Introduction
Knee osteoarthritis (KOA) is a chronic disease
(1–3), during which patients suffer from
persistent painful arthritis. KOA is characterized by progressive
destruction of articular cartilage, synovial inflammation,
fibrosis, osteophyte formation and subchondral bone changes, which
can lead to pain, stiffness and chronic disability (4–6).
Approximately 250 million people are currently affected by KOA
worldwide, and there is no effective drug for KOA treatment
(7,8).
It has been reported that inflammation serves a key
role in the pathogenesis of KOA (1,9–12).
The activation of the NOD-like receptor protein 3 (NLRP3)
inflammasome initiates the inflammatory cascade, and it is closely
related to a number of types of chronic inflammation (13). Thus, inhibition of NLRP3
inflammasome activation has been demonstrated to ameliorate a
variety of fibrotic diseases, including synovial fibrosis in KOA
(14). The NLRP3 inflammasome
consists of a sensor (NLRP3), an adaptor [apoptosis-related
speckle-like protein (ASC)/PYCARD] and an effector (caspase-1)
(15–17). When cells are stimulated, ASC
interacts with the caspase recruitment domain to assemble into a
macromolecular complex, which assembles the NLRP3 inflammasome
(18,19). Subsequently, the complex cleaves
caspase-1, leading to maturation of caspase-1. Subsequently, active
caspase-1 cleaves gasdermin-D (GSDMD) and induces the secretion of
interleukin (IL)-1β and IL-18, which results in cartilage
degeneration and synovial membrane inflammation (20,21).
Recent research has revealed that inhibiting NLRP3 activation
reduces synovial inflammation and fibrosis in KOA (22–24).
Coumarins comprise a large class of natural phenolic
compounds found in traditional medicinal herbs, such as Rutaceae,
Umbelliferae, Compositae and Leguminosae, which have been reported
to show antioxidant and anti-inflammatory activity (25,26).
As one of the derivatives in coumarins, dicoumarol was first
discovered from the spoilage of Melilotus officinalis (L.)
Pall. Due to its molecular structural similarity to vitamin K,
dicoumarol has been used as an anticoagulant and can reversibly
compete with vitamin K to prevent the formation of thrombi
(25,27,28).
However, there are no studies reporting the relationship between
dicoumarol and inflammation. Therefore, the present study aimed to
explore whether dicoumarol has a potential protective effect
against KOA based on its various biological activities.
Materials and methods
Compounds
Dicoumarol was purchased from Shanghai Aladdin
Biochemical Technology Co., Ltd. Dicoumarol was dissolved in
dimethyl sulfoxide (DMSO) as a stock solution, stored at −20°C, and
freshly diluted with medium to the final concentration used in
vitro studies. The final concentration of DMSO was
<0.1%.
Rat KOA model and experimental
design
A total of 26 3-month-old Sprague-Dawley (SD) male
rats weighing from 280 to 320 g (Beijing Vital River Laboratory
Animal Technology Co., Ltd.) were housed in a specific
pathogen-free (SPF)-grade environment at 25±2°C with a relative
humidity of 50–60%, and provided with food and water ad
libitum. Rats were divided into the following three groups:
Normal (vehicle) group (n=7), KOA group (n=7) and KOA + dicoumarol
group (n=7). All animal experiments were performed according to the
National Institute of Health Animal Care and Use Guidelines
(29), and the protocol was
approved by the committee for the Ethics of Animal Experiments,
Wuxi Hospital of Traditional Chinese Medicine (Wuxi, China;
approval no. SWJWQNXM2020033102). Before the operation, the SD rats
were fasted and deprived of water for 12 h. Animals were
anesthetized with 2% isoflurane mixed with oxygen via inhalation
and were maintained on the same concentration of anesthetic
throughout the entirety of the procedure. Briefly, a syringe was
used to inject a suspension of monosodium iodoacetate (MIA; 5
mg/ml; MilliporeSigma) into the knee joint cavity (30). Postoperatively, the rats were
closely monitored to ensure their comfort and wellbeing, with
prompt identification of any signs of discomfort or complications.
Drug administration commenced on day 7 post-modeling, and knee
joint diameter was measured every 7 days thereafter. The normal
group and the KOA group were administered normal saline via
intragastric administration as the control, and each rat in the KOA
+ dicoumarol group was treated with 10 mg/kg dicoumarol every day
via intragastric administration. Doses were chosen with reference
to previous studies (31). In
addition, several pre-experiments were performed to select the dose
of dicoumarol, with the selection criteria being both significant
relief of KOA in rats and achieving good compliance in rats (data
not shown). On day 28, the rats were anesthetized with 2%
isoflurane mixed with oxygen via inhalation and were maintained on
the same concentration of anesthetic throughout the entirety of the
procedure the rats were euthanized and separate knee joint tissues
for further experiments. Animals were sacrificed by intraperitoneal
administration of an overdose of 1–1.5 ml pentobarbitone (150–200
mg/kg), which amounted to 64.8 mg/ml pentobarbitone. Death was
confirmed by checking for cardiac arrest, after which the animal
was observed for ~5 min.
Cell preparation and treatment
For FLS isolation, knee joint tissues removed from
healthy SD rats (weight, 280–320 g; age, 3 months; Beijing Vital
River Laboratory Animal Technology Co., Ltd.) were snipped into 1–3
mm3 pieces, homogenized in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) and incubated for 1 h at 37°C with 1 mg/ml type I
collagenase (MilliporeSigma). The samples were then filtered
through a 100-µm cell strainer. After dissociation, the FLSs were
pelleted via centrifugation at 300 × g at ~25°C for 5 min and
plated in DMEM supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% antibiotics (penicillin and streptomycin;
Gibco; Thermo Fisher Scientific, Inc.). Cells were cultured at 37°C
in a humidified atmosphere with 95% air and 5% CO2.
Primary FLSs from passages 3–5 were used for subsequent
experiments. All animal experiments were performed according to the
National Institute of Health Animal Care and Use Guidelines
(29), and the protocol was
approved by the Committee for the Ethics of Animal Experiments,
Wuxi Hospital of Traditional Chinese Medicine (Wuxi, China;
approval no. SWJWQNXM2020033102).
FLSs were initially stimulated with
lipopolysaccharide (LPS; 10 ng/ml) for 12 h at 37°C, followed by a
3-h incubation with ATP (5 mM) at 37°C, to simulate the
inflammatory environment of KOA and to activate the NLRP3
inflammasome. Subsequently, the FLSs were treated with dicoumarol
(30 µM) for an additional 12 h before proceeding with subsequent
experiments. The doses of LPS and ATP were chosen with reference to
previous studies (14,32). LPS and ATP were obtained from
MilliporeSigma.
Degradation assay
Cycloheximide (CHX), MG132 and NH4Cl were
obtained from MilliporeSigma, and concentrations were chosen as
described previously (33).
Compounds used in assays were dissolved in DMSO and kept as 10 mM
stock solutions for in vitro studies (33). The final concentration of DMSO was
<0.1%.
Briefly, FLSs were pretreated with dicoumarol (30
µM) or DMSO for 12 h, followed by the addition of CHX (10 µM) for
0, 2, 4, 8, 12 and 24 h at 37°C, after which the cells were
harvested. In addition, FLSs were stimulated with LPS (10 ng/ml)
for 12 h, followed by a 3-h stimulation with ATP (5 mM). After a 12
h treatment with dicoumarol (30 µM), the cells were then treated
with NH4Cl (10 µM) or MG132 (10 µM) for 8 h and the
cells were collected.
Cellular thermal shift assay
(CETSA)
First step: FLSs were initially treated with DMSO or
dicoumarol. For each group, 3×107 cells were harvested
after 1 h of culturing with DMSO or dicoumarol (50 µM).
Subsequently, the cells were transferred to EP tubes and were heat
shocked for 3 min each at 43.5, 44.5, 45.9, 48, 50, 52.7, 55, 57.2,
59.5, 61.5, 62.7 and 63.5°C. The samples were then subjected to
three freeze-thaw cycles. For each cycle, cells were exposed to
liquid nitrogen for 1 min and placed in a heating block at 25°C.
The sample-containing tubes were then centrifuged at 15,000 × g for
15 min at 4°C to precipitate the cell debris. The soluble
supernatant was used for western blotting. Second step: Next, for
determination of the isothermal concentration-response fingerprint
for NLRP3, cells were incubated with DMSO or different
concentrations of dicoumarol for 30 min at 37°C. Cells were then
heated at 57.2°C, as calculated in the first step, for 3 min and
placed in an aluminum block at room temperature for 3 min. Western
blotting was then performed on the supernatant.
Sirius Red staining
Synovial tissues sections from rats in the Control,
KOA and KOA + dicoumarol groups underwent staining. The frozen knee
joint tissues sections (5 µm) were removed from the −20°C freezer
and restored to room temperature, they were then fixed with 4%
paraformaldehyde for 15 min at room temperature and rinsed with
running water. The sections were stained with 100% Sirius Red
staining solution (Wuhan Servicebio Technology Co., Ltd.) for 8 min
at room temperature. The sections were sequentially soaked in 70,
90 and 100% ethanol for 10 sec at room temperature for dehydration,
followed by 5 min in xylene. Finally, neutral resin was applied to
the center of the sections and a coverslip was added. Tissue
changes were observed under a light microscope (ZEISS Axio Vert.
A1; Carl Zeiss AG).
Hematoxylin and eosin (H&E)
staining
Synovial tissues sections from rats in the Control,
KOA and KOA + dicoumarol groups underwent staining. The frozen knee
joint tissues sections (5 µm) were removed from the −20°C freezer
and restored to room temperature, they were then fixed with 4%
paraformaldehyde for 15 min at room temperature and rinsed with
running water. The sections were stained with 100% hematoxylin
(Wuhan Servicebio Technology Co., Ltd.) for 5 min at room
temperature. Subsequently, the sections. The sections were
sequentially soaked in 85 and 95% ethanol for 5 min at RT for
dehydration. Next, the sections were stained with 100% eosin
staining solution (Wuhan Servicebio Technology Co., Ltd.) for 5 min
at room temperature. Tissue changes were observed under a light
microscope (ZEISS Axio Vert. A1).
Immunohistochemistry
Synovial tissues sections from rats in the Control,
KOA and KOA + dicoumarol groups underwent immunohistochemistry
analysis. The synovial tissues were fixed with 4% formalin for 20 h
at room temperature, embedded in paraffin and sections were
prepared (7 µm). The consecutive serial sections were
deparaffinized with xylene and rehydrated in an alcohol gradient.
The sections were immersed in sodium citrate and heated in a
microwave at 800 W for 3 min and then left for 5 min. Next, heat
was again applied at 800 W for 3 min and then left again for 5 min.
Finally, heat was applied at 200 W for 1 min, before the slices
were left to cool to room temperature. The sections were then
blocked for 1 h at room temperature with 3% hydrogen peroxide
methanol solution and BSA (MilliporeSigma), before incubation with
the following primary antibodies: Anti-TGF-β (1:200; cat. no.
A25313; ABclonal Biotech Co., Ltd.), anti-NLRP3 (1:200; cat. no.
A5652; ABclonal Biotech Co., Ltd,) and anti-IL-1β (1:200; cat. no.
ab315084; Abcam) overnight at 4°C. Subsequently, the sections were
washed with PBS and incubated with ready-to-use secondary
antibodies from an immunohistochemistry kit (cat. no. PV-6000;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for 30 min at
37°C. The sections were then stained with DAB Substrate Kit
(Beijing Zhongshan Jinqiao Biotechnology Co. LTD., Beijing, China,
cat. no. ZLI-9019) for 20 min at room temperature and with
hematoxylin (Sangon Biotech, Shanghai, China) for 10 sec at room
temperature. The slides were observed and scanned with an
orthogonal fluorescence microscope (ZEISS Axio Vert. A1).
Nuclear plasma separation
experiment
FLSs cells were collected and resuspended with 100
µl buffer A [10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA and 0.12%
NP-40] and incubated for 15 min at 4°C before centrifugation at
1,000 × g for 5 min at 4°C to remove the supernatant and storage at
−80°C (cytoplasmic). The precipitate was washed three times with
buffer A and washed with 150 µl buffer B [20 mM HEPES (pH 7.9), 0.4
mM NaCl, 1 mM EDTA, 1 mM EGTA and 0.5% NP-40]. After centrifugation
at 1,000 × g for 5 min at 4°C, the supernatant was collected and
stored at −80°C (cytoplasmic). Samples were subsequently subjected
to western blotting. Histone was used as a control for nuclear
proteins and GAPDH as a control for plasma proteins.
Western blotting
The FLSs or synovial tissues from rats in the
Control, KOA and KOA + dicoumarol groups were lysed with RIPA
Solution (Thermo Fisher Scientific, Inc.) and western blotting was
performed as previously described (34). The BCA assay was used for protein
quantification and the mass of proteins loaded per lane was 20 µg.
Subsequently, protein samples were separated by SDS-PAGE on 15%
gels and were transferred onto nitrocellulose membranes. The
membranes were then blocked with non-fat milk at room temperature
for 1 h and incubated at 4°C for 24 h with primary antibodies.
Subsequently, the membranes were incubated with secondary
antibodies at room temperature for 1 h. Blot visualization was
performed according to the manufacturer's instructions using the
High-sig ECL Western Blot Substrate (cat. no. 180-5001) and Fully
Automatic Chemiluminescence Image Analysis System (both from Tanon
Science and Technology Co., Ltd.).
The following primary antibodies were used:
Anti-TGF-β (1:1,000; cat. no. A2124), anti-TIMP1 (1:1,000; cat. no.
A4959), anti-COL1A1 (1:1,000; cat. no. A1352), anti-IL-1β (1:1,000;
cat. no. A16288), anti-IL-18 (1:1,000; cat. no. A1115), anti-NLRP3
(1:1,000; cat. no. A12694), anti-GSDMD (1:1,000; cat. no. A20728;
both cleaved and total GSDMD), anti-ASC (1:1,000; cat. no. A16672),
anti-NF-κB (1:1,000; cat. no. A22279), anti-Histone (1:100,000;
cat. no. A2348), anti-GAPDH (1:100,000; cat. no. AC001) and
anti-β-actin (1:100,000; cat. no. AC026). The following secondary
antibodies were used: HRP Goat Anti-Rabbit IgG (H+L) (cat. no.
AS014) and HRP Goat Anti-Mouse IgG (H+L) (cat. no. AS003). All
antibodies were purchased from ABclonal Biotech Co., Ltd. The HRP
Goat Anti-Rabbit IgG (H+L) was used to detect TGF-β, TIMP1, COL1A1,
IL-1β, IL-18, NLRP3, GSDMD, ASC, NF-κB, Histone and GAPDH. The HRP
Goat Anti-Mouse IgG (H+L) was used to detect β-actin.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from FLSs with
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
RNA (1 µg) was used to generate cDNA using the Reverse
Transcriptase kit (Vazyme Biotech Co., Ltd.) according to the
manufacturer's protocol. Subsequently, SYBR green-based qPCR assay
(Vazyme Biotech Co., Ltd.) was used to detect the transcriptional
levels of Il1b, Il18, Tgfb, Timp, Nlrp3, Col1a and
Gapdh. Briefly, 2X AceQ qPCR SYBR Green Master Mix, primers,
cDNA and ddH2O were combined in a sterile, nuclease-free
tube at a final reaction volume of 20 µl. qPCR was performed using
the Applied Biosystems 7500 Fast RT-PCR (Becton, Dickinson and
Company) under the following conditions: Initial denaturation at
95°C for 5 min; followed by 40 cycles at 95°C for 10 sec and 60°C
for 30 sec. The mRNA expression levels of the individual genes were
normalized to Gapdh and calculated using the
2−ΔΔCq data analysis method (35). Samples were normalized relative to
the expression of the endogenous control gene Gapdh. Primer
sequences are shown in Table
I.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR assay. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR assay.
| Gene | Forward | Reverse |
|---|
| Gapdh |
5′-TTCACCACCATGGAGAAGGC-3′ |
5′-CTCGTGGTTCACACCCATCA-3′ |
| Il1b |
5′-ACAGCAGCATCTCGACAAGAGC-3′ |
5′-CCACGGGCAAGACATAGGTAGC-3′ |
| Il18 |
5′-TCTGTAGCTCCATGCTTTCCG-3′ |
5′-GATCCTGGAGGTTGCAGAAGA-3′ |
| Tgfb |
5′-GACTCTCCACCTGCAAGACC-3′ |
5′-GGACTGGCGAGCCTTAGTTT-3′ |
| Timp |
5′-CAGCTTTCTGCAACTCGGAC-3′ |
5′-CAGCGTCGAATCCTTTGAGC-3′ |
| Nlrp3 |
5′-GAGCTGGACCTCAGTGACAATGC-3′ |
5′-ACCAATGCGAGATCCTGACAACAC-3′ |
| Col1a |
5′-GATCCTGGAGGTTGCAGAAGA-3′ |
5′-AAGTTCCGGTGTGACTCGTG-3′ |
Cell viability assay
Cell Counting Kit (CCK)-8 was used to detect the
effects of dicoumarol on the viability of FLSs. Cells were cultured
in 96-well plates. When the cell density reached 85–90%, they were
treated with different concentrations of dicoumarol (0, 0.4, 0.78,
1.56, 3.13, 6.25, 12.5, 25, 50 and 100 µM) for 24 h at 37°C. Then,
10 µl CCK-8 solution (Shanghai Yeasen Biotechnology Co., Ltd.) was
added to each well and the cells were placed in an incubator for 2
h at 37°C. The optical density of the wells was detected at 450 nm
using a microplate spectrophotometer (BioTek Instruments, Inc.).
The IC50 values were calculated by non-linear regression
analysis with the sigmoidal dose response with variable slope
equation (GraphPad Prism 8.0; Dotmatics): Y=1/[1 +
10^(logIC50- X)(Hillslope)].
Flow cytometry
Apoptosis was detected using the Annexin V-FITC/PI
Apoptosis Detection Kit (Vazyme Biotech Co., Ltd.). After
treatment, FLSs were double stained with 1% Annexin V dye and PI
dye for 20 min at room temperature. Apoptotic cells were
subsequently analyzed using a BD FACS ARIA II SORP (BD
Biosciences). The ModFit LT 5.0 (Verity Software House, Inc.) was
used to measure the total apoptosis rate in the present study.
Statistical analysis
The statistical analysis was performed using
GraphPad Prism 8. Data are presented as the mean ± standard
deviation from at least three independent experiments. Statistical
normality and variance homogeneity was assessed, and significance
was determined by unpaired Student's t-test or one-way ANOVA with
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Dicoumarol protects rat synoviocytes
from fibrosis and inflammation
FLSs have been identified as key drivers of
inflammatory joint destruction in OA (36). Therefore, primary FLSs were
separated from rat synovial tissues and validated by microscopic
observation of cell morphology. To test the inhibitory effect of
dicoumarol (the structure of which is shown in Fig. 1A) on cell viability, a CCK-8 assay
was performed. Dicoumarol inhibited the viability of FLSs in a
concentration-dependent manner, and the IC50 of
dicoumarol was ~95.21 µM (Fig.
1B). To exclude the influence of dicoumarol on viability, a
concentration of 30 µM was used in the subsequent experiments. To
simulate inflammation during KOA progression, the combination of
LPS and ATP was used to trigger the activation of inflammasomes and
apoptosis. The results showed that the combination of LPS and ATP
could significantly induce the apoptosis of FLSs; the apoptosis
rate was increased to 35.77%. By contrast, dicoumarol could
alleviate apoptosis and the apoptosis rate was decreased to 8.25%
(Fig. 1C). In KOA, inflammation
can increase the deposition of extracellular matrix and lead to
synovial fibrosis. Therefore, western blot analysis was performed
to evaluate the expression levels of fibrosis-related biomarkers.
Western blot analysis showed that the expression levels of TGF-β
(relative expression, 1.51), TIMP1 (2.20) and COL1A1 (1.49) were
significantly elevated in the LPS + ATP-treated group compared with
the expression of TGF-β (0.68), TIMP1 (0.84) and COL1A1 (0.72) in
the control group (Fig. 1D).
Notably, the expression levels of these proteins could be
suppressed by dicoumarol, and the protein expression levels of
TGF-β, TIMP1 and COL1A1 decreased to 0.61, 1.07 and 0.70,
respectively. The NLRP3 inflammasomes are key regulators that
promote the secretion of proinflammatory cytokines, such as IL-1β
and IL-18 (37,38). Western blot analysis showed that
the expression levels of IL-β (0.98) and IL-18 (1.43) were
significantly elevated in the LPS + ATP-treated group compared with
the expression of IL-β (0.50) and IL-18 (0.66) in the control
group, and the expression could be decreased to 0.59 and 0.70 by
dicoumarin, respectively (Fig.
1E). Subsequently, the mRNA expression levels of these fibrotic
and inflammatory markers were detected by RT-qPCR. The combination
of LPS and ATP could increase mRNA expression levels of Tgfb
(1.28), Timp (1.57), Col1a1 (3.11), Il1b
(2.38) and Il18 (2.11), while they were all reduced after
dicoumarol treatment (Fig. 1F).
The mRNA expression levels of Tgfb, Timp, Col1a1, Il1b and
Il18 in dicoumarol-treated FLSs were 1.02, 0.77, 1.62, 0.77
and 1.02, respectively. These results indicated that dicoumarol
could inhibit fibrosis and inflammation in KOA.
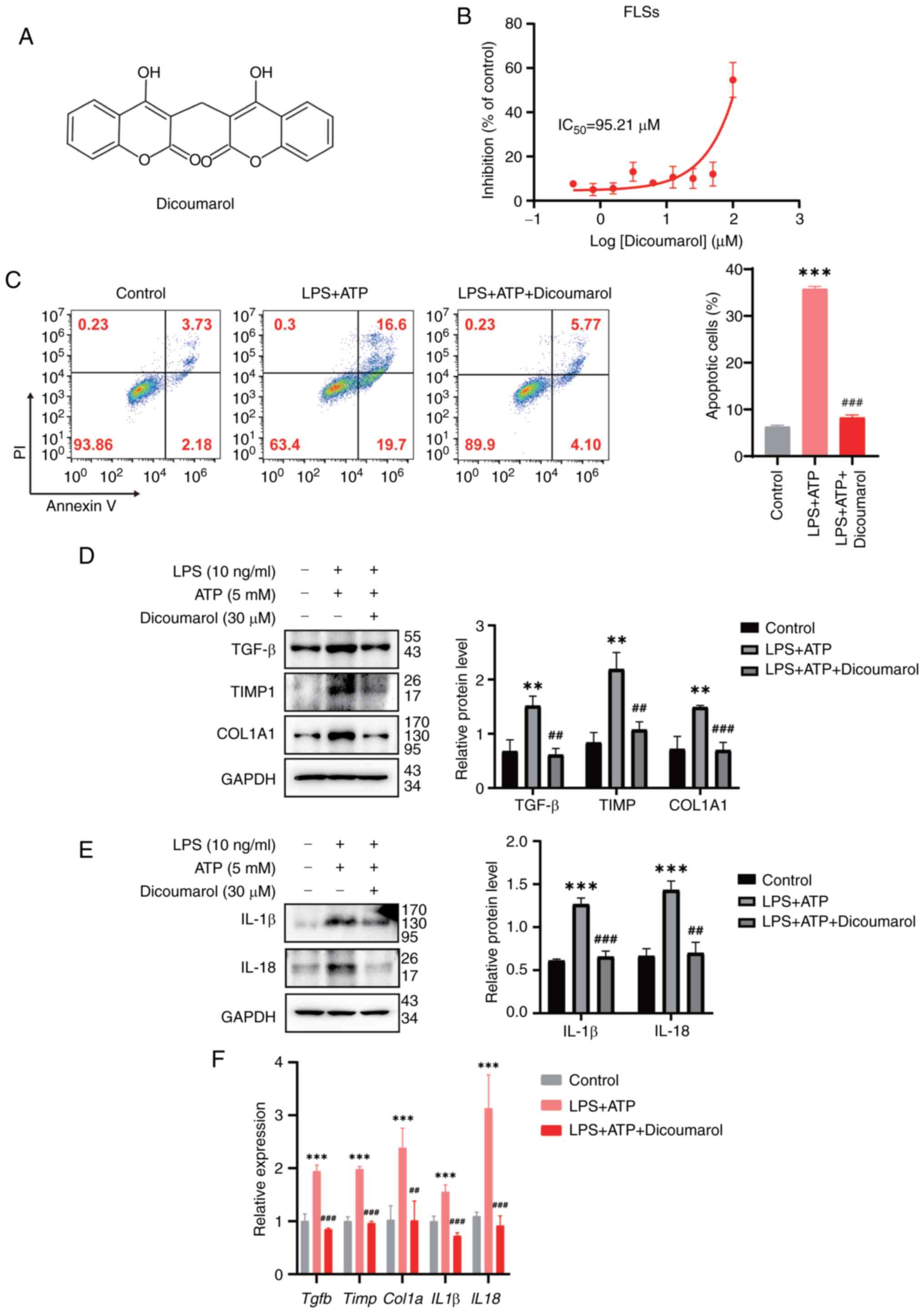 | Figure 1.Dicoumarol protects rat synoviocytes
from fibrosis and inflammation. (A) Chemical structure of
dicoumarol. (B) FLSs were treated with the indicated concentrations
of dicoumarol for 72 h. Cell viability was detected by Cell
Counting Kit-8 assay. This part of the experiment was repeated
three times. (C-F) FLSs were initially stimulated with LPS (10
ng/ml) for 12 h, followed by a 3-h incubation with ATP (5 mM), and
were subsequently treated with dicoumarol (30 µM) for an additional
12 h. This part of the experiment was repeated three times. (C)
Cells were stained with Annexin-V FITC/PI and flow cytometry was
carried out to assess apoptosis. Western blot analysis of (D)
fibrosis-related proteins and (E) inflammation-related proteins.
(F) Quantification of mRNA expression levels of Tgfb, Timp,
Col1α, Il1b and Il18. **P<0.01, ***P<0.001 vs.
control group; ##P<0.01, ###P<0.001 vs.
LPS + ATP group. FLS, fibroblast-like synoviocyte; IL, interleukin;
LPS, lipopolysaccharide. |
Dicoumarol specifically binds to NLRP3
to inhibit its expression
The present study aimed to uncover the mechanism
underlying the effects of dicoumarol on KOA. Since dicoumarol was
demonstrated to inhibit the secretion of proinflammatory cytokines,
it was hypothesized that dicoumarol could directly bind to the
NLRP3 inflammasome and decrease its activation to inhibit
apoptosis. Firstly, to determine the binding ability between
dicoumarol and NLRP3 in FLSs, a CETSA was performed. Western blot
analysis showed that the protein stability of NLRP3 in FLSs
decreased with increasing temperature (Fig. 2A). At 57.2°C, the protein
expression levels of NLRP3 in the dicoumarol-treated group (0.803)
were markedly higher than those (0.541) in the control group,
indicating that the interaction between dicoumarol and NLRP3
enhances the thermostability of NLRP3. Subsequently, FLSs were
treated with various concentrations of dicoumarol at 52°C. The
results revealed that the protein expression levels of NLRP3
increased from 0.012 to 0.869 as the concentration of dicoumarol
increased (Fig. 2B). The present
study also revealed that the combination of LPS and ATP could
increase the expression levels of NLRP3 (1.51) and cleaved-GSDMD
(1.41) compared with the expression of NLRP3 (1.06) and
cleaved-GSDMD (1.10) in the control group, whereas the expression
levels of ASC (adaptor protein of NLRP3) were not changed (Fig. 2C). Notably, dicoumarol could reduce
the elevated expression levels of NLRP3 (0.98) and cleaved-GSDMD
(0.94). To investigate whether the effect of dicoumarol was at the
transcriptional level, the mRNA expression levels of Nlrp3
were assessed. However, various concentrations of dicoumarol did
not inhibit the mRNA expression levels of Nlrp3; the
relative expression levels of Nlrp3 were 1.00, 0.96 and 0.91
in response to 12.5, 25 and 50 µM dicoumarol, respectively
(Fig. 2D). In summary, dicoumarol
could interact with NLRP3 to enhance its thermostability, inhibit
its expression and ultimately block cell apoptosis.
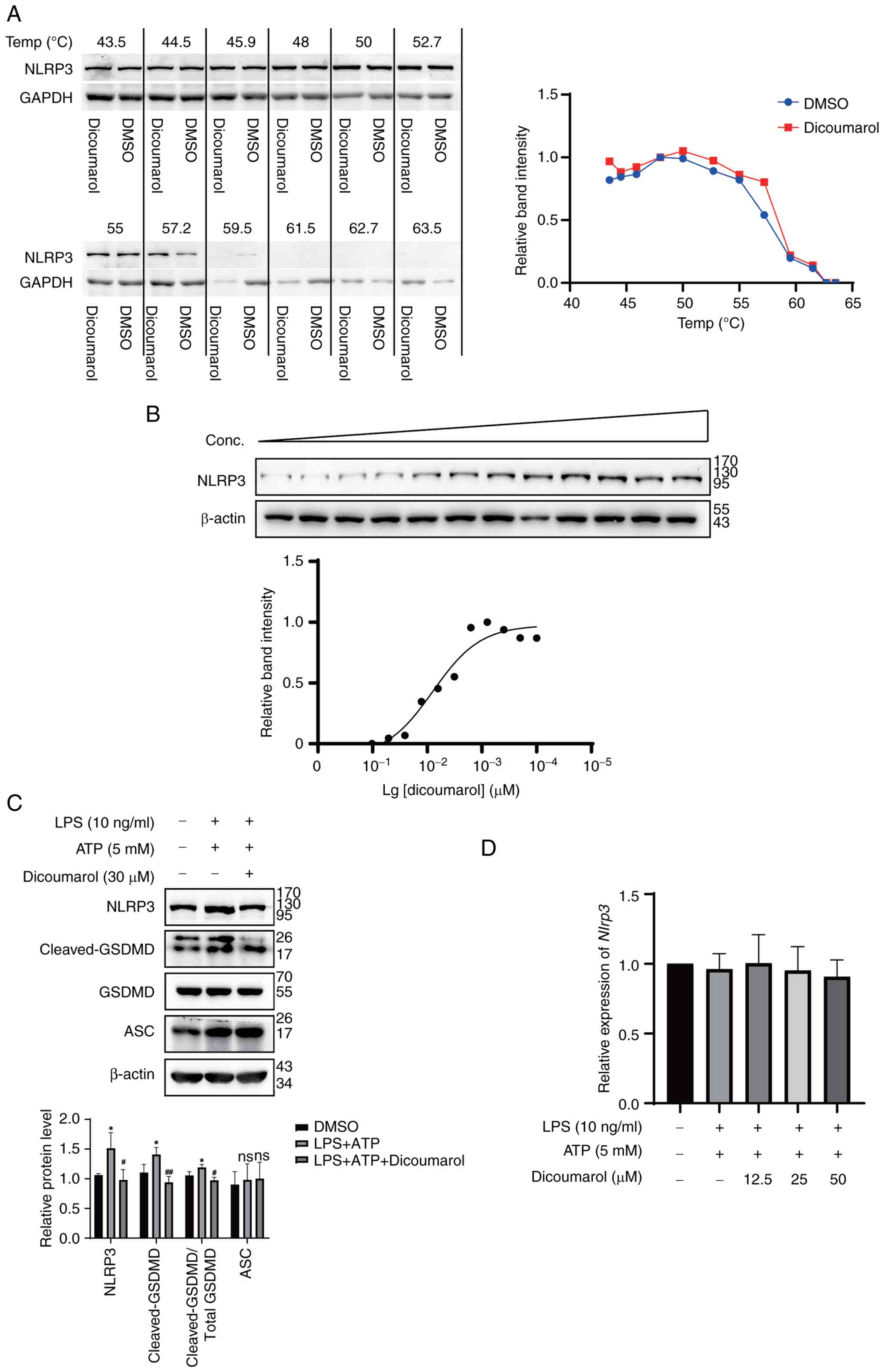 | Figure 2.Dicoumarol binds to NLRP3 to inhibit
its expression. (A) FLSs were treated with dicoumarol (30 µM) for 1
h. After shocking at the indicated temperatures and freeze-thaw
cycles, the thermostability of NLRP3 was determined by western blot
analysis. (B) FLSs were treated with different concentrations of
dicoumarol for 1 h. NLRP3 expression was detected by western blot
analysis. (C and D) FLSs were initially stimulated with LPS (10
ng/ml) for 12 h, followed by a 3-h incubation with ATP (5 mM), and
were subsequently treated with dicoumarol (30 µM) for an additional
12 h. (C) Western blot analysis of NLRP3, GSDMD, cleaved-GSDMD and
ASC. (D) Quantification of mRNA expression levels of Nlrp3.
*P<0.05 vs. control group; #P<0.05,
##P<0.01 vs. LPS + ATP group. ASC, apoptosis-related
speckle-like protein; FLS, fibroblast-like synoviocyte; GSDMD,
gasdermin-D; LPS, lipopolysaccharide; NLRP3, NOD-like receptor
protein 3; ns, not significant. |
Dicoumarol promotes NLRP3 degradation
through the ubiquitin-proteasome system
NF-κB serves an important role in the response to
inflammatory stress and the activation of NLRP3 (13,39).
Since coumarin compounds often inhibit NF-κB signaling, the present
study aimed to determine whether dicoumarol could inhibit NLRP3
expression by blocking the NF-κB pathway. The results revealed that
dicoumarol could not suppress the nuclear translocation of NF-κB
p65, which was induced by combination of LPS and ATP (Fig. 3A). Subsequently, the present study
aimed to determine whether dicoumarol inhibited NLRP3 expression by
promoting its degradation. Thus, FLSs were treated with CHX, a
protein synthesis inhibitor, at the specified time points with DMSO
or dicoumarol. In the control group, the protein expression levels
of NLRP3 started to markedly decrease at 12 h, whereas in the
dicoumarol-treated group, the protein expression levels of NLRP3
obviously decreased at 2 h (Fig.
3B). Dicoumarol could reduce the protein half-life of NLRP3
from 26.1 to 4.3 h, suggesting that dicoumarol promoted the
degradation of NLRP3. To reveal the detailed ways in which
dicoumarol promoted NLRP3 degradation, FLSs were treated with
MG132, a proteasome inhibitor, to block the proteasomal degradation
pathway. The western blot analysis showed that the expression
levels of NLRP3 (1.90) in the LPS + ATP-treated group were
increased compared with those (1.16) in the control group, whereas
dicoumarol decreased the expression to 0. 98 (Fig. 3C). When MG132 was added to block
the proteasomal degradation pathway, dicoumarol could not further
decrease the NLRP3 expression that was elevated by LPS and ATP.
Subsequently, FLSs were treated with NH4Cl, a lysosomal
inhibitor, to block the lysosomal degradation pathway. Western
blotting showed that the expression levels of NLRP3 (1.96) in the
LPS + ATP-treated group were increased compared with those (1.36)
in the control group, and dicoumarol decreased the expression to
1.06 (Fig. 3D). When
NH4Cl was added to block the proteasomal degradation
pathway, there was no impact on the aforementioned results. NLRP3
expression (1.77) in the LPS + ATP-treated group was still
increased compared with that (1.16) in the control group, and
dicoumarol decreased the expression to 1.04. Therefore, the
degradation of NLRP3 was obstructed by MG132, but not
NH4Cl.
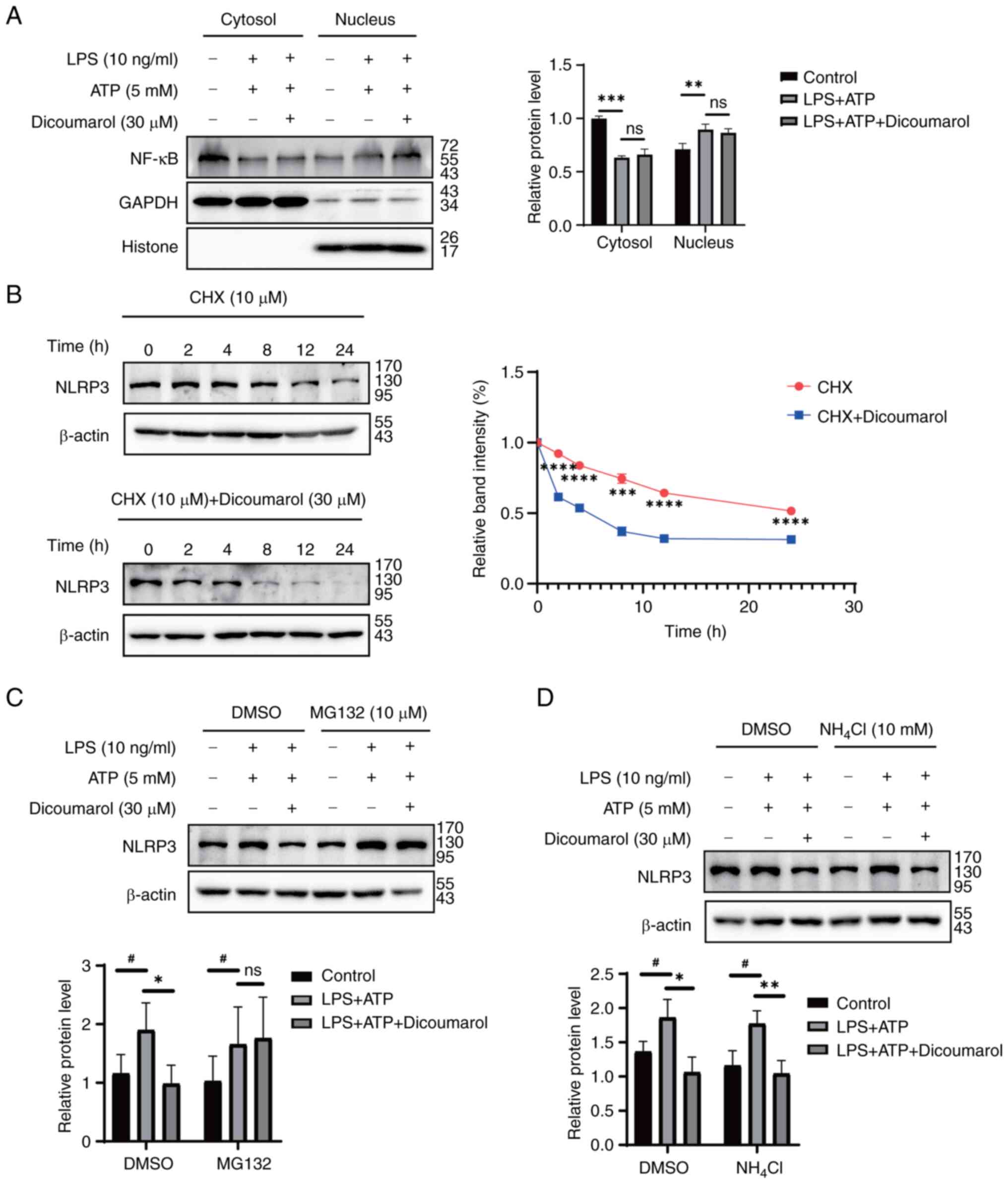 | Figure 3.Dicoumarol promotes NLRP3 degradation
through the ubiquitin-proteasome system. (A) FLSs were initially
stimulated with LPS (10 ng/ml) for 12 h, followed by a 3-h
incubation with ATP (5 mM), and were subsequently treated with
dicoumarol (30 µM) for an additional 12 h. Western blot analysis of
the expression of NF-κB in the cytoplasmic and nuclear extracts.
(B) FLSs were pretreated with dicoumarol (30 µM) or DMSO for 12 h,
followed by the addition of CHX (10 µg/ml) for various durations
(0, 2, 4, 8, 12 and 24 h), after which the cells were harvested and
western blot analysis of NLRP3 was performed. ***P<0.001,
****P<0.0001 vs. CHX + dicoumarol group. FLSs were stimulated
with LPS (10 ng/ml) for 12 h, followed by a 3-h stimulation with
ATP (5 mM). After a 12-h treatment with dicoumarol (30 µM), the
cells were treated with (C) MG132 (10 µM) or (D) NH4Cl
(10 mM) for 8 h, the cells were collected and western blot analysis
of NLRP3 was performed. *P<0.05, **P<0.01 (LPS + ATP group
vs. LPS + ATP + dicoumarol group); #P<0.05 (control
vs. LPS + ATP group). CHX, cycloheximide; FLS, fibroblast-like
synoviocyte; LPS, lipopolysaccharide; NLRP3, NOD-like receptor
protein 3; ns, not significant. |
Dicoumarol relieves synovitis induced
by MIA
To study the in vivo effect of dicoumarol on
KOA, the rat model of MIA-induced KOA was used. On day 28, the knee
joint diameter in the control group was 9.04 mm and the knee joint
diameter in the model group was 11.03 mm, which was significantly
larger than that in the control group (Fig. 4A). Dicoumarol significantly reduced
the knee joint diameter (9.93 mm) compared with that in the KOA
group (Fig. 4A). Moreover, the
anatomical characteristics and pathological sections of synovial
tissue were observed to assess synovial fibrosis in rats. As
determined by H&E and Sirius Red staining, intima formation,
resident cell proliferation and inflammatory infiltration were
reduced in dicoumarol-treated rats (Fig. 4B). Subsequently, synovial tissues
were collected and prepared for western blotting and
immunohistochemistry. Western blot analysis showed that the
expression levels of IL-β (1.57) and IL-18 (1.22) were elevated in
the model group compared with the expression of IL-β (0.45) and
IL-18 (0.53) in the vehicle group, and the expression could be
decreased to 0.49 and 0.44 by dicoumarol, respectively (Fig. 4C). In addition, western blot
analysis showed that the expression levels of NLRP3 (2.62), TIMP1
(2.19) and COL1A1 (1.26) were significantly elevated in the model
group compared with the expression of NLRP3 (0.54), TIMP1 (0.84)
and COL1A1 (0.34) in the vehicle group; however, their expression
could be suppressed by dicoumarol, and the protein expression
levels of NLRP3, TIMP1 and COL1A1 decreased to 0.59, 0.92 and 0.31,
respectively (Fig. 4D).
Furthermore, the protein expression levels of NLRP3, TGF-β and
IL-1β in the synovium of rats were measured by immunohistochemistry
(Fig. 4E). Similarly, dicoumarol
reduced the expression levels of NLRP3 in the synovium of rats
compared with those in the KOA group, which resulted in reduced
protein levels of the fibrosis-related biomarker TGF-β and the
inflammation-related biomarker IL-1β.
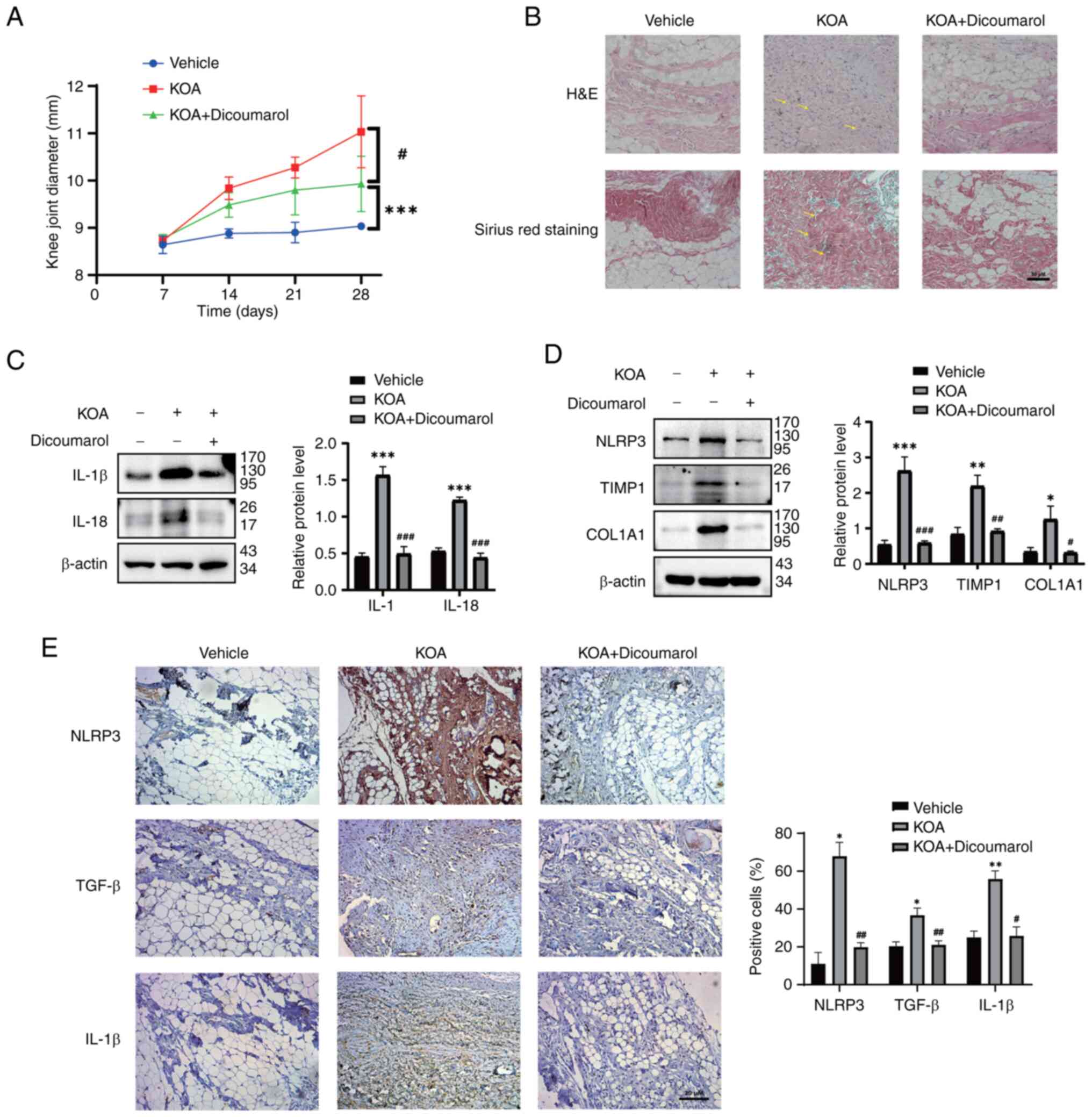 | Figure 4.Dicoumarol relieves synovitis induced
by MIA. (A-E) Rats were divided into the vehicle group (n=7), KOA
group (n=7) and KOA + dicoumarol group (n=7). After the rat KOA
model was established via the injection of a suspension of MIA, the
rats were treated with normal saline or 10 mg/kg dicoumarol every
day. (A) Diameters of right knees were evaluated to assess the
severity of synovial fibrosis. #P<0.05 vs. control
group. ***P<0.01 compared with KOA group. (B) Anatomical changes
of each group. Representative synovial tissues of each group
stained with H&E or Sirius Red. Scale bar, 50 µm. The lesion
area is indicated by yellow arrows. Expression levels of (C) IL-1β
and IL-18, and (D) NLRP3, TIMP1 and COL1A1 of synovial tissues in
each group were detected. (E) Immunohistochemical staining of
NLRP3, TGF-β and IL-1β in synovial tissues of each group. Scale
bar, 20 µm. *P<0.05, **P<0.01, ***P<0.001 vs. normal
group; #P<0.05, ##P<0.01,
###P<0.001 vs. KOA group. H&E, hematoxylin and
eosin; IL, interleukin; KOA, knee osteoarthritis; MIA, monosodium
iodoacetate; NLRP3, NOD-like receptor protein 3. |
Discussion
NLRP3 is a polyprotein oligomer composed of
caspase-1, ASC and NLRP3. After activation, NLRP3 interacts with
ASC to bridge NLRP3 to procaspase-1, which activates caspase-1
(40). Activated caspase-1 cleaves
the original forms of IL-1β and IL-18 into mature and active forms
(41). Notably, IL-1β and IL-18
are key inflammatory factors in the pathological process of
synovitis. The results of the present study demonstrated that
dicoumarol could protect against inflammasome-induced cell death
through decreasing NLRP3 expression, which consistently reduced the
expression of IL-1β and IL-18. Therefore, treatments that reduce
the secretion of these two inflammatory factors may be a reliable
method to treat synovial inflammation and fibrosis to delay the
progression of KOA.
KOA has become a common disorder in an increasingly
aging society (42,43). Several pathogenic factors of KOA
have been discovered; however, the pathogenesis is still unclear.
The occurrence of KOA is related to age, obesity, inflammation,
trauma and genetic factors (1,5). The
main features of KOA include synovitis, cartilage destruction and
osteophyte formation, which results in a serious burden to the
daily life of patients (44–46).
Patients with severe KOA may even be permanently disabled,
negatively affecting the physical and mental health of these
patients, which can produce a burden on the health system and
social economy. In the present study, it was revealed that
dicoumarol inhibited the progression of KOA through improving
inflammation and fibrosis. To the best of our knowledge, this
effect has not been previously reported in the study of dicoumarol.
Due to the key role of NLRP3 in inflammatory cascade amplification,
inhibiting NLRP3 expression has become the focus of
anti-inflammatory therapy. The NLRP3 inflammasome is closely
related to the pathogenesis of KOA, and is involved in cartilage
destruction and synovitis in KOA (30). Various herbal extracts have been
shown to block activation of the NLRP3 inflammasome with fewer side
effects (47). For example,
coumarins have been reported to exert anti-inflammatory effects by
inhibiting NLRP3 activation (26,48).
However, fewer compounds could alleviate inflammation by inhibiting
NLRP3. The present study found that dicoumarol directly interacted
with NLRP3 in FLSs, which offers novel compounds for specific
inhibitors of NLRP3. However, the affinity between dicoumarol and
NLRP3 was not further tested; therefore, our future studies aim to
advance the related research and optimize the structure of
dicoumarol.
The present study demonstrated that dicoumarol
inhibited the protein expression levels of NLRP3 and cleaved-GSDMD
in FLSs, rather than mRNA expression, suggesting that dicoumarol
suppressed NLRP3 expression at the post-transcriptional level.
Hence, the protein degradation pathway of NLRP3 was examined.
MG132, but not NH4Cl, could block the degradation of
NLRP3 induced by dicoumarol, which suggested that the
ubiquitin-proteasome system was involved in the process of
dicoumarol-decreased NLRP3 expression. The main pathological
features of KOA include cartilage matrix synovitis and chondrocyte
reduction (49). In in vivo
experiments, it was revealed that dicoumarol reduced the knee joint
diameter during the progression of KOA. Meanwhile, dicoumarol
inhibited collagen deposition and inflammatory cell infiltration.
Under pathological conditions, inflammatory cells can infiltrate
the joint synovium, and then release a large number of inflammatory
factors, chemokines and proteases, which can also be reduced by
dicoumarol in vivo. Although the inhibitory effect of
dicoumarol on OA is clearly defined in the present study, the
potential toxicity and pharmacokinetic profile have not been
studied, which are important factors for the application of
dicoumarol in clinical treatments.
Collectively, the present study demonstrated that
dicoumarol can alleviate the development of KOA in vivo and
in vitro. Furthermore, it was confirmed that dicoumarol can
interact with NLRP3 and degrade NLRP3 through the
ubiquitin-proteasome pathway. However, the present study has some
limitations. The mechanism by which dicoumarol inhibit fibrosis
needs to be further investigated. In addition, the specific effects
of dicoumarol in humans are still uncertain and need to be studied
further. In future studies, we aim to use more animal models to
further confirm the pharmacological effects of dicoumarol from the
aspects of in vitro cell and animal experiments, and provide
more reliable experimental data for its clinical application.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from the Youth Project of
Wuxi Health Commission (grant no. Q201916).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WG designed experiments, and wrote and edited the
manuscript. XZ and QW performed cell studies. JM and PJ performed
animal studies. JC directed this project and analyzed the
experimental data. WG and JC confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed according to
the National Institute of Health Animal Care and Use Guidelines.
The protocol was approved by the committee for the Ethics of Animal
Experiments, Wuxi Hospital of Traditional Chinese Medicine
(approval no. SWJWQNXM2020033102).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lv Z, Yang YX, Li J, Fei Y, Guo H, Sun Z,
Lu J, Xu X, Jiang Q, Ikegawa S and Shi D: Molecular classification
of knee osteoarthritis. Front Cell Dev Biol. 9:7255682021.
View Article : Google Scholar
|
|
2
|
Mintarjo JA, Poerwanto E and Tedyanto EH:
Current non-surgical management of knee osteoarthritis. Cureus.
15:e409662023.PubMed/NCBI
|
|
3
|
Onuora S: Osteoarthritis: OA chondrocytes
made senescent by genomic DNA damage. Nat Rev Rheumatol. 8:5022012.
View Article : Google Scholar
|
|
4
|
Dell'Isola A, Allan R, Smith SL, Marreiros
SS and Steultjens M: Identification of clinical phenotypes in knee
osteoarthritis: A systematic review of the literature. BMC
Musculoskelet Disord. 17:4252016. View Article : Google Scholar
|
|
5
|
Du X, Liu ZY, Tao XX, Mei YL, Zhou DQ,
Cheng K, Gao SL, Shi HY, Song C and Zhang XM: Research progress on
the pathogenesis of knee osteoarthritis. Orthop Surg. 15:2213–2224.
2023. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peloso P, Chen W, Lin HL, Gates D, Straus
W and Moore R: (363) Pain improvement in osteoarthritis (OA)
predicts improved functioning. J Pain. 15 (Suppl):S662014.
View Article : Google Scholar
|
|
7
|
Li R, Sun J, Hu H, Zhang Q, Sun R, Zhou S,
Zhang H and Fang J: Research trends of acupuncture therapy on knee
osteoarthritis from 2010 to 2019: A bibliometric analysis. J Pain
Res. 13:1901–1913. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Georgiev T and Angelov AK: Modifiable risk
factors in knee osteoarthritis: Treatment implications. Rheumatol
Int. 39:1145–1157. 2019. View Article : Google Scholar
|
|
9
|
Zhang J, Fan F, Liu A, Zhang C, Li Q,
Zhang C, He F and Shang M: Icariin: A potential molecule for
treatment of knee osteoarthritis. Front Pharmacol. 13:8118082022.
View Article : Google Scholar
|
|
10
|
Yang X, Thudium CS, Bay-Jensen AC, Karsdal
MA, van Santen J, Arden NK, Perry TA and Kluzek S: Association
between markers of synovial inflammation, matrix turnover and
symptoms in knee osteoarthritis: A cross-sectional study. Cells.
10:18262021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Mei W, Huang Z, Zhang L, Zhang L, Xu
B, Shi X, Xiao Y, Ma Z, Liao T, et al: Casticin suppresses
monoiodoacetic acid-induced knee osteoarthritis through inhibiting
HIF-1α/NLRP3 inflammasome signaling. Int Immunopharmacol.
86:1067452020. View Article : Google Scholar
|
|
12
|
Tan Q, Cai Z, Li J, Li J, Xiang H, Li B
and Cai G: Imaging study on acupuncture inhibiting inflammation and
bone destruction in knee osteoarthritis induced by monosodium
iodoacetate in rat model. J Pain Res. 15:93–103. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jo EK, Kim JK, Shin DM and Sasakawa C:
Molecular mechanisms regulating NLRP3 inflammasome activation. Cell
Mol Immunol. 13:148–159. 2016. View Article : Google Scholar
|
|
14
|
Zhang L, Li X, Zhang H, Huang Z, Zhang N,
Zhang L, Xing R and Wang P: Agnuside alleviates synovitis and
fibrosis in knee osteoarthritis through the inhibition of HIF-1α
and NLRP3 inflammasome. Mediators Inflamm. 2021:55346142021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma BR and Kanneganti TD: NLRP3
inflammasome in cancer and metabolic diseases. Nat Immunol.
22:550–559. 2021. View Article : Google Scholar
|
|
16
|
Xu J and Núñez G: The NLRP3 inflammasome:
Activation and regulation. Trends Biochem Sci. 48:331–344. 2023.
View Article : Google Scholar
|
|
17
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaufmann FN, Costa AP, Ghisleni G, Diaz
AP, Rodrigues ALS, Peluffo H and Kaster MP: NLRP3
inflammasome-driven pathways in depression: Clinical and
preclinical findings. Brain Behav Immun. 64:367–383. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-κB and the
NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017. View Article : Google Scholar
|
|
20
|
Conway R and McCarthy GM:
Calcium-containing crystals and osteoarthritis: An unhealthy
alliance. Curr Rheumatol Rep. 20:132018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar
|
|
22
|
Ding L, Liao T, Yang N, Wei Y, Xing R, Wu
P, Li X, Mao J and Wang P: Chrysin ameliorates synovitis and
fibrosis of osteoarthritic fibroblast-like synoviocytes in rats
through PERK/TXNIP/NLRP3 signaling. Front Pharmacol.
14:11702432023. View Article : Google Scholar
|
|
23
|
Zhao LR, Xing RL, Wang PM, Zhang NS, Yin
SJ, Li XC and Zhang L: NLRP1 and NLRP3 inflammasomes mediate
LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol Med Rep.
17:5463–5469. 2018.PubMed/NCBI
|
|
24
|
Ma Z, Huang Z, Zhang L, Li X, Xu B, Xiao
Y, Shi X, Zhang H, Liao T and Wang P: Vanillic acid reduces
pain-related behavior in knee osteoarthritis rats through the
inhibition of NLRP3 inflammasome-related synovitis. Front
Pharmacol. 11:5990222021. View Article : Google Scholar
|
|
25
|
Sun C, Zhao W, Wang X, Sun Y and Chen X: A
pharmacological review of dicoumarol: An old natural anticoagulant
agent. Pharmacol Res. 160:1051932020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chkhikvishvili I, Mamniashvili T, Gogia N,
Enukidze M, Machavariani M and Sanikidze T: Antioxidant,
anti-inflammatory activity of georgian leguminous crops cultures.
Georgian Med News. 147–153. 2017.
|
|
27
|
Timson DJ: Dicoumarol: A drug which hits
at least two very different targets in vitamin K metabolism. Curr
Drug Targets. 18:500–510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
PERONNET, . Anticoagulants, heparin and
dicoumarol. Fr Med. 11:3–8. 1948.(In French).
|
|
29
|
Council NJP: Guide for the Care and Use of
Laboratory Animals. 8th edition. National Academies Press;
Washington, DC: pp. 963–965. 2010
|
|
30
|
Liao T, Ding L, Wu P, Zhang L, Li X, Xu B,
Zhang H, Ma Z, Xiao Y and Wang P: Chrysin attenuates the NLRP3
inflammasome cascade to reduce synovitis and pain in KOA rats. Drug
Des Devel Ther. 14:3015–3027. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng ST, Hu JL, Ren JH, Yu HB, Zhong S,
Wai Wong VK, Kwan Law BY, Chen WX, Xu HM, Zhang ZZ, et al:
Dicoumarol, an NQO1 inhibitor, blocks cccDNA transcription by
promoting degradation of HBx. J Hepatol. 74:522–534. 2021.
View Article : Google Scholar
|
|
32
|
Shi J, Zhao W, Ying H, Zhang Y, Du J, Chen
S, Li J and Shen B: Estradiol inhibits NLRP3 inflammasome in
fibroblast-like synoviocytes activated by lipopolysaccharide and
adenosine triphosphate. Int J Rheum Dis. 21:2002–2010. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Xu J, Zhou S, Yao F, Zhang R, You
W, Dai J, Yu K, Zhang Y, Baheti T, et al: Endothelial DGKG promotes
tumor angiogenesis and immune evasion in hepatocellular carcinoma.
J Hepatol. 80:82–98. 2024. View Article : Google Scholar
|
|
34
|
Nàger M, Sallán MC, Visa A, Pushparaj C,
Santacana M, Macià A, Yeramian A, Cantí C and Herreros J:
Inhibition of WNT-CTNNB1 signaling upregulates SQSTM1 and
sensitizes glioblastoma cells to autophagy blockers. Autophagy.
14:619–636. 2018. View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bhattaram P and Chandrasekharan U: The
joint synovium: A critical determinant of articular cartilage fate
in inflammatory joint diseases. Semin Cell Dev Biol. 62:86–93.
2017. View Article : Google Scholar
|
|
37
|
Toldo S, Mezzaroma E, Buckley LF, Potere
N, Di Nisio M, Biondi-Zoccai G, Van Tassell BW and Abbate A:
Targeting the NLRP3 inflammasome in cardiovascular diseases.
Pharmacol Ther. 236:1080532022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shao BZ, Xu ZQ, Han BZ, Su DF and Liu C:
NLRP3 inflammasome and its inhibitors: A review. Front Pharmacol.
6:2622015. View Article : Google Scholar
|
|
39
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar
|
|
40
|
Lu A, Magupalli VG, Ruan J, Yin Q,
Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H and Egelman
EH: Unified polymerization mechanism for the assembly of
ASC-dependent inflammasomes. Cell. 156:1193–1206. 2014. View Article : Google Scholar
|
|
41
|
Elliott EI and Sutterwala FS: Initiation
and perpetuation of NLRP3 inflammasome activation and assembly.
Immunol Rev. 265:35–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shimizu H, Shimoura K, Iijima H, Suzuki Y
and Aoyama T: Functional manifestations of early knee
osteoarthritis: A systematic review and meta-analysis. Clin
Rheumatol. 41:2625–2634. 2022. View Article : Google Scholar
|
|
43
|
Rannou F and Poiraudeau S:
Non-pharmacological approaches for the treatment of osteoarthritis.
Best Pract Res Clin Rheumatol. 24:93–106. 2010. View Article : Google Scholar
|
|
44
|
Geng R, Li J, Yu C, Zhang C, Chen F, Chen
J, Ni H, Wang J, Kang K, Wei Z, et al: Knee osteoarthritis: Current
status and research progress in treatment (Review). Exp Ther Med.
26:4812023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Okada K, Yamaguchi S, Sato Y, Enomoto T,
Ogawa Y, Ohtori S, Tahara M and Sasho T: Comparison of meniscal
extrusion and osteophyte formation at the intercondylar notch as a
predictive biomarker for incidence of knee osteoarthritis-data from
the osteoarthritis initiative. J Orthop Sci. 24:121–127. 2019.
View Article : Google Scholar
|
|
46
|
Whittaker JL, Runhaar J, Bierma-Zeinstra S
and Roos EM: A lifespan approach to osteoarthritis prevention.
Osteoarthritis Cartilage. 29:1638–1653. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang M, Liu L, Zhang CS, Liao Z, Jing X,
Fishers M, Zhao L, Xu X and Li B: Mechanism of traditional Chinese
medicine in treating knee osteoarthritis. J Pain Res. 13:1421–1429.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Place DE and Kanneganti TD: Recent
advances in inflammasome biology. Curr Opin Immunol. 50:32–38.
2018. View Article : Google Scholar
|
|
49
|
Samadfam R, Chouinard L, Norton K and
Smith S: Pain assessment in monosodium iodoacetate (MIA)-induced
osteoarthritis (OA) model. J Pharmacol Toxicol Methods. 68:e392013.
View Article : Google Scholar
|


















