Introduction
Gouty arthritis is a non-infectious autoinflammatory
disease caused by persistently high levels of serum uric acid (UA),
leading to the deposition of monosodium urate (MSU) crystals in the
joints and surrounding tissues (1). Over recent decades, the incidence and
prevalence of gout have steadily increased, driven by lifestyle and
dietary changes, as well as an aging population (2). Statistics indicate that the global
prevalence of gout ranges between 1 and 4%, with a male to female
ratio ranging between 3:1 and 10:1, impacting the quality of life
(3). Gouty arthritis commonly
affects obese postmenopausal women, older men and individuals of
middle age (4). The disease is
associated with several factors, including disruptions in purine
metabolism (5), decreased UA
excretion and excessive UA production (6,7).
According to the European League Against Rheumatism, gout can be
categorized into the following four stages based on disease
progression: Asymptomatic hyperuricemia, acute gouty arthritis
attack, intercritical gouty arthritis and chronic gouty arthritis
(8). From the second to the fourth
stage of gout, the primary treatment strategies include
anti-inflammatory measures and serum UA reduction. Common
medications include allopurinol (9), febuxostat (10) and non-steroidal anti-inflammatory
drugs, such as celecoxib and ibuprofen (11). However, long-term use of these
drugs is inevitably accompanied by serious toxic side effects. For
example, allopurinol can lead to kidney damage (12), febuxostat can increase the risk of
cardiovascular diseases (13), and
ibuprofen is associated with symptoms such as dizziness and
drowsiness (14). Addressing gouty
arthritis has become a global challenge, and identifying potential
effective ingredients for its treatment holds significant promise
for overcoming this issue (15).
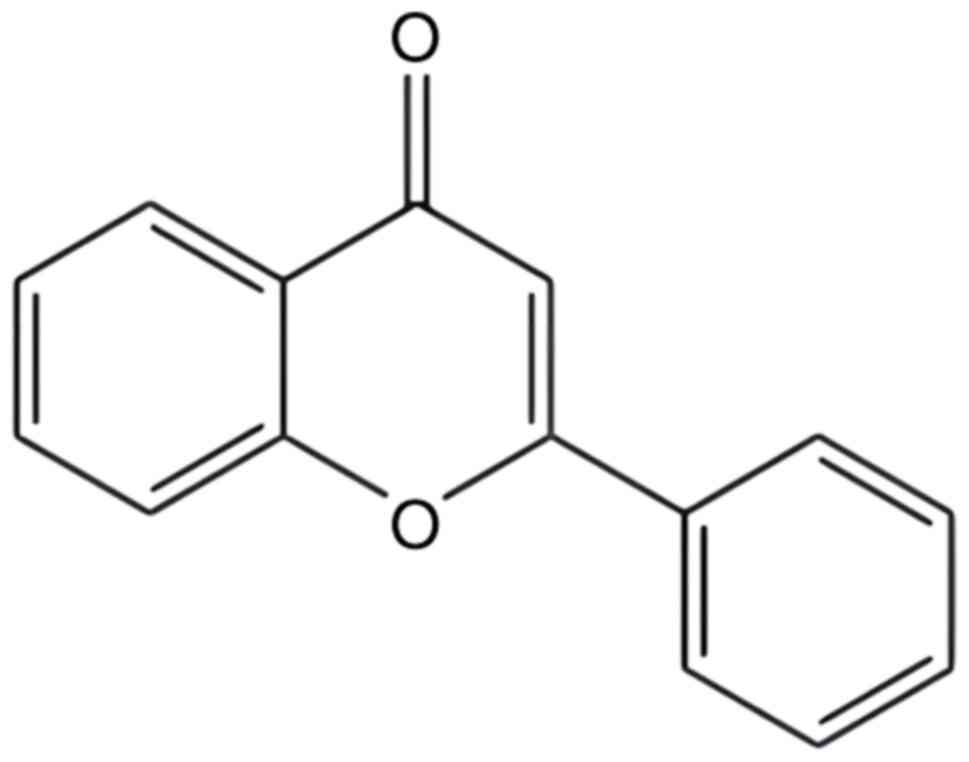
Flavonoids are significant secondary metabolites in
plants, characterized by a basic chemical structure comprising two
benzene rings linked by three carbon atoms, forming a C6-C3-C6
structure (Fig. 1) (16). These compounds are known for their
pronounced antitumor (17),
antioxidant (18) and
antibacterial (19) properties,
making them widely utilized in clinical research. Notably,
flavonoids have been identified to alleviate gouty arthritis. For
instance, Morin (2′,3′,4′,5,7-pentahydroxyflavone), found in figs,
apples, guava leaves, onions, tea and grains, is recognized for its
potential in treating gouty arthritis; it is particularly effective
in inhibiting inflammation triggered by MSU crystals (20).
The present study systematically reviewed the in
vivo and in vitro animal studies on flavonoids from
herbal medicines for the treatment of gouty arthritis that have
been previously published in the PubMed (https://pubmed.ncbi.nlm.nih.gov), ScienceDirect
(http://www.sciencedirect.com), Google
Scholar (http://scholar.google.cz) and China
National Knowledge Infrastructure databases (http://www.cnki.net) between 2000 and 2023. We
searched using the keywords ‘gouty arthritis’, ‘flavonoids’ and
‘mechanism study’. The literature inclusion criteria for this study
were that the study was a mechanistic study of flavonoids in the
treatment of gouty arthritis; and the study model was a gouty
arthritic animal receiving flavonoid treatment. The study excluded
repetitive studies, unfinished studies, studies with no available
data or incomplete data, literature with too low a quality rating
and literature with only abstracts and no access to full text.
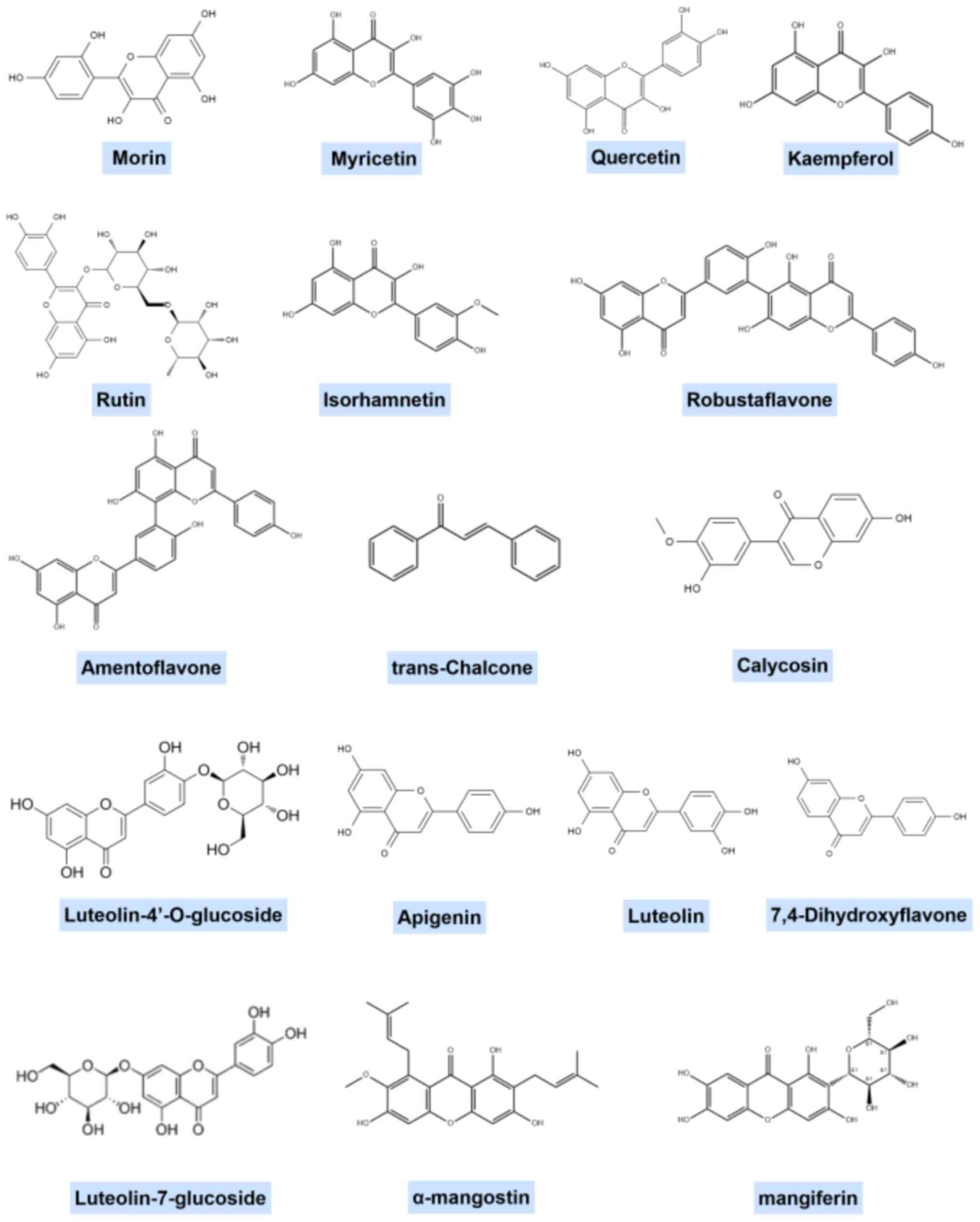
Given the extensive variety of flavonoid structural
classifications, it is challenging to generalize the structural
features of flavonoids that may be effective against gouty
arthritis. The representative structural formulae of a number of
flavonoids are shown in Fig.
2.
Extensive research has demonstrated that flavonoids
derived from natural herbs can markedly decrease UA levels
(21–23). More crucially, their therapeutic
benefits in managing gouty arthritis are attributed to various
mechanisms. These include reducing xanthine oxidase (XOD) activity
(24), regulating UA transporters
to promote UA excretion (25),
alleviating the inflammatory response (25,26)
and reducing oxidative stress (27). Such findings are fundamentally
important for the screening and identification of medications for
gouty arthritis from the natural chemical components found in
herbs. Table I outlines the
mechanisms of action of these flavonoids.
 | Table I.Pathways involved in
flavonoid-mediated improvement of gouty arthritis. |
Table I.
Pathways involved in
flavonoid-mediated improvement of gouty arthritis.
| Mechanisms | Source | Name | Models | Regulated
targets |
|---|
| Inhibition of
XOD | Maclura
cochinchinensis (Lour.) | Morin | Mice, PO | XOD |
| activity | Corner
heartwood |
|
|
|
|
| Morus alba
L. | Myricetin,
quercetin, rutin, | Mice, PO | XOD |
|
|
| kaempferol,
isorhamnetin |
|
|
|
| Gnaphalium
pensylvanicum Willd. |
Luteolin-7-glucoside | Mice, MSU | XOD |
|
| Pseudognaphalium
affine (D. Don) Anderb. |
Luteolin-4′-O-glucoside | Mice, MSU | XOD |
| Regulation of
the | Gnaphalium
affine D. Don |
7,4-Dihydroxyflavone | Mice, PO | mURAT1, mGLUT9 |
| uric acid
transporter | Gnaphalium
pensylvanicum Willd. |
Luteolin-7-glucoside | Mice, MSU | GLUT9, OAT1,
URAT1 |
|
| Garcinia
mangostana L. | α-Mangostin | Mice, PO | GLUT9 |
|
| Anemarrhena
asphodeloides Bge. | Mangiferin | Mice, PO | mURAT1, mGLUT9,
mOAT1 |
| Inhibition of
inflammation |
|
|
|
|
|
NLRP3 | Cunninghamia
lanceolata (Lamb.) Hook. | Amentoflavone | Mice, MSU | IL-1β,
caspase-1 |
|
inflammasome | Cunninghamia
lanceolata (Lamb.) Hook. | Robustaflavone | Mice, MSU | IL-1β, caspase-1,
ASC, NLRP3 |
|
| Angelica
keiskei Koidz. | trans-Chalcone | Mice, MSU | IL-1β, TNF-α, IL-6,
TGF-β, |
|
|
|
|
| NLRP3, ASC,
pro-caspase-1, |
|
|
|
|
| pro-IL-1β,
NF-κB |
|
| Ruta
graveolens L. | Rutinum | Quail, high purine
diet | NLRP3 |
|
TLR4/MyD88/ | Astragalus
membranaceus (Fisch.) Bge. | Calycosin | Mice, MSU | AIM2, Keap1,
p-p65, |
|
NF-κB |
|
|
| p-IκBα, p62 |
|
|
|
| PBMCs and
THP-1 | IL-1β, IL-6, TNF-α,
IL-10, AIM2, |
|
|
|
| cells | Keap1, p-p65,
p-IκBα, p62 |
|
| Lagotis
brachystachys Maxim | Luteolin | Rats, MSU | TLR4, MyD88,
NF-κB |
|
| Lagotis
brachystachys Maxim |
Luteolin-4′-O-glucoside | Rats, MSU | TLR4, MyD88,
NF-κB |
|
| Lagotis
brachystachys Maxim | Apigenin | Rats, MSU | TLR4, MyD88,
NF-κB |
| Improvement of | Apocynum
lancifolium Rus. | Quercetin | Rats, MSU | MDA |
| oxidative
stress | Ruta
graveolens L. | Rutinum | Quail, high purine
diet | ROS |
|
|
|
| Rats, MSU | MDA, NO, SOD,
GSH-PX, CAT |
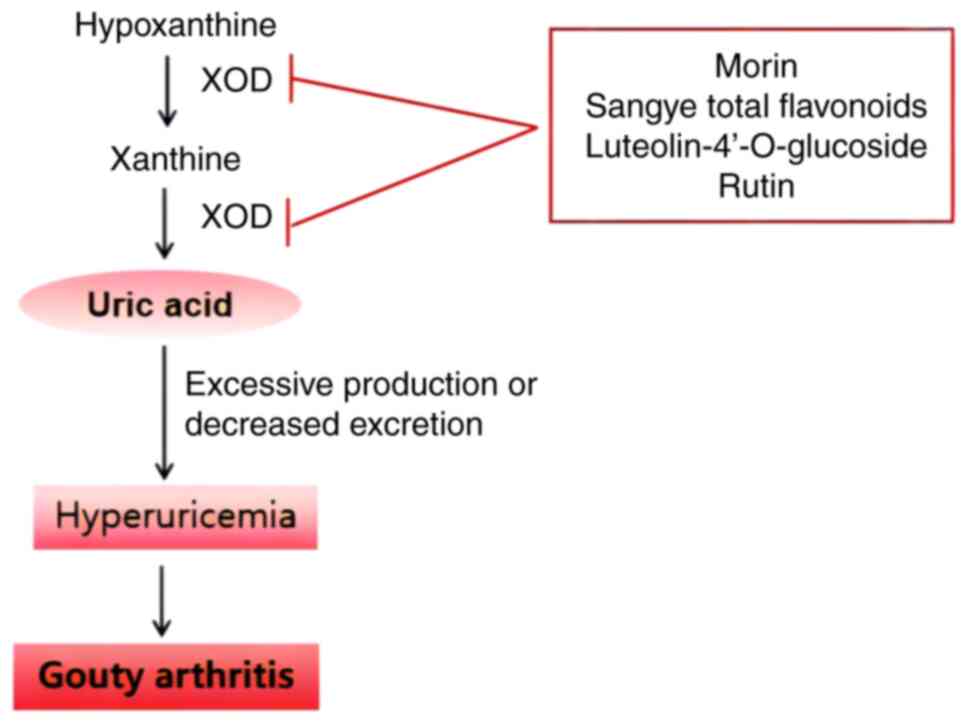
XOD and gouty arthritis
Abnormal XOD activity
XOD serves a crucial role in UA metabolism within
the body, promoting the oxidation of hypoxanthine to xanthine and
then further catalyzing the oxidation of xanthine to UA. Elevated
UA concentrations can lead to hyperuricemia, potentially triggering
attacks of gouty arthritis (28).
Inhibiting XOD activity reduces UA
production
In mouse models treated with intraperitoneal
injections of potassium oxonate and oral administration of
xanthine, Morin, a principal component of Gouji [Maclura
cochinchinensis (Lour.) Corner heartwood] extract, can inhibit
XOD activity in a non-competitive manner, lowering serum UA levels
(29). Sangye (Morus alba
L.), the leaf of the mulberry tree, contains flavonoids as its main
bioactive components (30). A
previous study (24) has indicated
that the key constituents of Sangye total flavonoids include
myricetin, quercetin, rutin, kaempferol and isorhamnetin. Serum UA
levels in hyperuricemic mice induced by potassium oxonate decreased
after 1 week of administration of Sangye total flavonoids. In the
same study, after 3 weeks of administration of Sangye total
flavonoids, serum UA approached normal levels in rats, and XOD
activity was reduced by 25.01% compared with that in the model
group. Furthermore, serum triglyceride and free fatty acid levels
were lowered, while total flavonoids of Sangye reduced the abnormal
liver and heart coefficient caused by adenine in mice (24). This suggests that Sangye total
flavonoids may regulate serum UA levels by inhibiting XOD and
managing lipid disorders. A study has also shown that luteolin
4′-O-glucoside and its aglycones, two primary flavonoid compounds
in Pseudognaphalium affine (D. Don) Anderb., can inhibit the
activity of XOD in hyperuricemic mice, thereby preventing UA
synthesis, and reducing the swelling and inflammation caused by MSU
crystals (31). In animal
research, a gouty arthritis rat model induced by MSU crystals
revealed reductions in XOD activity and improvements in gouty
arthritis symptoms after 5 days of rutin administration (Fig. 3) (25).
Furthermore, XOD generates a vast array of reactive
oxygen species (ROS), including H2O2 and
O2, through cascade reactions, using molecular oxygen as
an electron acceptor. These ROS are intricately linked to cell
damage, inflammation, carcinogenesis and aging (32). Thus, inhibiting XOD activity not
only potentially prevents gouty arthritis but also blocks
inflammatory pathways, reduces pro-inflammatory cytokines, and
offers protection against kidney injury, cardiovascular disease,
aging and cancer (33).
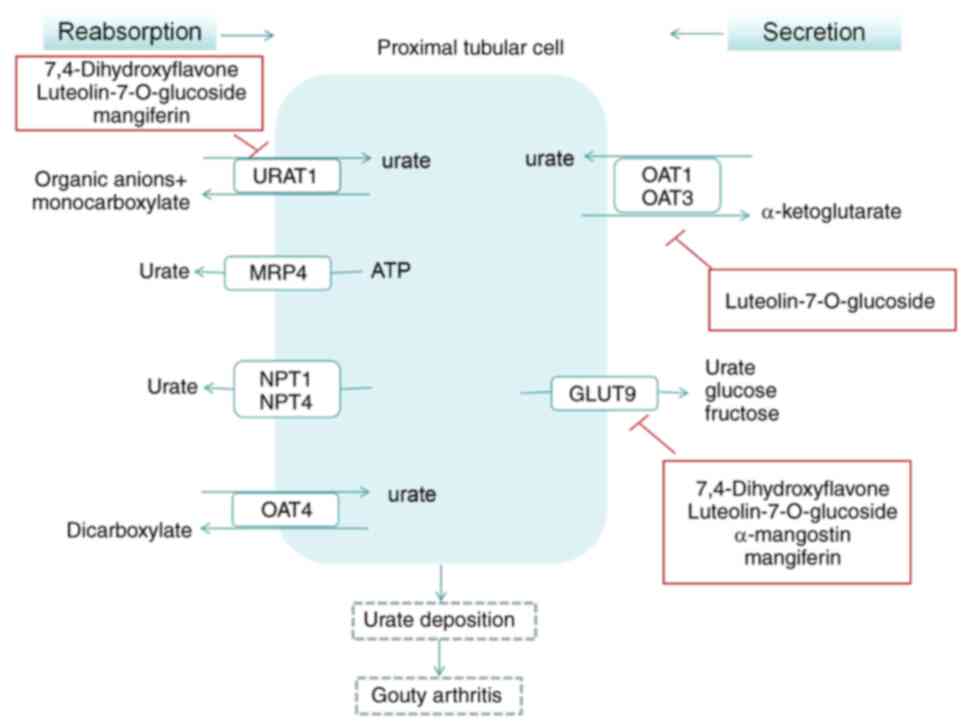
UA transporter and gouty arthritis
UA transporter disorder
The excretion of UA primarily occurs through UA
transporters in the kidneys, which are responsible for the
reabsorption and secretion of UA. The regulation of recombinant
urate transporter 1 (URAT1) and recombinant ATP binding cassette
transporter G2 promotes UA excretion and serves a crucial role in
the treatment of elevated UA levels (34). The kidney is a key organ in UA
excretion, and this process is divided into four stages (35): i) In total, >99% of serum UA is
filtered by the glomerulus; ii) 98% of the filtered UA is actively
reabsorbed in the S1 segment, the initial part of the proximal
renal tubules; iii) the active reabsorption of UA gradually
decreases in the S2 segment of the curvature of the proximal renal
tubules, and 50% of UA is secreted into the renal tubules; and iv)
in the straight S3 segment of the proximal renal tubules, the
concentration of UA in the renal tubules exceeds that in the
surrounding capillaries, resulting in passive reabsorption of UA
into the surrounding capillaries. Various transporters are involved
in this process. URAT1 is specifically expressed in human kidneys
and is located on the luminal side of the proximal tubular
epithelial cells in the renal cortex, mainly participating in the
reabsorption of urate by the proximal renal tubules (36). Glucose transporter 9 (GLUT9) is
primarily expressed in the kidney and liver, with two subtypes
(GLUT9L and GLUT9S); GLUT9L is found in the basement membrane of
proximal renal tubule cells and GLUT9S is located in the lateral
membrane of the proximal renal tubule (37). Studies have indicated that GLUT9
serves a crucial role in the transport of UA from intracellular to
extracellular spaces and participates in urate reabsorption at the
apical membrane of renal proximal tubules (38,39).
Furthermore, organic anion transporter (OAT)1 has been demonstrated
to be involved in the transport of UA in a dose- and time-dependent
manner, and it has been suggested to serve a role in the first step
of urate secretion, specifically, the uptake of urate from the
peritubular space into renal tubular cells (40,41).
OAT3 is predominantly expressed in proximal curved tubules, thick
ascending limbs of medullary loops and collecting ducts, and is
involved in urate transport. While the precise mechanism of OAT3 in
urate transport remains unclear, based on its expression site, it
is speculated that it may participate in the uptake of urate in
peripheral tubules, contributing to urate secretion, or in moving
urate from the basement membrane side into the peritubular
capillaries, thus engaging in urate reabsorption (42–44).
The aberrant transport of UA in the kidneys is a significant
pathogenic factor in gouty arthritis (45).
Regulation of UA transporters
In a mouse model of hyperuricemia induced by
oteracil potassium, the extract of Gnaphalium affine D. Don,
specifically 7,4-dihydroxyflavone, could regulate murine (m)URAT1
and mGLUT9 to reduce serum UA levels. This also assisted in
inhibiting the increase of urea nitrogen and creatinine levels
(46). Gnaphalium
pensylvanicum Willd., a traditional folk medicine used for
relieving inflammation, coughs and rheumatoid arthritis, contains a
high concentration of luteolin-7-O-glucoside, as identified by
ultra-performance liquid chromatography-electrospray tandem mass
spectrometry in prior studies (31,47).
Extracts from Gnaphalium pensylvanicum Willd. have been
demonstrated to alleviate foot swelling symptoms induced by MSU
crystals and reduce the infiltration of inflammatory cells
(48). Furthermore, western
blotting results indicated that the extract primarily decreased
serum UA by influencing GLUT9, OAT1 and URAT1, and by inhibiting
XOD activity in mice (48). Corn
silk, the style and stigma of the gramineous plant Zea mays
L., also known as Yu Shu Li Rui, is both a traditional food and
medicine in China, with flavonoids being its most effective
components (49). These
flavonoids, found in high concentrations in all parts of the corn
plant (50), can reduce UA levels
in hyperuricemia, effectively treating gout and gouty arthritis
(51). In an in vitro
experiment using HK-2 human renal tubular epithelial cells, the
impact of total flavonoids from corn silk on UA absorption and
related gene expression in HK-2 cells was assessed. After 48 h of
incubation, each concentration of total flavonoids from corn silk
inhibited UA absorption in HK-2 cells to varying degrees, with the
inhibition rate increasing with increasing concentration.
Furthermore, total flavonoids were able to reduce the UA-induced
apoptosis rate in HK-2 cells. There was a marked decrease in GLUT9
mRNA expression, and an increase in OAT1 and OAT3 mRNA expression
(52). α-Mangostin, the primary
active component in mangosteen peel extract, was shown to decrease
the serum UA level in hyperuricemic mice in a dose-dependent and
time-dependent manner, and increased the UA clearance rate in
hyperuricemic rats, indicative of the promotion of UA excretion in
the kidney. The study also revealed a reduction in the expression
levels of GLUT9 mRNA and protein in the kidneys of hyperuricemic
mice, suggesting the involvement of α-mangostin in the
downregulation of GLUT9 protein expression (53). Similarly, mangiferin, an active
component found in Anemarrhena asphodeloides Bge., was
capable of downregulating the mRNA and protein expression levels of
urate transporters mURAT1 and mGLUT9 in mice with renal
hyperuricemia induced by potassium oxonate. Mangiferin also
upregulated the expression levels of mOAT1, indicating that it may
promote UA excretion in hyperuricemic mice by inhibiting renal UA
reabsorption and increasing UA secretion, thus reducing serum UA
levels (54). These findings
suggest that the regulation of UA transporters is one of the
mechanisms through which flavonoids can improve hyperuricemia and
gouty arthritis (Fig. 4).
Immunoinflammatory disorders and gouty
arthritis
Immunoinflammatory disorders
The elevation of body UA levels due to abnormal
purine metabolism, surpassing the normal physiological serum UA
concentration, leads to a supersaturated state. This results in the
precipitation of MSU crystals, which accumulate in the joints and
surrounding tissues, causing inflammation (55). Inflammation and damage to the
joints and surrounding tissues are driven by the release of
inflammatory factors, which are regulated by numerous immune cells
and signaling pathways (56).
Phagocytes recognize the MSU crystals deposited in the joints
through various mechanisms, including the formation of immune
antibody complexes with the MSU crystals, promoting phagocytosis
through fragment crystallizable (Fc) receptors. MSU crystals can
also be directly recognized and phagocytosed by cell surface
receptors. Key receptors involved in recognizing MSU crystals
include CD16, CD11b, Toll-like receptor (TLR)2, TLR4 and CD14. The
interactions between these receptors and MSU crystals activate
downstream signaling pathways that mediate inflammation (57–59).
It has been suggested that MSU crystals can also directly bind to
cell membranes, causing tissue inflammatory damage (60,61).
Following phagocytosis and recognition of MSU crystals in the
joints, IL-1β expression is induced by signaling pathways such as
TLR, NOD-like receptor 3 (NLRP3), P2X purinoceptor 7 and
mitogen-activated protein kinase pathways, among others, leading to
an inflammatory response. Activation of these signaling pathways
results in the release of activated IL-1β into the cell,
subsequently attracting and activating inflammatory cells, such as
neutrophils, and releasing more inflammatory factors (62–64).
This process triggers an inflammatory cascade amplification
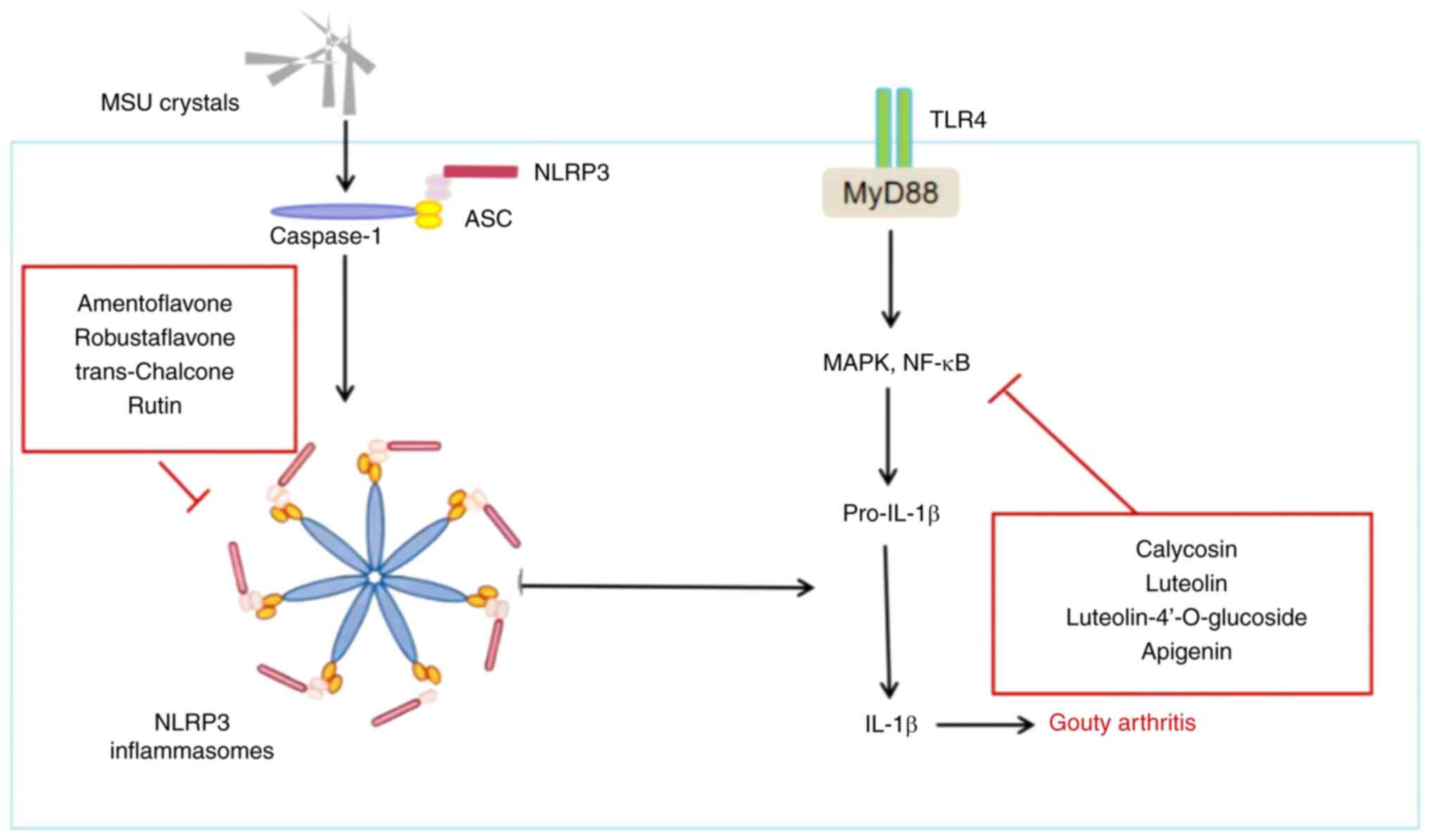
reaction (Fig. 5) (65).
Anti-inflammatory response
i) NLRP3 inflammasome. The NLRP3 inflammasome
comprises the effector protein pro-caspase-1, the receptor protein
NLRP3 and the adaptor protein apoptosis-associated speck-like
protein containing a CARD (ASC). The inflammasome can be activated
through various stimuli, including danger-associated molecular
patterns and pathogen-associated molecular patterns (66). Upon detection of a specific
activator, NLRP3 undergoes a conformational change, which then
promotes ASC oligomerization to form ASC ‘spots’ (67). Serving as a platform for
macromolecular signaling, ASC attracts pro-caspase-1 through its
CARD domain, enabling pro-caspase-1 to be cleaved and produce
active caspase-1. Caspase-1 then processes pro-IL-18 and pro-IL-1β
into mature IL-18 and IL-1β, respectively, leading to tissue damage
and inflammation (68,69). The abnormal activation of the NLRP3
inflammasome is associated with various diseases, including gouty
arthritis, cardiovascular disease and diabetes (70). A recent study (71) has demonstrated that amentoflavone
(AM) and its total flavonoid (TF) extract from the Chinese fir
[Cunninghamia lanceolata (Lamb.) Hook.] exhibited inhibitory
effects on foot thickness, lymphocyte infiltration, synovial injury
and cartilage destruction in mouse models of gouty arthritis.
Further investigation revealed that AM and TF reduced IL-1β
secretion and caspase-1 cleavage in a dose-dependent manner,
suggesting that they inhibit NLRP3 inflammasome activation.
Additionally, TF treatment notably decreased the formation of ASC
spots, indicating that TF could prevent the assembly of the NLRP3
inflammasome, characterized by the formation of ASC spots and
reduced NLRP3 expression (71,72).
trans-Chalcone, a precursor to flavonoids found mainly in herbs
such as licorice (Glycyrrhiza uralensis Fisch.) (73), exhibits anti-inflammatory and
antioxidant biological activities. In an experiment investigating
its protective effects in mice with gouty arthritis, trans-Chalcone
pre-treatment was administered to mice that were then injected in
the joints with MSU. This treatment was observed to inhibit
MSU-induced edema, mechanical hyperalgesia, leukocyte recruitment
and inflammatory cell recruitment in a dose-dependent manner.
Additionally, it reduced the in vivo production of IL-1β,
TNF-α and IL-6, while increasing the production of TGF-β. Notably,
trans-Chalcone also decreased nuclear factor κB (NF-κB) activation
and the mRNA expression of inflammasome components such as ASC,
NLRP3, pro-IL-1β and pro-caspase-1 (74). Similarly, quail models with
endogenous gout induced by a high-purine diet were treated with
rutinum for 10 days. The results indicated that rutin could exert
an anti-inflammatory effect by inhibiting the activation of the
NLRP3 inflammasome (26).
ii) TLR4/myeloid differentiation factor 88
(MyD88)/NF-κB pathway. In models of gouty arthritis, TLR4 in the
synovial tissue of rats is notably increased due to disturbances in
purine metabolism. Such disturbances lead to elevated UA levels,
which in turn activate the TLR4-mediated signaling pathway,
promoting the production of inflammatory cytokines and chemokines
(75). The TLR4/MyD88/NF-κB
signaling pathway involves TLR4, MyD88 and NF-κB, serving a key
role in immune and inflammatory responses (76). Activation by lipopolysaccharide
leads TLR4 to recruit MyD88, further activating the IL-1
receptor-associated kinase, which associates with TNF
receptor-associated factor 6. This sequence activates TGF-activated
kinase 1, leading to the phosphorylation of the inhibitor of κB
kinase, degradation of IκB, release of NF-κB and its translocation
into the nucleus to regulate the expression of various inflammatory
responses (77).
NF-κB is a crucial mediator of the inflammatory
response, linking extracellular stimuli with intracellular
signaling pathways, influencing the progression of gouty arthritis
(78). Inhibiting NF-κB activation
presents a valid strategy for improving gouty arthritis. Studies
(79,80) have demonstrated that calycosin
reduces knee joint swelling and neutrophil infiltration in a mouse
model of gouty arthritis induced by MSU. Inflammatory markers such
as IL-1β, IL-6, IL-10 and TNF-α showed notable decreases in
peripheral blood mononuclear cells and THP-1 cells induced by 0.2
mg/ml MSU after a 24-h pre-treatment with calycosin. Further
investigation revealed that calycosin decreased the levels of
interferon-inducible protein AIM2 (AIM2), Kelch-like ECH-associated
protein 1 (Keap1), phosphorylated (p-)p65 and p-IκBα proteins in
MSU-challenged cells in vitro, and increased the protein
expression levels of p62 (79).
These findings suggest that calycosin may exert a protective role
in gouty arthritis by inhibiting the AIM2 inflammasome-mediated
inflammatory response via the NF-κB and p62-Keap1 pathways. Similar
to the effects of calycosin, silencing of AIM2 also reversed
MSU-induced apoptosis in monocytes and macrophages, indicating that
calycosin can suppress apoptosis by deactivating the AIM2
inflammasome via certain pathways, thereby impacting MSU-induced
gouty arthritis (79). Lagotis
brachystachys Maxim. is recognized as an essential herb in the
clinical treatment of ‘Huang-shui’ disease, symptoms of which are
similar to those of arthritis, as understood in traditional Chinese
medicine (81). In Tibet, China,
this herb has been traditionally utilized for its anti-inflammatory
properties, particularly in conditions such as gouty arthritis and
alcoholic liver injury (82,83).
Research indicates that its antigout effects are achieved by
downregulating the expression levels of TLR4, MyD88 and NF-κB
proteins in the synovial tissue of rats. Three active flavonoids,
namely luteolin, luteolin-4′-O-glucoside and apigenin, have been
isolated from Lagotis brachystachys Maxim. (84). These active flavonoids have been
shown to exhibit anti-inflammatory activities in vivo
(85–87). Recent studies have suggested that
luteolin can reduce the inflammatory response in acute gouty
arthritis by inhibiting the TLR/MyD88/NF-κB pathway, reducing the
joint swelling index (88,89). Similarly, luteolin-4′-O-glucoside
was demonstrated to reduce foot swelling in rats by lowering serum
pro-inflammatory cytokines in MSU crystal-induced gouty arthritis
(31). Previous research has also
revealed that luteolin (90),
luteolin-4′-O-glucoside (91) and
apigenin (92) inhibit the TLR4
signaling pathway. Furthermore, a study employing molecular docking
techniques to evaluate the binding effects of luteolin,
luteolin-4′-O-glucoside and apigenin on TLR4 (93) found that these compounds interact
with TLR4 through hydrophobic interactions and hydrogen bonding,
with binding energy results less than-7 kcal/mol. This suggests a
high affinity of luteolin, luteolin-4′-O-glucoside and apigenin
with TLR4, indicating their potential therapeutic effects in
inhibiting inflammation.
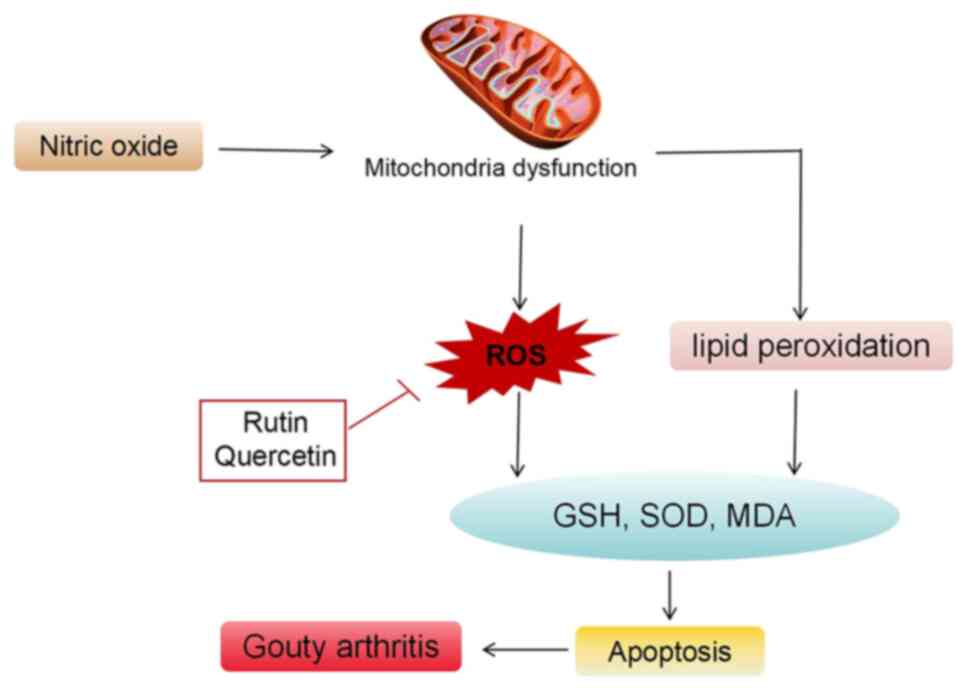
Oxidative stress and gouty arthritis
Abnormal oxidative stress
Under typical conditions, the body maintains a
balanced and gradual oxidation equilibrium. However, certain
stimuli can disrupt the antioxidant system of the body, leading to
oxidative stress reactions triggered by factors such as ROS,
resulting in localized or systemic damage (94). Currently, abnormal number and
function of T lymphocyte subpopulations, the activation of
inflammatory cytokines and the pathological loss of cell histology
in the pathogenesis of gouty arthritis are closely linked to the
extensive release of free radicals following oxidative stress. This
connection indicates that oxidative stress serves an important role
in the development of autoimmune diseases (95). Research has demonstrated that the
accumulation and deposition of MSU crystals in the joint cavity can
enhance oxidative stress, releasing large quantities of oxidants
such as ROS, nitric oxide (NO) and malondialdehyde (MDA), while
suppressing the activity of antioxidants such as superoxide
dismutase (SOD) and glutathione (GSH). This exacerbates joint
damage and causes symptoms such as redness, swelling, warmth, pain
and restricted joint mobility (96). Catalase is abundantly found in the
synovial cells of patients with gouty arthritis, with the
inflammatory response it triggers being a result of oxidative
stress. The marked increase in cellular NO−, Hydroxyl
radical (OH−), Peroxyl Radical (ROO−) and
Alkoxyl group (RO−) leads to an inflammatory response
induced by ROS. In gouty arthritis, UA crystals enter endothelial
cells through anion transporters in an exogenous
pathogen-associated molecular pattern, becoming pro-oxidants and
swiftly inducing oxidative stress. This promotes NO−
production by activating XOD and reduced nicotinamide adenine
dinucleotide phosphate oxidase (97,98).
The interaction between NO− and O2 releases
peroxynitrite anion (ONOO−), affecting cell
proliferation, leading to the degradation of connective tissue and
joint tissue deterioration (Fig.
6) (98).
Anti-oxidative stress
An animal study demonstrated that the administration
of quercetin in a rat model of gouty arthritis, induced by
injecting MSU crystal suspension into the right hind leg ankle,
alleviated edema in a dose-dependent manner and reduced acute
inflammatory histological characteristics in the treated animals.
Quercetin treatment was found to inhibit leukocyte aggregation,
decrease chemokine levels, lower the levels of MDA, a lipid
peroxidation end product, and enhance the activity of antioxidant
enzymes (27).
In rodents, such as rats and mice, UA resulting from
purine metabolism is further degraded by uricase into allantoin,
which has a higher solubility compared with UA and is excreted
through urine. Quail, similar to humans, lack uricase in their UA
synthesis and metabolism processes. The nucleic acids produced from
nucleotide proteolytic hydrolysis are degraded into purine
substances, which are then converted into UA by XOD and excreted as
UA (99,100). An animal experiment in quail,
involving a model of endogenous gout induced by a high-purine diet,
examined administration of rutin for 10 days. The results indicated
that rutin could improve gouty arthritis in quail by reducing XOD
activity and UA levels. Rutin restored the oxidative stress balance
by inhibiting the production of ROS and served a crucial
anti-inflammatory role (26).
Another study on a rat model of gouty arthritis,
induced by MSU crystals and followed by a 5-day administration of
rutin, found that rutin reduced ankle swelling, and the levels of
MDA and NO, and improved the activities of GSH-peroxidase, SOD and
catalase in rats (25). These
findings suggest that rutin could reduce gouty arthritis induced by
MSU crystals in rats, likely through its anti-oxidative stress
effects (25).
Conclusion and prospects
Flavonoid compounds hold a significant position in
the treatment of gout and gouty arthritis, although the
pathogenesis of these conditions is multifaceted. The present
review delves into the pathogenesis of gouty arthritis and the
mechanisms through which flavonoids reduce the condition, primarily
by inhibiting UA synthase activity and reducing UA production.
Flavonoids regulate the expression of renal UA transporters and
promote UA excretion; they also inhibit oxidative stress by
suppressing the production of ROS, MDA and other oxidants, while
boosting the activity of antioxidants such as SOD and GSH.
Furthermore, they regulate the expression of proteins in
inflammatory signaling pathways such as the TLR/MyD88/NF-κB and
NLRP3 pathways, reducing the release of inflammatory factors, as
illustrated in Fig. 7. Thus,
flavonoids serve a therapeutic role in managing hyperuricemia and
gouty arthritis. Based on evidence-based guidelines for the
diagnosis and treatment of gouty arthritis, the present review
suggests the use of flavonoids in symptomatic treatment according
to the stages of gout: During gouty arthritis attacks, flavonoids
manage oxidative stress and inflammatory signaling pathways to
exert anti-inflammatory effects, while in stages of asymptomatic
hyperuricemia, intermittent gouty arthritis and chronic gouty
arthritis, flavonoids inhibit UA synthase activity and regulate UA
transporter expression to reduce serum UA levels (101).
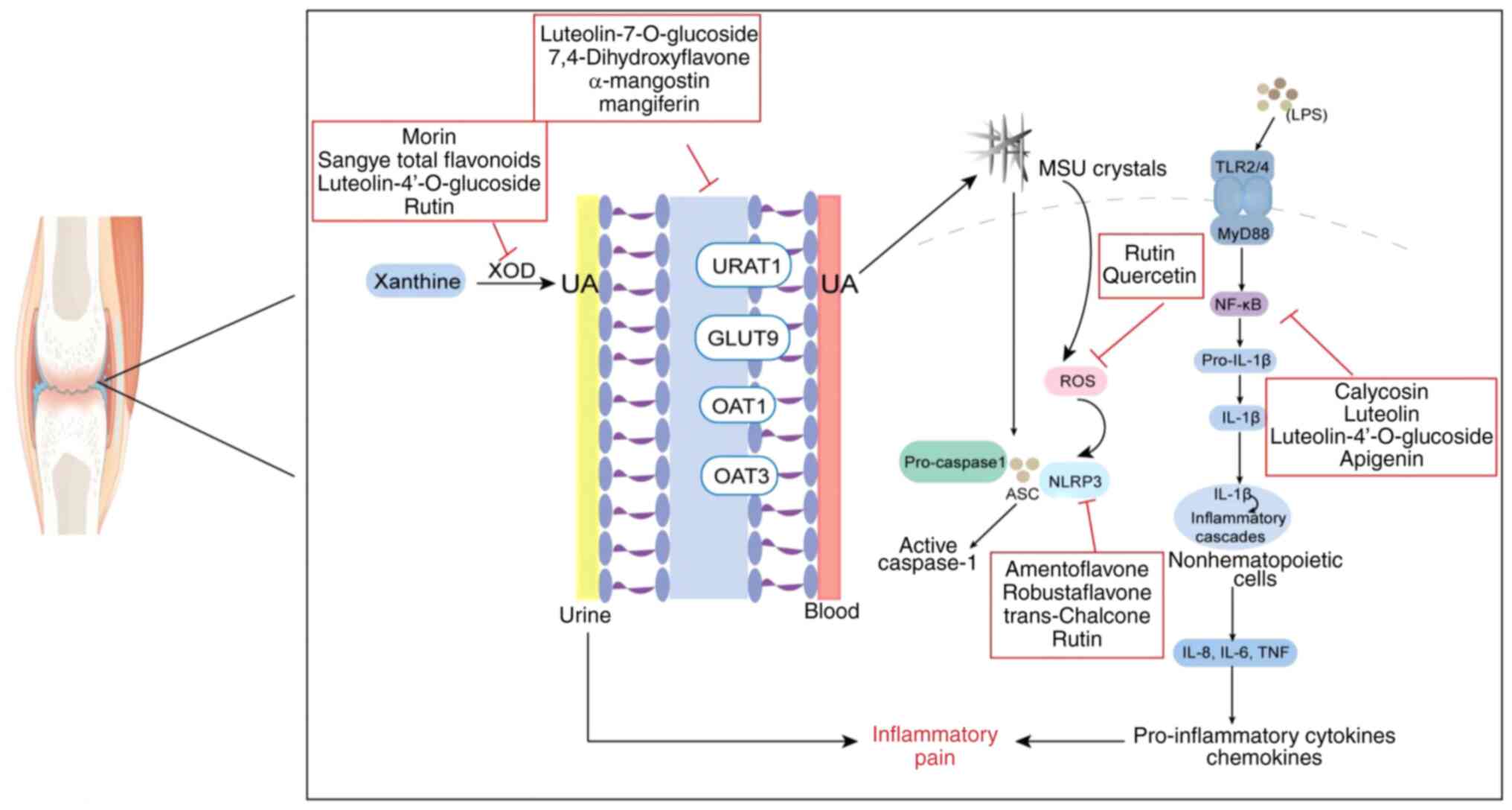 | Figure 7.Different targets of flavonoid action
in gouty arthritis. XOD, xanthine oxidase; UA, uric acid; URAT1,
recombinant urate transporter 1; OAT, organic anion transporter;
GLUT9, glucose transporter 9; NLRP3, NOD-like receptor 3; ASC,
apoptosis-associated speck-like protein containing a CARD; ROS,
reactive oxygen species; TLR, Toll-like receptor; MyD88, myeloid
differentiation factor 88; NF-κB, nuclear factor κB; LPS,
lipopolysaccharide; MSU, monosodium urate. |
Currently, research into the anti-gouty arthritis
mechanism of flavonoids is predominantly conducted in animal
studies, with relatively few clinical trials. In a randomized
controlled clinical study (102),
40 patients were treated with self-compatible Fuling (Smilax glabra
Roxb.) total flavone decoction plus conventional treatment regimen,
while the control group received a conventional treatment regimen
only. Routine treatment includes: i) Preventive treatment of diet
and lifestyle; and ii) non-steroidal anti-inflammatory drugs should
be used locally, and uricotropin drugs should be used depending on
the patient's condition. Clinical symptom self-rating scale was
used to evaluate the improvement of curative effect. The results
showed that the clinical symptoms of patients in both the
experimental group and the control group had improved to a certain
extent, but the clinical improvement effect of pain in the
experimental group was more significant compared with that in the
control group, and the serum uric acid level of patients in the
experimental group was prominently lower compared with that of the
control group (102). The present
review has certain limitations, including an incomplete
understanding of the pharmacological effects of flavonoids in
treating gouty arthritis. Besides reducing XOD activity, inhibiting
UA synthesis, regulating UA transporters, promoting UA excretion,
alleviating inflammation and improving oxidative stress, it remains
to be seen whether flavonoids alleviate gouty arthritis through
additional mechanisms. Furthermore, toxicological studies on
flavonoid treatment for gouty arthritis are scarce, with limited
reports on adverse reactions, complications, recurrence rates in
patients treated with flavonoids and the specific drug metabolism
process within the body. Addressing these gaps necessitates further
investigation and analysis in future studies.
At present, the clinical treatment of gouty
arthritis aims to reduce UA and inhibit inflammation as the main
method, and the treatment mechanism is singular, with patients
often needing to take multiple drugs at the same time to control
the disease (103). In the
treatment of gouty arthritis, herbal flavonoids have advantages of
multi-target and multi-pathway synergistic actions. In terms of
short-term efficacy, they can alleviate the symptoms of acute gouty
arthritis through anti-inflammatory effects. In terms of long-term
efficacy, they can serve a role in the treatment of gouty arthritis
by reducing serum UA levels and good safety (104), which can make up for the
shortcomings of modern medicine in the treatment of gouty
arthritis. Therefore, the present review can provide theoretical
support and direction for the treatment of gouty arthritis using
flavonoids from herbs used in traditional Chinese medicine in the
future, and provides an improved basis for the clinical development
of drugs for the treatment of gouty arthritis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Regional Foundation of
National Natural Science Foundation of China (grant no. 82360895),
the Yunnan Provincial Science and Technology Department Basic
Research Program of Traditional Chinese Medicine Joint Special
(grant no. 2019FF002-028), the Key Laboratory of Formula Granule of
Yunnan Province (grant no. 202105AG070014), the Yunnan Provincial
Department of Education Science Research Fund Project (grant no.
2024Y371) and the National Administration of Traditional Chinese
Medicine High-level Key Discipline Construction Project ‘Dai
Pharmacy’ (grant no. zyyzdxk-2023192).
Availability of data and materials
Not applicable.
Authors' contributions
PG and FL provided the concept of this article. PG,
FL, YB, YW, JH, YX and QL wrote, revised and finalized the article.
All authors have read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cleophas MCP, Crişan TO, Klück V,
Hoogerbrugge N, Netea-Maier RT, Dinarello CA, Netea MG and Joosten
LAB: Romidepsin suppresses monosodium urate crystal-induced
cytokine production through upregulation of suppressor of cytokine
signaling 1 expression. Arthritis Res Ther. 21:502019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roddy E and Choi HK: Epidemiology of gout.
Rheum Dis Clin North Am. 40:155–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mbuyi N and Hood C: An update on gout
diagnosis and management for the primary care provider. Nurse
Pract. 45:16–25. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ragab G, Elshahaly M and Bardin T. Gout:
An old disease in new perspective-a review. J Adv Res. 8:495–511.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dewulf JP, Marie S and Nassogne MC:
Disorders of purine biosynthesis metabolism. Mol Genet Metab.
136:190–198. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu YR, Wang JQ and Li J: Role of NLRP3 in
the pathogenesis and treatment of gout arthritis. Front Immunol.
14:11378222023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Sun W, Gao F, Lu J, Li K, Xu Y,
Li Y, Li C and Chen Y: Changes of serum uric acid level during
acute gout flare and related factors. Front Endocrinol (Lausanne).
14:10770592023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richette P, Doherty M, Pascual E, Barskova
V, Becce F, Castaneda J, Coyfish M, Guillo S, Jansen T, Janssens H,
et al: 2018 updated European league against rheumatism
evidence-based recommendations for the diagnosis of gout. Ann Rheum
Dis. 79:31–38. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu AM and Brown JN: Comparative effect of
allopurinol and febuxostat on long-term renal outcomes in patients
with hyperuricemia and chronic kidney disease: A systematic review.
Clin Rheumatol. 39:3287–3294. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rasheed Kayani R, Shamim R, Sultana Munir
S, Sultana M, Nazir SUR, Riaz H, Nazir T, Maaz Ali M and Islam A:
Medicinal plants and nonsteroidal anti-inflammatory drugs (NSAIDs)
in treatment of arthritis: A literature review. Altern Ther Health
Med. 28:58–64. 2022.PubMed/NCBI
|
|
11
|
Hainer BL, Matheson E and Wilkes RT:
Diagnosis, treatment, and prevention of gout. Am Fam Physician.
90:831–836. 2014.PubMed/NCBI
|
|
12
|
Lucas G and Droney L: Severe adverse drug
reaction to allopurinol. Aust Prescr. 45:130–131. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Febuxostat, . Updated advice suggests
caution in patients with a history of cardiovascular disease. React
Wkly 1960. 52023.
|
|
14
|
Ali S, Drendel AL, Rosychuk RJ, May SL,
McGrath P, Carleton B and Johnson WD: LO049: Ibuprofen or
oxycodone? An observational cohort study of post-emergency
department discharge management of children's fracture pain. CJEM.
18 (Suppl 1):S472016. View Article : Google Scholar
|
|
15
|
Keller SF and Mandell BF: Management and
cure of gouty arthritis. Rheum Dis Clin North Am. 48:479–492. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Atrahimovich D, Avni D and Khatib S:
Flavonoids-macromolecules interactions in human diseases with focus
on Alzheimer, atherosclerosis and cancer. Antioxidants (Basel).
10:4232021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li G, Ding K, Qiao Y and Zhang L, Zheng L,
Pan T and Zhang L: Flavonoids regulate inflammation and oxidative
stress in cancer. Molecules. 25:56282020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chagas MDSS, Behrens MD, Moragas-Tellis
CJ, Penedo GXM, Silva AR and Gonçalves-de-Albuquerque CF: Flavonols
and flavones as potential anti-inflammatory, antioxidant, and
antibacterial compounds. Oxid Med Cell Longev. 2022:99667502022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Sun C, Zhou S, Zhao W, Wang L,
Sheng L, Yi J, Liu T, Yan J, Ma X and Fang B: Recent advances in
chemistry and bioactivity of Sargentodoxa cuneata. J
Ethnopharmacol. 270:1138402021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhanasekar C and Rasool M: Morin, a
dietary bioflavonol suppresses monosodium urate crystal-induced
inflammation in an animal model of acute gouty arthritis with
reference to NLRP3 inflammasome, hypo-xanthine phospho-ribosyl
transferase, and inflammatory mediators. Eur J Pharmacol.
786:116–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang C, Zhao M, Jiang B, Yu J, Hao Q, Liu
W, Hu Z, Zhang Y and Song C: Extraction optimization, structural
characterization and potential alleviation of hyperuricemia by
flavone glycosides from celery seeds. Food Funct. 13:9832–9846.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng S, Wu S, Xie F, Yang CS and Shao P:
Natural compounds lower uric acid levels and hyperuricemia:
Molecular mechanisms and prospective. Trends Food Sci Tech.
123:87–102. 2022. View Article : Google Scholar
|
|
23
|
Altunayar-Unsalan C and Unsalan O:
Molecular structure, antioxidant potential, and pharmacokinetic
properties of plant flavonoid blumeatin and investigating its
inhibition mechanism on xanthine oxidase for hyperuricemia by
molecular modeling. ACS Omega. 9:13284–13297. 2024.PubMed/NCBI
|
|
24
|
Li J, Li S, Song Q, Ma E and Aimaijiang M:
Mechanism of total flavonoids from Ampelopsis grossedentata against
gouty arthritis based on multi-level interactive network and in
vivo experimental validation. Zhongguo Zhong Yao Za Zhi.
47:4733–4743. 2022.(In Chinese). PubMed/NCBI
|
|
25
|
Huang J, Song Y, Zhao P, Feng Y and Liu Y:
Experimental Study of Rutin in the Treatment of Acute Gouty
Arthritis. Mil Med Joint Logist. 27:533–535+539. 2013.
|
|
26
|
Wu H, Wang Y, Huang J, Li Y, Lin Z and
Zhang B: Rutin ameliorates gout via reducing XOD activity,
inhibiting ROS production and NLRP3 inflammasome activation in
quail. Biomed Pharmacother. 158:1141752023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang J, Zhu M, Tao Y, Wang S, Chen J, Sun
W and Li S: Therapeutic properties of quercetin on monosodium urate
crystal-induced inflammation in rat. J Pharm Pharmacol.
64:1119–1127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qian X and Jiang Y, Luo Y and Jiang Y: The
anti-hyperuricemia and anti-inflammatory effects of atractylodes
macrocephala in hyperuricemia and gouty arthritis rat models. Comb
Chem High Throughput Screen. 26:950–964. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato VH, Chewchinda S, Parichatikanond W
and Vongsak B: In vitro and in vivo evidence of hypouricemic and
anti-inflammatory activities of Maclura cochinchinensis
(Lour.) Corner heartwood extract. J Tradit Complement Med.
10:85–94. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nematbakhsh M, Hajhashemi V, Ghannadi A,
Talebi A and Nikahd M: Protective effects of the Morus alba
L. leaf extracts on cisplatin-induced nephrotoxicity in rat. Res
Pharm Sci. 8:71–77. 2013.PubMed/NCBI
|
|
31
|
Lin Y, Liu PG, Liang WQ, Hu YJ, Xu P, Zhou
J, Pu JB and Zhang HJ: Luteolin-4′-O-glucoside and its aglycone,
two major flavones of Gnaphalium affine D. Don, resist
hyperuricemia and acute gouty arthritis activity in animal models.
Phytomedicine. 41:54–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang AH, Jin Y, Wu Y, Cheng XF, Tian QH,
Xie Q and Liu W: Research progress on treatment of gout by xanthine
oxidase inhibitor in traditional Chinese medicine. Tianjin J Tradit
Chin Med. 36:1241–1245. 2019.
|
|
33
|
Mudgal R and Singh S: Xanthine
oxidoreductase in the pathogenesis of endothelial dysfunction: An
update. Curr Hypertens Rev. Feb 2–2024.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bardin T and Richette P: Novel
uricosurics. Rheumatology (Oxford). 57 (Suppl 1):i42–i46. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu QH, Zhu JX, Ning LI and Miao MX: Effect
of jasminoidin on potassium oxonate-induced hyperuricemia in mice
and its mechanism. Cent S Pharm. 11:721–725. 2013.
|
|
36
|
Cheng Y and Li F: Current status of
research on uric acid transporter proteins. J Hubei Univ Med.
36:470–473+486. 2017.
|
|
37
|
George RL and Keenan RT: Genetics of
hyperuricemia and gout: Implications for the present and future.
Curr Rheumatol Rep. 15:3092013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Anzai N, Ichida K, Jutabha P, Kimura T,
Babu E, Jin CJ, Srivastava S, Kitamura K, Hisatome I, Endou H and
Sakurai H: Plasma urate level is directly regulated by a
voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans.
J Biol Chem. 283:26834–26838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
So A and Thorens B: Uric acid transport
and disease. J Clin Invest. 120:1791–1799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Johnson RJ, Sanchez-Lozada LG and Nakagawa
T: The effect of fructose on renal biology and disease. J Am Soc
Nephrol. 21:2036–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wikoff WR, Nagle MA, Kouznetsova VL,
Tsigelny IF and Nigam SK: Untargeted metabolomics identifies
enterobiome metabolites and putative uremic toxins as substrates of
organic anion transporter 1 (Oat1). J Proteome Res. 10:2842–2851.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bush KT, Wu W, Lun C and Nigam SK: The
drug transporter OAT3 (SLC22A8) and endogenous metabolite
communication via the gut-liver-kidney axis. J Biol Chem.
292:15789–15803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nigam SK and Bhatnagar V: The systems
biology of uric acid transporters: The role of remote sensing and
signaling. Curr Opin Nephrol Hypertens. 27:305–313. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Woodward OM, Köttgen A, Coresh J,
Boerwinkle E, Guggino WB and Köttgen M: Identification of a urate
transporter, ABCG2, with a common functional polymorphism causing
gout. Proc Natl Acad Sci USA. 106:10338–10342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luo S, Cui X and Li X: Uric acid
transporter in the kidney. Prog Physiol Sci. 50:231–235. 2019.
|
|
46
|
Zhang HJ, Li LN, Zhou J, Yang QQ, Liu PG,
Xu P, Liang WQ, Cheng L, Zhang YQ, Pu JB, et al: Effects of
Gnaphalium affine D. Don on hyperuricemia and acute gouty
arthritis. J Ethnopharmacol. 203:304–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Caporali S, De Stefano A, Calabrese C,
Giovannelli A, Pieri M, Savini I, Tesauro M, Bernardini S, Minieri
M and Terrinoni A: Anti-inflammatory and active biological
properties of the plant-derived bioactive compounds luteolin and
luteolin 7-glucoside. Nutrients. 14:11552022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jiang Y, Lin Y, Hu YJ, Song XJ, Pan HH and
Zhang HJ: Caffeoylquinic acid derivatives rich extract from
Gnaphalium pensylvanicum willd. Ameliorates hyperuricemia
and acute gouty arthritis in animal model. BMC Complement Altern
Med. 17:3202017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li P, Ren G, Sun Y, Jiang D and Liu C:
Extraction optimization, preliminary identification, and
bioactivities in corn silk. Evid Based Complement Alternat Med.
2023:56851742023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xv G: Determination on the contents of the
flavonoids and the nutritive components in different parts of three
corns. J Henan Univ Technol (Natural Science Edition). 82–84.
2001.
|
|
51
|
Li P, Song J, Li Q, Zhang Q, Cui H, Guan
B, Zhao Y and Song Z: Curative effect analysis of flavone extract
from Stigma Maydis on rats of modified acute gouty arthritis model.
China Mod Med. 25:8–11. 2018.
|
|
52
|
Chi X, Ye H, Ma C, Yue H, Guo J, Lin Z,
Sun J, Ye D, Huang X and Lu G: Effect of total flavonoids in corn
stigma on uric acid uptake and related gene expression in HK-2
cells. Pharmacol Clini Chin Mater Med. 36:95–100. 2020.(In
Chinese).
|
|
53
|
Niu Y, Li Q, Tu C, Li N, Gao L, Lin H,
Wang Z, Zhou Z and Li L: Hypouricemic actions of the pericarp of
mangosteen in vitro and in vivo. J Nat Prod. 86:24–33. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu QH, Zhang X, Wang Y and Kong LD:
Mangiferin promotes uric acid excretion and kidney function
improvement and modulates related renal transporters in
hyperuricemic mice. Yao Xue Xue Bao. 45:1239–1246. 2010.(In
Chinese). PubMed/NCBI
|
|
55
|
Cobo I, Cheng A, Murillo-Saich J, Coras R,
Torres A, Abe Y, Lana AJ, Schlachetzki J, Liu-Bryan R, Terkeltaub
R, et al: Monosodium urate crystals regulate a unique JNK-dependent
macrophage metabolic and inflammatory response. Cell Rep.
38:1104892022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee YM, Cho SN, Son E, Song CH and Kim DS:
Apamin from bee venom suppresses inflammation in a murine model of
gouty arthritis. J Ethnopharmacol. 257:1128602020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cui R, Li M, Tuerxun G, Li Y and Xie S:
Research on the role of toll-like receptor 2 and toll-like receptor
4 and its signal pathway in the pathogenesis of primary gout
arthritis. Matrix Sci Pharma. 4:12020. View Article : Google Scholar
|
|
58
|
Jeong JH, Hong S, Kwon OC, Ghang B, Hwang
I, Kim YG, Lee CK and Yoo B: CD14+ cells with the
phenotype of infiltrated monocytes consist of distinct populations
characterized by anti-inflammatory as well as pro-inflammatory
activity in gouty arthritis. Front Immunol. 8:12602017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Akahoshi T: Pathological mechanisms of
gouty arthritis. Nihon Rinsho. 66:705–710. 2008.(In Japanese).
PubMed/NCBI
|
|
60
|
Cronstein BN and Sunkureddi P: Mechanistic
aspects of inflammation and clinical management of inflammation in
acute gouty arthritis. J Clin Rheumatol. 19:19–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Luo H, Tan J, Wei G, Huang L and JL:
Advances in the pathogenesis, diagnosis and treatment of gout.
Intern Med. 14:47–50. 2019.
|
|
62
|
Dai X, Fang X, Xia Y, Li M and Li X, Wang
Y, Tao J and Li X: ATP-activated P2X7R promote the attack of acute
gouty arthritis in rats through activating NLRP3 inflammasome and
inflammatory cytokine production. J Inflamm. 15:1237–1248. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xue Y, Li R, Fang P, Ye ZQ, Zhao Y, Zhou
Y, Zhang KQ and Li L: NLRP3 inflammasome inhibitor cucurbitacin B
suppresses gout arthritis in mice. J Mol Endocrinol. 67:27–40.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Han J, Shi G, Li W, Xie Y, Li F and Jiang
D: Preventive effect of dioscin against monosodium urate-mediated
gouty arthritis through inhibiting inflammasome NLRP3 and
TLR4/NF-κB signaling pathway activation: An in vivo and in vitro
study. J Nat Med. 75:37–47. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ou X, Ding T, Yang H, et al: Research
progress of signal pathway related to pathogenesis of gouty
arthritis. Pharmacol Clin Chin Mater Med. 37:234–240. 2021.
|
|
66
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20:33282019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hulse J and Bhaskar K: Crosstalk between
the NLRP3 inflammasome/ASC speck and amyloid protein aggregates
drives disease progression in Alzheimer's and Parkinson's disease.
Front Mol Neurosci. 15:8051692022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang M, Zhu Y, Zhao H and Zhao HF:
Moxibustion intervention improves synovitis by down-regulating
NLRP3/Caspase-1/IL-1β signaling of synovial tissue in rats with
adjuvant arthritis. Zhen Ci Yan Jiu. 48:1111–1116. 2023.(In
English, Chinese). PubMed/NCBI
|
|
69
|
Li X and Yang N: Exosome miR-223-3p in the
bone marrow-derived mesenchymal stem cells alleviates the
inflammation and airway remodeling through NLRP3-induced
ASC/Caspase-1/GSDMD signaling pathway. Int Immunopharmacol.
123:1107462023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Z, Guo J and Bi L: Role of the NLRP3
inflammasome in autoimmune diseases. Biomed Pharmacother.
130:1105422020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang X, Liu Y, Deng G, Huang B, Kai G,
Chen K and Li J: A purified biflavonoid extract from selaginella
moellendorffii alleviates gout arthritis via NLRP3/ASC/Caspase-1
axis suppression. Front Pharmacol. 12:6762972021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rong S, Wan D, Fan Y, Liu S, Sun K, Huo J,
Zhang P, Li X, Xie X, Wang F and Sun T: Amentoflavone affects
epileptogenesis and exerts neuroprotective effects by inhibiting
NLRP3 inflammasome. Front Pharmacol. 10:8562019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Çevik D, Erdogan S, Serttas R, Kan Y and
Kırmızıbekmez H: Cytotoxic and antimigratory activity of
retrochalcones from Glycyrrhiza echinata L. on human cancer
cells. Chem Biodivers. 20:e2022005892023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Staurengo-Ferrari L, Ruiz-Miyazawa KW,
Pinho-Ribeiro FA, Fattori V, Zaninelli TH, Badaro-Garcia S, Borghi
SM, Carvalho TT, Alves-Filho JC, Cunha TM, et al: Trans-chalcone
attenuates pain and inflammation in experimental acute gout
arthritis in mice. Front Pharmacol. 9:11232018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sun X, Li P, Qu X and Liu W: Isovitexin
alleviates acute gouty arthritis in rats by inhibiting inflammation
via the TLR4/MyD88/NF-κB pathway. Pharm Biol. 59:1326–1333. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hu N, Wang C, Dai X, Zhou M, Gong L, Yu L,
Peng C and Li Y: Phillygenin inhibits LPS-induced activation and
inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J
Ethnopharmacol. 248:1123612020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Takeda K and Akira S: Toll-like receptors.
Curr Protoc Immunol. 109:14.12.1–14.12.10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zaninelli TH, Fattori V, Saraiva-Santos T,
Badaro-Garcia S, Staurengo-Ferrari L, Andrade KC, Artero NA, Ferraz
CR, Bertozzi MM, Rasquel-Oliveira F, et al: RvD1 disrupts
nociceptor neuron and macrophage activation and neuroimmune
communication, reducing pain and inflammation in gouty arthritis in
mice. Br J Pharmacol. 179:4500–4515. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tian J, Zhou D, Xiang L, Xie B, Wang B, Li
Y and Liu X: Calycosin represses AIM2 inflammasome-mediated
inflammation and pyroptosis to attenuate monosodium urate-induced
gouty arthritis through NF-κB and p62-Keap1 pathways. Drug Dev Res.
83:1654–1672. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang F, Cao J, Li Y, Ren F, Bai J, Dong Q
and Guo J: Study of quality markers of antiuric acid formula by
grey relational analysis. SN Appl Sci. 3:6612021. View Article : Google Scholar
|
|
81
|
Xiong W, Zhang H, Wen L, Wang X, Zhong G,
Shi Y, Du X and Zhu J: Effect of Lagotis brachystachys Maxim
extract on xanthine oxidase and renal urate transporters in
hyperuricemia mice. Chin J New Drugs. 27:1538–1543. 2018.
|
|
82
|
Shan J, Ouyang X, Yang H, Wei R, Liu Y,
Zhong G, Liu H and Zhu J: Study on the effective parts of
Lagotis brachystachys Maxim against acute gouty arthritis in
rats. Tradit Chin Drug Res Clin Pharmacol. 32:492–498. 2021.(In
Chinese).
|
|
83
|
Shi Y, Li X, Wen L, Zeng J, Zhong G, Yao
X, Mu Z, Wang X and Zhu J: Anti-acute alcoholic liver injure
effects and mechanism of Lagotis brachystachy and lagotis
brevituba. Tradit Chin Drug Res Clin Pharmacol. 28:600–605.
2017.(In Chinese).
|
|
84
|
Wang L, Zhang H, Shi Y, Li M, Mu Z, Zhong
G, Zhu J and Wang H: Chemical constituents from Lagotis
brachystachy. Chin Tradit Patent Med. 42:2926–2930. 2020.(In
Chinese).
|
|
85
|
Nishitani Y, Yamamoto K, Yoshida M, Azuma
T, Kanazawa K, Hashimoto T and Mizuno M: Intestinal
anti-inflammatory activity of luteolin: role of the aglycone in
NF-κB inactivation in macrophages co-cultured with intestinal
epithelial cells. Biofactors. 39:522–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Luan RL, Meng XX and Jiang W: Protective
effects of apigenin against paraquat-induced acute lung injury in
mice. Inflammation. 39:752–758. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li Q, Tian Z, Wang M, Kou J, Wang C, Rong
X, Li J, Xie X and Pang X: Luteoloside attenuates neuroinflammation
in focal cerebral ischemia in rats via regulation of the
PPARγ/Nrf2/NF-κB signaling pathway. Int Immunopharmacol.
66:309–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ouyang X, Li NZ, Guo MX, Zhang MM, Cheng
J, Yi LT and Zhu JX: Active flavonoids from Lagotis
brachystachya attenuate monosodium urate-induced gouty
arthritis via inhibiting TLR4/MyD88/NF-κB pathway and NLRP3
expression. Front Pharmacol. 12:7603312021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shen R, Ma L and Zheng Y:
Anti-inflammatory effects of luteolin on acute gouty arthritis rats
via TLR/MyD88/NF-κB pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
45:115–122. 2020.(In English, Chinese). PubMed/NCBI
|
|
90
|
Lee MN, Lee Y, Wu D and Pae M: Luteolin
inhibits NLRP3 inflammasome activation via blocking ASC
oligomerization. J Nutr Biochem. 92:1086142021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang Z, Chen W, Li Y, Zhang S, Lou H, Lu X
and Fan X: Reduning injection and its effective constituent
luteoloside protect against sepsis partly via inhibition of
HMGB1/TLR4/NF-κB/MAPKs signaling pathways. J Ethnopharmacol.
270:1137832021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao F, Dang Y, Zhang R, Jing G, Liang W,
Xie L and Li Z: Apigenin attenuates acrylonitrile-induced
neuro-inflammation in rats: Involved of inactivation of the
TLR4/NF-κB signaling pathway. Int Immunopharmacol. 75:1056972019.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhu JX, Yang HY, Hu WQ, Cheng J, Liu Y, Yi
LT and Cheng HY: Active components from Lagotis
brachystachya maintain uric acid homeostasis by inhibiting
renal TLR4-NLRP3 signaling in hyperuricemic mice.
Inflammopharmacology. 29:1187–1200. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Newsholme P, Cruzat VF, Keane KN, Carlessi
R and de Bittencourt PIH Jr: Molecular mechanisms of ROS production
and oxidative stress in diabetes. Biochem J. 473:4527–4550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wójcik P, Gęgotek A, Žarković N and
Skrzydlewska E: Oxidative stress and lipid mediators modulate
immune cell functions in autoimmune diseases. Int J Mol Sci.
22:7232021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cheng JJ, Ma XD, Ai GX, Yu QX, Chen XY,
Yan F, Li YC, Xie JH, Su ZR and Xie QF: Palmatine protects against
MSU-induced gouty arthritis via regulating the NF-κB/NLRP3 and Nrf2
pathways. Drug Des Devel Ther. 16:2119–2132. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zeng D, Yin C, Wei H, Li Y, Yang Y, Nie H,
Pan Y, Xu R, Tai Y, Du J, et al: Activation of Nrf2 antioxidant
signaling alleviates gout arthritis pain and inflammation. Biomed
Pharmacother. 170:1159572024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zamudio-Cuevas Y, Hernández-Díaz C, Pineda
C, Reginato AM, Cerna-Cortés JF, Ventura-Ríos L and López-Reyes A:
Molecular basis of oxidative stress in gouty arthropathy. Clin
Rheumatol. 34:1667–1672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu H, Wang Y, Ren Z, Li Y, Huang J, Lin Z
and Zhang B: Overnutrition-induced gout: An immune response to
NLRP3 inflammasome dysregulation by XOD activity increased in
quail. Front Immunol. 13:10748672022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Maiuolo J, Oppedisano F, Gratteri S,
Muscoli C and Mollace V: Regulation of uric acid metabolism and
excretion. Int J Cardiol. 213:8–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kiltz U, Alten R, Fleck M, Krüger K,
Manger B, Müller-Ladner U, Nüsslein H, Reuss-Borst M, Schwarting A,
Schulze-Koops H, et al: Evidence-based recommendations for
diagnostics and treatment of gouty arthritis in the specialist
sector : S2e guidelines of the German society of rheumatology in
cooperation with the AWMF. Z Rheumatol. 76:118–124. 2017.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhao S: Clinical efficacy of traditional
Chinese medicine soup in the treatment of gout with damp-heat
stasis and the pharmacological effects of total flavonoids of the
monarch extract Poria cocos (Poria cocos). Capital Food Med.
26:187–188. 2019.
|
|
103
|
Engel B, Just J, Bleckwenn M and
Weckbecker K: Treatment options for gout. Dtsch Arztebl Int.
114:215–222. 2017.PubMed/NCBI
|
|
104
|
Levy RM, Pillai L and Burnett PB:
Nutritional benefits of flavocoxid in patients with osteoarthritis:
Efficacy and safety. Nutr Diet Suppl. 2:27–38. 2010. View Article : Google Scholar : PubMed/NCBI
|