Introduction
Cervical cancer is the most common gynecological
malignancy and ranks second among female malignancies worldwide
(1). The pathogenesis of cervical
cancer is complex, and human papilloma virus (HPV) is the main
cause of cervical cancer with ~95% of cervical cancer cases
reported to be caused by HPV infection (2). Once HPV infects cervical epidermal
cells, the expression of the oncogenes E6 and E7 inactivates p53
and pRb, which ultimately leads to malignant proliferation of
cervical cells (2). In addition,
factors such as family heredity, obesity and an unhealthy lifestyle
are risk factors for cervical cancer (3). In recent years, the incidence of
cervical cancer has been gradually increasing, with a trend towards
younger women, which has seriously affected the lives of female
patients and even led to death (4,5).
Therefore, early detection and active prevention of cervical cancer
are important research topics for gynecological malignant
tumors.
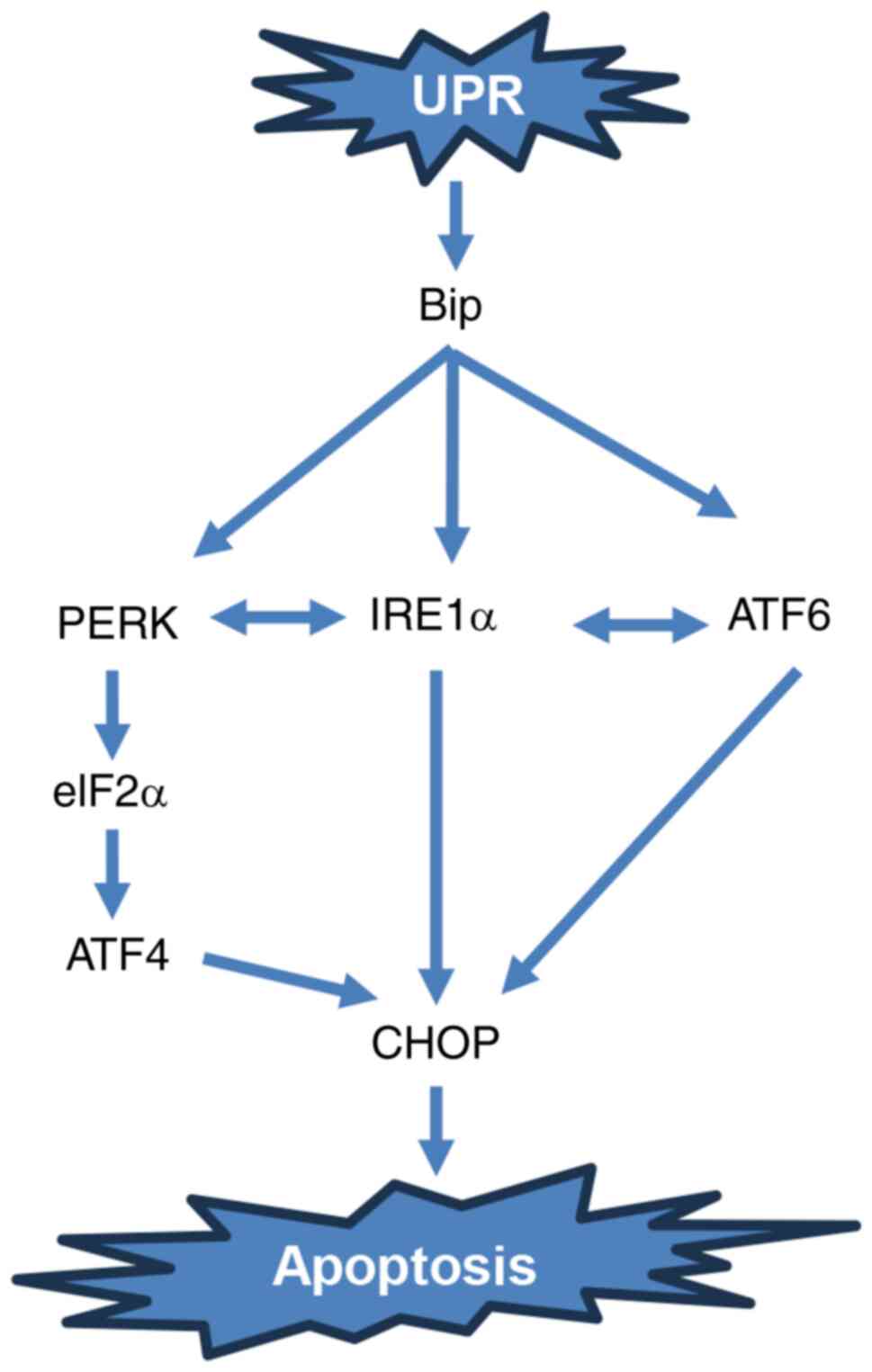
The endoplasmic reticulum (ER) is an organelle in
eukaryotic cells that plays an important role in the maintenance of
calcium homeostasis, lipid synthesis, and protein folding and
modification (6). The homeostasis
of the ER is essential for ensuring that proteins are correctly
folded, whereas slow-folding or unfolded proteins are retained in
the ER and degraded via the proteasome pathway, which is known as
the unfolded protein response (UPR) (7). As unfolded proteins accumulate in the
ER, ER transmembrane proteins, whose N-termini are in the ER lumen
and whose C-termini are in the cytoplasm, connect the ER to the
cytoplasm (8). Normally, the N
termini of these ER transmembrane proteins are regulated by the ER
chaperone protein Grp78 (Bip), which organizes their aggregation
(9). However, when unfolded
proteins accumulate in the ER, Bip is released, allowing these
transmembrane signaling proteins to aggregate and transmit UPR
signals (10). Protein kinase
R-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and
activating transcription factor (ATF) 6 are important molecules in
the three ER stress signaling pathways (11). PERK is a serine/threonine protein
kinase with a catalytic structural domain that is highly homologous
to kinases of the eukaryotic initiation factor 2α (eIF2α) family
(12). Once Bip is released, PERK
begins to oligomerize during ER membrane activation, inducing
autophosphorylation and activating the kinase structural domain to
an inactivated state, thus blocking the translation of downstream
mRNAs and reducing the protein load in the ER (Fig. 1) (12). However, when eIF2α expression is
restricted, several mRNAs, including ATF4, containing open reading
frames at the 5′ end begin to be translated (13). ATF4 drives transcription factor
C/EBP homologous protein (CHOP), which regulates the expression of
apoptosis-related genes (13). It
is well known that persistent activation of ER stress can directly
regulate tumor cell death, including apoptosis, by initiating a
variety of related signaling pathways (11).
Cantharidin is a major anticancer component of the
traditional Chinese medicine Zanthoxylum, but it is highly toxic to
the urinary system (14).
Norcantharidin (NCTD) is a novel compound formed by removing two
methyl groups on the basis of cantharidin, and the former
significantly reduces the side effects of the latter; this compound
has been widely used in the clinic (14). The antitumor effects of NCTD have
been widely reported (15,16). For example, in bladder cancer
cells, NCTD inhibited the malignant proliferation of bladder cancer
stem cell-like cells by targeting CDC6 (16). In non-small cell lung cancer types,
NCTD induces cell death in a mitophagy-dependent manner (15). However, whether NCTD inhibits
cervical cancer has not been reported. The aim of the present study
was to investigate whether NCTD inhibits the malignant progression
of cervical cancer and the underlying molecular mechanism through
the discovery of new effective drugs for the early prevention and
treatment of cervical cancer.
Materials and methods
Cell culture
The human cervical cancer cell lines C-33A and HeLa
were purchased from Procell Life Science & Technology Co., Ltd.
C-33A and HeLa cells were cultured in MEM (HyClone; Cytiva)
supplemented with 10% fetal bovine serum (HyClone; Cytiva) in an
incubator at 37°C with 5% CO2.
Western blotting
A total of 100 mg of tumor tissue and cervical
cancer cells were collected, RIPA buffer (high; Beijing Solarbio
Science & Technology Co., Ltd.) was added, and the samples were
centrifuged at 10,000 × g for 15 min at room temperature to collect
the supernatant. Subsequently, a BCA protein assay kit (Beijing
Solarbio Science & Technology Co., Ltd.) was used to measure
the protein concentration in the supernatants. In the present
study, 10% SDS-PAGE was used to separate the protein samples (20
µg/lane). After electrophoresis, the proteins were transferred to a
PVDF membrane (Thermo Fisher Scientific, Inc.). After which, the
PVDF membrane was rinsed with PBST (PBS supplemented with 0.1%
Tween-20, Beijing Solarbio Science & Technology Co., Ltd.) and
blocked with 8% skim milk (Thermo Fisher Scientific, Inc.) at room
temperature for 2 h. After the PVDF membranes were rinsed, they
were incubated with the following primary antibodies: Anti-ATF4
(cat. no. 11815), anti-CHOP (cat. no. 2895), anti-Bip (cat. no.
3177), anti-p-IRE1α (cat. no. ab124945), anti-IRE1α (cat. no.
3294), anti-p-PERK (cat. no. ab192591), anti-PERK (cat. no. 5683)
and anti-GAPDH (cat. no. 5174; all antibodies were purchased from
Cell Signaling Technology, Inc.; anti-p-IRE1α and p-PERK were from
Abcam; 1:1,000) at 4°C overnight. After sufficient washing with
PBST, the membrane was co-incubated with goat anti-rabbit or
anti-mouse IgG/HRP (cat. nos. SE134 and SE131, respectively;
1:5,000; Beijing Solarbio Science & Technology Co., Ltd.) at
room temperature for 2 h. Subsequently, ECL western blotting
substrate (Beijing Solarbio Science & Technology Co., Ltd.) was
added to detect the protein signal and GAPDH was used as an
internal reference. The relative protein intensity was determined
using ImageJ (V1.8.0; NIH).
Intracellular ROS detection
After C-33A and HeLa cells were processed, the
DCFH-DA fluorescent probe was diluted 1:1,000 in serum-free medium
according to the manufacturer's instructions (Beijing Solarbio
Science & Technology Co., Ltd.) and co-incubated with the cells
at 37°C for 20 min (the final concentration of DCFH-DA was 10 µM).
The cells were collected and detected via flow cytometry (BD FACS;
BD Biosciences). The data were analyzed using FlowJo software
(v10.10.0; FlowJo LLC).
Cell viability assay
A Cell Counting Kit-8 (CCK-8) (Beijing Solarbio
Science & Technology Co., Ltd.) was used to detect the
viability of the C-33A and HeLa cells. A total of 103
cells were inoculated in 96-well plates and cultured overnight,
after which different concentrations (0, 10, 20, 40, 80, 160 and
320 µM) of NCTD (Selleck Chemicals) were added to each well for
treatment. Next, 10 µl of CCK-8 reagent was added to each well and
incubated at 37°C for 2 h. The absorbance was measured at OD450 nm
using a Thermo Scientific Multiskan MK3 Enzyme Mark Instrument
(Thermo Fisher Scientific, Inc.). The 50% inhibitory concentration
(IC50) was calculated using GraphPad Prism 10
(Dotmatics).
To explore which type of cell death could be induced
by NCTD in cervical cancer cells, the cells were preincubated with
apoptosis inhibitor, z-VAD-FMK (ZVAD; 20 µM; MedChemExpress), the
ER stress inhibitor 4-phenylbutyric acid (4-PBA; 10 mM;
MedChemExpress), autophagy inhibitor, chloroquine (CQ; 20 µM;
MedChemExpress), for 1 h at 37°C. Then, the cells were further
treated with 40 µM NCTD for 37 h. After which, cell viability was
determined as aforementioned.
Cell cycle assay
After C-33A and HeLa cells were processed, the cells
were collected and washed well with PBS. Subsequently, a DNA
Content Quantitation Assay (Cell Cycle; cat. no. CA1510; Beijing
Solarbio Science & Technology Co., Ltd.) was used to detect
cell cycle changes. Briefly, the prepared cell suspension was fixed
with 70% pre-cooled ethanol for 2 h, and the cells were resuspended
by adding 100 µl of RNase A solution to the cell pellet in a water
bath at 37°C for 30 min. Subsequently, 400 µl of PI was added to
each group of cells for 30 min at 4°C in the dark, after which the
proteins were detected via flow cytometry (BD FACS; BD
Biosciences). The data were analyzed using FlowJo software
(v10.10.0; FlowJo LLC).
EdU staining
C-33A and HeLa cells were incubated with 100 µl of
EdU staining solution (Wuhan Servicebio Technology Co., Ltd.) at
37°C for 2 h. After sufficient washing with PBS, the cells were
fixed with 4% paraformaldehyde at room temperature for 30 min
followed by addition of 100 µl of PBS containing 0.5% Triton X-100
at room temperature for 15 min. Finally, 10 µl of DAPI was added to
the sections, which were incubated at room temperature for 10 min.
Subsequently, the sections were blocked with an anti-fluorescence
quenching sealer (Wuhan Servicebio Technology Co., Ltd.) and placed
under a fluorescence microscope (Keyence Corporation) for
observation.
DNA damage assay
The effect of NCTD on DNA damage in C-33A and HeLa
cells in the present study was assessed via a DNA damage assay kit
(cat. no. C2035S; Beyotime Institute of Biotechnology), and the
specific steps were carried out according to the manufacturer's
instructions. The relative fluorescence density was observed under
a fluorescence microscope (Keyence Corporation).
Intracellular Ca2+ level
assay
An intracellular Ca2+ Assay Kit (F04
method; cat. no. HR8229; Beijing Biolab Technology Co., Ltd.) was
used to detect changes in the intracellular calcium ion
concentration. Briefly, C-33A and HeLa cells were washed thoroughly
with HBSS, and F04 staining working solution was subsequently added
according to the manufacturer's instructions. Afterwards, the cells
were incubated at 37°C for 30 min before the fluorescence intensity
of Ca2+ was detected under a fluorescence microscope
(Keyence Corporation).
Mitochondrial membrane potential (MMP)
assay
MMP detection was performed using a Mitochondrial
Membrane Potential Assay Kit with JC-1 (Beijing Solarbio Science
& Technology Co., Ltd.). C-33A and HeLa cells were inoculated
in six-well plates, and after treatment with 40 µM NCTD at 37°C for
24 h, the cells were washed three times with PBS. Subsequently, the
cells were collected, stained according to the manufacturer's
instructions and recorded under a fluorescence microscope (Keyence
Corporation). When the MMP is high, JC-1 aggregates in the matrix
of mitochondria and forms polymers, which can produce red
fluorescence; when the mitochondrial membrane potential is low,
JC-1 cannot aggregate in the matrix of mitochondria, and at this
time, JC-1 is a monomer that can produce green fluorescence.
Tumor model in nude mice
The present study was approved by The Animal Ethics
Committee at Tongliao City Hospital (Tongliao, China; approval no.
2023-TLAJ34). Male nude mice aged 6–7 weeks (20±4 g) used in the
present study and were purchased from SPF (Beijing) Biotechnology,
Co., Ltd. All mice were housed in a temperature-(20–24°C) and
humidity-controlled (45–55%) environment. A 12/12 h light/dark
cycle was maintained in the animal housing rooms. All mice had free
access to food and water. A total of 106 HeLa cells (100
µl) were injected subcutaneously into the nude mice through the
right lower abdomen to construct the nude mouse subcutaneous tumor
model. Subsequently, the model mice were randomly divided into 2
groups (n=8/group) and given subcutaneous injections of PBS or 3
mg/kg NCTD every 2 days for 5 consecutive treatments, after which
the body weights of the mice and the growth of the subcutaneous
tumors were recorded. In order to anesthetize the mice, 4%
isoflurane was used. The mice were deeply anesthetized when they
breathed evenly, had no splinting pain reflex and had no corneal
reflex. Subsequently, cervical dislocation was used to execute the
mice, and the mice were judged to be dead when their heartbeat
stopped. At the end of treatment, the subcutaneous tumor tissues of
the mice in each group were removed and weighed, and the tumor
tissues were subjected to pathological section analysis.
H&E staining
Tissue specimens were fixed in 4% paraformaldehyde
(Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature for 24 h. The fixed tissues were then fully dehydrated
and paraffin embedded. Sections were cut into 5 µm sections, which
were then baked and deparaffinized at 70°C. Sections were stained
with hematoxylin staining solution (H&E; Beijing Solarbio
Science & Technology Co., Ltd.) as well as 0.5% eosin (Beijing
Solarbio Science & Technology Co., Ltd.) for 2 min at room
temperature, and after gradient dehydration with ethanol and
treatment with xylene, the sections were oven-dried and treated
with neutral gum (Beijing Solarbio Science & Technology Co.,
Ltd.). The pathological morphology of the tissues was observed
under a light microscope (Keyence Corporation) and recorded.
Immunohistochemistry (IHC)
Tissue specimens were fixed in 4% paraformaldehyde
(Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature for 24 h. The fixed tissues were then fully dehydrated
and paraffin embedded. Sections were cut into 5 µm sections, which
were then baked and deparaffinized at 70°C. After that, the
sections were incubated with 10% BSA at 37°C for 1 h, anti-CHOP
(cat. no. 2895; 1:50; CST Biological Reagents Co., Ltd.) or
anti-Bip (cat. no. 3177; 1:50; CST Biological Reagents Co., Ltd.)
were added to the sections and incubated at 4°C overnight. After
rinsing with PBS for 3 h, 60 µl of biotin-labeled Goat Anti-Mouse
or Anti-Rabbit IgG (H+L; cat. nos. A0286 and A0277, respectively;
1:50; Beyotime Institute of Biotechnology) were added to the
sections, which were incubated at 37°C for 1 h. Then, the sections
were color developed by adding an appropriate amount of DAB
Horseradish Peroxidase Color Development Kit (cat. no. P0202;
Beyotime Institute of Biotechnology) for 10 min at room
temperature, washed with tap water three times, and immediately
stained with hematoxylin stain for 5 min at room temperature. After
sufficient dehydration and transparency, the sections were closed
with neutral gum (Beijing Solarbio Science & Technology Co.,
Ltd.) and subsequently observed under a microscope (Keyence
Corporation).
Hematoxylin and Eosin Staining Kit (cat. no. C0105S,
Beyotime, Shanghai, China) was used for the detection of liver and
kidney pathological changes. After the slides were prepared as
described above, the slides were stained with hematoxylin at room
temperature for 5 min, rinsed in tap water, and continued to be
stained with eosin for 1 min. Sections were sealed according to the
above procedure and observed under the microscope (Keyence
Corporation).
Statistical analysis
All the data in the present study are expressed as
the mean ± standard deviation. GraphPad Prism 10 (Dotmatics) was
used for statistical analysis. Comparisons between two groups were
made using unpaired Student's t-tests, while comparisons between
more than three groups were made using one-way ANOVA followed by
post hoc analysis (Tukey's HSD). P<0.05 was considered to
indicate a statistically significant difference.
Results
NCTD inhibits the proliferation of
cervical cancer cells
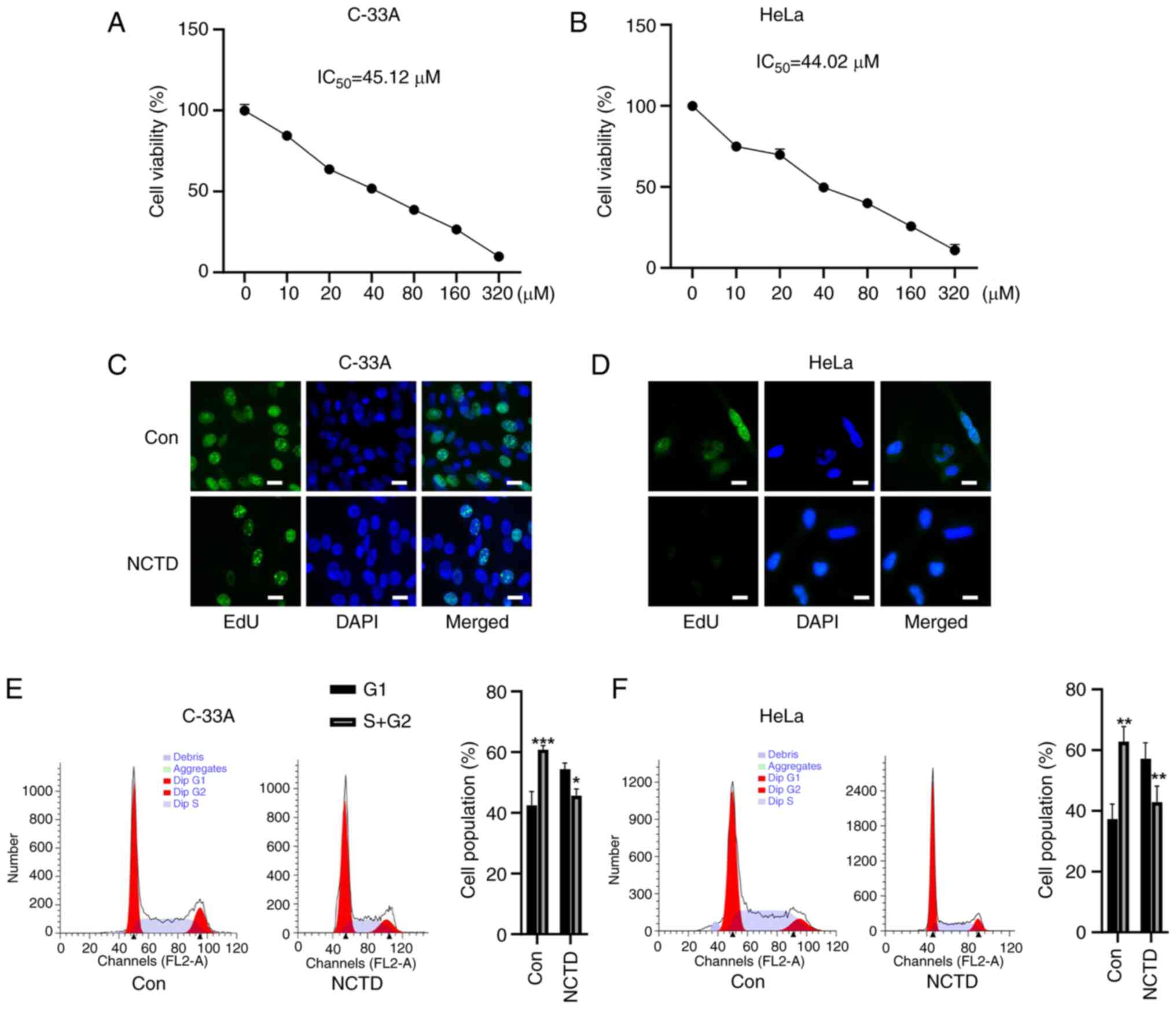
Whether NCTD inhibited the proliferation of cervical
cancer cells was first tested. NCTD reduced the viability of C-33A
and HeLa cells in a concentration-dependent manner (Fig. 2A and B). The IC50 values
of these compounds in C-33A and HeLa cells were 45.12 and 44.02 µM,
respectively. Therefore, 40 µM NCTD was selected for subsequent
experiments. Next, the effect of NCTD on the proliferation of
cervical cancer cells was detected by EdU staining. Compared with
that in the control group, the EdU staining of C-33A and HeLa cells
was attenuated after NCTD treatment (Fig. 2C and D). Flow cytometry revealed
that NCTD significantly blocked the cell cycle progression of C-33A
and HeLa cells, as evidenced by an increase in the cell population
in G1 phase and a decrease in the cell population in S+G2 phase
(Fig. 2E and F). These results
suggested that NCTD can inhibit the malignant proliferation of
cervical cancer cells.
ER stress inhibitor 4-phenylbutyric
acid reverses NCTD-induced cervical cancer cell death
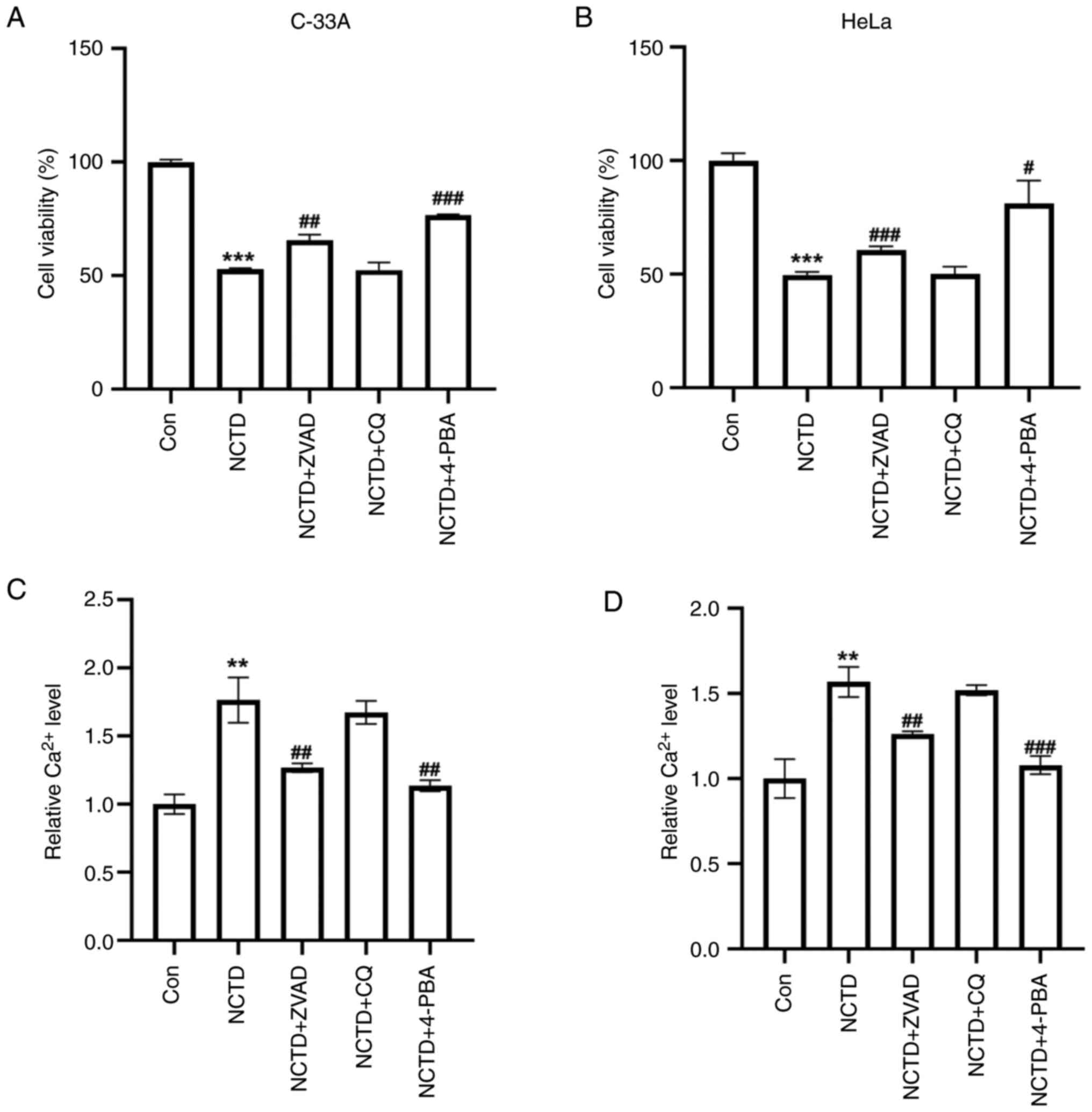
The mechanism by which NCTD actually causes cervical
cancer cell death was further explored. The NCTD-induced reduction
in the viability of C-33A and HeLa cells was reversed by the
apoptosis inhibitor, ZVAD and the ER stress inhibitor, 4-PBA, but
the autophagy inhibitor CQ had no significant effect (Fig. 3A and B). Compared with apoptosis
inhibitors, 4-PBA appeared to have a more pronounced ability to
reverse the decrease in NCTD-cell viability. Additionally, the
intracellular Ca2+ concentration was examined. Compared
with those in the controls, the Ca2+ levels in the
NCTD-treated C-33A and HeLa cells were significantly elevated
(Fig. 3C and D). Moreover, ZVAD
and 4-PBA reversed the NCTD-induced increase in Ca2+
levels, but CQ did not have this effect (Fig. 3C and D). These results suggested
that ER stress may play an important role in the NCTD-induced
decrease in the proliferative capacity of cervical cancer
cells.
ER stress inhibitor 4-PBA attenuates
NCTD-induced MMP reduction in cervical cancer cells
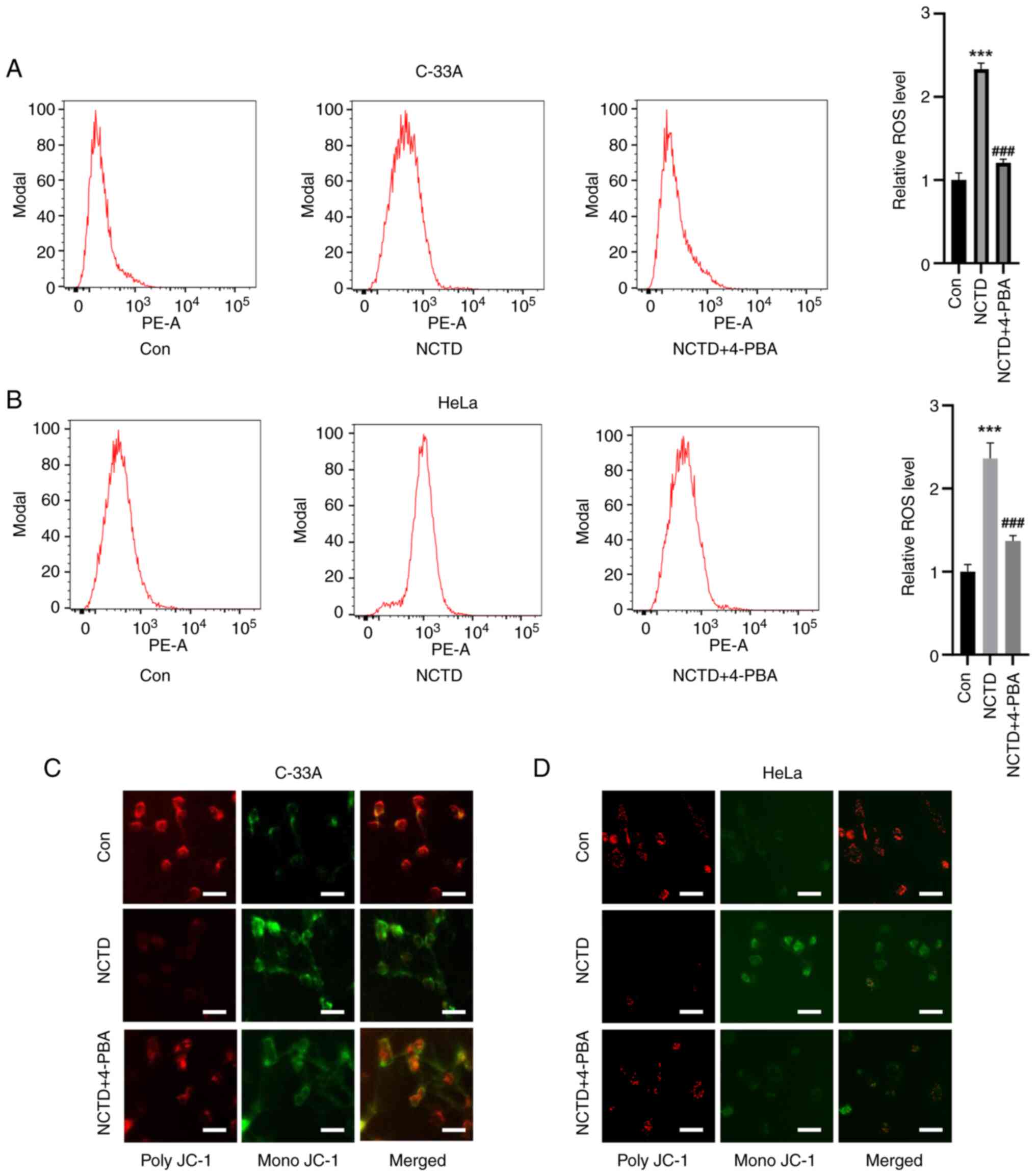
Mitochondrial damage is an important step in cell
death. Therefore, the effect of NCTD on ROS accumulation in
cervical cancer cells was also examined. Flow cytometry assays
showed that, compared with the control treatment, NCTD treatment
significantly increased the level of ROS in C-33A and HeLa cells,
while 4-PBA pre-treatment attenuated NCTD-induced ROS accumulation
(Fig. 4A and B). Unlike in the
control group, in the NCTD treatment group, the MMP was
significantly decreased, as evidenced by an increase in the level
of mono-JC-1. The NCTD-induced decrease in the MMP was ameliorated
to some extent by 4-PBA pre-treatment (Fig. 4C and D). These results suggested
that the NCTD-induced decrease in mitochondrial function can be
ameliorated by 4-PBA, an inhibitor of ER stress.
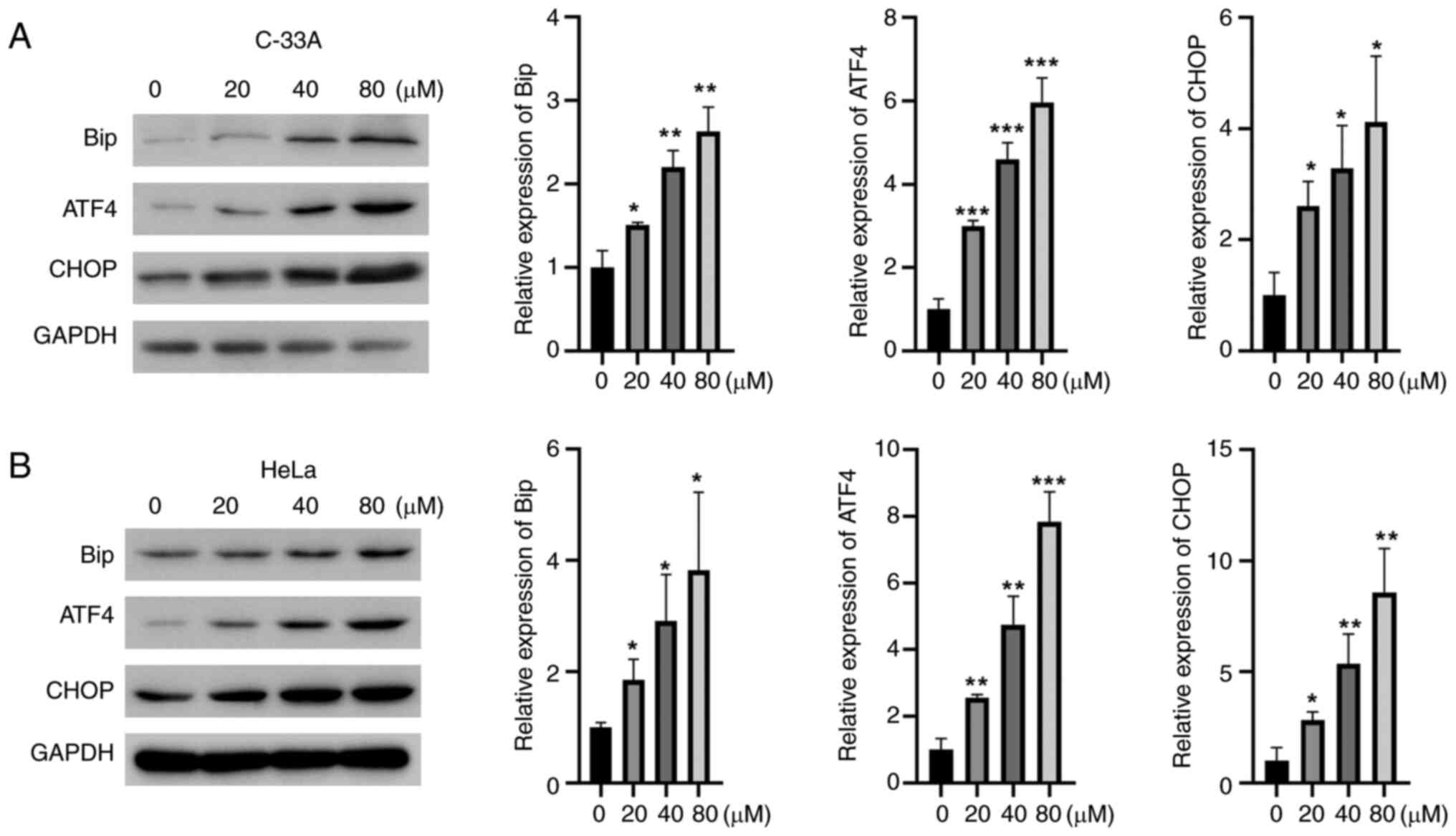
NCTD induces apoptosis in cervical
cancer cells via the ATF4-CHOP pathway
Next, the effect of NCTD on the ER stress-related
apoptotic pathway was examined. NCTD increased the protein
expression of Bip, ATF4 and CHOP in a concentration-dependent
manner in C-33A and HeLa cells (Fig.
5A and B). These results suggested that NCTD could induce
apoptosis in cervical cancer cells by activating ER stress.
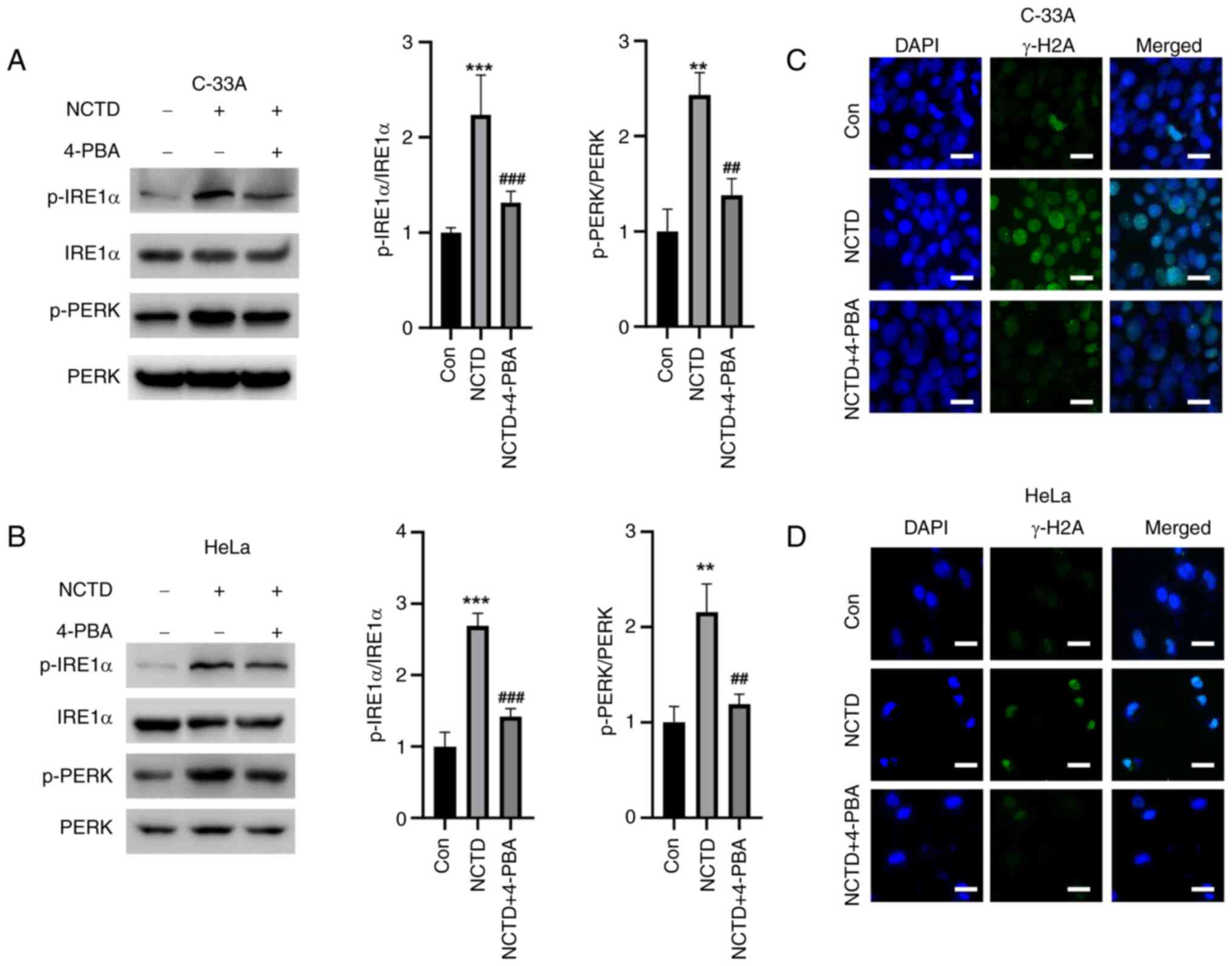
ER stress inhibitor 4-PBA reverses
NCTD-induced IRE1α and PERK activation
In addition to examining the effects of ATF6, the
effects of NCTD on the activation of two other UPR pathways, IRE1α
and PERK were examined. NCTD significantly elevated the
phosphorylation levels of IRE1α and PERK in cervical cancer cells,
whereas 4-PBA pre-treatment significantly reduced the NCTD-induced
increase in p-IRE1α and p-PREK (Fig.
6A and B). Moreover, NCTD increased the IF intensity of γ-H2A
in cervical cancer cells, while 4-PBA attenuated the IF intensity
of γ-H2A (Fig. 6C and D). These
results suggested that NCTD-induced ER stress and DNA damage in
cervical cancer cells can be reversed by 4-PBA.
NCTD attenuates tumor growth in mice
in vivo
Next, whether NCTD attenuated tumor growth in
vivo was tested. NCTD did not have side effects on the liver or
kidney, indicating that it is safe for these organs (Fig. 7A and B). NCTD significantly reduced
the weight and volume of the tumors (Fig. 7C and D). Additionally, the
expression of CHOP and Bip in the tumor tissues of the mice was
detected by IHC (Fig. 7E and F).
Compared with those in the control group, the levels of CHOP and
Bip in mouse tumor tissues were greater in the NCTD group.
Moreover, three UPR-related pathways, namely, the ATF6, p-IREα/IREα
and p-PERK/PERK pathways, were activated by NCTD in tumor tissues
(Fig. 7G). These results suggested
that NCTD can inhibit tumor growth in mice in vivo.
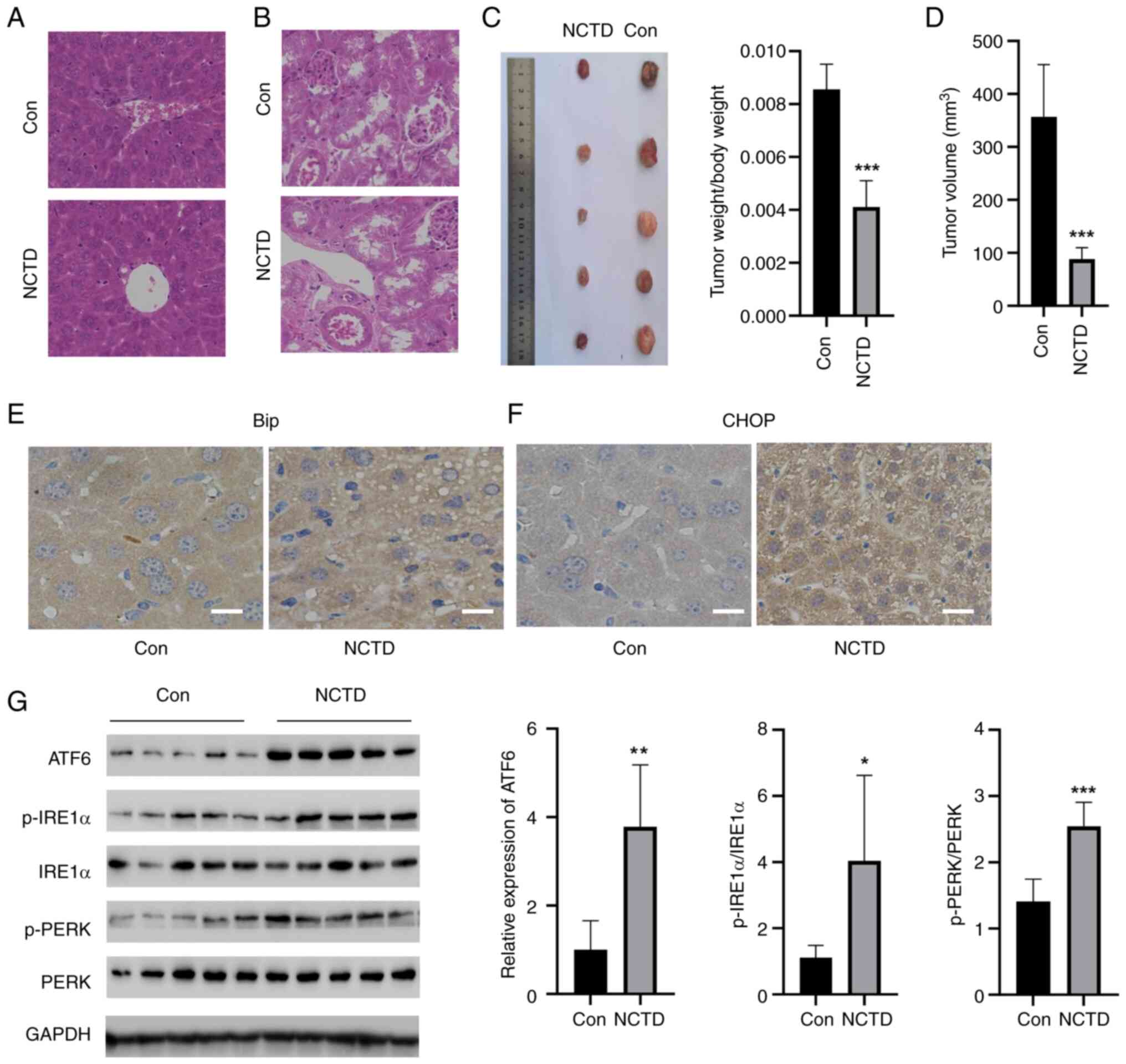 | Figure 7.NCTD attenuated tumor growth in mice
in vivo. H&E staining showed that NCTD did not have side
effects on the (A) liver or (B) kidney. NCTD significantly reduced
the (C) weight (Left, tumor images; Right, tumor weight) and (D)
volume of the tumors. Immunohistochemical staining showed that NCTD
significantly increased the levels of (E) Bip and (F) CHOP in the
tumor tissues of mice. (G) NCTD increased the levels of ATF6,
p-IREα/IREα and p-PERK/PERK in tumor tissues. *P<0.05,
**P<0.01, ***P<0.001 vs. Con. Con, control; NCTD,
norcantharidin; PERK, protein kinase R-like ER kinase; IRE1α,
inositol-requiring enzyme 1α; p-, phosphorylated; ATF6, activating
transcription factor 6; CHOP, C/EBP homologous protein. |
Discussion
ER stress is a double-edged sword (17). On the one hand, ER stress is
important for tumor microenvironmental homeostasis and tumor growth
inhibition; on the other hand, sustained ER stress can initiate
multiple signaling pathways to promote tumor cell death (18). Therefore, targeting ER
stress-related signals to regulate tumor cell death may constitute
a potential strategy for tumor treatment and intervention (18). NCTD is a novel synthetic antitumor
drug with clear antitumor and leukocyte-enhancing effects (19), but its efficacy in treating
cervical cancer alone has not been reported.
In the present study, to the best of our knowledge,
for the first time it was found that NCTD significantly inhibited
the proliferation of cervical cancer cells in a
concentration-dependent manner. Further studies revealed that the
ER stress inhibitor 4-PBA and the apoptosis inhibitor ZVAD could
reverse the NCTD-induced reduction in cervical cancer cell
viability to some extent. Disturbed Ca2+ homeostasis is
also a major manifestation of ER stress (17). Therefore, the effect of NCTD on
Ca2+ levels was evaluated. NCTD increased
Ca2+ levels in cervical cancer cells, whereas 4-PBA
reduced NCTD-induced Ca2+ elevation, suggesting that ER
stress is likely involved in the NCTD-mediated reduction in
cervical cancer cell viability.
ROS can react directly with proteins and their
precursors, affecting protein assembly and modification and leading
to a large accumulation of misfolded proteins as well as unfolded
proteins in the ER, which is an important factor in inducing ER
stress (20). It was clarified
that NCTD can significantly promote ROS generation in cervical
cancer cells and that this ROS accumulation is positively
associated with a decrease in cervical cancer cell viability.
Mitochondria and the ER are structurally related, and the two can
be directly connected at the outer mitochondrial membrane and form
transfer channels to facilitate the transfer of Ca2+
between the ER and mitochondria to regulate mitochondrial metabolic
homeostasis and apoptotic pathways (20,21).
Therefore, mitochondria are also important regulatory target
organelles of ER stress. In the present study, it was found that
NCTD disrupted mitochondrial function, which was manifested by
increased ROS generation and decreased MMP, suggesting that
mitochondria are important targets through which NCTD kills
cervical cancer cells. An inhibitor of ER stress, 4-PBA, reversed
the disruption of mitochondrial function induced by NCTD,
suggesting that NCTD affects mitochondrial function in cervical
cancer cells by regulating ER stress.
ER stress refers to a series of cellular adaptive
responses and regulatory pathways caused by the disruption of
protein folding and modification in the ER when cells are subjected
to a variety of physiological and pathological factors, as well as
nutritional deficiencies (6,22).
ER stress promotes cell survival by activating a series of adaptive
mechanisms called the UPR, which provides an improved
microenvironment for the survival of tumor cells (23). However, sustained ER stress can
directly regulate cell death, providing an important strategy for
selective antitumor therapy (7,24).
ER stress can activate intracellular PERK, IRE1α and ATF6, which in
turn promotes the transcription of the apoptosis-related protein
CHOP (10,24). In the present study, it was shown
that NCTD can increase the expression of the apoptosis-related
proteins Bip, ATF4 and CHOP in a concentration-dependent manner. In
addition, it was also found that NCTD significantly increased the
phosphorylation of PERK and IRE1α and that the ER-specific
inhibitor 4-PBA significantly ameliorated the effects of NCTD on ER
stress-related proteins in cervical cancer cells, suggesting that
NCTD regulates tumor cell apoptosis by activating ER stress in
cervical cancer cells. Although HPV is the main cause of cervical
cancer, there are still some cervical cancers that are not caused
by HPV infection (25). To
investigate whether NCTD has a generalized inhibitory effect on
different types of cervical cancer, two cell lines, an HPV-negative
C-33A cell line and an HPV-positive HeLa cell line, were used in
the present study. The data showed that NCTD significantly induced
apoptosis in cervical cancer cells through the activation of ER
stress in both cell lines. Accordingly, it was hypothesized that
NCTD has a therapeutic effect on different types of cervical
cancer.
In vivo experiments showed that NCTD
significantly inhibited the growth of subcutaneous tumors in mice.
H&E staining showed that NCTD treatment reduced the malignant
proliferation of cells in mouse tumor tissues. Moreover, the
expression of ER stress-related molecules and apoptosis-related
proteins increased significantly after NCTD treatment. Therefore,
in vivo experiments demonstrated that NCTD could induce ER
stress to inhibit tumor growth. It was also noted that NCTD
increased the vacuolization of xenograft tumors in nude mice. It
was hypothesized that NCTD induces ER stress and expansion, which
leads to the emergence and fusion of ER-derived vacuoles. ER
expansion is a typical feature of paraptosis (26). Thus, NCTD induced sustained ER
pressure and inhibited the malignant progression of cervical
cancer. However, whether NCTD is an ER stress inducer with specific
therapeutic effects on cervical cancer cells remains to be
explored.
The following limitations were also present in the
present study. The antitumor effects of NCTD have been widely
reported, but NCTD also plays a wide range of roles in other
diseases. For example, NCTD ameliorates renal tubulointerstitial
fibrosis by blocking TGF-β1/Smad signaling as well as the NF-κB
pathway (27). In
lipopolysaccharide-induced macrophages, NCTD downregulates hepcidin
expression through the inhibition of IL-6/JAK2/STAT3 signaling,
resulting in a reduction in iron in the mouse liver and spleen
(28). In a mouse model of
systemic lupus erythematosus, NCTD blockade of STAT3 signaling
inhibited Th17 cell differentiation and attenuated the inflammatory
response (29). Therefore, it is
not known whether NCTD curbs the development of cervical cancer
through other signaling pathways and thus, this requires further
exploration. In future studies, whether NCTD inhibits cervical
cancer through other molecular mechanisms will be investigated.
Moreover, to promote the potential clinical application of NCTD,
the number of samples in animal experiments will be increased to
determine its efficacy and safety.
In conclusion, NCTD regulates cell death by inducing
the ER stress pathway in cervical cancer cells. Therefore, NCTD
could be a potential ER stress inducer and thus a potential
strategy for anticancer cancer therapy. The present study revealed
to the best of our knowledge for the first time that NCTD is a
potential therapeutic agent for the treatment of cervical cancer,
but its actual clinical application needs to be confirmed by
additional experiments.
Acknowledgments
Not applicable.
Funding
This work was supported by Natural Science Foundation of Inner
Mongolia Autonomous Region (grant no. 2019BS08001).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZZ performed the experiments and analyzed the data.
BS, JL PB, YS, YL performed the animal experiments. ZZ designed the
experiment, analyzed the data and gave final approval of the
version to be published. ZZ, BS, JL PB, YS and YL confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by The Animal Ethics
Committee at Tongliao City Hospital (approval no. 2023-TLAJ34;
Tongliao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Buskwofie A, David-West G and Clare CA: A
review of cervical cancer: Incidence and disparities. J Natl Med
Assoc. 112:229–232. 2020.PubMed/NCBI
|
|
2
|
Kusakabe M, Taguchi A, Sone K, Mori M and
Osuga Y: Carcinogenesis and management of human papillomavirus-
associated cervical cancer. Int J Clin Oncol. 28:965–974. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Revathidevi S, Murugan AK, Nakaoka H,
Inoue I and Munirajan AK: APOBEC: A molecular driver in cervical
cancer pathogenesis. Cancer Lett. 496:104–116. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hill EK: Updates in cervical cancer
treatment. Clin Obstet Gynecol. 63:3–11. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mayadev JS, Ke G, Mahantshetty U, Pereira
MD, Tarnawski R and Toita T: Global challenges of radiotherapy for
the treatment of locally advanced cervical cancer. Int J Gynecol
Cancer. 32:436–445. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumor and its microenvironment. Nat
Rev Cancer. 21:71–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marciniak SJ, Chambers JE and Ron D:
Pharmacological targeting of endoplasmic reticulum stress in
disease. Nat Rev Drug Discov. 21:115–140. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi Z and Chen L: Endoplasmic Reticulum
Stress and Autophagy. Adv Exp Med Biol. 1206:167–177. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren J, Bi Y, Sowers JR, Hetz C and Zhang
Y: Endoplasmic reticulum stress and unfolded protein response in
cardiovascular diseases. Nat Rev Cardiol. 18:499–521. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Groenendyk J, Agellon LB and Michalak M:
Calcium signaling and endoplasmic reticulum stress. Int Rev Cell
Mol Biol. 363:1–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oakes SA: Endoplasmic reticulum stress
signaling in cancer cells. Am J Pathol. 190:934–946. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Shi C, He M, Xiong S and Xia X:
Endoplasmic reticulum stress: Molecular mechanism and therapeutic
targets. Signal Transduct Target Ther. 8:3522023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Y, Zhou X, Cao T, Chen E, Li Y, Lei
W, Hu Y, He B and Liu S: Endoplasmic reticulum stress and oxidative
stress in inflammatory diseases. DNA Cell Biol. 41:924–934. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng L and Tang S: Norcantharidin analogs:
A patent review (2006–2010). Expert Opin Ther Pat. 21:1743–53.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Z, Li B, Cao M and Jiang J:
Norcantharidin triggers apoptotic cell death in non-small cell lung
cancer via a mitophagy-mediated autophagy pathway. Ann Transl Med.
9:9712021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi X, Chen S, Zhang Y, Xie W, Hu Z, Li H,
Li J, Zhou Z and Tan W: Norcantharidin inhibits the DDR of bladder
cancer stem-like cells through cdc6 degradation. Onco Targets Ther.
12:4403–4413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu D, Liu Z, Liang MX, Fei YJ, Zhang W, Wu
Y and Tang JH: Endoplasmic reticulum stress targeted therapy for
breast cancer. Cell Commun Signal. 20:1742022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao T, Du J and Zeng H: Interplay between
endoplasmic reticulum stress and noncoding RNAs in cancer. J
Hematol Oncol. 13:1632020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhai BT, Sun J, Shi YJ, Zhang XF, Zou JB,
Cheng JX, Fan Y, Guo DY and Tian H: Review targeted drug delivery
systems for norcantharidin in cancer therapy. J Nanobiotechnology.
20:5092022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wadgaonkar P and Chen F: Connections
between endoplasmic reticulum stress-associated unfolded protein
response, mitochondria, and autophagy in arsenic-induced
carcinogenesis. Semin Cancer Biol. 76:258–266. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Senft D and Ronai ZA: UPR, autophagy, and
mitochondria crosstalk underlies the ER stress response. Trends
Biochem Sci. 40:141–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wiseman RL, Mesgarzadeh JS and Hendershot
LM: Reshaping endoplasmic reticulum quality control through the
unfolded protein response. Mol Cell. 82:1477–1491. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hetz C, Zhang K and Kaufman RJ:
Mechanisms, regulation and functions of the unfolded protein
response. Nat Rev Mol Cell Biol. 21:421–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernandes A, Viveros-Carreño D, Hoegl J,
Ávila M and Pareja R: Human papillomavirus-independent cervical
cancer. Int J Gynecol Cancer. 32:1–7. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo MJ, Lee DM, Kim IY, Lee D, Choi MK,
Lee JY, Park SS, Jeong SY, Choi EK and Choi KS: Gambogic acid
triggers vacuolization-associated cell death in cancer cells via
disruption of thiol proteostasis. Cell Death Dis. 10:1872019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Ge Y, Liu FY, Peng YM, Sun L, Li J,
Chen Q, Sun Y and Ye K: Norcantharidin, a protective therapeutic
agent in renal tubulointerstitial fibrosis. Mol Cell Biochem.
361:79–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng J, Qian ZM, Sun YX and Bao YX:
Downregulation of hepcidin by norcantharidin in macrophage. Nat
Prod Res. 38:673–678. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du LJ, Feng YX, He ZX, Huang L, Wang Q,
Wen CP and Zhang Y: Norcantharidin ameliorates the development of
murine lupus by inhibiting the generation of IL-17 producing cells.
Acta Pharmacol Sin. 43:1521–1533. 2022. View Article : Google Scholar : PubMed/NCBI
|