice (n=10). (F-H) HOMA-β, ΔI30/ΔG30, and MBCI of WT, AAV-control
and AAV–VEGFB186 mice (n=10). *P<0.05 vs. WT;
#P<0.05 vs. AAV-control. VEGFB, vascular endothelial
growth factor B; STZ, streptozocin; WT, wild type; KO, knockout;
AAV, adeno-associated virus. |
The morphological changes of pancreatic islets were
observed by H&E staining. It is not easy to distinguish
multiple cell types in the islets under H&E staining. The
endocrine cells in the islets mainly include A cells (~20% of the
total islet cells), B cells (~75% of the total islet cells), D
cells (~5% of the total islet cells), and others such as PP cells
and D1 cells (17,18). The islets of mice in the WT group
were round or oval with clear boundaries and close arrangement
between islet cells. The size of islets in T2DM mice was not
homogeneous, and some islets showed atrophy and volume reduction. β
cells, with a large number, were in the center of the islet, while
α cells, with a small number, were in the periphery of the pancreas
islet. The nucleus of β cells in WT was circular and intact, and
secretory vesicles could also be observed. The volume of β cells
and the number of mitochondria decreased in T2DM mice (Fig. 4A). After the injection of
AAV–VEGFB186, the size of the islet was improved
(Fig. 4B).
 | Figure 4.Effect of VEGFB on the islet, β cells
and insulin secretory vesicles of mice. (A and B) The morphology of
islets by light microscope (magnification, ×400; scale bar, 50 µm),
immunofluorescence (magnification, ×400; scale bar, 50 µm), and
electron microscopy (magnification, ×8,000; scale bar, 2 µm). The
arrows stand for the islet. (C) Number of islet cells, (D) density
of β cells, and the density of (E) mature and (F) immature insulin
secretory vesicles in β cells of WT, STZ-KO and STZ-WT mice (n=6).
*P<0.05 vs. WT; #P<0.05 vs. STZ-WT. (G-J) The
number of (G) islet cells, (H) the density of β cells, and the
density of (I) mature and (J) immature insulin secretory vesicles
in β cells of WT, AAV-control and AAV–VEGFB186 mice
(n=6). *P<0.05 vs. WT; #P<0.05 vs. AAV-control.
VEGFB, vascular endothelial growth factor B; STZ, streptozocin; KO,
knockout; WT, wild-type; AAV, adeno-associated virus. |
The number of islet cells and the density of β cells
in STZ-KO mice were lower than those of STZ-WT mice, and meanwhile,
the density of mature and immature secretory vesicles of STZ-KO
mice decreased significantly (Fig.
4C-F). Compared with AAV-control, the number of islet cells,
the density of β cells, and secretory vesicles in
AAV–VEGFB186 mice were higher (Fig. 4G-J).
VEGFB regulates insulin secretion
through Ca2+/CaMK2 and its association with PPP3CA
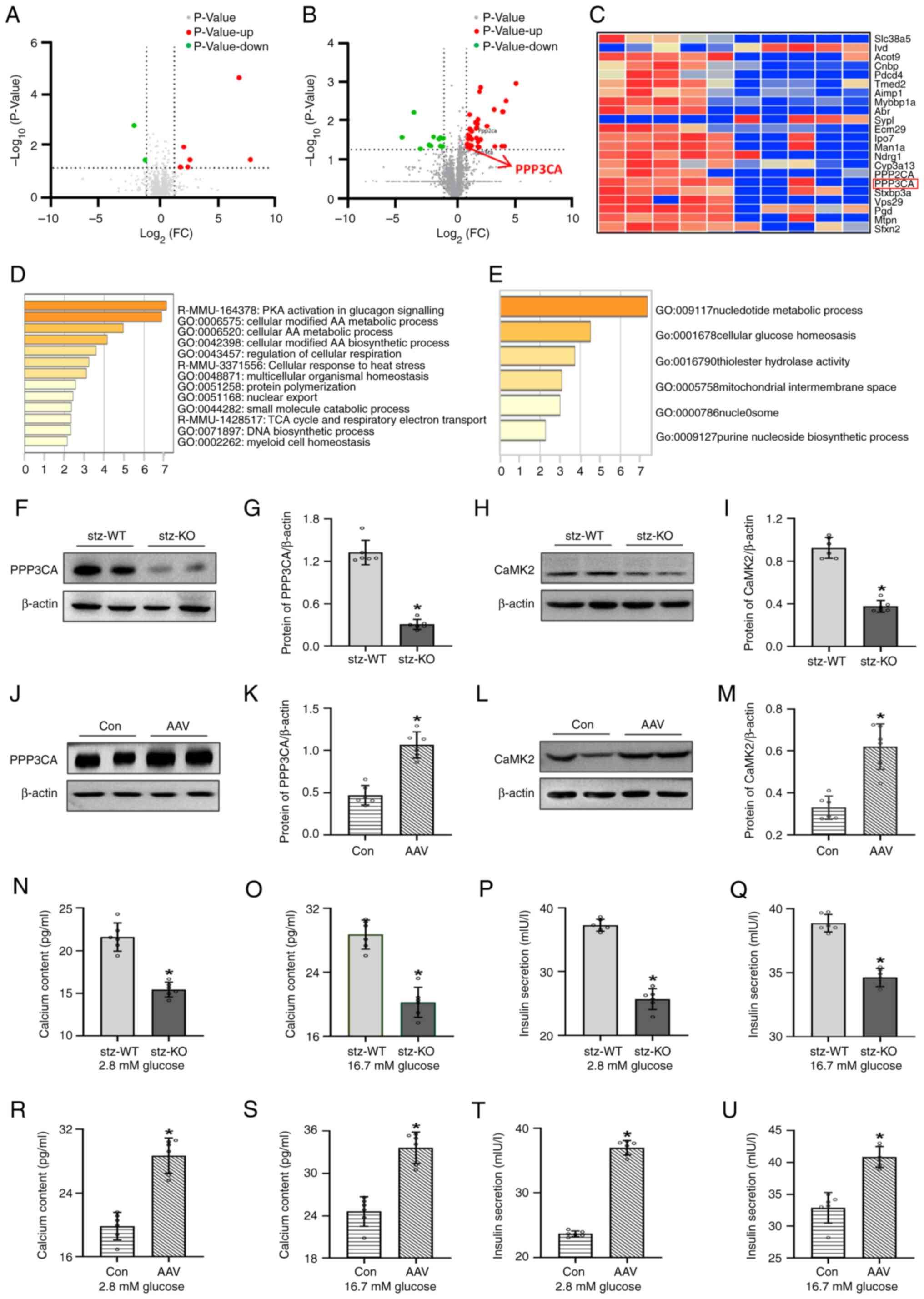
A total of 2,034 proteins were identified in the
islets of VEGFB+/+ and VEGFB−/− mice, of
which 100 proteins were different between the two groups. A total
of 34 upregulated and 12 downregulated proteins were analyzed among
these differential proteins in the islets of mice (Fig. 5A). A total of 1,722 proteins were
identified in the islets of STZ-WT and STZ-KO mice, of which 43
proteins were different between the two groups among these
differential proteins (Fig. 5B).
The heatmap showed that PPP3CA was associated with VEGFB in
differential proteins (Fig. 5C).
The differential proteins were analyzed by interpretative
phenomenological analysis (IPA) method after VEGFB KO, which was
mainly involved in cellular glucose homeostasis (Fig. 5D and E).
The protein expression of PPP3CA and CaMK2 was
decreased in STZ-KO mice, while in AAV–VEGFB186 mice,
the expression of PPP3CA and CaMK2 was increased (Fig. 5F-M). The intracellular
Ca2+ and insulin were detected, and their contents in
STZ-KO mice were lower than those of STZ-WT mice (Fig. 5N-Q), while in
AAV–VEGFB186 mice, Ca2+ and insulin levels
increased after glucose stimulation (Fig. 5R-U).
VEGFB/VEGFR1 affects the content of
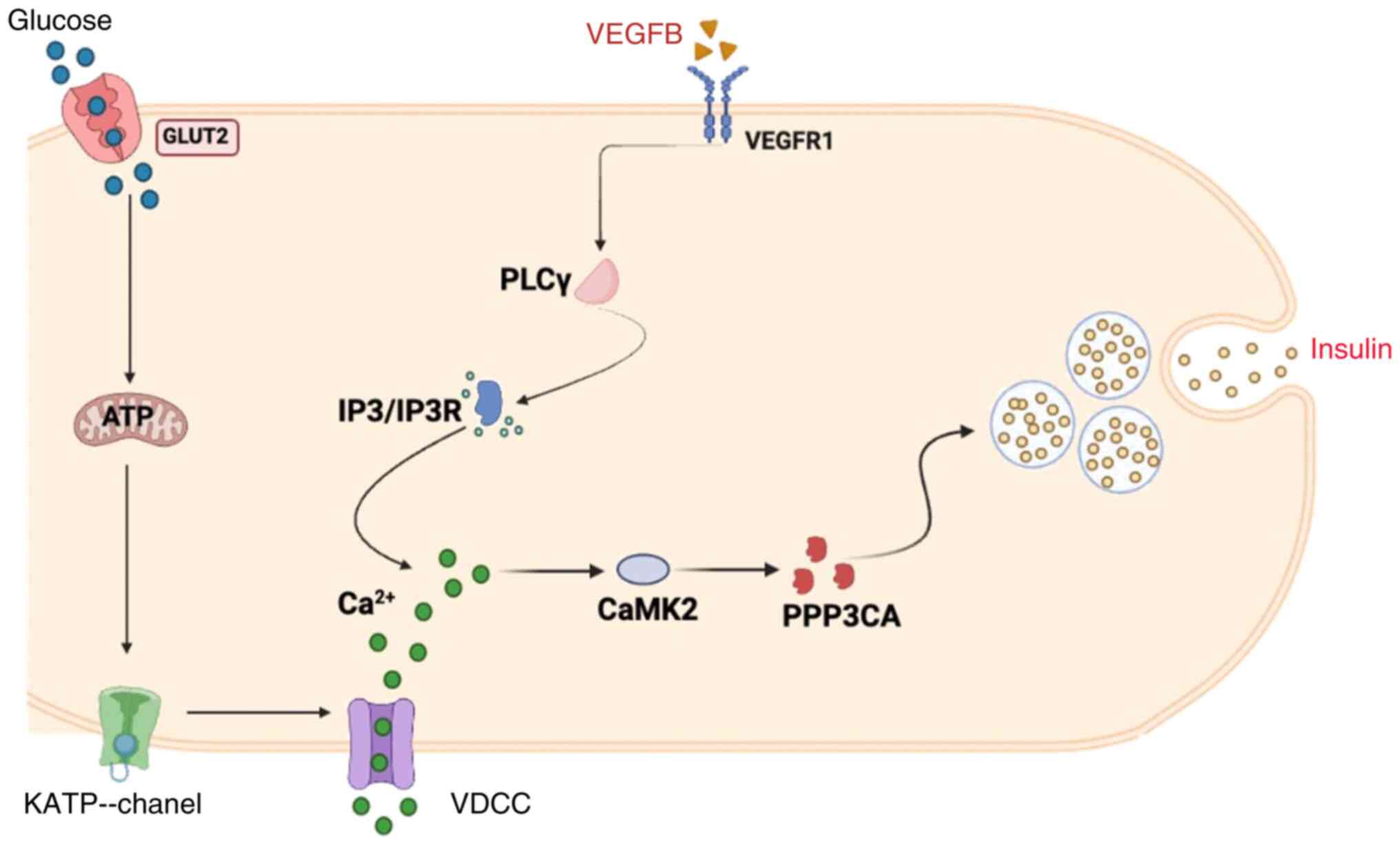
Ca2+ via the PLCγ/IP3R signaling pathway
In order to detect the effects of VEGFB on the
PLCγ/IP3R signaling pathway, the expression of VEGFB, VEGFR1, PLCγ
and IP3R was examined by western blot analysis. The results
revealed that VEGFR1 protein expression was decreased as the VEGFB
was knocked out, which suppressed the expression of the downstream
proteins PLCγ and IP3R in STZ-KO mice (Fig. 6A-H). Moreover, the expression of
VEGFR1, PLCγ and IP3R proteins were elevated in
AAV–VEGFB186 mice with overexpressed VEGFB gene
(Fig. 6I-P).
VEGFB/VEGFR1 affects the content of
Ca2+ and insulin secretion via VEGFA in physiological
state
In VEGFB+/+ and VEGFB−/− mice,
the serum glucose content was decreased and serum insulin was
increased after the VEGFB was knocked out (Fig. S1A and B). The calcium content and
insulin secretion of islet cells in VEGFB−/− mice
significantly increased after stimulation of 2.8 and 16.7 mM
glucose (Fig. S1C-F). The
expression of the VEGFR1 protein declined following the loss of the
VEGFB gene while the expression of VEGFA and VEGFR2 protein
increased. siRNA transfection in MIN6 was performed to detect the
insulin secretion and calcium content as the expression of VEGFB
was suppressed at the protein and mRNA levels (Fig. S1J and K). The ATP, calcium and
insulin secretion were increased in the SI group with the
stimulation of 2.8 and 16.7 mM glucose (Fig. S1L-Q). The expression of VEGFR1 was
reduced and the expression levels of VEGFA and VEGFR2 were elevated
in the SI group (Fig. S1R-T).
Discussion
VEGFB) is a glycoprotein with high metabolic
activity, which has been a late discovery factor in VEGF families
(19). However, its role in
promoting angiogenesis is not ascertained (19,20).
It was previously reported that VEGFB can regulate free fatty acid
uptake in endothelial cells by adjusting fatty acid transporters
(21). The expression of VEGFB and
fatty acid transporters increased after binding with VEGFR1, which
resulted in hyperglycemia (22).
Paradoxically, a previous study reported that VEGFB suppressed
inflammation related to obesity and ameliorated lipid homeostasis
since it was transduced into obese mice (23). An increasing number of researchers
were interested in deciphering the peculiar regulatory effect of
VEGFB on lipid metabolism due to this controversial phenomenon.
Numerous studies have identified that VEGFB can inhibit lipid
deposition and improve lipid metabolism (24). VEGFB-deficient mice had white fat
swelling and increased lipid accumulation. Fat-specific VEGFB
inhibition could promote lipid deposition (25). However, the combination of VEGFB
and IL22 proteins could reduce lipid deposition by suppressing
fatty acid transporters (26). The
findings of the present study were similar to the aforementioned
studies. T2DM mice gained weight and elevated serum TC and TG after
VEGFB KO. VEGFB186 overexpression improved lipid
metabolism in T2DM mice.
The increase in TG causes ectopic lipid deposition,
damages β cell function and affects insulin secretion (27,28).
A correlation was found between VEGFB and TC, TG and blood glucose
in patients with T2DM. The plasma VEGFB levels in newly diagnosed
patients with T2DM was closely related to glucose metabolism and
insulin level (3). The VEGFB
expression in the renal tissue of patients with diabetic
nephropathy was positively correlated with the content of
gamma-hydroxybutyric acid (26).
The present study illustrated that blood glucose increased after
VEGFB KO, as the growth of blood lipids in T2DM mice increased.
VEGFB overexpression can improve glucolipid metabolism in T2DM
mice. The regulatory effect of VEGFB on glucose metabolism may be
related to the reduction of TG in T2DM mice.
The markers of T2DM pathogenesis include islet
dysfunction and a reduced number of β cells (28). Glucotoxicity damages the β cell and
impairs insulin secretion function (29). Decreased β cell function will
influence insulin secretion although the number of β cells has a
certain impact on T2DM (30).
Hyperglycemia will worsen β cell damage, affecting insulin
secretion (31). The present study
revealed that loss of VEGFB exacerbated β cell population reduction
in T2DM mice. However, VEGFB overexpression attenuates β cell
injury in T2DM mice. These results of the present study indicated
that VEGFB can alleviate β cell damage to insulin secretion. The
insulin of T2DM mice decreased after VEGFB KO, and the evaluation
index of insulin secretion decreased, indicating that VEGFB has an
effect on basic and early insulin secretion function.
Insulin is released from insulin secretory vesicles
in pancreatic β cells. Insulin secretory vesicles are divided into
immature and mature vesicles (32). Proinsulin is encapsulated into
immature insulin secretory vesicles with low electron density and
has to undergo through a series of tight regulatory procedures to
develop into mature insulin secretory vesicles (33). The mature secretory vesicles are
composed of insulin, zinc and calcium crystals, containing dense
core vesicles (34). Mature
insulin secretory vesicles are stored in the vesicle pool or
transported near the cell membrane (35). Insulin secretory vesicles fuse with
the cell membrane to release insulin when the blood glucose level
increases. The present study revealed that VEGFB can regulate
insulin synthesis by affecting the number of immature vesicles in β
cells. Concurrently, VEGFB can regulate insulin secretion by
affecting the number of mature vesicles.
VEGFR, a specific VEGF receptor, elicits a variety
of biological functions through a combination of corresponding
VEGF. At present, the VEGFR family contains five members, and
VEGFR1, VEGFR2 and VEGFR3 belong to receptor tyrosine kinases
(36). The function of VEGFB on
lipid homeostasis is strongly dependent on VEGFR1 (37). VEGFB could enhance fatty acid
uptake by endothelial cells through VEGFR1 (21). VEGFR1 KO in mice with obesity and
insulin resistance could decrease insulin secretion (23). The results of the present study
revealed consistent VEGFR1 expression with VEGFB expression after
VEGFB KO or overexpression in T2DM mice. Consequently, it was
revealed that VEGFB combines with VEGFR1 to regulate insulin
secretion in T2DM mice.
The intracellular VEGFR-mediated signal transduction
is a complex process. The mechanism of the VEGFR1-mediated
signaling pathway is not clear and remains a current research
hotspot. The VEGFR1-mediated signaling pathway can activate
numerous biological reactions. A previous study revealed that the
VEGFR1-mediated signal transduction pathway could activate
intracellular MAPK signal transduction by binding with PIGF
(38). VEGFR1 suppression could
inhibit the expression of its upstream PI3K/AKT signaling pathway
(39). VEGFR1 is involved in the
PKGI signaling pathway (40).
Additionally, the combination of VEGFB and VEGFR1 could activate
the intracellular PLCγ signal transduction (41). PLCγ activation produces IP3
(42). PLCγ and IP3 combination
could effectively stimulate calcium efflux. PLCγ activation
releases Ca2+, promotes β cell function, and improves
insulin secretion to prevent the occurrence of hyperglycemia
(43). It was observed in the
present study that VEGFB/VEGFR1 could affect Ca2+
content in β cells by activating the PLCγ/IP3 signaling pathway to
regulate insulin secretion.
Additionally, CaMK is the main mediator of calcium
(44). The CaMK expressed in β
cells is CaMK2 which is a multifunctional Ca2+/CaMK and
is activated by glucose and other insulin secretagogues (45). It has the function of
phosphorylating a variety of proteins and is crucial for insulin
secretion. Moreover, CaMK2 needs to supplement the reserve vesicle
pool in β cells after stimulation is completed (46). The present study is consistent with
those revelations, showing that VEGFB can promote Ca2+
by activating the CaMK2 to regulate insulin secretion.
PPP3CA was further analyzed and it was found that is
associated with VEGFB in differential proteins through proteomics
and bioinformatics. In 2008, Wang et al reported that PPP3CA
modulates the VEGF-stimulated cell proliferation and signaling
cascades in cells (47). PPP3CA is
a serine/threonine phosphatase regulated by Ca2+/CaM
(48,49). Gelernter et al found that
PPP3CA encodes a calcium-dependent, calmodulin-stimulated protein
phosphatase involved in calcium signaling (50). The secretion of Ca2+
depends on insulin resistance and type 2 diabetes. Insulin
secretion pathways were reported to be activated by upregulating
PPP3CA (51,52). PPP3CA can be involved in
complications of diabetes (53).
Therefore, it was confirmed that PPP3CA and CAMK2 variations are
consistent in VEGFB regulation of insulin secretion in T2DM mice,
which indicated that VEGFB may stimulate insulin secretion by
activating Ca2+/CaM to accelerate substrate protein
phosphorylation (Fig. 7).
Moreover, the present study revealed decreased blood
glucose and increased insulin secretion in VEGFB−/− mice
fed with SD in the 24th week, which was different from the
variation in T2DM mice with VEGFB KO. This may be associated with
the leverage function of VEGFB in maintaining homeostasis. VEGFB
does not play an obvious biological function in a physiological
state, while it plays as a safety guard in a pathological state
(54–56). Therefore, it was hypothesized that
the mechanisms of VEGFB that regulate insulin secretion are
different under physiological and pathological conditions. VEGFB
may participate in the regulation of insulin secretion through the
VEGFA/VEGFR1 signaling pathway under physiological conditions,
unlike the regulatory mechanism of VEGFB on insulin secretion in
T2DM mice. The signal system of the VEGF family is complex, and the
affinity and selectivity of members to different receptors are
different. VEGFA can combine with VEGFR1 and VEGFR2, while VEGFB
can only combine with VEGFR1 (57,58).
The present study supports that VEGFR2 plays a dominant role in all
receptors. Some researchers consider that VEGFR1 is a decoy
receptor. In general, it not only transmits mitogenic signals but
also blocks VEGF, thereby preventing VEGF from binding to VEGFR2.
VEGFR1 can negatively regulate the VEGFR2 signaling pathway and
promote VEGFR2 under certain pathological conditions (59). At present, the specific mechanism
is not completely clear. VEGFR1 expression was downregulated in the
present study, while VEGFA and VEGFR2 expression levels were
upregulated after the loss of VEGFB in the islets β cell of mice
fed with SD. Same results were acquired through the validation of
the Min6 cell line. It was indicated that the increase of insulin
secretion after VEGFB KO may be related to the VEGFA/VEGFR2
signaling pathway upregulation caused by the decreased VEGFR1
expression under physiological conditions (Fig. S1U). The specific mechanism remains
unclear although it was validated in Min6 cells in the present
study. In the future, the association between VEGFB and
VEGFA/VEGFR2 shall be further validated by the authors using
receptor blockers at the in vivo and in vitro
levels.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 31771284), the Basic Research
Project of Yantai Science and Technology Innovation and Development
Plan (grant no. 2022JCYJ026) and the Natural Science Foundation of
Shandong province (grant no. ZR202111250163).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The mass spectrometry proteomics data have been deposited
to the Proteome Xchange Consortium via the PRIDE partner repository
with the dataset identifier PXD043843 (https://www.ebi.ac.uk/pride/).
Authors' contributions
YQL, RRL and XL conceptualized and designed
experiments, analyzed and interpreted data and drafted the article.
FX, MZY, LHZ, QHW, WGJ and YNL designed and conducted experiments,
acquired, analyzed and interpreted the data, and revised the
article critically for intellectual content. All authors read and
approved the final version of the manuscript. WGJ and YNL
confirming the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Institutional Review Board of Binzhou Medical University (Yantai,
China). All procedures involving animals were reviewed and approved
(IACUC approval no. 2022-210) by the Institutional Animal Care and
Use Committee of the Medical Ethics Committee of Binzhou Medical
University (Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rorsman P and Braun M: Regulation of
insulin secretion in human pancreatic islets. Annu Rev Physiol.
75:155–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cernea S and Dobreanu M: Diabetes and beta
cell function: From mechanisms to evaluation and clinical
implications. Biochem Med (Zagreb). 23:266–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu J, Wei H, Qu H, Feng Z, Long J, Ge Q
and Deng H: Plasma vascular endothelial growth factor B levels are
increased in patients with newly diagnosed type 2 diabetes mellitus
and associated with the first phase of glucose-stimulated insulin
secretion function of β-cell. J Endocrinol Invest. 40:1219–1226.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ning FC, Jensen N, Mi J, Lindström W,
Balan M, Muhl L, Eriksson U, Nilsson I and Nyqvist D: VEGF-B
ablation in pancreatic beta-cells upregulates insulin expression
without affecting glucose homeostasis or islet lipid uptake. Sci
Rep. 10:9232020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shang R, Lal N, Lee CS, Zhai Y, Puri K,
Seira O, Boushel RC, Sultan I, Räsänen M, Alitalo K, et al:
Cardiac-specific VEGFB overexpression reduces lipoprotein lipase
activity 2 and improves insulin action in rat heart. Am J Physiol
Endocrinol Metab. 321:E753–E765. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rorsman P and Ashcroft FM: Pancreatic
β-cell electrical activity and insulin secretion: Of mice and men.
Physiol Rev. 98:117–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vishnu N, Hamilton A, Bagge A, Wernersson
A, Cowan E, Barnard H, Sancak Y, Kamer KJ, Spégel P, Fex M, et al:
Mitochondrial clearance of calcium facilitated by MICU2 controls
insulin secretion. Mol Metab. 51:1012392021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiederkehr A and Wollheim CB: Minireview:
Implication of mitochondria in insulin secretion and action.
Endocrinology. 147:2643–2649. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rask–Madsen C and King GL: Differential
regulation of VEGF signaling by PKC-alpha and PKC-epsilon in
endothelial cells. Arterioscler Thromb Vasc Biol. 28:919–924. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YH, Chang M and Davidson BL:
Molecular signatures of disease brain endothelia provide new sites
for CNS-directed enzyme therapy. Nat Med. 15:1215–1218. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Corbett BF, You JC, Zhang X, Pyfer MS,
Tosi U, Iascone DM, Petrof I, Hazra A, Fu CH, Stephens GS, et al:
ΔFosB regulates gene expression and cognitive dysfunction in a
mouse model of Alzheimer's Disease. Cell Rep. 20:344–355. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding H, Underwood R, Lavalley N and
Yacoubian TA: 14-3-3 inhibition promotes dopaminergic neuron loss
and 14-3-3theta overexpression promotes recovery in the MPTP mouse
model of Parkinson's disease. Neuroscience. 307:73–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Ibi D, Taniguchi K, Lee J, Herrema
H, Akosman B, Mucka P, Hernandez MA, Uyar MF, Park SW, et al:
Inflammation improves glucose homeostasis through IKKβ-XBP1s
interaction. Cell. 167:1052–1066. e10182016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Wu Y, Zhao J, Wang H, Tan J, Yang M,
Li Y, Deng S, Gao S, Li H, et al: Distinct cardiac energy
metabolism and oxidative stress adaptations between obese and
non-obese type 2 diabetes mellitus. Theranostics. 10:2675–2695.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun J, Fu X, Liu Y, Wang Y, Huo B, Guo Y,
Gao X, Li W and Hu X: Hypoglycemic effect and mechanism of honokiol
on type 2 diabetic mice. Drug Des Devel Ther. 9:6327–6342.
2015.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peterson QP, Veres A, Chen L, Slama MQ,
Kenty JHR, Hassoun S, Brown MR, Dou H, Duffy CD, Zhou Q, et al: A
method for the generation of human stem cell-derived alpha cells.
Nat Commun. 11:22412020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stuhlmann T, Planells-Cases R and Jentsch
TJ: LRRC8/VRAC anion channels enhance β-cell glucose sensing and
insulin secretion. Nat Commun. 9:19742018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lal N, Chiu AP, Wang F, Zhang D, Jia J,
Wan A, Vlodavsky I, Hussein B and Rodrigues B: Loss of VEGFB and
its signaling in the diabetic heart is associated with increased
cell death signaling. Am J Physiol Heart Circ Physiol.
312:H1163–H1175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dmytriyeva O, de Diego Ajenjo A, Lundo K,
Hertz H, Rasmussen KK, Christiansen AT, Klingelhofer J, Nielsen AL,
Hoeber J, Kozlova E, et al: Neurotrophic effects of vascular
endothelial growth factor B and novel mimetic peptides on neurons
from the central nervous system. ACS Chem Neurosci. 11:1270–1282.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hagberg CE, Falkevall A, Wang X, Larsson
E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L,
Vanwildemeersch M, et al: Vascular endothelial growth factor B
controls endothelial fatty acid uptake. Nature. 464:917–921. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McDonald DM: Tighter lymphatic junctions
prevent obesity. Science. 361:551–552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robciuc MR, Kivela R, Williams IM, de Boer
JF, van Dijk TH, Elamaa H, Tigistu-Sahle F, Molotkov D, Leppänen
VM, Käkelä R, et al: VEGFB/VEGFR1-induced expansion of adipose
vasculature counteracts obesity and related metabolic
complications. Cell Metab. 23:712–724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zafar MI, Zheng J, Kong W, Ye X, Gou L,
Regmi A and Chen LL: The role of vascular endothelial growth
factor-B in metabolic homoeostasis: Current evidence. Biosci Rep.
37:BSR201710892017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Zhao M, Wang C, Wen H, Zhang Y, Lu
M, Adlat S, Zheng T, Zhang M, Li D, et al: Adipose vascular
endothelial growth factor B is a major regulator of energy
metabolism. J Endocrinol. 244:511–521. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen Y, Chen W, Han L, Bian Q, Fan J, Cao
Z, Jin X, Ding T, Xian Z, Guo Z, et al: VEGF-B antibody and
interleukin-22 fusion protein ameliorates diabetic nephropathy
through inhibiting lipid accumulation and inflammatory responses.
Acta Pharm Sin B. 11:127–142. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su K, Yi B, Yao BQ, Xia T, Yang YF, Zhang
ZH and Chen C: Liraglutide attenuates renal tubular ectopic lipid
deposition in rats with diabetic nephropathy by inhibiting lipid
synthesis and promoting lipolysis. Pharmacol Res. 156:1047782020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herman-Edelstein M, Scherzer P, Tobar A,
Levi M and Gafter U: Altered renal lipid metabolism and renal lipid
accumulation in human diabetic nephropathy. J Lipid Res.
55:561–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weir GC: Glucolipotoxicity, β-Cells, and
diabetes: The emperor has no clothes. Diabetes. 69:273–278. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Campbell JE and Newgard CB: Mechanisms
controlling pancreatic islet cell function in insulin secretion.
Nat Rev Mol Cell Biol. 22:142–158. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li M, Abraham NG, Vanella L, Zhang Y,
Inaba M, Hosaka N, Hoshino S, Shi M, Ambrosini YM, Gershwin ME, et
al: Successful modulation of type 2 diabetes in db/db mice with
intra-bone marrow-bone marrow transplantation plus concurrent
thymic transplantation. J Autoimmun. 35:414–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nasteska D, Fine NHF, Ashford FB, Cuozzo
F, Viloria K, Smith G, Dahir A, Dawson PWJ, Lai YC, Bastidas-Ponce
A, et al: PDX1(LOW) MAFA(LOW) beta-cells contribute to islet
function and insulin release. Nat Commun. 12:6742021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu M, Wright J, Guo H, Xiong Y and Arvan
P: Proinsulin entry and transit through the endoplasmic reticulum
in pancreatic beta cells. Vitam Horm. 95:35–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Slepchenko KG, James CB and Li YV:
Inhibitory effect of zinc on glucose-stimulated zinc/insulin
secretion in an insulin-secreting beta-cell line. Exp Physiol.
98:1301–1311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Omar-Hmeadi M and Idevall-Hagren O:
Insulin granule biogenesis and exocytosis. Cell Mol Life Sci.
78:1957–1970. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu L, Shan Z, Wang F, Gao X and Tong Y:
Vascular endothelial growth factor B exerts lipid-lowering effect
by activating AMPK via VEGFR1. Life Sci. 276:1194012021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Golfmann K, Meder L, Koker M, Volz C,
Borchmann S, Tharun L, Dietlein F, Malchers F, Florin A, Büttner R,
et al: Synergistic anti-angiogenic treatment effects by dual FGFR1
and VEGFR1 inhibition in FGFR1-amplified breast cancer. Oncogene.
37:5682–5693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ling M, Quan L, Lai X, Lang L, Li F, Yang
X, Fu Y, Feng S, Yi X, Zhu C, et al: VEGFB promotes myoblasts
proliferation and differentiation through VEGFR1-PI3K/Akt signaling
pathway. Int J Mol Sci. 22:133522021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen Z, Zhang Z, Wang X and Yang K:
VEGFB-VEGFR1 ameliorates Ang II-induced cardiomyocyte hypertrophy
through Ca(2+)-mediated PKG I pathway. J Cell Biochem.
119:1511–1520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weddell JC, Chen S and Imoukhuede PI:
VEGFR1 promotes cell migration and proliferation through PLCgamma
and PI3K pathways. NPJ Syst Biol Appl. 4:12018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kadamur G and Ross EM: Mammalian
phospholipase C. Annu Rev Physiol. 75:127–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liang S, Zhao J, Wang Q, Yang M, Wang X,
Chen S, Chen M and Sun C: Carbon monoxide enhances calcium
transients and glucose-stimulated insulin secretion from pancreatic
β-cells by activating Phospholipase C signal pathway in diabetic
mice. Biochem Biophys Res Commun. 582:1–7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takemoto-Kimura S, Suzuki K, Horigane SI,
Kamijo S, Inoue M, Sakamoto M, Fujii H and Bito H: Calmodulin
kinases: Essential regulators in health and disease. J Neurochem.
141:808–818. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Choi SE, Shin HC, Kim HE, Lee SJ, Jang HJ,
Lee KW and Kang Y: Involvement of Ca2+, CaMK II and PKA in EGb
761-induced insulin secretion in INS-1 cells. J Ethnopharmacol.
110:49–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miyano R, Miki T and Sakaba T:
Ca-dependence of synaptic vesicle exocytosis and endocytosis at the
hippocampal mossy fibre terminal. J Physiol. 597:4373–4386. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang K, Song Y, Chen DB and Zheng J:
Protein phosphatase 3 differentially modulates vascular endothelial
growth factor- and fibroblast growth factor 2-stimulated cell
proliferation and signaling in ovine fetoplacental artery
endothelial cells. Biol Reprod. 79:704–710. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Panneerselvam S, Wang J, Zhu W, Dai H,
Pappas JG, Rabin R, Low KJ, Rosenfeld JA, Emrick L, Xiao R, et al:
PPP3CA truncating variants clustered in the regulatory domain cause
early-onset refractory epilepsy. Clin Genet. 100:227–233. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu J, Zheng C, Wang X, Yun S, Zhao Y, Liu
L, Lu Y, Ye Y, Zhu X, Zhang C, et al: MicroRNA-30 family members
regulate calcium/calcineurin signaling in podocytes. J Clin Invest.
125:4091–4106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gelernter J, Kranzler HR, Sherva R,
Koesterer R, Almasy L, Zhao H and Farrer LA: Genome-wide
association study of opioid dependence: Multiple associations
mapped to calcium and potassium pathways. Biol Psychiatry.
76:66–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fong CC, Wei F, Chen Y, Yu WK, Koon CM,
Leung PC, Fung KP, Lau CB and Yang M: Danshen-gegen decoction
exerts proliferative effect on rat cardiac myoblasts H9c2 via MAPK
and insulin pathways. J Ethnopharmacol. 138:60–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao Y, Xue Q, Su X, Xie L, Yan Y, Wang L
and Steinman AD: First identification of the toxicity of
microcystins on pancreatic islet function in humans and the
involved potential biomarkers. Environ Sci Technol. 50:3137–3144.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Atkin AS, Moin ASM, Nandakumar M,
Al-Qaissi A, Sathyapalan T, Atkin SL and Butler AE: Impact of
severe hypoglycemia on the heat shock and related protein response.
Sci Rep. 11:170572021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li X, Kumar A, Zhang F, Lee C and Tang Z:
Complicated life, complicated VEGF-B. Trends Mol Med. 18:119–127.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gao R, Zhu BH, Tang SB, Wang JF and Ren J:
Scutellarein inhibits hypoxia- and moderately-high glucose-induced
proliferation and VEGF expression in human retinal endothelial
cells. Acta Pharmacol Sin. 29:707–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Koch S, Tugues S, Li X, Gualandi L and
Claesson-Welsh L: Signal transduction by vascular endothelial
growth factor receptors. Biochem J. 437:169–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Anisimov A, Leppanen VM, Tvorogov D,
Zarkada G, Jeltsch M, Holopainen T, Kaijalainen S and Alitalo K:
The basis for the distinct biological activities of vascular
endothelial growth factor receptor-1 ligands. Sci Signal.
6:ra522013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Uemura A, Fruttiger M, D'Amore PA, De
Falco S, Joussen AM, Sennlaub F, Brunck LR, Johnson KT, Lambrou GN,
Rittenhouse KD and Langmann T: VEGFR1 signaling in retinal
angiogenesis and microinflammation. Prog Retin Eye Res.
84:1009542021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cudmore MJ, Hewett PW, Ahmad S, Wang KQ,
Cai M, Al-Ani B, Fujisawa T, Ma B, Sissaoui S, Ramma W, et al: The
role of heterodimerization between VEGFR-1 and VEGFR-2 in the
regulation of endothelial cell homeostasis. Nat Commun. 3:9722012.
View Article : Google Scholar : PubMed/NCBI
|