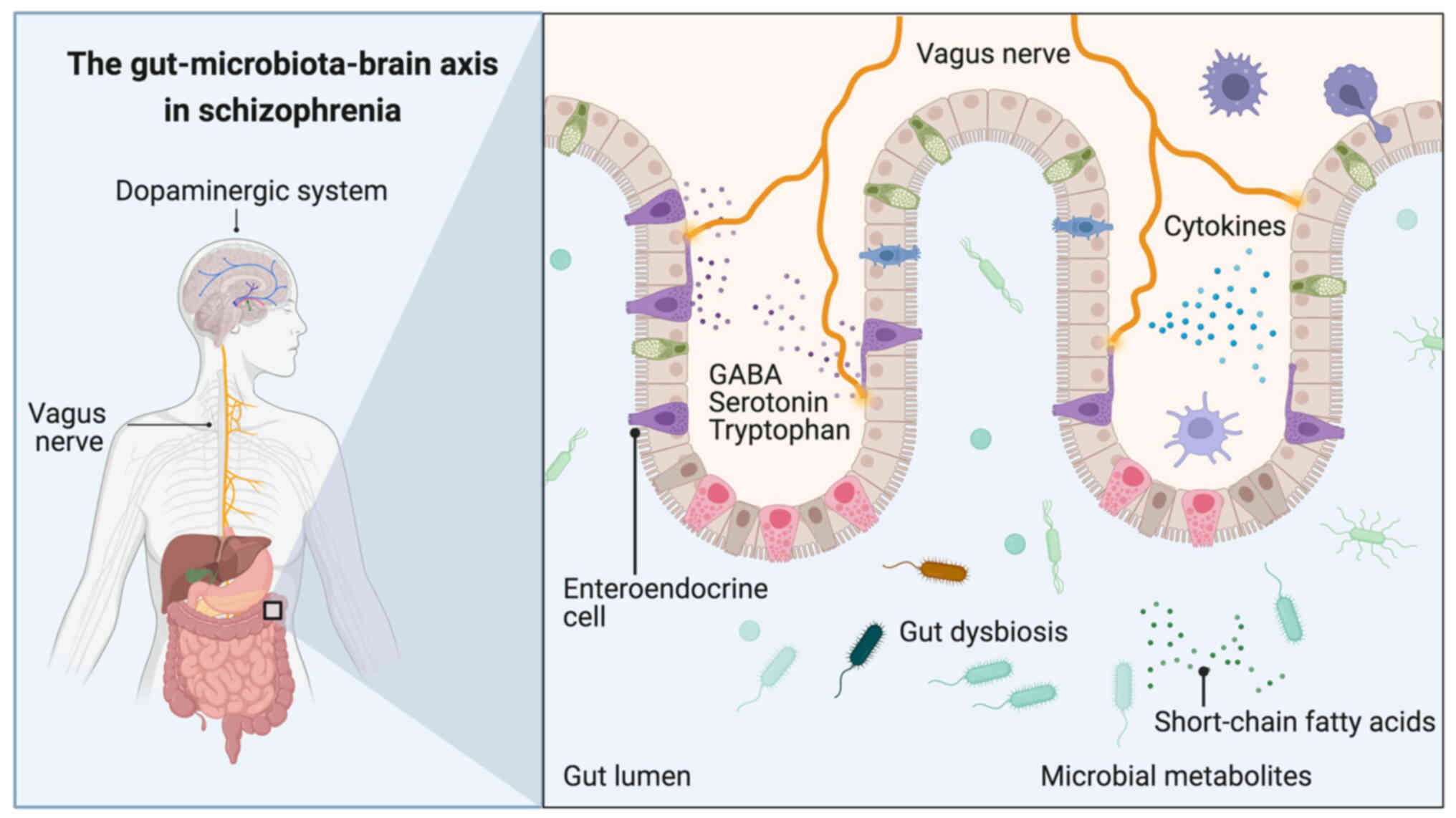
Changes in the balance, composition and diversity of
the gut microbiota (gut dysbiosis) have been found to be associated
with the occurrence of various neuropsychiatric disorders and
psychosis (1–3). Specifically, the gut-microbiota-brain
axis, is a complex bidirectional connection system linking the
gastrointestinal tract to the brain (Fig. 1), which passes through various
connecting roots, such as the neuroendocrine system, the vagus
nerve and the immune system (4).
Additionally, the gut microbiota is responsible for the generation
of short-chain fatty acids (SCFAs), neurotransmitters [dopamine,
norepinephrine, norepinephrine-gamma-aminobutyric acid (GABA),
glutamate and serotonin] and precursors of neurotransmitters. All
these substances have been found to affect brain processes
(5–7); for instance, SCFAs have been shown to
prevent blood-brain barrier dysfunction and promote neurogenesis,
angiogenesis and long-term memory storage (8).
Early-life stress and various stressors encountered
have been shown to be associated with gut dysbiosis, leading to
irregular immunological and neuroendocrine functions, which
potentially contribute to the occurrence of first-episode psychosis
(FEP) (9). The treatment of
psychosis could entail specific dietary interventions, along with
the use of probiotics, prebiotics and fecal microbiota
transplantation (10,11).
The gut microbiota has been found to be associated
with the occurrence of schizophrenia (SCH) (11,12).
Numerous bacterial species have been found to have
serotonin-synthesizing qualities (13), while abnormalities in the
conversion route of tryptophan to serotonin have been reported to
be linked to SCH (10). In the
study by Zhu et al (14),
the transfer of fecal microbiota from patients with SCH into
antibiotic-treated mice resulted in psychomotor hyperactivity,
diminished learning and memory, as well as in an increase in
tryptophan catabolism through the kynurenine (Kyn)-kynurenic acid
(Kyna) route in the peripheral and central nervous systems [Kyn and
Kyna are known to influence central glutamate and serotonin systems
(15)]. In the study by Zhu et
al (14), the Kyn/Kyna levels
were found to be associated with glutamate in the prefrontal
cortex, dopamine in the striatum, and glutamate and tryptophan in
the hippocampus; additionally, elevated basal levels of
extracellular dopamine were found in the prefrontal cortex and
5-hydroxytryptamine in hippocampus in mice receiving SCH fecal
microbiota (14).
Furthermore, it has been proposed that d-serine may
be associated with SCH; consequently, changes in the gut microbiota
could modulate the metabolism of d-amino acids (16,17).
Moreover, Lactobacillus and Bifidobacterium in the
gut may be able to produce GABA, a neurotransmitter that has been
shown to be associated with SCH (16). Within this context, alterations in
the glutamate glutamine-GABA cycle and reduced levels of glutamate
in the brain were proposed to be associated with NMDA receptor
hypofunction in SCH (18).
Furthermore, the utilization of NMDA receptor antagonists
(phencyclidine and ketamine) may elicit psychotic positive and
negative symptoms, along with cognitive dysfunction in healthy
subjects, patients with SCH and rodents (19,20).
The dysregulation of dopamine in the brain has also been found to
be associated with SCH. Staphylococcus can convert l-DOPA
into dopamine in the human gut (21). It is noteworthy that in the study
by Li et al (22), the
shortage of vitamin B6 decreased dopamine levels and produced
social deficits and excitation/inhibition abnormality in
EphB6-deficient mice.
Gut dysbiosis may play a role in the occurrence of
FEP and may be considered an objective for treatment (33). Below, 12 studies (presented in
Table I) are discussed, which
examined the effects of gut dysbiosis in treatment resistance and
the psychotic symptomatology of subjects with FEP.
It is worth noting that an increased intestinal
permeability, chronic inflammation and oxidative stress, observed
in subjects with SCH, have been proposed to be associated with
changes in the microbiome (1,25).
Moreover, the gut microbiota mediates the regulation of
pro-inflammatory cytokines; in fact, increased amounts of IL-6,
TNF-a, soluble IL-2 receptor, and elevated prostaglandin E2 levels
and COX activity have been observed in subjects with FEP (44,45).
Previous research using rats demonstrated that exposure to diverse
early-life stressors (e.g., maternal separation or social
isolation) could alter the hypothalamic-pituitary-adrenal (HPA)
axis and intensify the plasma corticosterone response following
acute stress, resulting in the elevated production of
pro-inflammatory cytokines (46,47).
Subsequently, Ko and Liu (48)
observed an increase in the levels of pro-inflammatory cytokines
(IL-1b, IL-6 and TNF-a) following 4 weeks of the rearing of rats in
isolation. In the study by Dunphy-Doherty et al (49), it was found that continuous social
isolation in rats (a confirmed animal model for SCH) resulted in
changes to the gut microbiota (increases in Actinobacteria and
decreases in the class Clostridia), decreased levels of hippocampal
IL-6 and IL-10, and modified neurogenesis (significantly fewer
BrdU/NeuN-positive cells in the dentate gyrus compared with the
controls).
The treatment of gut dysbiosis in patients with FEP
could entail specific dietary interventions, along with the use of
probiotics, prebiotics and fecal microbiota transplantation. The
administration of vitamin D and probiotics, including
Bifidobacterium bifidum, Lactobacillus acidophilus,
Lactobacillus fermentum and Lactobacillus reuteri has
been shown to result in improvements in the general and total PANSS
score, and in metabolic parameters, as well as in a decrease in
C-reactive protein levels in patients with SCH (67). In another study, patients with SCH
that were administered a probiotic with Bifidobacterium
breve A-1, exhibited elevated levels of IFN-γ, IL-22, IL-1R and
IL-10, and decreased TNF-α levels; these results were associated
with ameliorated hospital anxiety and depression scale scores and
positive and negative syndrome scale scores (68). In the study by Bravo et al
(69), non-vagotomized mice fed
Lactobacillus rhamnosus demonstrated decreased anxiety
levels, with a modified central GABA receptor expression. In the
study by Kao et al (70),
the prebiotic, Bimuno galactooligosaccharide™ (B-GOS®),
was administered to rats in combination with olanzapine; it
decreased the acetate concentrations, blocked weight gain and
improved cognitive function in psychosis. Fecal microbiota
transplantation is still at the experimental stage and there are
only a limited number of reports evaluating its efficacy in
psychosis (71,72). Optogenetic manipulations of the gut
brain axis in patients with SCH have been also proposed and are
under examination (73).
The present review discussed the instrumental role
of the gut microbiota in the brain and its influence on the
occurrence of FEP. There is great heterogeneity observed in the
changes in gut microbiota composition; however, the studies
presented herein demonstrate significant GM alterations in patients
with FEP vs. the controls, and underline the significant role of
gut dysbiosis in the occurrence, symptom intensity and treatment
efficacy in FEP. It should be noted that the composition of the gut
microbiota in subjects with FEP may be influenced by various
dietary, environmental and treatment factors; consequently,
investigating the gut microbiota in various populations may provide
the opportunity for the establishment of causal associations and
the possibility for personalized treatment strategies (74–76).
Within this context, further human studies are warranted, with a
sufficient number of subjects who are at risk of developing
psychosis, in order to further enable the evaluation of GM
alterations related to FEP.
Not applicable.
Funding: No funding was received.
Not applicable.
CT and ER made substantial contributions to the
conception and design of the study, and the acquisition, analysis
or interpretation of data to be included in the review. CT and ER
were also involved in the drafting of the manuscript, and in
revising it critically for important intellectual content. MIS, MD,
EA, KT, NS and DAS contributed to the design of the study. All
authors have read and approved the final version of the manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
|
1
|
Nguyen TT, Kosciolek T, Eyler LT, Knight R
and Jeste DV: Overview and systematic review of studies of
microbiome in schizophrenia and bipolar disorder. J Psychiatr Res.
99:50–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Ameringen M, Turna J, Patterson B,
Pipe A, Mao RQ, Anglin R and Surette MG: The gut microbiome in
psychiatry: A primer for clinicians. Depress Anxiety. 36:1004–1025.
2019. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu JCW, Gorbovskaya I, Hahn MK and Müller
DJ: The gut microbiome in schizophrenia and the potential benefits
of prebiotic and probiotic treatment. Nutrients. 13:11522021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gómez-Eguílaz M, Ramón-Trapero JL,
Pérez-Martínez L and Blanco JR: The microbiota-gut-brain axis and
its large projections. Rev Neurol. 68:111–117. 2019.(In Spanish).
PubMed/NCBI
|
|
5
|
Strandwitz P: Neurotransmitter modulation
by the gut microbiota. Brain Res. 1693:128–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Xu J and Chen Y: Regulation of
Neurotransmitters by the gut microbiota and effects on cognition in
neurological disorders. Nutrients. 13:20992021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stopińska K, Radziwoń-Zaleska M and
Domitrz I: The microbiota gut-brain axis as a key to
neuropsychiatric disorders: A mini review. J Clin Med. 10:46402021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michel L and Prat A: One more role for the
gut: Microbiota and blood brain barrier. Ann Transl Med.
4:152016.PubMed/NCBI
|
|
9
|
Giannopoulou I, Georgiades S, Stefanou MI,
Spandidos DA and Rizos E: Links between trauma and psychosis
(Review). Exp Ther Med. 26:3862023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang DW, Adams JB, Coleman DM, Pollard EL,
Maldonado J, McDonough-Means S, Caporaso JG and Krajmalnik-Brown R:
Long-term benefit of microbiota transfer therapy on autism symptoms
and gut microbiota. SciRep. 9:58212019.
|
|
11
|
Rantala MJ, Luoto S, Borraz-Leon JI and
Krams I: Schizophrenia: The new etiological synthesis. Neurosci
Biobehav Rev. 142:1048942022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Munawar N, Ahmad A, Anwar MA and Muhammad
K: Modulation of gut microbial diversity through non-pharmaceutical
approaches to treat schizophrenia. Int J Mol Sci. 23:26252022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roth W, Zadeh K, Vekariya R, Ge Y and
Mohamadzadeh M: Tryptophan metabolism and gut brain homeostasis.
Int J Mol Sci. 22:29232021. View Article : Google Scholar
|
|
14
|
Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q,
Sun Q, Fan Y, Xie Y, Yang Z, et al: Transplantation of microbiota
from drug-free patients with schizophrenia causes
schizophrenia-like abnormal behaviors and dysregulated kynurenine
metabolism in mice. Mol Psychiatry. 25:2905–2918. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Agus A, Planchais J and Sokol H: Gut
microbiota regulation of tryptophan metabolism in health and
disease. Cell Host Microbe. 23:716–724. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghorbani M, Rajandas H, Parimannan S,
Stephen Joseph GB, Tew MM, Ramly SS, Muhamad Rasat MA and Lee SY:
Understanding the role of gut microbiota in the pathogenesis of
schizophrenia. Psychiatr Genet. 31:39–49. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mikulska J, Juszczyk G, Gawrońska-Grzywacz
M and Herbet M: Brain sciences HPA Axis in the pathomechanism of
depression and schizophrenia: New therapeutic strategies based on
its participation. Brain Sci. 11:12982021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng P, Zeng B, Liu M, Chen J, Pan J, Han
Y, Liu Y, Cheng K, Zhou C, Wang H, et al: The gut microbiome from
patients with schizophrenia modulates the glutamate-glutamine-GABA
cycle and schizophrenia-relevant behaviors in mice. Sci Adv.
5:eaau83172019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krystal JH, Karper LP, Seibyl JP, Freeman
GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr and Charney
DS: Subanesthetic effects of the non-competitive NMDA antagonist,
ketamine, in humans: Psychotomimetic, perceptual, cognitive, and
neuroendocrine responses. Arch Gen Psychiatry. 51:199–214. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jodo E: The role of the
hippocampo-prefrontal cortex system in phencyclidine-induced
psychosis: A model for schizophrenia. J Physiol Paris. 107:434–440.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luqman A, Nega M, Nguyen MT, Ebner P and
Götz F: SadA: Expressing staphylococci in the human gut show
increased cell adherence and internalization. Cell Rep. 22:535–545.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Luo ZY, Hu YY, Bi YW, Yang JM, Zou
WJ, Song YL, Li S, Shen T, Li SJ, et al: The gut microbiota
regulates autism-like behavior by mediating vitamin B6 homeostasis
in EphB6-deficient mice. Microbiome. 8:1202020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Severance EG, Alaedini A, Yang S, Halling
M, Gressitt KL, Stallings CR, Origoni AE, Vaughan C, Khushalani S,
Leweke FM, et al: Gastrointestinal inflammation and associated
immune activation in schizophrenia. Schizophr Res. 138:48–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Severance EG, Gressitt KL, Yang S,
Stallings CR, Origoni AE, Vaughan C, Khushalani S, Alaedini A,
Dickerson FB and Yolken RH: Seroreactive marker for inflammatory
bowel disease and associations with antibodies to dietary proteins
in bipolar disorder. Bipolar Disord. 16:230–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Severance EG, Gressitt KL, Stallings CR,
Origoni AE, Khushalani S, Leweke FM, Dickerson FB and Yolken RH:
Discordant patterns of bacterial translocation markers and
implications for innate immune imbalances in schizophrenia.
Schizophr Res. 148:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Severance EG, Yolken RH and Eaton WW:
Autoimmune diseases, gastrointestinal disorders and the microbiome
in schizophrenia: More than a gut feeling. Schizophr Res.
176:23–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu F, Ju Y, Wang W, Wang Q, Guo R, Ma Q,
Sun Q, Fan Y, Xie Y, Yang Z, et al: Metagenome-Wide association of
gut microbiome features for schizophrenia. Nat Commun. 11:16122020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen Y, Xu J, Li Z, Huang Y, Yuan Y, Wang
J, Zhang M, Hu S and Liang Y: Analysis of gut microbiota diversity
and auxiliary diagnosis as a biomarker in patients with
schizophrenia: A cross-sectional study. Schizophr Res. 197:470–477.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Zhuo M, Huang X, Huang Y, Zho J,
Xiong D, Li J, Liu Y, Pan Z, Li H, et al: Altered gut microbiota
associated with symptom severity in schizophrenia. PeerJ.
8:e95742020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu R, Wu B, Liang J, He F, Gu W, Li K, Luo
Y, Chen J, Gao Y, Wu Z, et al: altered gut microbiota and mucosal
immunity in patients with schizophrenia. Brain Behav Immun.
85:120–127. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nguyen TT, Kosciolek T, Maldonado Y, Daly
RE, Martin AS, McDonald D, Knight R and Jeste DV: Differences in
gut microbiome composition between persons with chronic
schizophrenia and healthy comparison subjects. Schizophr Res.
204:23–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nocera A and Nasrallah HA: The association
of the gut microbiota with clinical features in schizophrenia.
Behav Sci (Basel). 12:892022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sen P, Prandovszky E, Honkanen JK, Chen O,
Yolken R and Suvisaari J: Dysregulation of microbiota in patients
with first-episode psychosis is associated with symptom severity
and treatment response. Biol Psychiatry. 95:370–379. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwarz E, Maukonen J, Hyytiäinen T,
Kieseppä T, Orešič M, Sabunciyan S, Mantere O, Saarela M, Yolken R
and Suvisaari J: Analysis of microbiota in first episode psychosis
identifies preliminary associations with symptom severity and
treatment response. Schizophr Res. 192:398–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He Y, Kosciolek T, Tang J, Zhou Y, Li Z,
Ma X, Zhu Q, Yuan N, Yuan L, Li C, et al: Gut microbiome and
magnetic resonance spectroscopy study of subjects at ultra-high
risk for psychosis may support the membrane hypothesis. Eur
Psychiatry. 53:37–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan X, Zhang P, Wang Y, Liu Y, Li X,
Kumar BU, Hei G, Lv L, Huang XF, Fan X and Song X: Changes in
metabolism and microbiota after 24-week risperidone treatment in
drug naïve, normal weight patients with first episode
schizophrenia. Schizophr Res. 201:299–306. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, Pan LY, Zhang Z, Zhou YY, Jiang
HY and Ruan B: Analysis of gut mycobiota in first-episode,
drug-naïve Chinese patients with schizophrenia: A pilot study.
Behav Brain Res. 379:1123742020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma X, Asif H, Dai L, He Y, Zheng W, Wang
D, Ren H, Tang J, Li C, Jin K, et al: Alteration of the gut
microbiome in first-episode drug-naïve and chronic medicated
schizophrenia correlate with regional brain volumes. J Psychiatr
Res. 123:136–144. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu C, Zheng M, Ali U, Xia Q, Wang Z,
Chenlong, Yao L, Chen Y, Yan J, Wang K, et al: Association between
abundance of haemophilus in the gut microbiota and negative
symptoms of schizophrenia. Front Psychiatry. 12:6859102021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuan X, Wang Y, Li X, Jiang J, Kang Y,
Pang L, Zhang P, Li A, Lv L, Andreassen OA, et al: Gut microbial
biomarkers for the treatment response in first-episode, drug-naïve
schizophrenia: A 24-week follow-up study. Transl Psychiatry.
11:4222021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Fan X, Yuan X, Pang L, Hu S, Wang Y,
Huang X and Song X: The role of butyric acid in treatment response
in drug-naïve first episode schizophrenia. Front Psychiatry.
12:7246642021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan X, Li X, Kang Y, Pang L, Hei G, Zhang
X, Wang S, Zhao X, Zhang S, Tao Q, et al: Gut mycobiota dysbiosis
in drug-naïve, first-episode schizophrenia. Schizophr Res.
250:76–86. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Z, Yuan X, Zhu Z, Pang L, Ding S, Li
X, Kang Y, Hei G, Zhang L, Zhang X, et al: Multiomics analyses
reveal microbiome-gut-brain crosstalk centered on aberrant
gamma-aminobutyric acid and tryptophan metabolism in drug-naïve
patients with first-episode schizophrenia. Schizophr Bull.
50:187–198. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
García-Bueno B, Bioque M, Mac-Dowell KS,
Barcones MF, Martínez-Cengotitabengoa M, Pina-Camacho L,
Rodríguez-Jiménez R, Sáiz PA, Castro C, Lafuente A, et al:
Pro-/anti-inflammatory dysregulation in patients with first episode
of psychosis: Toward an integrative inflammatory hypothesis of
schizophrenia. Schizophr Bull. 40:376–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
García-Bueno B, Bioque M, MacDowell KS,
Santabárbara J, Martínez-Cengotitabengoa M, Moreno C, Sáiz PA,
Berrocoso E, Gassó P, Fe Barcones M, et al: Pro-/antiinflammatory
dysregulation in early psychosis: Results from a 1-year
follow-upstudy. Int J Neuropsychopharmacol. 18:pyu0372014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
O'Mahony SM, Marchesi JR, Scully P,
Codling C, Ceolho AM, Quigley EM, Cryan JF and Dinan TG: Early life
stress alters behavior, immunity, and microbiota in rats:
implications for irritable bowel syndrome and psychiatric
illnesses. Biol Psychiatry. 65:263–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Plotsky PM, Thrivikraman KV, Nemeroff CB,
Caldji C, Sharma S and Meaney MJ: Long-term consequences of
neonatal rearing on central corticotropin releasing factor systems
in adult male rat offspring. Neuropsychopharmacology. 30:2192–2204.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ko CY and Liu YP: Isolation rearing
impaired sensorimotor gating but increased pro-inflammatory
cytokines and disrupted metabolic parameters in both sexes of rats.
Psychoneuroendocrinology. 55:173–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dunphy-Doherty F, O'Mahony SM, Peterson
VL, O'Sullivan O, Crispie F, Cotter PD, Wigmore P, King MV, Cryan
JF and Fone KCF: Post-weaning social isolation of rats leads to
long-term disruption of the gut microbiota-immune-brain axis. Brain
Behav Immun. 68:261–273. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Morgan C, Charalambides M, Hutchinson G
and Murray RM: Migration, ethnicity, and psychosis: Toward a
sociodevelop-mental model. Schizophr Bull. 36:655–664. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Davis EG, Humphreys KL, McEwen LM, Sacchet
MD, Camacho MC, MacIsaac JL, Lin DTS, Kobor MS and Gotlib IH:
Accelerated DNA methylation age in adolescent girls: Associations
with elevated diurnal cortisol and reduced hippocampal volume.
Transl Psychiatry. 7:e12232017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen Q, Li D, Jin W, Shi Y, Li Z, Ma P,
Sun J, Chen S, Li P and Lin P: Research progress on the correlation
between epigenetics and schizophrenia. Front Neurosci.
15:6887272021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Alameda L, Rodriguez V, Carr E, Aas M,
Trotta G, Marino P, Vorontsova N, Herane-Vives A, Gadelrab R,
Spinazzola E, et al: A systematic review on mediators between
adversity and psychosis: Potential targets for treatment. Psychol
Med. 50:1966–1976. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bayer TA, Falkai P and Maier W: Genetic
and non-genetic vulnerability factors in schizophrenia: The basis
of the ‘two hit hypothesis.’. J Psychiatr Res. 33:543–548. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Charmandari E, Kino T, Souvatzoglou E and
Chrousos GP: Pediatric stress: Hormonal mediators and human
development. Horm Res. 59:161–179. 2003.PubMed/NCBI
|
|
56
|
Lardinois M, Lataster T, Mengelers R, Van
Os J and Myin-Germeys I: Childhood trauma and increased stress
sensitivity in psychosis. Acta Psychiatr Scand. 23:28–35. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Walker EF, Brennan PA, Esterberg M,
Brasfield J, Pearce B and Compton MT: Longitudinal changes in
cortisol secretion and conversion to psychosis in at-risk youth. J
Abnorm Psychol. 119:401–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Walker EF, Trotman HD, Pearce BD,
Addington J, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH,
Perkins DO, Seidman LJ, et al: Cortisol levels and risk for
psychosis: Initial findings from the North American prodrome
longitudinal study. Biol Psychiatry. 74:410–417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sapolsky RM: Glucocorticoids and
hippocampal atrophy in neuropsychiatric disorders. Arch Gen
Psychiatry. 57:925–935. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Vita A, De Peri L, Silenzi C and Dieci M:
Brain morphology in first-episode schizophrenia: A meta-analysis of
quantitative magnetic resonance imaging studies. Schizophr Res.
82:75–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Thompson Ray M, Weickert CS, Wyatt E and
Webster MJ: Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in
the hippo-campus of individuals with schizophrenia and mood
disorders. J Psychiatry Neurosci. 36:195–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Daskalakis NP, De Kloet ER, Yehuda R,
Malaspina D and Kranz TM: Early life stress effects on
Glucocorticoid-BDNF interplay in the hippocampus. Front Mol
Neurosci. 8:682015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rizos EN, Rontos I, Laskos E, Arsenis G,
Michalopoulou PG, Vasilopoulos D, Gournellis R and Lykouras L:
Investigation of serum BDNF levels in drug-naive patients with
schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry.
32:1308–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rizos EN, Papathanasiou M, Michalopoulou
PG, Mazioti A, Douzenis A, Kastania A, Nikolaidou P, Laskos E,
Vasilopoulou K and Lykouras L: Association of serum BDNF levels
with hippocampal volumes in first psychotic episode drug-naive
schizophrenic patients. Schizophr Res. 129:201–204. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rizos EN, Michalopoulou PG, Siafakas N,
Stefanis N, Douzenis A, Rontos I, Laskos E, Kastania A, Zoumpourlis
V and Lykouras L: Association of serum brain-derived neurotrophic
factor and duration of untreated psychosis in first-episode
patients with schizophrenia. Neuropsychobiology. 62:87–90. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Theleritis C, Fisher HL, Shäfer I, Winters
L, Stahl D, Morgan C, Dazzan P, Breedvelt J, Sambath I, Vitoratou
S, et al: Brain derived Neurotropic Factor (BDNF) is associated
with childhood abuse but not cognitive domains in first episode
psychosis. Schizophr Res. 159:56–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ghaderi A, Banafshe HR, Mirhosseini N,
Moradi M, Karimi MA, Mehrzad F, Bahmani F and Asemi Z: Clinical and
metabolic response to vitamin D plus Probiotic in schizophrenia
patients. BMC Psychiatry. 19:772019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Okubo R, Koga M, Katsumata N, Odamaki T,
Matsuyama S, Oka M, Narita H, Hashimoto N, Kusumi I, Xiao J and
Matsuoka YJ: Effect of bifidobacterium breve A-1 on anxiety and
depressive symptoms in schizophrenia: A proof-of-concept study. J
Affect Disord. 245:377–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bravo JA, Forsythe P, Chew MV, Escaravage
E, Savignac HM, Dinan TG, Bienenstock J and Cryan JF: Ingestion of
Lactobacillus strain regulates emotional behavior and central GABA
receptor expression in a mouse via the vagus nerve. Proc Natl Acad
Sci USA. 108:16050–16055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kao ACC, Spitzer S, Anthony DC, Lennox B
and Burnet PWJ: Prebiotic attenuation of olanzapine-induced weight
gain in rats: Analysis of central and peripheral biomarkers and gut
microbiota. Transl Psychiatry. 8:662018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hinton R: A case report looking at the
effects of faecal microbiota transplantation in a patient with
bipolar disorder. Aust N Z J Psychiatry. 54:649–650. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Parker G, Spoelma MJ and Rhodes N: Faecal
microbiota transplantation for bipolar disorder: A detailed case
study. Bipolar Disord. 24:559–563. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Patrono E, Svoboda J and Stuchlík A:
Schizophrenia, the gut microbiota, and new opportunities from
optogenetic manipulations of the gut-brain axis. Behav Brain Funct.
17:72021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Nuncio-Mora L, Lanzagorta N, Nicolini H,
Sarmiento E, Ortiz G, Sosa F and Genis-Mendoza AD: The role of the
microbiome in first episode of psychosis. Biomedicines.
11:17702023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Aschner M, Skalny AV, Gritsenko VA,
Kartashova OL, Santamaria A, Rocha JBT, Spandidos DA, Zaitseva IP,
Tsatsakis A and Tinkov AA: Role of gut microbiota in the modulation
of the health effects of advanced glycation end-products (Review).
Int J Mol Med. 51:442023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tsamakis K, Galinaki S, Alevyzakis E,
Hortis I, Tsiptsios D, Kollintza E, Kympouropoulos S, Triantafyllou
K, Smyrnis N and Rizos E: Gut Microbiome: A brief review on its
role in schizophrenia and first episode of psychosis.
Microorganisms. 10:11212022. View Article : Google Scholar : PubMed/NCBI
|