Introduction
Excessive ethanol consumption can lead to alcoholic
fatty liver disease (AFLD), with severe cases of this disease
causing hepatitis, cirrhosis and liver cancer (1). Hepatic steatosis and lipid
accumulation are the earliest characteristic of AFLD and are
considered a factor responsible for the progression of alcoholic
liver disease (ALD) (1). A
previous study reported that excessive alcohol consumption could
promote liver lipid accumulation (2). Reducing fat accumulation may be
sufficient to prevent the steatosis of ALD (3). Hepatic steatosis, the accumulation of
lipid triglycerides (TGs) and cholesterol (CHO) in the cytoplasm of
hepatocytes, is a reversible pathological condition and an early
stage in the progression of ALD (2). The accumulation of excess lipids in
the cytoplasmic space of hepatocytes occupies this space, which
leads to the nucleus being squeezed off center, affecting the
metabolism of other nutrients, hormones and vitamins, and makes
these cells more vulnerable to external stimulants, such as
oxidative stress, cytokine and endotoxins (4,5).
Therefore, reducing hepatic steatosis may inhibit the occurrence
and development of ALD. The pathogenesis of hepatic steatosis is
complex and diverse, with alcohol-induced lipogenesis being an
important pathogenic factor of hepatic steatosis, which promotes
the synthesis of fatty acids and TG in hepatocytes (6,7).
Consuming ethanol induces oxidative damage and
inflammation in liver tissue, which leads to oxidative stress and
blocks lipid metabolism, contributing to ALD (8). A previous study reported that an
increase in liver fat is an important pathogenic mechanism in the
occurrence and development of hepatic steatosis (9). Nuclear erythroid 2-related factor 2
(Nrf2), a key transcription factor, is induced and activated by
oxidative stress, which regulates the adaptive response to the
oxidative stress of AFL injury and liver dysfunction (10). In addition, inflammation serves an
important role in AFL injury (11). Long-term alcohol consumption
damages the intestinal barrier, increases mucosa permeability and
allows bacterial endotoxin to enter the circulatory system
(11). Excessive endotoxin release
following long-term alcohol consumption induces the inflammatory
response and the progression of AFL. NF-κB is activated and
transported to the nucleus, which promotes pro-inflammatory
cytokine secretion (12).
Therefore, inhibiting liver inflammation and restoring liver
function could contribute to protection against ALF.
Agarwood is a rare and expensive fragrant wood
containing resin that can be used as a spice (13). Agarwood, a traditional Chinese
medicine ingredient, has been used for the treatment of various
diseases, such as gastric ulcers, enteritis, myocardial ischemia,
vomiting and asthma (14). Natural
products of agarwood have anti-inflammatory (15–17),
analgesic (18), antioxidant
(19) and other biological
activities, such as in sedation and improving immunity. These
effects support the use of agarwood for treating certain diseases,
such as hepatic disease, ulcers, angina, trauma and coughs
(13,14). Our previous studies reported that
agarwood extract protects against drug-induced liver injury
(20,21). Our previous study also reported
that agarwood has two main constituents of sesquiterpenes and
chromone and the components of agarwood extract comprise
sesquiterpenes (10.615%), chromone (31.678%), aromatics (31.831%)
and other known compounds (25.760%) (20). Therefore, agarwood may have a
protective effect against AFL by improving liver function,
pathological characteristics, anti-oxidation and anti-inflammation.
However, to our knowledge, it has not been previously reported that
AAE has a protective effect on AFL.
The present study aimed to investigate the potential
protective effect of AAE on the liver and explore its potential
mechanisms of action. In addition, the protective effects of
different types of AAEs on AFL were compared. The present study
provided a scientific basis for the study and development of
agarwood for the clinical prevention and treatment of liver
disease.
Materials and methods
Reagents
Biochemical kits for the detection of AST (cat. no.
C010-2-1), ALT (cat. no. C009-2-1), CHO (cat. no. A111-1-1), TG
(cat. no. A110-1-1), nitric oxide (NO; cat. no. A013-2-1),
H2O2 (cat. no. A064-1-1), lipid peroxide
(LPO; cat. no. A106-1-3), total peroxide content (T-AOC; cat. no.
A015-2-1), catalase (CAT; cat. no. A007-2-1), superoxide dismutase
(SOD; cat. no. A001-3-2) and BCA (cat. no. A045-4-2) were purchased
from the Nanjing Jiancheng Bioengineering Research Institute Co.,
Ltd. ELISA kits for the detection of TNF-α (cat. no. 8066441),
IFN-γ (cat. no. 8066449), IL-6 (cat. no. 8066455) and IL-33 (cat.
no. 8063899) were purchased from Bossbio Biotech. RIPA buffer (cat.
no. P0013B) was bought from Beyotime Institute of Biotechnology.
Nrf2 (cat. no. sc-365949), NF-κB (cat. no. sc-8008) and β-actin
(cat. no. sc-8008) antibodies were purchased from Santa Cruz
Biotechnology, Inc. The chemiluminescence kit (cat. no. P0018S) was
purchased from Beyotime Institute of Biotechnology. Anhydrous
ethanol and other chemicals purchased were of analytical grade.
Preparation of the agarwood
extract
The whole-tree agarwood-inducing technique (patent
no. ZL201010104119.5) was used to obtain artificially produced
agarwood by the infusion agarwood-inducing technique (specimen no.
JC2016112), wounding wild agarwood by causing damage via cuts to
the trunk similar to wild agarwood formation (13,22)
(specimen no. JC2016099) and burning agarwood via chisel-drilling
using traditional or primitive fire drilling agarwood (13,22)
(specimen no. JC2016076), which were purchased from the Guangdong
Huzhou Guolin Agarwood Planting Cooperative. The aforementioned
company was the experimental base of the agarwood-inducing
production technology utilized in the present study. Agarwood was
identified by Professor Jian-he Wei and stored in an
air-conditioned sample room at 16°C from purchase. Agarwood alcohol
extracts (AAEs) whole-tree agarwood-inducing technique alcohol
extract (WTAAE), wild agarwood induced by ace wounds alcohol
extract (WAAE) and burning-chisel-drilling agarwood alcohol extract
(FBAAE) were extracted and prepared according to extraction methods
reported in previous studies (20,22,23).
Agarwood (1,000 g) was naturally dried at 30°C for a week, smashed
and soaked in 95% ethanol (5 l) for 2 h. The resulting agarwood
ethanol extract was extracted using reflux extraction for 1 h and
filtered using 2-µm filter paper. The reflux extraction was
repeated twice. The resulting ethanol solutions were combined,
concentrated and dried in vacuo to obtain dark-brown WTAAE
(140 g; 14%), WAAE (105 g; 10.5%) and FBAAE (140 g; 14%) which were
stored in a freezer at −20°C.
Animals and experimental
procedure
Healthy, adult male C57 mice (n=80; weight, 20±2 g)
were purchased from the Vital River Laboratory Animal Technology
Co., Ltd. (SCXK (Jing) 2017-022, Beijing, China). Animals were
housed in a specific-pathogen-free laboratory animal room using a
12-h light/12-h dark cycle, with ad libitum access to food
and water, and were housed for 3 days prior to experiments.
The 80 mice were randomly divided into eight groups:
i) Normal; ii) model; iii) compound methionine choline tablets
(CMCT); iv) WAAE; v) FBAAE; vi) 0.71 g/kg WTAAE; vii) 1.42 g/kg
WTAAE; and viii) 2.84 g/kg WTAAE groups. The CMCT group was used as
a positive control group. CMCT is a compound preparation of
methionine choline used for the treatment of fatty liver and acute
or chronic hepatitis. The dosage of WAAE (20,21),
FBAAE (20,21) and WTAAEs (20,21)
were determined according to the previously published conversion of
human daily dosage of these compounds. The normal and model groups
were orally gavaged with 20 ml/kg of distilled water. The CMCT
group was orally garaged with 200 mg/kg CMCT daily. The WAAE and
FBAAE groups were orally gavaged with 2.84 g/kg of the respective
compounds daily. The WTAAE groups were orally gavaged with 0.71,
1.42 or 2.84 g/kg (equivalent to the mass of raw material for
agarwood) (20,21) of treatment daily, respectively. All
groups were fed a high-fat diet, except for the normal group, which
was fed a normal maintenance diet. The composition of high fat feed
included: 20% Sucrose, 15% lard, 1.2% cholesterol, 0.2% sodium
cholate and the remaining food was base feed. All groups, except
for the normal group, were orally gavaged with 30% ethanol, 20
ml/kg daily for 3 weeks (24). The
corresponding test drugs were orally administered at 20 ml/kg daily
from days 8–21. On the 21st day, the mice were fasted without water
for 12 h, then blood and liver tissues were collected for the
assessment of pharmacodynamic indexes. Animal care and experimental
protocols were approved by the Animal Care and Use Committee at the
Institute of Medicinal Plant Development, Chinese Academy of
Medical Sciences (ethical approval no. SLXD-20180809012).
Determination of liver and adipose
indexes
Mice were weighed 1 h after the final drug dose was
administered. Mice were briefly anesthetized with cotton balls
impregnated with ether. Mice were considered anesthetized when the
animals lay on their back, their heart rate and breathing were
even, muscles were relaxed, limbs were not moving, whiskers were
not moving and their pedal reflex disappeared. Then, 0.5-1 ml of
blood was collected from the retro-orbital sinus and centrifuged at
1,006.2 × g at 4°C for 15 min for serum analysis. Animals were
sacrificed by cervical dislocation immediately following blood
sample collection. The liver and adipose tissues were collected and
weighed. The liver index and fat indexes were calculated as
follows: Liver index (mg/g)=liver weight (mg)/animal weight (g);
and fat index (mg/g)=fat weight (mg)/animal weight (g).
Histopathological evaluation
Histopathological changes in the liver of mice were
analyzed using hematoxylin and eosin (H&E) staining (25). The liver tissues were fixed in 4%
paraformaldehyde solution for 24 h at room temperature, embedded in
paraffin, cut into 4-µm sections, and then stained with H&E for
25 min at room temperature. The specimens were imaged using a
fluorescence microscope at ×400 magnification (Olympus
Corporation). Histological damage was calculated using the
following score system (PDI score): Score 0, absent (no
destruction); score 0.5, few (0–20% destruction); score 1, mild
(20–50% destruction); score 2, moderate (50–80% destruction); score
3, severe (80–100% destruction) (21,25).
Assessment of oxidative stress in
tissues
The liver tissues were fully homogenized
mechanically with saline (1:9 w/v) and were centrifuged at 1,006.2
× g at 4°C for 15 min, the supernatant was collected and used for
measuring oxidative stress. The levels of NO,
H2O2, LPO, CAT, SOD and T-AOC were measured
using the respective biochemical kits according to the
manufacturer's instructions.
Assessment of TNF-α, IL-6, IL-33 and
IFN-γ levels in serum
Serum samples were collected to assess the levels of
inflammatory cytokines. The levels of TNF-α, IL-6, IL-33 and IFN-γ
were measured in serum using ELISA kits according to the
manufacturer's instructions.
Western blotting analysis of the
protein expression levels in liver tissue
Total liver proteins were extracted from liver
tissues using RIPA buffer. The protein concentration was measured
using a BCA protein assay. The protein was loaded onto a 10%
SDS-polyacrylamide gel (20 µg/lane), transferred onto a PVDF
membrane and blocked with 5% skimmed milk at room temperature for 2
h. Membranes were incubated with primary antibodies for Nrf2
(1:500), NF-κB (1:1,000) and β-actin (1:2,000) at 4°C overnight.
The membrane was then incubated with secondary antibodies (1:2,000;
goat anti-rabbit secondary antibody; cat. no. A0208) at room
temperature for 2 h. The membranes were washed three times using
TBST (0.05% Tween) and visualized using an enhanced
chemiluminescence kit. The membranes were imaged using a pro-gel
imaging system and protein expression levels were semi-quantified
using ImageJ Software V1.8 (National Institutes of Health).
Statistical analysis
Data were expressed as the mean ± standard deviation
and were analyzed using SPSS (version 17.0; SPSS, Inc.).
Differences between each group were analyzed by one-way analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
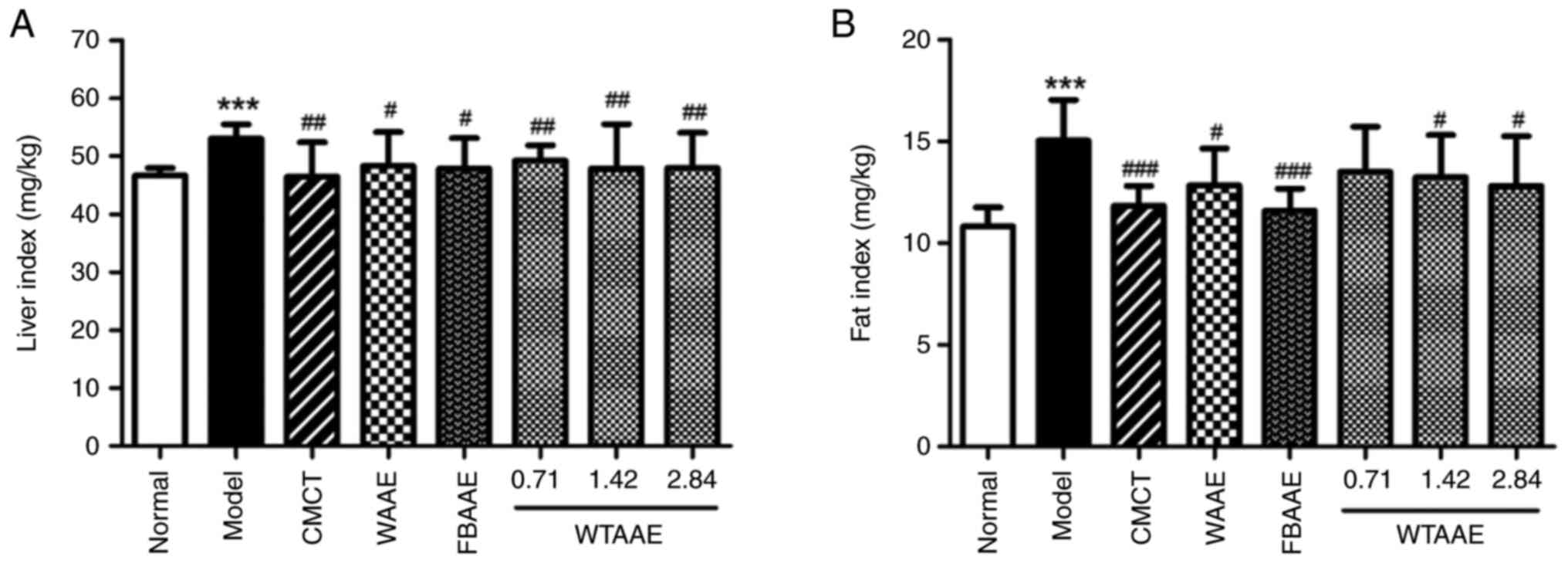
Effect of AAEs on liver and fat
indexes
Liver and fat indexes were determined and were both
significantly elevated in the model group compared with the normal
group (Fig. 1). High liver and fat
indexes are characterized by fat accumulation and liver edema,
therefore, the high fat diet and alcohol consumption may lead to
the accumulation of fat and the formation of fatty liver, which
suggested that the model of AFL was successfully constructed in the
present study. Pretreatment of mice with different AAEs
significantly reduced the liver and adipose indexes compared with
the model group for all AAEs, apart from pretreatment with 0.71
g/kg WTAAE which did not significantly impact the fat index. WTAAE
pretreatment demonstrated a dose-dependent effect at reduced the
liver and adipose indexes, which suggested that AAEs had a
significant effect on relieving hepatic steatosis.
Effect of AAEs on AST, ALT, TG and CHO
levels
The levels of liver metabolic enzymes and lipids,
such as AST, ALT, TG and CHO, are key markers used to evaluate
liver function (2,6,7). The
levels of AST, ALT, TG and CHO were significantly increased in mice
in the model group compared with the normal group, which suggested
the liver function was significantly impaired (Fig. 2). Pretreatment of mice with AAEs
significantly reduced the levels of the aforementioned enzymes and
lipids compared with the model group. In addition, WTAAE
dose-dependently reduced the liver and adipose indexes, which
suggested that AAE treatment demonstrated a marked effect on
restoring the liver function in AFL mice.
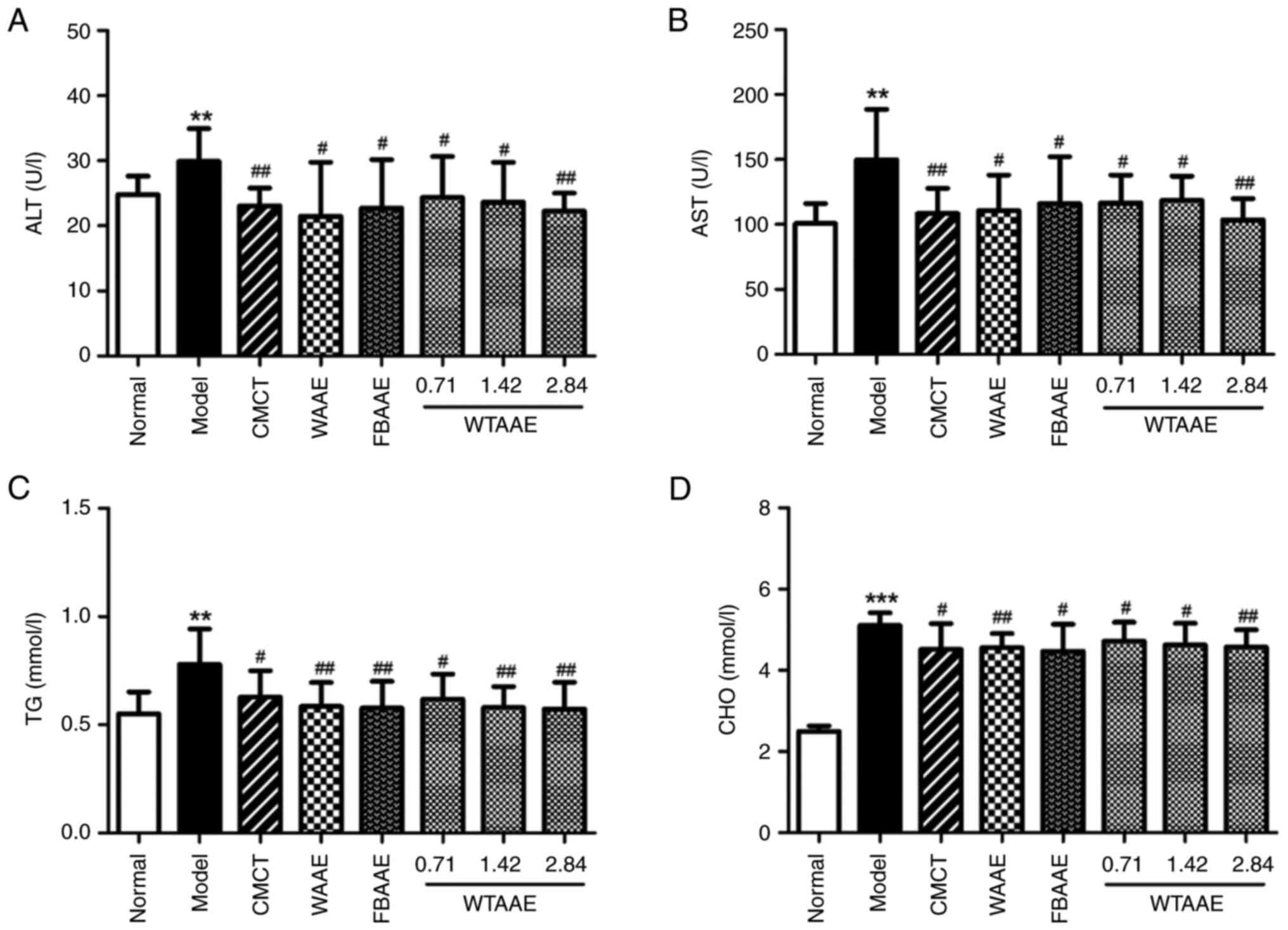 | Figure 2.Effects of agarwood alcohol extract
on AST, ALT, TG and CHO. Levels of (A) AST. (B) ALT. (C) TG. (D)
CHO in the liver. Data are presented as the mean ± standard
deviation (n=10). **P<0.01, ***P<0.001 vs. Normal group.
#P<0.05 and ##P<0.01 vs. Model group.
CMCT, compound methionine choline tablets; WAAE, wild agarwood
induced by axe wounds alcohol extract; FBAAE,
burning-chisel-drilling agarwood alcohol extract; WTAAE, whole-tree
agarwood-inducing technique alcohol extract; AST, aspartate
aminotransferase; ALT, alanine aminotransferase; TG, triglyceride;
CHO, cholesterol. |
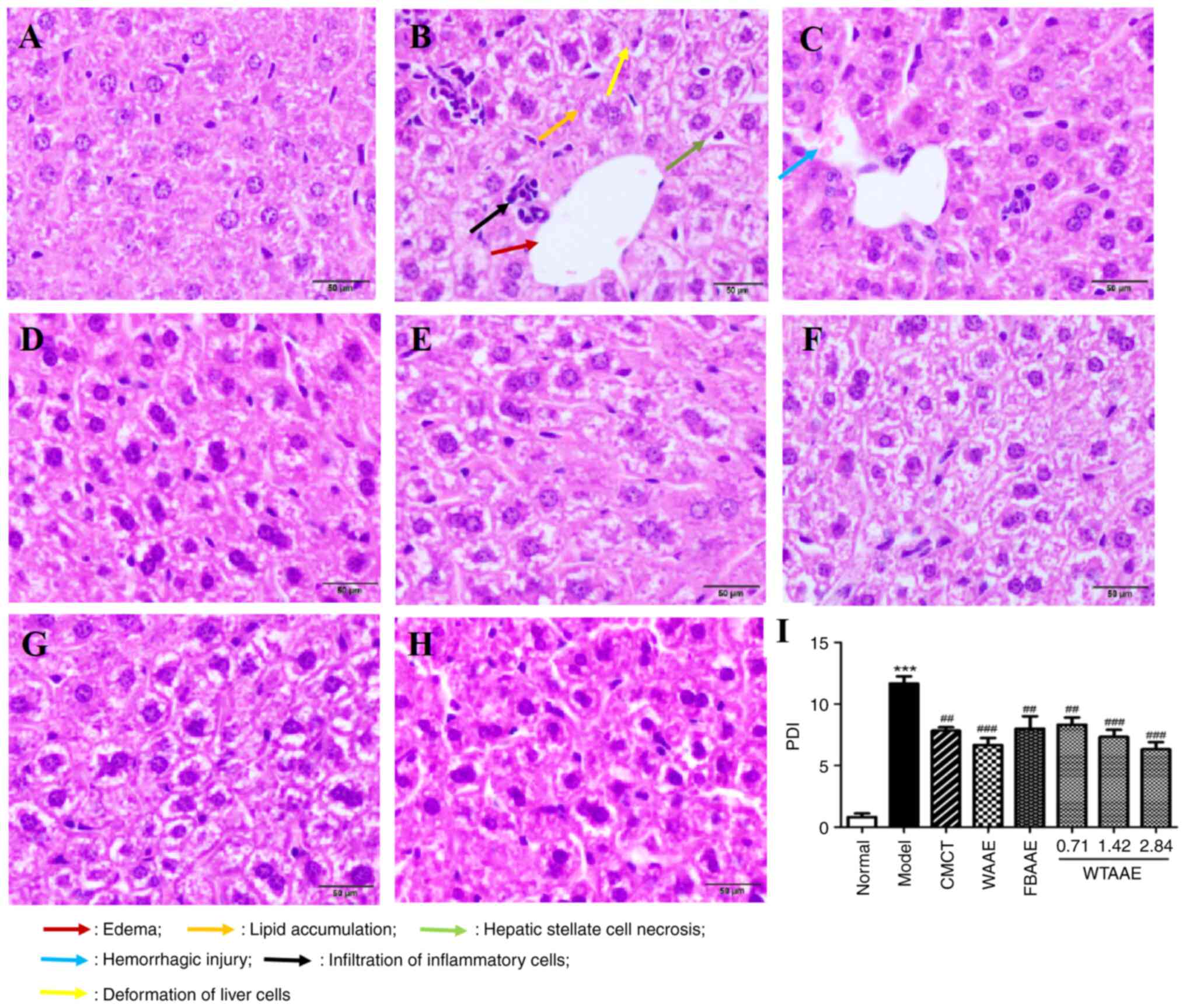
Effect of AAEs on histopathological
lesions
The ethanol and high fat diet induced marked
histopathological injuries, including lipid accumulation, edema,
deformation, hemorrhagic injury, hepatic stellate cell necrosis and
inflammatory cell infiltration (Fig.
3). AAEs markedly reduced this damage in liver tissues and
protected against liver histopathological lesions. Mice treated
with DBGTP, WAAE and FBAAE presented with less swelling and lower
numbers of inflammatory cells. WTAAE pretreatment demonstrated
dose-dependent protection against ALF. The pathological damage
index (PDI) could show the results more directly (Fig. 3I). WTAAE and WAAE pretreatment had
a similar effect at the 2.84 g/kg dose, which was more effective
than FBAAE. These data suggested that AAEs demonstrated a
significant protective effect on liver pathological injury in ALF
mice.
Anti-oxidative stress activity of
AAEs
Significantly increased levels of NO, LPO and
H2O2 in the model group demonstrated the
damage caused by oxidative stress compared with the normal group
(Fig. 4A-C). AAE treatment
significantly decreased the levels of lipid peroxidation. The
levels of CAT, SOD and T-AOC were significantly lower in the model
group compared with the normal group (Fig. 3D-F). The levels of CAT were
significantly increased with AAE pretreatment compared with the
model group, apart from in animals treated with 0.71 g/kg WTAAE.
SOD levels were significantly increased in mice treated with WAAE
and WTAAE at concentrations of 1.42 and 2.84 g/kg compared with the
model group. Levels of T-AOC were significantly increased in mice
pretreated with AAE compared with the model group. These data
suggested that AAEs have an anti-oxidant effect and the effect of
WTAAE was similar to WAAE and may be more effective than FBAAE at
the highest dose tested (2.84 g/kg).
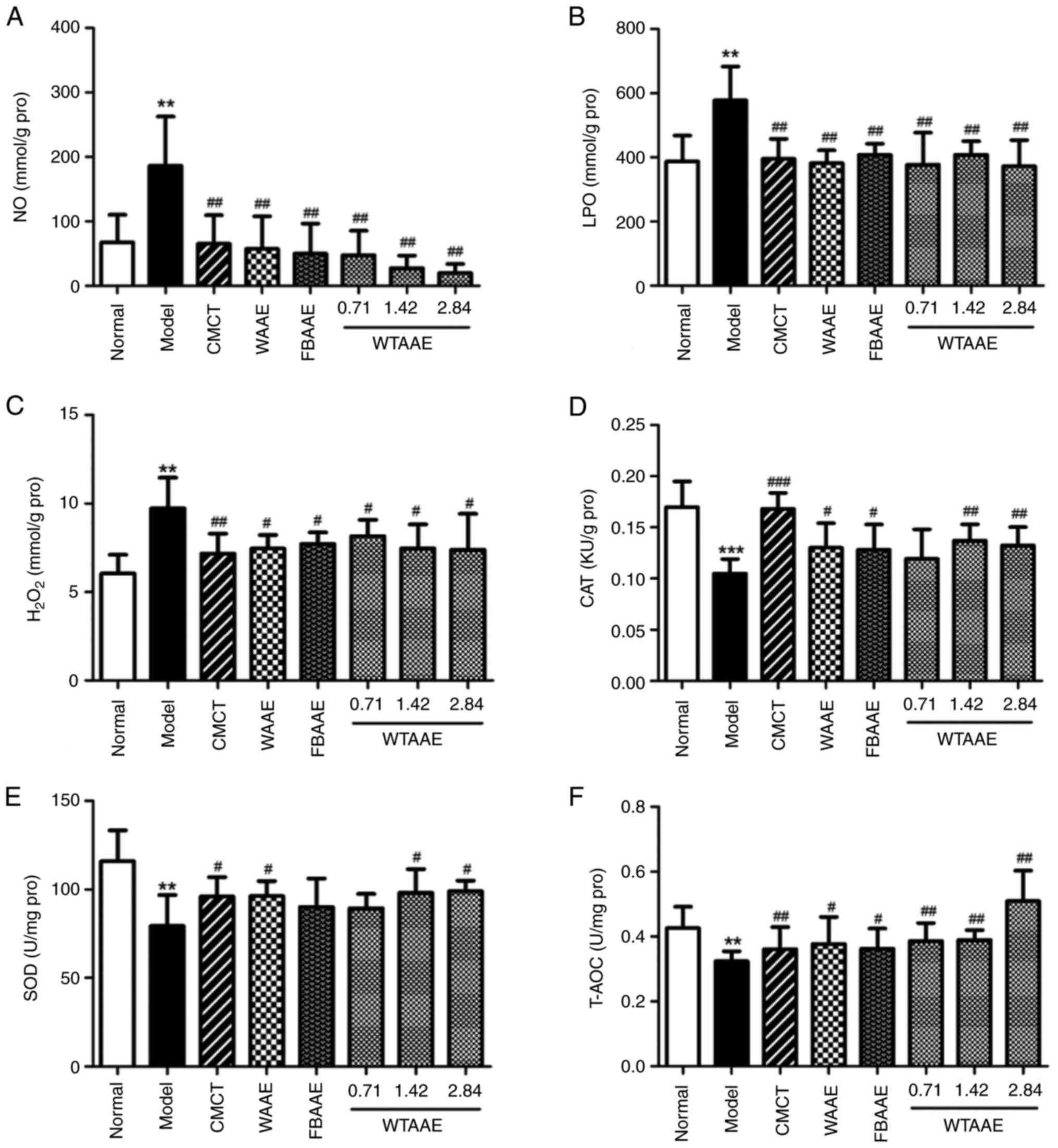 | Figure 4.Effects of agarwood alcohol extract
on NO, LPO, H2O2, CAT, SOD and T-AOC. Levels
of (A) NO. (B) LPO. (C) H2O2. (D) CAT. (E)
SOD. (F) T-AOC. Data are presented as the mean ± standard deviation
(n=10). **P<0.01, ***P<0.001 vs. Normal group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. Model group. CMCT, compound methionine
choline tablets; WAAE, wild agarwood induced by axe wounds alcohol
extract; FBAAE, burning-chisel-drilling agarwood alcohol extract;
WTAAE, whole-tree agarwood-inducing technique alcohol extract; LPO,
lipid peroxide; H2O2, hydrogen peroxide;
T-AOC, total peroxide content; CAT, catalase; SOD, superoxide
dismutase; Pro, protein. |
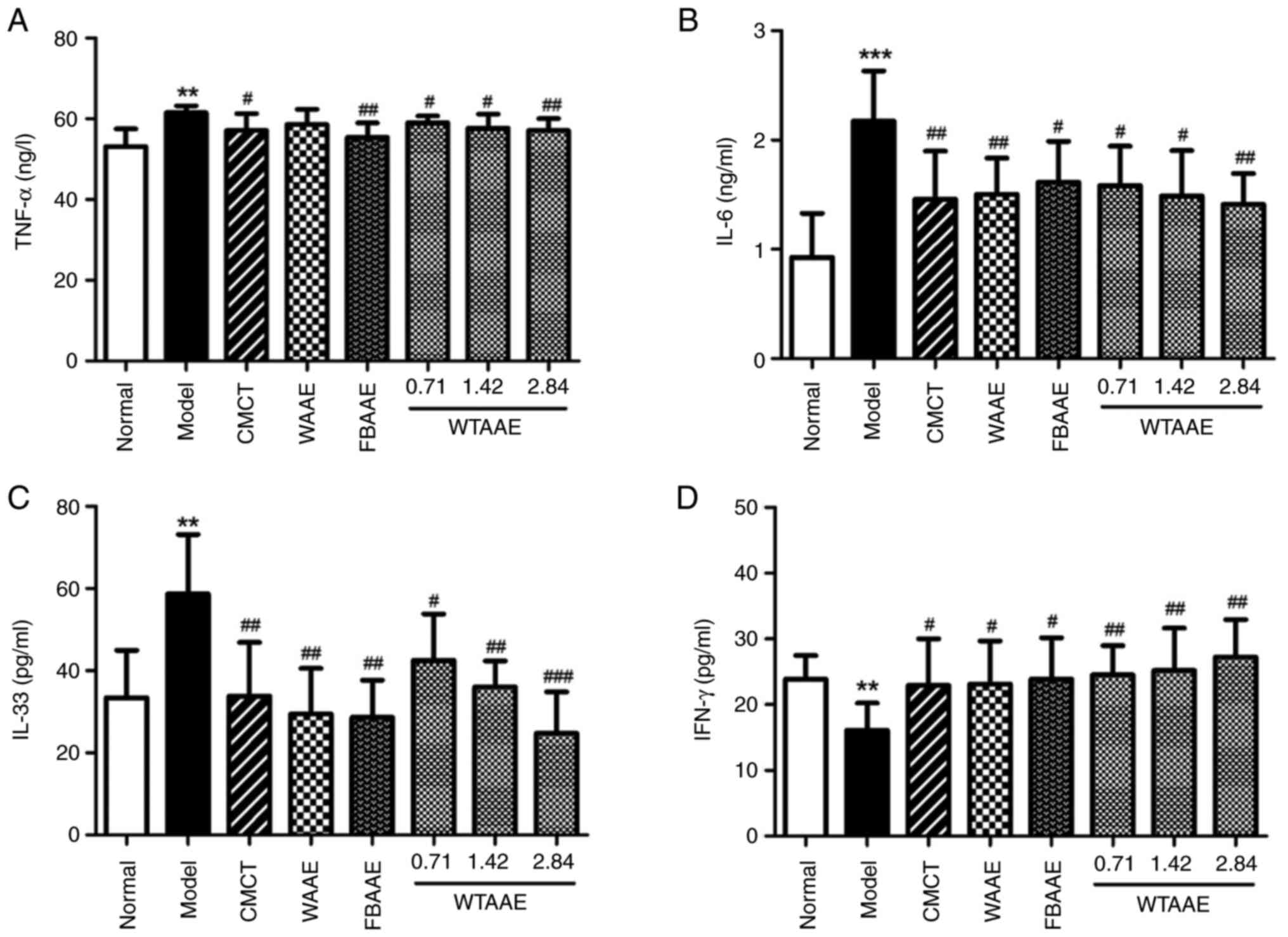
Cytokine expression following AAE
treatment
The levels of pro- and anti-inflammatory cytokines
were analyzed to evaluate the potential anti-inflammatory activity
of AAE treatment. The levels of TNF-α, IL-6 and IL-33 were
significantly increased in the AFL mice compared with normal mice
(Fig. 5A-C). AAEs significantly
decreased the levels of IL-6 and IL-33 in all treatment groups
compared with AFL mice. The levels of TNF-α were significantly
decreased by FBAAE and all concentrations of WTAAE tested compared
with the model group. WTAAE treatment at 2.84 g/kg appeared to be
the most effective treatment regime tested to reduce levels of the
aforementioned cytokines. In contrast, IFN-γ levels were
significantly decreased in the model group compared with the normal
group of mice (Fig. 5D). IFN-γ
levels were significantly increased in the WAAE, FBAAE and WTAAE
groups of mice and were highest with 2.84 g/kg WTAAE.
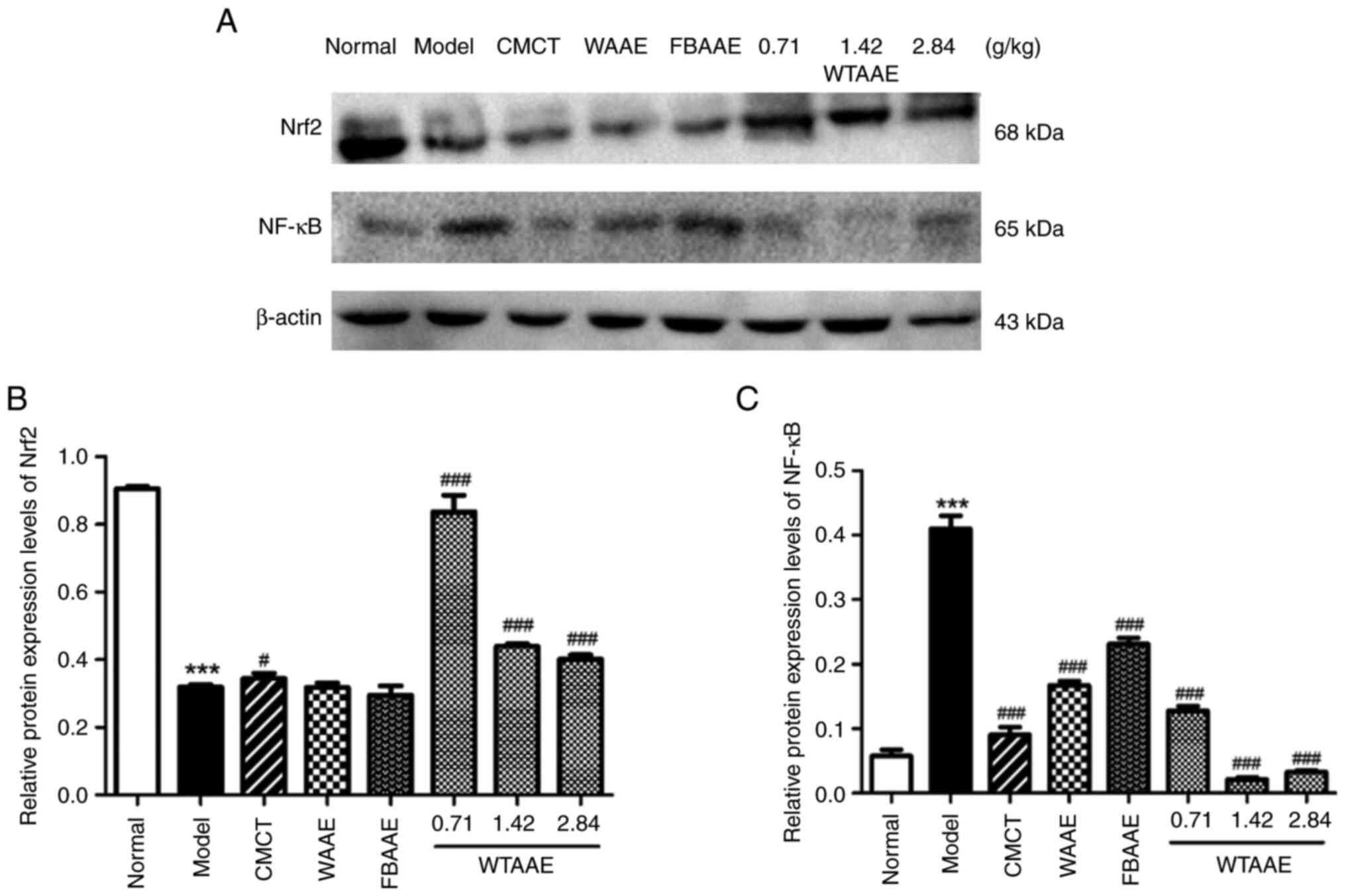
AAEs affect the protein expression
levels of Nrf2 and NF-κB
To further assess the underlying molecular
mechanisms of AAEs, the protein expression levels of Nrf2 and NF-κB
were assessed using western blotting (Fig. 6). The protein expression levels
were semi-quantified and it was demonstrated that the protein
expression levels of Nrf2 were significantly decreased in AFL mice
compared with the normal group. WTAAE pretreatment significantly
increased Nrf2 expression levels at each of the concentrations
tested compared with the model group. The protein expression levels
of NF-κB were significantly increased in the model group compared
with the normal group; however, AEE pretreatment significantly
decreased the protein expression levels of NF-κB compared with AFL
mice. These results suggested that AAEs may exert anti-oxidative
and anti-inflammatory effects.
Discussion
Agarwood, as an ingredient used in traditional
Chinese medicine, had been used for treating chest and abdominal
distension pain, stomach symptoms such as hiccupping and vomiting,
asthma, insomnia, anxiety and depression for >15 centuries
(13). Agarwood is an
over-exploited natural product that was on the verge of extinction;
in the 1980s, it was included in the national secondary key
protection of wild plants. Fortunately, agarwood produced by
artificial methods is high quality, therefore this ingredient can
continue to be used in medical and pharmacological research.
Moreover, the present study demonstrated that agarwood produced by
our previously published whole-tree agarwood-inducing technique
could be safely and effectively applied in the traditional Chinese
medicine clinic (13). Previous
studies reported that agarwood extracts have a protective effect
against liver injury (20,21). The present study demonstrated that
AAEs had a protective effect against an in vivo model of AFL
as treatment with AAEs relieved pathological liver damage and
decreased lipid peroxide and inflammatory cytokine levels. The
effect of WTAAE was similar to that with WAAE and was overall more
effective than FBAAE treatment. Long-term alcohol consumption and a
high fat diet was successfully used to induce an in vivo
model of AFL. AFL mice are characterized by hepatic tissue edema,
fatty degeneration necrosis, oxidative damage and inflammatory cell
infiltration (24,25). Liver and fat indexes, liver
metabolic enzymes and lipids and the presence of histological
lesions in the liver were used to assess the development of AFL in
the present study. The present study demonstrated that 30% ethanol
(0.2 ml/10 g) gavage and a high fat diet administered to mice for 3
weeks produced an AFL model. AAE treatment markedly reduced the
liver and fat indexes, the levels of liver metabolic enzymes and
lipids, and the degree of histological lesions in the liver.
Excessive accumulation of oxygen free radicals can
stimulate cell proliferation and induce oxidative damage of liver
tissue (26). Antioxidants such as
CAT, SOD and T-AOC can scavenge free radicals and protect the liver
from oxidative damage (27).
Reduced levels of CAT and SOD, and increased levels of NO, LPO and
H2O2 indicate the severity of damage caused
by oxidative stress in the liver as a result of excessive ethanol
consumption (27). The present
study demonstrated that ethanol and a high fat diet caused an
increase in the levels of NO, LPO and H2O2
and a decrease in levels of CAT, SOD and T-AOC in the model group,
which suggested that the livers of AFL mice were damaged.
Furthermore, AAE treatment reversed these indicators and
antioxidant effects by scavenging free radicals. Previous studies
reported that WTAAE has anti-myocardial ischemia and anti-gastric
ulcer effects and that agarwood improved tissue damage via an
antioxidant mechanism (22,23,28).
A previous study reported that AFLD was accompanied
by increased levels of pro-inflammatory cytokines, such as TNF-α,
IL-1β and IL-6 (22,29). The present study demonstrated that
AAE pretreatment significantly reduced the levels of the
pro-inflammatory cytokine TNF-α IL-6 and IL-33 and increased the
levels of the anti-inflammatory cytokine INF-γ in AFL mice, which
suggested that AAE protected against inflammation caused by AFL.
Previous studies have also reported that WTAAE inhibited
pathological effects, such as gastric ulcers and colitis and
alleviated tissue damage by anti-inflammatory effects in certain
diseases such as myocardial ischemia (22,23,30).
To further investigate the antioxidant and
anti-inflammatory mechanism and protective effect of agarwood on
the liver, the protein expression levels of Nrf2 and NF-κB were
detected by western blotting. Nrf2 is a key transcription factor
that clears free radicals and regulates the adaptive response to
oxidative stress (8,31). Nrf2 protein is induced and
activated by oxidative stress, serving a protective role in AFL
injury (32). A previous study
reported that inflammation served a critical role in AFL (32). NF-κB is an important transcription
factor in inflammatory responses, which induce upregulation of
pro-inflammatory cytokines and the inflammatory response (33). When liver cells stimulated by
ethanol, NF-κB is activated and triggers production of inflammatory
cytokines, which aggravates liver tissue damage (12,34).
In the present study, pre-treatment with AAEs inhibited NF-κB
protein activation and decreased the protein expression levels of
NF-κB, which contributed to inhibition of the inflammatory injury
caused by AFL. The effect of WTAAE was more potent compared with
WAAE and FBAAE.
To conclude, AAE treatment displayed protective
effects against AFL, with WTAAE found to be more effective compared
with WAAE and FBAAE. These data suggest that the cultivation of
artificial agarwood could replace the use of wild agarwood for
research into the development of medicines and the prevention of
clinical disease. The potential mechanisms of action of these
compounds may be due to their anti-oxidative and anti-inflammatory
properties. The present study provided experimental evidence for
further research and the clinical application of agarwood against
liver diseases such as liver steatosis, liver oxidative injury and
hepatitis. However, the pharmacodynamics and specific molecular
mechanisms of these substances needs to be further explored and
confirmed. Overall, agarwood has the potential to be used in the
future in the prevention and treatment of hepatic diseases.
Acknowledgements
Not applicable.
Funding
This research study was supported by the National Natural
Science Foundation of China (grant no. 82204657), the Maoming
Special Science and Technology Project (grant no. 2022S032), the
Key Research Project of Hainan Province (grant no.
ZDYF2022SHFZ030), the Maoming Laboratory Independent Research
Project (grant no. 2022KF015) and the Maoming City Provincial
Science and Technology Innovation Strategy Project (grant no.
2023S001007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and JW designed the research project and the
experimental scheme. CW, BG and YW performed the animal experiments
and data analysis. DP and YL prepared the agarwood extract. CW
prepared the manuscript. All authors read and approved the final
version of the manuscript. CW and JW revised the manuscript CW, BG,
DP, YL, YW and JW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Animal care and experimental protocols were approved
by the Animal Care and Use Committee at the Institute of Medicinal
Plant Development, Chinese Academy of Medical Sciences (approval
no. SLXD-20180809012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
TG
|
triglyceride
|
|
CHO
|
cholesterol
|
|
LPO
|
lipid peroxide
|
|
H2O2
|
hydrogen peroxide
|
|
T-AOC
|
total peroxide content
|
|
CAT
|
catalase
|
|
SOD
|
superoxide dismutase
|
|
CMCT
|
compound methionine choline
tablets
|
|
WTAAE
|
whole-tree agarwood-inducing technique
alcohol extract
|
|
WAAE
|
wild agarwood induced by axe wounds
alcohol extract
|
|
FBAAE
|
burning-chisel-drilling agarwood
alcohol extract
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
WB
|
western blotting
|
|
ALD
|
alcoholic liver disease
|
|
Nrf2
|
nuclear erythroid 2-related factor
2
|
|
NF-κB
|
nuclear factor kappa-B
|
|
H&E
|
hematoxylin and eosin
|
|
PDI
|
pathological damage index
|
References
|
1
|
Fan JG: Epidemiology of alcoholic and
nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol.
28 (Suppl 1):S11–S17. 2013. View Article : Google Scholar
|
|
2
|
Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun
W, Sun X, Yin X, Sun X, Kim S, et al: Chronic alcohol exposure
stimulates adipose tissue lipolysis in mice: Role of reverse
triglyceride transport in the pathogenesis of alcoholic steatosis.
Am J Pathol. 180:998–1007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lakshman MR: Some novel insights into the
pathogenesis of alcoholic steatosis. Alcohol. 34:45–48. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raghu R, Liu CT, Tsai MH, Tang X, Kalari
KR, Subramanian S and Sheen LY: Transcriptome analysis of
garlic-induced hepatoprotection against alcoholic fatty liver. J
Agric Food Chem. 60:11104–11119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sookoian S and Pirola CJ: Systems biology
elucidates common pathogenic mechanisms between nonalcoholic and
alcoholic-fatty liver disease. PLoS One. 8:e588952013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lieber CS: Alcoholic fatty liver: Its
pathogenesis and mechanism of progression to inflammation and
fibrosis. Alcohol. 34:9–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma D, Hu J, Xu W, Wang Y, Wang J, Li L,
Wang S, Zhou H, Li Y and Liu L: Phosphoesterase complex modulates
microflora and chronic inflammation in rats with alcoholic fatty
liver disease. Life Sci. 262:1185092020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo H, Sun J, Li D, Hu Y, Yu X, Hua H,
Jing X, Chen F, Jia Z and Xu J: Shikonin attenuates
acetaminophen-induced acute liver injury via inhibition of
oxidative stress and inflammation. Biomed Pharmacother.
112:1087042019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuge Q, Zhang Y, Liu B and Wu MJ:
Blueberry polyphenols play a preventive effect on alcoholic fatty
liver disease C57BL/6 J mice by promoting autophagy to accelerate
lipolysis to eliminate excessive TG accumulation in hepatocytes.
Ann Palliat Med. 9:1045–1054. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang M, Zhang XJ, Feng K, He C, Li P, Hu
YJ, Su H and Wan JB: Dietary α-linolenic acid-rich flaxseed oil
prevents against alcoholic hepatic steatosis via ameliorating lipid
homeostasis at adipose tissue-liver axis in mice. Sci Rep.
6:268262016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Mukhopadhyay P, Cao Z, Wang H,
Feng D, Haskó G, Mechoulam R, Gao B and Pacher P: Cannabidiol
attenuates alcohol-induced liver steatosis, metabolic
dysregulation, inflammation and neutrophil-mediated injury. Sci
Rep. 7:120642017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong L, Chen J, Ji X, Qin Q, Yang H, Liu
D, Li D and Sun M: Alcoholic fatty liver disease inhibited the
co-expression of Fmo5 and PPARα to activate the NF-κB signaling
pathway, thereby reducing liver injury via inducing gut microbiota
disturbance. J Exp Clin Cancer Res. 40:182021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Chen H, Yang Y, Zhang Z, Wei J,
Meng H, Chen W, Feng J, Gan B, Chen X, et al: Whole-tree
agarwood-inducing technique: An efficient novel technique for
producing high-quality agarwood in cultivated Aquilaria sinensis
trees. Molecules. 18:3086–3106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takagi K, Kimura M, Harada M and Otsuka Y:
Pharmacology of medicinal herbs in East Asia. Nanzando Tokyo.
41:187–188. 1982.
|
|
15
|
Zhu Z, Zhao Y, Huo H, Gao X, Zheng J, Li J
and Tu P: HHX-5, a derivative of sesquiterpene from Chinese
agarwood, suppresses innate and adaptive immunity via inhibiting
STAT signaling pathways. Eur J Pharmacol. 791:412–423. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Z, Gu Y, Zhao Y, Song Y, Li J and Tu
P: GYF-17, a chloride substituted 2-(2-phenethyl)-chromone,
suppresses LPS-induced inflammatory mediator production in RAW264.7
cells by inhibiting STAT1/3 and ERK1/2 signaling pathways. Int
Immunopharmacol. 35:185–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huo HX, Gu YF, Sun H, Zhang YF, Liu WJ,
Zhu ZX, Shi SP, Song YL, Jin HW, Zhao YF, et al: Anti-inflammatory
2-(2-phenylethyl)chromone derivatives from Chinese agarwood.
Fitoterapia. 118:49–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou M, Wang H, Suolangjiba Kou J and Yu
B: Antinociceptive and anti-inflammatory activities of Aquilaria
sinensis (Lour.) Gilg. Leaves extract. J Ethnopharmaco.
117:345–350. 2008. View Article : Google Scholar
|
|
19
|
Sattayasai J, Bantadkit J, Aromdee C,
Lattmann E and Airarat W: Antipyretic, analgesic and anti-oxidative
activities of Aquilaria crassna leaves extract in rodents. J
Ayurveda Integr Med. 3:175–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CH, Wang S, Peng DQ, Yu ZX, Guo P and
Wei JH: Protective effect of agarwood alcohol extracts produced by
whole-tree agarwood-inducing technique on the fluorouracil-induced
liver injury in mice. J Int Pharm Res. 45:187–197. 2018.
|
|
21
|
Wang CH, Wang S, Peng DQ, Liu YY, Guo P
and Wei JH: Protective effect of alcohol extract of agarwood on
acute liver injury induced by carbon tetrachloride in mice. Mod
Chin Med. 19:1091–1096. 2017.
|
|
22
|
Wang C, Peng D, Liu Y, Wu Y, Guo P and Wei
J: Agarwood alcohol extract protects against gastric ulcer by
inhibiting oxidation and inflammation. Evid Based Complement
Alternat Med. 2021:99446852021.PubMed/NCBI
|
|
23
|
Wang C, Peng D, Liu Y, Yu Z, Guo P and Wei
J: Agarwood alcohol extract ameliorates isoproterenol-induced
myocardial ischemia by inhibiting oxidation and apoptosis. Cardiol
Res Pract. 2020:36408152020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan P, Liang H, Nie J, Diao Y, He Q, Hou
B, Zhao T, Huang H, Li Y, Gao X, et al: Establishment of an
alcoholic fatty liver disease model in mice. Am J Drug Alcohol
Abuse. 43:61–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong Q, Chu F, Wu C, Huo Q, Gan H, Li X
and Liu H: Scutellaria baicalensis Georgi extract protects against
alcohol-induced acute liver injury in mice and affects the
mechanism of ER stress. Mol Med Rep. 13:3052–3062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeh MM and Brunt EM: Pathological features
of fatty liver disease. Gastroenterol. 147:754–764. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Park J, Han SJ, Park I, Huu TN,
Kim JS, Woo HA and Lee SR: The critical role of redox regulation of
PTEN and peroxiredoxin III in alcoholic fatty liver. Free Rad Bio
Med. 162:141–148. 2021. View Article : Google Scholar
|
|
28
|
Wang Z, Dou X, Li S, Zhang X, Sun X, Zhou
Z and Song Z: Nuclear factor (erythroid-derived 2)-like 2
activation-induced hepatic very-low-density lipoprotein receptor
overexpression in response to oxidative stress contributes to
alcoholic liver disease in mice. Hepatolo. 59:1381–1392. 2014.
View Article : Google Scholar
|
|
29
|
Park JH, Lee DH, Park MS, Jung YS and Hong
JT: C-C chemokine receptor type 5 deficiency exacerbates alcoholic
fatty liver disease through pro-inflammatory cytokines and
chemokines-induced hepatic inflammation. J Gastroentero Hepatolo.
32:1258–1264. 2017. View Article : Google Scholar
|
|
30
|
Wang C, Wang S, Peng D, Liu Y, Guo P and
Wei J: Agarwood extract mitigates intestinal injury in
fluorouracil-induced mice. Biol Pharm Bull. 42:1112–1119. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han X, Ding C, Zhang G, Pan R, Liu Y,
Huang N, Hou N, Han F, Xu W and Sun X: Liraglutide ameliorates
obesity-related nonalcoholic fatty liver disease by regulating
Sestrin2-mediated Nrf2/HO-1 pathway. Biochem Biophys Res Commun.
525:895–901. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao G, Xie Z, Li EW, Yuan Y, Fu Y, Wang P,
Zhang X, Qiao Y, Xu J, Hölscher C, et al: Dehydroabietic acid
improves nonalcoholic fatty liver disease through activating the
Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J Nat Med.
75:540–552. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao J, Wang Y, Wu X, Tong P, Yue Y, Gao
S, Huang D and Huang J: Inhibition of CCL19 benefits non-alcoholic
fatty liver disease by inhibiting TLR4/NF-κB-p65 signaling. Mol Med
Rep. 18:4635–4642. 2018.PubMed/NCBI
|
|
34
|
Gao X, Hong R and Li J: Gastroenterology
DO. Protective effect of silymarin on alcoholic fatty liver in rats
possibly via impairing nf-κb activation. Acta Univer Med Anhui.
2014.
|