Introduction
Hyperoxia therapy is often used to treat premature
infants and children with acute hypoxic respiratory failure, and
although it may improve their survival rate, it can also easily
cause acute and chronic lung injury (1,2).
Hyperoxia-induced lung injury (HILI) is a common neonatal emergency
and a major cause of severe permanent lung injury and death
(3,4). However, the exact pathogenesis of
HILI remains unclear, thus hindering the development of effective
treatments. HILI primarily affects alveolar epithelial cells,
especially alveolar type II epithelial cells (AECII), a subgroup
that functions as stem cells for growth and damage repair (5). Therefore, it is of significant
interest and clinical value to study the regeneration and repair of
AECII and to explore the underlying pathological mechanisms of
early intervention strategies for HILI.
Calcitonin gene-related peptide (CGRP) is a
neuropeptide transmitter that is synthesized primarily by neurons,
but AECII can also synthesize CGRP (6–9).
CGRP exerts its biological functions by binding to its specific
CGRP receptor (CGRPR), which is a G protein-coupled receptor (GPCR)
(10). CGRP is not only a potent
vasodilator (11), but it is also
involved in the occurrence and development of pain (12,13),
inflammation (14,15), cardiovascular disease (16,17)
and wound healing (18). It has
been reported that CGRP can alleviate lung injury by inhibiting
ischemia-reperfusion and lipopolysaccharide-induced inflammation
(19–21), but its role in the development and
progression of HILI remains unclear. Previous studies by the
authors revealed that CGRP plays an important role in repairing
damage caused by HILI by inhibiting apoptosis and promoting cell
proliferation in AECII (22–25),
but the underlying molecular mechanisms are largely unclear.
Therefore, further investigations on the role and mechanisms of
CGRP in the repair of damage caused by HILI may assist in the
development of effective strategies for the prevention and
treatment of HILI.
Transient receptor potential vanilloid 1 (TRPV1) is
a non-selective cationic channel protein that can be activated by
physical and chemical stimuli, such as traction stimulation (for
example, lung ventilation) or capsaicin, to facilitate the entry of
Ca2+-dominant cations, thus participating in a variety
of physiological or pathological processes (26,27).
Although several studies have revealed that activation of TRPV1
channels promotes Ca2+-dependent CGRP production and
release from sensory endings (28,29),
whether CGRP plays a role by inversely regulating TRPV1 channels is
unknown, to the best of the authors' knowledge. Furthermore,
although TRPV1 channels expressed in lung epithelial cells play a
protective role in ischemia-reperfusion injury (19,30),
its role in AECII has not been reported, to the best of the authors
knowledge, let alone its potential involvement in the pathogenesis
of HILI.
Therefore, the aim of the present study was to
explore the protective effects of CGRP against HILI and its
underlying molecular mechanisms, focusing on its downstream
signaling pathway. It was revealed that CGRP exerted a protective
role in HILI via a novel and unique CGRPR/TRPV1/Ca2+
signaling axis, highlighting a potential target for the prevention
and treatment of HILI.
Materials and methods
Cell culture and induction of
hyperoxia
Human alveolar A549 cells were purchased from
Haixing Biological Technology Co., Ltd. (cat. no. TCH-C116). Cells
were cultured in DMEM-HIGH Glucose medium supplemented with 10%
fetal bovine serum (AusGeneX Pty Ltd.), 100 U/ml penicillin and 0.1
mg/ml streptomycin (Beyotime Institute of Biotechnology). The cells
were plated in 6- or 96-well plates for subsequent experiments.
When the cell confluence reached 50–60%, the cells were treated
with 10 nM CGRP (cat. no. HY-P1548A; MedChemExpress) or 1 µM
capsaicin (cat. no. HY-10448; MedChemExpress), and then stimulated
with hyperoxia. Cells were pretreated with CGRP8-37
(cat. no. HY-P1014), SB-705498 (cat. no. HY-10633), BAPTA-AM (cat.
no. HY-100545), U-73122 (cat. no. HY-13419), U-73343 (cat. no.
HY-108630), or Go6976 (cat. no. HY-10183; all from MedChemExpress)
for 2 h before CGRP treatment. Hyperoxia exposure was defined as
cells cultured in a closed oxygen chamber (Billups-Rothenberg,
Inc.) supplied with 95 O2 and 5% CO2 for 24 h
(31). The cells of the control
group were cultured under normal air conditions. All cells were
cultured at a constant temperature of 37°C and supplied with 5%
CO2.
Lentivirus infection
Lentivirus infection was performed as previously
described (32). Lentivirus was
purchased from OBiO Technology Corp., Ltd. The TRPV1 shRNA and shNC
primer sequences are shown in Table
I. A549 cells were infected with lentivirus according to the
manufacturer's protocol, and stable cells were screened with
puromycin 72 h later.
 | Table I.Primer sequences for TRPV1 shRNA in
A549 cells. |
Table I.
Primer sequences for TRPV1 shRNA in
A549 cells.
| Gene name | Primer sequence
(5′→3′) |
|---|
| TRPV1-shRNA-1 | F:
CCGGCCGTTTCATGTTTGTCTACATCTCGAGATGTAGACAAACATGAAACGGTTTTTTG |
|
| R:
AATTCAAAAAACCGTTTCATGTTTGTCTACATCTCGAGATGTAGACAAACATGAAACGG |
| TRPV1-shRNA-2 | F:
CCGGGAAGTTTATCTGCGACAGTTTCTCGAGAAACTGTCGCAGATAAACTTCTTTTTTG |
|
| R:
AATTCAAAAAAGAAGTTTATCTGCGACAGTTTCTCGAGAAACTGTCGCAGATAAACTTC |
| TRPV1-shRNA-3 | F:
CCGGGCGCATCTTCTACTTCAACTTCTCGAGAAGTTGAAGTAGAAGATGCGCTTTTTTG |
|
| R:
AATTCAAAAAAGCGCATCTTCTACTTCAACTTCTCGAGAAGTTGAAGTAGAAGATGCGC |
| TRPV1-shNC | F:
CCGGCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTTG |
|
| R:
AATTCAAAAAACCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG |
Immunofluorescence assay
A549 cells were plated on 24-well coverslips at a
density of 5×104/ml. When the confluence reached 60–70%,
the cells were fixed with 4% paraformaldehyde at room temperature
for 15 min, and washed with PBS three times. Cells were blocked
with 1% bovine serum albumin (Beyotime Institute of Biotechnology)
at room temperature for 30 min, then incubated with anti-TRPV1
antibody (1:200; cat. no. ACC-030; Alomone Labs) for 2 h at 4°C or
anti-CGRPR antibody (1:200; cat. no. A8533; ABclonal) overnight at
4°C. After washing with PBS, cells were incubated with Alexa Fluor
488-labeled anti-rabbit secondary antibody (1:500; cat. no. A0423;
Beyotime Institute of Biotechnology) at room temperature for 1 h.
Cell nuclei were counterstained with 5 µg/ml DAPI at room
temperature for 5 min, and images were captured using a confocal
microscope (Nikon Corporation).
Cell counting kit-8 (CCK-8)
proliferation assay
Cell proliferation assay was performed as previously
described (31,32). Cell proliferation was measured
using a CCK-8 assay (cat. no. HY-K0301; MedChemExpress). A549 cells
were plated in 96-well plates at a density of 4×104/ml.
After 24 h, the media was replaced, the treatments were applied,
and cells were cultured in either normal air or hyperoxic
conditions for 24 h. Next, 100 µl medium containing 10% CCK-8
solution was added to each well and incubated for 1–2 h at 37°C.
The optical density was measured at 450 nm using a spectrometer.
Cell viability was calculated using the absorbance method to
express cell proliferation (33).
Apoptosis assay
Apoptosis assay was performed as previously
described (31). Apoptosis
detection kits were purchased from BD Biosciences (cat. no. 556547)
or Shanghai Yeasen Biotechnology Co., Ltd. (cat. no. 40302ES).
Cells were plated in 6-well plates for 24 h at 37°C and then
treated with the various drugs for another 24 h. The cells were
digested using EDTA-free trypsin for 2 min, transferred to flow
tubes, centrifuge at 300 × g for 5 min at room temperature, and
washed twice with PBS. Subsequently, cells were resuspended in 100
µl Annexin V Binding Buffer, and 5 µl FITC Annexin V and 5 µl PI
were added. Then, the cells were gently rocked and incubated for 15
min in the dark at room temperature, after which, 400 µl Binding
Buffer was added to the cell suspension. Apoptosis was measured
using a flow cytometer (Beckman Coulter Life, Inc.) and analyzed
using FlowJo software (version 10.8.1; FlowJo LLC). Apoptosis cells
(%)=Q2 (late apoptotic cells) + Q3 (early apoptotic cells).
Calcium measurement
A549 cells were plated on coverslips and incubated
with 5 µM Fura-2 AM (cat. no. F1221; Invitrogen; Thermo Fisher
Scientific, Inc.) in physiological salt solution (PSS) at 37°C for
60 min, then washed with PSS with or without antagonist for 20 min.
Cells on coverslips were then mounted in a standard perfusion
chamber on a Nikon microscope table. The fluorescence ratio of
Fura-2 (F340/380) was tracked over time at an excitation wavelength
of 340 or 380 nm and captured using an intensified CCD camera
(Hamamatsu Photonics K.K.) and a MetaFluor Imaging system (Version
7.10.4.407; Molecular Devices, LLC). The ratio of F340/380
represented the intracellular Ca2+ levels and was
quantified using Δ(F340/380), that is, the difference between the
baseline and maximum values after stimulation. The PSS for
Ca2+ measurement consisted of the following: 140 mM
NaCl, 5 mM KCl, 2 mM CaCl2, 10 mM HEPES and 10 mM
glucose at pH 7.3. The osmolality of the solution was ~300
mOsmol/kg H2O.
Electrophysiological recordings
Whole-cell membrane currents of A549 cells were
recorded with an EPC 10 USB Double Patch Clamp Amplifier (HEKA).
Data were digitized at 10 kHz and filtered at 5 kHz. The cell
voltage was fixed at 0 mV to inactivate voltage-gated calcium and
sodium channels, and a 100 ms linear ramp protocol (−100 to 100 mV)
was applied every 2 sec. The amplitude of the current was recorded.
The extracellular buffer contained 140 mM NaCl, 5 mM KCl, 2 mM
CaCl2, 2 mM MgCl2 and 10 mM HEPES at pH 7.3.
The pipette solution contained 140 mM CsCl, 5 mM EGTA, 3 mM Mg-ATP,
and 10 mM HEPES at pH 7.3. The osmolality of all the solutions was
~300 mOsmol/kg H2O.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed as previously described
(34). Total RNA was extracted
using an RNA isolation kit (cat. no. R0027; Beyotime Institute of
Biotechnology). Then, cDNA synthesis was performed using
PrimeScript™ RT MasterMix (cat. no. RR036A; Takara Bio,
Inc.) according to the manufacturer's instructions. Finally, using
1,000 ng cDNA as the template, SYBR Green qPCR MasterMix (cat. no.
HY-K0523; MedChemExpress) was added in a 10 µl reaction system for
qPCR. The amplification procedure was as follows: Heating at 95°C
for 5 min to activate the hot-start DNA polymerase, followed by 40
cycles of 10 sec at 95°C and 30 sec at 60°C. The data were
quantified using the 2−ΔΔCq relative quantification
method (35), and β-actin was used
as the internal control. The primers were designed and purchased
from Sangon Biotech Co., Ltd. The primer sequences are shown in
Table II.
 | Table II.Primer sequences for quantitative
PCR. |
Table II.
Primer sequences for quantitative
PCR.
| Gene name | Primer sequence
(5′→3′) |
|---|
| CGRPR | F:
ATGGAGAAAAAGTGTACCCTGT |
|
| R:
TGAATGGGGTCTTGCATAATCT |
| TRPV1 | F:
TGGTATTCTCCCTGGCCTTG |
|
| R:
CTTCCCGTCTTCAATCAGCG |
| Cyclin D1 | F:
GTCCTACTTCAAATGTGTGCAG |
|
| R:
GGGATGGTCTCCTTCATCTTAG |
| PCNA | F:
TAATTTCCTGTGCAAAAGACGG |
|
| R:
AAGAAGTTCAGGTACCTCAGTG |
| Bcl-2 | F:
GACTTCGCCGAGATGTCCAG |
|
| R:
GAACTCAAAGAAGGCCACAATC |
| Bax | F:
CGAACTGGACAGTAACATGGAG |
|
| R:
CAGTTTGCTGGCAAAGTAGAAA |
| β-actin | F:
CCTGGCACCCAGCACAAT |
|
| R:
GGGCCGGACTCGTCATAC |
Western blotting
RIPA Lysis Buffer (cat. no. HY-K1001;
MedChemExpress) containing 1% protease inhibitor cocktail (cat. no.
HY-K0010; MedChemExpress), 1% phosphatase inhibitor cocktail (cat.
no. HY-K0021; MedChemExpress) and 1% PMSF Solution (cat. no. ST507;
Beyotime Institute of Biotechnology) was used to extract proteins
from the pretreated cells. The protein concentration was determined
using a bicinchoninic acid assay (cat. no. P0010; Beyotime
Institute of Biotechnology), and then the total protein was mixed
with SDS-PAGE loading buffer and boiled for 10 min. Equal amounts
of 30 µg protein were loaded on a 10% SDS-PAGE gel (cat. no. PG112;
EpiZyme, Inc.) for electrophoretic separation, and transferred to
PVDF membranes (cat. no. ISEQ00010; MilliporeSigma). Blots were
blocked for 10 min at room temperature using rapid blot blocking
buffer (cat. no. P30500; New Cell & Molecular Biotech Co.,
Ltd.), and then incubated at 4°C overnight with the following
specific primary antibodies: anti-TRPV1 (1:500), anti-CGRPR
(1:500), anti-PCNA (1:1,000; cat. no. 13110; Cell Signaling
Technology, Inc.), anti-Cyclin D1 (1:1,000; cat. no. 55506; Cell
Signaling Technology, Inc.), anti-Bcl-2 (1:1,000; cat. no.
60178-1-Ig, ProteinTech Group, Inc.), anti-Bax (1:5,000; cat. no.
60267-1-Ig; ProteinTech Group, Inc.), anti-β-actin (1:1,000; cat.
no. 4970; Cell Signaling Technology, Inc.), or anti-GAPDH (1:1,000;
cat. no. 2118; Cell Signaling Technology, Inc.).
After the primary antibody was recovered, TBST
(0.05% Tween-20) was used to wash the membrane three times, 8 min
each time. Then the membranes were incubated with goat anti-rabbit
(cat. no. ZB-2301) or goat anti-mouse (cat. no. ZB-2305; ZSGB
Biotechnology, Inc.) secondary antibody for 1 h at room
temperature. The dilution ratio of the secondary antibodies was
1:5,000. Signals were visualized using enhanced chemiluminescence
reagent (MilliporeSigma) and densitometric analysis was performed
using ImageJ software (version Fiji; National Institutes of
Health).
ELISA
ELISA was performed as previously described
(34). The levels of CGRP in the
supernatant of A549 cells treated with air or hyperoxia were
detected using a human CGRP ELISA kit (cat. no. CB-E08210h; Cusabio
Technology, LLC) according to the manufacturer's protocol.
Statistical analysis
SPSS version 25.0 (IBM Corp.) was used to analyze
the data. All data are presented as the mean ± SD of at least three
repeats. The unpaired Student's t-test was used for comparison
between two groups. A one-way ANOVA was used for comparison among
multiple groups; if the variances were homogeneous, a Fisher's
least significant difference test was used for analysis; otherwise,
Dunnett's T3 analysis was used. P<0.05 was considered to
indicate a statistically significant difference.
Results
Hyperoxia downregulates CGRPR and
TRPV1 channels expression in A549 cells
Since CGRP exerts its biological effects by binding
to its specific CGRPR (10), to
confirm whether CGRP exerted its protective effects through TRPV1,
immunofluorescence was first performed to detect the expression and
localization of CGRPR and TRPV1 in A549 cells. CGRPR and TRPV1 were
expressed and were primarily located in the cytoplasm and cell
membrane (Fig. 1A). Whether
hyperoxia affects CGRP release is unknown, thus, A549 cells were
cultured either in normal air or hyperoxic conditions, after which,
the release of CGRP was detected. ELISA results revealed that there
was no difference in CGRP release between the air and hyperoxia
groups (Fig. 1B). At the same
time, the effect of hyperoxia on the expression of CGRPR and TRPV1
channels in A549 cells was examined. After incubation under
hyperoxic conditions, the mRNA and protein expression levels of
CGRPR were significantly decreased compared with cells cultured
with normal air (Fig. 1C and D).
Similarly, the mRNA and protein expression levels of TRPV1 were
also considerably reduced (Fig. 1E and
F). Therefore, these results showed that hyperoxia
downregulates the expression of CGRPR and TRPV1 channels in A549
cells without altering CGRP release.
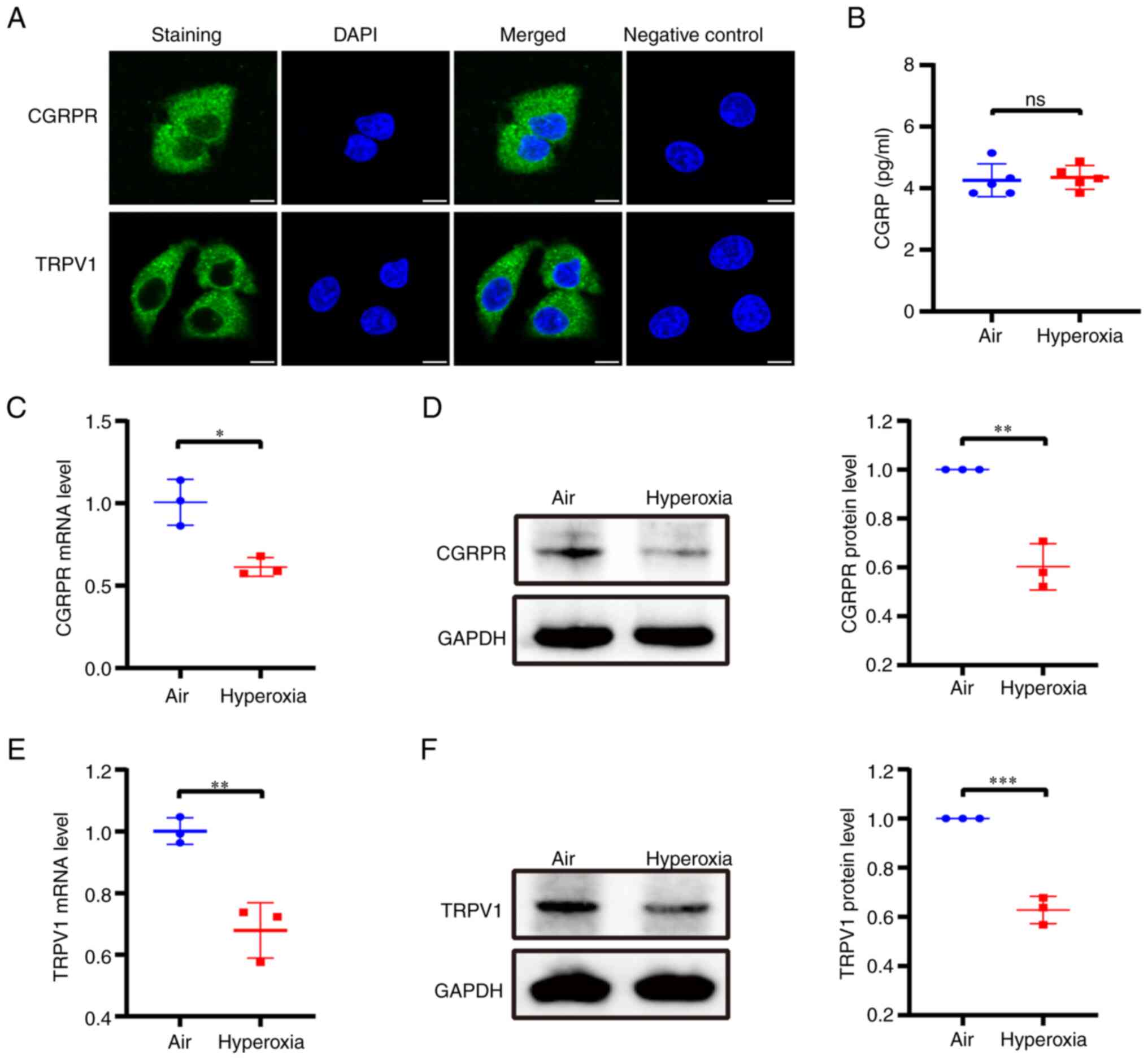 | Figure 1.Release of CGRP and expression of
CGRPR and TRPV1 in A549 cells under hyperoxic conditions. (A)
Immunofluorescence staining for CGRPR and TRPV1 in A549 cells.
Upper panel, CGRPR protein (green); lower panel, TRPV1 protein
(green), the nuclei of the cells were counterstained with DAPI
(blue). The negative control was not treated with the primary
antibody. Scale bar, 10 µm. (B) Changes in CGRP release in A549
cells cultured under hyperoxic conditions. (C and D) qPCR and
western blotting were used to determine the effects of hyperoxia on
CGRPR expression in A549 cells. (E and F) qPCR and western blotting
were used to determine the effects of hyperoxia on TRPV1 expression
in A549 cells. Data are presented as the mean ± SD of at least
three repeats. *P<0.05, **P<0.01 and ***P<0.001. CGRP,
calcitonin gene-related peptide; CGRPR, CGRP receptor; TRPV1,
transient receptor potential vanilloid 1; qPCR, quantitative PCR;
ns, not significant. |
CGRP/CGRPR and capsaicin/TRPV1 promote
cell proliferation but inhibit apoptosis under hyperoxic
conditions
Next, the effects of hyperoxia on the proliferation
and apoptosis of A549 cells were investigated. First, compared with
the normal air group, the proliferation of A549 cells was
significantly decreased in the hyperoxia group (Fig. 2A), consistent with a previous
report on HILI (36). It is well
established that capsaicin, a selective TRPV1 agonist (37,38),
induces CGRP release from nerve endings (39). Additionally, TRPV1 channels exert a
protective role in ischemia-reperfusion injury (19,30);
however, the role of TRPV1 channels in HILI is unknown. Therefore,
whether CGRP/CGRPR and capsaicin/TRPV1 exerted a protective effect
in hyperoxia-induced alveolar cell injury was investigated. Indeed,
CGRP and capsaicin enhanced cell proliferation of cells in both the
normal air and hyperoxia groups in a dose-dependent manner
(Fig. 2B and C). Based on these
findings, 10 nM CGRP and 1 µM capsaicin were used in subsequent
experiments. Moreover, hyperoxia significantly induced apoptosis in
A549 cells, which was attenuated by CGRP and capsaicin (Fig. 2D). Taken together, CGRP/CGRPR and
capsaicin/TRPV1 may play protective roles in hyperoxia-induced
alveolar cell injury by promoting cell proliferation and inhibiting
apoptosis.
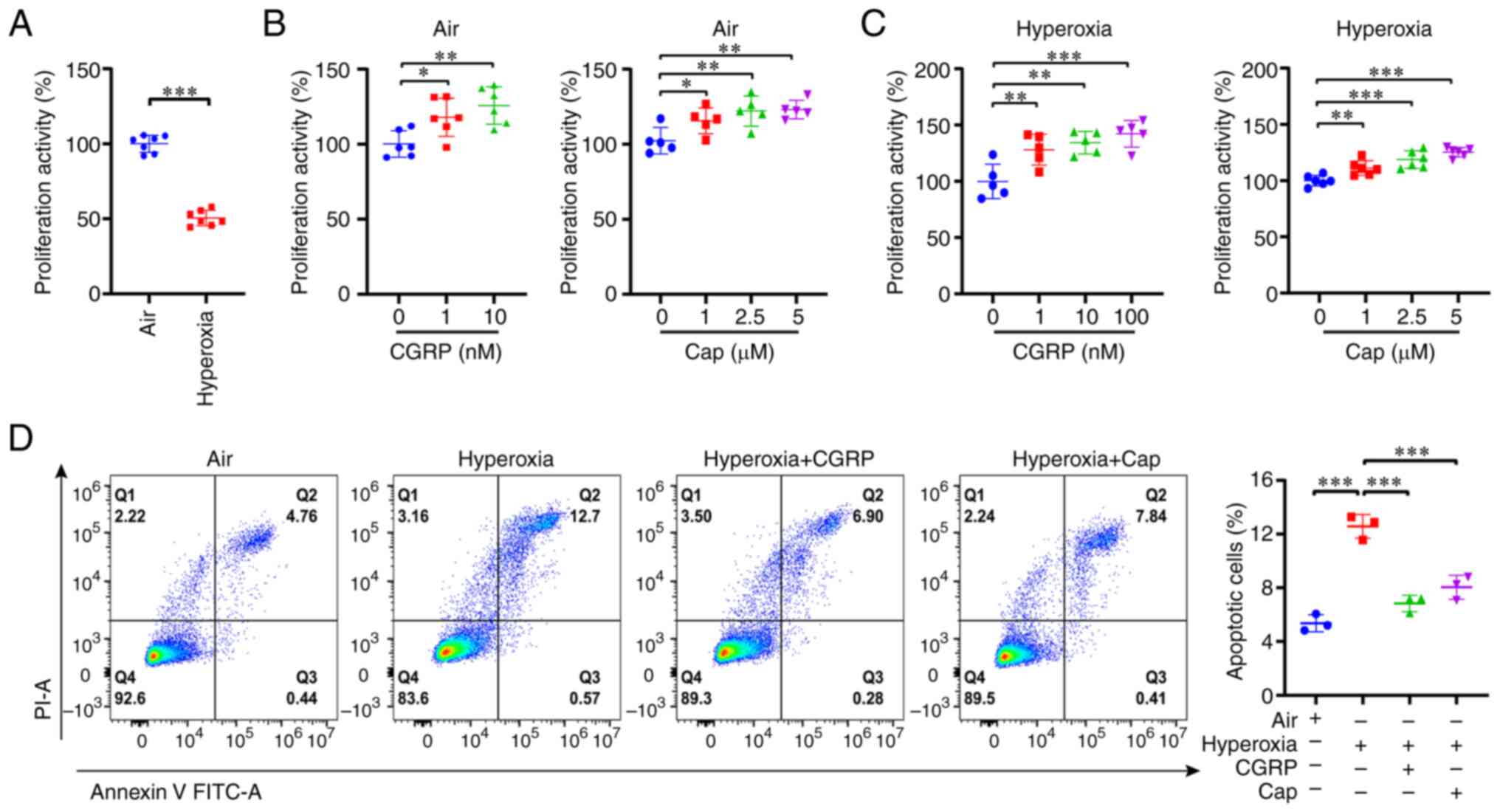 | Figure 2.CGRP and cap promote cell
proliferation but inhibit cell apoptosis in A549 cells grown under
hyperoxic conditions. (A) Cell Counting Kit-8 assay revealed that
hyperoxia inhibited A549 cell proliferation. (B) Different
concentrations of CGRP (1 or 10 nM) and cap (1, 2.5, or 5 µM)
promoted cell proliferation in the normal air group. (C) Different
concentrations of CGRP (1, 10, or 100 nM) and cap (1, 2.5, or 5 µM)
promoted cell proliferation under hyperoxia. (D) Flow cytometry
revealed that hyperoxia promoted apoptosis of A549 cells, and both
CGRP (10 nM) and cap (1 µM) reversed this. Data are presented as
the mean ± SD of at least three repeats. *P<0.05, **P<0.01
and ***P<0.001. CGRP, calcitonin gene-related peptide; cap,
capsaicin; PI, propidium iodine. |
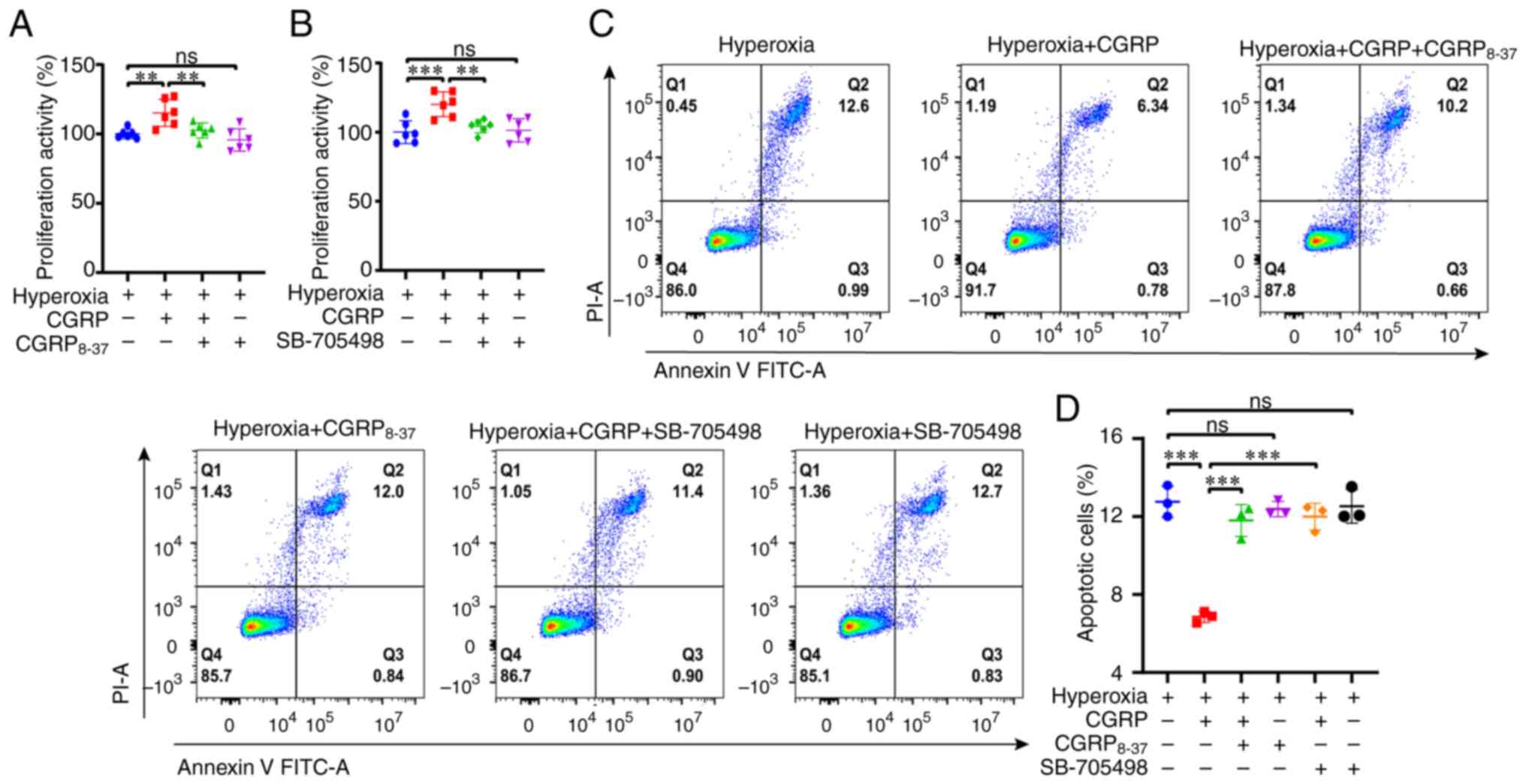
CGRP promotes proliferation but
inhibits apoptosis via a CGRPR/TRPV1 axis under hyperoxic
conditions
Since CGRP/CGRPR and capsaicin/TRPV1 exerted
protective effects in parallel, whether CGRP acted via a
CGRPR/TRPV1 axis was next assessed. First, it was revealed that
CGRP8-37 (100 nM), a selective CGRPR inhibitor,
inhibited CGRP-induced A549 cell proliferation with no toxic
effects (Fig. 3A). Second, the
selective TRPV1 channel blocker SB-705498 (10 µM) also attenuated
CGRP-induced cell proliferation (Fig.
3B). Third, CGRP8-37 and SB-705498 attenuated the
inhibitory effect of CGRP on apoptosis, and they themselves had no
significant effect on apoptosis (Fig.
3C and D). Therefore, CGRP may promote cell proliferation but
inhibit apoptosis of A549 cells via a CGRPR/TRPV1 axis.
The CGRPR/TRPV1 axis affects
transcription and protein expression levels of proliferation and
apoptotic factors
Since Cyclin D1 and PCNA are critical factors for
cell proliferation (40,41), while Bcl-2 and Bax are key targets
of apoptosis (42,43), whether they were involved in the
protective role of the CGRPR/TRPV1 axis in HILI was assessed. At
the transcriptional level, compared with the normal air group, the
mRNA expression levels of Cyclin D1 and PCNA in the hyperoxia group
were significantly downregulated, and this was rescued by CGRP.
However, CGRP8-37 or SB-705498 significantly attenuated
the CGRP-induced increase in Cyclin D1 and PCNA levels in the
hyperoxic cells (Fig. 4A).
Similarly, it was found that the mRNA expression levels of Bcl-2
were downregulated but Bax was upregulated in the hyperoxia group.
However, CGRP increased the Bcl-2 expression, and this was reversed
by either CGRP8-37 or SB-705498 (Fig. 4B, left panel). By contrast, CGRP
decreased the Bax expression, and this was reversed by either
CGRP8-37 or SB-705498 (Fig.
4B, right panel). Meanwhile, western blotting was performed to
detect the expression of proliferation-related and
apoptosis-related proteins after pretreatment with CGRP,
CGRP8-37 and SB-705498. The changes in these factors at
the protein level reflected what was observed at the transcription
level (Fig. 4C and D). Taken
together, these data suggested that CGRP promotes proliferation but
inhibits apoptosis of A549 cells via a CGRPR/TRPV1 axis.
 | Figure 4.The CGRPR/TRPV1 axis regulates the
mRNA and protein expression levels of proliferation and apoptosis
factors in A549 cells under hyperoxic conditions. (A and B)
quantitative PCR revealed the effects of 10 nM CGRP, 100 nM
CGRP8-37 and 10 µM SB-705498 on the expression of Cyclin
D1, PCNA, Bcl-2 and Bax in A549 cells cultured under hyperoxic
conditions. (C) Western blotting revealed the effects of CGRP and
CGRP8-37 on the expression levels of Cyclin D1, PCNA,
Bcl-2 and Bax in A549 cells cultured under hyperoxic conditions.
(D) Western blotting revealed the effects of CGRP and SB-705498 on
the expression levels of Cyclin D1, PCNA, Bcl-2 and Bax in A549
cells cultured under hyperoxic conditions. Data are presented as
the mean ± SD of at least three repeats. *P<0.05, **P<0.01
and ***P<0.001. CGRP, calcitonin gene-related peptide; CGRPR,
CGRP receptor; TRPV1, transient receptor potential vanilloid 1;
PCNA, proliferating cell nuclear antigen. |
Role of TRPV1 channels in
CGRP-mediated cell proliferation and apoptosis in hyperoxia
First, lentiviral infection was used to knock down
TRPV1 channels expression in A549 cells, and knockdown was
confirmed at both the mRNA and protein levels (Fig. 5A and B). The shTRPV1-2 sequence
exhibited the optimal result out of the three shRNAs used, and thus
it was used for all subsequent experiments. ShTRPV1 significantly
reduced CGRP-induced proliferation of A549 cells in hyperoxia
(Fig. 5C). Similarly, CGRP had no
inhibitory effect on apoptosis after shTRPV1 in hyperoxia (Fig. 5D). Finally, western blotting was
used to analyze the effect of shTRPV1 on the protein expression
levels of Cyclin D1, PCNA, Bcl-2 and Bax in hyperoxia. As
demonstrated in Fig. 5E, shTRPV1
significantly attenuated the CGRP-induced upregulation of Cyclin
D1, PCNA and Bcl-2 protein expression, and reversed the
CGRP-induced downregulation of Bax expression. The results
following the knockdown of TRPV1 in A549 cells were consistent with
those of the selective TRPV1 blocker treatment (Fig. 4D). Therefore, these data confirmed
the role of TRPV1 channels in CGRP-mediated proliferation and
apoptosis of A549 cells in hyperoxia.
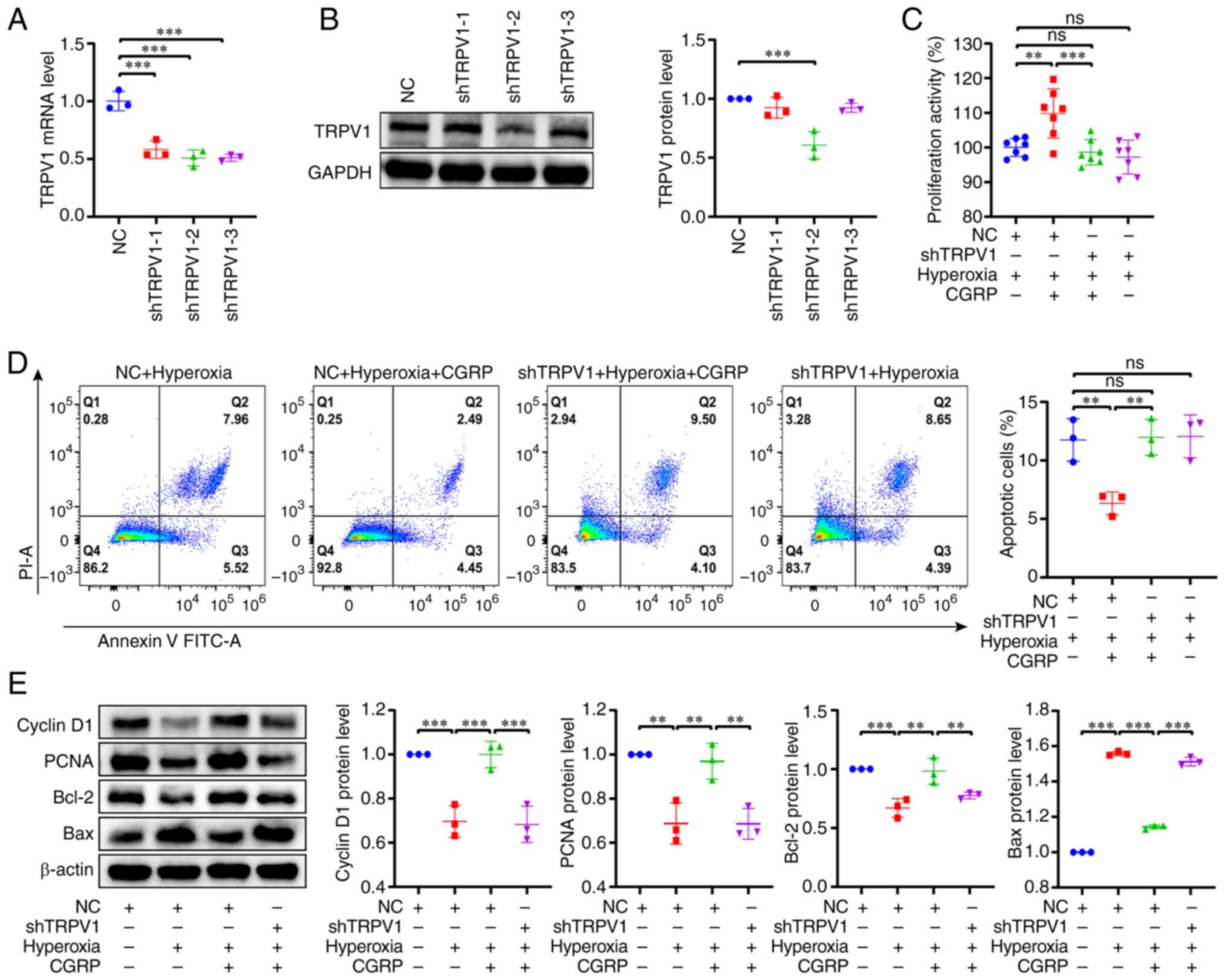 | Figure 5.Knockdown of TRPV1 confirms the role
of TRPV1 in CGRP-mediated cell proliferation and apoptosis of A549
cells cultured under hyperoxic conditions. (A and B) The mRNA and
protein expression levels of TRPV1. For knockdown of TRPV1, three
lentiviruses with different interference sequences were used.
ShTRPV1-2 was used for subsequent experiments. (C) shTRPV1
attenuated the CGRP-induced increase in proliferation of A549 cells
under hyperoxic conditions. (D) shTRPV1 attenuated the effect of
CGRP-induced decrease in apoptosis of A549 cells under hyperoxic
conditions. (E) Effects of CGRP on Cyclin D1, PCNA, Bcl-2 and Bax
expression in A549 shTRPV1 cells cultured under hyperoxic
conditions. Data are presented as the mean ± SD of at least three
repeats. **P<0.01 and ***P<0.001. TRPV1, transient receptor
potential vanilloid 1; CGRP, calcitonin gene-related peptide; m,
messenger; sh, short hairpin; PCNA, proliferating cell nuclear
antigen; NC, negative controls; ns, not significant; PI, propidium
iodine. |
CGRP induces Ca2+ entry via
TRPV1 channels
Since TRPV1 channels are plasma membrane
Ca2+-permeable channels (32,44),
whether CGRP protected against hyperoxia-induced alveolar cell
injury via the TRPV1/Ca2+ signaling pathway was
examined. Patch-clamp and calcium imaging were performed using A549
cells. As demonstrated in the left panel of Fig. 6A, the membrane currents increased
significantly with the addition of CGRP, while SB-705498 inhibited
the CGRP-induced membrane currents. The middle panel of Fig. 6A shows a voltage-current diagram of
the same cell, highlighting CGRP-induced membrane non-selective
cation currents after SB-705498 treatment. Summary data on the
right panel of Fig. 6A
demonstrates that SB-705498 significantly inhibited CGRP-induced
membrane currents. Subsequently, Ca2+ imaging
experiments were performed in A549 cells. As revealed in Fig. 6B and D, CGRP induced a significant
increase in intracellular Ca2+ signaling, which was
attenuated by SB-705498. To further examine the importance of
CGRP-mediated Ca2+ signaling in A549 cells, BAPTA-AM, a
cell-permeable calcium chelator was used. It significantly
attenuated the CGRP-induced intracellular Ca2+ signaling
(Fig. 6C and E). In addition, it
was found that BAPTA-AM (1 µM) attenuated the CGRP-induced
proliferation of A549 cells in hyperoxia, while BAPTA-AM itself did
not affect cell proliferation (Fig.
6F). By contrast, BAPTA-AM reversed the CGRP-induced decrease
in apoptosis under hyperoxic conditions (Fig. 6G and H). These data suggested that
CGRP protects against hyperoxia-induced alveolar cell injury via
the TRPV1/Ca2+ signaling pathway.
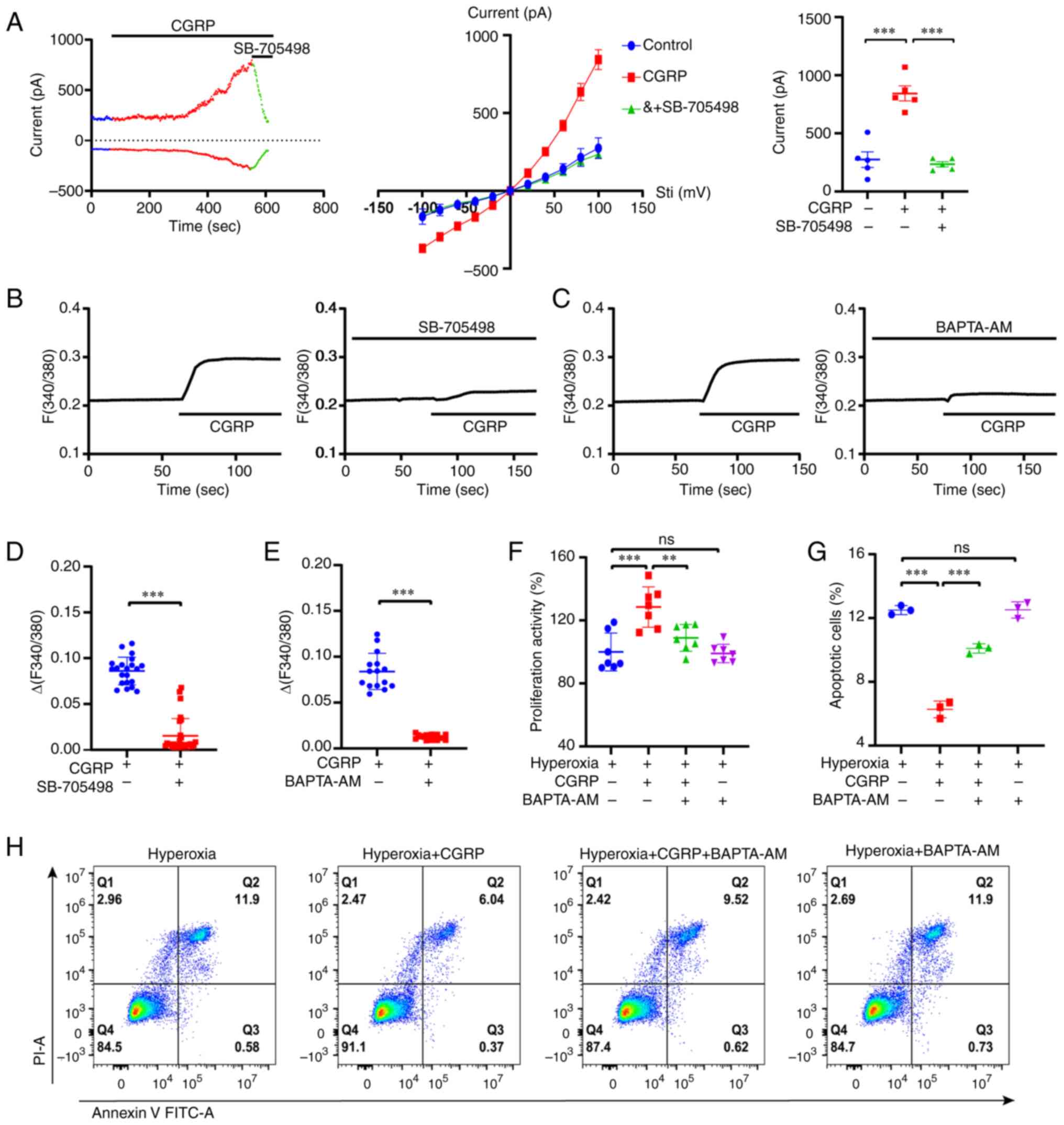 | Figure 6.CGRP regulates Ca2+ entry
into A549 cells via TRPV1 channels under hyperoxic conditions. (A)
Left panel, membrane currents were increased by treatment with 100
nM CGRP and inhibited by 10 µM SB-705498. Middle panel,
current-voltage curves in response to voltage stepping from −100 to
+100 mV in the presence of 100 nM CGRP or combination of 10 µM
SB-705498. Right panel, summary data of the membrane currents
measured at 100 mV (n=5). (B and D) Time courses of 100 nM
CGRP-induced intracellular Ca2+ signaling changes in the
presence or the absence of 10 µM SB-705498, measured based on the
Fura-2 ratio. Summary of the ∆Fura-2 fluorescence ratio (the peak
values) (n=20-30 cells per group). (C and E) Time courses of 100 nM
CGRP-induced intracellular Ca2+ signaling changes in the
presence or the absence of 1 µM BAPTA-AM, measured based on the
Fura-2 ratio. Summary of the ∆Fura-2 fluorescence ratio (the peak
values) (n=15-20 cells per group). (F) Cell Counting Kit-8 assay
revealed that 10 nM CGRP promoted A549 cell proliferation under
hyperoxic conditions, which was inhibited by BAPTA-AM. (G and H) 10
nM CGRP inhibited A549 cell apoptosis under hyperoxic conditions,
which was attenuated by BAPTA-AM. Data are presented as the mean ±
SD of at least three repeats. **P<0.01 and ***P<0.001; CGRP,
calcitonin gene-related peptide; TRPV1, transient receptor
potential vanilloid 1; ns, not significant; PI, propidium
iodine. |
The phospholipase C (PLC)/protein
kinase C (PKC) pathway is involved in CGRP-mediated
TRPV1/Ca2+ signaling
Activation of GPCR can stimulate TRPV1 channels to
mediate inflammation via the PLC/PKC pathway (45–47).
CGRPR acts as a GPCR, thus whether PLC/PKC was involved in the
CGRPR/TRPV1/Ca2+ axis in A549 cells was examined. First,
the role of PLC in CGRPR activation was examined. It was revealed
that the selective PLC inhibitor U-73122 (1 µM) reduced
CGRP-induced Ca2+ signaling, whereas the inactive analog
U-73343 did not reduce CGRP-induced Ca2+ signaling
(Fig. 7A). In addition, whether
PKC was involved in the activation of CGRPR/TRPV1 channels was
assessed. CGRP-induced Ca2+ signaling was attenuated by
the selective PKC inhibitor Go6976 (200 nM) (Fig. 7B). These results suggested that
PLC/PKC is involved in activating CGRPR/TRPV1 channels.
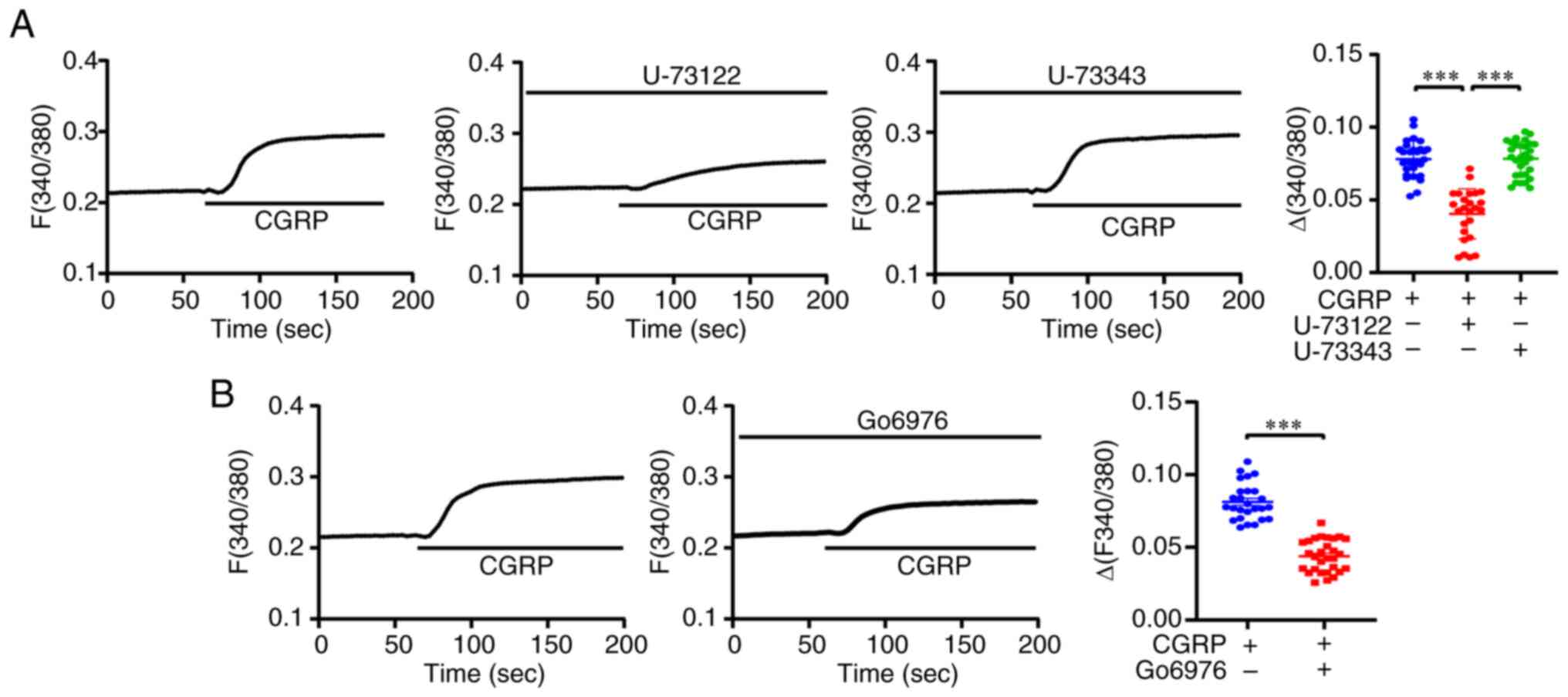 | Figure 7.The PLC/PKC pathway is involved in
CGRP-mediated TRPV1/Ca2+ signaling. (A) Left panel, time
courses of 100 nM CGRP-induced intracellular Ca2+
signaling changes in the presence or the absence of 1 µM U-73122 or
1 µM U-73343, measured based on the Fura-2 ratio. Right panel,
summary data of the ∆Fura-2 fluorescence ratio. n=20-30 cells per
group. (B) Left panel, time courses of CGRP-induced intracellular
Ca2+ signaling changes in the presence or the absence of
200 nM Go6976, measured based on the Fura-2 ratio. Right panel,
summary data of the ∆Fura-2 fluorescence ratio. n=20-30 cells per
group. Data are presented as the mean ± SD of at least three
repeats. ***P<0.001. PLC, phospholipase C; PKC, protein kinase
C; CGRP, calcitonin gene-related peptide; TRPV1, transient receptor
potential vanilloid 1. |
CGRP/CGRPR regulates
TRPV1/Ca2+ via the PLC/PKC pathway in hyperoxia
Further applying the PLC inhibitor U-73122 (1 µM)
and PKC inhibitor Go6976 (200 nM), it was revealed that both
inhibitors attenuated CGRP-induced cell proliferation in hyperoxia
(Fig. 8A and B). In addition, both
PLC and PKC inhibitors attenuated the CGRP-induced increase in
Cyclin D1, PCNA and Bcl-2 protein expression, but reversed the
CGRP-induced decrease in Bax expression (Fig. 8C-F). Therefore, these data
suggested that CGRPR activates TRPV1 channels via the PLC/PKC
pathway.
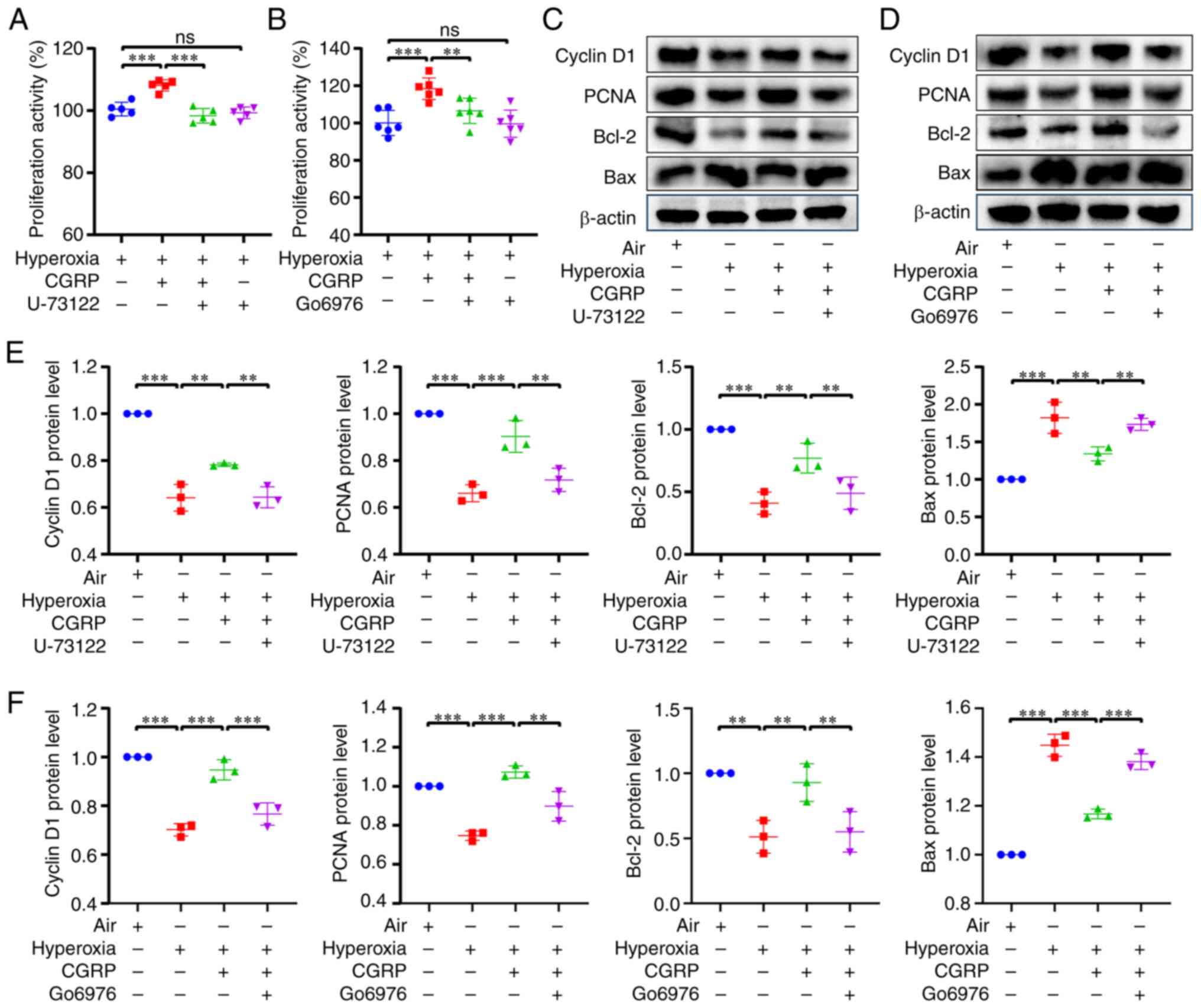 | Figure 8.CGRP/CGRPR regulates TRPV1 via the
PLC/PKC pathway in A549 cells cultured under hyperoxic conditions.
(A and B) Cell Counting Kit-8 assay revealed that 10 nM CGRP
promoted A549 cell proliferation under hyperoxic conditions, and
this was inhibited by both 1 µM U-73122 and 200 nM Go6976. (C and
D) Western blotting revealed CGRP increased the expression of
Cyclin D1, PCNA and Bcl-2 and attenuated the expression of Bax in
hyperoxia, whereas both U-73122 and Go6976 reversed the effects of
CGRP. (E and F) The densitometric analysis diagram of the western
blots. Data are presented as the mean ± SD of at least three
repeats. **P<0.01 and ***P<0.001; CGRP, calcitonin
gene-related peptide; CGRPR, CGRP receptor; TRPV1, transient
receptor potential vanilloid 1; PLC, phospholipase C; PKC, protein
kinase C; PCNA, proliferating cell nuclear antigen; ns, not
significant. |
Discussion
In the present study, the protective role of CGRP in
hyperoxia-induced human alveolar cell injury was verified, and the
underlying molecular mechanisms were elucidated. The primary
findings were as follows: i) Although hyperoxia treatment did not
alter CGRP release from human alveolar cells, it significantly
downregulated the mRNA and protein expression levels of CGRPR and
TRPV1 channels; ii) CGRP promoted proliferation but inhibited
apoptosis of human alveolar cells via the
CGRPR/TRPV1/Ca2+ signaling axis in hyperoxia; iii)
CGRP/CGRPR activated TRPV1 channels to induce Ca2+ entry
via the PLC/PKC pathway; iv) CGRP protected against
hyperoxia-induced human alveolar cell injury via regulation of
proliferation and apoptotic factors Cyclin D1, PCNA, Bcl-2 and
Bax.
It is well established that activation of TRPV1
channels at sensory nerve endings can induce the release of
Ca2+-dependent CGRP to exert biological effects by
acting on CGRPR, indicating that the TRPV1/Ca2+
signaling pathway plays a key role in regulating CGRP release from
sensory nerve endings (28,29).
The present study investigated whether CGRP acted inversely on
TRPV1 channels to exert a protective effect against HILI from the
perspective of CGRP. Previous studies have revealed that TRPV1
channels are functionally expressed in human bronchial epithelial
cells, with increased expression in patients with asthma (48,49).
However, there was no significant change in the expression of TRPV1
in pulmonary artery smooth muscle cells of rats under hypoxic
conditions (50). Since the
expression of TRPV1 under hyperoxia has not yet been reported to
the best of the authors' knowledge, in the present study it was
demonstrated for the first time that the expression of TRPV1 in
human alveolar cells was downregulated under hyperoxic treatment.
CGRPR expression is significantly downregulated in LPS-induced
acute lung injury in rats (20).
The expression of CGRPR under hyperoxic conditions was unknown
however, in the present study, it was also demonstrated that
hyperoxia downregulated CGRPR expression in A549 cells.
Although CGRP secretion from human alveolar cells
was not altered by hyperoxia treatment compared with the control
culture conditions, the exogenous addition of CGRP induced cell
proliferation and inhibited cell apoptosis under hyperoxia,
indicating that exogenous CGRP, but not endogenous CGRP, played a
protective role in hyperoxia-induced human alveolar cell injury.
Therefore, the protective role of exogenous CGRP and molecular
mechanisms involved were further assessed. First, it was revealed
that the promotion of cell proliferation by CGRP was attenuated by
selective inhibitors of CGRPR and TRPV1. In addition, CGRP
inhibited apoptosis in hyperoxia, but selective inhibitors for both
CGRPR and TRPV1 reversed this inhibitory effect of CGRP on
apoptosis. These results suggested that CGRPR/TRPV1 was involved in
CGRP-mediated alveolar cell proliferation and apoptosis in
hyperoxia. After applying selective inhibitors of CGRPR and TRPV1
channels or using shTRPV1 to knock down TRPV1 expression, the
proliferation and apoptotic factors Cyclin D1, PCNA, Bcl-2 and Bax
were assessed. Inhibition of CGRPR and TRPV1 channels reduced the
protective effects of CGRP against hyperoxia-induced alveolar cell
injury. Taken together, it was hypothesized that a CGRPR/TRPV1 axis
plays a critical role in protecting alveolar cells under hyperoxic
conditions.
It was also revealed that the TRPV1 agonist
capsaicin promoted alveolar cell proliferation, but inhibited
apoptosis under hyperoxia-induced injury, further highlighting the
protective role of TRPV1. Since TRPV1 channels are plasma membrane
Ca2+-permeable channels (32,44),
patch clamp and Ca2+ imaging were used to confirm that
CGRP induced an increase in membrane non-selective currents and
intracellular Ca2+ signaling, while SB-705498 inhibited
these changes. The intracellular calcium chelator BAPTA-AM was also
used to elucidate the role of TRPV1/Ca2+ signaling in
CGRP/CGRPR protection against hyperoxia-induced alveolar cell
injury.
CGRPR is a GPCR, its activation can regulate
Gs/Gi to promote or inhibit adenylate cyclase
to generate cAMP and can regulate the
Gq/11-Ca2+ pathway (51). Since the CGRPR/cAMP pathway has
been extensively studied (52–55),
a focus was placed on the largely undefined CGRPR/Ca2+
pathway. Since activation of the Gq/11 protein promotes PLC
activity (51), in the present
study it was revealed that a PLC inhibitor reduced CGRP-induced
intracellular Ca2+ signaling while attenuating the
effects of CGRP-mediated protection against cell proliferation and
apoptosis under hyperoxic conditions, suggesting that CGRP/CGRPR
regulates Gq/11-PLC pathway. Due to PLC activating the PKC pathway,
it was further confirmed that CGRP/CGRPR regulates PKC in alveolar
cells. It was revealed that a PKC selective inhibitor attenuated
the CGRP-induced increase in intracellular Ca2+
signaling in alveolar cells, while reversing the changes of
CGRP-mediated proliferation and apoptotic factors Cyclin D1, PCNA,
Bcl-2 and Bax. These data suggested that the PLC/PKC pathway plays
a role in CGRP-mediated protection against hyperoxia-induced
alveolar cell injury. However, the exact mechanisms of how the
CGRPR/TRPV1/Ca2+ axis regulates proliferation-related
and apoptosis-related factors need to be further elucidated.
Finally, the limitations of the present study will
be discussed. Since the present study was performed at the in
vitro cellular level, it lacks animal experiments or human
in vivo experiments; thus, further in vivo
experiments are required to verify the role of the identified
CGRPR/TRPV1/Ca2+ axis. In addition, the human alveolar
A549 cells were used to establish the cell model; not using primary
AECII is also a potential limitation of the present study. The
reasons for not using primary AECII are that primary human AECII
are difficult to isolate and culture; and primary AECII isolated
from rats have high background levels of apoptosis (56). Moreover, primary cultured AECII are
prone to mutation and are unsuitable for transfection. However, the
A549 cells, an AECII line, have similar biological properties to
AECII, are frequently used in studies on HILI, making them a
valuable research subject for hyperoxia (57–61).
In conclusion, CGRP protected against
hyperoxia-induced alveolar cell injury via a novel
CGRPR/TRPV1/Ca2+ axis (Fig.
9). CGRP/CGRPR activated TRPV1 channels via the PLC/PKC
pathway, inducing extracellular Ca2+ entry to promote
cell proliferation but inhibit apoptosis following hyperoxic
injury, ultimately protecting against HILI. Therefore, although
activation of TRPV1 channels promotes Ca2+-dependent
CGRP release from sensory endings (28,29),
in the present study it was revealed that exogenous CGRP could also
inversely regulate the function of TRPV1 channels in alveolar
cells. Importantly, the CGRPR/TRPV1/Ca2+ axis protected
against hyperoxia-induced alveolar cell injury, highlighting a
potential target for the management of HILI.
Since TRPV1 channels are located both on sensory
endings to promote CGRP release and on alveolar cells to protect
against hyperoxia-induced alveolar cell injury, the findings of the
present study strongly suggested that capsaicin is a potential
candidate to effectively prevent/treat HILI given its alveolar cell
protective and anti-inflammatory effects (62–64),
although this requires further study. Meanwhile, TRPV1 can be used
as a drug development target in future studies to explore its role
in the prevention and treatment of HILI.
Acknowledgements
Not applicable.
Funding
The present study was supported (grant no. 82273115) by research
grants from the National Natural Science Foundation of China.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FX conceived the study and designed some
experiments. HD designed all experiments, wrote and finalized the
manuscript. JL performed most experiments and data analysis, and
drafted the manuscript. HW, LW, FF and JW performed some
experiments and revising the manuscript. All authors read and
approved the final the manuscript. JL and FX confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CGRP
|
calcitonin gene-related peptide
|
|
CGRPR
|
CGRP receptor
|
|
GPCR
|
G protein-coupled receptor
|
|
TRPV1
|
transient receptor potential vanilloid
1
|
|
AECII
|
alveolar type II epithelial cells
|
|
HILI
|
hyperoxia-induced lung injury
|
|
PLC
|
phospholipase C
|
|
PKC
|
protein kinase C
|
References
|
1
|
Dias-Freitas F, Metelo-Coimbra C and
Roncon-Albuquerque R: Molecular mechanisms underlying hyperoxia
acute lung injury. Respir Med. 119:23–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim MJ, Ryu JC, Kwon Y, Lee S, Bae YS,
Yoon JH and Ryu JH: Dual Oxidase 2 in lung epithelia is essential
for Hyperoxia-Induced acute lung injury in mice. Antioxid Redox
Signal. 21:1803–1818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marseglia L, D'Angelo G, Granese R,
Falsaperla R, Reiter RJ, Corsello G and Gitto E: Role of oxidative
stress in neonatal respiratory distress syndrome. Free Radic Biol
Med. 142:132–137. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cannavò L, Perrone S, Viola V, Marseglia
L, Di Rosa G and Gitto E: Oxidative stress and respiratory diseases
in preterm newborns. Int J Mol Sci. 22:125042021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nabhan AN, Brownfield DG, Harbury PB,
Krasnow MA and Desai TJ: Single-cell wnt signaling niches maintain
stemness of alveolar type 2 cells. Science. 359:1118–1123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pinho-Ribeiro FA, Baddal B, Haarsma R,
O'Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB,
Wessels MR and Chiu IM: Blocking neuronal signaling to immune cells
treats streptococcal invasive infection. Cell. 173:1083–1097.e22.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonner K, Pease JE, Corrigan CJ, Clark P
and Kay AB: CCL17/thymus and activation-regulated chemokine induces
calcitonin gene-related peptide in human airway epithelial cells
through CCR4. J Allergy Clin Immunol. 132:942–950.e1-e3. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonner K, Kariyawasam HH, Ali FR, Clark P
and Kay AB: Expression of functional receptor activity modifying
protein 1 by airway epithelial cells with dysregulation in asthma.
J Allergy Clin Immunol. 126:1277–1283.e3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Hou L, Hua Z and Wang X:
Interleukin-1β induces β-calcitonin gene-related peptide secretion
in human type II alveolar epithelial cells. FASEB J. 18:1603–1605.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Jia L, Wang T, Sun W, Wu S and
Wang X: Endogenous calcitonin gene-related peptide protects human
alveolar epithelial cells through protein kinase Cepsilon and heat
shock protein. J Biol Chem. 280:20325–20330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Russell FA, King R, Smillie SJ, Kodji X
and Brain SD: Calcitonin Gene-related peptide: Physiology and
pathophysiology. Physiol Rev. 94:1099–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edvinsson L: Calcitonin gene-related
peptide (CGRP) is a key molecule released in acute migraine
attacks-Successful translation of basic science to clinical
practice. J Intern Med. 292:575–586. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Russo AF: Calcitonin Gene-related peptide
(CGRP): A new target for migraine. Annu Rev Pharmacol Toxicol.
55:533–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu W, Feng B, Liu J, Li Y, Liao Y, Wang S,
Tao S, Hu S, He W, Shu Q, et al: The CGRP/macrophage axis signal
facilitates inflammation recovery in the intestine. Clin Immunol.
245:1091542022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan K, Zheng J, Shen X, Wu Y, Han Y, Jin
X and Huang X: Sensory nerves promote corneal inflammation
resolution via CGRP mediated transformation of macrophages to the
M2 phenotype through the PI3K/AKT signaling pathway. Int
Immunopharmacol. 102:1084262022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brain SD and Grant AD: Vascular actions of
calcitonin Gene-related peptide and adrenomedullin. Physiol Rev.
84:903–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
MaassenVanDenBrink A, Meijer J, Villalón
CM and Ferrari MD: Wiping out CGRP: Potential cardiovascular risks.
Trends Pharmacol Sci. 37:779–788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wurthmann S, Nägel S, Hadaschik E, Schlott
S, Scheffler A, Kleinschnitz C and Holle D: Impaired wound healing
in a migraine patient as a possible side effect of calcitonin
gene-related peptide receptor antibody treatment: A case report.
Cephalalgia. 40:1255–1260. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Q, Wang W, Wang R and Cheng Y: TRPV1
and neuropeptide receptor immunoreactivity and expression in the
rat lung and brainstem after lung ischemia-reperfusion injury. J
Surg Res. 203:183–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang W, Xv M, Yang WC, Wang N, Zhang XZ
and Li WZ: Exogenous α-calcitonin gene-related peptide attenuates
lipopolysaccharide-induced acute lung injury in rats. Mol Med Rep.
12:2181–2188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong-Min F, Chun-Rong H, Rui Z, Li-Na S,
Ya-Jun W and Li L: CGRP 8–37 enhances lipopolysaccharide-induced
acute lung injury and regulating aquaporin 1 and 5 expressions in
rats. J Physiol Biochem. 73:381–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dang H, Yang L, Wang S, Fang F and Xu F:
Calcitonin Gene-related peptide ameliorates Hyperoxia-induced lung
injury in neonatal rats. Tohoku J Exp Med. 227:129–138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dang HX, Li J, Liu C, Fu Y, Zhou F, Tang
L, Li L and Xu F: CGRP attenuates hyperoxia-induced oxidative
stress-related injury to alveolar epithelial type II cells via the
activation of the Sonic hedgehog pathway. Int J Mol Med.
40:209–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu H, Zhang T, Huang R, Yang Z, Liu C, Li
M, Fang F and Xu F: Calcitonin gene-related peptide protects type
II alveolar epithelial cells from hyperoxia-induced DNA damage and
cell death. Exp Ther Med. 13:1279–1284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai Y, Fang F, Jiang J and Xu F: Extrinsic
calcitonin Gene-related peptide inhibits hyperoxia-induced alveolar
epithelial type II cells apoptosis, oxidative stress, and reactive
oxygen species (ROS) production by enhancing Notch 1 and
Homocysteine-Induced endoplasmic reticulum protein (HERP)
expression. Med Sci Monit. 23:5774–5782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Negri S, Faris P, Rosti V, Antognazza MR,
Lodola F and Moccia F: Endothelial TRPV1 as an emerging molecular
target to promote therapeutic angiogenesis. Cells. 9:13412020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: A
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riera CE, Huising MO, Follett P, Leblanc
M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C and
Dillin A: TRPV1 pain receptors regulate longevity and metabolism by
neuropeptide signaling. Cell. 157:1023–1036. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakanishi M, Hata K, Nagayama T, Sakurai
T, Nishisho T, Wakabayashi H, Hiraga T, Ebisu S and Yoneda T: Acid
activation of Trpv1 leads to an Up-Regulation of calcitonin
Gene-related peptide expression in dorsal root ganglion neurons via
the CaMK-CREB cascade: A potential mechanism of inflammatory pain.
Mol Biol Cell. 21:2568–2577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Xu Y, Cheng Y and Wang R: α7
nicotinic acetylcholine receptor contributes to the alleviation of
lung ischemia-reperfusion injury by transient receptor potential
vanilloid type 1 stimulation. J Surg Res. 230:164–174. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu X, Wang C, Wu D, Zhang C, Xiao C and Xu
F: Quantitative proteomics reveals the mechanisms of
hydrogen-conferred protection against hyperoxia-induced injury in
type II alveolar epithelial cells. Exp Lung Res. 44:464–475. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao N, Yang F, Chen S, Wan H, Zhao X and
Dong H: The role of TRPV1 ion channels in the suppression of
gastric cancer development. J Exp Clin Cancer Res. 39:2062020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou J, Jiang Y, Chen H, Wu Y and Zhang L:
Tanshinone I attenuates the malignant biological properties of
ovarian cancer by inducing apoptosis and autophagy via the
inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 53:e127392020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen X, Lu W, Lu C, Zhang L, Xu F and Dong
H: The CaSR/TRPV4 coupling mediates pro-inflammatory macrophage
function. Acta Physiol (Oxf). 237:e139262023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Z, Fang F and Xu F: Effects of
different states of oxidative stress on fetal rat alveolar type II
epithelial cells in vitro and ROS-induced changes in wnt signaling
pathway expression. Mol Med Rep. 7:1528–1532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jordt SE and Julius D: Molecular basis for
species-specific sensitivity to ‘hot’ chili peppers. Cell.
108:421–430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu SL, Wang ML, He YT, Guo SW, Li TT,
Peng WJ and Luo D: Capsaicin ameliorates intermittent high
glucose-mediated endothelial senescence via the TRPV1/SIRT1
pathway. Phytomedicine. 100:1540812022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin YT, Yu Z, Tsai SC, Hsu PH and Chen JC:
Neuropeptide FF receptor 2 inhibits capsaicin-induced CGRP
upregulation in mouse trigeminal ganglion. J Headache Pain.
21:872020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi L, Zhang S, Huang Z, Hu F, Zhang T,
Wei M, Bai Q, Lu B and Ji L: Baicalin promotes liver regeneration
after acetaminophen-induced liver injury by inducing NLRP3
inflammasome activation. Free Radic Biol Med. 160:163–177. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liao S, Chen H, Liu M, Gan L, Li C, Zhang
W, Lv L and Mei Z: Aquaporin 9 inhibits growth and metastasis of
hepatocellular carcinoma cells via Wnt/β-catenin pathway. Aging
(Albany NY). 12:1527–1544. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu YP, Yuan H, Xu Y, Liu RM, Luo Y and
Xiao JH: Protective effects of Ligularia fischeri root extracts
against ulcerative colitis in mice through activation of Bcl-2/Bax
signalings. Phytomedicine. 99:1540062022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Yang X, Ge X and Zhang F:
Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved
caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid
hemorrhage mice. Biomed Pharmacother. 109:726–733. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yuan J, Liu H, Zhang H, Wang T, Zheng Q
and Li Z: Controlled activation of TRPV1 channels on microglia to
boost their autophagy for clearance of Alpha-Synuclein and enhance
therapy of Parkinson's disease. Adv Mater. 34:21084352022.
View Article : Google Scholar
|
|
45
|
Than JYXL, Li L, Hasan R and Zhang X:
Excitation and modulation of TRPA1, TRPV1, and TRPM8
Channel-expressing sensory neurons by the pruritogen chloroquine. J
Biol Chem. 288:12818–12827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Minke B and Pak WL: The light-activated
TRP channel: The founding member of the TRP channel superfamily. J
Neurogenet. 36:55–64. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kumar R, Hazan A, Geron M, Steinberg R,
Livni L, Matzner H and Priel A: Activation of transient receptor
potential vanilloid 1 by lipoxygenase metabolites depends on PKC
phosphorylation. FASEB J. 31:1238–1247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McGarvey LP, Butler CA, Stokesberry S,
Polley L, McQuaid S, Abdullah H, Ashraf S, McGahon MK, Curtis TM,
Arron J, et al: Increased expression of bronchial epithelial
transient receptor potential vanilloid 1 channels in patients with
severe asthma. J Allergy Clin Immunol. 133:704–712.e4. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Grace MS, Baxter M, Dubuis E, Birrell MA
and Belvisi MG: Transient receptor potential (TRP) channels in the
airway: Role in airway disease. Br J Pharmacol. 171:2593–2607.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Parpaite T, Cardouat G, Mauroux M,
Gillibert-Duplantier J, Robillard P, Quignard JF, Marthan R,
Savineau JP and Ducret T: Effect of hypoxia on TRPV1 and TRPV4
channels in rat pulmonary arterial smooth muscle cells. Pflugers
Arch. 468:111–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cottrell GS: CGRP receptor signalling
pathways. Calcitonin Gene-Related peptide (CGRP) mechanisms. vol.
255. Brain SD and Geppetti P: Springer International Publishing;
Cham: pp. 37–64. 2018, View Article : Google Scholar
|
|
52
|
Zhang Y, Xu J, Ruan YC, Yu MK, O'Laughlin
M, Wise H, Chen D, Tian L, Shi D, Wang J, et al: Implant-derived
magnesium induces local neuronal production of CGRP to improve
bone-fracture healing in rats. Nat Med. 22:1160–1169. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Do TP, Deligianni C, Amirguliyev S,
Snellman J, Lopez CL, Al-Karagholi MA, Guo S and Ashina M: Second
messenger signalling bypasses CGRP receptor blockade to provoke
migraine attacks in humans. Brain. 146:5224–5234. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Villa I, Mrak E, Rubinacci A, Ravasi F and
Guidobono F: CGRP inhibits osteoprotegerin production in human
osteoblast-like cells via cAMP/PKA-dependent pathway. Am J Physiol
Cell Physiol. 291:C529–C537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hartopo AB, Emoto N, Vignon-Zellweger N,
Suzuki Y, Yagi K, Nakayama K and Hirata K: Endothelin-converting
Enzyme-1 gene ablation attenuates pulmonary fibrosis via
CGRP-cAMP/EPAC1 pathway. Am J Respir Cell Mol Biol. 48:465–476.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Geiser T, Ishigaki M, Van Leer C, Matthay
MA and Broaddus VC: H(2)O(2) inhibits alveolar epithelial wound
repair in vitro by induction of apoptosis. Am J Physiol Lung Cell
Mol Physiol. 287:L448–L453. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bao T, Liu X, Hu J, Ma M, Li J, Cao L, Yu
B, Cheng H, Zhao S and Tian Z: Recruitment of PVT1 enhances
YTHDC1-mediated m6A modification of IL-33 in Hyperoxia-induced lung
injury during bronchopulmonary dysplasia. Inflammation. Nov
2–2023.doi: 10.1007/s10753-023-01923-1 (Epub ahead of print).
View Article : Google Scholar
|
|
58
|
Yang M, Chen Y, Huang X, Shen F and Meng
Y: ETS1 Ameliorates Hyperoxia-Induced bronchopulmonary dysplasia in
mice by activating Nrf2/HO-1 mediated ferroptosis. Lung.
201:425–441. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang X, Chu X, Gong X, Zhou H and Cai C:
The expression of miR-125b in Nrf2-silenced A549 cells exposed to
hyperoxia and its relationship with apoptosis. J Cell Mol Med.
24:965–972. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
He F, Wang QF, Li L, Yu C, Liu CZ, Wei WC,
Chen LP and Li HY: Melatonin protects against hyperoxia-induced
apoptosis in alveolar epithelial type II cells by activating the
MT2/PI3K/AKT/ETS1 signaling pathway. Lung. 201:225–234. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang X, Huo R, Liang Z, Xu C, Chen T, Lin
J, Li L, Lin W, Pan B, Fu X and Chen S: Simvastatin Inhibits NLRP3
inflammasome activation and ameliorates lung injury in
Hyperoxia-Induced bronchopulmonary dysplasia via the KLF2-Mediated
mechanism. Oxid Med Cell Longev. 2022:83360702022.PubMed/NCBI
|
|
62
|
Wan H, Chen XY, Zhang F, Chen J, Chu F,
Sellers ZM, Xu F and Dong H: Capsaicin inhibits intestinal
Cl-secretion and promotes Na+ absorption by blocking TRPV4 channels
in healthy and colitic mice. J Biol Chem. 298:1018472022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen YS, Lian YZ, Chen WC, Chang CC,
Tinkov AA, Skalny AV and Chao JCJ: Lycium barbarum polysaccharides
and capsaicin inhibit oxidative stress, inflammatory responses, and
pain signaling in rats with dextran sulfate sodium-induced colitis.
Int J Mol Sci. 23:24232022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang Q, Luo P, Xia F, Tang H, Chen J,
Zhang J, Liu D, Zhu Y, Liu Y, Gu L, et al: Capsaicin ameliorates
inflammation in a TRPV1-independent mechanism by inhibiting
PKM2-LDHA-mediated Warburg effect in sepsis. Cell Chem Biol.
29:1248–1259.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|