Introduction
Breast cancer, characterized by malignant solid
tumors originating from mammary gland epithelium, is a major cause
of mortality among women worldwide (1). Notably, the 5-year overall survival
rate remains poor, primarily owing to the metastasis of cancer
cells to vital organs such as the lungs, brain, and bones (2–4).
Therefore, elucidating the molecular mechanisms underlying breast
cancer metastasis is imperative.
The metastatic cascade involves a complex array of
biological processes, including extracellular matrix (ECM)
degradation; ECM, a crucial noncellular tissue component, provides
structural and biochemical support essential for cellular functions
(5,6). Degradation of ECM requires the
involvement of several extracellular proteinases, among which
matrix metalloproteases (MMPs), a family of zinc-dependent
proteinases, play pivotal roles in pathological processes,
including breast cancer (7).
Notably, MMP-9 is a key player in cancer cell invasion and
metastasis. MMP-9 expression is induced through the activation of
various intracellular signaling proteins through the stimulation of
probol esters, inflammatory cytokines, epidermal growth factors
(EGFs), or phorbol esters such as
12-O-tetradecanoylphorbol-13-acetate (TPA) (8–10).
TPA, a selective activator of protein kinase C
(PKC), orchestrates a cascade of events leading to the production
of reactive oxygen species (ROS) either upstream or downstream
(11–13). Consequently, ROS modulate several
intracellular signaling pathways, including protein kinase B (AKT)
and mitogen-activated protein kinases (MAPKs) (14–16),
thereby influencing the activation of transcription factors such as
activator protein-1 (AP-1) and nuclear factor-kappa B (NF-κB),
which are intricately associated with MMP expression during tumor
invasion and metastasis (15–19).
Nicotinamide adenine dinucleotide phosphate (NADPH)
oxidases (NOXs) are a family of membrane proteins involved in
intracellular ROS production and facilitate electron transfer
across biological membranes. NOXs catalyze superoxide generation at
the plasma membrane, subsequently releasing it into the
extracellular space, where it is converted into ROS, such as
hydrogen peroxide and superoxide anions (12,13).
NOXs are closely associated with phosphatidylinositol 3-OH kinase
(PI3K) signaling; PKC, a downstream molecule of PI3K, is essential
for superoxide generation via NOXs (14,20,21).
PKC mediates the activation of NOXs (22); however, the mechanism underlying
NOX activation via PKC during breast cancer invasion remains
unclear.
In this study, we investigated whether PKC regulates
ROS production through NOXs during cell invasion of MCF-7 cells, a
human breast cancer cell. Furthermore, we confirm the significance
of NOXs in TPA-induced MMP-9 expression and cell invasion in these
cells. We believe that our findings would help broaden our
understanding of the molecular mechanisms underlying breast cancer
metastasis.
Materials and methods
Cells and reagents
MCF-7 cells (cat. no. HTB-22) were procured from the
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with heat-inactivated 10% fetal bovine serum (FBS) and
1% antibiotics. The cell culture was maintained in a controlled
environment with a temperature of 37°C in a 5% CO2
atmosphere in an incubator. β-actin antibody (cat. no. A5441),
bovine serum albumin (BSA), skim milk, TPA, diphenyleneiodonium
chloride (DPI), and apocynin (APO) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). MMP-9 (cat. no. SC-12759) and
goat anti-mouse IgG-HRP (cat. no. sc-2005) antibodies were obtained
from Santa Cruz Biotechnology (Dallas, TX, USA). GF109203X (PKC
inhibitor; GF) was purchased from Abcam (Cambridge, MA, USA).
Phosphate-buffered saline (PBS), FBS, and DMEM were purchased from
Gibco-BRL (Gaithersburg, MD, USA).
RNA interference
NOX1-5-specific and control siRNAs were purchased
from BIONEER (Daejeon, Korea). The siRNA sets used for
amplification were as follows: NOX1, sense:
5′-GAGCAUGAAUGAGAGUCAU-3′, antisense: 5′-AUGACUCUCAUUCAUGCUC-3′;
NOX2, sense: 5′-GUAAUGUCAGUGGAAGUUA-3′, antisense:
5′-UAACUUCCACUGACAUUAC-3′; NOX3, sense: 5′-CACCAUGUUUUCAUCGUCU-3′,
antisense: 5′-AGACGAUGAAAACAUGGUG-3′; NOX4, sense:
5′-CAGAGUUUACCCAGCACAA-3′, antisense: 5′-UUGUGCUGGGUAAACUCUG-3′;
NOX5, sense: 5′-GUGACUACUUGUAUCUGAA-3′, antisense:
5′-UUCAGAUACAAGUAGUCAC-3′; control siRNA, sense:
5′-UUCUCCGAACGUGUCACGU-3′, antisense: 5′-ACGUGACACGUUCGGAGAA-3′.
The cells were transfected with NOX1 (30 pmol) and NOX3 (30 pmol)
siRNAs for 24 h and with NOX2 (30 pmol), NOX4 (100 pmol), and NOX5
(100 pmol) siRNAs for 48 h. Control siRNA transfections were also
conducted for each respective time point. All siRNA transfections
in MCF-7 cells were performed using Lipofectamine™ RNAiMAX
(Invitrogen, San Diego, CA, USA), in accordance with the
manufacturer's forward transfection protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cells using
TRIzol® (Life Technologies, Grand Island, NY, USA)
following the manufacturer's protocol. RNA concentration and purity
were calculated using BioSpec-nano (Shimadzu, Kyoto, Japan). cDNA
was synthesized using 1 µg total RNA using a PrimeScript™RT reagent
Kit (cat. no. RR047A; TaKaRa, Shiga, Japan). The RT-qPCR cycling
protocol comprised an initial denaturation step at 95°C for 10 min,
followed by 40 cycles of amplification consisting of denaturation
at 95°C for 15 sec and annealing/extension at 60°C for 1 min.
Subsequently, a melting curve analysis was conducted with
temperature ramping from 95°C for 15 sec, followed by
annealing/extension at 60°C for 1 min, and concluding with a final
denaturation step at 95°C for 15 sec. The mRNA expression levels of
MMP-9, NOX1, NOX5, and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) were analyzed using the ABI PRISM 7900 sequence detection
system and SYBR Green (Applied Biosystems, Foster City, CA, USA).
Relative quantitation was performed using the comparative
2−ΔΔCq method (23).
The primer sets used for amplification were as follows: MMP-9 (NM
004994), forward primer: 5′-CCTGGAGACCTGAGAACCAATCT-3′, reverse
primer: 5′-CCACCCGAGTGTAACCATAGC-3′; GAPDH (NM 002046), forward
primer: 5′-ATGGAAATCCCATCACCATCTT-3′, reverse primer:
5′-CGCCCCACTTGATTTTGG-3′; Primers for NOX1 (NM_007052, cat. no.
PPH06068A) and NOX5 (NM_024505, cat. no. PPH17569A) were obtained
from QIAGEN (Hilden, Germany). To account for variations in mRNA
concentration, the expression levels of MMP9, NOX1, and NOX5 were
normalized to that of the housekeeping gene, GAPDH. Relative
quantification was analyzed using the comparative 2−ΔΔCq
method, following the manufacturer's instructions.
Quantification of intracellular
ROS
The intracellular ROS was detected using an
oxidation-sensitive fluorescent probe dye (CM-H2DCFDA;
Invitrogen). MCF-7 cells were stimulated with 20 nM TPA for 24 h,
after which the cells were incubated with 10 µM
CM-H2DCFDA at 37°C for 30 min. CM-H2DCFDA was
oxidized to the green fluorescent dichlorofluorescein (DCF) using
hydrogen peroxide. The DCF fluorescence was measured using a
FACSCalibur flow cytometer (BD Biosciences, San Diego, CA, USA).
ROS production was expressed as the mean fluorescence intensity and
analyzed using the CellQuest software (BD Biosciences).
Western blot analysis
The cells (7×105) were incubated with 20
nM TPA for 24 h at 37°C. Following the treatments, cells were lysed
with ice-cold radioimmunoprecipitation assay (RIPA) buffer (Thermo
Scientific, Rockford, IL, USA) for 30 min on ice, and the protein
concentration in the resulting lysates was determined using a Bio
Spec-nano (Shimadzu). Subsequently, 20 µg of protein samples were
resolved using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to Hybond™
polyvinylidene fluoride (PVDF) membranes (GE Healthcare Life
Sciences, Buckinghamshire, UK) through western blotting. Following
the blocking with 5% BSA or skim milk for 2 h at 4°C, the membranes
were cropped around 70 kDa. Subsequently, the upper and lower
portions of the cropped membranes were incubated overnight at 4°C
with the primary antibody (1:2,500) targeting MMP-9 (92 kDa) and
β-actin (45 kDa), respectively, HRP-conjugated IgG (1:2,500) served
as the secondary antibody for 1 h at 4°C. Protein expression levels
were determined through signal analysis using a MINI HD6 image
analyzer (UVITEC, Cambridge, UK).
Matrigel invasion assay
The invasion of MCF-7 cells was assessed using a
24-well cell culture insert (8-µm pore size) coated with rehydrated
20 µl Matrigel™ (Corning Life Sciences, Corning, NY, USA) in
culture medium for 30 min. Suspended cells (4×105) and
chemical attractant, 20 nM of TPA, in 0.5 ml culture medium
(supplemented with 10% FBS and 1% antibiotics) were transferred
into the upper and lower chambers. Following 24 h incubation in a
5% CO2 incubator at 37°C, the cells on the upper side of
the membrane were gently removed using cotton swabs. The cells that
invaded the lower chamber through the membrane were fixed with a
10% formalin solution for 30 min at room temperature. Following
fixation, they were stained with 0.2% crystal violet for an
additional 30 min at room temperature. Subsequently, the invading
cells were counted in five random areas of the membrane under a
light microscope.
Gelatin zymography assay
MCF-7 cells were pre-treated with DPI (5 µM) or APO
(300 µM) for 1 h and then stimulated with TPA (20 nM) for 24 h at
37°C in serum-free DMEM medium. The collected culture medium was
suspended in a zymography sample buffer. Electrophoresis was
performed under non-reducing conditions using 10% sodium dodecyl
sulfate-polyacrylamide gel containing 0.1% (w/v) gelatin. Following
electrophoresis, the gel was washed with 2.5% Triton X-100 for 30
min under gentle agitation. Subsequently, the gel was incubated
overnight at 37°C in a developing solution (50 mM Tris-HCl, 5 mM
CaCl2, 100 mM NaCl, 0.02% Brij-35; pH 7.5). After
incubation, the gel was stained with 0.25% Coomassie blue R-250 at
room temperature for 30 min and washed with a destaining solution
(40% methanol and 7% acetic acid) until the bands were visible. The
proteolytic activity of MMP-9 was measured by comparing the
transparent bands resulting from the decomposition of gelatin.
Bands were visualized using a MINI HD6 image analyzer (UVITEC).
NOX activity assay
Cells were washed twice in ice-cold PBS and then
scraped from the plate using the same solution. Subsequently, the
cells were centrifuged at 4,000 rpm at 4°C for 10 min, and the
resulting pellet was suspended in a buffer containing 20 mM
KH2PO4, 1 mM EGTA, 150 mM sucrose, and a
protease inhibitor mixture. The cell suspension was lysed on ice
for 30 min. For the assay, 20 µl of lysate was mixed with 180 µl of
an assay buffer containing 250 mM HEPES (pH 7.4), 250 µM lucigenin,
1.2 mM MgSO4 (7H2O), 120 mM NaCl, 1.75 mM
CaCl2 (2H2O), 11 mM glucose, 0.5 mM EDTA, 5.9
mM KCl, and 200 µM NADPH for a duration of 10 sec. Photoemission in
terms of RLU was measured using the GloMax®-Multi Jr
Detection System (Promega, Madison, Wisconsin, USA) every minute
for 15 min.
Membrane fractionation
MCF-7 cells (5×107) were pre-treated with
GF for 1 h followed by incubation with TPA for an additional hour
at 37°C. Subsequently, the cells were suspended in homogenization
buffer (composed of 20 mM Tris-HCl, 2 mM EDTA, 5 mM EGTA, 5 mM DTT,
and protease inhibitor; pH 7.5) and homogenized using a sonicator
(5 times of 10 sec each at 10% amplitude), followed by a 30-min
incubation on ice. The resulting cell lysate was then subjected to
centrifugation at 16,000 × g for 15 min at 4°C to separate the
soluble (cytosolic) fraction from the pellet (membrane) fraction.
The pellet fraction was further treated with solubilization buffer
(homogenization buffer supplemented with 1% NP-40) for 30 min on
ice, followed by another centrifugation step at 16,000 × g for 15
min at 4°C.
Statistical analyses
Statistical analyses were performed using GraphPad
Prism version 8.0 (GraphPad Software, San Diego, CA, USA). Data are
presented as the mean and standard error of the mean. An unpaired
Student's t-test was used to compare two groups, and one-way
analysis of variance with Tukey's post hoc test was used to compare
independent multiple groups. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed at least in triplicate.
Results
Inhibition of NOX suppresses
TPA-induced cell invasion and MMP-9 expression in MCF-7 cells
In our previous study, we reported that stimulation
of MCF-7 cells with 20 nM TPA induces the expression of MMP-9 and
cell invasion, which occurs through various intracellular signaling
pathways (24,25). In addition, according to previous
reports, breast cancer cells are treated with DPI at concentrations
from 1 to 10 µM and APO at concentrations from 100 to 500 µM.
Therefore, in our study, we used intermediate concentrations of 5
µM DPI and 300 µM APO (26–30).
To investigate the impact of NOXs on TPA-induced MMP-9 expression
and cell invasion, MCF-7 cells were pre-treated with 5 µM DPI and
300 µM APO for 1 h, followed by stimulation with 20 nM TPA for 24
h. Western blotting and zymography revealed that NOX inhibitor
treatment effectively inhibited the upregulation of TPA-induced
MMP-9 protein expression and exocytosis in MCF-7 cells (Fig. 1A). RT-qPCR revealed that TPA
increased MMP9 levels in MCF-7 cells, whereas NOX inhibitors
mitigated TPA-induced MMP9 mRNA upregulation (Fig. 1B). Additionally, the Matrigel
invasion assay confirmed that pretreatment with NOX inhibitors
mitigated the increase in TPA-induced cell invasion (Fig. 1C). Therefore, the inhibition of NOX
expression suppresses TPA-induced breast cancer cell invasion by
inhibiting MMP-9 expression.
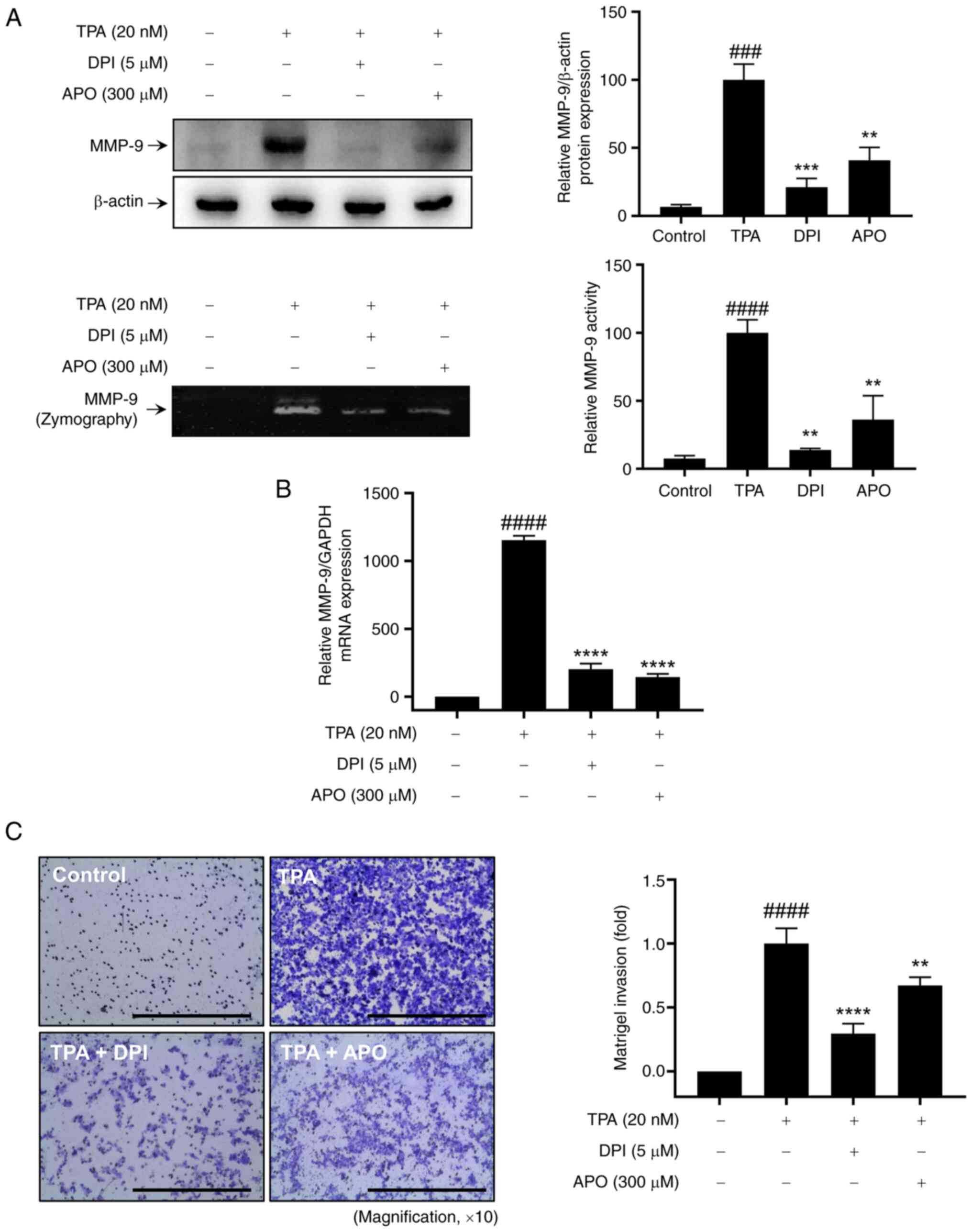 | Figure 1.Effect of NOX inhibitors on
TPA-induced MMP-9 expression and Matrigel invasion of MCF-7 cells.
(A) Western blot analysis (upper panel) and gelatin zymography
assay (lower panel) were performed to assess MMP-9 expression and
activity, respectively. (B) Reverse transcription-quantitative PCR
was used to analyze MMP9 mRNA expression. (C) Matrigel
invasion assay was conducted to evaluate cell invasion, with DPI or
APO added to the lower chamber along with 20 nM TPA for 24 h. Scale
bar, 100 µm. Results represent mean ± SEM of three independent
experiments. ###P<0.0005, ####P<0.0001
vs. control; **P<0.005, ***P<0.0005, ****P<0.0001 vs. TPA.
TPA, 12-O-tetradecanoylphorbol-13-acetate; MMP, matrix
metalloprotease; DPI, diphenyleneiodonium chloride; APO, apocynin;
SEM, standard error of the mean. |
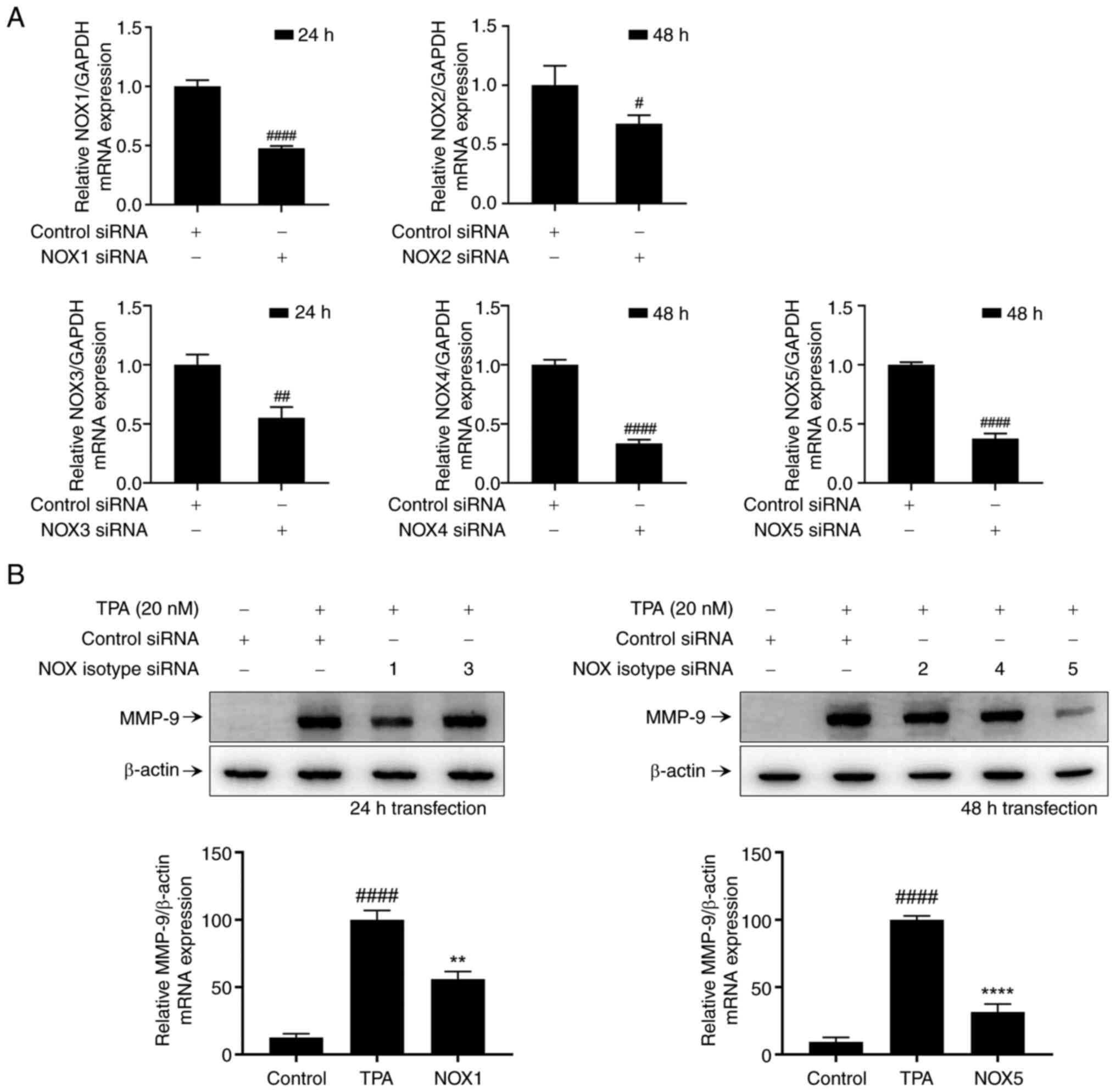
Role of NOX isotypes in TPA-induced
expression in MCF-7 cells
NOXs contribute to breast cancer cell invasion by
modulating MMP-9 expression and activity (Fig. 1). Among the members of the NOX
family, we investigated which isotypes (NOX1-5) were implicated in
TPA-induced MMP-9 protein expression in MCF-7 cells. For accurate
comparison, we identified conditions wherein the expression of NOX
mRNA decreased by approximately 50% following cell transfection
with NOX-specific small interfering RNA (siRNA) (Fig. 2A). We also identified a decrease in
protein expression by western blot analysis (Fig. S1). However, we could not detect
the NOX3 band under our conditions, so we conducted the experiment
based on mRNA expression levels. To assess the contribution of NOX
isotypes in TPA-induced MMP-9 expression, MCF-7 cells were
stimulated with TPA for 24 h following each condition of NOX
isotype transfection. Western blot analysis revealed that
transfection with NOX1 and NOX5 siRNA attenuated TPA-induced MMP-9
expression in MCF-7 cells (Fig.
2B). Therefore, TPA-induced MMP-9 expression in MCF-7 cells is
mediated by NOX1 and NOX5.
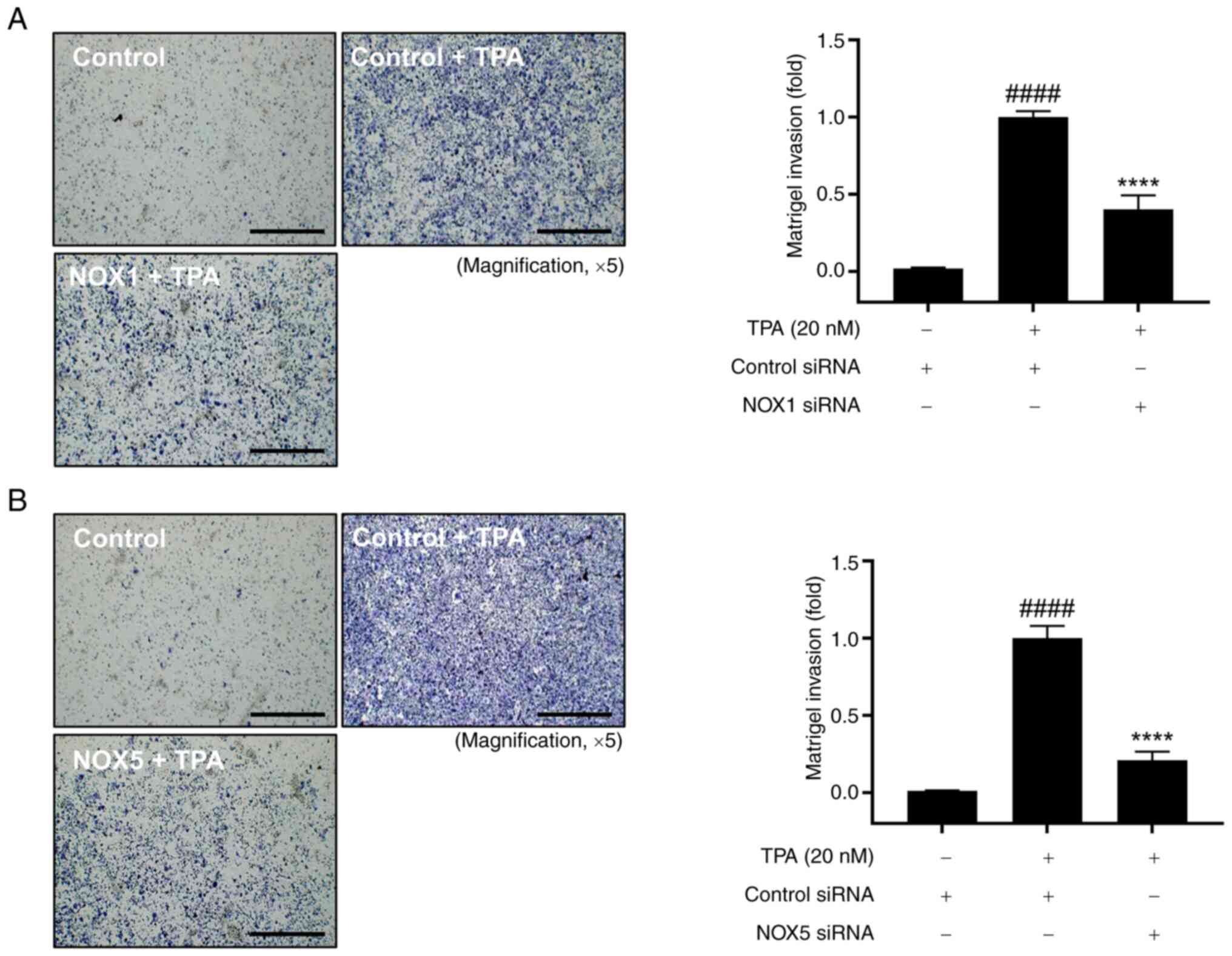
Validation of NOX1 and NOX5 roles in
cell invasion and ROS regulation in TPA-induced MCF-7 cells
We further validated the roles of NOX1 and NOX5 in
cell invasion. Cell invasion was significantly increased in
TPA-treated cells compared with that in control cells, and this
increase was significantly reduced by the knockdown of NOX1 and
NOX5 (Fig. 3). Furthermore, we
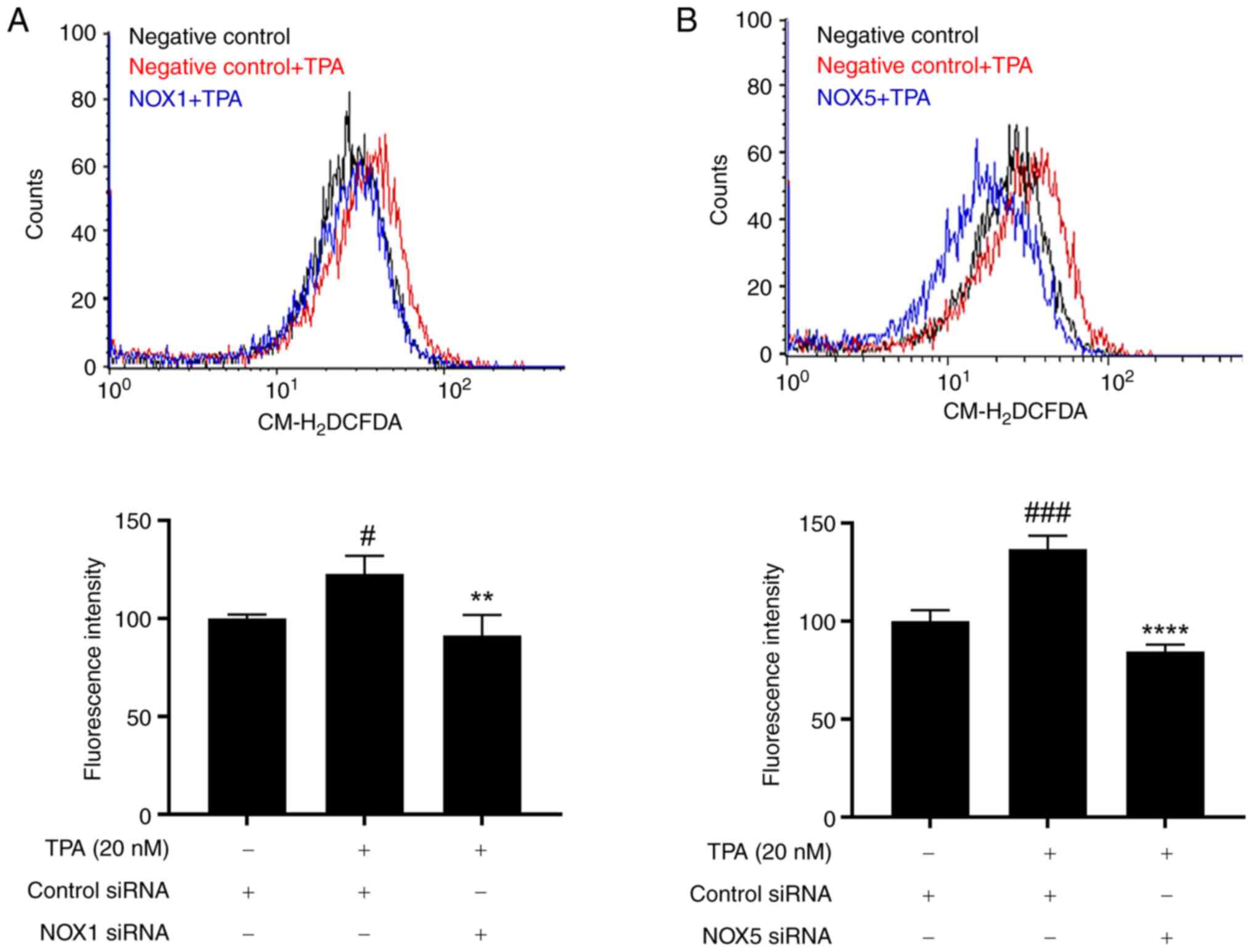
investigated whether NOX1 and NOX5 knockdown mediates ROS
regulation. The
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
(CM-H2DCFDA) fluorescence assay helped confirm that TPA
treatment (20 nM) induced ROS production in MCF-7 cells, this
effect was mitigated by knockdown of NOX1 and NOX5 (Fig. 4). Therefore, NOX1 and NOX5 inhibit
TPA-induced cell invasion by regulating ROS production.
PKC modulation of NOX activity and
MMP-9 expression in TPA-induced MCF-7 cells
TPA selectively activates PKC, acting as an upstream
or downstream regulator to generate ROS (11–13).
Therefore, we used GF109203X (GF), a widely used PKC inhibitor, to
confirm the involvement of PKC in TPA-induced ROS generation, MMP-9
expression, and MCF-7 cell activation. It is known that the
activation of PKC by TPA involves the translocation of PKC isoforms
from the cytosol to the plasma membrane (31). Pretreatment with GF was found to
inhibit the membrane translocation of the PKC isoforms α, β, and δ,
which was induced by TPA stimulation (Fig. S2). DPI was used as a positive
control in this experiment as a representative NOX inhibitor
(32). We observed that GF
effectively inhibited TPA-induced ROS production (Fig. 5A) and MMP-9 expression and
activation (Fig. 5B) in MCF-7
cells. Additionally, GF treatment attenuated the increase in
TPA-induced NOX activity in MCF-7 cells (Fig. 5C). Therefore, NOX activity is
mediated by PKC, which plays a pivotal role in regulating
TPA-induced MMP-9 expression and invasion in MCF-7 cells.
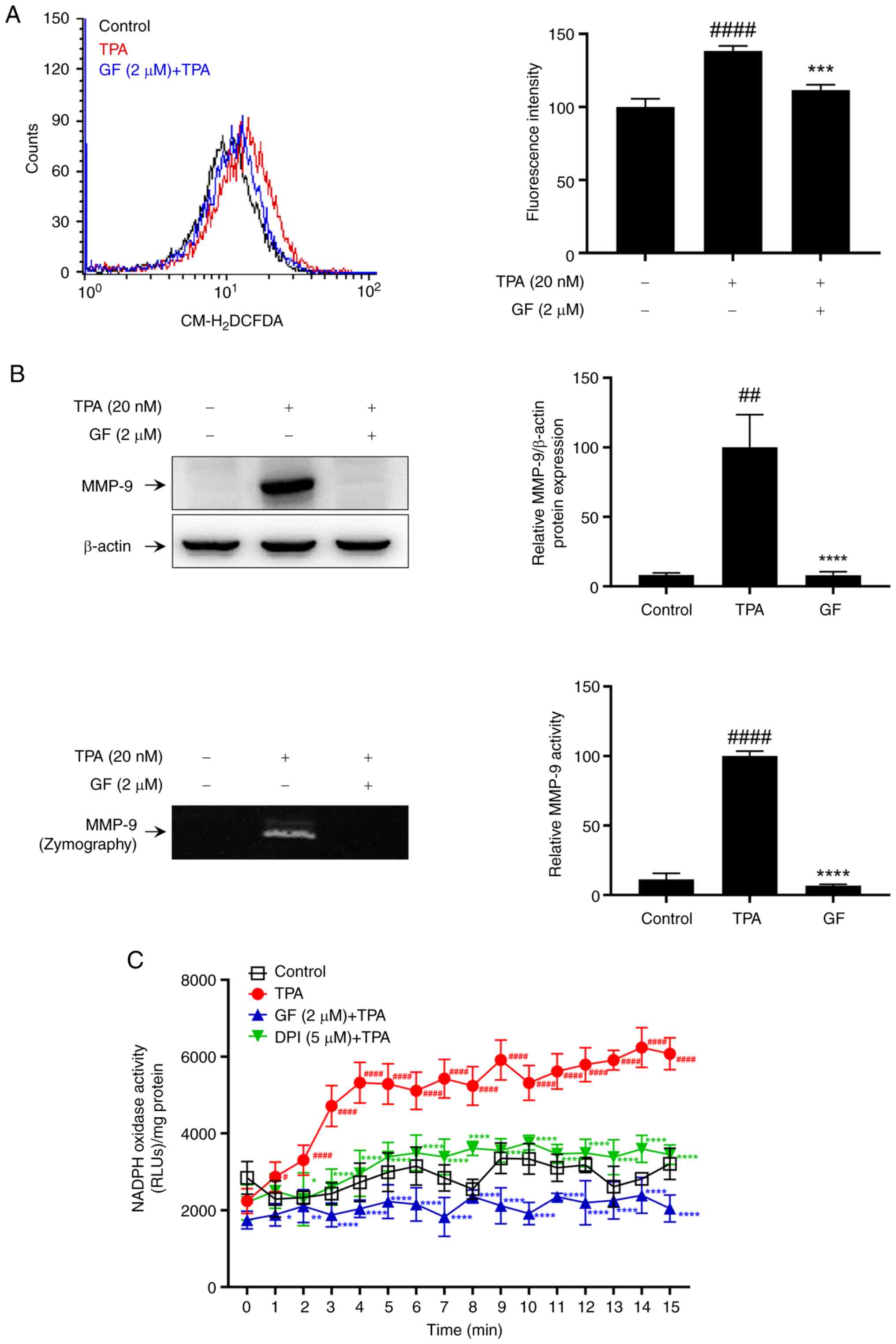 | Figure 5.Effect of PKC inhibitors on
TPA-induced intracellular ROS levels, MMP-9 expression, and NOX
activity in MCF-7 cells. (A) DCF fluorescence was measured to
assess ROS levels after treatment with a 2 µM PKC inhibitor (GF)
and 20 nM TPA stimulation. (B) Cells pre-treated with GF for 1 h
and stimulated with TPA for 24 h. MMP-9 expression was analyzed as
western blotting. Secreted MMP-9 activity was confirmed by gelatin
zymography assay. (C) NAPDH oxidase activity was evaluated using a
luminometer after GF treatment and 20 nM TPA stimulation. Results
represent mean ± SEM of three independent experiments.
#P<0.05, ##P<0.005,
####P<0.0001 vs. control; *P<0.05, **P<0.005,
***P<0.0005, ****P<0.0001 vs. TPA. TPA,
12-O-tetradecanoylphorbol-13-acetate; MMP, matrix metalloprotease;
PKC, protein kinase C; ROS, reactive oxygen species; GF, GF109203X;
SEM, standard error of the mean. |
Discussion
Breast cancer is a malignant tumor that is the
leading cause of mortality among women (33). Metastasis to diverse organs,
including bones, lungs, liver, brain, and kidneys, accounts for
most breast cancer-related deaths (34). The initial event in cancer cell
invasion and migration involves a decrease in the ECM, which poses
biochemical and mechanical barriers to cell movement (6,35).
The most important factors in ECM degradation are MMP expression
and activity, which play pivotal roles in breast cancer (6,36).
MMP is a family of proteases that play important roles in the
development and progression of cancer; among these, MMP-9 is vital
in tumor invasion and metastasis owing to its collagenase activity
in ECM degradation (37). Breast
cancer cells, stimulated by various factors, including TPA,
increase MMP-9 expression by activating several intracellular
signaling pathways (36,38). Following MMP-9 expression and
activation, ECM loss in blood vessels or lymphatic walls
facilitates cancer cell invasion into these systems, leading to
metastasis to other organs. Therefore, regulation of MMP-9
expression is pivotal in controlling cancer metastasis.
Mitochondria and the NOX family (NOX1-5 and DUOX1/2)
constitute two important sources of ROS production in cancer cells
(39). NOX, a protein facilitating
electron transport across biological membranes, generates
superoxide in the plasma membrane, which is converted into hydrogen
peroxide; this facilitates its entry into the cell in the form of
superoxide or hydrogen peroxide, thus impacting various
intracellular signaling mechanisms (40,41).
NOX family members play crucial roles in various human cancer
tissues (42,43). However, the role of NOX isotypes
(NOX1-5) in TPA-induced breast cancer cell invasion remains
unexplored. NOX1 is overexpressed in various human solid tumors,
including colon cancer, prostate cancer, and melanoma (44,45),
and it contributes to the regulation of cell invasion by regulating
MMP-9 production and cell migration (46). NOX5 expression is increased in
tumor tissues of patients with breast cancer, and it promotes
breast cancer cell proliferation and metastasis (47,48).
However, we lack reports on the importance of NOX1 and NOX5 in
breast cancer invasion. We confirmed that the previously known NOX
inhibitors, DPI and APO, inhibit MMP-9 expression and cell invasion
in MCF-7 cells (Fig. 1).
Furthermore, we confirmed that the inhibition of NOX1 and NOX5
among the NOXs is involved in MMP-9 expression and cell invasion in
MCF-7 cells (Figs. 2 and 3).
TPA induces multiple signaling pathways in a
PKC-dependent manner (49); PKC
activation promotes tumor development and is associated with
special cell functions, such as adhesion, invasion, and metastasis
(50). PKC activation in breast
cancer is strongly associated with increased invasion through the
production and secretion of MMP-9 (24,25,51).
Furthermore, ROS generation induces TPA-mediated migration and
invasion (52). PKC activates
NADPH oxidases, leading to the production of ROS (22,53–57).
Therefore, we confirmed the inhibitory effects of NOX1 and NOX5
suppression on TPA-induced ROS generation in MCF-7 cells (Fig. 4). Finally, we confirmed the
inhibitory effects of PKC inhibitors on TPA-induced ROS production,
MMP-9 expression, and NOX activation in MCF-7 cells (Fig. 5).
However, the current study has limitations as it
lacks evidence from animal experiments and does not explore the
role of DUOX1 and DUOX2 in breast cancer metastasis. Therefore,
future studies incorporating animal models and investigating the
involvement of additional NOX isoforms could provide a more
comprehensive understanding of the mechanisms underlying breast
cancer invasion.
In conclusion, our findings confirm that NOX1 and
NOX5 mediate TPA-induced invasion of MCF-7 cells by regulating
MMP-9 expression and activation; this is achieved mainly by
modulating ROS generation via PKC. To the best of our knowledge,
this is the first study to demonstrate that TPA-induced
PKC-dependent-MCF-7 cell invasion is modulated by NOXs. Despite
some limitations in our study, our findings highlight potential
strategies for treating breast cancer metastasis via NOX1 and NOX5
regulation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by Wonkwang University in 2022.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YRL and HJY designed and conceptualized the
experiments. HKS, EMN and JMK performed the experiments and data
collection. HKS wrote the original draft and revised the
manuscript. JMK analyzed and generated the figures. YRL and HJY
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anne N, Sulger E and Pallapothu R: Primary
squamous cell carcinoma of the breast: A case report and review of
the literature. J Surg Case Rep. 2019:rjz1822019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zagelbaum NK, Ward MF II, Okby N and
Karpoff H: Invasive ductal carcinoma of the breast with
osteoclast-like giant cells and clear cell features: A case report
of a novel finding and review of the literature. World J Surg
Oncol. 14:2272016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berman AT, Thukral AD, Hwang WT, Solin LJ
and Vapiwala N: Incidence and patterns of distant metastases for
patients with early-stage breast cancer after breast conservation
treatment. Clin Breast Cancer. 13:88–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woessner JF Jr: Matrix metalloproteinases
and their inhibitors in connective tissue remodeling. FASEB J.
5:2145–2154. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizutani K, Kofuji K and Shirouzu K: The
significance of MMP-1 and MMP-2 in peritoneal disseminated
metastasis of gastric cancer. Surg Today. 30:614–621. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeigler ME, Chi Y, Schmidt T and Varani J:
Role of ERK and JNK pathways in regulating cell motility and matrix
metalloproteinase 9 production in growth factor-stimulated human
epidermal keratinocytes. J Cell Physiol. 180:271–284. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hozumi A, Nishimura Y, Nishiuma T, Kotani
Y and Yokoyama M: Induction of MMP-9 in normal human bronchial
epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am J
Physiol Lung Cell Mol Physiol. 281:L1444–L1452. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weng CJ, Chau CF, Hsieh YS, Yang SF and
Yen GC: Lucidenic acid inhibits PMA-induced invasion of human
hepatoma cells through inactivating MAPK/ERK signal transduction
pathway and reducing binding activities of NF-kappaB and AP-1.
Carcinogenesis. 29:147–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HB, Yu MR, Song JS and Ha H: Reactive
oxygen species amplify protein kinase C signaling in high
glucose-induced fibronectin expression by human peritoneal
mesothelial cells. Kidney Int. 65:1170–1179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu WS, Tsai RK, Chang CH, Wang S, Wu JR
and Chang YX: Reactive oxygen species mediated sustained activation
of protein kinase C alpha and extracellular signal-regulated kinase
for migration of human hepatoma cell Hepg2. Mol Cancer Res.
4:747–758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Traore K, Sharma RB, Burek CL and Trush
MA: Role of ROS and MAPK in TPA-induced ICAM-1 expression in the
myeloid ML-1 cell line. J Cell Biochem. 100:1010–1021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang L, Lin H, Chen Q, Yu L and Bai D:
MPPa-PDT suppresses breast tumor migration/invasion by inhibiting
Akt-NF-κB-dependent MMP-9 expression via ROS. BMC Cancer.
19:11592019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee GH, Jin SW, Kim SJ, Pham TH, Choi JH
and Jeong HG: Tetrabromobisphenol A induces MMP-9 expression via
NADPH Oxidase and the activation of ROS, MAPK, and Akt pathways in
human breast cancer MCF-7 Cells. Toxicol Res. 35:93–101. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu TC, Young MR, Cmarik J and Colburn NH:
Activator protein 1 (AP-1)- and nuclear factor kappaB
(NF-kappaB)-dependent transcriptional events in carcinogenesis.
Free Radic Biol Med. 28:1338–1348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Q, Shen HM and Ong CN: Inhibitory
effect of emodin on tumor invasion through suppression of activator
protein-1 and nuclear factor-kappaB. Biochem Pharmacol. 68:361–371.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Savaraj N, Wei Y, Unate H, Liu PM, Wu CJ,
Wangpaichitr M, Xia D, Xu HJ, Hu SX and Tien Kuo M: Redox
regulation of matrix metalloproteinase gene family in small cell
lung cancer cells. Free Radic Res. 39:373–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
Subunit of NF-kappa B through utilization of the Ikappa B kinase
and activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brandes RP, Weissmann N and Schroder K:
Nox family NADPH oxidases: Molecular mechanisms of activation. Free
Radic Biol Med. 76:208–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JM, Park J, Noh EM, Song HK, Kang SY,
Jung SH, Kim JS, Park BH, Lee YR and Youn HJ: Bruton's
agammaglobulinemia tyrosine kinase (Btk) regulates TPA-induced
breast cancer cell invasion via PLCγ2/PKCβ/NF-κB/AP-1-dependent
matrix metalloproteinase-9 activation. Oncol Rep. 45:562021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noh EM, Park YJ, Kim JM, Kim MS, Kim HR,
Song HK, Hong OY, So HS, Yang SH, Kim JS, et al: Fisetin regulates
TPA-induced breast cell invasion by suppressing matrix
metalloproteinase-9 activation via the PKC/ROS/MAPK pathways. Eur J
Pharmacol. 764:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piszczatowska K, Przybylska D, Sikora E
and Mosieniak G: Inhibition of NADPH oxidases activity by
diphenyleneiodonium chloride as a mechanism of senescence induction
in human cancer cells. Antioxidants (Basel). 9:12482020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren G, Luo W, Sun W, Niu Y, Ma DL, Leung
CH, Wang Y, Lu JJ and Chen X: Psoralidin induced reactive oxygen
species (ROS)-dependent DNA damage and protective autophagy
mediated by NOX4 in breast cancer cells. Phytomedicine. 23:939–947.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nasimian A, Farzaneh P, Tamanoi F and
Bathaie SZ: Cytosolic and mitochondrial ROS production resulted in
apoptosis induction in breast cancer cells treated with Crocin: The
role of FOXO3a, PTEN and AKT signaling. Biochem Pharmacol.
177:1139992020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Anneo A, Carlisi D, Emanuele S, Buttitta
G, Di Fiore R, Vento R, Tesoriere G and Lauricella M: Parthenolide
induces superoxide anion production by stimulating EGF receptor in
MDA-MB-231 breast cancer cells. Int J Oncol. 43:1895–1900. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaikai S, Lu F, Xie J, Wu M, Cai B, Liu Y,
Zhang H, Tan H, Pan Y and Xu H: Cambogin exerts anti-proliferative
and pro-apoptotic effects on breast adenocarcinoma through the
induction of NADPH oxidase 1 and the alteration of mitochondrial
morphology and dynamics. Oncotarget. 7:50596–50611. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jacobson PB, Kuchera SL, Metz A,
Schächtele C, Imre K and Schrier DJ: Anti-inflammatory properties
of Gö 6850: A selective inhibitor of protein kinase C. J Pharmacol
Exp Ther. 275:995–1002. 1995.PubMed/NCBI
|
|
32
|
Augsburger F, Filippova A, Rasti D,
Seredenina T, Lam M, Maghzal G, Mahiout Z, Jansen-Dürr P, Knaus UG,
Doroshow J, et al: Pharmacological characterization of the seven
human NOX isoforms and their inhibitors. Redox Biol. 26:1012722019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Redig AJ and McAllister SS: Breast cancer
as a systemic disease: A view of metastasis. J Intern Med.
274:113–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang WG, Sanders AJ, Katoh M, Ungefroren
H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P,
et al: Tissue invasion and metastasis: Molecular, biological and
clinical perspectives. Semin Cancer Biol. 35 (Suppl):S244–S275.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duffy MJ, Maguire TM, Hill A, McDermott E
and O'Higgins N: Metalloproteinases: Role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Scorilas A, Karameris A, Arnogiannaki N,
Bassilopoulos P, Trangas T and Talieri M: Overexpression of
matrix-metalloproteinase-9 in human breast cancer: A potential
favourable indicator in node-negative patients. Br J Cancer.
84:1488–1496. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan C and Boyd DD: Regulation of matrix
metalloproteinase gene expression. J Cell Physiol. 211:19–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Landry WD and Cotter TG: ROS signalling,
NADPH oxidases and cancer. Biochem Soc Trans. 42:934–938. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang F, Zhang Y and Dusting GJ: NADPH
oxidase-mediated redox signaling: Roles in cellular stress
response, stress tolerance, and tissue repair. Pharmacol Rev.
63:218–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lambeth JD: NOX enzymes and the biology of
reactive oxygen. Nat Rev Immunol. 4:181–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kamata T: Roles of Nox1 and other Nox
isoforms in cancer development. Cancer Sci. 100:1382–1388. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Block K and Gorin Y: Aiding and abetting
roles of NOX oxidases in cellular transformation. Nat Rev Cancer.
12:627–637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deep G, Kumar R, Jain AK, Dhar D,
Panigrahi GK, Hussain A, Agarwal C, El-Elimat T, Sica VP, Oberlies
NH and Agarwal R: Graviola inhibits hypoxia-induced NADPH oxidase
activity in prostate cancer cells reducing their proliferation and
clonogenicity. Sci Rep. 6:231352016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shinohara M, Adachi Y, Mitsushita J,
Kuwabara M, Nagasawa A, Harada S, Furuta S, Zhang Y, Seheli K,
Miyazaki H and Kamata T: Reactive oxygen generated by NADPH oxidase
1 (Nox1) contributes to cell invasion by regulating matrix
metalloprotease-9 production and cell migration. J Biol Chem.
285:4481–4488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Juhasz A, Ge Y, Markel S, Chiu A,
Matsumoto L, van Balgooy J, Roy K and Doroshow JH: Expression of
NADPH oxidase homologues and accessory genes in human cancer cell
lines, tumours and adjacent normal tissues. Free Radic Res.
43:523–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dho SH, Kim JY, Lee KP, Kwon ES, Lim JC,
Kim CJ, Jeong D and Kwon KS: STAT5A-mediated NOX5-L expression
promotes the proliferation and metastasis of breast cancer cells.
Exp Cell Res. 351:51–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lim PS, Sutton CR and Rao S: Protein
kinase C in the immune system: From signalling to chromatin
regulation. Immunology. 146:508–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Parekh DB, Ziegler W and Parker PJ:
Multiple pathways control protein kinase C phosphorylation. EMBO J.
19:496–503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim JM, Park J, Noh EM, Song HK, Kang SY,
Jung SH, Kim JS, Youn HJ and Lee YR: Downregulation of matriptase
suppresses the PAR-2/PLCγ2/PKC-mediated invasion and migration
abilities of MCF-7 breast cancer cells. Oncol Rep. 46:2472021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu WS: The signaling mechanism of ROS in
tumor progression. Cancer Metastasis Rev. 25:695–705. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Eid BG, Abu-Sharib AT, El-Bassossy HM,
Balamash K and Smirnov SV: Enhanced calcium entry via activation of
NOX/PKC underlies increased vasoconstriction induced by
methylglyoxal. Biochem Biophys Res Commun. 506:1013–1018. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cui X, Li X, He Y, Yu J, Fu J, Song B and
Zhao RC: Combined NOX/ROS/PKC signaling pathway and metabolomic
analysis reveals the mechanism of TRAM34-Induced endothelial
progenitor cell senescence. Stem Cells Dev. 30:671–682. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen F, Yu Y, Haigh S, Johnson J, Lucas R,
Stepp DW and Fulton DJ: Regulation of NADPH oxidase 5 by protein
kinase C isoforms. PLoS One. 9:e884052014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee TH, Chen JL, Liu PS, Tsai MM, Wang SJ
and Hsieh HL: Rottlerin, a natural polyphenol compound, inhibits
upregulation of matrix metalloproteinase-9 and brain astrocytic
migration by reducing PKC-delta-dependent ROS signal. J
Neuroinflammation. 17:1772020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brandes RP and Schroder K: NOXious
phosphorylation: Smooth muscle reactive oxygen species production
is facilitated by direct activation of the NADPH oxidase Nox1. Circ
Res. 115:898–900. 2014. View Article : Google Scholar : PubMed/NCBI
|