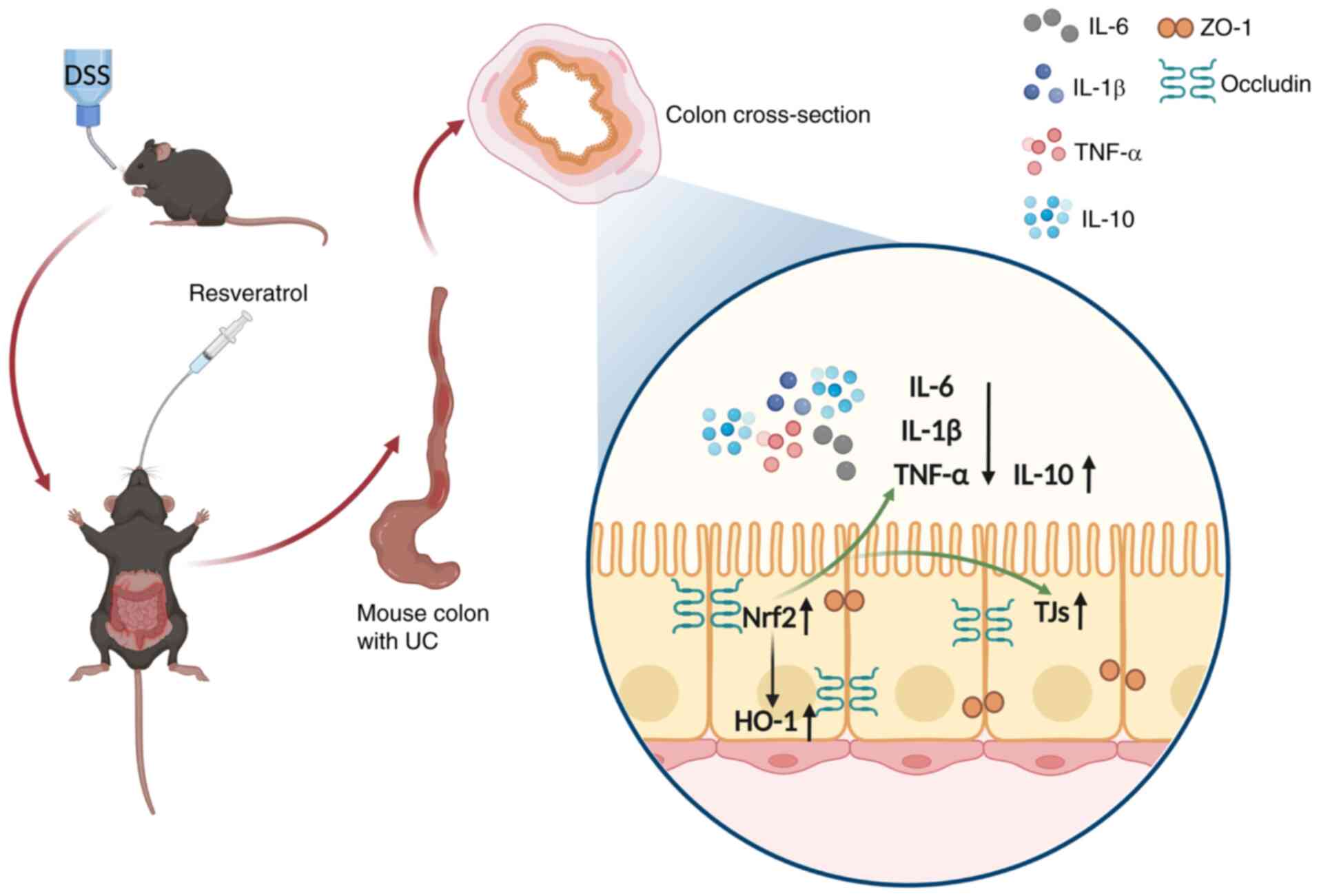
Introduction
Inflammatory bowel disease (IBD) encompasses a group
of chronic, recurrent and idiopathic inflammatory disorders
affecting the gastrointestinal tract, including Crohn's disease and
ulcerative colitis (UC). UC is considered a precancerous condition
for colorectal cancer but its causes and development remain unclear
(1). The primary symptoms include
abdominal pain, diarrhea and hematochezia. Due to the combined
effects of susceptibility genes and environmental factors, the
intestinal mucosa initiates a prolonged and intense immune response
to various stimuli, leading to emergence and progression of
inflammation (2). A previous
epidemiological survey reported that UC affects ~10 million people
globally, coinciding with changes in dietary habits and circadian
rhythms (3). Current
pharmacological treatments such as 5-aminosalicylic acid,
corticosteroids and immunosuppressants, have limited effectiveness.
Long-term use can lead to side effects, including diarrhea, nausea,
abdominal pain, pancreatitis and renal function damage, imposing
psychological and economic burdens on patients. Additionally,
patient non-responsiveness to systemic steroids remains a challenge
in IBD management (4–6). Therefore, understanding the
pathogenesis of UC and developing novel intervention and treatment
strategies is key.
Previous studies have highlighted the key role of
intestinal mucosal barrier integrity in the pathophysiology of
various diseases (7,8). The intestinal mucosal barrier,
separating the external and internal environments, is the largest
and most important defense against the external environment.
Maintaining an intact intestinal barrier is key for protecting
against microorganisms and toxins, serving as the primary defense
in the gastrointestinal tract against external pathogens. The
functional integrity of this barrier is primarily determined by the
epithelium and tight junctions (TJs) that seal the paracellular
space. Key TJ proteins, including intracellular protein zonula
occludens-1 (ZO-1) and transmembrane proteins occludin and
claudins, are essential for maintaining the integrity of the
intestinal mucosal barrier. Numerous studies suggest that
dysfunction of the intestinal mucosal barrier contributes to
worsening of IBD, particularly in patients with UC (9,10).
Therapeutic strategies that strengthen the intestinal mucosal
barrier may provide new pathways for treating UC.
In recent years, phyto-polyphenolic extracts have
been used in treating and preventing various types of diseases due
to their comprehensive anti-inflammatory and antioxidant properties
(11,12). Resveratrol (Res), a non-flavonoid
polyphenolic compound primarily found in grape leaves and skins, is
a key bioactive component in wine and grape juice. In vivo
and in vitro studies have shown that Res has antioxidative,
anti-inflammatory, anti-neoplastic and cardioprotective effects,
along with benefits such as cost-effectiveness, minimal side
effects, easy oral absorption and renal and fecal excretion
following metabolism (13,14). Empirical studies suggest that
dietary Res supplementation improves clinical outcomes and life
quality in patients with UC, partly by decreasing inflammation and
oxidative stress (15,16). However, the mechanism behind these
effects is not fully understood.
An array of natural phyto-polyphenols improves
oxidative stress levels by enhancing the nuclear factor
erythroid-2-related factor 2 (Nrf2) pathway (17). Nrf2 is a key target in recent
oxidative stress defense mechanism research (18). Nrf2 controls the expression of
detoxifying enzymes and antioxidant proteins, such as: NAD(P)H,
quinone oxidoreductase 1 (NQO1), glutamate cysteine ligase
catalytic subunit and heme oxygenase 1 (HO-1), via antioxidant
response elements thereby combating oxidative stress (19,20).
HO-1, an effector protein downstream of Nrf2, breaks heme into
ferrous ion (Fe2+), carbon monoxide (CO) and biliverdin.
The metabolism of the heme group is beneficial to preventing
oxidation. As a necessary endogenous gas messenger molecule, CO has
anti-tinflammatory, vasodilator and microcirculation metabolic
roles. Biliverdin and the product of its metabolism, bilirubin, not
only have powerful antioxidant and anti-inflammatory effects but
also effectively scavenge reactive oxygen species activity to
defend against peroxide, peroxynitrite, hydroxyl and superoxide
free radicals (21). Previous
in vitro study has showed that Res increases Nrf2 and HO-1
expression, reducing dextran sulfate sodium (DSS)-induced
intestinal epithelial cell (IEC) cytotoxicity, inflammation,
barrier dysfunction and TJ protein loss (22). Therefore, Res might also protect
the intestinal mucosal barrier by modulating the Nrf2/HO-1
signaling pathway in vivo.
The present study aimed to assess the modulation of
the Nrf2/HO-1 signaling pathway by Res and whether this can
alleviate DSS-induced experimental UC in mice, offering a novel
perspective for Res treatment and prevention of UC and intestinal
mucosal barrier-associated pathology.
Materials and methods
Chemicals and reagents
DSS (molecular weight, 36,000-50,000 Da) was
purchased from Shanghai Yeasen Biotechnology Co., Ltd. Res (purity
≥98%) was purchased from Shanghai Aladdin Biochemical Technology
Co., Ltd. ELISA kits included IL-6 (cat. No. CHE0006-048, Beijing
4A Biotech Co., Ltd.), IL-1β (CHE0015-048, Beijing 4A Biotech Co.,
Ltd.), TNF-α (CHE0004-048, Beijing 4A Biotech Co., Ltd.) and IL-10
(CHE0016-048, Beijing 4A Biotech Co., Ltd.). RIPA buffer, protease
and phosphatase inhibitors, and enhanced BCA Protein Assay kit were
purchased from Beyotime Institute of Biotechnology. Antibodies used
included rabbit anti-ZO-1 (cat. no. GB111402; Wuhan Servicebio
Technology Co., Ltd.), anti-occludin (cat. no. 91131; Cell
Signaling Technology, Inc.), anti-Nrf2 (cat. no. 16396-1-AP;
Proteintech Group, Inc.), anti-HO-1 (cat. no. 82551; Cell Signaling
Technology, Inc.) and anti-β-actin (cat. no. 380624; Chengdu
Zen-Bioscience Co., Ltd.) and HRP-conjugated goat anti-rabbit IgG
(cat. no. 7074; Cell Signaling Technology, Inc.).
Animals and experimental protocol
A total of 30 male C57BL/6J mice (age, 8 weeks;
weight, 20–22 g), were purchased from SPF (Beijing) Biotechnology
Co., Ltd. Mice were housed in individual ventilated cages with
controlled temperature (23±1°C) and humidity-controlled (45–55%)
(12 h light/dark cycle) and standard laboratory chow and water
ad libitum. They were allowed to acclimatize for ≥1 week.
All animal care and experimental procedures were in accordance with
the guidelines of the Animal Welfare Ethics Committee of Dali
University (Dali, China; approval no. 2023-PZ-194).
According to previous studies, the safe dose of Res
for relieving UC in mice is 10–100 mg/kg (23–25).
To reflect the concentration-effect relationship according to Singh
et al (26), Res was used
low (10 mg/kg), medium (50 mg/kg) and high (100 mg/kg) doses. The
mice were divided randomly into five groups (n=6/group) as follows:
Control; model (DSS-only); Res low dose (RLD); Res medium dose
(RMD) and Res high dose (RHD).
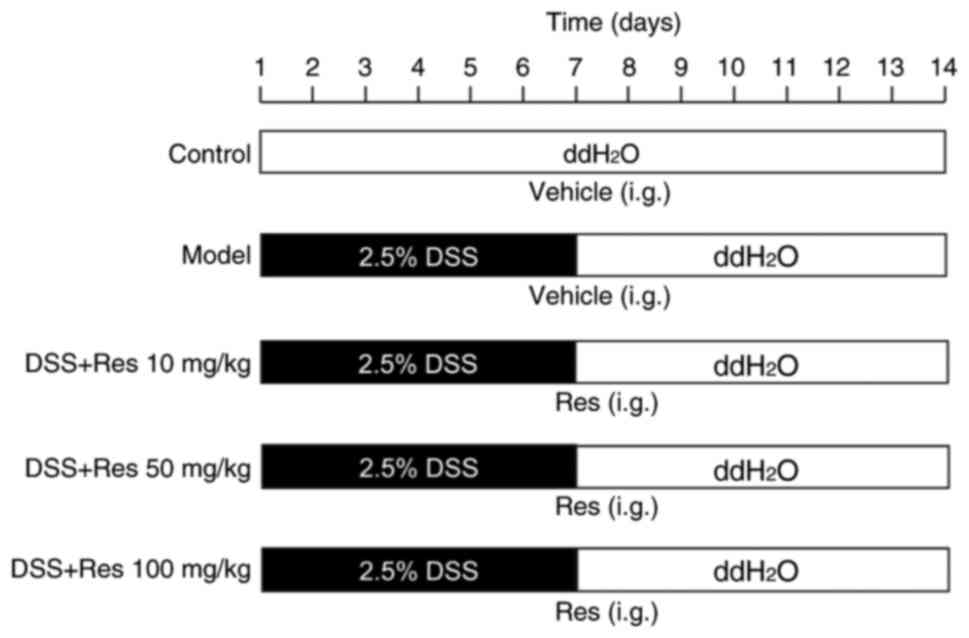
To induce the experimental UC model, mice received
2.5% DSS in filtered drinking ddH2O for 7 days. On day
8, the DSS solution was replaced with filtered ddH2O.
The control group received only filtered ddH2O for 14
days. Res was dissolved in 0.5% carboxymethyl cellulose sodium
(CMC-Na). Res (10, 50 or 100 mg/kg) or vehicle (0.5% CMC-Na) was
administered intragastrically for 14 days, starting concurrently
with DSS exposure (Fig. 1).
The general condition and body weight change of mice
were monitored and recorded daily. The study employed humane
endpoints according to AVMA Guidelines for the Euthanasia of
Animals (27), including when the
mice showed an inability to obtain food or water on their own, had
a weight loss of >20% of their starting body weight, difficulty
moving, were depressed in the absence of anesthesia, or their body
temperature was persistently below 37°C. All animal experiments
were performed in accordance with the regulations of the ARRIVE
guidelines (28). No mice died or
were euthanized before the end of the experiment. On day 15, mice
were anesthetized with 1% sodium pentobarbital (60 mg/kg,
intraperitoneal). A single collection of orbital venous blood (300
µl/mouse) was performed with an aseptic capillary glass tube. After
centrifugation at 1,500 × g for 10 min at 4°C, the serum was
collected. Spleen was collected and weighed, and the spleen index
was calculated as the ratio of spleen weight to body weight. The
entire colon from cecum to anus were then collected, weighed, and
length measured. A portion of colon tissue was fixed in 4%
paraformaldehyde at 4°C for 6 h for histopathological examination
and immunohistochemistry; remaining tissue and serum were stored at
−80°C for further analysis.
Disease activity index (DAI)
score
DAI score was calculated based on a previous study
(29). Briefly, DAI score is the
average of body weight change, diarrhea condition and bloody stool
test scores (Table I).
 | Table I.DAI scoring system. |
Table I.
DAI scoring system.
| DAI score | Body weight change
(%) | Stool
condition |
Occult/hematochezia |
|---|
| 0 | <1 | Normal | Normal |
| 1 | 1-5 | Loose | Hemoccult
positive |
| 2 | 6-10 | Loose | Hemoccult
positive |
| 3 | 11-15 | Diarrhea | Bloody stool |
| 4 | >15 | Diarrhea | Bloody stool |
Histopathological examination
The fixed colon samples were dehydrated with graded
ethanol solution, tissues were embedded in paraffin wax. A serial
frontal section was cut at intervals for 5 µm and stained with
hematoxylin and eosin (H&E) staining for at room temperature
(hematoxylin staining for 3 min and eosin staining for 15 sec) for
histopathology. The pathological changes in the colon mucosa were
observed under a light microscope. Histological damage was graded
following Dieleman's criteria (30) (Table
II).
 | Table II.Histological scoring system. |
Table II.
Histological scoring system.
| Score | Pathological
changes of colonic mucosa |
|---|
| 0 | None |
| 1 | Crypt basal 1/3
damaged |
| 2 | Crypt basal 2/3
damaged |
| 3 | Crypt lost, only
surface epithelium intact, |
|
| inflammatory
infiltration |
| 4 | Crypt lost, mucosal
erosion and ulcer, severe inflammatory infiltration |
Immunohistochemistry
Immunohistochemistry was conducted to assess ZO-1
and occludin protein expression in colon tissue. Briefly, slides
were de-paraffinized at room temperature, washed with xylene and
rehydrated in descending ethanol series. The colon sections were
subjected to antigen retrieval with 0.01 M sodium citrate buffer
and their endogenous peroxidase activity was blocked at room
temperature for 30 min in 1% hydrogen peroxide/phosphate-buffered
saline solution. Tissues were blocked for 1 h at room temperature
with 5% goat serum (Wuhan Servicebio Technology Co., Ltd.). Slides
were incubated overnight at 4°C with primary antibodies against
ZO-1 (1:500) and occludin (1:300) and incubated for 1 h at room
temperature with HRP-conjugated goat anti-rabbit IgG secondary
antibody (1:3,000). Staining was performed with
3,3-diaminobenzidine for 5 min at room temperature and
counterstained with hematoxylin for 3 min at room temperature.
Images were captured under a light microscope at ×50 and ×400
magnification. Staining results were quantified using the following
equation: H-Score=(percentage of weak intensity ×1) + (percentage
of moderate intensity ×2) + (percentage of strong intensity ×3)
(31–34).
ELISA
The serum concentrations of IL-6, IL-1β, TNF-α and
IL-10 were measured using ELISA kits according to the
manufacturer's instructions. Absorbance for each well was recorded
at a wavelength of 450 nm using a microplate reader.
Western blotting
Protein levels of ZO-1, occludin, Nrf2 and HO-1 in
colon tissue were determined using western blotting. A total of ~20
mg tissue was homogenized in 200 µl ice-cold RIPA buffer
supplemented with 1% protease and phosphatase inhibitors for 30 min
before centrifugation at 15,616 × g for 10 min at 4°C to obtain
supernatant. The loading buffer was mixed in a 1:4 ratio, and the
mixture was boiled at 100°C for 10 min to denature the protein.
Protein concentration was determined using an enhanced BCA Protein
Assay kit. 20 µg protein/lane was separated by SDS-PAGE on a 10%
gel and electro-transferred onto a PVDF membrane (Merck KGaA), then
blocked with 5% non-fat milk for 2 h at room temperature and
incubated overnight at 4°C with primary antibodies against ZO-1
(1:1,000), occludin (1:1,000), Nrf2 (1:6,000), HO-1 (1:1,000) and
β-actin (1:10,000). The blots were incubated with HRP-conjugated
goat anti-rabbit IgG secondary antibody (1:5,000) for 1 h at room
temperature. β-actin was used as a loading control. The signal was
detected using BeyoECL Moon kit (Beyotime Institute of
Biotechnology) and a ImageQuant LAS 4000 mini chemiluminescence
imaging system (Cytiva) and protein expression was quantified with
Image J v1.51 (National Institutes of Health).
Assessment of pharmacokinetic (PK)
parameters
PK study examine the absorption, distribution,
metabolism and excretion (ADME) processes of drugs, including oral
bioavailability (OB), Caco-2 permeability, blood-brain barrier
(BBB) permeability and drug-likeness (DL) (35). PK parameters of Res were sourced
from Traditional Chinese Medicine systems pharmacology database and
analysis platform (TCMSP; tcmsp-e.com/) database, a phytochemical
database for TCM active ingredients.
Potential targets of Res and UC
The potential targets of Res were obtained from
TCMSP, DrugBank (go.drugbank.com/) and PubChem
(pubchem.ncbi.nlm.nih.gov/) databases. Genes associated with UC
were identified through the GeneCards (genecards.org/), MalaCards
(malacards.org/) and NCBI-gene (ncbi.nlm.nih.gov/) databases. After
removing duplicate target genes, the online tool Venny2.1 (36) (bioinfogp.cnb.csic.es/tools/venny)
was used to identify overlaps between Res targets and UC
targets.
Functional enrichment analysis
Database for Annotation, Visualization and
Integrated Discovery (37)
(7eg7d.ncifcrf.gov/) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway (38)
(genome.jp/7eg/) databases were used for Gene Ontology (GO)
function enrichment and KEGG pathway analyses. The analyzed data
were visualized using the R Project 4.2.3 (Shorstop Beagle).
Protein-protein interaction (PPI)
network analysis
Potential compound-disease target genes were
uploaded to Search Tool for the Retrieval of Interaction Gene
(STRING) (cn.string-db.org/) to construct the PPI network.
Parameters set included ‘Homo sapiens’ as the organism and ‘medium
confidence (0.400)’. The Cytoscape 3.7.0 (Cytoscape 3.7.0 Release
Notes) was used to visualize the interaction network.
Molecular docking
Target protein crystal structures were sourced from
Protein Data Bank (rcsb.org/) database and preprocessed, including
hydrogenation, amino acid modification, energy optimization and
force field parameter adjustment, as previously described (39). Next, two-dimensional structure of
the active compound was downloaded from the PubChem database. After
optimizing for minimum free energy and obtaining a low energy
conformation, the protein structure was docked with the ligand
structure (39). AutoDock Vina
1.2.2 (vina.scripps.edu/) was used for hydrogenation and charge
balancing and PyRx 0.8 (pyrx.sourceforge.io/) was used for docking.
PyMOL 2.3 (pymol.org/2/) was used for visualization before analysis
using Discovery Studio 4.5 (Dassault systemes).
Statistical analysis
All data are presented as the mean ± SD of three
independent experiments. The measurement data obeying normal
distribution between two groups were analyzed by unpaired t test
and one-way analysis of variance was used for comparison among
multiple comparisons followed by Tukey's post hoc test. All
statistical analyses were performed using GraphPad Prism 9.0
(Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
Res attenuates DSS-induced UC in
mice
To determine the effect of Res in experimental UC,
physiological parameters of mice were monitored and assessed,
including body weight, diarrhea and hematochezia, daily during DSS
intervention. No pathological manifestations were observed in the
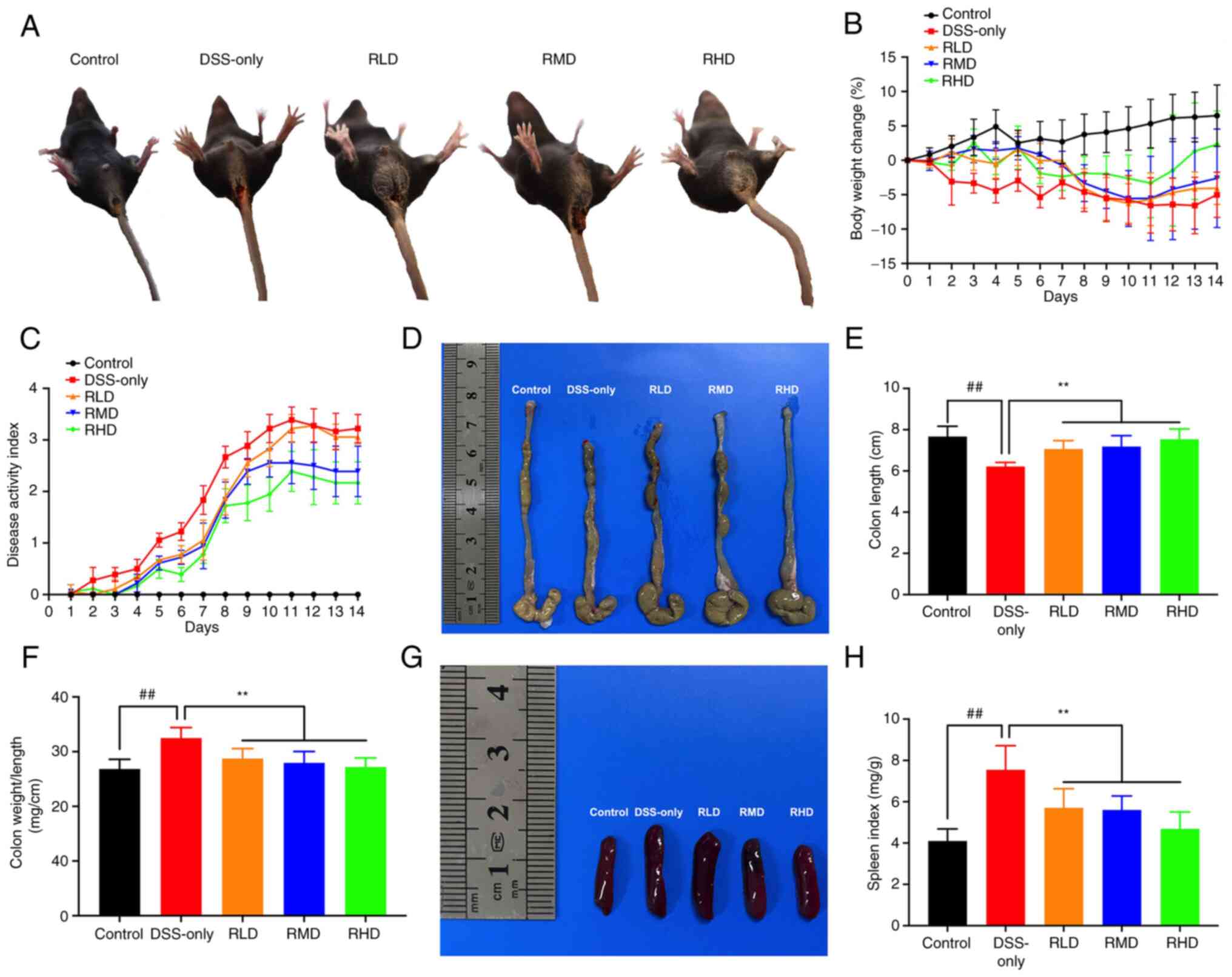
control group (Fig. 2). However,
the DSS-only group exhibited a marked reduction in body weight
starting from the 2nd day post −2.5% DSS administration (Fig. 2B). From day 5, mice in the DSS-only
group showed symptoms such as loose stool, fecal occult blood,
decreased food intake, unkempt fur and decreased responsiveness to
external stimuli; concurrently, DAI score increased compared with
the control group. These symptoms were moderately decreased in mice
treated with Res compared with the DSS-only group. DAI score in Res
treatment groups were reduced compared with the DSS-only group
(Fig. 2C).
Furthermore, in the DSS-only group there was
significant shortening of the colon, significant increase in
colonic weight-to-length ratio and spleen index and notable spleen
enlargement compared with the control. These changes in the colon
and spleen were significantly decreased in all Res treatment groups
compared with the DSS-only group, suggesting that Res alleviated
clinical manifestations of experimental UC in mice (Fig. 2D-H).
Res alleviates intestinal mucosal
histopathological injury in DSS-induced UC mice
Histopathological examination was conducted to
evaluate colonic injury. The DSS-only group showed extensive
mucosal erosion, crypt depletion, destruction of mucosal epithelium
and intestinal glandular architecture, connective tissue
proliferation and infiltration of granulocytes and lymphocytes
(Fig. 3A).
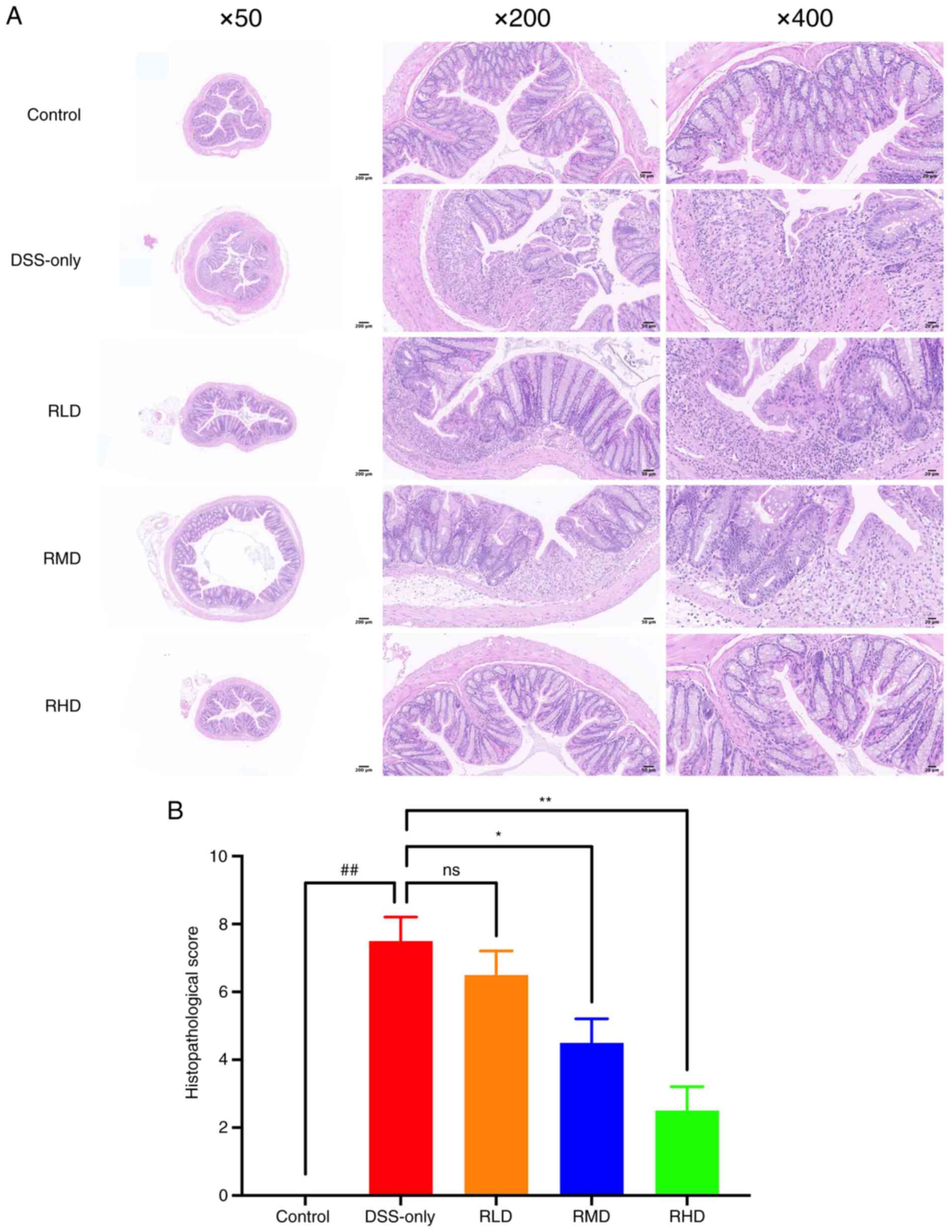 | Figure 3.Effect of Res on histological changes
of mice with DSS-induced UC. (A) Hematoxylin and eosin staining
(magnification, ×50, ×200 and ×400). The DSS group showed extensive
mucosal erosion, crypt depletion, destruction of mucosal epithelium
and intestinal glandular architecture, connective tissue
proliferation and infiltration of granulocytes and lymphocytes. The
degree of colonic injury and intestinal inflammation in the
Res-treated groups was less severe compared with the UC group. (B)
Histological score. ##P<0.01 vs. control; *P<0.05
and **P<0.01 vs. DSS. Res, resveratrol; DSS, dextran sulfate
sodium; RLD, Res low dose; RMD, Res medium dose; RHD, Res high
dose; UC, ulcerative colitis; ns, not significant. |
The degree of colonic injury and intestinal
inflammation in the Res-treated groups was less severe compared
with the UC group (Fig. 3A). The
histological score also showed that the pathological changes of
colonic mucosa in Res group were alleviated (Table II). The histological scores for
the RMD and RHD groups were significantly lower compared with
DSS-only group, although there was no significant difference
between RLD and DSS-only group (Fig.
3B). These results indicated that middle and high dosages of
Res effectively decreased intestinal mucosal damage induced by DSS
in experimental UC.
Res decreases secretion of
inflammatory cytokines in DSS-induced UC mice
The production of pro-inflammatory cytokines in
serum and colonic tissue was quantified using ELISA. The levels of
IL-6, IL-1β, and TNF-α were significantly increased in DSS-only
group compared with the control in both serum and colonic tissue.
In the RLD, RMD and RHD groups, levels of these cytokines were
significantly decreased compared with DSS-only group (Fig. 4A-C, E-G).
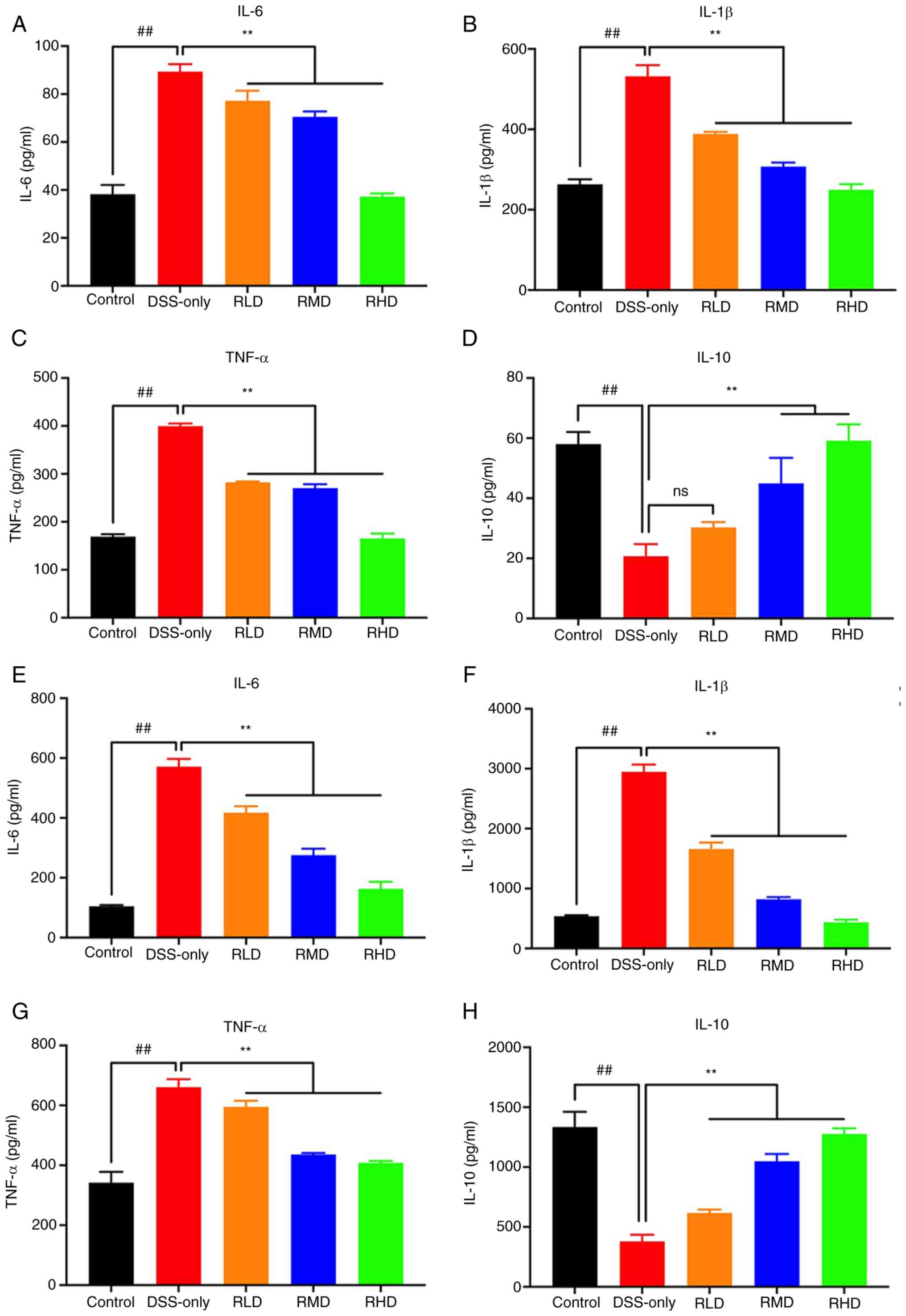 | Figure 4.Inflammatory cytokine expression in
serum and colon tissue of UC mice determined by enzyme-linked
immunosorbent assay. The levels of (A) IL-6, (B) IL-1β, (C) TNF-α
and (D) IL-10 in serum of mice. The levels of (E) IL-6, (F) IL-1β,
(G) TNF-α and (H) IL-10 in colon tissue of mice.
##P<0.01 vs. control; **P<0.01 vs. DSS. Res,
resveratrol; DSS, dextran sulfate sodium; RLD, Res low dose; RMD,
Res medium dose; RHD, Res high dose; UC, ulcerative colitis; ns,
not significant. |
The levels of IL-10 were significantly decreased in
DSS-only group compared with the control, and restored by Res, but
no significant different in the serum RLD group. (Fig. 4D, H). These findings suggested that
Res decreased inflammation induced by DSS in experimental UC
mice.
Res enhances TJ protein expression in
DSS-induced UC mice
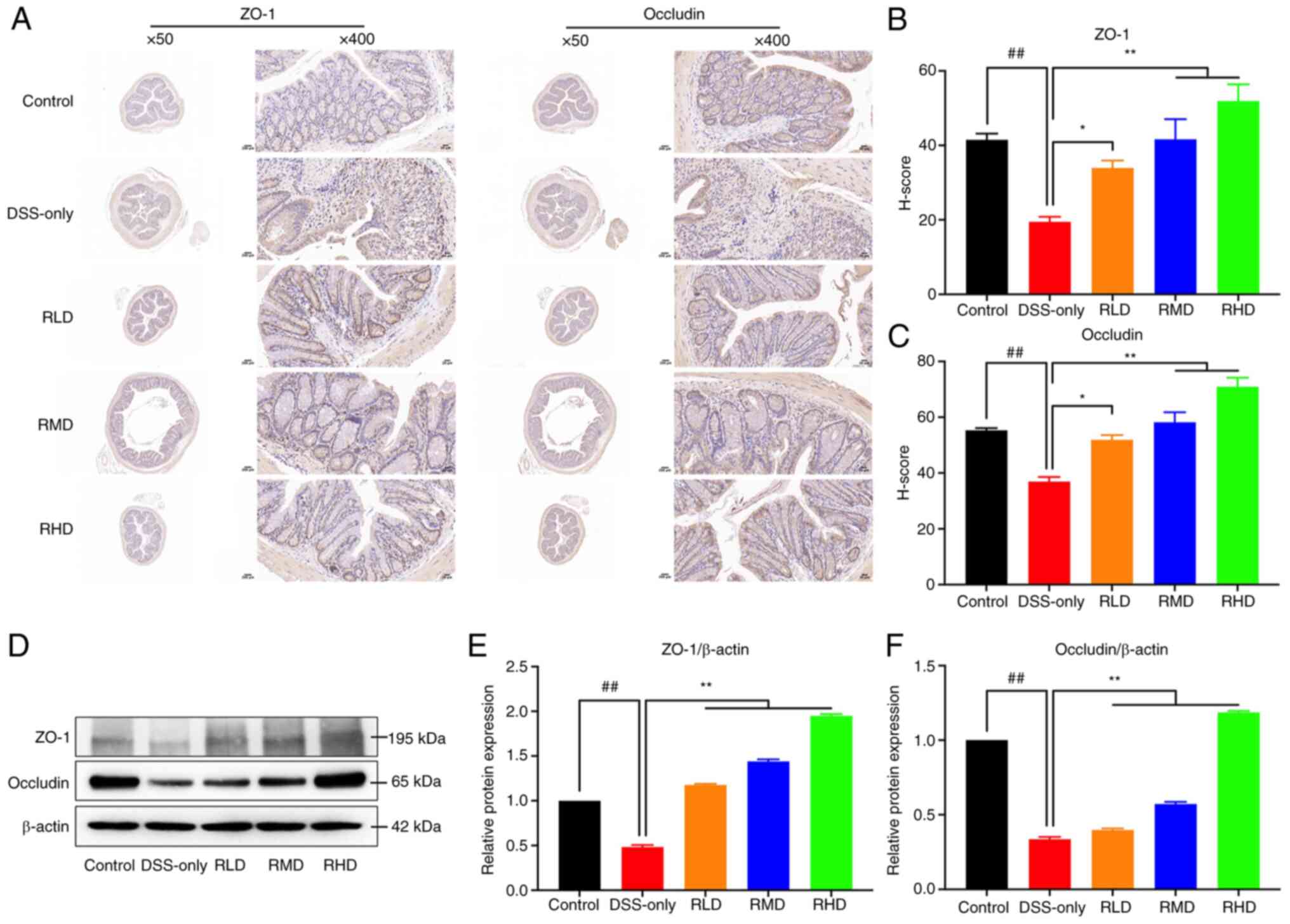
ZO-1 and occludin, key TJ proteins, serve a key role
in maintaining intestinal mucosal barrier integrity (40). In the present study, colonic tissue
of mice was subjected to immunohistochemical staining and western
blotting. DSS significantly decreased protein expression of ZO-1
and occludin in colonic tissues of experimental UC mice compared
with the control. The results of H-score quantitative
immunohistochemical staining showed that ZO-1 and occludin H-score
decreased significantly following DSS induction. Compared with
DSS-only group, the protein expression and H-score of ZO-1 and
occludin in RLD, RMD and RHD groups were significantly upregulated,
particularly at a dosage of 100 mg/kg (Fig. 5). Collectively, these results
suggested that Res treatment enhanced integrity of the intestinal
mucosa.
PK parameters of Res
The Res profile across 12 principal drug
characterization and evaluation parameters is presented in Table III. OB, the fraction of orally
administered dose that reaches systemic circulation, reflects the
integration of the ADME process. DL is used to evaluate the
‘drug-like’ qualities of potential compounds (41). Res had an OB of 19.07% and a DL of
0.11. Res also had a low molecular weight and was classified as
moderately permeable (−0.3< BBB <0.3).
 | Table III.Pharmacokinetic properties of
resveratrol. |
Table III.
Pharmacokinetic properties of
resveratrol.
| Molecular
formula | MW, Da | AlogP | Hdon | Hacc | OB/% | Caco-2 | BBB | DL | FASA- | TPSA | RBN | HL |
|---|
|
C14H12O3 | 228.26 | 3.01 | 3 | 3 | 19.07 | 0.8 | −0.01 | 0.11 | 0.49 | 60.69 | 2 | - |
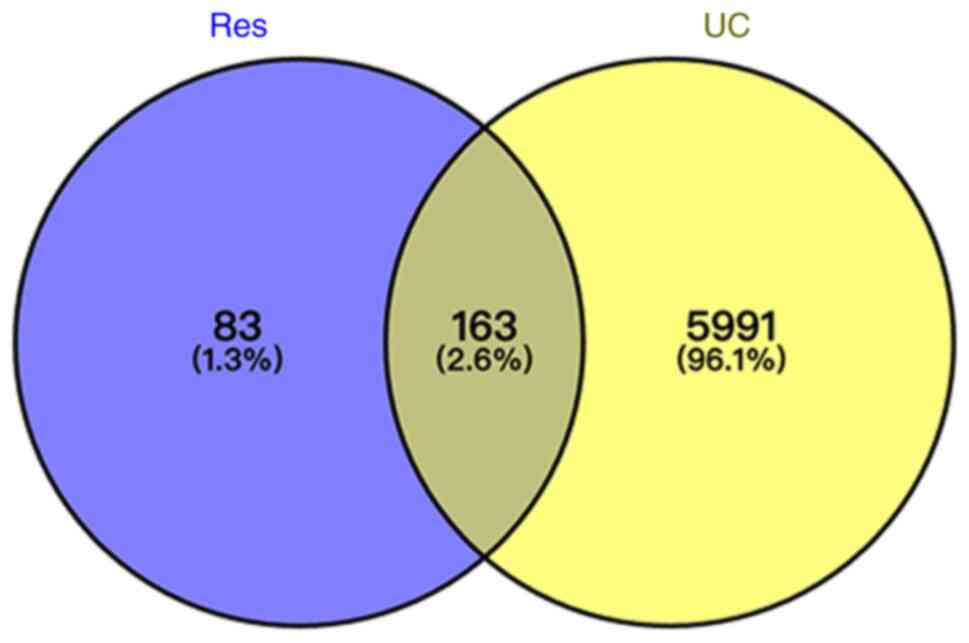
Potential targets of Res and UC
Using TCMSP, DrugBank and PubChem databases, a total
of 246 unique targets were obtained after removing duplicates.
UC-associated target genes were sourced from GeneCards, MalaCards
and NCBI-gene databases. A total of 6,154 unique targets were
identified after duplicate removal. Venny2.1 was used to determine
the intersection, identifying 163 overlapping genes between
compound and disease targets (Fig.
6).
GO enrichment and KEGG pathway
analysis
GO enrichment and KEGG pathway analyses were
conducted on 163 identified target genes to understand the function
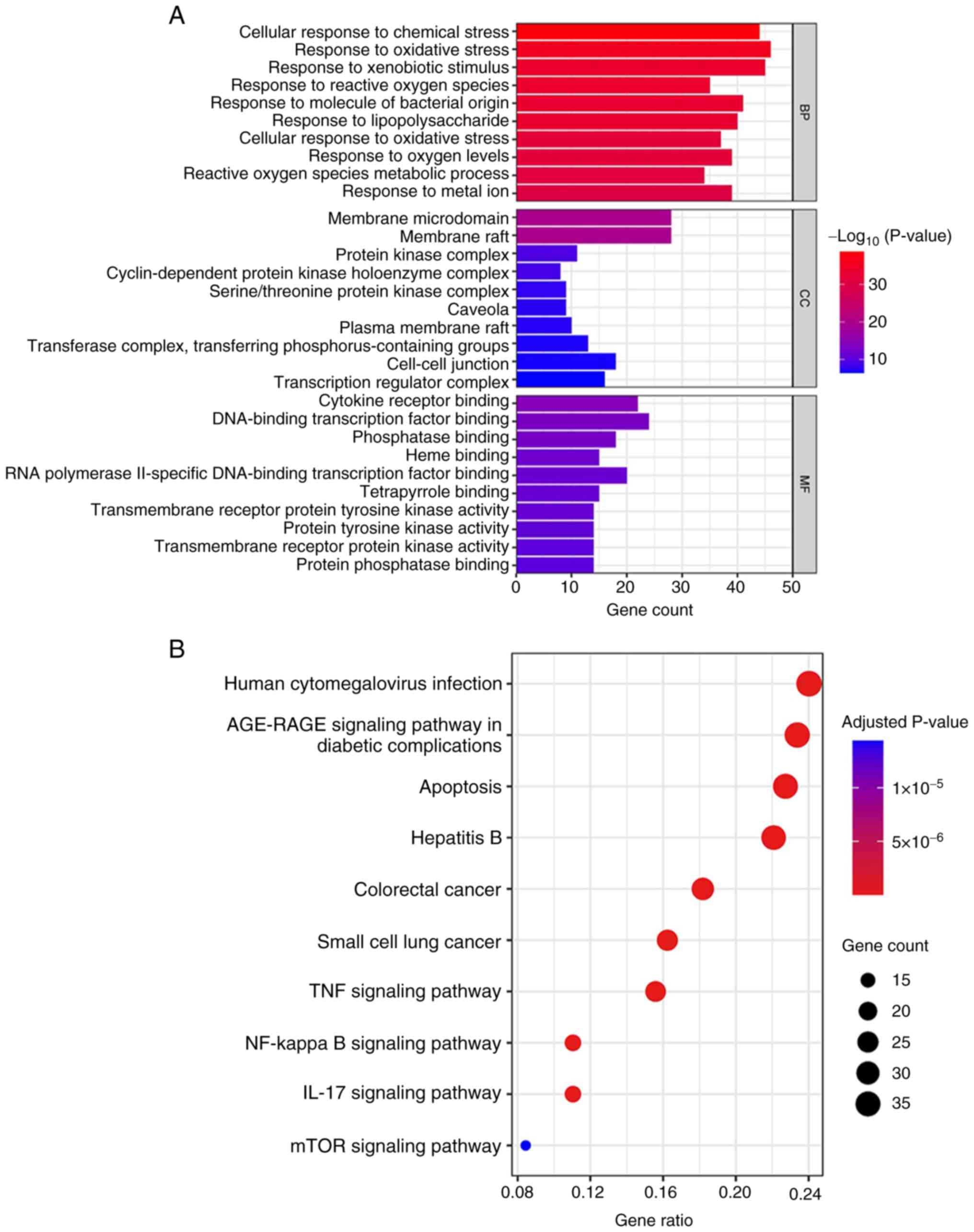
and pharmacological mechanism of Res. According to GO analysis, the
overlapping genes were involved in biological processes associated
with ‘response to oxidative stress’, ‘response to xenobiotic
stimulus’, ‘cellular response to chemical stress’, ‘response to
molecule of bacterial origin’ and ‘response to lipopolysaccharide’.
In terms of cellular components (CCs), the target genes were
primarily associated with ‘membrane microdomain’ and ‘membrane
raft’. The genes enriched in molecular functions (MFs) were
associated with ‘DNA-binding transcription factor binding’,
‘cytokine receptor binding’, ‘RNA polymerase II-specific
DNA-binding transcription factor binding’, ‘phosphatase binding’
and ‘heme binding’ (Fig. 7A). KEGG
analysis revealed that ‘apoptosis’, ‘colorectal cancer’, ‘TNF
signaling pathway’, ‘NF-kappa B signaling pathway’, and ‘IL-17
signaling pathway’ might be involved in regulating UC (Fig. 7B).
PPI network and key target
screening
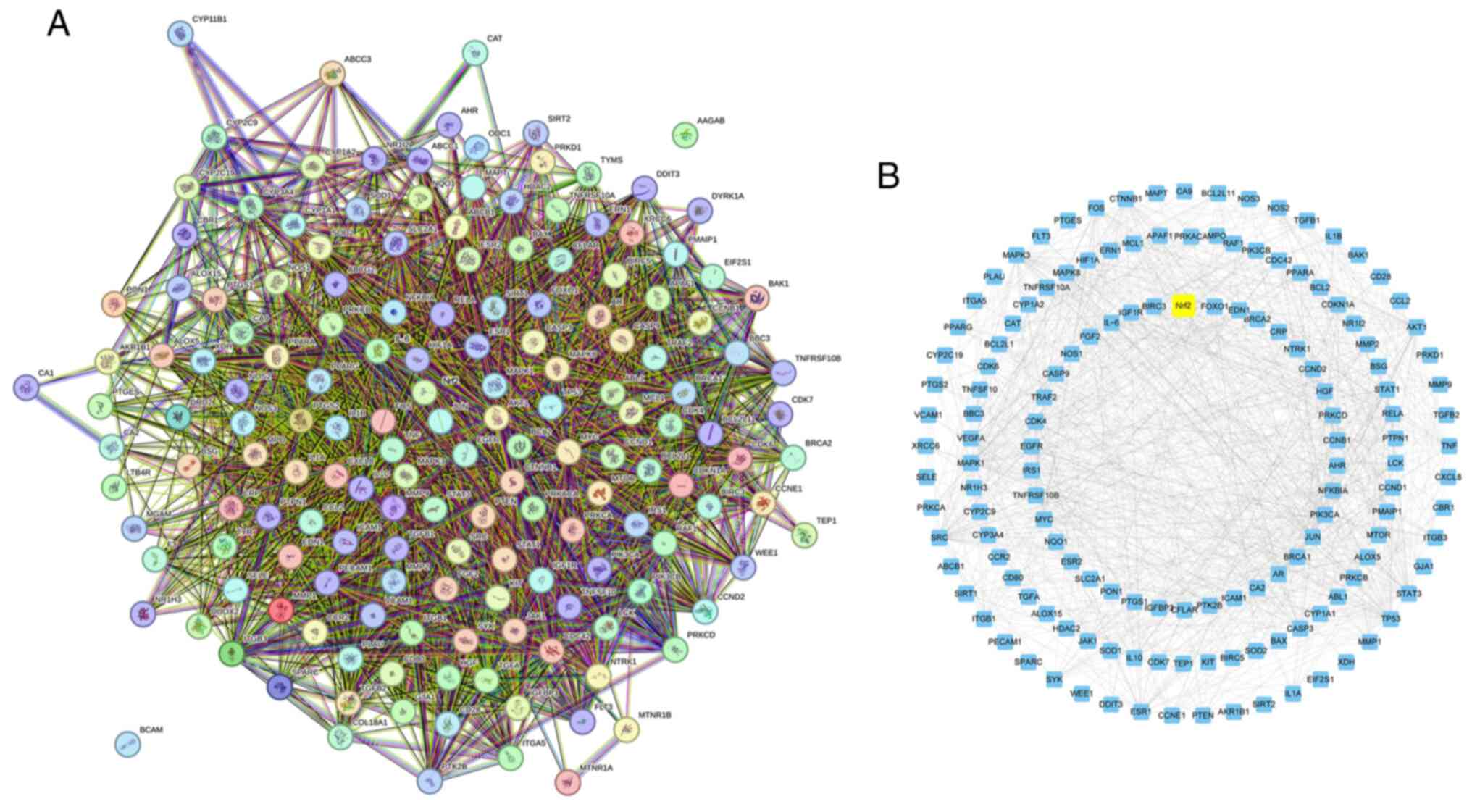
The common targets of Res and UC association were
analyzed using the STRING program, selecting for medium confidence
with a score >0.400 to construct the PPI network. Cytoscape was
then used for visualization (Fig.
8). Nrf2 was identified as having a strong association with the
potential molecular mechanism of Res alleviating UC, functioning as
a regulator of anti-inflammatory and antioxidant capacity both
in vivo and in vitro. Therefore, it was hypothesized
that Res may protect the intestinal mucosal barrier and improve UC
by modulating anti-inflammatory and antioxidant pathways associated
with Nrf2.
Molecular docking
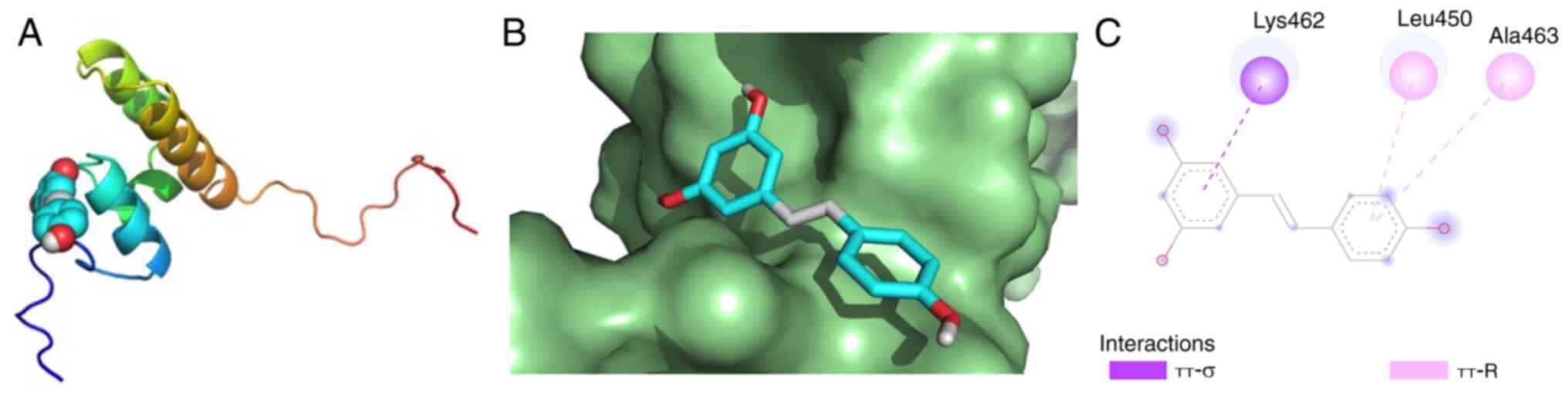
Based on the aforementioned analysis, it was
hypothesized that Res could counteract UC by influencing the
antioxidant pathway involving Nrf2. Dock Res to the hydrophobic
pocket of Nrf2 to calculate affinity. Binding affinity (kcal/mol)
indicates the stability between receptor and ligand; a lower
affinity suggests greater stability (42). A binding energy <-5 kcal/mol is
indicative of strong affinity (43). Res established π-σ interactions
with Nrf2 Lys462 and π-R interactions with Nrf2 Leu450 and Ala463.
The molecular docking results demonstrated that Res and Nrf2 had
strong affinity (Fig. 9; Table IV).
 | Table IV.Molecular docking affinity of
resveratrol. |
Table IV.
Molecular docking affinity of
resveratrol.
| Receptor | Ligand | Affinity,
kcal/mol |
|---|
| Nrf2 | Resveratrol | −5.6 |
Res attenuates DSS-induced
experimental UC via Nrf2/HO-1 pathway
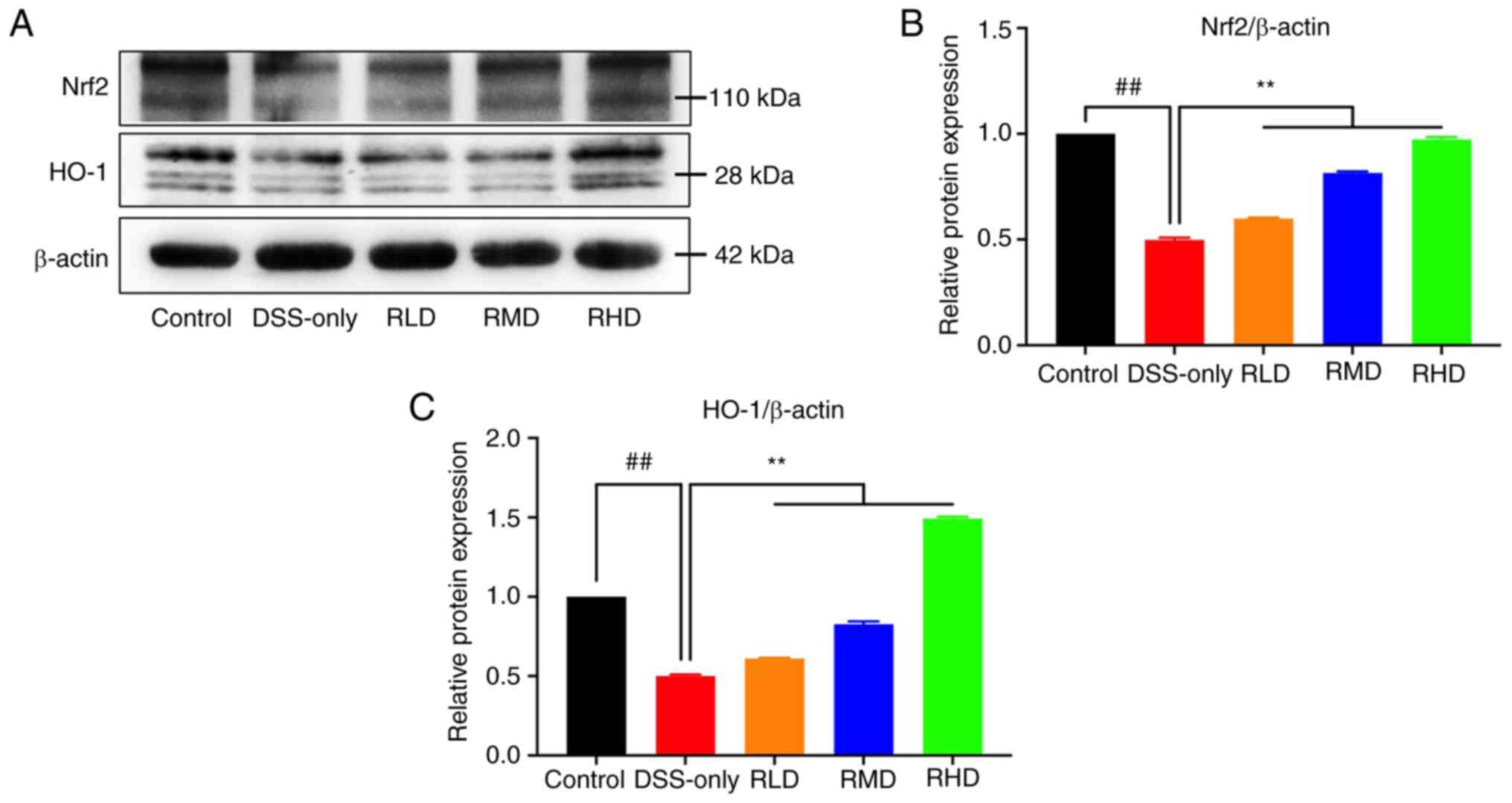
Protein expression of Nrf2 and its downstream target
HO-1 was assessed by western blotting. Nrf2 and HO-1 expression in
colon tissue was significantly reduced in the DSS-only group
compared with the control, while Res treatment significantly
increased Nrf2 and HO-1 levels (Fig.
10). These results suggested that Res exerted a protective
effect on DSS-induced experimental UC via the Nrf2/HO-1
pathway.
Discussion
UC is a complex condition characterized by
inflammation and ulceration of the rectum and colon mucosa, with
severity that can increase over time (44). Determining risk of UC progression
and identifying the most effective treatment strategy pose clinical
challenges, particularly as a number of patients experience adverse
reactions to treatment (45).
Thus, identifying novel targets and intervention methods is
crucial. The present study aimed to evaluate the mechanism by which
Res decreases experimental UC in mice. Res improved intestinal
mucosal barrier dysfunction caused by experimental UC model, with
this protective effect predominantly mediated by activation of the
Nrf2/HO-1 signaling pathway (Fig.
11).
In the present study, an experimental UC mouse model
was established using DSS solution consumption, a method known to
resemble human UC symptoms (46).
Mice in the model group exhibited clinical symptoms such as weight
loss, diarrhea and hematochezia. Pathological changes such as colon
shortening, splenomegaly, intestinal mucosal hyperemia, edema,
ulceration, crypt destruction and inflammatory infiltration
confirmed that DSS induced acute intestinal injury in mice. The
changes in colon and spleen suggested that intestinal mucosal
inflammation led to interstitial fibrosis, which may have caused
abnormal smooth muscle function, decreased mucosal motility and
colon shortening. The spleen enlargement was likely due to
hyperemia, edema and lymphocytosis, resulting in increased spleen
index. Similar symptoms were observed in another study using a
DSS-induced chronic UC model (47). To assess inflammation in
experimental UC mice, pro-inflammatory and anti-inflammatory
cytokine levels in serum and colon tissues were quantified.
Inflammation is key in maintaining the integrity of
the intestinal mucosal barrier (48). IL-6 and IL-1β levels in serum from
patients with IBD positively correlate with inflammation severity
(49,50). TNF-α, a key initiator of intestinal
mucosal barrier injury, activates NF-κB, triggering production of
various inflammatory cytokines and exacerbating barrier impairment
(51). Cui et al (52) reported that Res alleviates the
inflammatory stress state of UC mice by regulating CD3+
T cells that express TNF-α and decreasing the percentage of
neutrophils in mesenteric lymph nodes and lamina propria. This
decreases the incidence of colon cancer associated with colitis. By
contrast, IL-10, an anti-inflammatory cytokine, decreases
inflammation and protects the intestinal barrier by improving TJ
permeability (53).
In the present study, DSS increased IL-6, IL-1β and
TNF-α levels and reduced IL-10 protein expression in serum and
colon tissue of experimental UC mice. However, Res administration
(50 and 100 mg/kg) effectively decreased the secretion of
pro-inflammatory cytokines (IL-6, IL-1β and TNF-α) and promoted the
secretion of anti-inflammatory cytokine (IL-10), thus alleviating
DSS-induced inflammation, suggesting a direct link between level of
inflammation and the integrity of the intestinal mucosal
barrier.
In previous studies, the role of the intestinal
barrier in the pathogenesis and progression of UC has received
considerable attention. The intestinal barrier comprises IECs and
TJs between these cells. TJ proteins, including the cytoplasmic
protein ZO family and transmembrane proteins occludin and claudins,
are crucial components of the intestinal mucosal barrier,
protecting against invasion of bacteria, pathogens, endotoxins and
other harmful substances (54,55).
The present study confirmed that DSS compromised integrity of the
intestinal mucosal barrier. However, 10, 50 and 100 mg/kg Res
increased the protein expression of ZO-1 and occludin, reduced the
histopathological score of colon tissues and improved DSS-induced
intestinal injury in mice. Pan et al (47) reported similar findings, where Res
significantly counteracted decreased levels of TJ proteins and
increased the expression of inflammatory mediators, suggesting that
Res exerts an anti-inflammatory effect by preserving integrity of
the intestinal mucosal barrier. This conclusion is consistent with
research using diquat-induced piglets, indicating the protective
role of Res in another animal model (56).
To validate the hypothesis that Res may protect
experimental UC mice by modulating the Nrf2/HO-1 pathway, network
pharmacological analysis was conducted. PK merits priority in drug
research, which describes the ADME process of drugs in vivo
and the variation of drug concentration with time (57). A total of 12 PK characteristics of
Res were obtained from TCMSP database. Among these, OB is a key
parameter in evaluating drug distribution to systemic circulation.
OB ≥30% is considered ‘drug-like’. Although the OB of Res is only
19.07%, there is growing evidence of the therapeutic potential of
Res for intestinal health (58).
But the relatively low OB and rapid metabolism of Res may limit its
use in humans (59). However,
other properties of Res exhibit primary drug features. Lipinski's
rule of five, which includes criteria such as molecular weight
<500 Da, Hdon <5, Hacc <10, AlogP <5 and RBN <10,
indicates suitability for oral administration in small molecular
compounds (60,61). Hdon and Hacc represent the number
of hydrogen-bonded donors and receptors respectively, indicating
the hydrogen bonding ability of the molecules. AlogP represents
logarithmic value of lipid-water partition coefficient, which is
necessary for measuring the hydrophobicity of molecules. RBN is the
number of keys that allow themselves to rotate freely and is used
to descrive molecular flexibility. Res met these criteria,
highlighting its potential for drug development.
A total of 163 overlapping genes were identified by
aligning potential targets of Res with UC-associated genes. To
assess the interactions among overlapping genes, GO enrichment and
KEGG pathway analyses were performed. GO enrichment showed that
target genes were primarily associated with cellular responses to
chemical and oxidative stress. The most prominent MF ontologies
included ‘cytokine receptor binding’ and ‘DNA-binding transcription
factor binding’. In terms of CC, a significant proportion of genes
were related to cell membrane raft and microdomain. Specifically,
in vivo studies have illustrated that Res decreases
expression of inflammatory cytokines via inducible nitric oxide
synthase (iNOS)/NF-κB and SIRT1/NF-κB signaling pathways,
respectively, thereby mitigating UC and colitis-associated tumors
(23,26).
The present KEGG pathway analysis highlighted that
the TNF and NF-κB signaling pathways may be key pathways in UC.
Differential expression genes were evaluated using a PPI network.
The proteins with high association in the inner circle could be
categorized into five groups based on primary function: Regulation
of cell cycle and death, including forkhead box o1 (FOXO1), cyclin
D2 (CCND2), caspase 8 and fas-associating protein with a novel
death domain like apoptosis regulator and caspase 9 (CASP9);
hormonal regulation, including insulin-like growth factor
binding-protein-3 (IGFBP3), prostaglandin-endoperoxide synthase 1
(PTGS1), estrogen receptor beta (ESR2) insulin receptor substrate 1
(IRS1); cancer susceptibility genes, including, BRCA2) and
myelocytoma oncogene; inflammatory response regulation, including
C-reative protein (CRP), nuclear factor kappa-B subunit 1 alpha,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
(PIK3CA) and IL-6 and oxidative stress regulation, including NQO1
and NOS1. According to the results of network pharmacology, Res was
suggested to target UC via anti-inflammatory and antioxidant
pathways. However, among potential targets, except for Nrf2, other
proteins were either not highly associated or cannot regulate
anti-inflammatory and antioxidant processes at the same time.
Molecular docking showed that Nrf2 had a high
affinity for Res. Western blotting further suggested that Res
decreased DSS-induced inflammation and intestinal mucosal barrier
injury by upregulating expression of Nrf2/HO-1 pathway proteins.
According to Zheng et al (62), Nrf2 is necessary for Res mediated
antioxidant effects in UC and Nrf2−/− mice are more
likely to exhibit DSS-induced colitis and subsequent colon cancer.
The aforementioned study provides support for the results of the
present study and further corroborates that Res positively impacts
UC via anti-inflammatory and antioxidant activity.
In summary, intragastric administration of Res
decreased intestinal mucosal barrier injury in DSS-induced
experimental UC mice. This improvement was evidenced by enhanced
colon tissue morphology, decreased inflammation and increased
expression of TJ proteins and antioxidant capacity in colon
tissues. Through network pharmacology and empirical studies, it was
determined that the beneficial effects of Res on UC were at least
partially associated with the Nrf2/HO-1 pathway. This proposed
mechanism provides a foundation for future research on Res as a
potential anti-inflammatory and antioxidant therapeutic agent.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81960371).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XY and LG conceived the study. XY, XL and LG
designed the experiments. XY, XL, YX, JZ and YL performed the
experiments. YL and YZ collected and analyzed the data. XY drafted
the manuscript. LG revised the manuscript and acquired funding. All
authors have read and approved the final manuscript. XL and LG
confirm the authenticity of all the raw dara.
Ethics approval and consent to
participate
The animal study was reviewed and approved by the
Dali University's Animal Welfare Ethics Committee (Dali, China;
approval no. 2023-PZ-194).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah SC and Itzkowitz SH: Colorectal
cancer in inflammatory bowel disease: Mechanisms and management.
Gastroenterol. 162:715–730.e3. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tili E, Michaille JJ, Piurowski V, Rigot B
and Croce CM: MicroRNAs in intestinal barrier function,
inflammatory bowel disease and related cancers-their effects and
therapeutic potentials. Curr Opin Pharmacol. 37:142–150. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kobayashi T, Siegmund B, Le Berre C, Wei
SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L
and Hibi T: Ulcerative colitis. Nat Rev Dis Primers. 6:742020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ungaro R, Mehandru S, Allen PB,
Peyrin-Biroulet L and Colombel JF: Ulcerative colitis. Lancet.
389:1756–1770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garcia-Planella E, Mañosa M, Van Domselaar
M, Gordillo J, Zabana Y, Cabré E, López San Román A and Domènech E:
Long-term outcome of ulcerative colitis in patients who achieve
clinical remission with a first course of corticosteroids. Dig
Liver Dis. 44:206–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoffmann P, Wehling C, Krisam J,
Pfeiffenberger J, Belling N and Gauss A: Performance of tacrolimus
in hospitalized patients with steroid-refractory acute severe
ulcerative colitis. World J Gastroenterol. 25:1603–1617. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vindigni SM, Zisman TL, Suskind DL and
Damman CJ: The intestinal microbiome, barrier function, and immune
system in inflammatory bowel disease: A tripartite
pathophysiological circuit with implications for new therapeutic
directions. Therap Adv Gastroenterol. 9:606–625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Odenwald MA and Turner JR: The intestinal
epithelial barrier: A therapeutic target? Nat Rev Gastroenterol
Hepatol. 14:9–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keane TJ, Dziki J, Sobieski E, Smoulder A,
Castleton A, Turner N, White LJ and Badylak SF: Restoring mucosal
barrier function and modifying macrophage phenotype with an
extracellular matrix hydrogel: Potential therapy for ulcerative
colitis. J Crohns Colitis. 11:360–368. 2017.PubMed/NCBI
|
|
10
|
Sina C, Kemper C and Derer S: The
intestinal complement system in inflammatory bowel disease: Shaping
intestinal barrier function. Semin Immunol. 37:66–73. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chagas MDSS, Behrens MD, Moragas-Tellis
CJ, Penedo GXM, Silva AR and Gonçalves-de-Albuquerque CF: Flavonols
and flavones as potential anti-inflammatory, antioxidant, and
antibacterial compounds. Oxid Med Cell Longev. 2022:99667502022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HH, Jeong SH, Park MY, Bhosale PB,
Abusaliya A, Kim HW, Seong JK, Kim DI, Lee SJ, Park KI and Kim GS:
Potential antioxidant and anti-inflammatory properties of
polyphenolic compounds from cirsium japonicum extract. Int J Mol
Sci. 25:7852024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Javid AZ, Hormoznejad R, Yousefimanesh HA,
Haghighi-Zadeh MH and Zakerkish M: Impact of resveratrol
supplementation on inflammatory, antioxidant, and periodontal
markers in type 2 diabetic patients with chronic periodontitis.
Diabetes Metab Syndr. 13:2769–2774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zimmermann-Franco DC, Esteves B, Lacerda
LM, Souza IdO, Santos JAD, Pinto NdCC, Scio E, da Silva AD and
Macedo GC: In vitro and in vivo anti-inflammatory properties of
imine resveratrol analogues. Bioorg Med Chem. 26:4898–4906. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Samsami-Kor M, Daryani NE, Asl PR and
Hekmatdoost A: Anti-inflammatory effects of resveratrol in patients
with ulcerative colitis: A randomized, double-blind,
placebo-controlled pilot study. Arch Med Res. 46:280–285. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samsamikor M, Daryani NE, Asl PR and
Hekmatdoost A: Resveratrol supplementation and
oxidative/anti-oxidative status in patients with ulcerative
colitis: A randomized, double-blind, placebo-controlled pilot
study. Arch Med Res. 47:304–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stefanson AL and Bakovic M: Dietary
regulation of Keap1/Nrf2/ARE pathway: Focus on plant-derived
compounds and trace minerals. Nutrients. 6:3777–3801. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang W, Zhong Y, Gao B, Zheng B and Liu
Y: Nrf2-mediated therapeutic effects of dietary flavones in
different diseases. Front Pharmacol. 14:12404332023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korytina GF, Akhmadishina LZ, Aznabaeva
YG, Kochetova OV, Zagidullin NS, Kzhyshkowska JG, Zagidullin SZ and
Viktorova TV: Associations of the NRF2/KEAP1 pathway and
antioxidant defense gene polymorphisms with chronic obstructive
pulmonary disease. Gene. 692:102–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Somparn N, Prawan A, Senggunprai L,
Kukongviriyapan U, Jetsrisuparb A, Lee MH, Kim DH, Kukongviriyapan
V and Surh YJ: Cellular adaptation mediated through Nrf2-induced
glutamate cysteine ligase up-regulation against oxidative stress
caused by iron overload in β-thalassemia/HbE patients. Free Radic
Res. 53:791–799. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Ni P, Tang M, Song Y, Liu C and
Zhao B: Dapagliflozin alleviates renal podocyte pyroptosis via
regulation of the HO-1/NLRP3 axis. Mol Med Rep. 28:2002023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu X, Wang Y, Xu Y, Li X, Zhang J, Su Y
and Guo L: Resveratrol attenuates intestinal epithelial barrier
dysfunction via Nrf2/HO-1 pathway in dextran sulfate sodium-induced
Caco-2 cells. Immun Inflamm Dis. 12:e11932024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Youn J, Lee JS, Na HK, Kundu JK and Surh
YJ: Resveratrol and piceatannol inhibit iNOS expression and
NF-kappaB activation in dextran sulfate sodium-induced mouse
colitis. Nutr Cancer. 61:847–854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bilotta S, Arbogast J, Schart N, Frei M
and Lorentz A: Resveratrol treatment prevents increase of mast
cells in both murine OVA enteritis and IL-10−/− colitis.
Int J Mol Sci. 23:12132022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alrafas HR, Busbee PB, Nagarkatti M and
Nagarkatti PS: Resveratrol downregulates miR-31 to promote T
regulatory cells during prevention of TNBS-induced colitis. Mol
Nutr Food Res. 64:e19006332020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh UP, Singh NP, Singh B, Hofseth LJ,
Price RL, Nagarkatti M and Nagarkatti PS: Resveratrol
(trans-3,5,4′-trihydroxystilbene) induces silent mating type
information regulation-1 and down-regulates nuclear transcription
factor-kappaB activation to abrogate dextran sulfate sodium-induced
colitis. J Pharmacol Exp Ther. 332:829–839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
American Veterinary Medical Association
(AVMA), . AVMA guidelines for the euthanasia of animals. Available
from. https://www.avma.org/resources-tools/avma-policies/avma-guidelines-euthanasia-animals
|
|
28
|
Animal Research, . Reporting of in vivo
experiments (ARRIVE). ARRIVE guidelines. Available from:.
https://arriveguidelines.org/
|
|
29
|
Chen W, Da W, Li C, Fan H, Liang R, Yuan
J, Huang X, Yang R, Zhang J and Zhu J: Network pharmacology-based
identification of the protective mechanisms of taraxasterol in
experimental colitis. Int Immunopharmacol. 71:259–266. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dieleman LA, Palmen MJ, Akol H, Bloemena
E, Peña AS, Meuwissen SG and Van Rees EP: Chronic experimental
colitis induced by dextran sulphate sodium (DSS) is characterized
by Th1 and Th2 cytokines. Clin Exp Immunol. 114:385–391. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maclean A, Bunni E, Makrydima S,
Withington A, Kamal AM, Valentijn AJ and Hapangama DK: Fallopian
tube epithelial cells express androgen receptor and have a distinct
hormonal responsiveness when compared with endometrial epithelium.
Hum Reprod. 35:2097–2106. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jurmeister P, Glöß S, Roller R, Leitheiser
M, Schmid S, Mochmann LH, Payá Capilla E, Fritz R, Dittmayer C,
Friedrich C, et al: DNA methylation-based classification of
sinonasal tumors. Nat Commun. 13:71482022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Paschalis A, Sheehan B, Riisnaes R,
Rodrigues DN, Gurel B, Bertan C, Ferreira A, Lambros MBK, Seed G,
Yuan W, et al: Prostate-specific membrane antigen heterogeneity and
DNA repair defects in prostate cancer. Eur Urol. 76:469–478. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo R, Berry LD, Aisner DL, Sheren J,
Boyle T, Bunn PA Jr, Johnson BE, Kwiatkowski DJ, Drilon A, Sholl LM
and Kris MG: MET IHC is a poor screen for MET amplification or MET
exon 14 mutations in lung adenocarcinomas: data from a
tri-institutional cohort of the lung cancer mutation consortium. J
Thorac Oncol. 14:1666–1671. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Meng Q, Yang M, Liu D, Hou X, Tang
L, Wang X, Lyu Y, Chen X, Liu K, et al: Current trends in drug
metabolism and pharmacokinetics. Acta Pharm Sin B. 9:1113–1144.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun L, Dong S, Ge Y, Fonseca JP, Robinson
ZT, Mysore KS and Mehta P: DiVenn: An interactive and integrated
web-based visualization tool for comparing gene lists. Front Genet.
10:4212019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: Kegg: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45(D1): D353–D361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li H, Hung A and Yang AWH: Herb-target
virtual screening and network pharmacology for prediction of
molecular mechanism of Danggui Beimu Kushen Wan for prostate
cancer. Sci Rep. 11:66562021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuo WT, Odenwald MA, Turner JR and Zuo L:
Tight junction proteins occludin and ZO-1 as regulators of
epithelial proliferation and survival. Ann N Y Acad Sci.
1514:21–33. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi XQ, Yue SJ, Tang YP, Chen YY, Zhou GS,
Zhang J, Zhu ZH, Liu P and Duan JA: A network pharmacology approach
to investigate the blood enriching mechanism of Danggui buxue
decoction. J Ethnopharmacol. 235:227–242. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jarmoskaite I, AlSadhan I, Vaidyanathan PP
and Herschlag D: How to measure and evaluate binding affinities.
Elife. 9:e572642020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang T, Jiang X, Ruan Y, Zhuang J and Yin
Y: Based on network pharmacology and in vitro experiments to prove
the effective inhibition of myocardial fibrosis by Buyang Huanwu
decoction. Bioengineered. 13:13767–13783. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choi CHR, Al Bakir I, Ding NSJ, Lee GH,
Askari A, Warusavitarne J, Moorghen M, Humphries A,
Ignjatovic-Wilson A, Thomas-Gibson S, et al: Cumulative burden of
inflammation predicts colorectal neoplasia risk in ulcerative
colitis: A large single-centre study. Gut. 68:414–422. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schirmer M, Denson L, Vlamakis H, Franzosa
EA, Thomas S, Gotman NM, Rufo P, Baker SS, Sauer C, Markowitz J, et
al: Compositional and temporal changes in the gut microbiome of
pediatric ulcerative colitis patients are linked to disease course.
Cell Host Microbe. 24:600–610.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eichele DD and Kharbanda KK: Dextran
sodium sulfate colitis murine model: An indispensable tool for
advancing our understanding of inflammatory bowel diseases
pathogenesis. World J Gastroenterol. 23:6016–6029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pan HH, Zhou XX, Ma YY, Pan WS, Zhao F, Yu
MS and Liu JQ: Resveratrol alleviates intestinal mucosal barrier
dysfunction in dextran sulfate sodium-induced colitis mice by
enhancing autophagy. World J Gastroenterol. 26:4945–4959. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Al-Sadi R, Boivin M and Ma T: Mechanism of
cytokine modulation of epithelial tight junction barrier. Front
Biosci (Landmark Ed). 14:2765–2778. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Reinecker HC, Steffen M, Doehn C, Petersen
J, Pflüger I, Voss A and Raedler A: Proinflammatory cytokines in
intestinal mucosa. Immunol Res. 10:247–248. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Reinisch W, Gasché C, Tillinger W, Wyatt
J, Lichtenberger C, Willheim M, Dejaco C, Waldhör T, Bakos S,
Vogelsang H, et al: Clinical relevance of serum interleukin-6 in
Crohn's disease: Single point measurements, therapy monitoring, and
prediction of clinical relapse. Am J Gastroenterol. 94:2156–2164.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Clark IA: How TNF was recognized as a key
mechanism of disease. Cytokine Growth Factor Rev. 18:335–343. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cui X, Jin Y, Hofseth AB, Pena E, Habiger
J, Chumanevich A, Poudyal D, Nagarkatti M, Nagarkatti PS, Singh UP
and Hofseth LJ: Resveratrol suppresses colitis and colon cancer
associated with colitis. Cancer Prev Res (Phila). 3:549–559. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Howe KL, Reardon C, Wang A, Nazli A and
McKay DM: Transforming growth factor-beta regulation of epithelial
tight junction proteins enhances barrier function and blocks
enterohemorrhagic Escherichia coli o157:H7-induced increased
permeability. Am J Pathol. 167:1587–1597. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kaminsky LW, Al-Sadi R and Ma TY: IL-1β
and the intestinal epithelial tight junction barrier. Front
Immunol. 12:7674562021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shen L, Su L and Turner JR: Mechanisms and
functional implications of intestinal barrier defects. Dig Dis.
27:443–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xun W, Fu Q, Shi L, Cao T, Jiang H and Ma
Z: Resveratrol protects intestinal integrity, alleviates intestinal
inflammation and oxidative stress by modulating AhR/Nrf2 pathways
in weaned piglets challenged with diquat. Int Immunopharmacol.
99:1079892021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang YF, Huang Y, Ni YH and Xu ZM:
Systematic elucidation of the mechanism of geraniol via network
pharmacology. Drug Des Devel Ther. 13:1069–1075. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang S, Xu W, Feng L, Zhang C, Yan C,
Zhang J, Lai J, Yan T, He Z, Du X, et al: Resveratrol improves the
digestive ability and the intestinal health of siberian sturgeon.
Int J Mol Sci. 23:119772022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang W, Wang S, Liu T, Ma Y, Huang S, Lei
L, Wen A and Ding Y: Resveratrol: Multi-targets mechanism on
neurodegenerative diseases based on network pharmacology. Front
Pharmacol. 11:6942020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lipinski CA, Lombardo F, Dominy BW and
Feeney PJ: Experimental and computational approaches to estimate
solubility and permeability in drug discovery and development
settings. Adv Drug Deliv Rev. 46:3–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lipinski CA: Lead- and drug-like
compounds: The rule-of-five revolution. Drug Discov Today Technol.
1:337–341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zheng Z, Chen Y, Huang J, Deng H, Tang X
and Wang XJ: Mkp-1 is required for chemopreventive activity of
butylated hydroxyanisole and resveratrol against colitis-associated
colon tumorigenesis. Food Chem Toxicol. 127:72–80. 2019. View Article : Google Scholar : PubMed/NCBI
|