Introduction
Carboxyl-terminal modulator protein 1 (CTMP1), also
known as thioesterase superfamily member 4 (THEM4), was initially
identified through yeast two-hybrid analysis as a protein kinase Bα
(PKBα)-binding protein that inhibits the phosphorylation of PKBα.
CTMP1 reverts the phenotype of viral-PKB-transformed cells
(1). It is a crucial component of
the PKB signaling pathway, which is involved in a wide range of
biological processes including insulin signaling, cell survival,
growth and metabolism (2–4). CTMP1, a mitochondrial protein, is
synthesized in the nucleus and then translocates to the
mitochondria. There, it undergoes maturation through cleavage of
its mitochondrial localization signal at the N-terminus by
mitochondrial peptidases. In addition, CTMP1 can be phosphorylated
at Ser37/Ser38 (5,6). CTMP1 has been linked to several
important health-related conditions, including cancer (7–17),
drug resistance (18,19), brain injury (20–26),
diabetic metabolism (27–29) and fibrosis-related diseases
(29,30). CTMP2, also termed THEM5, is a CTMP1
paralog and a component of the mitochondrial proteome. CTMP2 has a
vital role in cardiolipin remodeling and the development of fatty
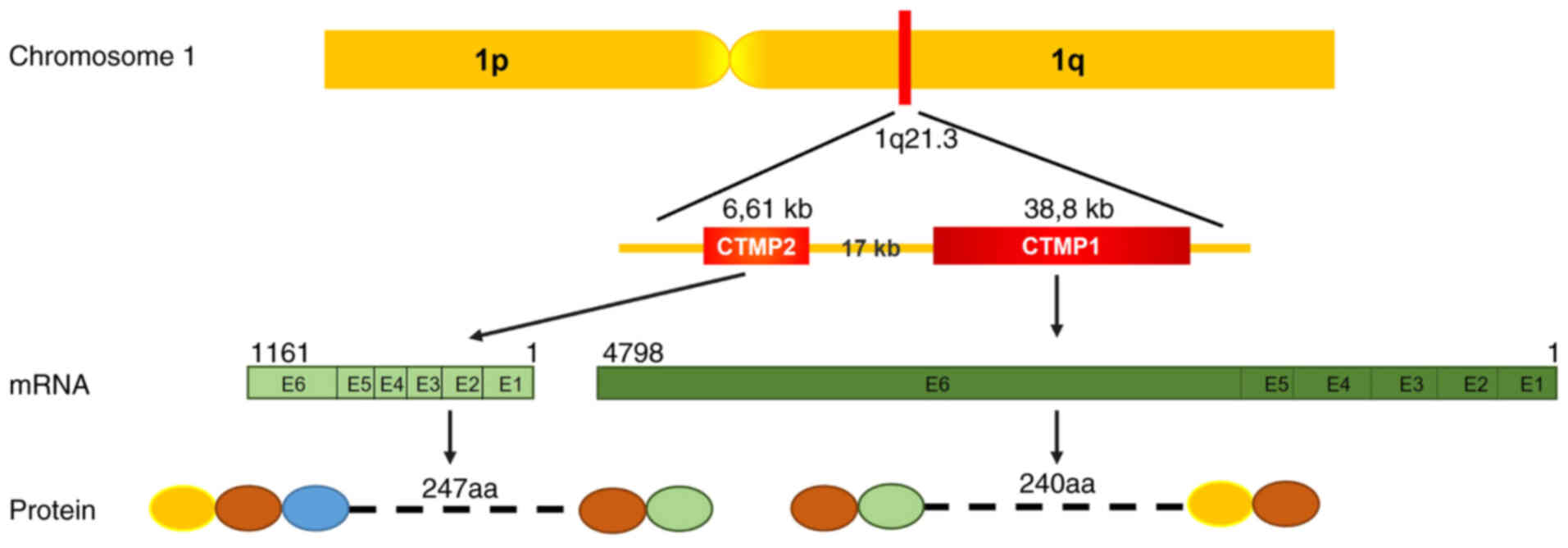
liver disease. CTMP1 and CTMP2 are located next to each other in
human chromosome 1q21.3 and have six exons in mouse chromosome 3
(Fig. 1). Although CTMP1 is found
in lower eukaryotes, including yeast, CTMP2 is only present in
mammals (31). The functions of
CTMP2 have not been fully revealed and fewer studies have focused
on CTMP2 than on CTMP1. Thus, the findings of both are
controversial and diverse.
The present review provides an overview of CTMP1 and
CTMP2 regulation and discusses how CTMP1 and/or CTMP2 are involved
in PKB signaling, mitochondrial function and vital conditions and
processes including cancer, brain injury, mitochondrial function
and lipid metabolism. The summaries provide additional insight,
offer promising avenues for research on CTMP-related subjects and
underscore key findings.
Classification and structural analysis of
CTMP
Thioesterases are widely distributed enzymes that
can be found in bacteria, archaea and eukaryotes. They catalyze the
cleavage of thioester bonds in a variety of substrates, including
activated fatty acyl-coenzyme A (CoA) substrates, acyl carrier
proteins and glutathione (32).
Substrates bonded to CoA are involved in various biosynthetic
pathways, such as the synthesis of fatty acids and cholesterol, as
well as in catabolic processes, such as fatty acid oxidation and
the tricarboxylic acid cycle. Acyl-CoA thioesterases (ACOTs) have a
vital role in regulating lipid metabolic functions, including
energy expenditure, hepatic gluconeogenesis and neuronal function
(33). These enzymes are located
within various cellular compartments, including peroxisomes,
mitochondria and the cytosol. ACOTs are categorized into two
distinct types based on their enzymatic activities: Type I and II.
Type I ACOTs belong to the α/β-hydrolase protein family, while type
II ACOTs are part of the ‘hotdog’ fold family (34). Among the six human type II gene
products, CTMP1 (THEM4) and CTMP2 (THEM5) share identical
structural features of type II (Fig.
2) but lack sequence homology with other members of the hotdog
superfamily. Research on the structure of CTMP1 has focused on its
carboxyl-terminal domain, which consists of ~100 amino acids that
include a hotdog-fold thioesterase subunit, conferring thioesterase
activity (35,36). More is known about the functional
attributes of the C-terminal domain than the N-terminal domain,
which remains less explored. X-ray analysis of human CTMP1 revealed
its binding with undecan-2-one-CoA, and analysis of the N-terminal
domain showed an irregular and flexible secondary structure,
suggesting its potential role as a protein-binding domain (36). However, there is no evidence to
suggest that CTMP1 inhibits PKB in the regulation of thioesterase
activity (35). In their crystal
forms, CTMP1 and CTMP2 exhibit the classic hotdog-fold structure
and form distinct homodimers (31). However, CTMP1 and CTMP2 may form
higher-order oligomers in mitochondria in response to specific
stimuli or environmental conditions. Overall, the architecture of
CTMP highlights its function as an acyl-CoA thioesterase, whereas
its widely acknowledged role as a PKB inhibitor is not supported by
current research findings. This indicates that the interaction
between CTMP and PKB may depend on the specific subcellular
locations or colocalization of each.
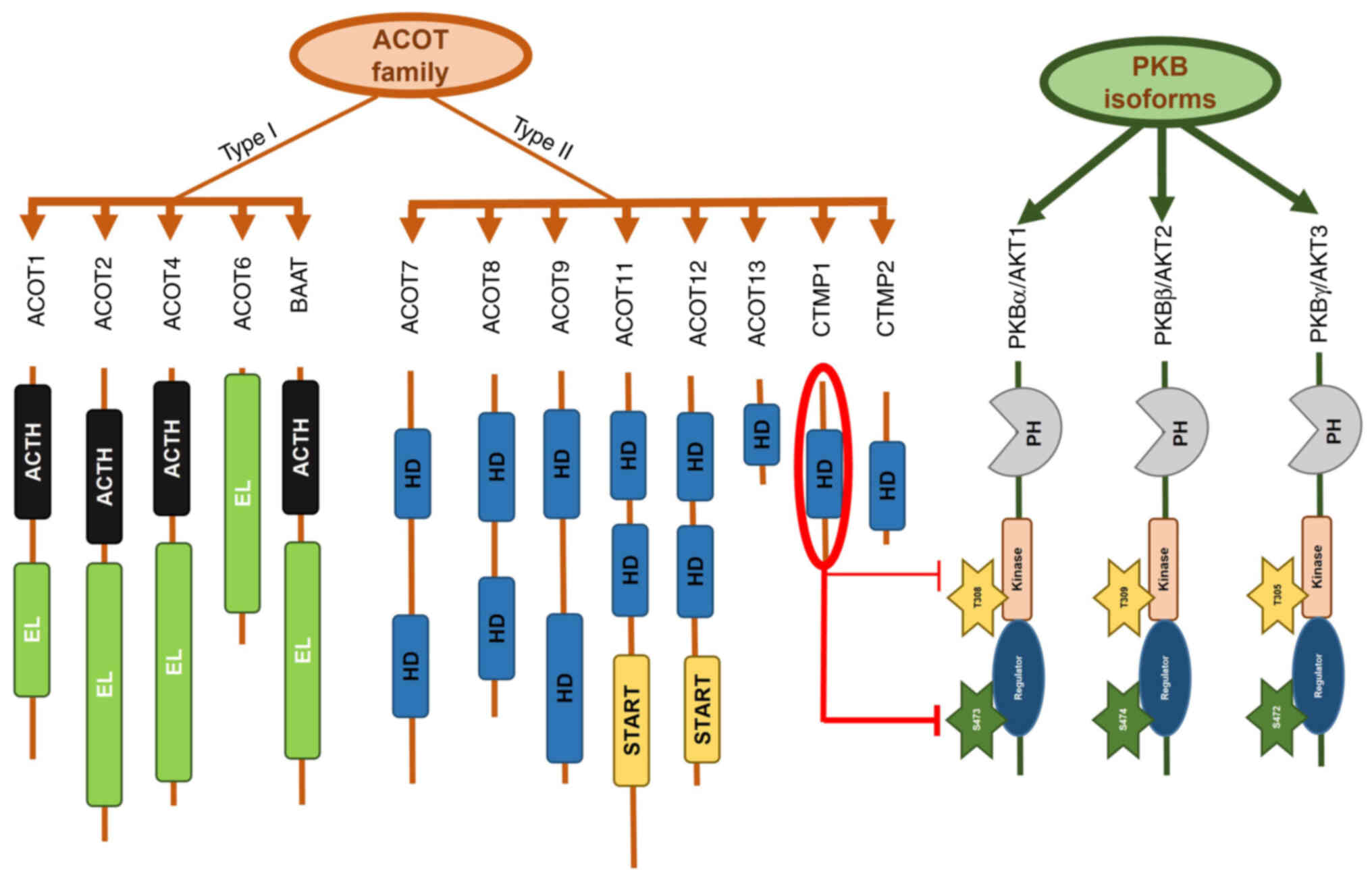 | Figure 2.ACOTs family members and PKB isoforms
in terms of the correlation between CTMP1 and PKBα. The ACOTs
family comprises type I and II ACOTs. CTMP1 and CTMP2 are type II
ACOTs and share the same ‘HD. CTMP1 inhibits the phosphorylation of
PKBα in residue Ser473 and less so in residue Thr308 by binding
directly to carboxyl terminal ends (1). Others sharing significant sequences
such as the HD, EL, BAAT, ACTH and START between ACOTs family type
I and II are briefly shown. CTMP, carboxyl-terminal modulator
protein; PKB, protein kinase B; ACOT, acyl-coenzyme A thioesterase;
ACTH, acyl-CoA thioester hydrolase domain; BAAT, bile acid-coenzyme
A: amino acid N-acyltransferase; EL, esterase-lipase domain; HD,
‘hot dog’ fold domain; START, steroidogenic acute regulatory
protein-related lipid transfer domain. |
Role of CTMP in PKB signaling pathway
PKB, also known as α serine/threonine-protein kinase
(AKT), is a serine/threonine kinase that includes three isoforms
and belongs to the cyclic adenosine monophosphate (cAMP)-dependent
protein kinases A, G and C superfamily. These isoforms share
structural homology within their catalytic domains and have similar
mechanisms of activation. In mammals, three PKB isoforms have been
identified: PKBα or AKT1 (37),
PKBβ or AKT2 (38) and PKBγ or
AKT3 (39) (Fig. 2). These isoforms are located on
chromosomes 14q32, 19q13 and 1q44, respectively (40). PKBα is phosphorylated to regulate a
variety of cellular proteins involved in metabolism, apoptosis and
proliferation (41). Dysregulation
of PKBα is associated with the pathogenesis of cancer, diabetes and
multiple ocular diseases (42).
PKBα activation occurs through site-specific phosphorylation at
Thr308 and Ser473 on the plasma membrane, facilitated by the
binding of its pleckstrin homology domain to
phosphatidylinositol-3,4,5-trisphosphate (43). Specifically, phosphorylation of
PKBα primarily occurs at the activation T-loop on Thr308 by
phosphoinositide-dependent kinase 1 (PDK1) via PDK1-directed
phosphorylation (44). Another
PKBα phosphorylation site is Ser473, located in the C-terminus, a
noncatalytic region of the enzyme within the hydrophobic motif
(45). In 2005, research showed
that the mammalian target of rapamycin (mTOR)/rapamycin-insensitive
companion of mTOR complex directly phosphorylates PKB on Ser473 and
enables phosphorylation of Thr308 by PDK1 (46).
THEM4 and THEM5 were named based on their structures
and original family association. However, understanding why THEM4
is also referred to as CTMP1 requires further exploration into the
origin of CTMP1. CTMP1 was initially identified as a PKBα inhibitor
in 2001 (1). During this
identification, a yeast two-hybrid assay was conducted using the
COOH-terminal regulatory domain of PKBα, which includes the
hydrophobic motif and residue Ser473, as bait. This led to the
discovery of a protein comprising 240 amino acids with a molecular
weight of 27 kDa, termed carboxyl-terminal modulator protein 1
(CTMP1). As a result, THEM4 became known as CTMP1. The designations
CTMP1 and CTMP2 were then introduced to differentiate between THEM4
and THEM5 (47).
CTMP1 exerts its inhibitory effect on PKB signaling
through the carboxyl-terminal regulatory domain of PKBα at the
plasma membrane, inhibiting PKBα activity by preventing its
phosphorylation at Ser473 and, to a lesser extent, at Thr308
residues (1) (Fig. 2). As PKB is a kinase, a reduction
in its active form results in decreased activity and subsequent
phosphorylation of downstream substrates, such as glycogen synthase
kinase 3β (GSK3β) (1).
Colocalization of CTMP1 and PKBα as an endogenous complex is
observed in the plasma membrane of serum-starved cells and within
cell fractions (48). Conversely,
the absence of CTMP1 binding may facilitate PKB phosphorylation by
allowing access to the hydrophobic motif at Ser473.
No reports to date have disclosed a relationship
between CTMP2 and PKB. In 2013, however, a doctoral thesis by
Zhuravleva (47) at the University
of Basel demonstrated that in CTMP2(−/-) mice subjected to an
insulin challenge, phosphorylation of PKB was increased in the
liver and adipose tissues (both white and brown), while no
differences were observed in the levels of phosphorylated PKB in
muscle, heart or brain tissues. In addition to the increase in PKB
phosphorylation, a decrease in the phosphorylation of AMP-activated
protein kinase at residue Thr172 was observed (47).
By contrast, a report in 2007 demonstrated an
inverse relationship between CTMP1 and PKB, showing that
overexpression of CTMP1 increased PKB phosphorylation, while
knockdown of CTMP1 decreased PKB phosphorylation (49). In that study, Ono et al
(49) conducted experiments on
Cos-1, HepG2, HeLa and NIH3T3 cells, yielding comparable results.
Although they observed an inhibitory effect of CTMP1 on PKB,
consistent with other research, the discrepancies between their
study and others may be attributed to the use of different cell
lines or sublines. This suggests that the effect of CTMP1 on
certain cell lines may be unexpectedly complex. Such findings
present both advantages and challenges for researchers focusing on
CTMP1 and CTMP more broadly. Thus, given the complexities in the
interaction between CTMP and PKB activity, all evidence indicates
that CTMP (particularly CTMP1) has a crucial role in regulating PKB
and its downstream effects.
Impact of CTMP on metabolic syndrome
In a study from 2013 examining transformed
lymphocytes from 190 Caucasian and African-American individuals,
researchers aimed to identify functional variants linked to type 2
diabetes susceptibility in the chromosome 1q21-24 region. They
found that CTMP1 expression in adipocytes was significantly higher
in individuals with the T allele (P=0.005), correlating with
glucose homeostasis traits in the MAGIC dataset (28). In addition, in mice fed a high-fat
diet, the CTMP1 protein level was higher in white adipose tissue
(27) and bone marrow macrophages
from mice with diet-induced obesity (50). Conversely, the leucine
zipper/EF-hand-containing transmembrane protein 1 (LETM1), known to
bind CTMP1 and exhibit anti-cancer effects (51), was downregulated. LETM1 also
negatively affects the role of CTMP1 in PKB activation in obese
conditions. Specifically, CTMP1 upregulation in obesity enhances
its inhibitory effect on PKB activation, contributing to insulin
resistance, a hallmark of obesity. Interestingly, the negative
impact of LETM1, acting as a CTMP1 inhibitor, is diminished; this
leads to increased CTMP1 expression (27).
CTMP1 levels are lower in mice with diabetic kidney
disease than in normal mice (29).
In the human renal proximal tubular epithelial cell line HKC, CTMP1
expression decreases in response to high-glucose stimuli (29). Following a high-fat diet, CTMP1
expression in the hippocampus is increased while PKB
phosphorylation is decreased (52). In addition, CTMP1 has been shown to
regulate the synthesis of branched-chain fatty acids in lamb liver
(53). Furthermore, CTMP1 is
positively associated with the human serum metabolite 3-hydroxyl
decanoate, a hydroxyl-saturated medium-chain fatty acid anion
(54). The effects of CTMP2 in the
regulation of phosphorylated PKB inhibition are limited to adipose
tissue and liver; CTMP2 does not impact the heart, muscle or brain
in this regard (47). Overall, the
influence of CTMP on lipid metabolism, diabetic status or
high-glucose conditions varies across different organs, primarily
through the modulation of PKB activity.
Regulation of CTMP in apoptosis and
mitochondrial function
Both membrane-bound CTMP1 and a free pool of mature
CTMP1 are present in the inter-membrane space of mitochondria. Upon
apoptosis, CTMP1 is rapidly released from the mitochondria into the
cytosol. This release is associated with increased mitochondrial
membrane depolarization and enhanced cleavage of caspase-3 and
polyADP-ribose polymerase (PARP), all of which are linked to CTMP1
overexpression. Conversely, knockdown of CTMP1 significantly
reduces caspase-3 and PARP activation and mitigates loss of the
mitochondrial membrane potential, as observed in 293 cells and HeLa
cell lines (5). In A549 cells,
CTMP1 promotes apoptosis through inhibition of anti-apoptotic
heat-shock protein 27 (Hsp27) (55). In HeLa cells, CTMP1 binds to Hsp70,
inhibiting the Hsp70-apoptotic protease activating factor-1 complex
and thus promoting apoptosis (6).
In addition, CTMP1 affects mitochondrial morphology by inhibiting
OPA1 mitochondrial dynamin-like GTPase (simply known as OPA1),
which is necessary for mitochondrial fusion (55). Furthermore, LETM1, a protein that
binds to CTMP1, contributes to mitochondrial fragmentation via OPA1
cleavage (56). A defect in the
N-terminus of CTMP1 or loss of the full length of CTMP1 results in
clustering of spherical mitochondria, indicating the role of CTMP1
in mitochondrial fission (57).
CTMP2 has also been identified as a mitochondrial
protein. Bioinformatics analysis has revealed a mitochondrial
targeting sequence at the N-terminal end of the human CTMP2 protein
and in the CTMP2 orthologs of other species. Translocase of outer
mitochondrial membrane 20 (TOMM20) is a mitochondrial membrane
protein. In one study, immunofluorescence staining of CTMP2 and
TOMM20 showed overlapping localizations in the cytoplasm of U2OS
cells (31). Further analyses
indicated that CTMP2 localizes within the mitochondrial matrix. The
absence of CTMP2 results in variations in mitochondrial morphology
and function (31). Therefore,
both CTMP1 and CTMP2 are mitochondrial proteins that may influence
apoptosis and mitochondrial functions in various ways.
Various functions of CTMP in cancer and drug
resistance
The expressions of CTMP1 and CTMP2 in cancer may
vary, being either higher or lower than physiological levels
depending on the cancer type (Fig.
3A). The relationship between CTMP1 and cancer is more
established than that between CTMP2 and cancer (Fig. 3A). The data were analyzed using
Gene Expression Profiling Interactive Analysis (GEPIA2; http://gepia2.cancer-pku.cn/#index). Therefore,
the following sections will summarize the latest research on the
role of CTMP1 in regulating various types of cancer (Fig. 3B) and on the role of CTMP2
specifically in lung cancer.
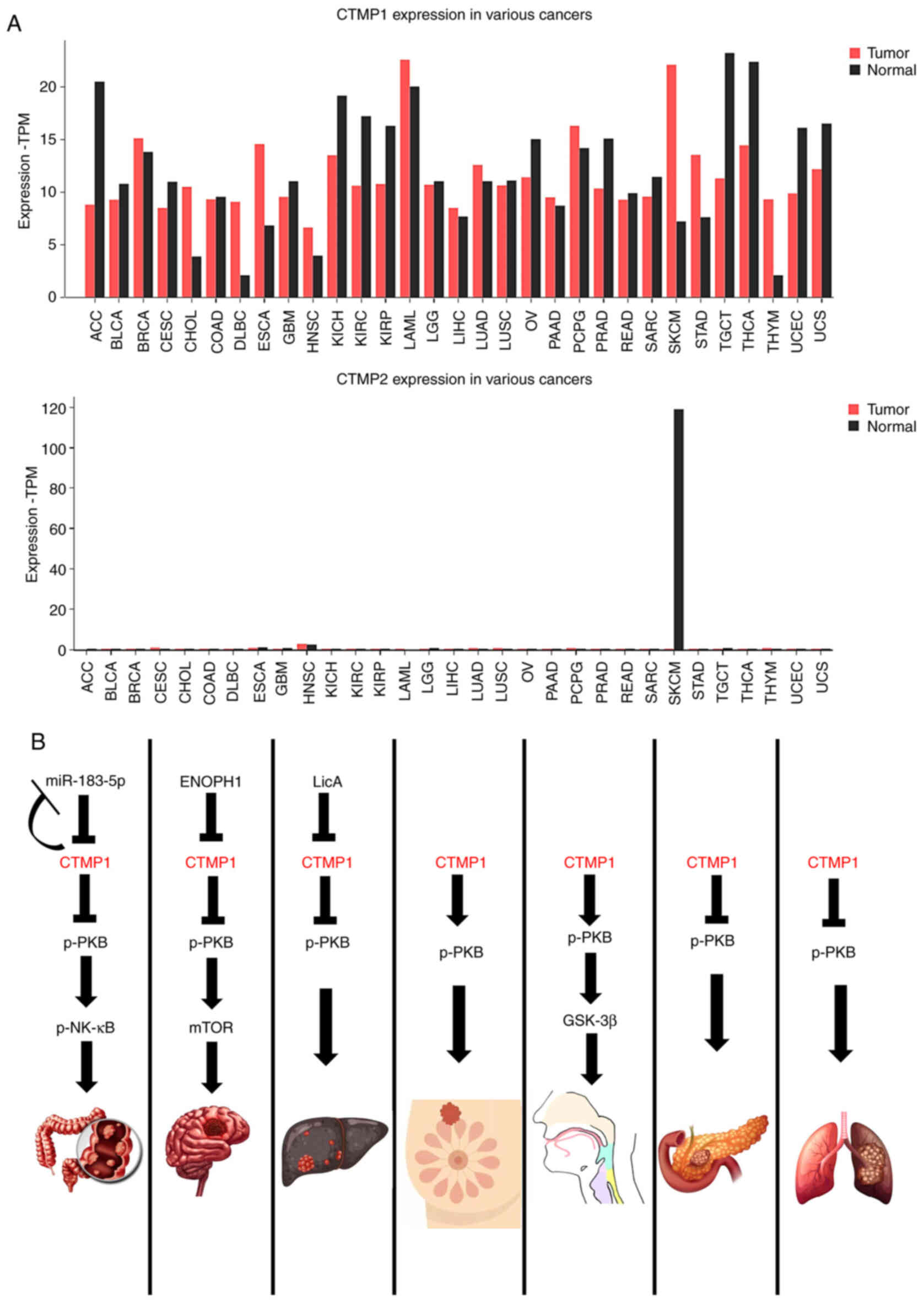 | Figure 3.CTMP functions in several types of
cancer. (A) Different expressions of CTMP1 and CTMP2 compared
between normal tissue and cancer tissue. CTMP1 expression has been
more thoroughly researched than CTMP2. While the expression of
CTMP1 in various cancers is revealed, CTMP2 expression in cancer is
not well-known. CTMP1 tends to be elevated in most cancers. (B)
Mechanism of CTMP1 in the regulation of seven specific cancers
(colon cancer, glioma, hepatocellular carcinoma, breast cancer,
head and neck squamous cell carcinoma, pancreatic adenocarcinoma
and lung cancer). CTMP1 can promote tumorigenesis through
inhibiting or facilitating phosphorylated PKB. CTMP,
carboxyl-terminal modulator protein; TPM, transcripts per million;
ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma;
BRCA, breast invasive carcinoma; CESC, cervical squamous cell
carcinoma and endocervical adenocarcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid
neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma;
GBM, glioblastoma multiforme; HNSC, head and neck squamous cell
carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell
carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute
myeloid leukemia; LGG, brain lower grade glioma; LIHC, liver
hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung
squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous
cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG,
pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma;
READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous
melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell
tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine
corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM,
uveal melanoma; miR, microRNA. |
Colon cancer
In the context of colon cancer, there is a notable
increase in the level of microRNA (miR)-183-5p in M2-polarized
tumor-associated macrophages. The subsequent overexpression of
CTMP1 reduces the carcinogenic effects mediated by miR-183-5p, and
this is accompanied by inactivation of the PKB and NF-κB pathways
in colon cancer cells (Fig. 3B).
Therefore, CTMP1 may serve as a target for miR-183-5p to modulate
the progression of colon cancer (17).
Glioma
Similar to other cancers, in glioma, the different
expression between tumor and normal tissues is a foundational
finding for a potential biomarker (58). In glioma, CTMP1 interacts with
enolase-phosphatase 1, a newly identified enzyme involved in
L-methionine biosynthesis. This interaction, proven through
immunoprecipitation and western blot analyses, regulates the
PI3K/AKT/mTOR signaling pathway (Fig.
3B), thereby affecting glioma cell growth and invasion
(59). Furthermore, the level of
CTMP1 mRNA was found to be decreased in glioblastoma and six glioma
cell lines. This decrease in mRNA is associated with downregulation
of CTMP1 transcription and hypermethylation of the CTMP1 promoter
in glioblastoma (11,60,61).
Monochemosensitive
choriocarcinoma
Analysis of the expression of the 760-gene panel in
the PanCancer Pathway, which is related to oncogenesis and immune
tolerance in tissue samples of complete hydatidiform moles and
gestational choriocarcinoma, showed that CTMP1 expression was
higher in monochemoresistant than monochemosensitive
choriocarcinoma (15).
Hepatocellular carcinoma (HCC)
To improve the prognostic prediction of HCC,
numerous biomarkers have been found (62). A total of 365 samples of HCC were
taken from The Cancer Genome Atlas database and least absolute
shrinkage and selection operator analysis was conducted to examine
HCC mRNA expression; CTMP1 was one of nine mRNAs identified as a
prognostic indicator or risk factor (16). In addition, when linking CTMP1 and
its LETM1 binding partner and overexpressing them, tumorigenesis in
a mouse model of HCC was reduced and mitochondria-mediated
apoptosis was induced through morphological changes and defects of
mitochondrial function (51).
Fenofibrate and activated peroxisome proliferator-activated
receptor α can elevate CTMP1 expression in liver cancer cells (Huh7
cell line); additionally, CTMP1 exerts an inhibitory effect on PKB
(63). In HepG2 cells, CTMP1 acts
downstream of licochalcone A, a novel chemotherapy drug that
induces apoptosis by inhibiting Bcl-2. In addition, licochalcone A
promotes the generation of reactive oxygen species, leading to
induction of autophagy, with CTMP1 playing a role in this process
(64) (Fig. 3B).
Breast cancer and triple-negative
breast cancer (TNBC)
TNBC accounts for ~15–-20% of all cases of breast
cancer (65). CTMP1 is upregulated
in both specimens and cell lines of breast cancer (13) and TNBC (14). CTMP1 is overexpressed in breast
cancer and knockdown experiments have shown that CTMP1 functions as
an oncogene. It enhances cell proliferation and tumorigenic
properties by promoting PKB phosphorylation (13). Furthermore, in TNBC metastasis,
CTMP1 enhances migration and invasion abilities by elevating PKB
activity (14) (Fig. 3B).
PKB activation and loss of CTMP1 occur in
tamoxifen-resistant human breast cancer cell lines, indicating that
CTMP1 acts as an inhibitory factor for PKB (19). CTMP1 adopts several different roles
in regulating PKB phosphorylation due to various genomic
aberrations in the tamoxifen resistance model.
Head and neck squamous cell
carcinoma
CTMP1 exhibits higher expression at the protein and
mRNA levels in head and neck squamous cell carcinoma (both tumor
tissue and cell lines) than in normal tissues and is associated
with lymph node metastasis (12).
CTMP1 is also associated with PKB/GSK3β phosphorylation, increased
Snail levels and decreased E-cadherin levels, indicative of
epithelial-to-mesenchymal transition. These findings suggest an
oncogenic role of CTMP1 in head and neck squamous cell carcinoma
(12) (Fig. 3B).
Pancreatic adenocarcinoma
PKB-related genes are rarely mutated in pancreatic
adenocarcinoma. When PKB activity was inhibited with a
cell-permeable peptide targeting the predicted N-terminal region of
CTMP1, both human and murine pancreatic adenocarcinoma cell lines
underwent apoptosis. In addition, these cell lines displayed
smaller tumors in allograft models (10) (Fig.
3B). In another study, CTMP1 was not detectable in pancreatic
cancer cell lines compared with a three-dimensional culture system
of pancreatic duct epithelial cells (66).
Endometrial cancer
CTMP1 and its binding partner LETM1 show higher
protein expression in endometrial cancer tissues than in atypical
hyperplastic tissues and in atypical hyperplastic tissues than in
normal tissues. The correlation of CTMP1 and LETM1 serves as an
oncogenic factor in endometrial cancer cells (KLE cell line)
(9).
Lung cancer
In mice with lung cancer subjected to a diet high in
inorganic phosphate, CTMP1 expression was reduced, while PKB kinase
activity was increased, leading to enhanced progression of lung
tumors (7). In addition, in a
mouse model of lung cancer utilizing lentiviral vector-CTMP1
administered as an aerosol, downregulation of PKB phosphorylation
resulted in reduced pulmonary tumorigenesis (8) (Fig.
3B). Using short-term interventions (30 min) (8) or long-term interventions (30 min
twice a week for 4 weeks) (67)
yielded similar results, suggesting that viral delivery of CTMP1
can be a practical tool for lung cancer treatment (67). CTMP2 antisense RNA1 (C2CD4D-AS1)
was found to be predominantly upregulated in lung adenocarcinoma
tissues and cell lines, and this upregulation was induced by ETS
translocation variant 4. In addition, ablation of C2CD4D-AS1
suppressed cell proliferation, migration, invasion and apoptosis of
lung adenocarcinoma (68).
In summary, CTMP1 and CTMP2 exhibit different
functions and expressions in various cancer types through
dissimilar mechanisms, suggesting the high potential for CTMP to
serve as a novel component of anti-cancer therapy.
Emerging role of CTMP1 in regulation of
fibrosis
CTMP1 protein expression is decreased in the kidneys
of diabetic mice. In addition, the kidneys of such mice exhibit an
increase in transforming growth factor β1 (TGFβ1) and α-smooth
muscle actin (αSMA) (29), both of
which are major regulators of extracellular matrix metabolism in
various tissues (29,69). An increase in the expression of
phospho-PKB (Ser473) has also been observed in the kidneys of
diabetic mice, suggesting that CTMP1 mitigates renal extracellular
matrix accumulation by modulating phospho-PKB, TGFβ1 and αSMA in
these mice (29). In the HKC cell
line, high glucose levels were found to reduce CTMP1 protein
expression (29). Conversely,
enhancement of CTMP1 expression in mice through tail vein injection
of the pYr-ads-4-musCTMP vector counteracted the elevations in
TGFβ1 and αSMA.
In the heart, CTMP1 serves as a regulator that
attenuates cardiac hypertrophy and fibrosis (30). CTMP1 protein expression is lower in
human hearts affected by dilated cardiomyopathy and hypertrophic
cardiomyopathy than in normal hearts (30). Increased expression of CTMP1
lessens the severity of cardiac hypertrophy and fibrosis induced by
pressure overload, whereas the absence of CTMP1 exacerbates these
conditions. This is evidenced by mRNA expression of fibrosis
markers such as collagen Iα, collagen III and connective tissue
growth factor, as well as Sirius red staining for collagen
deposition (30). As expected,
Ser473 and downstream genes, including mTOR, GSK3β and ribosomal
protein S6 kinase β-1 (p70S6K), also showed increased expression
(34). Mechanistically, CTMP1 is
thought to alleviate pathological cardiac hypertrophy by blocking
the PKB pathway (30).
Idiopathic pulmonary fibrosis is a chronic,
progressive, fibrotic interstitial lung disease predominantly
affecting older individuals (70).
Bleomycin is widely recognized for its ability to induce pulmonary
fibrosis in mice (71). In a
bleomycin-induced lung fibrosis model, CTMP1(−/-) mice exhibited
increased collagen deposition and enhanced fibrosis. Specifically,
these knock-out mice showed elevated levels of collagen type I α1
chain and α-SMA expression. In addition, epithelial-to-mesenchymal
transition was evidenced by a significant increase in mesenchymal
markers such as fibronectin and a decrease in the epithelial marker
E-cadherin at both the protein and mRNA levels (unpublished data)
(Fig. 4). In summary, CTMP1 has
been shown to impact fibrosis in the kidneys, heart and lungs by
inhibiting PKB phosphorylation. However, the effects of CTMP1 on
other organs remain elusive, necessitating further investigation
(Fig. 4).
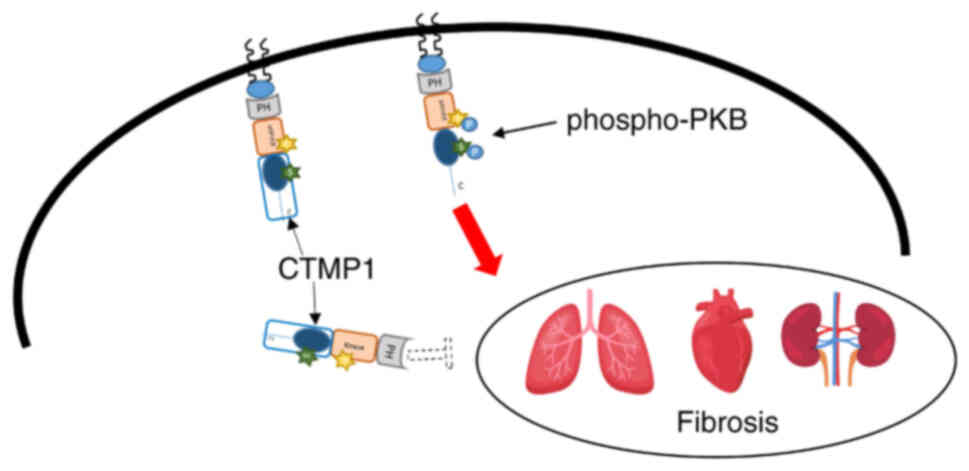 | Figure 4.Role of CTMP1 in the regulation of
fibrosis in lung, heart and kidney through PKB phosphorylation.
CTMP1 alleviates lung, heart and kidney fibrosis by inhibiting
phosphorylated PKB and then downregulating other substrates of PKB
signaling. Conversely, CTMP1 ablation enhances fibrosis in these
organs. CTMP, carboxyl-terminal modulator protein; PKB, protein
kinase B, PH, Pleckstrin homology domain; S, Ser473; T, Thr308; C,
C-terminal end. |
Adverse effects of CTMP on brain injury
Astrocytes from the hippocampi of mice with kainic
acid-induced neurodegeneration exhibit an increase in CTMP1
expression and suppression of PKB activity, negatively affecting
astrocyte activation (72). CTMP1
inhibits PKB, thereby negatively impacting the mobilization of
functional calcium-activated potassium channels in developing
parasympathetic neurons. This inhibition hinders the mobilization
evoked by β-neuregulin-1 and TGFβ1 (73). In addition, CTMP1 may be related to
the mechanism by which isoflurane ameliorates neurological
outcomes. This is because isoflurane can prevent neurological
complications when administered with a normal diet, but not with a
high-fat diet. In addition, a high-fat diet has been shown to
increase CTMP1 levels and reduce PKB activity in the hippocampus
(52). The CTMP1 mRNA level
rapidly increases following ischemic cerebral infarction but only
partially recovers after reperfusion. CTMP1 transcription is
suppressed by activating transcription factor 3 (ATF3), leading to
the protection of neurons from hypoxic insult by indirectly
enhancing PKB activity (22).
Inhibition of CTMP1 reduces hypoxic neuronal apoptosis by
increasing phospho-PKB levels, while upstream ATF3 levels remain
constant (23). This reinforces
the endogenous neuroprotective ATF3-CTMP1 signaling cascade,
representing a potential therapeutic target for ischemic brain
injury. Outside the hippocampus, CTMP1 also shows higher expression
in type 2 diabetic mice with focal cerebral ischemia (25) and in vulnerable hippocampal neurons
(26), leading to suppressed PKB
activity and negatively impacting ischemia outcomes (25,26).
Sevoflurane serves as an additional neuroprotective
target by enhancing PKB activity and GSK3β. However, the
neuroprotective benefits of its preconditioning are diminished in
the presence of CTMP1 overexpression. In addition, a correlation
has been observed between CTMP1 and reduced PKB expression after
ischemic events (21). CTMP1
expression is increased in tissues of the ischemic penumbra,
suggesting that the rise in CTMP1 levels with age may have a role
in the decreased ischemic tolerance of the brain (20). Furthermore, in mice subjected to
traumatic brain injury, phospho-PKB levels initially peak to
provide neuroprotection before diminishing, while CTMP1 levels peak
and remain stable after injury. This indicates that during
traumatic brain injury, CTMP1 activation serves to inhibit PKB
phosphorylation. In Parkinson's disease, the second most common
neurodegenerative disorder, CTMP2 has been observed to reduce the
use of the full-length transcript containing 247 amino acids
(Fig. 1) while increasing the use
of a shorter transcript containing 119 amino acids (74).
This variation led to an intriguing discovery: The
full-length isoform of CTMP2 localizes within mitochondria, whereas
the shorter isoform is more likely to be found in the extracellular
space than in the mitochondria. This suggests that in Parkinson's
disease, mitochondrial CTMP2 is downregulated along with a decrease
in the full-length transcript (75). Furthermore, CTMP2 has been found to
be significantly associated with cisplatin-induced peripheral
neuropathy, in which higher expression of CTMP2 is correlated with
this side effect of cisplatin chemotherapy (76). Overall, CTMP appears to exert a
negative effect on neuroprotection. Inhibiting CTMP may enhance the
recovery of neurological function by increasing phosphorylation of
PKB.
Other factors
Age
In a study of autoimmune thyroiditis, one of the
most prevalent endocrine autoimmune diseases, the methylation
levels of CTMP1 cDNA were analyzed and compared between patients
and controls exposed to varying levels of iodine in water. This
comparison was performed to assess the impact of genes related to
the PI3K-AKT signaling pathway. The study showed a negative
correlation between the CTMP1 methylation level and age (77). In addition, in rat brains, an
increase in CTMP1 expression was observed alongside a decrease in
activated PKB as a function of aging (20).
Vitamin D
The active form of vitamin D, namely
1,25-dihydroxyvitamin D [1,25(OH)2D], alleviates local inflammation
by upregulating CTMP1, which in turn reduces the phosphorylation of
both PKB and its downstream target, IκBα. Conversely, knockdown of
CTMP1 diminishes the inhibitory effect of 1,25(OH)2D on macrophages
(78).
Malaria
Malaria is a deadly parasitic disease and its
underlying mechanisms are not fully understood. Analysis of the
GSE1124 dataset revealed that CTMP1 was positively associated with
asymptomatic Plasmodium falciparum infection, a
classification of malaria (79).
Muscle atrophy and myogenesis,
amyotrophic lateral sclerosis (ALS)
CTMP1 has a negative role in hypertrophy of both
skeletal and cardiac muscles. It does so by enhancing muscle
atrophy after nerve injury, primarily through inhibition of PKB
activity and other downstream factors (80). CTMP1 interacts with N-Myc
downstream-regulated gene 4, which hinders its ability to bind to
PKB. This interference increases PKB phosphorylation and
subsequently enhances cAMP response element-binding protein
activity during differentiation of C2C12 myoblasts. This process
leads to a boost in the expression of myogenic genes (81). Furthermore, in both ALS model mice
and differentiated C2C12 cells, a significant increase in CTMP1 at
the protein level was observed in the hindlimb skeletal muscle.
This increase was associated with a decrease in the level of
phosphorylated PKB (82).
Lung hypertension
CTMP1 ablation enhances PKB activity during the
development of pulmonary hypertension. This enhancement is
attributed to the ability of asymmetric dimethylarginine to
increase peroxynitrite generation and promote the mitochondrial
translocation of endothelial nitric oxide synthase. This
translocation is essential for regulating mitochondrial function
(83).
Acute rejection after
transplantation
In cases of acute rejection following renal
transplantation, CTMP2 is notably downregulated. It shows a
negative correlation with naive B cells but a positive association
with M2 macrophages, resting mast cells, memory B cells and resting
natural killer cells (84).
Future perspectives
The recent discoveries regarding CTMP1 and CTMP2
have led to several key points, which are summarized as follows and
also in Table I.
 | Table I.Summary comparison of CTMP1 and
CTMP2′s functions. |
Table I.
Summary comparison of CTMP1 and
CTMP2′s functions.
| Function | CTMP1 | CTMP2 |
|---|
| Role of CTMP in PKB
signaling pathway | CTMP1 inhibits PKBα
activity by preventing its phosphorylation at Ser473 and Thr308
residues | CTMP2′s
relationship with PKB is unclear |
| Impact of CTMP on
metabolic syndrome | CTMP1 upregulation
in obesity contributes to insulin resistance | CTMP2′s role is
limited to adipose tissue and liver |
| Regulation of CTMP
in apoptosis and mitochondria | CTMP1 promotes
apoptosis by inhibiting anti-apoptotic proteins and affects
mitochondrial morphology | CTMP2 influences
mitochondrial function |
| Various functions
of CTMP in cancer | CTMP1 exhibits
varied expression in different cancers and impacts tumor
progression | CTMP2′s role is
less understood, particularly in cancer |
| Emerging role of
CTMP1 in regulation of fibrosis | CTMP1 mitigates
renal and cardiac fibrosis, while exacerbating pulmonary
fibrosis | Effect of CTMP2 in
fibrosis is elusive |
| Adverse effects of
CTMP on brain injury | CTMP1 negatively
affects astrocyte activation, neuroprotection and neurological
recovery post-injury | CTMP2 may
contribute to Parkinson's disease and cisplatin-induced
neuropathy |
| Other factors | CTMP1 expression is
influenced by age, vitamin D, malaria, muscle atrophy, lung
hypertension and acute rejection post-transplantation | CTMP2 is associated
with fatty liver disease and cardiolipin remodeling |
CTMP1 primarily functions as an inhibitor of PKB,
playing a regulatory role in lipid metabolism, cancer, fibrosis and
neurodegeneration. However, there are certain exceptions to this.
CTMP1 has been shown to increase PKB phosphorylation, leading to
opposite conclusions. Therapeutically targeting CTMP1 in cancer and
fibrosis presents a promising avenue for future treatment. However,
adopting a rigid mindset in researching CTMP1 or CTMP2 is
discouraged. In addition, CTMP1 and its binding partner LETM1 are
often correlated with apoptosis, lipid metabolism and cancer.
The expression of CTMP1 under obesity-related
conditions across different organs remains controversial. However,
its effects on organs may be categorized into two groups: i)
Adipose tissues and liver, in which lipid metabolism is
predominant; and ii) other organs, such as the kidneys, heart and
lungs. Further investigation into the effects on these remaining
organs is required.
The knowledge of CTMP2 is comparably scarce,
particularly in the context of cancer, with no findings related to
CTMP2 in this area. The sole functions of CTMP2 identified thus far
pertain to fatty liver disease and cardiolipin remodeling. The
possibility that CTMP2 may share similar functions with CTMP1
cannot be dismissed. Furthermore, the correlation between CTMP2 and
PKB is not well established, suggesting a novel area of
exploration.
These findings offer fascinating opportunities for
further research. Thorough exploration is required to fully
understand the roles of CTMP1 and CTMP2 and their potential for
therapeutic applications.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the research fund of
Chungnam National University (2022; to SK), by the National
Research Foundation of Korea (NRF) grant funded by the Korean
Government (MEST; grant nos. NRF-2021R1A2C1008492,
NRF-2020R1F1A1049801 and NRF-2018R1A6A1A03023718) and by the
Starting growth Technological R&D Program (TIPS Program; grant
no. S3198556) funded by the Ministry of Small and Medium-sized
Enterprises (SMEs) and Micro Enterprises (Ministry of SMEs and
Startups, Korea) in 2021.
Availability of data and materials
Not applicable.
Authors' contributions
HN, SG, UJ, WJ, SHK, SK and JP contributed to the
conception and design of the study. HN, UJ, SG, QH, SL, BL and JP
were involved in the literature search, selection and analysis. HN,
UJ, SG and WJ contributed to the analysis in the GEPIA database.
SHK, SK and JP contributed to acquiring funding and performing the
final revision of the manuscript. All authors agreed to be
accountable for all aspects of the work and all authors read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maira SM, Galetic I, Brazil DP, Kaech S,
Ingley E, Thelen M and Hemmings BA: Carboxyl-terminal modulator
protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the
plasma membrane. Science. 294:374–380. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glaviano A, Foo ASC, Lam HY, Yap KCH,
Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al:
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies
in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z: Regulation of cell cycle
progression by growth factor-induced cell signaling. Cells.
10:33272021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parcellier A, Tintignac LA, Zhuravleva E,
Cron P, Schenk S, Bozulic L and Hemmings BA: Carboxy-terminal
modulator protein (CTMP) is a mitochondrial protein that sensitizes
cells to apoptosis. Cell Signal. 21:639–650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piao L, Li Y, Yang KJ, Park KA, Byun HS,
Won M, Hong J, Kim JL, Kweon GR, Hur GM, et al: Heat shock protein
70-mediated sensitization of cells to apoptosis by
carboxyl-terminal modulator protein. BMC Cell Biol. 10:532009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin H, Xu CX, Lim HT, Park SJ, Shin JY,
Chung YS, Park SC, Chang SH, Youn HJ, Lee KH, et al: High dietary
inorganic phosphate increases lung tumorigenesis and alters Akt
signaling. Am J Respir Crit Care Med. 179:59–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang SK, Kwon JT, Park SJ, Chang SH, Lee
ES, Chung YS, Beck GR Jr, Lee KH, Piao L, Park J and Cho MH:
Lentivirus-mediated carboxyl-terminal modulator protein gene
transfection via aerosol in lungs of K-ras null mice. Gene Ther.
14:1721–1730. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niu F, Duan Y, Man Y, Liu W, Dai T, Zhang
H, Li C and Wei D: Mitochondrial protein LETM1 and its-mediated
CTMP are potential therapeutic targets for endometrial cancer.
Anticancer Drugs. 33:632–641. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simon PO Jr, McDunn JE, Kashiwagi H, Chang
K, Goedegebuure PS, Hotchkiss RS and Hawkins WG: Targeting AKT with
the proapoptotic peptide, TAT-CTMP: A novel strategy for the
treatment of human pancreatic adenocarcinoma. Int J Cancer.
125:942–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knobbe CB, Reifenberger J, Blaschke B and
Reifenberger G: Hypermethylation and transcriptional downregulation
of the carboxyl-terminal modulator protein gene in glioblastomas. J
Natl Cancer Inst. 96:483–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang JW, Jung SN, Kim JH, Shim GA, Park
HS, Liu L, Kim JM, Park J and Koo BS: Carboxyl-terminal modulator
protein positively acts as an oncogenic driver in head and neck
squamous cell carcinoma via regulating Akt phosphorylation. Sci
Rep. 6:285032016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YP, Liao WC, Ger LP, Chen JC, Hsu TI,
Lee YC, Chang HT, Chen YC, Jan YH, Lee KH, et al: Carboxyl-terminal
modulator protein positively regulates Akt phosphorylation and acts
as an oncogenic driver in breast cancer. Cancer Res. 73:6194–6205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin CH, Lin WD, Huang YC, Chen YC, Loh ZJ,
Ger LP, Lin FC, Li HY, Cheng HC, Lee KH, et al: Carboxyl-terminal
modulator protein facilitates tumor metastasis in triple-negative
breast cancer. Cancer Gene Ther. 30:404–413. 2023.PubMed/NCBI
|
|
15
|
Bolze PA, Lopez J, Allias F, Hajri T,
Patrier S, Devouassoux-Shisheboran M, Massardier J, You B, Golfier
F and Mallet F: Transcriptomic and immunohistochemical approaches
identify HLA-G as a predictive biomarker of gestational
choriocarcinoma resistance to monochemotherapy. Gynecol Oncol.
158:785–793. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ni FB, Lin Z, Fan XH, Shi KQ, Ao JY, Wang
XD and Chen RC: A novel genomic-clinicopathologic nomogram to
improve prognosis prediction of hepatocellular carcinoma. Clin Chim
Acta. 504:88–97. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang S, Li D, Zhao M, Yang F, Sang C, Yan
C, Wang Z and Li Y: Exosomal miR-183-5p shuttled by M2 polarized
tumor-associated macrophage promotes the development of colon
cancer via targeting THEM4 mediated PI3K/AKT and NF-κB pathways.
Front Oncol. 11:6726842021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen YC, Li HY, Liang JL, Ger LP, Chang
HT, Hsiao M, Calkins MJ, Cheng HC, Chuang JH and Lu PJ: CTMP, a
predictive biomarker for trastuzumab resistance in HER2-enriched
breast cancer patient. Oncotarget. 8:29699–29710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Block M, Grundker C, Fister S, Kubin J,
Wilkens L, Mueller MD, Hemmerlein B, Emons G and Günthert AR:
Inhibition of the AKT/mTOR and erbB pathways by gefitinib,
perifosine and analogs of gonadotropin-releasing hormone I and II
to overcome tamoxifen resistance in breast cancer cells. Int J
Oncol. 41:1845–1854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Shan W and Zuo Z: Age-related
upregulation of carboxyl terminal modulator protein contributes to
the decreased brain ischemic tolerance in older rats. Mol
Neurobiol. 55:6145–6154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Nie H, Tian L, Tong L, Deng J,
Zhang Y, Dong H and Xiong L: Sevoflurane preconditioning-induced
neuroprotection is associated with Akt activation via
carboxy-terminal modulator protein inhibition. Br J Anaesth.
114:327–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kao MH, Huang CY, Cheung WM, Yan YT, Chen
JJ, Ho YS, Hsu CY and Lin TN: Activating transcription factor 3
diminishes ischemic cerebral infarct and behavioral deficit by
downregulating carboxyl-terminal modulator protein. Int J Mol Sci.
24:23062023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang CY, Chen JJ, Wu JS, Tsai HD, Lin H,
Yan YT, Hsu CY, Ho YS and Lin TN: Novel link of anti-apoptotic ATF3
with pro-apoptotic CTMP in the ischemic brain. Mol Neurobiol.
51:543–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao S, Fu J, Liu F, Rastogi R, Zhang J
and Zhao Y: Small interfering RNA directed against CTMP reduces
acute traumatic brain injury in a mouse model by activating Akt.
Neurol Res. 36:483–490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Cai M, Deng J, Tian L, Wang S,
Tong L, Dong H and Xiong L: Elevated expression of carboxy-terminal
modulator protein (CTMP) aggravates brain ischemic injury in
diabetic db/db Mice. Neurochem Res. 41:2179–2189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyawaki T, Ofengeim D, Noh KM,
Latuszek-Barrantes A, Hemmings BA, Follenzi A and Zukin RS: The
endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced
neuronal death. Nat Neurosci. 12:618–626. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park J, Li Y, Kim SH, Yang KJ, Kong G,
Shrestha R, Tran Q, Park KA, Jeon J, Hur GM, et al: New players in
high fat diet-induced obesity: LETM1 and CTMP. Metabolism.
63:318–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mondal AK, Sharma NK, Elbein SC and Das
SK: Allelic expression imbalance screening of genes in chromosome
1q21-24 region to identify functional variants for Type 2 diabetes
susceptibility. Physiol Genomics. 45:509–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen N, Hao J, Li L, Li F, Liu S and Duan
H: Carboxy-terminal modulator protein attenuated extracellular
matrix deposit by inhibiting phospho-Akt, TGF-β1 and α-SMA in
kidneys of diabetic mice. Biochem Biophys Res Commun. 474:753–760.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Yang Q, Zhu LH, Liu J, Deng KQ, Zhu
XY, Liu Y, Gong J, Zhang P, Li S, et al: Carboxyl-terminal
modulator protein ameliorates pathological cardiac hypertrophy by
suppressing the protein kinase B signaling pathway. J Am Heart
Assoc. 7:e0086542018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhuravleva E, Gut H, Hynx D, Marcellin D,
Bleck CK, Genoud C, Cron P, Keusch JJ, Dummler B, Esposti MD and
Hemmings BA: Acyl coenzyme A thioesterase Them5/Acot15 is involved
in cardiolipin remodeling and fatty liver development. Mol Cell
Biol. 32:2685–2697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Swarbrick CMD, Nanson JD, Patterson EI and
Forwood JKL: Structure, function, and regulation of thioesterases.
Prog Lipid Res. 79:1010362020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tillander V, Alexson SEH and Cohen DE:
Deactivating fatty acids: Acyl-CoA thioesterase-mediated control of
lipid metabolism. Trends Endocrinol Metab. 28:473–484. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brocker C, Carpenter C, Nebert DW and
Vasiliou V: Evolutionary divergence and functions of the human
acyl-CoA thioesterase gene (ACOT) family. Hum Genomics. 4:411–420.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao H, Martin BM, Bisoffi M and
Dunaway-Mariano D: The Akt C-terminal modulator protein is an
acyl-CoA thioesterase of the Hotdog-Fold family. Biochemistry.
48:5507–5509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao H, Lim K, Choudry A, Latham JA,
Pathak MC, Dominguez D, Luo L, Herzberg O and Dunaway-Mariano D:
Correlation of structure and function in the human hotdog-fold
enzyme hTHEM4. Biochemistry. 51:6490–6492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jones PF, Jakubowicz T and Hemmings BA:
Molecular cloning of a second form of rac protein kinase. Cell
Regul. 2:1001–1009. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng JQ, Godwin AK, Bellacosa A, Taguchi
T, Franke TF, Hamilton TC, Tsichlis PN and Testa JR: AKT2, a
putative oncogene encoding a member of a subfamily of
protein-serine/threonine kinases, is amplified in human ovarian
carcinomas. Proc Natl Acad Sci USA. 89:9267–9271. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brodbeck D, Cron P and Hemmings BA: A
human protein kinase Bgamma with regulatory phosphorylation sites
in the activation loop and in the C-terminal hydrophobic domain. J
Biol Chem. 274:9133–9136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ruan GX and Kazlauskas A: Focus on
molecules: Akt (PKB). Exp Eye Res. 93:570–571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Andjelkovic M, Alessi DR, Meier R,
Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM and
Hemmings BA: Role of translocation in the activation and function
of protein kinase B. J Biol Chem. 272:31515–31524. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stephens L, Anderson K, Stokoe D,
Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB,
McCormick F, Tempst P, et al: Protein kinase B kinases that mediate
phosphatidylinositol 3,4,5-trisphosphate-dependent activation of
protein kinase B. Science. 279:710–714. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Alessi DR, Andjelkovic M, Caudwell B, Cron
P, Morrice N, Cohen P, Morrice N, Cohen P and Hemmings BA:
Mechanism of activation of protein kinase B by insulin and IGF-1.
EMBO J. 15:6541–6551. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhuravleva E: Structural and functional
characterization of novel mitochondrial acyl-CoA thioesterase
Them5/CTMP2 (Doctoral Thesis). University of Basel; 2013
|
|
48
|
Brazil DP, Park J and Hemmings BA: PKB
binding proteins. Getting in on the Akt. Cell. 111:293–303. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ono H, Sakoda H, Fujishiro M, Anai M,
Kushiyama A, Fukushima Y, Katagiri H, Ogihara T, Oka Y, Kamata H,
et al: Carboxy-terminal modulator protein induces Akt
phosphorylation and activation, thereby enhancing antiapoptotic,
glycogen synthetic, and glucose uptake pathways. Am J Physiol Cell
Physiol. 293:C1576–C1585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou Q, Leeman SE and Amar S: Signaling
mechanisms involved in altered function of macrophages from
diet-induced obese mice affect immune responses. Proc Natl Acad Sci
USA. 106:10740–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shin JY, Chung YS, Kang B, Jiang HL, Yu
DY, Han K, Chae C, Moon JH, Jang G and Cho MH: Co-delivery of LETM1
and CTMP synergistically inhibits tumor growth in H-ras12V liver
cancer model mice. Cancer Gene Ther. 20:186–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu H, Deng J and Zuo Z: High-fat diet
reduces neuroprotection of isoflurane post-treatment: Role of
carboxyl-terminal modulator protein-Akt signaling. Obesity (Silver
Spring). 22:2396–2405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhao Y, Zhang Y, Khas E, Bai C, Cao Q and
Ao C: Transcriptome analysis reveals candidate genes of the
synthesis of branched-chain fatty acids related to mutton flavor in
the lamb liver using Allium mongolicum Regel extract. J Anim Sci.
100:skac2562022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu B, Zheng Y, Alexander D, Morrison AC,
Coresh J and Boerwinkle E: Genetic determinants influencing human
serum metabolome among African Americans. PLoS Genet.
10:e10042122014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hwang SK, Minai-Tehrani A, Yu KN, Chang
SH, Kim JE, Lee KH, Park J, Beck GR Jr and Cho MH:
Carboxyl-terminal modulator protein induces apoptosis by regulating
mitochondrial function in lung cancer cells. Int J Oncol.
40:1515–1524. 2012.PubMed/NCBI
|
|
56
|
Piao L, Li Y, Kim SJ, Sohn KC, Yang KJ,
Park KA, Byun HS, Won M, Hong J, Hur GM, et al: Regulation of
OPA1-mediated mitochondrial fusion by leucine
zipper/EF-hand-containing transmembrane protein-1 plays a role in
apoptosis. Cell Signal. 21:767–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Parcellier A, Tintignac LA, Zhuravleva E,
Dummler B, Brazil DP, Hynx D, Cron P, Schenk S, Olivieri V and
Hemmings BA: The carboxy-terminal modulator protein (CTMP)
regulates mitochondrial dynamics. PLoS One. 4:e54712009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vo TT, Kong G, Kim C, Juang U, Gwon S,
Jung W, Nguyen H, Kim SH and Park J: Exploring scavenger receptor
class F member 2 and the importance of scavenger receptor family in
prediagnostic diseases. Toxicol Res. 39:341–353. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang B, Xu X, Liu X, Wang D, Zhuang H, He
X, Han T and Hong J: Enolase-phosphatase 1 acts as an oncogenic
driver in glioma. J Cell Physiol. 236:1184–1194. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tews B, Roerig P, Hartmann C, Hahn M,
Felsberg J, Blaschke B, Sabel M, Kunitz A, Toedt G, Neben K, et al:
Hypermethylation and transcriptional downregulation of the CITED4
gene at 1p34.2 in oligodendroglial tumours with allelic losses on
1p and 19q. Oncogene. 26:5010–5016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Knobbe CB, Trampe-Kieslich A and
Reifenberger G: Genetic alteration and expression of the
phosphoinositol-3-kinase/Akt pathway genes PIK3CA and PIKE in human
glioblastomas. Neuropathol Appl Neurobiol. 31:486–490. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gao S, Gang J, Yu M, Xin G and Tan H:
Computational analysis for identification of early diagnostic
biomarkers and prognostic biomarkers of liver cancer based on GEO
and TCGA databases and studies on pathways and biological functions
affecting the survival time of liver cancer. BMC Cancer.
21:7912021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yamasaki D, Kawabe N, Nakamura H,
Tachibana K, Ishimoto K, Tanaka T, Aburatani H, Sakai J, Hamakubo
T, Kodama T and Doi T: Fenofibrate suppresses growth of the human
hepatocellular carcinoma cell via PPARalpha-independent mechanisms.
Eur J Cell Biol. 90:657–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Niu Q, Zhao W, Wang J, Li C, Yan T, Lv W,
Wang G, Duan W, Zhang T, Wang K and Zhou D: LicA induces autophagy
through ULK1/Atg13 and ROS pathway in human hepatocellular
carcinoma cells. Int J Mol Med. 41:2601–2608. 2018.PubMed/NCBI
|
|
65
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gutierrez-Barrera AM, Menter DG,
Abbruzzese JL and Reddy SA: Establishment of three-dimensional
cultures of human pancreatic duct epithelial cells. Biochem Biophys
Res Commun. 358:698–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hwang SK, Lim HT, Minai-Tehrani A, Lee ES,
Park J, Park SB, Beck GR Jr and Cho MH: Repeated aerosol delivery
of carboxyl-terminal modulator protein suppresses tumor in the
lungs of K-rasLA1 mice. Am J Respir Crit Care Med. 179:1131–1140.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang B, Cai Y, Li X, Kong Y, Fu H and Zhou
J: ETV4 mediated lncRNA C2CD4D-AS1 overexpression contributes to
the malignant phenotype of lung adenocarcinoma cells via
miR-3681-3p/NEK2 axis. Cell Cycle. 20:2607–2618. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gao S, Tan H and Li D: Oridonin suppresses
gastric cancer SGC-7901 cell proliferation by targeting the
TNF-alpha/androgen receptor/TGF-beta signalling pathway axis. J
Cell Mol Med. 27:2661–2674. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Strykowski R and Adegunsoye A: Idiopathic
pulmonary fibrosis and progressive pulmonary fibrosis. Immunol
Allergy Clin North Am. 43:209–228. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gul A, Yang F, Xie C, Du W, Mohammadtursun
N, Wang B, Le J and Dong J: Pulmonary fibrosis model of mice
induced by different administration methods of bleomycin. BMC Pulm
Med. 23:912023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shin N, Yi MH, Kim S, Baek H, Triantafillu
UL, Park J and Kim DW: Astrocytic expression of CTMP following an
excitotoxic lesion in the mouse hippocampus. Exp Neurobiol.
26:25–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chae KS, Martin-Caraballo M, Anderson M
and Dryer SE: Akt activation is necessary for growth factor-induced
trafficking of functional K(Ca) channels in developing
parasympathetic neurons. J Neurophysiol. 93:1174–1182. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lampropoulos IC, Malli F, Sinani O,
Gourgoulianis KI and Xiromerisiou G: Worldwide trends in mortality
related to Parkinson's disease in the period of 1994–2019: Analysis
of vital registration data from the WHO mortality database. Front
Neurol. 13:9564402022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dick F, Nido GS, Alves GW, Tysnes OB,
Nilsen GH, Dolle C and Tzoulis C: Differential transcript usage in
the Parkinson's disease brain. PLoS Genet. 16:e10091822020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dolan ME, El Charif O, Wheeler HE, Gamazon
ER, Ardeshir-Rouhani-Fard S, Monahan P, Feldman DR, Hamilton RJ,
Vaughn DJ, Beard CJ, et al: Clinical and genome-wide analysis of
cisplatin-induced peripheral neuropathy in survivors of adult-onset
cancer. Clin Cancer Res. 23:5757–5768. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ren B, Wan S, Wu H, Qu M, Chen Y, Liu L,
Jin M, Zhou Z and Shen H: Effect of different iodine levels on the
DNA methylation of PRKAA2, ITGA6, THEM4 and PRL genes in PI3K-AKT
signaling pathway and population-based validation from autoimmune
thyroiditis patients. Eur J Nutr. 61:3571–3583. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang Q, He Y, Shen Y, Zhang Q, Chen D, Zuo
C, Qin J, Wang H, Wang J and Yu Y: Vitamin D inhibits COX-2
expression and inflammatory response by targeting thioesterase
superfamily member 4. J Biol Chem. 289:11681–1194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nambou K, Nie X, Tong Y and Anakpa M:
Weighted gene co-expression network analysis and drug-gene
interaction bioinformatics uncover key genes associated with
various presentations of malaria infection in African children and
major drug candidates. Infect Genet Evol. 89:1047232021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang J, Tierney L, Wilson C, Phillips V,
Goldman L, Mumaw C, Muang E and Walker CL: Carboxyl-terminal
modulator protein (CTMP) deficiency mitigates denervation-induced
skeletal muscle atrophy. Biochem Biophys Res Commun. 644:155–161.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhu M, Zheng R, Guo Y, Zhang Y and Zuo B:
NDRG4 promotes myogenesis via Akt/CREB activation. Oncotarget.
8:101720–10134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang J, Fry CME and Walker CL:
Carboxyl-terminal modulator protein regulates Akt signaling during
skeletal muscle atrophy in vitro and a mouse model of amyotrophic
lateral sclerosis. Sci Rep. 9:39202019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sun X, Kellner M, Desai AA, Wang T, Lu Q,
Kangath A, Qu N, Klinger C, Fratz S, Yuan JX, et al: Asymmetric
dimethylarginine stimulates Akt1 phosphorylation via heat shock
protein 70-facilitated carboxyl-terminal modulator protein
degradation in pulmonary arterial endothelial cells. Am J Respir
Cell Mol Biol. 55:275–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lu Z, Tang F, Li Z, Xie Z, Zheng H, Zhang
J, Gao Y, Lu Z, Cai Y, Lai Y and He Z: Characteristic genes and
immune infiltration analysis for acute rejection after kidney
transplantation. Dis Markers. 2022:65750522022. View Article : Google Scholar : PubMed/NCBI
|