Introduction
Itching, commonly known as an itch sensation, is a
sensory experience that is universally felt by most animals,
including humans. It serves as a protective mechanism against
irritants and serves as a defense mechanism against parasites and
pests (1). However, when itching
becomes uncontrollable and excessive, as often seen in conditions
such as atopic dermatitis and allergies, it can lead to skin
damage, sleep disturbances and a significant reduction in the
quality of life (2). Historically,
studies on itching have primarily focused on histamine-related
mechanisms. However, in the search for solutions to chronic itching
that is not alleviated by antihistamines, research has been
conducted on non-histaminergic itching. The field of itch studies
has expanded to the central nervous system (CNS) (3). Itch signals can be transmitted from
the skin to astrocytes of the spinal dorsal horn (SDH) through
primary afferent nerves. Histamine-independent itch can develop
(4). Therefore, it is necessary to
find substances that can regulate itching not only in the skin, but
also in the CNS.
Microglia under normal physiological conditions
serve a central role as intrinsic immune cells of the CNS (5), promoting the proliferation and
survival of neuronal precursor cells and protecting neurons with
limited regenerative abilities (6). However, upon exposure to pathological
stimuli, activated microglia can release IL-6 and inflammatory
mediators such as nitric oxide, prostaglandin E2, IL-1β
and TNF-α known to contribute to itching (7). Spinal microglia serve a crucial role
in both histamine-dependent and histamine-independent itching
(8), with a recent study
emphasizing microglia-neuron interactions involving the
gastrin-releasing peptide receptor (GRPR) in itch sensation
(9).
Astrocytes comprising 20–40% of the glial cells in
the CNS traditionally provide structural and nutritional support to
neurons (10). Historically,
astrocytes have been primarily associated with pain in
neurodegenerative conditions and inflammation (11,12).
However, their relationship with acute or chronic itching remains
an uncharted territory. Emerging findings from rodent models of
atopic dermatitis and contact dermatitis have now shed light on the
activation of astrocytes within the SDH region, indicating their
involvement in persistent itching by releasing gastrin-releasing
peptide (GRP) (13).
Diospyros lotus, a member of the Ebenaceae
family and a deciduous tree native to various parts of Asia
including South Korea, has a rich history of traditional use in
food, folk and traditional medicine (14). Different parts of D. lotus,
including its fruit, seeds, leaves and bark, have been used for
various therapeutic purposes due to their anti-inflammatory
(14), sedative (14) and antimicrobial (15) effects. They can also relieve
biliousness, diabetes, cancer and fevers (16). In addition, they can provide relief
from back pain (17). We have
previously identified several key compounds in D. lotus
extract (DLE), including gallic acid, caffeic acid, chlorogenic
acid, myricetin-3-O-galactoside, myricitrin, astragalin, quercetin
and myricetin, with myricitrin standing out as notably abundant
among these compounds (18,19).
Our previous studies have also demonstrated multifaceted effects of
DLE, including anti-inflammatory (18), photoprotective (18), liver-protective (19), anti-obesity (20) and notably, anti-atopic effects
(21,22). Particularly, our previous research
has demonstrated DLE's efficacy in alleviating itching by
inhibiting the activity of SDH astrocytes (23). Despite these promising findings,
previous studies have not thoroughly investigated the influence of
DLE on cytokine production related to itch in microglia, nor have
they explored the complex interplay between microglia and
astrocytes in relation to itch mechanisms. Therefore, the primary
objective of the present study was to investigate effects of DLE
and its major component myricitrin (MC) on microglia when
stimulated by lipopolysaccharide (LPS) in relation to itchiness.
Additionally, the present study aimed to examine the impact of
conditioned media from LPS-treated microglia on astrocytes,
particularly in relation to itch mechanisms.
Materials and methods
Plant materials
On June 13, 2022, D. lotus leaves were
collected from Cheonjam mountain, Jeonju-si, Jeollabuk-do, Republic
of Korea. The identification and authentication of the plant were
conducted by Professor Hong-Jun Kim (College of Oriental Medicine,
Woosuk University, Jeonbuk, Republic of Korea). A voucher specimen
(no. 2022-06-04) was deposited in the Department of Health
Management, College of Medical Science, Jeonju University
(Jeollabuk-do, Republic of Korea). Leaves were washed five times
with water and then dried in a well-ventilated shaded area. Dried
leaves (100 g) were extracted with 2 l of 70% (v/v) ethanol at room
temperature for 48 h. The resulting extract was filtered using a 5
µm filter paper, vacuum-concentrated and then freeze-dried to
obtain a powdered form of the DLE.
Reagents and materials
The following reagents and materials were purchased
from the specified suppliers: LPS, Griess reagent and protease
inhibitors from MilliporeSigma; myricitrin from Tokyo Chemical
Industry; Quanti-MAX WST-8 Cell Viability Assay Kit and WestGlow
FEMTO Chemiluminescent substrate from Biomax Ltd.;
radio-immunoprecipitation assay (RIPA) buffer, IL-33, inositol
1,4,5-trisphosphate receptor 1 (IP3R1), lipocalin-2 (LCN2), glial
fibrillary acidic protein (GFAP), goat anti-rabbit IgG Alexa Fluor
488 antibodies and goat anti-mouse IgG Alexa Fluor 488 from Thermo
Fisher Scientific, Inc.; IL-31 antibodies from Abcam; β-actin,
phosphorylated (p-)IκBα, IκBα, p-NF-κB, NF-κB, p-JNK, JNK, p-p38,
p38, oncostatin M receptor (OSMR), Toll-like receptor 4 (TLR4),
IL-6, interleukin 31 receptor a (IL31RA) antibodies and IL-31 short
interfering (si)RNA from Santa Cruz Biotechnology, Inc.; and
ProLong Gold Antifade Reagent with DAPI, p-Ikk, Ikk, p-JAK1, JAK1
p-STAT3, STAT3, p-ERK and ERK antibodies from Cell Signaling
Technology, Inc.
Cell culture
Mouse-origin microglia (CRL-3265) and astrocytes
(CRL-2541) were obtained from ATCC. Microglia were cultured and
maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 5% horse serum, 100
U/ml penicillin and 100 µg/ml streptomycin (all from Thermo Fisher
Scientific, Inc.) in a 5% CO2 incubator at 37°C.
Astrocytes were cultured and maintained in DMEM supplemented with
10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin under the
same incubation conditions.
IL-6 and IL-31 gene silencing
To silence IL-6 and IL-31 genes in microglia, IL-6
siRNA (cat no. sc-39628), IL-31 siRNA (cat no. sc-146219), control
siRNA (cat no. sc-37007), siRNA transfection medium (cat no.
sc-36868) and siRNA transfection reagent (cat no. sc-29528) from
Santa Cruz Biotechnology, Inc. were used. Gene silencing was
performed according to the manufacturer's protocol without
modification. Microglia (2×105 cells/ml) were cultured
in 6-well cell culture plate at 37°C until 60–80% confluence was
reached. siRNA duplexes were diluted to a final concentration of 50
pmol in 100 µl of siRNA transfection medium. Separately, 5 µl of
siRNA transfection reagent was diluted in 100 µl of the same
medium. The two solutions were combined and incubated at room
temperature for 30 min to form transfection complexes. Cells were
washed once with siRNA transfection medium and the transfection
complexes were overlaid onto the cells. After a 6 h incubation
period at 37°C, 1 ml of normal growth medium (DMEM) containing
double the normal concentration of serum and antibiotics was added
without removing the transfection mixture. Cells were further
incubated for 24 h and used for experiments within 48 h after
transfection. Control siRNAs with scrambled sequences were used to
ensure specificity of the siRNA-mediated gene silencing. The
manufacturer confirmed the following:
sc-39628: IL-6 siRNA (m) is a pool of 3 different
siRNA duplexes:
sc-39628A: Sense: GGCAAUUCUGAUUGUAUGAtt; antisense:
UCAUACAAUCAGAAUUGCCtt
sc-39628B: Sense: CCAAGACCAUCCAAUUCAUtt; antisense:
AUGAAUUGGAUGGUCUUGGtt
sc-39628C: Sense: CCAGAUGGUUUCUUGGAAUtt; antisense:
AUUCCAAGAAACCAUCUGGtt
sc-146219: IL-31 siRNA (m) is a pool of 3 different
siRNA duplexes:
sc-146219A: Sense: GAACUACAAUUGACCUCUUtt; antisense:
AAGAGGUCAAUUGUAGUUCtt
sc-146219B: Sense: CAGGCUAAGGACAAUACUAtt; antisense:
UAGUAUUGUCCUUAGCCUGtt
sc-146219C: Sense: GGUCAUUACUAGUCAUGUUtt; antisense:
AACAUGACUAGUAAUGACCtt.
Preparation of LPS-stimulated
microglia culture medium (LSMCM)
Microglia (2×105 cells/ml) were cultured
in 60-mm cell culture dishes at 37°C for 24 h. Cells were then
stimulated with LPS (1 µg/ml) at 37°C for 3 h. Afterward, the
culture medium was replaced with fresh culture medium and cells
were cultured for an additional 24 h. Subsequently, the culture
medium was collected and centrifuged at 300 × g at 4°C for 2 min,
followed by storage at 4°C.
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of IL-6 and IL-31 contained in
LSMCM were analyzed using an ELISA kit. ELISA analysis was
performed according to the manufacturer's instructions (IL-6; cat.
no. M6000B; R&D Systems, Inc. and IL-31; cat. no. ab243681;
Abcam). The optical density was measured at 450 nm using a
microplate reader (Tecan Group Ltd.).
Protein extraction and western
blotting
Microglia (2×105 cells/ml) were cultured
in 60-mm cell culture dishes at 37°C for 24 h. Cells were then
treated with either DLE (50 and 100 µg/ml) or MC (10 and 20 µM) and
further incubated at 37°C for 1 h. Subsequently, cells were
stimulated with LPS (1 µg/ml) at 37°C for 30 min or 24 h.
Astrocytes (1×105 cells/ml) were cultured in 60-mm cell
culture dishes at 37°C for 24 h. Cells were then treated with
either DLE (50 and 100 µg/ml) or MC (10 and 20 µM) and further
incubated at 37°C for 1 h. Subsequently, cells were stimulated by
LSMCM (mixed with the existing culture medium at a 1:1 ratio) at
37°C for 30 min or 24 h. Total protein was extracted from each
sample using RIPA buffer treated with protease/phosphatase
inhibitors. The protein concentration was determined using the
Bradford assay. SDS-PAGE was performed using 7.5, 10, or 12% gels
to separate proteins (50 µg of proteins per lane). Separated
proteins were transferred to a polyvinylidene fluoride membrane.
Subsequently, membranes were blocked with 5% skimmed milk dissolved
in TBST buffer (Tris-buffered saline with 1% Tween-20) for 1 h at
room temperature to prevent non-specific binding during subsequent
immunoblotting steps. After three washes with TBST for 10 min each,
membranes were incubated overnight with primary antibodies against
IL-6 (1:500; cat. no. sc-57315), IL-31 (1:2,000; cat. no.
ab102750), IL-33 (1:1,000; cat no. MA5-15773), p-ERK (1:500; cat
no. sc-81492), ERK (1:500; cat no. sc-514302), p-p38 (1:500; cat
no. sc-166182), p38 (1:500; cat no. sc-1972), p-JNK (1:500; cat no.
sc-293136), JNK (1:500; cat no. sc-7345), p-STAT3 (1:1,000; cat no.
9145S), STAT3 (1:1,000; cat no. 9139S), p-JAK1 (1:1,000; cat no.
44-422G), JAK1 (1:1,000; cat no. 3344S), p-IKK (1:1,000; cat no.
2697S), IKK (1:1,000; cat no. 2682S), p-IκB (1:500; cat no.
sc-52943), IκB (1:500; cat no. sc-1643), p-NF-κB (1:500; cat no.
sc-271908), NF-κB (1:500; cat no. sc-8414), OSMR (1:500; cat. no.
sc-271695), IL-31RA (1:500; cat. no. sc-515465), IP3R1 (1:1,000;
cat no. PA1-901), LCN2 (1:1,000; cat no. PA5-79590), GFAP (1:1,000;
cat no. 13-0300) and β-actin (1:1,000; cat no. sc-8432) at 4°C.
Following five washes with TBST, membranes were incubated with
HRP-conjugated secondary antibodies anti-mouse (1:5,000; cat no.
sc-525409) and anti-rabbit (1:5,000; cat no. sc-8432) in 5% skimmed
milk for 2 h at room temperature. Subsequently, membranes were
washed three times with TBST solution for 10 min each and
visualized using an imaging system (ALLIANCE LD4; UVITEC) and
WestGlow FEMTO (Biomax). Band densities were analyzed using ImageJ
1.53e (National Institutes of Health) with β-actin serving as the
loading control.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Microglia (2×105 cells/ml) were cultured
in 60 mm cell culture dishes for 24 h. Cells were then treated with
either DLE (50 and 100 µg/ml) or MC (10 and 20 µM) and further
incubated at 37°C for 1 h. Subsequently, cells were stimulated with
LPS (1 µg/ml) at 37°C for 3 h. Cells were used to extract total
RNAs using an RNA-spin Total RNA Extraction Kit obtained from
iNtRON Biotechnology. Subsequently, cDNAs were synthesized from
extracted RNAs using an iScript cDNA Synthesis Kit (Bio-Rad
Laboratories, Inc.). and a T100TM Bio-Rad Thermal Cycler (Bio-Rad
Laboratories, Inc.). Resulting cDNAs were then amplified using a
SYBR kit from TOYOBO. To quantify gene expression levels, reverse
transcription PCR was conducted using a StepOne Real-Time PCR
system from Thermo Fisher Scientific, Inc. Sequences of primers
used for reverse transcription PCR in the present study are listed
in Table I. The thermal profile
consisted of an initial denaturation step at 95°C for 5 min,
followed by 30 cycles of amplification at 95°C for 30 sec and 60°C
for 30 sec. Expression levels were normalized to GAPDH using the
2−ΔΔCq method (24).
All protocols were conducted in accordance with the manufacturer's
instructions without any modifications.
 | Table I.List of primer sequences used for
reverse transcription PCR in the present study. |
Table I.
List of primer sequences used for
reverse transcription PCR in the present study.
| Primer | Forward | Reverse |
|---|
| Mouse IL-6 |
5′-TCCATCCAGTTGCCTTCTTG-3′ |
5′-AAGCCTCCGACTTGTGAAGTG-3′ |
| Mouse IL-31 |
5′-CCTACCCTGGTGCTGCTTTG-3′ |
5′-CTGACATCCCAGATGCCTGC-3′ |
| Mouse IL-33 |
5′-ACTGTGGTGCCTGCTCTTCT-3′ |
5′-TTGGCTTACGATGTTGTGGA-3′ |
| Mouse GAPDH |
5′-GGCTACACTGAGGACCAGGT-3′ |
5′-TCCACCACCCTGTTGCTGTA-3′ |
Immunofluorescence staining
Microglia (2×105 cells/ml) were cultured
on cell culture slides at 37°C for 24 h. These cells were then
treated with either DLE (50 and 100 µg/ml) or MC (10 and 20 µM) and
further incubated at 37°C for 1 h. Subsequently, cells were
stimulated with LPS (1 µg/ml) at 37°C for 30 min. Slides were fixed
with 4% formaldehyde at room temperature for 15 min. Slides were
then rinsed three times with PBS for 5 min each. Subsequently,
slides were blocked with a solution containing 5% FBS and 0.3%
Triton X-100 in PBS for 1 h. They were then incubated with a
primary antibody against p-NF-κB (1:500; cat no. sc-271908) or
p-STAT3 (1:1,000; cat no. 9145S), which were diluted in PBS
containing 1% bovine serum albumin (BSA) and 0.3% Triton X-100, at
4°C overnight. Afterward, slides were rinsed three times with PBS
for 5 min each and incubated with secondary antibodies anti-mouse
(1:2,000; cat no. A-11001) or anti-rabbit (1:1,000; cat no.
A-11008) purchased from Thermo Fisher Scientific, Inc., which were
diluted in antibody buffer at room temperature for 2 h. Finally,
slides were covered with a coverslip using a mounting solution
containing DAPI. Afterwards, the slide was incubated for 24 h at
room temperature in the dark. Immunofluorescence images were
observed at ×400 magnification using a Zeiss Axioskop 50 microscope
and images were captured with an AxioCam ICm1 camera (Carl Zeiss
AG).
High-performance liquid chromatography
(HPLC) analysis
An Agilent 1100 series (Agilent Technologies, Inc.)
was employed for the identification of primary active compounds in
DLE using an HPLC method. The setup included a binary pump delivery
system, a degasser (cat. no. G1379A), an autosampler (cat. no.
G1313A) and a diode array detector (cat. no. G1315B; all from
Agilent Technologies, Inc.). The separation was carried out using
an Agilent Eclipse XDB-C18 column (Agilent Technologies, Inc.;
4.6×250 mm; 5 mm particles) through gradient elution with 0.5%
aqueous formic acid (A) and acetonitrile (B). The gradient elution
profile was: 0 min, 5% B; 10 min, 10% B; 50 min, 40% B; 54 min,
100% B and then hold for 10 min before returning to initial
conditions. The gradient elution involved varying the proportion of
solvent A (water, containing 0.2% acetic acid) to solvent B
(acetonitrile). The mobile phase flow rate was set at 1 ml/min. UV
detection was performed at a wavelength of 280 nm. The column
temperature was maintained at 30°C and the sample injection volume
was 20 ml. Identification of the standard was based on retention
time. The concentration of the main isolated compound was
determined by comparing its peak area with that of a standard.
Standard stock solutions (1,000 ppm) were prepared using methanol
and a calibration curve was constructed using six different
concentrations (0, 20, 40, 60, 80, 100 ppm). The integration of
each component on the chromatogram was processed using Agilent
Chemstation software (Agilent Technologies, Inc.).
Statistical analysis
All statistical analyses were performed using SPSS
version 26.0 (IBM Corp.). Data are presented as mean ± standard
deviation (n=3). For statistical analysis, one-way ANOVA followed
by Tukey's post hoc test was performed for comparisons involving
three or more groups, while independent samples t-tests were used
for comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Quantitative analysis of MC content in
DLE using HPLC
HPLC analysis was conducted to measure the content
of MC, known to be the main active compound of DLE. As a result, MC
(retention time: 16.013 min) was detected. The concentration of MC,
the main peak, was determined by establishing a strong linear
regression between the peak area and the concentration of
myricitrin standard. Based on this analysis, the concentration of
myricitrin was found to be 86.68±3.36 µg/mg (Fig. 1).
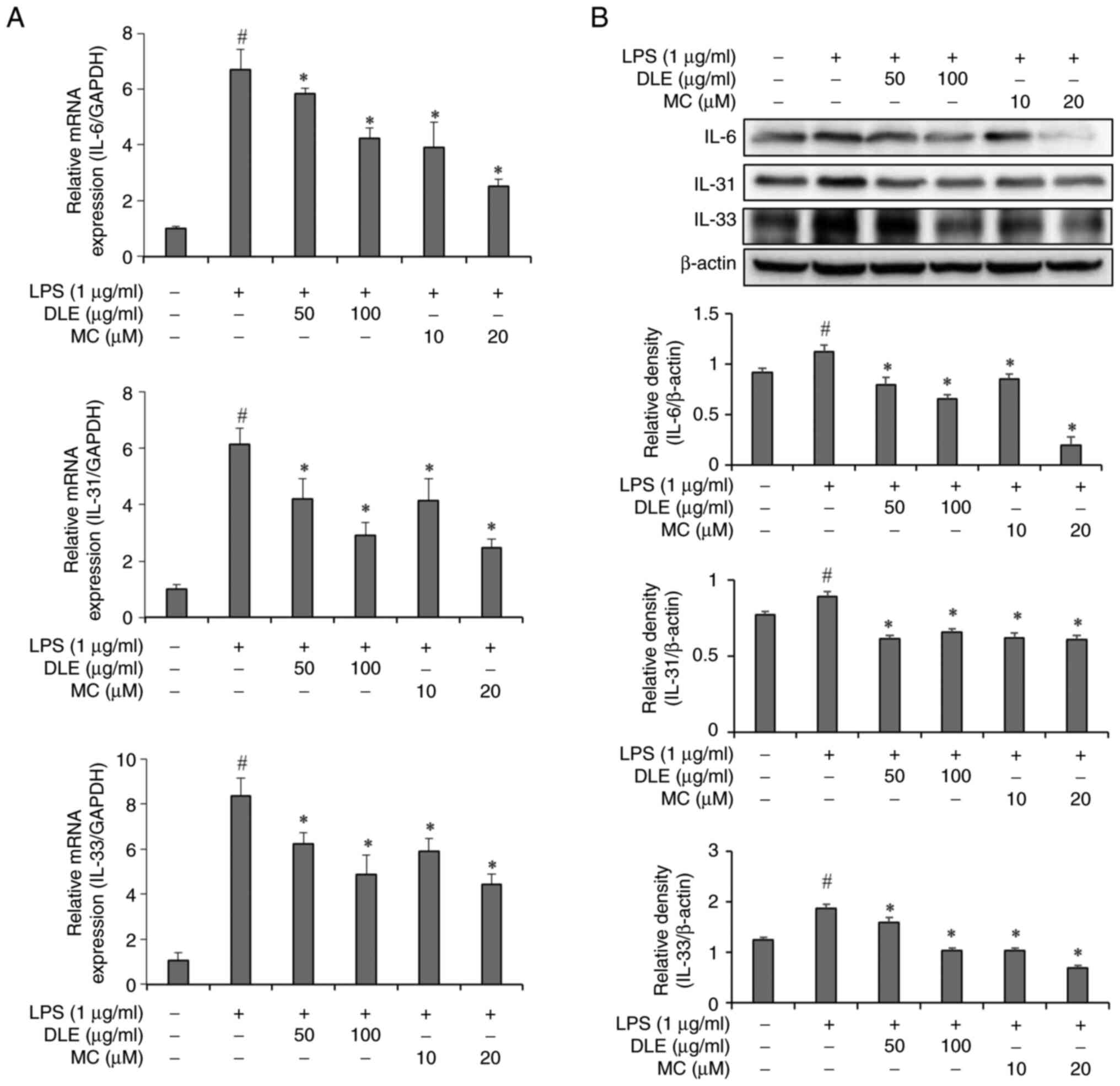
Effects of DLE and MC on cytokine
expression in LPS-stimulated microglia
To investigate effects of DLE on cytokine production
related to itch in activated microglia, PCR and western blot
analysis was conducted. Results showed that mRNA levels of IL-6,
IL-31 and IL-33 were significantly increased in the in microglia
after 3 h of LPS treatment. However, pre-treatment with DLE or MC
before LPS stimulation led to significant reductions of IL-6, IL-31
and IL-33 mRNA expression levels (Fig.
2A). Following 24 h of LPS stimulation, intracellular protein
expression levels of IL-6, IL-31 and IL-33 were significantly
increased. Pre-treatment with DLE or MC before LPS exposure
resulted in significant decreases in the expression of these
proteins across all treatment concentrations (Fig. 2B).
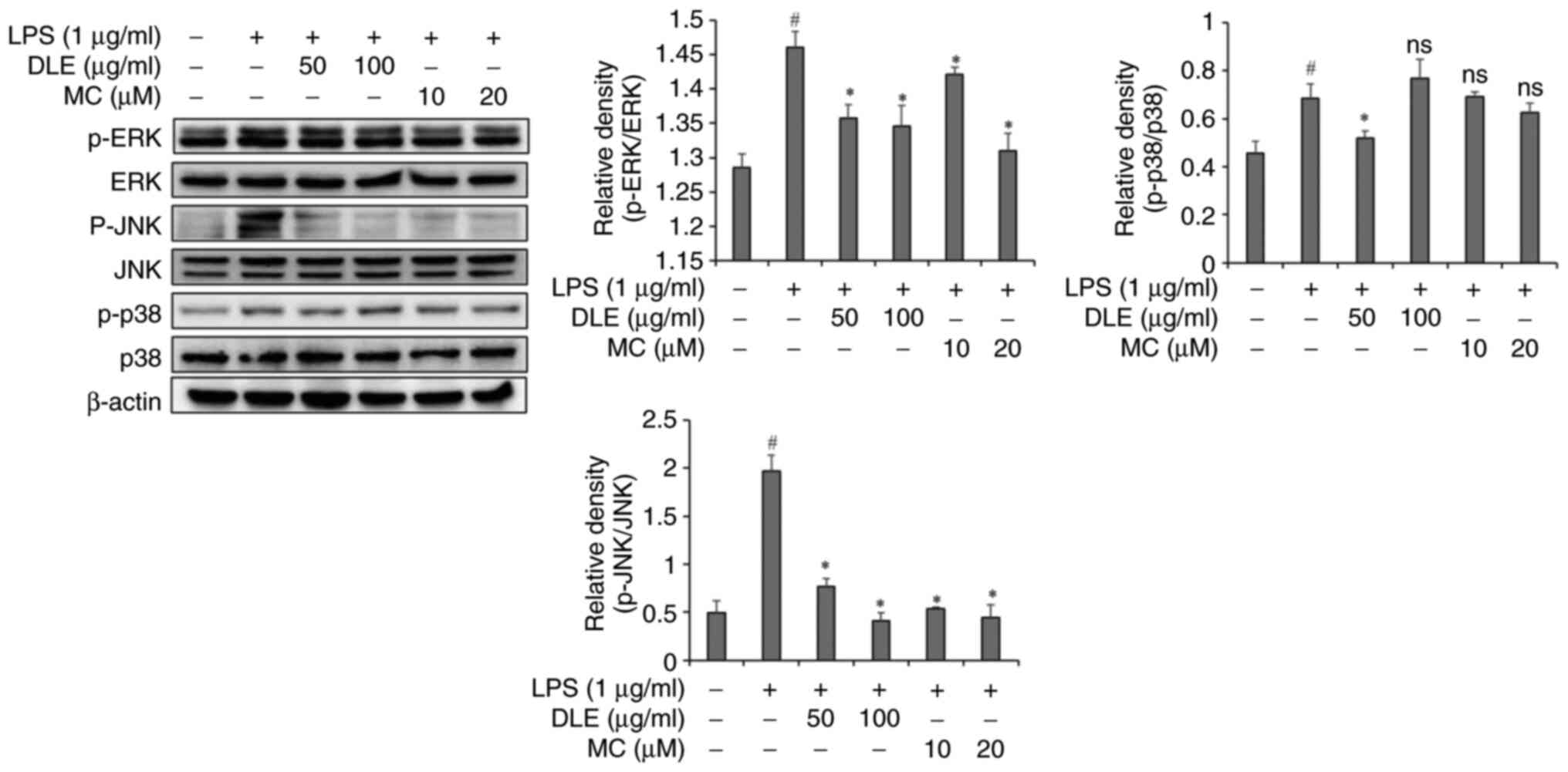
Effects of DLE and MC on MAPKs
activation in LPS-stimulated microglia
DLE and MC effectively suppressed cytokine
production in LPS-stimulated microglia. To delve into its mechanism
of action, an experiment was conducted into the influence of DLE
and MC on phosphorylation of MAPKs using western blot analysis. LPS
treatment significantly increased the phosphorylation of ERK, JNK
and p38 in microglia (Fig. 3).
However, pre-treatment with DLE or MC before LPS exposure led to
significant reductions of the phosphorylation of ERK and JNK.
Notably, both DLE and MC demonstrated effective inhibition of JNK
phosphorylation (Fig. 3). However,
phosphorylation of p38 did not significantly decrease except in the
group treated with DLE at 50 µg/ml.
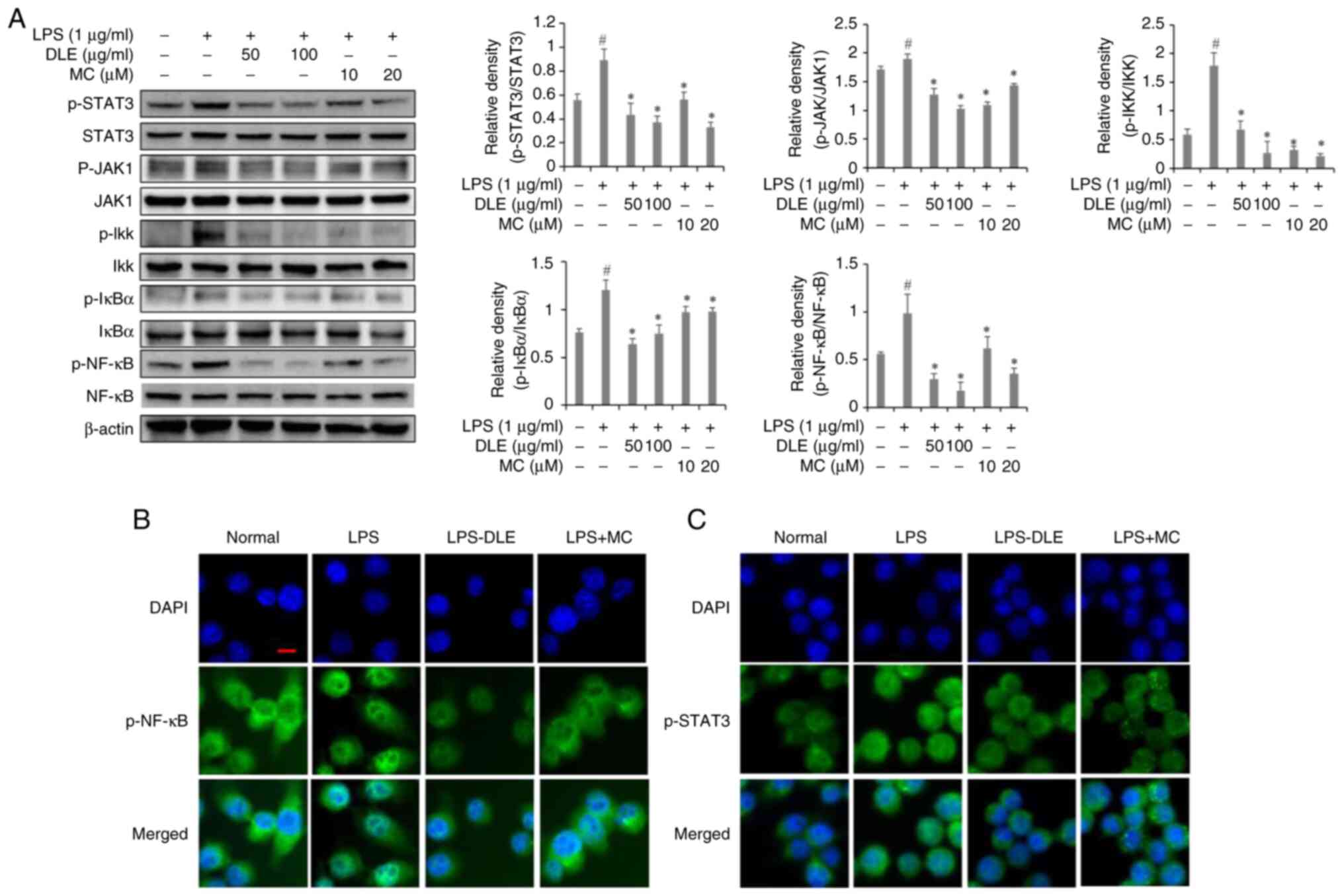
Effects of DLE and MC on STAT3 and
NF-κB Signaling pathways in LPS-stimulated microglia
Since DLE inhibited the phosphorylation of MAPKs in
LPS-stimulated microglia, the effect of DLE on cellular signaling
molecules associated with MAPKs was investigated. A 30 min LPS
treatment significantly increased the phosphorylation of STAT3,
AKT, IKK, IκB and NF-κB in microglia (Fig. 4A). However, pre-treatment with DLE
or MC effectively suppressed the phosphorylation of these factors.
To investigate the inhibition of NF-κB and STAT3 nuclear
translocation, immunofluorescence staining was performed (Fig. 4B and C). Results showed that
stimulation with LPS led to nuclear translocation of NF-κB and
STAT3. However, pretreatment with DLE and MC resulted in a
reduction of their nuclear translocation.
Effects of DLE and MC on itch-related
receptor expression in LSMCM-stimulated astrocytes
Levels of IL-6 and IL-31 in LSMCM were measured
using an ELISA kit. Results indicated that IL-6 level was ~345
pg/ml, while IL-31 level was measured at ~78 pg/ml (Fig. S1). Whether cytokines produced by
activated microglia activated astrocytes was investigated and the
effect of DLE and MC on this process in LSMCM-stimulated astrocytes
assessed. Expression levels of itch signaling receptors OSMR,
IL-31RA and IP3R1 were significantly increased in LSMCM-stimulated
astrocytes. However, pre-treatment with DLE and MC led to decreased
expression levels of these receptors (Fig. 5).
 | Figure 5.Effects of DLE and MC on expression
of OSMR, IL-31RA and IP3R1 in LSMCM-stimulated astrocytes.
Astrocytes were pretreated with DLE or MC at indicated
concentrations and stimulated with LSMCM for 24 h. Relative
expression levels of OSMR, IL-31RA and IP3R1 were evaluated by
western blot analysis. Each bar represents the mean ± SD (n=3).
#P<0.05 vs. untreated cells; *P<0.05 vs. LSMCM
alone treated cells; ns, no significant difference compared to
LSMCM alone treated cells. DLE, Diospyros lotus leaf
extract; MC, myricitrin; LSMCM, LPS-stimulated microglia culture
medium; LPS, lipopolysaccharide; OSMR, oncostatin M receptor;
IL-31RA, interleukin 31 receptor a; IP3R1, inositol
1,4,5-trisphosphate receptor 1. |
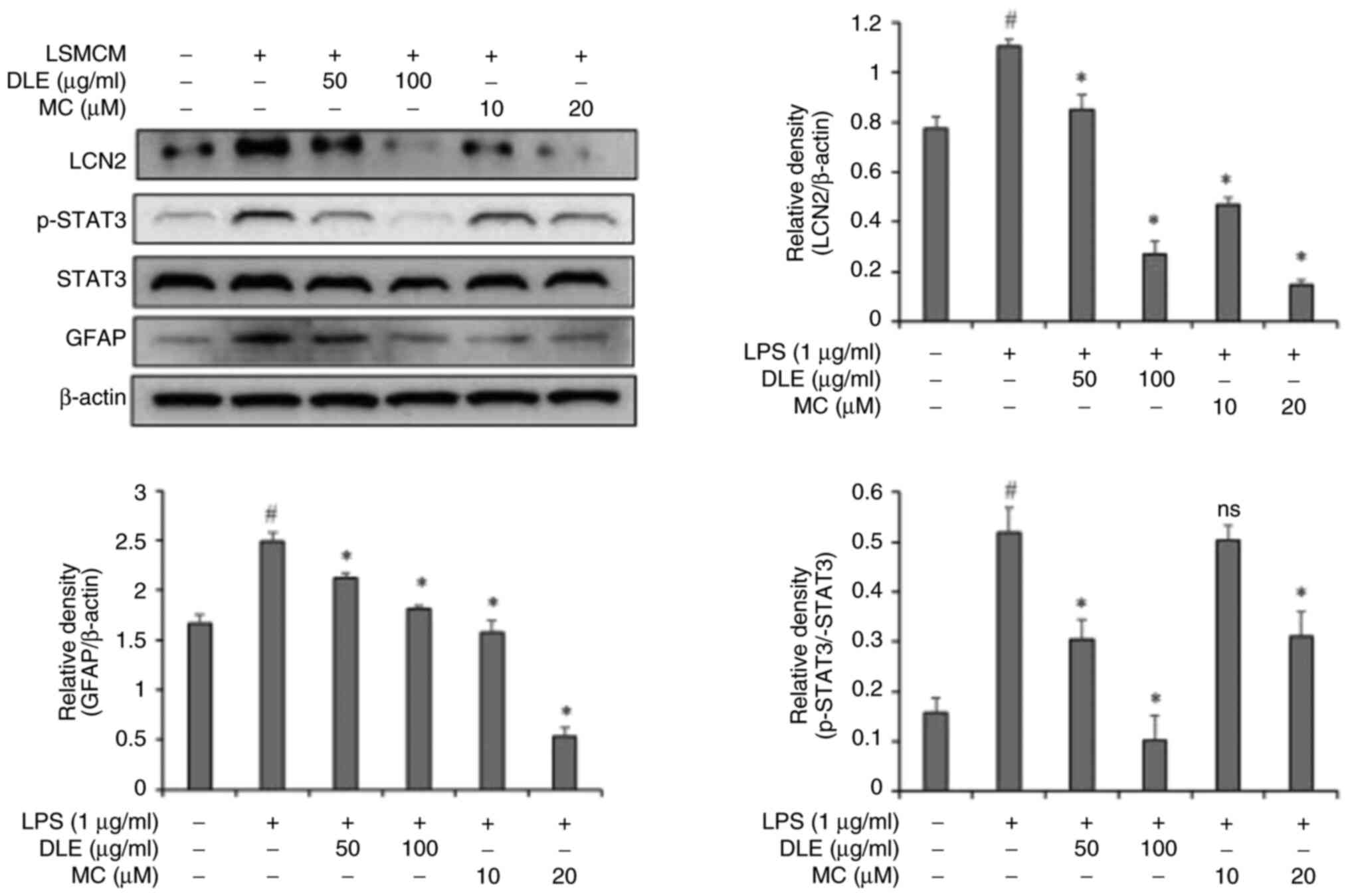
Effects of DLE and MC on LCN2, STAT3
and GFAP expression in LSMCM-stimulated astrocytes
To further investigate the effect of DLE and MC,
downstream signaling and associated proteins were assessed in
LSMCM-stimulated astrocytes. Expression levels of LCN2, STAT3 and
GFAP were significantly increased in LSMCM-stimulated astrocytes.
However, in groups pre-treated with DLE and MC, expression levels
of LCN2, STAT3 and GFAP were decreased by DLE and MC in a
concentration-dependent manner (Fig.
6).
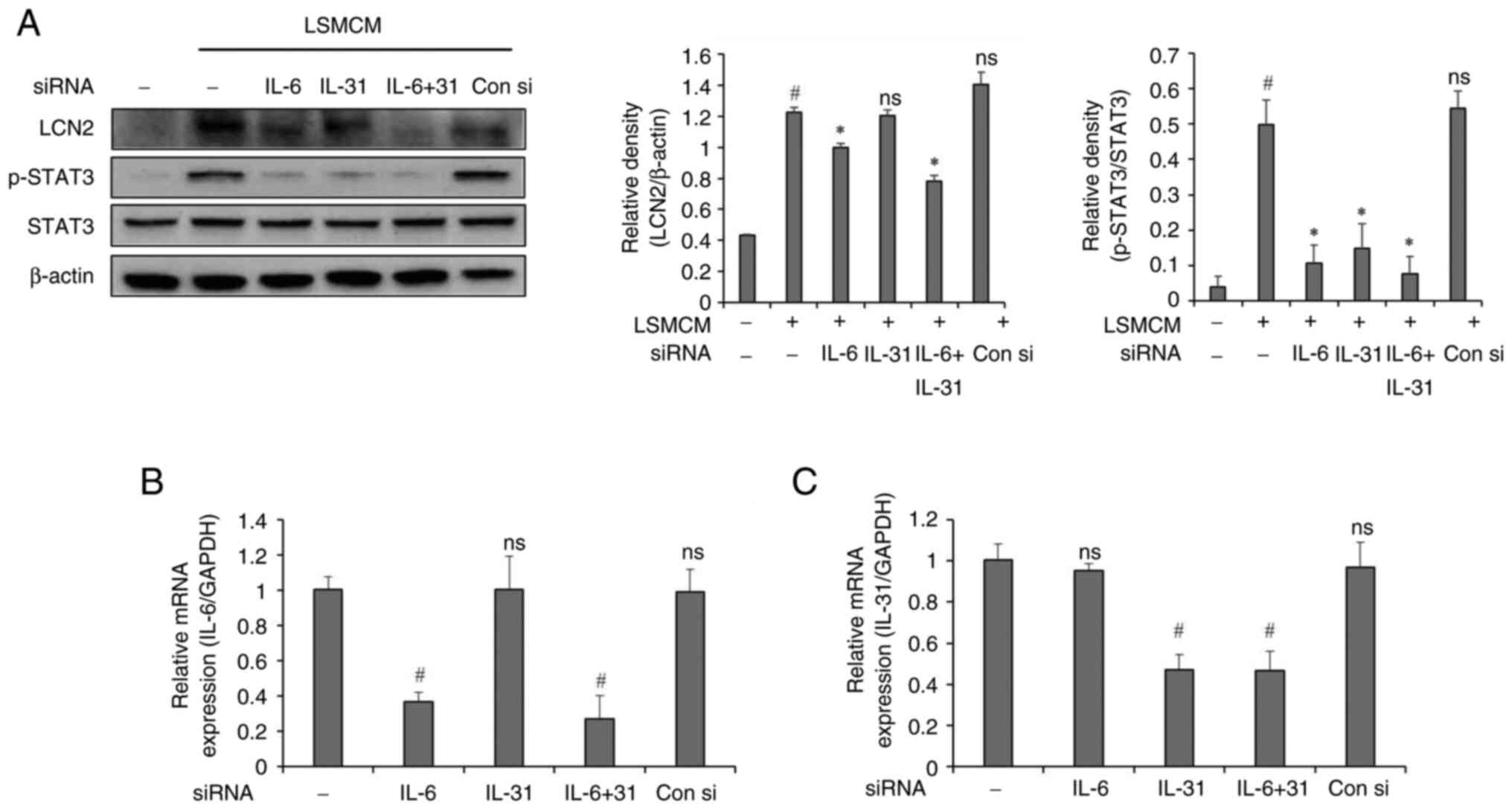
Effects of IL-6 and IL-31 gene
silencing in microglia on astrocyte activation
The present study conducted experiments using
conditioned media from microglia in which IL-6 and IL-31 genes were
silenced to investigate whether the activation of astrocytes by
LSMCM was mediated by cytokines IL-6 and IL-31 known to be
associated with itching. Astrocytes cultured in LSMCM exhibited
activation of LCN2 and STAT3. However, in groups with IL-6 or IL-6
plus IL-31 gene silenced, LCN2 expression was suppressed, while it
showed no significant decrease in the IL-31 alone inhibition group.
STAT3 activity was inhibited in all gene-silenced groups, although
it showed no significant changes in the control siRNA group
(Fig. 7).
Discussion
LPS is a well-established macrophage stimulant. It
has been widely used to study molecular mechanisms underlying
microglial activation (25,26).
The current study found that LPS induced the activation of
microglia, leading to the generation of cytokines IL-6, IL-31 and
IL-33. This observation agreed with our earlier research, where we
initially demonstrated that LPS stimulation led to the production
of IL-31 and IL-33 by microglia (27). In chronic itching, an increase in
IL-6 has been observed, although its precise function remains to be
elucidated (28). However, it has
been reported that IL-6 causes itching by acting on dorsal root
ganglion (DRG) in uremic itching (29) and that upregulation of IL-6 can
improve the pruritic response of SDH astrocytes in a chronic
itching mouse model (30). IL-31,
a significant contributor to itching in conditions such as atopic
dermatitis, acts as a crucial neuroimmune link between T helper 2
cells (Th2) and sensory neurons (31). IL-33, on the other hand, has been
associated with the activation of Th2 cells, mast cells and
eosinophils, leading to the production of various Th2 cytokines and
mediating allergic immune responses (32). Based on these findings, it can be
inferred that DLE and MC may alleviate pruritic conditions by
inhibiting the production of IL-6, IL-31 and IL-33.
To elucidate the mechanisms underlying inhibitory
effects of DLE and MC on cytokine production in microglia, the
present study investigated key signaling pathways associated with
cytokine production. The NF-κB pathway is well-known for
upregulating the expression of inflammatory cytokines. It is
traditionally considered a classical inflammatory signaling pathway
(33). Following LPS stimulation,
IκB, an NF-κB inhibitor, is phosphorylated and degraded by IKK,
leading to nuclear translocation of NF-κB and subsequent
transcription of target genes (34). In the present study, DLE and MC
effectively inhibited the phosphorylation of IKK and IκB,
ultimately suppressing nuclear translocation of NF-κB.
Additionally, the MAPKs pathway encompassing JNK, ERK and p38
serves a crucial role in regulating the production of cytokines and
chemokines in LPS-stimulated microglia (35,36).
Activation of MAPKs can stimulate kinase proteins, leading to
nuclear translocation of NF-κB (37). DLE and MC were able to
significantly inhibit the phosphorylation of ERK and JNK. However,
suppression of p38 phosphorylation was not observed. These results
suggested that inhibitory effects of DLE and MC on LPS-induced
NF-κB nuclear translocation are attributed to suppression of the
MAPK signaling pathway excluding p38. Cytokine production signaling
is also mediated through the JAK/STAT pathway (38). LPS can bind to TLR4 on the cell
membrane, leading to phosphorylation of JAK1, which subsequently
phosphorylates STAT3, ultimately inducing transcriptional responses
and increasing the expression of relevant inflammatory mediators
(39,40). To further verify the effect of DLE
and MC on the inhibition of NF-κB and STAT3 phosphorylation,
immunofluorescence staining was performed. Consistent with the
western blotting results, translocation of NF-κB and STAT3 to the
nucleus was observed upon LPS stimulation, as evidenced by overlap
of green fluorescence and nuclear staining. These results are
consistent with the results of other previous studies (41,42).
By contrast, treatment with DLE or MC significantly reduced the
fluorescence intensity in the nuclear location, indicating that
translocation of NF-κB and STAT3 was inhibited. This suggests that
DLE and MC may reduce the production of inflammatory mediators by
inhibiting the activation of NF-κB and STAT3 in microglia.
DLE and MC were found to suppress the expression of
OSMR, IL-31RA, IP3R1, phosphorylated STAT3 and LCN2 in
LSMCM-stimulated astrocytes (Fig.
5). IL-31 mediates itch-related signaling through the
heterodimeric receptor composed of OSMR and IL-31RA (43). Considering the reported involvement
of IL31RA/OSMR through SDH in sensitizing CNS-related itching and
spinal neurons (44), DLE and MC
might be able to alleviate CNS-related itching. Additionally, DLE
and MC suppressed the phosphorylation of STAT3 and the expression
of LCN2 in LSMCM-stimulated astrocytes. In chronic itching, SDH
astrocytes exhibit sustained STAT3 activation due to IL-6, leading
to upregulation of LCN2, which can exacerbate itch (13). GFAP, a target gene of STAT3, is
commonly used as a marker for the activation status of astrocytes
(45). Reports of increased GFAP
expression in the SDH of itch-inducing mice suggest that GFAP
activation serves as an indicator of itch (13,46,47).
DLE and MC were found to inhibit the expression of GFAP in
LSMCM-stimulated astrocytes. These inhibitory effects are
considered to result from the suppression of STAT3. Considering
these results, it is hypothesized that DLE and MC can suppress the
activity of astrocytes by microglia in relation to itching.
Additionally, the present study demonstrated the
role of microglial IL-6 and IL-31 in the activation of STAT3 and
LCN2 in astrocytes. Treatment with IL-6-silenced LSMCM
significantly reduced the activation of LCN2 and STAT3 in
astrocytes. This agreed with previous research reporting the
crucial role of IL-6 in the activation of STAT3 and LCN2 in
astrocytes (13). Although
silencing the IL-31 gene did not significantly inhibit the
expression of LCN2, it did suppress the activation of STAT3.
Considering that IL-31 activates STAT3 and subsequently increases
GRPR activity in DRG neurons (48), it is plausible to assume that IL-31
also enhances the activity of molecules associated with itch in
astrocytes. Translating these findings into in vivo models
will be crucial to validating the relevance of the in vitro
results of the present study in a more physiological setting.
Future studies should aim to explore effects of IL-6 and IL-31 gene
silencing in animal models, allowing for a more comprehensive
understanding and implications of itching-related neuroinflammatory
responses.
In our previous research, various flavonoid
compounds within DLE, including the most abundant MC, were
identified (18,19). MC has been reported to alleviate
carbon tetrachloride-induced toxicity in rats (49), inhibit periodontitis (50) and alleviate acute lung injury in
rats by blocking the JAK/STAT pathway (51). Based on these preceding studies, it
is hypothesized that the demonstrated itchy cytokine inhibition
effect and mechanism of action of DLE in the present study are
attributable to its high content of MC. However, considering that
DLE is a complex mixture of various flavonoids as plant extracts,
it is worth investigating whether MC interacts with other compounds
within DLE to exert anti-inflammatory effects, taking into account
the possibility of synergistic effects among these compounds.
Through such investigation, it is expected that effects of DLE can
be expanded beyond MC alone.
In conclusion, the present study demonstrated that
DLE and MC can exert significant inhibitory effects on the
production of key pruritic cytokines, IL-6 and IL-31, in
LPS-stimulated microglia. This suggested a potential therapeutic
role of DLE and MC in alleviating pruritic conditions.
Mechanistically, the findings of the present study revealed that
DLE and MC could modulate critical signaling pathways involved in
cytokine production, such as the NF-κB, MAPK and JAK/STAT pathways.
The present study also extended to the role of DLE and MC in
astrocyte activation, a crucial component in CNS-related itching.
DLE and MC were found to suppress the expression of receptors and
signaling molecules associated with itching, such as OSMR, IL-31RA,
phosphorylated STAT3 and LCN2, in LSMCM-stimulated astrocytes. This
inhibitory effect suggested that DLE and MC might alleviate
CNS-related itching by modulating astrocyte activity (Fig. 8). Moreover, the present study
elucidated the involvement of microglial IL-6 and IL-31 in the
activation of STAT3 and LCN2 in astrocytes, providing insights into
intricate neuroinflammatory responses underlying itching. In
relation to this, subsequent research is required to apply it to
in vivo models and validate the relevance of these in
vitro results.
 | Figure 8.Schematic diagram of the mechanism
involved in the anti-itch effect of DLE and MC on astrocytes and
microglia. DLE, Diospyros lotus leaf extract; MC,
myricitrin; gp130, glycoprotein 130; TLR4, toll-like receptor 4;
JAK1, Janus kinase; p38, p38 mitogen-activated protein kinases;
LSMCM, LPS-stimulated microglia-conditioned media; IP3R1, inositol
1,4,5-trisphosphate receptor 1, TRPC, transient receptor potential
canonical; IL-31RA, interleukin 31 receptor a; OSMR, oncostatin M
receptor; LCN2, lipocalin-2; GFAP, glial fibrillary acidic
protein. |
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant (grant no.
NRF-2022R1F1A1064419) of the National Research Foundation funded by
the Korean government.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JYS contributed to the conceptualization,
methodology, validation, writing the original draft and
visualization. BOC contributed to the conceptualization and
validation. JHP, ESK, JHK and HYH contributed to performing the
experiments and analyzing the data. YSK contributed to the
conceptualization, validation and writing the review and editing.
SIJ contributed to the conceptualization, validation, review and
editing, supervision, project administration and funding
acquisition. JYS and SIJ confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bourane S, Duan B, Koch SC, Dalet A, Britz
O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q and Goulding M:
Gate control of mechanical itch by a subpopulation of spinal cord
interneurons. Science. 350:550–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel T and Yosipovitch G: Therapy of
pruritus. Expert Opin Pharmacother. 11:1673–1682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lay M and Dong X: Neural mechanisms of
itch. Annu Rev Neurosci. 43:187–205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun YG and Chen ZF: A gastrin-releasing
peptide receptor mediates the itch sensation in the spinal cord.
Nature. 448:700–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colonna M and Butovsky O: Microglia
function in the central nervous system during health and
neurodegeneration. Annu Rev Immunol. 35:441–468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muzio L, Viotti A and Martino G: Microglia
in neuroinflammation and neurodegeneration: From understanding to
therapy. Front Neurosci. 15:7420652021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tjalkens RB, Popichak KA and Kirkley KA:
Inflammatory activation of microglia and astrocytes in manganese
neurotoxicity. Adv Neurobiol. 18:159–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Y, Mou B, Zhang QR, Zhao HX, Zhang
JY, Yun X, Xiong MT, Liu Y, Liu YU, Pan H, et al: Microglia are
involved in regulating histamine-dependent and non-dependent itch
transmissions with distinguished signal pathways. Glia.
71:2541–2558. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Wang Y, Zeng Y, Wang D, Wen Y, Fan
L, He Y, Zhang J, Sun W, Liu Y and Tao A: Microglia-neuron
interactions promote chronic itch via the NLRP3-IL-1β-GRPR axis.
Allergy. 78:1570–1584. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herculano-Houzel S: The glia/neuron ratio:
How it varies uniformly across brain structures and species and
what that means for brain physiology and evolution. Glia.
62:1377–1391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grace PM, Hutchinson MR, Maier SF and
Watkins LR: Pathological pain and the neuroimmune interface. Nat
Rev Immunol. 14:217–231. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Svensson CI and Brodin E: Spinal
astrocytes in pain processing: Non-neuronal cells as therapeutic
targets. Mol Interv. 10:25–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiratori-Hayashi M, Yamaguchi C, Eguchi
K, Shiraishi Y, Kohno K, Mikoshiba K, Inoue K, Nishida M and Tsuda
M: Astrocytic STAT3 activation and chronic itch require
IP3R1/TRPC-dependent Ca2+ signals in mice. J Allergy
Clin Immunol. 147:1341–1353. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uddin G, Rauf A, Siddiqui BS, Muhammad N,
Khan A and Shah SUA: Anti-nociceptive, anti-inflammatory and
sedative activities of the extracts and chemical constituents of
Diospyros lotus L. Phytomedicine. 21:954–959. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uddin G, Rauf A, Siddiqui B, Arfan M,
Rahman I and Khan I: Proximate chemical composition and
antimicrobial activities of fixed oils from Diospyros lotus
L. Med Chem. 3:282–285. 2013.
|
|
16
|
Yin H, Yan HH, Qin CQ, Li HR, Li X and Ren
DF: Protective effect of fermented Diospyros lotus L.
extracts against the high glucose-induced apoptosis of MIN6 cells.
J Food Biochem. 45:e136852021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loizzo MR, Said A, Tundis R, Hawas UW,
Rashed K and Menichini F, Frega NG and Menichini F: Antioxidant and
antiproliferative activity of Diospyros lotus L. extract and
isolated compounds. Plant Food Hum Nutr. 64:264–270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho BO, Che DN, Shin JY, Kang HJ, Kim JH,
Kim HY, Cho WG and Jang SI: Ameliorative effects of Diospyros
lotus leaf extract against UVB-induced skin damage in BALB/c
mice. Biomed Pharmacother. 95:264–274. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho BO, Yin HH, Fang CZ, Kim SJ, Jeong SI
and Jang SI: Hepatoprotective effect of Diospyros lotus leaf
extract against acetaminophen-induced acute liver injury in mice.
Food Sci Biotechnol. 24:2205–2212. 2015. View Article : Google Scholar
|
|
20
|
Che DN, Kang HJ, Cho BO, Shin JY and Jang
SI: Combined effects of Diospyros lotus leaf and grape stalk
extract in high-fat-diet-induced obesity in mice. Food Sci
Biotechnol. 28:1207–1215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho BO, Che DN, Yin HH, Shin JY and Jang
SI: Diospyros lotus leaf and grapefruit stem extract
synergistically ameliorate atopic dermatitis-like skin lesion in
mice by suppressing infiltration of mast cells in skin lesions.
Biomed Pharmacother. 89:819–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho BO, Shin JY, Kim JS, Kim JS, Che DN,
Kang HJ, Kang HJ, Oh H, Kim YS and Jang SI: Enzyme-treated date
plum leave extract ameliorates atopic dermatitis-like skin lesion
in hairless mice. Asian Pac J Trop Biomed. 10:239–247. 2020.
View Article : Google Scholar
|
|
23
|
Shin JY, Cho BO, Park JH, Kang ES, Kim YS
and Jang SI: Diospyros lotus leaf extract and its main
component myricitrin regulate pruritus through the inhibition of
astrocyte activation. Exp Ther Med. 26:3232023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng LT, Ryu GM, Kwon BM, Lee WH and Suk
K: Anti-inflammatory effects of catechols in
lipopolysaccharide-stimulated microglia cells: Inhibition of
microglial neurotoxicity. Eur J Pharmacol. 588:106–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rivest S: Regulation of innate immune
responses in the brain. Nat Rev Immunol. 9:429–439. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Che DN, Cho BO, Kim JS, Shin JY, Kang HJ
and Jang SI: Effect of luteolin and apigenin on the production of
IL-31 and IL-33 in lipopolysaccharides-activated microglia cells
and their mechanism of action. Nutrients. 12:8112020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Storan ER, O'Gorman SM, McDonald ID and
Steinhoff M: Role of cytokines and chemokines in itch. Handb Exp
Pharmacol. 226:163–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keshari S, Sipayung AD, Hsieh CC, Su LJ,
Chiang YR, Chang HC, Yang WC, Chuang TH, Chen CL and Huang CM:
Il-6/P-Btk/P-Erk signaling mediates calcium phosphate-induced
pruritus. FASEB J. 33:12036–12046. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shiratori-Hayashi M and Tsuda M: Spinal
glial cells in itch modulation. Pharmacol Res Perspect.
9:e007542021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cevikbas F, Wang X, Akiyama T, Kempkes C,
Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, et
al: A sensory neuron-expressed IL-31 receptor mediates T helper
cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin
Immunol. 133:448–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yasuoka S, Kawanokuchi J, Parajuli B, Jin
S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T and Suzumura A:
Production and functions of IL-33 in the central nervous system.
Brain Res. 1385:8–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lawrence T: The nuclear factor NF-κB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-κB and IκB proteins: Implications
in cancer and inflammation. Trends Biochem Sci. 30:43–52. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park HY, Han MH, Park C, Jin CY, Kim GY,
Choi IW, Kim ND, Nam TJ, Kwon TK and Choi YH: Anti-inflammatory
effects of fucoidan through inhibition of NF-κB, MAPK and Akt
activation in lipopolysaccharide-induced BV2 microglia cells. Food
Chem Toxicol. 49:1745–1752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vermeulen L, De Wilde G, Van Damme P,
Berghe WV and Haegeman G: Transcriptional activation of the NF-κB
p65 subunit by mitogen-and stress-activated protein kinase-1
(MSK1). EMBO J. 22:1313–1324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marrero MB, Venema VJ, He H, Caldwell RB
and Venema RC: Inhibition by the JAK/STAT pathway of IFNγ-and
LPS-stimulated nitric oxide synthase induction in vascular smooth
muscle cells. Biochem Biophys Res Commun. 252:508–512. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samavati L, Rastogi R, Du W, Hüttemann M,
Fite A and Franchi L: STAT3 tyrosine phosphorylation is critical
for interleukin 1 beta and interleukin-6 production in response to
lipopolysaccharide and live bacteria. Mol Immunol. 46:1867–1877.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morris R, Kershaw NJ and Babon JJ: The
molecular details of cytokine signaling via the JAK/STAT pathway.
Protein Sci. 27:1984–2009. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ren Y, Yue B, Ren G, Yu Z, Luo X, Sun A,
Zhang J, Han M, Wang Z and Dou W: Activation of PXR by
alantolactone ameliorates DSS-induced experimental colitis via
suppressing NF-κB signaling pathway. Sci Rep. 9:166362019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pan S, Liu R, Wu X, Ma K, Luo W, Nie K,
Zhang C, Meng X, Tong T, Chen X, et al: LncRNA NEAT1 mediates
intestinal inflammation by regulating TNFRSF1B. Ann Transl Med.
9:7732021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Q, Putheti P, Zhou Q, Liu Q and Gao
W: Structures and biological functions of IL-31 and IL-31
receptors. Cytokine Growth Factor Rev. 19:347–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Datsi A, Steinhoff M, Ahmad F, Alam M and
Buddenkotte J: Interleukin-31: The ‘itchy’ cytokine in inflammation
and therapy. Allergy. 76:2982–2997. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ceyzériat K, Abjean L, Carrillo-de Sauvage
MA, Haim LB and Escartin C: The complex STATes of astrocyte
reactivity: How are they controlled by the JAK–STAT3 pathway?
Neurosci. 330:205–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Green D and Dong X: Supporting itch: A new
role for astrocytes in chronic itch. Nat Med. 21:841–842. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shiratori-Hayashi M, Koga K, Tozaki-Saitoh
H, Kohro Y, Toyonaga H, Yamaguchi C, Hasegawa A, Nakahara T,
Hachisuka J, Akira S, et al: STAT3-dependent reactive astrogliosis
in the spinal dorsal horn underlies chronic itch. Nat Med.
21:927–931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nemmer JM, Kuchner M, Datsi A, Oláh P,
Julia V, Raap U and Homey B: Interleukin-31 signaling bridges the
gap between immune cells, the nervous system and epithelial
tissues. Front Med (Lausanne). 8:6390972021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Domitrović R, Rashed K, Cvijanović O,
Vladimir-Knežević S, Škoda M and Višnić A: Myricitrin exhibits
antioxidant, anti-inflammatory and antifibrotic activity in carbon
tetrachloride-intoxicated mice. Chem Biol Interact. 230:21–29.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shimosaki S, Tsurunaga Y, Itamura H and
Nakamura M: Anti-allergic effect of the flavonoid myricitrin from
Myrica rubra leaf extracts in vitro and in vivo. Nat Prod Res.
25:374–380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qi S, Feng Z, Li Q, Qi Z and Zhang Y:
Myricitrin modulates NADPH oxidase-dependent ROS production to
inhibit endotoxin-mediated inflammation by blocking the JAK/STAT1
and NOX2/p47 phox pathways. Oxid Med Cell Longev. 2017:97387452017.
View Article : Google Scholar : PubMed/NCBI
|