Introduction
Obesity is caused by an imbalance between energy
intake and expenditure, resulting in the accumulation of excessive
white adipose tissue (WAT) (1).
The coronavirus disease 2019 pandemic led to a higher prevalence of
obesity, due to restricted outdoor physical activities (2) and unhealthy lifestyle changes
(3). Furthermore, obesity
predisposes patients with severe acute respiratory syndrome
coronavirus-2 infection to severe outcomes (4). The association between obesity,
infection and various metabolic diseases including insulin
resistance or coronary heart disease is well known (5). Since obesity is caused by immoderate
lipid deposition and adipose tissue expansion, inhibiting
proliferation and hypertrophy of adipocyte may solve obesity and
other metabolic complications (6).
As one of the most complex organs in the human body,
adipose tissue consists of lipid-rich cells called adipocytes,
which interact with the entire body to maintain metabolic
homeostasis (7). Hypertrophy and
hyperplasia of the adipose tissue contribute to adipose tissue
dysfunction (8). Pathological
changes in adipose tissue are reflected by abnormal adipokine
secretion, insulin resistance, or prolonged inflammation related to
obesity and its associated comorbidities (9). Therefore, understanding the molecular
mechanisms underlying adipocyte differentiation, physiology,
morphological changes and related factors is necessary to determine
the effects of adipocytes. Specifically, adenosine
monophosphate-activated protein kinase (AMPK) is an energy sensor
that negatively regulates white adipogenesis in the body (10). The AMPK pathway activation inhibits
adipocyte proliferation by regulating adipogenic transcription
factors, such as sterol regulatory element-binding protein
(SREBP)-1 and peroxisome proliferator-activator (PPAR)-γ (10). SREBP-1 is a member of a
transcription factor family that regulates lipid homeostasis and
metabolism, thereby controlling the synthesis of endogenous
cholesterol, fatty acids, triacylglycerol and phospholipids
(11). PPARs are a group of
proteins required for fatty acid oxidation and energy metabolism
(12). One of the three subtypes
of PPARs, PPAR-γ, contributes to energy balance, and lipid and
glucose homeostasis regulation as a nuclear receptor superfamily
member (13).
Drynaria rhizome is the dried root of Drynaria
fortunei, a herbaceous perennial plant (14), which has been used to improve bone
health by promoting trauma recovery and treating bone fractures
(15). As a medicinal herb, the
rhizome is classified among the ‘Yang-tonifying’ or
‘kidney-tonifying’ herbs specific for bone-related diseases,
including osteoporosis or bone fractures, that can regulate bone
formation or bone resorption (16–18).
Compounds of Drynaria rhizome, such as flavonoids, have exhibited
protective effects against osteoarthritis by enhancing bone
regeneration (19,20). Since numerous studies have reported
on the association between bone health and obesity, it is necessary
to investigate therapeutic candidates that can regulate these
(21–24). Since obesity may induce an increase
in bone density affected by higher mechanical loads or higher
17β-estradiol levels protecting bone (25,26),
it seems to be worth to investigate the effects of Drynaria rhizome
which is known for bone-related diseases. Previous studies have
demonstrated the promoting effects of Drynaria rhizome and its
flavonoids on osteoblast differentiation in MC3T3-E1 cells and
ovariectomized Spragues-Dawley rats (27–29).
Given the increasing evidence of the close relationship between
weight loss and bone health (30,31),
evaluating the anti-obesity effects of existing reliable medicines
for bone disease is important. Therefore, the present study
investigated the effects of Drynaria rhizome extract (DRE)
supplemented with a high-fat diet (HFD) on HFD-induced obese
mice.
Materials and methods
Antibodies
The phosphorylated (p)-AMPK (cat. no. 2535) and AMPK
(cat. no. 2532) antibodies were obtained from Cell Signaling
Technology, Inc. PPAR-γ (cat. no. sc-7273), SREBP1 (cat. no.
sc-13551) and β-actin (cat. no. sc-81178) antibodies were obtained
from Santa Cruz Biotechnology, Inc.
Preparation of DRE
The root of the herb Drynaria fortunei was
purchased from Nanum Pharmaceutical Company (cat. no.
HA1800100101). The herb (400 g) was extracted in 4 l hot water at
100°C for 4 h. The extract was freeze-dried and the yield was
calculated at 17.5%; [dried extract weight (38.465 g)/dry starting
material weight (219.8 g)] ×100 (%).
HFD-induced obese mouse model and
treatment
The present study followed the methods of Park et
al (32). DRE powder was
lyophilized, extracted with water at 100°C for 4 h and purified
using filter papers under a vacuum rotary evaporator (EYELA-Tokyo
Rikakikai Co., Ltd.); the residual powder was stored at −20°C until
needed. The powder was used to generate a supplemented diet
containing 10% DRE and 45% HFD (Research Diets, Inc.); 20 g DRE
powder was mixed with 180 of HFD. Male C57BL/6 N mice
(specific-pathogen-free grade; age, 8 weeks; weight, 20±2 g) were
purchased from Dae Han Bio Link Co., Ltd. The mice were adapted to
modified conditions for 1 week, and 30 healthy mice were then used
in the present study. The mice were randomly distributed into the
following three groups (n=10/group): Normal diet (CON), 45%
HFD-induced (HFD), and 45% HFD-induced and 10% DRE-administered
(HFD + DRE) groups. The mice were provided ad libitum access
to food and water. Mice in the HFD + DRE group were provided a HFD
for 4 weeks leading to HFD-induced obesity. Subsequently, the mice
were fed a HFD supplemented with 10% DRE from week 5. Mice in the
CON and HFD groups were fed normal diet and HFD, respectively for 9
weeks. The mice were maintained under a 12/12 h light/dark cycle,
at a constant temperature of 22±2°C and relative humidity of 55±9%.
Body weight and food intake were measured weekly. For sacrifice,
mice were placed in a 9 l container; 100% of carbon dioxide was
supplied to the container at a volume displacement rate of 30% per
min (~3 l/min). The flow was continued until 1 min after breathing
or heartbeat stopped. Subsequently, cervical dislocation was
performed to ensure the animal was sacrificed. All procedures were
conducted following the National Institutes of Health guidelines
(33) and the present study was
approved by the Ethical Committee for Animal Care and the Use of
Laboratory Animals of Sangji University (approval no. 2019-11;
Wonju, South Korea). At the end of the 10-week period, liver and
adipose tissues were obtained, rinsed, weighed and stored at −80°C
until further analysis.
Food efficiency ratio
After sacrifice, body weight gain was divided with
food intake. And the value was expressed as percentage).
Weight of eWAT tissues
Relative epididymal white adipose tissues were
calculated epididymal WAT weight divided to body weight.
Western blot analysis
Segments of liver or epididymal WAT (eWAT) were
suspended in PRO-PREP™ protein extraction solution (Intron
Biotechnology, Inc.) and incubated for 20 min at 4°C. Cell debris
was removed via microcentrifugation (20,784 × g, 4°C for 30 min)
followed by quick freezing of the supernatant. Protein
concentration was determined using the Bio-Rad protein assay
reagent (Bio-Rad Laboratories, Inc.) according to the
manufacturer's instructions. Cellular proteins (30 µg) from
homogenized adipose tissue were separated by 10–12% SDS-PAGE and
electro-blotted onto a polyvinylidene fluoride membrane. The
membrane was then incubated for 1 h with blocking solution (5% skim
milk) at room temperature, followed by overnight incubation with
primary antibodies (1:1,000) at 4°C. Blots were washed three times
with 0.1% Tween 20/Tris-buffered saline (T/TBS) and were then
incubated with horseradish peroxidase-conjugated secondary
antibodies [Peroxidase AffiniPure Rabbit Anti-Mouse IgG (H+L); cat.
no. 315-035-003; Peroxidase AffiniPure Goat Anti-Rabbit IgG (H+L);
cat. no. 111-035-003; both from Jackson ImmunoResearch Laboratories
Inc. 1:2,500 dilution] for 2 h at room temperature. Blots were
again washed three times with T/TBS, and then developed via
enhanced chemiluminescence (GE Healthcare). Densitometric analysis
was performed using ImageJ ver 1.53a software (National Institutes
of Health).
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the liver tissue and
eWAT using the EASY BLUE RNA extraction kit (iNtRON Biotechnology),
according to the manufacturer's instructions. The RNA was then
reverse transcribed into cDNA using an Maxime RT PreMix (Random
primer, iNtRON Biochnology) according to the manufacturer's
protocol. It was conducted using a GeneAmp PCR System 9700 (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The synthesized cDNA
was 200 bp in size. A StepOnePlus Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used for
amplification with Power-SYBR Green PCR Master Mix (Applied
Biosystems). The qPCR thermocycling conditions were as follows:
Preheating at 92°C for 2 min, followed by 50 cycles at 92°C for 30
sec, 60°C for 30 sec and 68°C for 30 sec. The expression data were
calculated from the quantification cycle (Cq) value using the
2−ΔΔCq method (34).
GAPDH was used for normalization. The primer sequences used for
RT-qPCR are listed in a previous study (35), as follows: AMPKα, forward (F)
5′-AGAGGGCCGCAATAAAAGAT-3′, reverse (R) 5′-TGTTGTACAGGCAGCTGAGG-3′;
SREBP1, F 5′-ATCGCAAACAAGCTGACCTG-3′, R 5′-AGATCCAGGTTTGAGGTGGG-3′;
PPARγ, F 5′-ATCGAGTGCCGAGTCTGTGG-3′; R 5′-GCAAGGCACTTCTGAAACCG-3′;
GAPDH, F 5′-GACGGCCGCATCTTCTTGT-3′ and R
5′-CACACCGACCTTCACCATTTT-3′.
Biochemical analysis
Briefly, 1.0–1.2 ml total blood was collected from
each mouse using cardiac puncture following sacrifice. The
collected blood was immediately centrifuged (1,000 × g for 30 min
at 4°C) to obtain plasma. The plasma levels of triglycerides (TG)
and total cholesterol (TC) were measured using commercial kits
(AM157S-K for TG and AM 202-K for TC) (Asan Pharmaceutical, Co.,
Ltd.).
Histological analysis
The liver tissue and eWAT from mice in each group
were fixed in 10% buffered formalin for 5–10 min for eWAT as well
as 20–30 min for liver at 37°C, embedded in paraffin and cut into
8-µm sections. Sections were stained for 6 h at 60–70°C with
hematoxylin and eosin (H&E) for histological examination of
lipid droplets. Adipocyte cell size was determined by measuring 10
randomly selected adipocytes per area of respective tissue and
images were acquired using an Olympus SZX10 light stereomicroscope
(Olympus Corporation).
Statistical analysis
Data are presented as the mean ± standard deviation
of triplicate experiments. The experimental data were analyzed
using one-way analysis of variance followed by Dunnett's post hoc
test (GraphPad PRISM 5; Dotmatics). P<0.05 was considered to
indicate a statistically significant difference.
Results
DRE reduces the body weight and serum
biochemical parameters of HFD-fed obese mice
Changes in body weight were tracked weekly for 10
weeks. The body weight of the HFD group was significantly increased
after 4 weeks compared with that in the CON group (Fig. 1A). DRE was administered via the
diet as 10% DRE supplemented in the HFD. The body weight of mice in
the HFD + DRE group was lower than that in the HFD group. DRE
induced a significant change in body weight at 6 weeks caused by
adaptation of mice to the new diet. After 10 weeks, the body weight
of mice in the HFD group was significantly increased (35.50±2.76 g)
compared with that in the CON group (28.85±0.97 g). In addition,
the HFD + DRE group exhibited reduced body weight (31.85±1.95 g)
compared with that in the HFD group. Mice were allowed ad
libitum access to the diet, and food intake was evaluated,
showing no differences between the groups (Fig. 1B). To calculate the food efficiency
ratio, the equation used to determine the protein efficiency ratio
(PER; %) was adapted as follows: Body weight gain (g)/food intake
(g) ×100 (36). Instead of
evaluating the protein quality in food, the present study aimed to
assess total food efficiency, which was significantly increased in
the HFD group compared to the CON group as well as significantly
decreased in the HFD + DRE group comparing to the HFD group
(Fig. 1C). At the end of the
experiment, after 10 weeks, anatomical examination confirmed the
reduced body fat mass in the HFD + DRE group compared with that in
the HFD group (Fig. 1D). In
addition, after 9 weeks, the levels of serum biochemical markers,
TG and TC, were significantly reduced in the HFD + DRE group
compared with those in the HFD group and were significantly
increased in the HFD group compared with those in the CON group
(Fig. 1E and F).
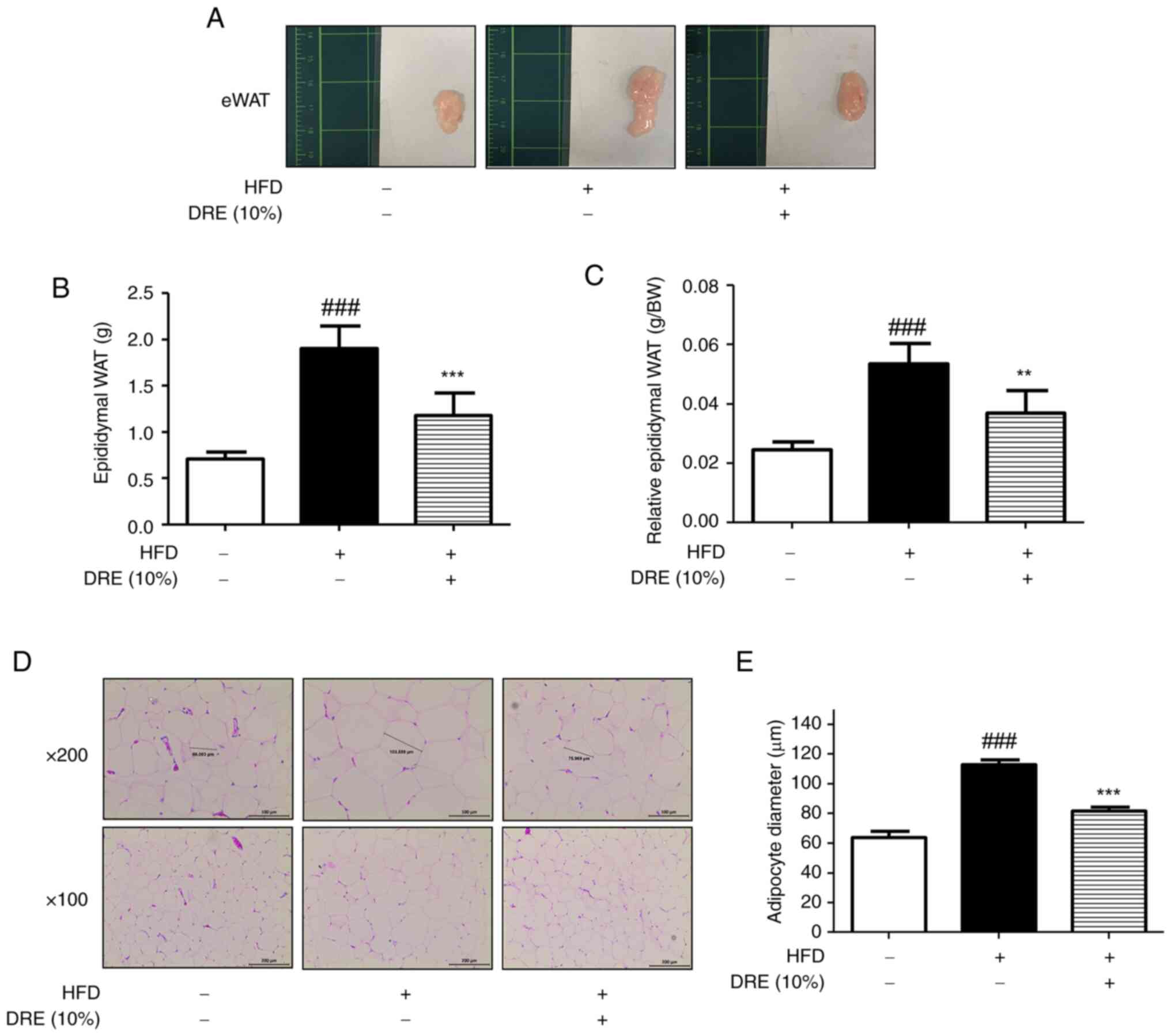
DRE suppresses lipid accumulation in
eWAT in HFD-fed obese model mice
Adipose tissue grows via two processes, hypertrophy
and hyperplasia (37). In the
present study, eWAT exhibited marked changes in size (Fig. 2A) and weight (Fig. 2B). The average weight of eWAT in
the HFD group (1.90±0.32 g) was significantly increased compared
with that in the CON group (0.70±0.09 g). This increase was
suppressed by DRE (1.17±0.32 g) in the HFD + DRE group. This
tendency was verified by determining relative eWAT weight (tissue
weight/body weight) (Fig. 2C). To
determine the effects of DRE on adipose tissue growth and lipid
accumulation, H&E staining was performed on eWAT (Fig. 2D). As shown in Fig. 2D and E, the HFD group exhibited an
increase in adipocyte size (113.22±4.57 µm) compared with that in
the CON group (63.86±6.30 µm); however, mice in the HFD + DRE group
exhibited decreased adipocyte diameter (81.93±3.61 µm) compared
with that in the HFD group.
DRE downregulates the expression of
adipogenic markers in the eWAT of HFD-induced obese mice
Western blotting was conducted to clarify the effect
of DRE on the expression levels of adipogenic transcription factors
in eWAT. The HFD + DRE group exhibited restored protein expression
of p-AMPK-α compared with that in the HFD group, which was
significantly decreased compared with the CON group (Fig. 3A and B). Given the regulatory
effect of DRE on p-AMPK-α (Fig. 3A and
B), the protein expression levels of adipogenic transcription
factors, SREBP-1 and PPAR-γ, were determined. The increased protein
expression levels induced by HFD were mitigated by DRE dietary
treatment (Fig. 3C-E). To assess
the mRNA expression levels of these factors, RT-qPCR analysis was
performed. The mRNA expression levels were consistent with the
protein expression levels. DRE upregulated AMPK activation, and a
downregulatory effect on SREBP-1 or PPAR-γ expression (Fig. 3F).
 | Figure 3.Effect of DRE on the expression
levels of adipogenic transcription factors in the eWAT of
HFD-induced obese mice. (A) Representative western blot and (B)
semi-quantification of p-AMPK-α protein expression levels in eWAT.
P-AMPK expression was normalized to AMPK using ImageJ v1.50i. (C)
Representative western blot, and semi-quantification of (D) SREBP-1
and (E) PPAR-γ protein expression levels in eWAT. Expression was
normalized to β-actin using ImageJ v1.50i. (F) AMPK-α, SREBP-1 and
PPAR-γ mRNA expression levels were detected in eWAT using reverse
transcription-quantitative polymerase chain reaction. Relative mRNA
expression levels were normalized to GAPDH. Data are presented as
the mean ± standard deviation. ##P<0.01 and
###P<0.001 vs. CON group; *P<0.05, **P<0.01 and
***P<0.001 vs. HFD group. AMPK-α, adenosine
monophosphate-activated protein kinase-α; CON, control; DRE,
Drynaria rhizome extract; eWAT, epididymal white adipose tissue;
HFD, high-fat diet; p-, phosphorylated; PPAR-γ, peroxisome
proliferator-activated receptor-γ; SREBP-1, sterol regulatory
element binding protein-1. |
DRE ameliorates lipid accumulation in
the liver of HFD-fed obese model mice
Excessive fat accumulation in the liver leads to
fatty liver diseases, such as nonalcoholic fatty liver disease
(38). Therefore, the present
study observed the size and color of liver tissue and compared its
weight between groups. Bigger liver tissue of HFD group compared to
CON group was presented and brighter liver tissue of HFD + DRE
group comparing to CON or HFD group was shown (Fig. 4A), there was no statistically
significant difference in liver weight among the three groups
(Fig. 4B). However, histological
examination with H&E confirmed that HFD-induced lipid
accumulation was alleviated by DRE, resulting in smaller and less
frequent lipid droplets, as indicated with black arrows (Fig. 4C).
DRE suppresses adipogenic marker
expression in the liver in HFD-induced obese mice
The present study assessed the mRNA and protein
expression levels of adipogenesis factors via RT-qPCR and western
blot analysis, respectively. Unlike the changes in AMPK activation
observed in eWAT and protein expression of liver, the difference in
the mRNA expression levels of AMPK among the groups was not
significant (Fig. 5A, B and F).
Also, DRE treatment didn't significantly recover the protein
expression of AMPK phosphorylation comparing to HFD group whereas
apparently decreased phosphorylated AMPK expression in HFD group.
However, the adipogenic transcription factor SREBP-1, and its
direct target PPAR-γ, were markedly induced by HFD and suppressed
by DRE dietary treatment (Fig.
5C-F). In Fig. 5B, D, and E,
the relative density of protein expression was presented.
 | Figure 5.Effect of DRE on adipogenesis in the
liver in HFD-induced obese mice. (A) Representative western blot
and (B) semi-quantification of p-AMPK-α protein expression levels
in liver tissue. P-AMPK expression was normalized to AMPK using
ImageJ v1.50i. (C) Representative western blot, and
semi-quantification of (D) SREBP-1 and (E) PPAR-γ protein
expression levels in liver tissue. Expression was normalized to
β-actin using ImageJ v1.50i. (F) AMPK-α, SREBP-1 and PPAR-γ mRNA
expression levels in the liver were determined using reverse
transcription-quantitative polymerase chain reaction. Relative mRNA
expression levels were normalized to GAPDH. Data are presented as
the mean ± standard deviation. ###P<0.001 vs. CON
group; *P<0.05 and ***P<0.001 vs. HFD group. AMPK-α,
adenosine monophosphate-activated protein kinase-α; CON, control;
DRE, Drynaria rhizome extract; eWAT, epididymal white adipose
tissue; HFD, high-fat diet; p-, phosphorylated; PPAR-γ, peroxisome
proliferator-activated receptor-γ; SREBP-1, sterol regulatory
element binding protein-1. |
Discussion
In drug development, interest in drug repurposing
has increased, with the aim of uncovering novel therapeutic
indications of proven drugs (39).
Drynaria rhizome has been used for bone health and blood
revitalization, and is mainly prescribed for bone formation or
fractures due to its warm (nature) and bitter taste (40–43).
The compounds of Drynaria rhizome have been suggested to treat
osteoporosis (44). In addition,
it is known that Drynaria rhizome has no obvious toxic side effects
(41). Drynaria rhizome contains
various bioactive compounds with the capacity for alleviating
obesity, such as (−)-epicatechin or narigin (45,46).
Recent studies have focused on the complex relationship between
bone health and obesity, including the effect of adipokines on bone
cells and bone metabolism in type 2 diabetes (22,26,47).
Therefore, the present study evaluated the effects of the root of
D. fortunei on obesity.
A HFD is the primary promotor of obesity by inducing
an imbalance in energy expenditure and intake (48). Therefore, the HFD-induced obesity
mouse model is a well-established in vivo experimental model
for research on obesity (49). The
present study used a 45% HFD-induced obese C57BL/6 mouse model. To
mimic the influence of food and minimize stress in mice, the
experimental preparation (DRE) was mixed with the HFD at a
concentration of 10%. The dosage and method of DRE administration
were selected according to the recommendations of previous studies
(35,50,51).
Body weight was measured weekly and obesity was revealed to be
significantly induced after 5 weeks. Differential weight loss by
DRE after 8 weeks of the administration. Despite similar food
intake across the groups, obesity was significantly alleviated in
the DRE-supplemented group. Nutritional factors affected by DRE
were determined by modified PER, which represents the weight gain
of a subject divided by the intake of dietary protein during the
experimental period (52).
Furthermore, it seems to be valuable to determine the effects of
DRE on the efficiency of food digestion and absorption to
evaluation intestinal capacity for further study. The increased
body fat mass was determined based on the appearance of eWAT in the
abdominal cavity.
As the abnormal expansion and accumulation of eWAT
are considered characteristics of obesity, the present study
investigated the histological and molecular changes in eWAT
(53). A HFD induced a significant
increase in the weight and size of eWAT, which indicated adipocyte
expansion and hyperplasia. These morphological changes were
histologically confirmed under a microscope via H&E staining
indicating lipid accumulation (54,55).
Additionally, DRE alleviated the increase in adipocyte diameter
induced by HFD. Furthermore the protein and mRNA expression levels
of lipogenesis-related markers, AMPK, SREBP-1 and PPAR-γ, were
altered by DRE dietary supplementation. AMPK is a protein kinase
involved in metabolism, which regulates adipogenic transcription
factors, including SREBP-1 and PPAR-γ (56), and suppresses insulin resistance
(57). Furthermore, it has been
reported that total flavonoids of Drynaria rhizome exhibit efficacy
in treating osteoarthritis via the AMPK/NF-κB pathway (58). In the present study, DRE
upregulated p-AMPK expression under HFD-induced obesity conditions.
AMPK and its downstream proteins maintain energy homeostasis in
adipose tissues (59). The effects
of DRE on the molecular level of AMPK-α, SREBP-1, or PPAR-γ were
assessed using western blotting and RT-qPCR. DRE treatment induced
up-regulated protein and mRNA expression of AMPK-α comparing to the
ones in HFD group. Also, SREBP-1 and PPAR-γR were showed suppressed
protein and mRNA expression by DRE treatment comparing to the ones
in HFD group.
The effects of DRE were also notable on liver
tissue. Even though the differences in weight and AMPK
phosphorylation in the liver were not significant among the groups,
the activation and expression of adipogenic transcription factors
were suppressed in the DRE-supplemented group compared with that in
the HFD group. Since AMPK is considered to improve lipid metabolic
disorders by inhibiting SREBP activity, the present study expected
to observe a recovery effect of DRE on the decreased p-AMPK
expression in the eWAT from HFD-induced obese mice (60). However, the mRNA expression of AMPK
exhibited no significant changes among the groups in the liver,
unlike those detected in eWAT. This may be due to the involvement
of other molecular biomarkers, or differences among the tissues,
liver and eWAT, such as CCAAT/enhancer-binding protein or other
subunits of AMPK, including AMPK-β or AMPK-γ (61,62).
To identify the exact signaling pathways in the future, it is
necessary to perform in vitro studies on the effects of DRE
on preadipocytes, their differentiation and other adipogenic
transcription factors. In addition, it is necessary to investigate
the association between the molecular mechanisms and different
tissues for further study such as fatty liver diseases (61).
In conclusion, despite limited data on the
mechanism, the present study demonstrated through an in vivo
model that DRE exerts its effects on HFD-induced obesity by
downregulating related transcription factors (Fig. 6), providing insight into the
potential role of herbal medicines in alleviating both obesity and
improving bone health.
Acknowledgements
Not applicable.
Funding
This research was supported by a National Research Foundation of
Korea (NRF) grant funded by the Korea Government Ministry of
Science and ICT (grant no. NRF-2021R1A2C3011862).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TYG, JP and HJA conceived and designed the
experiments. YJP, HYK and DCC performed the experiments. TYG and JP
analyzed the data. HJA contributed reagents, materials and analysis
tools, and was involved in revisiting the manuscript critically for
crucial intellectual contents. TYG and HJA wrote the paper. HJA and
TYG confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures followed the National Institute of
Health guidelines and the present study was approved by the Ethical
Committee for Animal Care and the Use of Laboratory Animals of
Sangji University (approval no. 2019-11).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hall KD, Farooqi IS, Friedman JM, Klein S,
Loos RJF, Mangelsdorf DJ, O'Rahilly S, Ravussin E, Redman LM, Ryan
DH, et al: The energy balance model of obesity: Beyond calories in,
calories out. Am J Clin Nutr. 115:1243–1254. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michelini E, Bortoletto N and Porrovecchio
A: Outdoor physical activity during the first wave of the COVID-19
Pandemic. A comparative analysis of Government Restrictions in
Italy, France, and Germany. Front Public Health. 9:6157452021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holly JMP, Biernacka K, Maskell N and
Perks CM: Obesity, Diabetes and COVID-19: an infectious disease
spreading from the East Collides With the Consequences of an
Unhealthy Western Lifestyle. Front Endocrinol (Lausanne).
11:5828702020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sattar N, McInnes IB and McMurray JJV:
Obesity is a risk factor for severe COVID-19 Infection: Multiple
potential mechanisms. Circulation. 142:4–6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engin A: The definition and prevalence of
obesity and metabolic syndrome. Adv Exp Med Biol. 960:1–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Longo M, Zatterale F, Naderi J, Parrillo
L, Formisano P, Raciti GA, Beguinot F and Miele C: Adipose tissue
dysfunction as determinant of obesity-associated metabolic
complications. Int J Mol Sci. 20:23582019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choe SS, Huh JY, Hwang IJ, Kim JI and Kim
JB: Adipose tissue remodeling: Its role in energy metabolism and
metabolic disorders. Front Endocrinol (Lausanne). 7:302016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Tienen FH, Laeremans H, van der Kallen
CJ and Smeets HJ: Wnt5b stimulates adipogenesis by activating
PPARgamma, and inhibiting the beta-catenin dependent Wnt signaling
pathway together with Wnt5a. Biochem Biophys Res Commun.
387:207–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmad B, Serpell CJ, Fong IL and Wong EH:
Molecular mechanisms of adipogenesis: The Anti-adipogenic Role of
AMP-Activated protein kinase. Front Mol Biosci. 7:762020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eberle D, Hegarty B, Bossard P, Ferre P
and Foufelle F: SREBP transcription factors: Master regulators of
lipid homeostasis. Biochimie. 86:839–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hernandez-Quiles M, Broekema MF and
Kalkhoven E: PPARgamma in metabolism, immunity, and cancer: Unified
and diverse mechanisms of action. Front Endocrinol (Lausanne).
12:6241122021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang QA, Zhang F, Jiang L, Ye R, An Y,
Shao M, Tao C, Gupta RK and Scherer PE: Peroxisome
proliferator-activated receptor gamma and its role in adipocyte
homeostasis and thiazolidinedione-mediated insulin sensitization.
Mol Cell Biol. 38:e00677–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ettinger B, Genant HK and Cann CE:
Postmenopausal bone loss is prevented by treatment with low-dosage
estrogen with calcium. Ann Intern Med. 106:40–45. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu YP: Chinese Materia Medica: Chemistry,
Pharmacology and Applications. CRC Press; Boca Raton, FL, USA: pp.
593–609. 1998
|
|
16
|
Zhou L, Wong KY, Poon CC, Yu W, Xiao H,
Chan CO, Mok DK and Wong MS: Water extract of rhizoma drynaria
selectively exerts estrogenic activities in ovariectomized rats and
estrogen receptor-positive cells. Front Endocrinol (Lausanne).
13:8171462022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong RW and Rabie AB: Systemic effect of
crude extract from rhizome of Drynaria fortunei on bone formation
in mice. Phytother Res. 20:313–315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen LL, Lei LH, Ding PH, Tang Q and Wu
YM: Osteogenic effect of Drynariae rhizoma extracts and Naringin on
MC3T3-E1 cells and an induced rat alveolar bone resorption model.
Arch Oral Biol. 56:1655–1662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Y, Cai X, Sun J, Bi W and Yu Y:
Active components and mechanisms of total flavonoids from Rhizoma
Drynariae in enhancing cranial bone regeneration: An investigation
employing serum pharmacochemistry and network pharmacology
approaches. J Ethnopharmacol. 319((Pt 3)): 1172532024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song S, Gao Z, Lei X, Niu Y, Zhang Y, Li
C, Lu Y, Wang Z and Shang P: Total Flavonoids of drynariae rhizoma
prevent bone loss induced by hindlimb unloading in rats. Molecules.
22:10332017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rinonapoli G, Pace V, Ruggiero C,
Ceccarini P, Bisaccia M, Meccariello L and Caraffa A: Obesity and
Bone: A Complex Relationship. Int J Mol Sci. 22:136622021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao P, Xu A and Leung WK: Obesity, bone
loss, and periodontitis: The Interlink. Biomolecules. 12:8652022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gkastaris K, Goulis DG, Potoupnis M,
Anastasilakis AD and Kapetanos G: Obesity, osteoporosis and bone
metabolism. J Musculoskelet Neuronal Interact. 20:372–381.
2020.PubMed/NCBI
|
|
24
|
Pedersen BK and Febbraio MA: Muscles,
exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev
Endocrinol. 8:457–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiao D, Li Y, Liu X, Zhang X, Qian X,
Zhang H, Zhang G and Wang C: Association of obesity with bone
mineral density and osteoporosis in adults: A systematic review and
meta-analysis. Public Health. 180:22–28. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou J, He C, He W, Yang M, Luo X and Li C:
Obesity and bone health: A complex link. Front Cell Dev Biol.
8:6001812020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Y, Mu P, Ma X, Shi J, Zhong Z and Huang
L: Rhizoma drynariae total flavonoids combined with calcium
carbonate ameliorates bone loss in experimentally induced
Osteoporosis in rats via the regulation of Wnt3a/β-catenin pathway.
J Orthop Surg Res. 16:7022021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Zhou H, Hu C, Yang J, Ye J, Zhou Y,
Li Z, Chen L and Zhou Q: Total Flavonoids of Rhizoma drynariae
promotes differentiation of osteoblasts and growth of bone graft in
induced membrane partly by activating wnt/β-catenin signaling
pathway. Front Pharmacol. 12:6754702021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeong JC, Lee JW, Yoon CH, Kim HM and Kim
CH: Drynariae Rhizoma promotes osteoblast differentiation and
mineralization in MC3T3-E1 cells through regulation of bone
morphogenetic protein-2, alkaline phosphatase, type I collagen and
collagenase-1. Toxicol In Vitro. 18:829–834. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Tan C and Tan W: BMI,
socioeconomic status, and bone mineral density in U.S. adults:
Mediation analysis in the NHANES. Front Nutr. 10:11322342023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pinar-Gutierrez A, Garcia-Fontana C,
Garcia-Fontana B and Munoz-Torres M: Obesity and bone health: A
complex relationship. Int J Mol Sci. 23:83032022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park YJ, Lee GS, Cheon SY, Cha YY and An
HJ: The anti-obesity effects of Tongbi-san in a high-fat
diet-induced obese mouse model. BMC Complement Altern Med.
19:12019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Institute of Laboratory Animal Resources
(US) and the Committee on Care, Use of Laboratory Animals, . Guide
for the Care and Use of Laboratory Animals. US Department of Health
and Human Services and Public Health Service, National Institutes
of Health; Bethseda, MD, USA: pp. 46–68. 1986
|
|
34
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2^(−delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
35
|
Park YJ, Seo DW, Gil TY, Cominguez DC, Lee
H, Lee DS, Han I and An HJ: Pharmacological Properties of a
Traditional Korean Formula Bojungchiseup-tang on 3T3-L1
preadipocytes and high-fat diet-induced obesity mouse model. Biomed
Res Int. 2020:88510102020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Balogun JK, Auta J, Abdullahi SA and
Agboola OE: Potentials of castor seed meal (Ricinus communis
L.) as feed ingredient for Oreochromis niloticus. Proceedings
of the 19th Annual Conference of the Fisheries Society of Nigeria
(FISON): Ilorin, 29th November-3rd December, 2004. Fisheries
Society of Nigeria Publications. 838–843. 2005.
|
|
37
|
Ciesielska K and Gajewska M: Fatty acids
as potent modulators of autophagy activity in white adipose tissue.
Biomolecules. 13:2552023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geisler CE and Renquist BJ: Hepatic lipid
accumulation: Cause and consequence of dysregulated glucoregulatory
hormones. J Endocrinol. 234:R1–R21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krishnamurthy N, Grimshaw AA, Axson SA,
Choe SH and Miller JE: Drug repurposing: A systematic review on
root causes, barriers and facilitators. BMC Health Serv Res.
22:9702022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jeong JC, Lee BT, Yoon CH, Kim HM and Kim
CH: Effects of Drynariae rhizoma on the proliferation of human bone
cells and the immunomodulatory activity. Pharmacol Res. 51:125–136.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen SQ, Liang W, Zhang XM, Li X, Zhan ZL,
Guo LP, Huang LQ, Zhang XM and Gao WY: Research progress on
chemical compositions and pharmacological action of Drynariae
Rhizoma. Zhongguo Zhong Yao Za Zhi. 46:2737–2745. 2021.(In
Chinese). PubMed/NCBI
|
|
42
|
Wong RW and Rabie AB: Traditional Chinese
medicines and bone formation-a review. J Oral Maxillofac Surg.
64:828–837. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wong RW, Rabie B, Bendeus M and Hagg U:
The effects of rhizoma curculiginis and rhizoma drynariae extracts
on bones. Chin Med. 2:132007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Jiang J, Shen H, Chai Y, Wei X
and Xie Y: Total flavonoids from Rhizoma Drynariae (Gusuibu) for
treating osteoporotic fractures: implication in clinical practice.
Drug Des Devel Ther. 11:1881–1890. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bettaieb A, Cremonini E, Kang H, Kang J,
Haj FG and Oteiza PI: Anti-inflammatory actions of (−)-epicatechin
in the adipose tissue of obese mice. Int J Biochem Cell Biol.
81((Pt B)): 383–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee HS, Heo CU, Song YH, Lee K and Choi
CI: Naringin promotes fat browning mediated by UCP1 activation via
the AMPK signaling pathway in 3T3-L1 adipocytes. Arch Pharm Res.
46:192–205. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kirk B, Feehan J, Lombardi G and Duque G:
Muscle, bone, and fat crosstalk: The biological role of myokines,
osteokines, and adipokines. Curr Osteoporos Rep. 18:388–400. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Romieu I, Dossus L, Barquera S, Blottière
HM, Franks PW, Gunter M, Hwalla N, Hursting SD, Leitzmann M,
Margetts B, et al: Energy balance and obesity: what are the main
drivers? Cancer Causes Control. 28:247–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
de Moura E, Dias M, Dos Reis SA, da
Conceicao LL, Sediyama CMNO, Pereira SS, de Oliveira LL, Gouveia
Peluzio MDC, Martinez JA and Milagro FI: Diet-induced obesity in
animal models: Points to consider and influence on metabolic
markers. Diabetol Metab Syndr. 13:322021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
An HJ, Rim HK, Suh SE, Jeong HJ, Um JY,
Hong SH and Kim HM: Gamiwalbitang, composed of four herbs, controls
body weight increase and lipid level elevation induced by a
high-fat diet in mice. Immunopharmacol Immunotoxicol. 32:307–312.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
An HJ, Chung HS, Kim NH, Hong SH, Park EJ,
Baek SH and Kim HM: Regulatory effect of sense line diet on
cholesterol and body weight in mice fed a high-fat diet. Ann Nutr
Metab. 48:398–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Funk MD, Lee M, Vidoni ML and Reininger
BM: Weight loss and weight gain among participants in a
community-based weight loss Challenge. BMC Obes. 6:22019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang X, Zhao Y, Zhou D, Tian Y, Feng G and
Lu Z: Gab2 deficiency suppresses high-fat diet-induced obesity by
reducing adipose tissue inflammation and increasing brown adipose
function in mice. Cell Death Dis. 12:2122021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lizardo K, Ayyappan JP, Oswal N, Weiss LM,
Scherer PE and Nagajyothi JF: Fat tissue regulates the pathogenesis
and severity of cardiomyopathy in murine chagas disease. PLoS Negl
Trop Dis. 15:e00089642021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cui A, Hu Z, Han Y, Yang Y and Li Y:
Optimized analysis of in vivo and in vitro hepatic steatosis. J Vis
Exp. ((121)): 551782017.PubMed/NCBI
|
|
56
|
Canto C and Auwerx J: PGC-1alpha, SIRT1
and AMPK, an energy sensing network that controls energy
expenditure. Curr Opin Lipidol. 20:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Garcia D, Hellberg K, Chaix A, Wallace M,
Herzig S, Badur MG, Lin T, Shokhirev MN, Pinto AFM, Ross DS, et al:
Genetic Liver-Specific AMPK activation protects against
diet-induced obesity and NAFLD. Cell Rep. 26:192–208. e62019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen GY, Liu XY, Yan XE, Yu X, Liu Y, Luo
J and Tao QW: Total flavonoids of rhizoma drynariae treat
osteoarthritis by inhibiting arachidonic acid metabolites through
AMPK/NFκB pathway. J Inflamm Res. 16:4123–4140. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ix JH and Sharma K: Mechanisms linking
obesity, chronic kidney disease, and fatty liver disease: The roles
of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 21:406–412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X,
Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al: AMPK
phosphorylates and inhibits SREBP activity to attenuate hepatic
steatosis and atherosclerosis in diet-induced insulin-resistant
mice. Cell Metab. 13:376–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Clain DJ and Lefkowitch JH: Fatty liver
disease in morbid obesity. Gastroenterol Clin North Am. 16:239–252.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang Q, Liu S, Zhai A, Zhang B and Tian G:
AMPK-Mediated regulation of lipid metabolism by phosphorylation.
Biol Pharm Bull. 41:985–993. 2018. View Article : Google Scholar : PubMed/NCBI
|