Introduction
Human embryo implantation is a highly regulated
process. It begins with the implantation of trophoblast cells from
a competent blastocyst onto the maternal endometrium. This process
is pivotal for a successful pregnancy (1). This adjustment occurs during the
‘window of implantation (WOI)’, a short period of uterine
receptivity corresponding to the mid-secretory phase of the
menstrual cycle. Successful embryo implantation is highly dependent
on the endometrial cycle, ovarian steroid hormones and endometrial
receptive adhesion molecules. Receptive adhesion molecules, such as
integrins, cadherins and selectins, are differentially expressed in
endometrial epithelial cells throughout the menstrual cycle and
play important roles during implantation (1).
Adiponectin, an important adipokine secreted from
the adipose tissues, exerts diverse physiological effects and
influences carbohydrate metabolism, lipid metabolism, insulin
sensitization, anti-inflammatory responses and cardiovascular
health (2). In the human
endometrium, adiponectin functions as a hormonal regulator of
energy homeostasis and has anti-inflammatory effects associated
with events such as implantation and endometriosis (3,4).
Mice lacking adiponectin show impaired fertility potential and
ovarian folliculogenesis (5).
Adiponectin exerts its effects through the activation of two
receptors; adiponectin receptor 1 (ADIPOR1), which is highly
expressed in the skeletal muscle and acts via AMP-activated protein
kinase (AMPK) and adiponectin receptor 2 (ADIPOR2), which is mostly
expressed in the liver and acts through the proliferator-activated
receptor α (PPAR α) pathway to regulate glucose and lipid
metabolism (6,7). These two adiponectin receptors are
expressed in the epithelial and stromal cells of the endometrium,
markedly increasing in the mid-secretory phase and aligning with
the period of receptivity of the endometrium to the embryo.
Additionally, adiponectin receptors are downregulated in the
endometria of women with recurrent implantation failure compared
with those in fertile women (8).
AMPK comprises three subunits; the catalytic α (α1 and α2) subunit
and the regulatory β (β1 and β2) and γ (γ1, γ2 and γ3) subunits,
forming a heterotrimeric complex. The AMPK α subunit is activated
via phosphorylation by liver kinase B1 (LKB1) or
calmodulin-dependent protein kinase. Activated AMPK regulates the
cellular metabolism (9,10). A previous study suggested that AMPK
is a mediator of endometrial receptivity due to elevated estrogen
expression and delayed embryo implantation in mice lacking AMPK
(11). The relationship between
AMPK and energy metabolism is critical in uterine receptivity
(12). AMPK activity is closely
associated with placental development (13,14).
E-cadherin, a type of cadherin, is crucial for cell-cell contact
via adherens junctions in epithelial cells. Its fundamental
molecular function is implicated in the epithelial-mesenchymal
transition. E-cadherin serves as a biomarker of endometrial
receptivity and is closely linked to implantation, facilitating the
initial communication between mothers and fetuses. It actively
contributes to early adhesion during human embryo implantation
(15). Adhesion molecules, pivotal
proteins in embryo implantation, also play a crucial role in cancer
progression. In endometrial cancer, the L1 cell adhesion molecule
is particularly significant for predicting prognosis and recurrence
due to its association with invasiveness and metastatic potential
(16,17). However, advancements in studies on
endometrial receptivity to improve pregnancy success are impeded by
the lack of knowledge of the regulation of various signaling
pathways.
The present study explored the effects of ADIPOR1 on
the regulation of endometrial receptivity in human endometrial
tissues and cell lines (RL95-2 and AN3CA) with different
receptivity. The results showed that ADIPOR1 regulated AMPK
activity and E-cadherin expression, which are closely related to
endometrial receptivity. ADIPOR1 downregulation attenuated JAr
spheroid attachment in vitro.
Materials and methods
Reagents
Dorsomorphin dihydrochloride, an AMPK inhibitor and
5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an AMPK
activator, were obtained from Tocris Bioscience and puromycin
dihydrochloride was purchased from InvivoGen, Inc.
Collection of human endometrial
tissues
Human endometrial tissues were obtained from fertile
participants in the proliferative [9-11 menstrual cycle days (mcd);
n=11; mean age, 36.7±2.8 years] and secretory (20–24 mcd; n=9; mean
age, 37.8±2.6 years) phases between April and June 2019 at the
Konyang University Hospital. Samples from patients with infertility
were collected from the secretory phase (20–22 mcd; n=7; mean age,
38.9±3.5 years) between April 2018 and April 2020 at the MizMedi
Hospital (Seoul, South Korea). Patients with infertility did not
receive any medication, including hormone treatments, during the
sampling period. Endometrial sampling was performed using a
disposable uterine sampler (Rampipella; Ri.mos. (S.r.l.). The
menstrual stages of the samples were determined using the Noyes
criteria by an experienced gynecological pathologist (18). The present study was approved by
the Bioethics Committee of Konyang University Hospital
[Institutional Review Board (IRB) file no. KYUH 2018-11-007] and
MizMedi Hospital (IRB file no. MMIRB 2018-3). Signed informed
consent was obtained from each patient.
Cell culture
Human endometrial cell lines (RL95-2 and AN3CA) were
acquired from the American Type Culture Collection. RL95-2 and
AN3CA cells were cultured in DMEM/F-12 and MEM (HyClone; Cytiva)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (HyClone; Cytiva).
Mycoplasma contamination tests were performed with MycoStrip
(InvivoGen, Inc.).
Human choriocarcinoma JAR cells were obtained from
the South Korean Cell Line Bank and cultured in RPMI-1640 medium
(HyClone; Cytiva) supplemented with 10% heat-inactivated FBS and 1%
penicillin/streptomycin. All cells were maintained at 37°C in a
humidified atmosphere containing 5% CO2. RL95-2 cells
were treated with dorsomorphin dihydrochloride for 24 h. AN3CA
cells were treated with AICAR (AMPK activators; Tocris Bioscience)
for 24 h.
Transfection and transduction
For the generation of lentivirus particles, 293T
cells were transfected with 400 ng of either a control (pLKO1;
SHC016) or a short hairpin (sh)RNA targeting adiponectin receptor 1
(shADIPOR1; sense: 5′-CGTCTATTGTCATTCAGAGAA-3′, antisense:
5′-TTCTCTGAATGACAATAGACG-3′) expression vector (MilliporeSigma)
alongside 400 ng pLP1, 100 ng pLP2 and 300 ng pLP/VSVG vectors (all
from Thermo Fisher Scientific, Inc.) based on a 3rd generation
system using Lipofectamine® 3,000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
recommended protocol. Lentiviral particles targeting ADIPOR1
and control lentiviral particles were collected from media
following transfection for 48 h. Subsequently, RL95-2 cells were
transduced with 10 MOI control lentiviral particles or those
carrying shRNA against the ADIPOR1 mRNA. RL95-2 cells with
ADIPOR1 knockdown were isolated and selected using 0.5 µg/ml
puromycin dihydrochloride.
RNA isolation and reverse
transcription-quantitative (RT-q) PCR
Total RNA extraction from both 1×106
AN3CA or 2×106 RL95-2 cells and endometrial tissues was
performed using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's protocol.
Complementary DNA (cDNA) was synthesized from 2 µg RNA for cells
and 5 µg for tissues using Moloney Murine Leukemia Virus reverse
transcriptase (Promega Corporation) according to the manufacturer's
recommended protocol.
RT-qPCR was performed to analyze mRNA expression
using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc.) on a CFX
Connect Real-time PCR Detection System (Bio-Rad Laboratories, Inc.)
according to the manufacturer's recommended protocol. Specific gene
primers and their annealing temperatures are listed in Table I. The following amplification
conditions were used for select genes encoding ADIPOR1, ADIPOR2,
ITGB5, L-Selectin, E-Selectin, E-cadherin, AMPK and
β-actin: An initial denaturation step at 95°C for 3 min,
followed by 49 cycles of denaturation at 95°C for 30 sec, annealing
at 58°C for 15 sec and extension at 72°C for 15 sec. Gene
expression levels were normalized to those of β-actin, an internal
control. Relative expression was calculated using the
2−ΔΔCq method (19) and
fold change was evaluated compared with the control.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer
sequences | PCR product size,
bp | Annealing
temperature, °C |
|---|
| ADIPOR1 | Forward
5′ACGTTGGAGGGTCATCCCATA 3′ | 175 | 56 |
|
| Reverse
5′AAACAGCACGAAACCAAGCAG 3′ |
|
|
| ADIPOR2 | Forward
5′CTGGATGGTACACGAAGAGGT 3′ | 176 | 56 |
|
| Reverse
5′TGGGCTTGTAAGAGAGGGGAC 3′ |
|
|
| E-SEL | Forward
5′CCGAGCGAGGCTACATGAAT 3′ | 122 | 56 |
|
| Reverse
5′GCCACATTGGAGCCTTTTGG 3′ |
|
|
| L-SEL | Forward
5′ATTTCCTGGCACATCATG 3′ | 95 | 56 |
|
| Reverse
5′ATTGTCTCGGCAGAATCT 3′ |
|
|
| ITGB5 | Forward
5′ACCTGGAACAACGGTGGAGA 3′ | 217 | 60 |
|
| Reverse
5′AAAAGATGCCGTGTCCCCAA 3′ |
|
|
|
E-cadherin | Forward
5′GGCCTGAAGTGACTCGTAACG 3′ | 201 | 60 |
|
| Reverse
5′TCAGACTAGCAGCTTCGGAACC 3′ |
|
|
| AMPK | Forward
5′AGGAAGAATCCTGTGACAAGCAC 3′ | 145 | 56 |
|
| Reverse
5′CCGATCTCTGTGGAGTAGCAGT 3′ |
|
|
| β-Actin | Forward
5′CAAGAGATGGCCACGGCTGCT 3′ | 275 | 56 or 60 |
|
| Reverse
5′TCCTTCTGCATCCTGTCGGCA 3′ |
|
|
Immunoblot analysis
Cells were lysed using ice-cold
radioimmunoprecipitation assay (RIPA) buffer on ice (JuBiotech),
supplemented with protease and phosphatase inhibitors (Roche
Diagnostics GmbH), and protein concentration was quantified using
the bicinchoninic acid assay (Thermo Fisher Scientific, Inc.).
Subsequently, 50 µg (for p-AMPK, AMPK, E-cadherin, integrin β5 and
β-actin detection) or 70 µg (for ADIPOR1, ADIPOR2, L-selectin,
E-selectin, E-cadherin and β-actin detection) proteins were
separated by sodium dodecyl sulphate-polyacrylamide gel
electrophoresis on 8 or 10% gels and transferred onto
polyvinylidene difluoride membranes. The blots were blocked with 5%
skimmed milk (Difco; BD Biosciences) for 2 h at room temperature,
washed with 1X TBS containing Tween 20 buffer (Biosesang) and then
incubated with primary antibodies against ADIPOR1 (1:500; cat. no.
ab70362; Abcam), ADIPOR2 (1:500; cat. no. ab77612; Abcam),
L-selectin (1:1,000; cat. no. PAA086Hu01; Cloud-Clone Corp.),
E-selectin (1:1,000; cat. no. ab18981; Abcam), integrin β5
(1:1,000; cat. no. ab184312; Abcam), Phospho-AMPKα (Thr172)
(1:1,000; cat. no. 2531; Cell Signaling Technology, Inc.), AMPKα
(1:1,000; cat. no. 2532; Cell Signaling Technology, Inc.),
E-cadherin (1:1,000; cat. no. 3195; Cell Signaling Technology,
Inc.) and β-actin (1:3,000; cat. no. 4967; Cell Signaling
Technology, Inc.) were incubated overnight at 4°C. The following
day, blots were probed with horseradish peroxidase-conjugated
secondary antibodies (1:3,000; cat. no. AP132P; MilliporeSigma) for
2 h at room temperature and bands were visualized using an Enhanced
Chemiluminescence Kit (Thermo Fisher Scientific, Inc.).
Quantification of western blot images was performed using the
ImageJ software 1.50b (National Institutes of Health).
In vitro assay for JAr spheroid
implantation
JAr cell spheroids were prepared by seeding onto a
V-bottom microplate (Greiner Bio One Ltd.) and incubating in DMEM
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin (HyClone; Cytiva) for 24 h in a
humidified atmosphere with 5% CO2. Endometrial cells
were cultured in 24-well plates until they reached 90% confluency.
The harvested spheroids were co-cultured with either RL95-2 cell
monolayers (for 1 h) or AN3CA cell monolayers (for 2 h), These
cells were pre-treated with either dorsomorphin, AICAR,
ADIPOR1 shRNA vector, or respective controls. The attached
spheroids were quantified by inverting the microplate and
centrifuging at 10 × g for 10 min. The attached spheroids were then
counted under a microscope (Olympus Corporation). The percentage of
spheroid attachment was calculated by dividing the number of
attached spheroids after centrifugation by the total number of
spheroids. The implantation assay was conducted at least three
times for validation.
Statistical analysis
Data are presented as mean ± standard error of the
mean. The results were analysed using unpaired Student's t-test or
one-way ANOVA with Tukey's post hoc test with GraphPad Prism 5
(Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
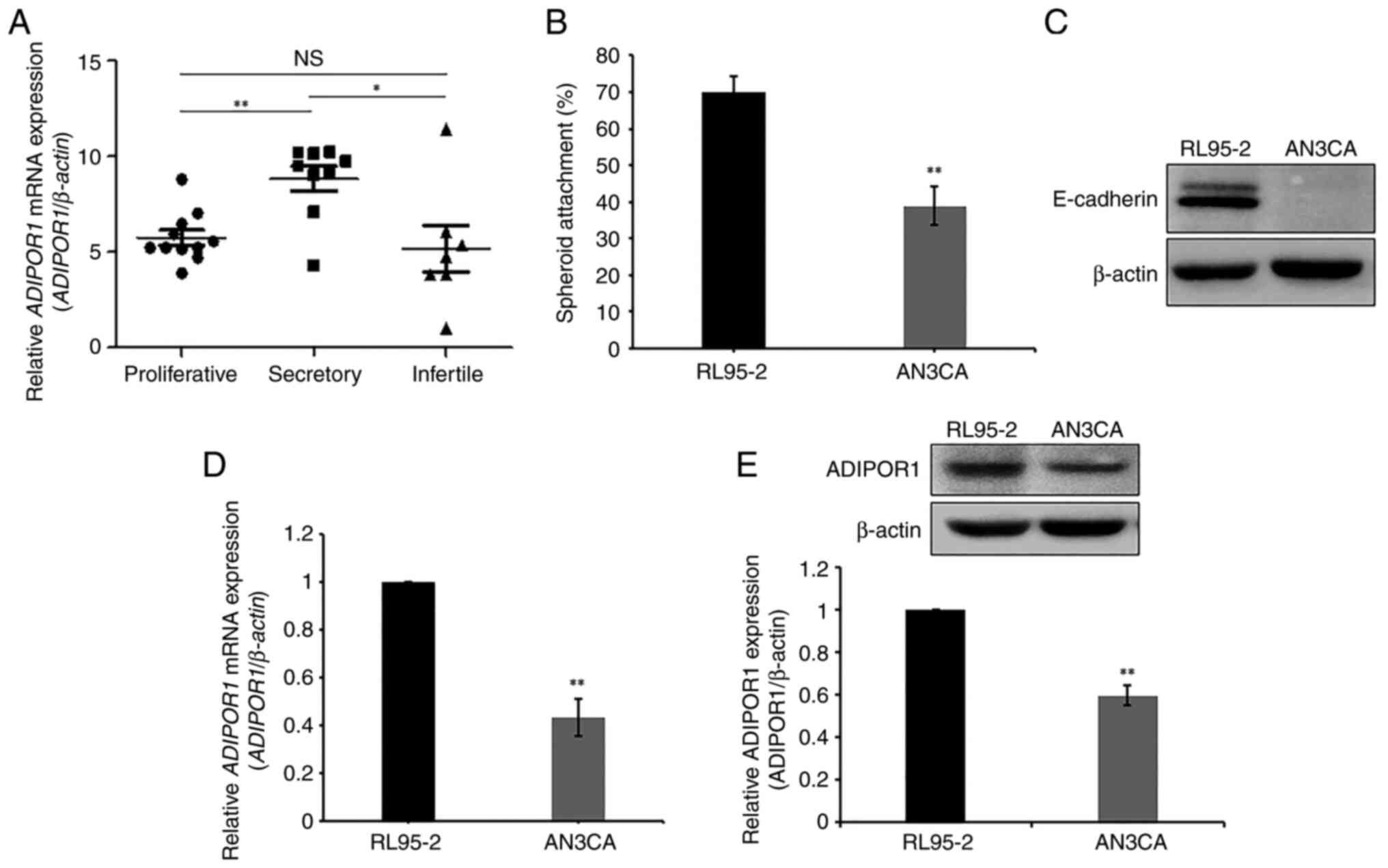
Examination of the dynamics of ADIPOR1
expression and its role in endometrial receptivity
To investigate the role of ADIPOR1 in the
endometrial cycle, the present study first quantified its
expression in the endometria during the proliferative and secretory
phases of fertile women as well as the secretory phase of infertile
women. The mRNA levels of ADIPOR1 were markedly higher in
the secretory phase compared with the proliferative phase in
fertile women. ADIPOR1 expression was markedly reduced
during the secretory phase in infertile women compared with that in
fertile women (Fig. 1A). Based on
these results, to explore the function of ADIPOR1 in the
endometrial receptivity process, the receptive RL95-2 and
non-receptive AN3CA cell lines were selected. Endometrial
receptivity between RL95-2 and AN3CA cell lines were confirmed
using an in vitro JAr spheroid attachment model. The
frequency of spheroid attachment was higher in receptive RL95-2
cells compared with non-receptive AN3CA cells, consistent with
previous data (20) (Fig. 1B). For further validation, the
expression patterns of proteins that were biomarkers of endometrial
receptivity were examined. The expression levels of E-selectin,
L-selectin and ITGB5 were significantly higher in the receptive
RL95-2 cells compared with the non-receptive AN3CA cells (data not
shown). In particular, the expression of E-cadherin was
significantly higher in receptive RL95-2 cells compared with
non-receptive AN3CA cells (Fig.
1C). Based on these results, the expression pattern of ADIPOR1
in cells with different receptivity were investigated. Lower mRNA
levels of ADIPOR1 were observed in non-receptive AN3CA cells
compared with receptive RL95-2 cells (Fig. 1D). ADIPOR1 protein levels were
reduced in AN3CA cells (Fig. 1E).
By contrast, ADIPOR2 was more highly expressed in the endometria of
infertile women compared with those of fertile women and in
non-receptive epithelial cells compared with receptive epithelial
cells in the present study (Fig.
S1). These results suggested that differences in ADIPOR1
expression may be associated with endometrial receptivity.
Role of ADIPOR1 in regulating
endometrial receptivity-related biomarkers
Due to the differences in ADIPOR1 expression in
cells with different receptivity, the changes in endometrial
receptivity caused by ADIPOR1 downregulation were investigated in
relatively upregulated recipient RL95-2 cells. Stable cells with
downregulated ADIPOR1 (shADIPOR1) were established using
shRNA-mediated gene silencing. The expression levels of ADIPOR1
were examined to verify the establishment of stable cell lines. The
mRNA levels of ADIPOR1 and corresponding protein levels were
significantly lower in shADIPOR1 cells compared with the
pLKO1 control cells (Fig. 2A and
B). Next, an in vitro implantation assay using the JAr spheroid
attachment method was performed to investigate the relationship
between the changes in ADIPOR1 expression and embryo implantation.
As shown in Fig. 2C, ADIPOR1
downregulation attenuated spheroid attachment in JAr cells compared
with that in the control cells. The E-cadherin protein is important
for successful embryo implantation (21). Therefore, the effect of
ADIPOR1 knockdown on E-cadherin expression was examined.
E-cadherin mRNA expression was reduced in shADIPOR1 cells
compared with that in the control cells (Fig. 2D). Consistent with the changes in
mRNA expression, E-cadherin protein levels were reduced in
shADIPOR1-treated cells (Fig. 2E).
E-selectin, L-selectin and ITGB5 expression was reduced following
ADIPOR1 knockdown (Fig. S2).
These results suggest that ADIPOR1 positively regulates E-cadherin
expression and may play an important role in the regulation of
endometrial receptivity during embryo implantation.
ADIPOR1 regulates AMPK activity in
endometrial epithelial cells
The present study examined the AMPK phosphorylation
state in receptive and non-receptive cells showing differential
ADIPOR1 expression to investigate the role of the association
between ADIPOR1 and AMPK activity in regulating endometrial
receptivity. AMPK phosphorylation was lower in non-receptive AN3CA
cells compared with receptive RL95-2 cells (Fig. 3A). Knockdown of ADIPOR1 induced
decreased AMPK phosphorylation in RL95-2 cells (Fig. 3B). These results indicated that
ADIPOR1-mediated AMPK regulation may be associated with the
receptivity of endometrial epithelial cells.
AMPK inhibition via dorsomorphin
attenuates endometrial receptivity
The present study used dorsomorphin, which inhibits
AMPK activity, to investigate whether the AMPK pathway regulates
the receptivity of endometrial epithelial cells. Dorsomorphin did
not affect cell viability in cytotoxicity tests. The inhibition of
AMPK phosphorylation was also confirmed via western blotting in
RL95-2 cells treated with various concentrations (Fig. S3A). AMPK phosphorylation was
reduced upon dorsomorphin treatment in a dose-dependent manner
(Fig. 4A). Next, JAr cell spheroid
attachment-mediated endometrial receptivity in RL95-2 cells
following dorsomorphin treatment was investigated.
Dorsomorphin-induced reduction in spheroid attachment was
proportional to the inhibition of AMPK phosphorylation (Fig. 4B). The expression patterns of
biomarkers associated with endometrial receptivity was confirmed.
Silencing AMPKa1 triggers the disruption of cell-cell adhesion via
the Twist/E-cadherin pathway in breast cancer (22). Therefore, E-cadherin expression in
receptive RL95-2 cells treated with dorsomorphin was examined. The
mRNA expression of E-cadherin decreased in the cells treated
with dorsomorphin (Fig. 4C).
Dorsomorphin treatment also reduced E-cadherin protein expression
in a dose-dependent manner (Fig.
4D). These results suggest that AMPK inhibition is closely
associated with decreased endometrial receptivity.
AMPK activation via AICAR improves
endometrial receptivity
The AMPK activator AICAR was used to further
investigate the relationship between AMPK activity and endometrial
receptivity. The toxicity following AICAR treatment was assessed
using the MTT assay for cell viability and the phosphorylation
state of AMPK was verified via immunoblot analysis at different
concentrations in non-receptive AN3CA cells (Fig. S3B). The phosphorylation level of
AMPK increased proportionally with AICAR (250 and 500 µM) (Fig. 5A). The rate of JAr cell-spheroid
attachment was significantly increased following AICAR treatment,
consistent with enhanced AMPK phosphorylation (Fig. 5B). Next, E-cadherin
expression in non-receptive AN3CA cells following AN3CA treatment
was examined. AICAR treatment significantly increased the mRNA
expression of E-cadherin in a dose-dependent manner (Fig. 5C). E-cadherin protein levels
increased in a dose-dependent manner in the AICAR-treated cells
(Fig. 5D). These results strongly
suggested that AMPK activity affects endometrial receptivity by
regulating E-cadherin expression.
Discussion
The endometrium undergoes morphological and
functional changes throughout the phases of menstrual cycle.
Specifically, the WOI represents a distinct period that leads to a
dynamic transition from a non-receptive endometrium to a receptive
endometrium for embryo implantation. Numerous researchers have
explored the differences between the endometria of fertile and
infertile women during WOI, aiming to identify key factors
associated with endometrial receptivity and propose potential
biomarkers for WOI (23,24). However, advancements in studies on
endometrial receptivity to improve pregnancy success are impeded by
the lack of knowledge of the regulation of various signaling
pathways.
The present study investigated the correlation
between endometrial receptivity and adiponectin signaling.
Adiponectin is a potential regulator of reproductive system
function. While plasma adiponectin was negatively associated with
progressive gestational age in non-obese women, no significant
difference was observed in plasma adiponectin levels between
non-obese and overweight pregnant women (25). Another study found no significant
difference in adiponectin expression between the endometria of
fertile and infertile groups (26). However, both ADIPOR1 and ADIPOR2
are markedly upregulated in the endometrium during the
mid-secretory phase, a critical stage of embryo implantation during
the menstrual cycle (3).
Furthermore, both receptors are expressed at lower levels in the
endometria of the infertile group than in those of the fertile
group (8).
The present study specifically focused on the
potential association between the two adiponectin signaling pathway
receptors and endometrial receptivity. It confirmed that ADIPOR1
was consistently upregulated in the endometrium during the
mid-secretory phase compared with that in the proliferative phase,
as previously reported (8).
However, ADIPOR1 was expressed at lower levels in the endometria of
infertile women. Additionally, ADIPOR1 expression was higher in
receptive epithelial cells compared with non-receptive epithelial
cells, as evident from the in vitro implantation assay.
Therefore, the correlation between ADIPOR1
expression and endometrial receptivity was examined. Stable cell
lines with diminished ADIPOR1 expression caused by shRNA
showed attenuated JAr spheroid attachment in an in vitro
implantation assay compared with that in the control cells.
Moreover, shADIPOR1 downregulated E-cadherin, a vital marker
of endometrial receptivity. These findings indicate that ADIPOR1
may be important in regulating endometrial receptivity during
embryo implantation.
In liver cells, adiponectin enhances AMPK activity
through the ADIPOR1-mediated signaling pathway and PPARa activity
through its other receptor ADIPOR2-mediated signaling pathway
(7). Adiponectin receptors
activate multiple signaling pathways and play various regulatory
roles in cellular functions (27).
ADIPOR1 stimulates the phosphorylation of AMPK, directly regulating
glucose metabolism and insulin sensitivity in skeletal muscle cells
(28,29). Kim et al (30) demonstrated a reduction in AMPK
phosphorylation in the brains, livers, kidneys and spleens of mice
with ADIPOR1 knockdown injected with an ADIPOR1 shRNA
mixture. Therefore, the present study investigated the correlation
between ADIPOR1 and AMPK expression in the endometrial epithelial
cells. The phosphorylation level of AMPK was lower in non-receptive
AN3CA cells with downregulated ADIPOR1 compared with receptive
RL95-2 cells and stable cells with ADIPOR1 knockdown showed a
decrease in AMPK phosphorylation. These results demonstrated that
ADIPOR1 mediated AMPK signaling in endometrial epithelial
cells.
AMPK is intricately involved in steroid hormone
signaling within the uterus and its activity, regulated by
progesterone, is crucial in uterine receptivity for embryo
implantation through the regulation of energy or glycogen in
endometrial epithelial cells (11,31).
In addition to its role in uterine receptivity, AMPK is vital in
placental and embryonic development (32). Recent reports have indicated that
the suppression of AMPKa1 induces breast cancer metastasis through
the disruption of cell-cell adhesion mediated by E-cadherin. In
contrast, the elevation of AMPKa1 expression induces E-cadherin
expression in cervical cancer cells (22,33).
AMPKa2 plays a role in regulating E-cadherin expression. When PHD
finger protein 2 is phosphorylated by AMPKa2, it enhances
demethylation activity, resulting in reduced methylation of
E-cadherin at H3K9me2 (34). These
observations indicate that AMPK activity may affect endometrial
receptivity by regulating E-cadherin expression, which is vital in
endometrial receptivity. Therefore, the present study examined the
association between the AMPK/E-cadherin pathway and endometrial
receptivity through the regulation of AMPK activity using two
pharmacological agents; dorsomorphin and AICAR. Dorsomorphin
reduced in vitro endometrial receptivity as well as
E-cadherin expression by inhibiting AMPK phosphorylation in
receptive RL95-2 cells. AICAR induced in vitro endometrial
receptivity and E-cadherin expression through the activation of
AMPK phosphorylation in non-receptive AN3CA cells. Accordingly, it
was hypothesized that AMPK activity plays an important role in
endometrial receptivity by regulating E-cadherin expression.
AICAR is a pharmacological agent that exhibits
numerous beneficial effects on metabolism, hypoxia, exercise and
cancer in the human body, with the potential for further research
(35). Turner et al
(36) reported that AICAR reduces
the LPS-stimulated inflammatory response in bovine endometrial
tissue. AICAR has been shown to prevent hypoxia-induced fetal
growth restriction by improving the uterine artery blood flow
during murine pregnancy (37).
Therefore, it was hypothesized that AICAR treatment of
non-receptive endometrium may improve endometrial receptivity.
However, it is essential to validate this hypothesis through in
vivo experiments because the present investigation focused
solely on in vitro endometrial receptivity.
The present study revealed the intricate interplay
among endometrial receptivity, the adiponectin signaling pathway
and AMPK activity. ADIPOR1 is a key regulator of endometrial
receptivity in successful embryo implantation. The
ADIPOR1/AMPK/E-cadherin axis mediates key cellular processes in
endometrial epithelial cells. Therefore, the findings of the
present study provided insights into the molecular mechanisms
underlying endometrial receptivity and can be used to design
potential therapeutic approaches to improve pregnancy success.
However, the present study has some limitations. The sample size
for comparing ADIPOR1 expression between fertile and infertile
women was small and tissue heterogeneity may have limited the
applicability of the findings. Further validation using in
vivo experiments is crucial to bridge the gap between these
findings and their clinical applications.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Priority Research Centers
Program through the National Research Foundation of Korea (NRF),
the Ministry of Education (grant no. NRF-2021R1I1A3059211) and
Konyang University Myunggok Research Fund (grant no. 2023-03).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author
Authors' contributions
BES, SLY and JK were responsible for
conceptualization, validation, investigation, data curation and
formal analysis. HJJ, DUJ and SRP were responsible for formal
analysis. BES and SLY wrote, reviewed and edited the manuscript.
THK, SKL and ARH were responsible for providing human endometrial
samples and formal analysis. JK was responsible for project
administration and funding acquisition. SLY and JK confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Bioethics
Committee of Konyang University Hospital [Institutional Review
Board (IRB) file no. KYUH 2018-11-007] and MizMedi Hospital (IRB
file no. MMIRB 2018-3). Signed informed consent was obtained from
each patient.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Achache H and Revel A: Endometrial
receptivity markers, the journey to successful embryo implantation.
Hum Reprod Update. 12:731–746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen TMD: Adiponectin: Role in
physiology and pathophysiology. Int J Prev Med. 11:1362020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takemura Y, Osuga Y, Yamauchi T, Kobayashi
M, Harada M, Hirata T, Morimoto C, Hirota Y, Yoshino O, Koga K, et
al: Expression of adiponectin receptors and its possible
implication in the human endometrium. Endocrinology. 147:3203–3210.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takemura Y, Osuga Y, Harada M, Hirata T,
Koga K, Morimoto C, Hirota Y, Yoshino O, Yano T and Taketani Y:
Serum adiponectin concentrations are decreased in women with
endometriosis. Hum Reprod. 20:3510–3513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng L, Shi H, Jin Y, Li X, Pan J, Lai Y,
Lin Y, Jin Y, Roy G, Zhao A and Li F: Adiponectin deficiency leads
to female subfertility and ovarian dysfunctions in mice.
Endocrinology. 157:4875–4887. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamauchi T, Kamon J, Ito Y, Tsuchida A,
Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et
al: Cloning of adiponectin receptors that mediate antidiabetic
metabolic effects. Nature. 423:762–769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamauchi T, Nio Y, Maki T, Kobayashi M,
Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T,
et al: Targeted disruption of AdipoR1 and AdipoR2 causes abrogation
of adiponectin binding and metabolic actions. Nat Med. 13:332–339.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dos Santos E, Serazin V, Morvan C, Torre
A, Wainer R, de Mazancourt P and Dieudonné MN: Adiponectin and
leptin systems in human endometrium during window of implantation.
Fertil Steril. 97:771–778.e771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riek U, Scholz R, Konarev P, Rufer A,
Suter M, Nazabal A, Ringler P, Chami M, Müller SA, Neumann D, et
al: Structural properties of AMP-activated protein kinase:
Dimerization, molecular shape, and changes upon ligand binding. J
Biol Chem. 283:18331–18343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ross FA, MacKintosh C and Hardie DG:
AMP-activated protein kinase: A cellular energy sensor that comes
in 12 flavours. FEBS J. 283:2987–3001. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Griffiths RM, Pru CA, Behura SK, Cronrath
AR, McCallum ML, Kelp NC, Winuthayanon W, Spencer TE and Pru JK:
AMPK is required for uterine receptivity and normal responses to
steroid hormones. Reproduction. 159:707–717. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi Y, Wang X, Hou S, Wu Z, Xu X and Pang
C: Intracavitary physiotherapy combined with acupuncture mediated
AMPK/mTOR signalling to improve endometrial receptivity in patients
with thin endometrium. Eur J Obstet Gynecol Reprod Biol. 277:32–41.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carey EA, Albers RE, Doliboa SR, Hughes M,
Wyatt CN, Natale DR and Brown TL: AMPK knockdown in placental
trophoblast cells results in altered morphology and function. Stem
Cells Dev. 23:2921–2930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Waker CA, Albers RE, Pye RL, Doliboa SR,
Wyatt CN, Brown TL and Mayes DA: AMPK knockdown in placental
labyrinthine progenitor cells results in restriction of critical
energy resources and terminal differentiation failure. Stem Cells
Dev. 26:808–817. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jha RK, Titus S, Saxena D, Kumar PG and
Laloraya M: Profiling of E-cadherin, beta-catenin and Ca(2+) in
embryo-uterine interactions at implantation. FEBS Lett.
580:5653–5660. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giannini A, D'Oria O, Corrado G, Bruno V,
Sperduti I, Bogani G, Laganà AS, Chiantera V, Caserta D and Vizza
E: The role of L1CAM as predictor of poor prognosis in stage I
endometrial cancer: A systematic review and meta-analysis. Arch
Gynecol Obstet. 309:789–799. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vizza E, Bruno V, Cutillo G, Mancini E,
Sperduti I, Patrizi L, Certelli C, Zampa A, Giannini A and Corrado
G: Prognostic role of the removed vaginal cuff and its correlation
with L1CAM in low-risk endometrial adenocarcinoma. Cancers (Basel).
14:342021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noyes RW, Hertig AT and Rock J: Reprint
of: Dating the endometrial biopsy. Fertil Steril. 112:e93–e115.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu SL, Kang Y, Jeong DU, Lee DC, Jeon HJ,
Kim TH, Lee SK, Han AR, Kang J and Park SR: The miR-182-5p/NDRG1
axis controls endometrial receptivity through the
NF-κB/ZEB1/E-cadherin pathway. Int J Mol Sci. 23:123032022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rahnama F, Thompson B, Steiner M, Shafiei
F, Lobie PE and Mitchell MD: Epigenetic regulation of E-cadherin
controls endometrial receptivity. Endocrinology. 150:1466–1472.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yi Y, Chen D, Ao J, Zhang W, Yi J, Ren X,
Fei J, Li F, Niu M, Chen H, et al: Transcriptional suppression of
AMPKα1 promotes breast cancer metastasis upon oncogene activation.
Proc Natl Acad Sci USA. 117:8013–8021. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Messaoudi S, El Kasmi I, Bourdiec A,
Crespo K, Bissonnette L, Saint CL, Bissonnette F and Kadoch IJ: 15
years of transcriptomic analysis on endometrial receptivity: What
have we learnt? Fertil Res Pract. 5:92019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rubin SC, Abdulkadir M, Lewis J,
Harutyunyan A, Hirani R and Grimes CL: Review of endometrial
receptivity array: A personalized approach to embryo transfer and
its clinical applications. J Pers Med. 13:7492023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nien JK, Mazaki-Tovi S, Romero R, Erez O,
Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et
al: Plasma adiponectin concentrations in non-pregnant, normal and
overweight pregnant women. J Perinat Med. 35:522–531. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pandey N, Kriplani A, Yadav RK, Lyngdoh BT
and Mahapatra SC: Peritoneal fluid leptin levels are increased but
adiponectin levels are not changed in infertile patients with
pelvic endometriosis. Gynecol Endocrinol. 26:843–849. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barbe A, Bongrani A, Mellouk N, Estienne
A, Kurowska P, Grandhaye J, Elfassy Y, Levy R, Rak A, Froment P and
Dupont J: Mechanisms of adiponectin action in fertility: An
overview from gametogenesis to gestation in humans and animal
models in normal and pathological conditions. Int J Mol Sci.
20:15262019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kadowaki T and Yamauchi T: Adiponectin and
adiponectin receptors. Endocr Rev. 26:439–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X,
Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, et al: Adiponectin
activates AMP-activated protein kinase in muscle cells via
APPL1/LKB1-dependent and phospholipase
C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent
pathways. J Biol Chem. 284:22426–22435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim MW, Abid NB, Jo MH, Jo MG, Yoon GH and
Kim MO: Suppression of adiponectin receptor 1 promotes memory
dysfunction and Alzheimer's disease-like pathologies. Sci Rep.
7:124352017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nie L, Zhang LX, Wang YC, Long Y, Ma YD,
Liao LC, Dai XH, Cui ZH, Liu H, Wang ZQ, et al: Consistency and
synchronization of AMPK-glycogen in endometrial epithelial cells
are critical to the embryo implantation. Reproduction. 163:293–307.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaufman MR and Brown TL: AMPK and
placental progenitor cells. Exp Suppl. 107:73–79. 2016.PubMed/NCBI
|
|
33
|
Konieczny P, Adamus T, Sułkowski M,
Skrzypek K and Majka M: Impact of AMPK on cervical carcinoma
progression and metastasis. Cell Death Dis. 14:432023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong Y, Hu H, Zhang X, Zhang Y, Sun X,
Wang H, Kan W, Tan MJ, Shi H, Zang Y and Li J: Phosphorylation of
PHF2 by AMPK releases the repressive H3K9me2 and inhibits cancer
metastasis. Signal Transduct Target Ther. 8:952023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Višnjić D, Lalić H, Dembitz V, Tomić B and
Smoljo T: AICAr, a widely used AMPK activator with important
AMPK-independent effects: A systematic review. Cells. 10:10952021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Turner ML, Cronin JG, Noleto PG and
Sheldon IM: Glucose availability and AMP-activated protein kinase
link energy metabolism and innate immunity in the bovine
endometrium. PLoS One. 11:e01514162016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lane SL, Houck JA, Doyle AS, Bales ES,
Lorca RA, Julian CG and Moore LG: AMP-activated protein kinase
activator AICAR attenuates hypoxia-induced murine fetal growth
restriction in part by improving uterine artery blood flow. J
Physiol. 598:4093–4105. 2020. View Article : Google Scholar : PubMed/NCBI
|