Introduction
Mesenchymal stem cells (MSCs) have been widely
studied for their potential applications in various medical fields,
including regenerative medicine and cancer therapy (1–4).
MSCs are multipotent stromal cells that can differentiate into a
variety of cell types, including bone cells, cartilage cells and
adipose cells. They also possess immunomodulatory properties,
making them attractive candidates for therapeutic applications
(5). The potential of MSCs in
cancer treatment is an area of active research, but the outcomes
and involved mechanisms are complex and can vary depending on
several factors, including the type of cancer, the microenvironment
and the specific characteristics of the MSCs used (6). Studies have suggested that MSCs can
promote tumor growth and metastasis under certain conditions
(7). The supportive roles of MSCs
in the tumor microenvironment may be related to their interactions
with cancer cells, immune cells and the extracellular matrix.
However, some research suggests that MSCs may exert antitumor
effects (8,9). They may inhibit cancer cell growth
through mechanisms such as inducing apoptosis (programmed cell
death) or modulating immune responses to target cancer cells
(7,10).
Extracellular vesicles (EVs) carry various bioactive
molecules, including proteins, nucleic acids and lipids. They are
involved in cell-to-cell communication and have been frequently
investigated for potential therapeutic applications (11). MSC-derived EVs (MSC-EVs) are a
heterogeneous group of lipid-coated nanovesicles that mainly serve
as paracrine mediators of MSCs. Equipped with the characteristics
of MSCs, MSC-EVs show a strong tendency to migrate to tumor sites
in vivo and as anticancer therapeutic candidates, they are
considered superior to MSCs in a number of ways, such as being
cell-free and tiny, nonimmunogenic, easy to manipulate, and
biologically genetically safe (12). A number of studies have shown that
MSC-EVs are involved in tumor regulation by affecting various steps
of tumor growth and metastasis through positive or negative
mechanisms (12–14). It seems that whether MSC-EVs
promote or suppress tumor progression is determined by the origin
of the MSC and the type of tumor (6). The potential anticancer effects of
EVs derived from mesenchymal stem cells on various types of cancer
are unclear.
Breast cancer is the most prevalent cancer type and
the leading cause of cancer-related mortality among women (15,16).
Despite the improvements in cancer treatment throughout recent
decades, a large percentage of patients still experience
disappointing results. New creative approaches that can increase
the effectiveness of treatment are urgently needed. As
aforementioned, the physiological roles of EVs derived from
mesenchymal stem cells in cancer are unclear. The human placenta, a
temporary vascular organ that occurs during fetal development, has
emerged as an alternative and highly attractive source of MSCs and
MSC-released EVs (17). Therefore,
the potent effects of EVs derived from human placenta mesenchymal
stem cells (hPMSC-EVs) on breast cancer progression were validated
in the present study and it demonstrated that hPMSC-EVs can inhibit
the proliferation, migration and colony formation of the 4T1 breast
cancer cell line, decrease the tube formation potential of human
umbilical vein endothelial cells (HUVECs) in vitro, and
inhibit tumor growth and angiogenesis in models derived from the
4T1 cell line.
Materials and methods
Cell culture
The mouse breast cancer cell line 4T1 was purchased
from ATCC and cultured in RPMI 1640 medium supplemented with 10%
FBS (cat. no. SV30087.02; HyClone; Cytiva), 1%
penicillin-streptomycin solution (cat. no. 15140122; Gibco; Thermo
Fisher Scientific, Inc.) and 1% nonessential amino acid solution
(cat. no. 11140050; Gibco; Thermo Fisher Scientific, Inc.). The
human breast cancer cell line MCF-7 was purchased from ATCC and
cultured in Dulbecco's modified Eagle's medium (DMEM; cat. no.
11995065; Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS and 1% penicillin-streptomycin solution (cat. no. 15140122;
Gibco; Thermo Fisher Scientific, Inc.). HUVECs (passage 3–4), human
placenta-derived MSCs (hPMSCs; passage 4–6) and human umbilical
cord-derived MSCs (hUC-MSCs; passage 4–6) were donated by
AmCellGene Co. Ltd. and preserved at Nankai University (18). For the tracking of transplanted
tumor cells in vivo, 4T1 cells were transduced with a
self-activating lentiviral vector that carried a 5′LTR
promoter-driven double fusion (DF) reporter gene that contained
firefly luciferase and enhanced green fluorescence protein
(Fluc-eGFP) as previously reported (19,20).
Isolation and characterization of
EVs
EVs were isolated by ultracentrifugation as
previously described (11,18). The supernatant of MSCs was
successively centrifuged at 500 × g for 10 min, 2,000 × g for 30
min and 10,000 × g for 30 min at 4°C to exclude cell debris and
apoptotic bodies. After that, the EV pellet was collected by
ultracentrifugation at 100,000 × g for 70 min and washed by a
second ultracentrifugation at 100,000 × g for 2 h in
phosphate-buffered saline (PBS). All the above ultracentrifugation
steps were performed at 4°C. Finally, the purified EV pellet was
resuspended in 200 µl PBS and stored at −80°C for further
experiments. Transmission electron microscopy (TEM; HT7700;
Hitachi, Ltd.) was used to observe the morphology of the isolated
EVs (18). Briefly, 2 µg/µl EVs
were loaded onto a carbon film (Zhongjingkeyi Technology),
incubated for 5 min at room temperature and was subsequently
stained with 2% phosphotungstic acid for 2 min at room temperature.
Excess liquid was removed by filter paper. The samples were allowed
to air dry for at least 10 min at room temperature and then
observed using TEM at an acceleration voltage of 120 kV. The size
distribution of EVs was determined using a Malvern Particle Size
Analyzer (Zeta sizer Nano ZS; Malvern Instruments, Ltd.). The
protein concentration was quantified using a BCA protein assay kit
(Promega Corporation).
Western blotting
In the present study, the proteins expressed in MSCs
and MSC-EVs, including tumor susceptibility gene 101 (TSG101),
ALG-2 interacting protein X (ALIX) and CD63, were identified as
previously reported (21). In
brief, 30 µg of EVs lysates in 100 µl radioimmunoprecipitation
assay (RIPA) buffer (cat. no. R0010; Beijing Solarbio Science &
Technology Co., Ltd.), determined by BCA protein assay kit (Promega
Corporation), were subjected to western blotting analysis. Proteins
were heat-denaturized in 5X SDS-PAGE loading buffer (cat. no.
CW0027S; CWBIO), fractionated on 10% SDS-polyacrylamide gels, and
electro-transferred onto polyvinylidene fluoride membranes
(MilliporeSigma). Blocked with 5% non-fat milk (cat. no. A600669;
Beyotime Institute of Biotechnology) for 2 h at room temperature,
proteins were respectively immunoblotted with the indicated
antibodies. Briefly, they were incubated with primary antibodies at
4°C overnight and subsequently identified by second antibodies
through a second incubation step of 2 h at room temperature. The
following antibodies were used according to the manufacturer's
instructions: ALIX (1:2,000; cat. no. WL03063; Wanleibio Co.,
Ltd.), TSG101 (1:2,000; cat. no. EPR7131; Abcam) and CD63 (1:2,000;
cat. no. WL02549; Wanleibio Co., Ltd.). The secondary antibody
applied in this study was HRP-labeled Goat Anti-Rabbit IgG (H+L;
1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology). The
blot bands were visualized with enhanced chemiluminescence
detection reagent (cat. no. WP20005; Thermo Fisher Scientific,
Inc.) and analyzed by ImageJ software (version 1.54j; National
Institutes of Health).
Cell apoptosis and proliferation
analysis
To determine whether hPMSC-EVs could induce
apoptosis in 4T1 cells, Annexin V/propidium iodide (PI)
fluorescence-activated cell sorting (FACS) analysis was performed
with a FACS instrument (FACSCalibur; BD Biosciences). Single-cell
suspension of 4T1 cells was harvested after 24 h of EV treatment
and stained using an Annexin V/PI kit (cat. no. CA1040; Beijing
Solarbio Science & Technology Co., Ltd.) to assess the
apoptotic proportion of 4T1 cells. According to the manufacturer's
protocol, 100 µl of 4T1 cell suspension at a concentration of
1–5×106/ml was firstly stained with 5 µl Annexin V for 5
min at room temperature. Then, 5 µl PI (20 µg/ml) and 400 µl
phosphate-buffered saline (PBS) were added and detected
immediately. The PBS-treated 4T1 cells were used as control, and
the Annexin V−/PI− cells were identified as
live cells. The Annexin V+/PI− cells were
identified as early apoptotic cells, Annexin
V+/PI+ cells were identified as late
apoptotic cells, and Annexin V−/PI+ cells
were scored as necrotic cells. Data were analyzed by FlowJo 10
(FlowJo LLC). To investigate whether hPMSC-EVs affect cell
proliferation, 4T1 cells were treated with hPMSC-EVs and evaluated
by bioluminescence imaging.
Collection of conditioned medium
(CM)
For the collection of 4T1 CM, 4T1 cells were
primitively cultured in 1640 medium. When the confluence reached
40%, the old medium was replaced with a fresh complete medium
supplemented with 5 mg EVs. After 48 h, the supernatants were
harvested and stored at −80°C. For the collection of HUVEC-CM,
which served as a control in the present study, the complete HUVEC
medium was replaced with 7 ml of EGM-2 per T25 flask (Corning,
Inc.), with HUVECs also reaching 40% confluence. After 48 h, the
supernatants were collected and stored at −80°C.
Cell migration assay
Transwell assays were performed using Transwell
chambers through a filter membrane with the pore size of 8 µm
(MilliporeSigma). Briefly, 1×105 Fluc-GFP-labeled 4T1
cells immersed in 200 µl of RPMI 1640 medium supplemented with 2%
FBS were seeded in the upper inserts. RPMI 1640 supplemented with
10% FBS was added to the lower chamber. The low concentration of
FBS in the upper inserts prevents cell proliferation, consistent
with previous reports (22). After
24 h, non-migrated cells on the upper side of the filter were
removed with a cotton swab. The migrated cells were counted under a
confocal microscope at a magnification of 200× and five randomly
chosen fields were selected for each insert. The experiments were
repeated at least three times. For the wound healing assay, cells
were seeded in 6-well plates at a density of 2×105 per
well and grown to 90% confluence. Sterile 10 µl tips (Corning Life
Sciences) were used to create 2–3 straight wounds in each well,
followed by medium replacement and treatment with 100 µg/ml EV. In
this assay, similar to the Transwell assay, 10% FBS from RPMI 1640
was replaced with 2% low-concentration FBS to suppress 4T1 cell
proliferation. The images were captured and reported temporally,
with an interval of 12 h. The number of cells that migrated to the
wound area was calculated using ImageJ software (Version 1.54j;
National Institutes of Health).
Tube formation assay
HUVECs treated with hPMSC-EVs were subjected to a
tube formation assay. In brief, precooled Matrigel (BD Biosciences)
was pipetted into a 48-well plate, which was maintained at 4°C
overnight. Before the HUVECs were seeded in Matrigel-treated
48-well plates, they were placed in an incubator and rewarmed to
37°C. Afterwards, the HUVECs were seeded into 48-well plates at a
density of 5×104 cells/well, which were randomly divided
into two groups and cultured with EGM-2 medium. In particular, CM
from 4T1 cells treated with hPMSC-EVs was added to the medium of
HUVECs in the EV group. After culturing for 6 h, tube formation was
observed under a microscope (ECLIPSE Ti-U; Nikon Corporation); five
fields were chosen randomly and analyzed using ImageJ software
(Version 1.54j; National Institutes of Health).
Colony formation assay
4T1 cells were seeded into a six-well plate at 1,000
cells per well and subsequently treated with hPMSC-EVs at a
concentration of 100 µg/ml and an equal volume of PBS was also
repeatedly added to the control group every other day. After two
weeks, the cells were washed with PBS and then stained with crystal
violet and the colonies with more than 50 cells were counted and
analyzed.
Animal model
In the present study, 12 BALB/c mice (female, 8–12
weeks old) were purchased from Biocytogen. To monitor the
angiogenic effects of EVs in vivo in real time, transgenic
mice expressing firefly luciferase under the promoter of VEGFR2
(VEGFR2-luc) were obtained from Xenogen Corporation. All
experimental procedures were conducted according to institutional
guidelines for the Care and Use of Laboratory Animals at Nankai
University, Tianjin, China (23)
and in accordance with the National Institutes of Health (NIH)
Guide for the Care and Use of Laboratory Animals, 8th edition
(24). The mice were housed in a
specific pathogen-free environment, the temperature and humidity of
which were maintained at 21–23°C and 45–50%, respectively. With a
12-h light/dark cycle (lights on 07:00-19:00 h), the housing
facility supplied the mice with sufficient food and water. The
4T1-derived xenograft model was established according to the
following protocol. Mice were anesthetized with isoflurane, 2.0%
induction for 4 min, 1.5% maintenance and 1l/min oxygen inhalation
(19). For the animal model of the
4T1-derived xenograft model, BALB/c mice were inoculated with
1×106 4T1 (Fluc-eGFP) cells in the fourth pair of
mammary fat pads. Once the tumors were palpable, which could be
judged after ~4 days of tumor inoculation (day 0), the mice were
randomly divided into two groups (six mice per group).
In the experimental group, 100 µg of MSC-EVs were
peri-injected into the tumors on days 0, 2 and 4. In the control
group, a peritumoral injection of PBS was administered on days 0, 2
and 4. All mice were observed for tumor progression and treatment
effects by BLI for up to 14 days prior to sacrifice and tumor
tissues from the two groups were collected for histological
analysis. Under the same conditions, the VEGFR2-luc mice were
inoculated with unlabeled 4T1 cells (15,25)
and BLI was also performed on days 0, 4, 7, 10 and 14. On day 14,
mice were sacrificed by cervical dislocation. The tumors were
removed for further histological analysis. The humane endpoints
(e.g., severe distress, inability to eat or drink, significant
weight loss, behavioral abnormalities indicating suffering) were
used to determine whether animals should be sacrificed before the
end of the study.
In vivo BLI
BLI was performed in all experimental mice at
specific time points using the IVIS Lumina imaging system (Xenogen
Corporation). Fluc imaging of DF-labeled 4T1 cells was performed to
observe tumor progression and metastasis and assess the therapeutic
effects of hPMSC-EVs. For angiogenesis experiments in VEGFR2
transgenic mice, Fluc imaging was used to visualize VEGFR2
expression. Mice were anesthetized with isoflurane, 2.0% induction
for 4 min, 1.5% maintenance and 1l/min oxygen inhalation (19). After intraperitoneal injections of
reporter probe D-Luciferin (150 mg of luciferin/kg), animals were
imaged for 2 sec to 2 min. The BLI signal was quantified by the
average radiance of the region of interest over the xenograft tumor
using Living Image Software (version 4.7; PerkinElmer, Inc.)
(15,25).
Immunofluorescence staining
Immunofluorescence staining was performed on frozen
sections of 4T1 ×enograft tumor samples to detect neovasculature in
4T1 tumor tissue. Sections were incubated with primary antibodies
against CD31 (1:200; cat. no. 550274; BD Biosciences), Ki67 (1:200;
cat. no. 571599; BD Biosciences) for 2 h at room temperature,
followed by incubation with Alexa Fluor 594-labeled secondary
antibodies (1:500; cat. no. A21209; Invitrogen; Thermo Fisher
Scientific, Inc.) for 30 min at room temperature. The nuclei were
stained with DAPI for 5 min at room temperature. Fluorescence
images (magnification, ×200) were acquired with a microscope
(FV1000; Olympus Corporation) and analyzed with ImageJ software
(Version 1.54j; National Institutes of Health).
Reverse transcription-quantitative
(RT-q) PCR
To assess the mRNA expression levels of the analyzed
genes, total RNA was extracted from HUVECs at a confluence of
80–90% and tumor tissues treated with TRIzol® reagent
(cat. no. 15596018CN; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. First-strand cDNA was synthesized
using reverse transcriptase-mediated oligo-dT primers (cat. no.
11151ES60; Yeasen Biotech). Subsequently, RT-qPCR was performed on
an Opticon System (Bio-Rad Laboratories, Inc.) in 20 µl reaction
volumes. The level of mRNA expression was quantified using the
TransStart Top Green qPCR SuperMix Kit (cat. no. AQ131-01; TransGen
Biotech Co., Ltd.). The 2−ΔΔCq method was used to
determine the relative changes in mRNA folding (26). The primers and amplification
conditions are listed in the Tables
SI and SII.
Statistical analysis
All experiments were performed in triplicate for
each condition and repeated at least three times. The data are
presented as the means ± standard deviations (SDs). For comparisons
between two groups, two-tailed paired Student's t-tests were
performed. For comparisons among multiple groups, one-way ANOVA
followed by the least significant difference post hoc test was
used. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of hPMSC-derived EVs
(hPMSC-EVs)
The phenotype of hPMSCs was confirmed by surface
marker expression via FACS analysis (Fig. S1). EVs were isolated from the
supernatant of hPMSCs by ultracentrifugation and then characterized
by TEM, nanoparticle tracking analysis (NTA) and western blotting.
The enriched particles exhibited a typical cup-shaped morphology
under TEM (Fig. 1A) and relatively
heterogeneous sizes of ~120 nm in diameter (Fig. 1B). Western blot analysis confirmed
the expression of classical EV markers (TSG101, ALIX and CD63) in
hPMSC-EVs (Figs. 1C and S2), which is consistent with a previous
report (20).
hPMSCs-EVs inhibit cell proliferation
but not apoptosis
A recent study reported that MSCs and their EVs can
be tumorigenic or antitumorigenic by affecting multiple steps of
tumor progression (6). To
determine the effects of hPMSC-EVs on breast cancer, 4T1 cells were
labeled with an Fluc-GFP double fusion reporter gene (Fig. S3) and apoptosis and proliferation
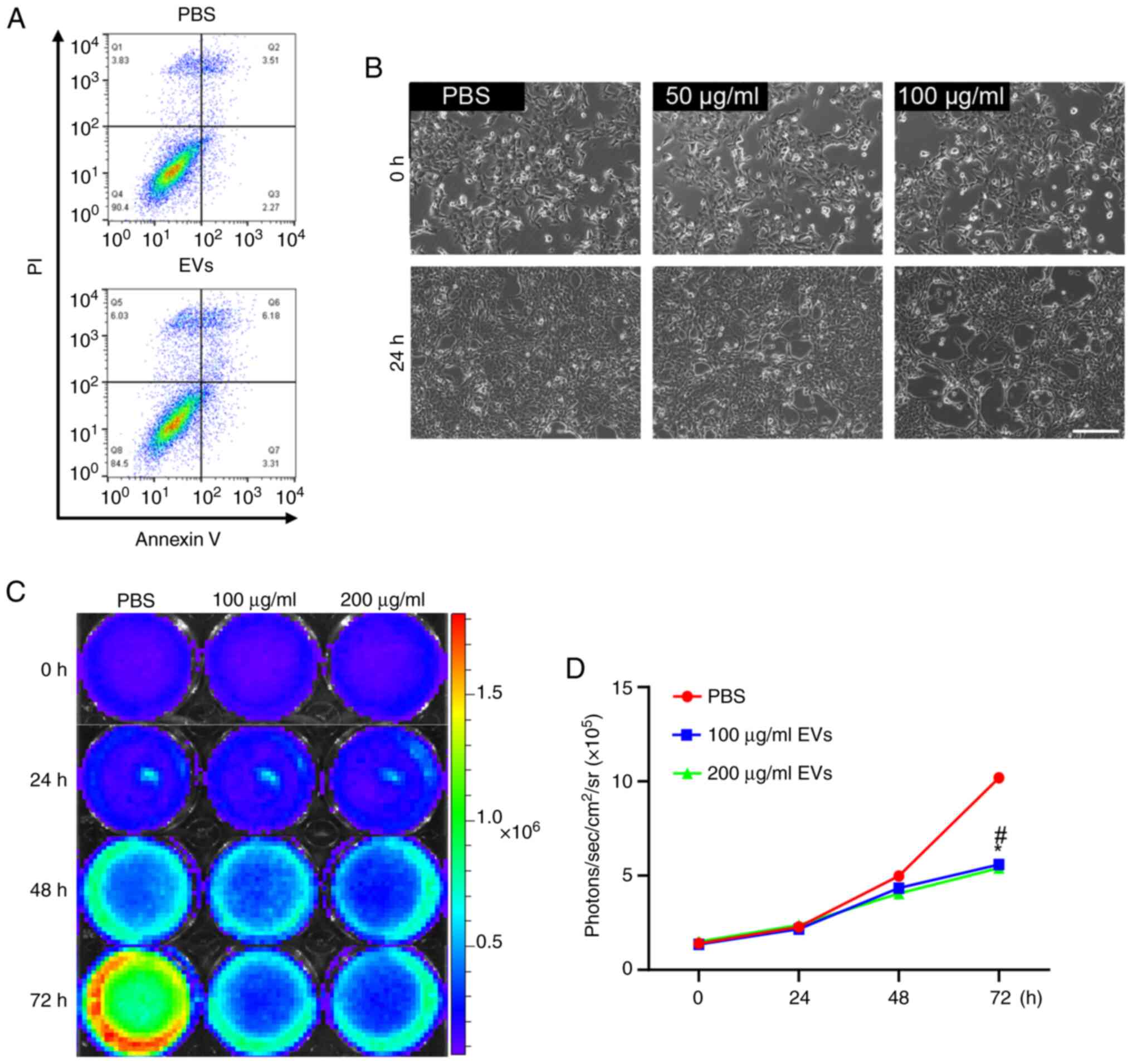
were assessed after treatment with MSC-EVs. Annexin V and PI FACS
analysis was performed (Fig. 2A),
which revealed that hPMSC-EV treatment did not induce apoptosis in
cultured 4T1 cells; however, changes in cell morphology, the
elongation of some cells with filamentous ends and a decrease in
cell number, were detected via brightfield microscopy, indicating
that cell status was adversely affected by EV treatment (Fig. 2B). The morphology of 4T1 cells
induced by EVs needs to be clarified to exclude the possibility
that it is induced by cell-cell contact. Under identical
experimental conditions, control group exhibited rapid 4T1 cell
proliferation and increased cell-cell contact. According to the
results, the effect of cell-cell contacts during proliferation
tended to make the cells more tightly packed. The proportion of
cells with elongated, filamentous ends was significantly higher in
the EV group compared with the control group. The EV group
exhibited significantly lower cell density and confluence compared
with the control group, suggesting a diminished role of cell-cell
contact in influencing 4T1 cell morphology. In summary, the
morphological changes in 4T1 cells may be the result of both
cell-cell contact and EV treatment and the role of EVs may be the
primary factor involved. Concurrently, the hPMSC-EV-mediated
suppression of the proliferation of other breast cancer cells, such
as MCF-7 cells, was also validated. Consistent results were
revealed using EVs from human umbilical cord MSCs (hUCMSCs), which
validated the universality of the present study (Figs. S4 and S5). Furthermore, under BLI, EV treatment
led to a significantly reduced Fluc signal, indicating that cell
viability was decreased and that cellular proliferation was
inhibited (Fig. 2C and D).
CM from hPMSC-EV-treated 4T1 cells
weakens the angiogenic potential of HUVECs
Angiogenesis is the basis for solid tumor growth and
metastasis. Blocking the formation of new blood vessels in solid
tumors has proven to be a highly efficient anticancer strategy. The
present study explored the effects of hPMSC-EVs on tumor
angiogenesis using HUVECs, a widely used model system for
researches related to tumor vascular biology. First, the
supernatant of 4T1 cells that had been pretreated with EV for 24 h
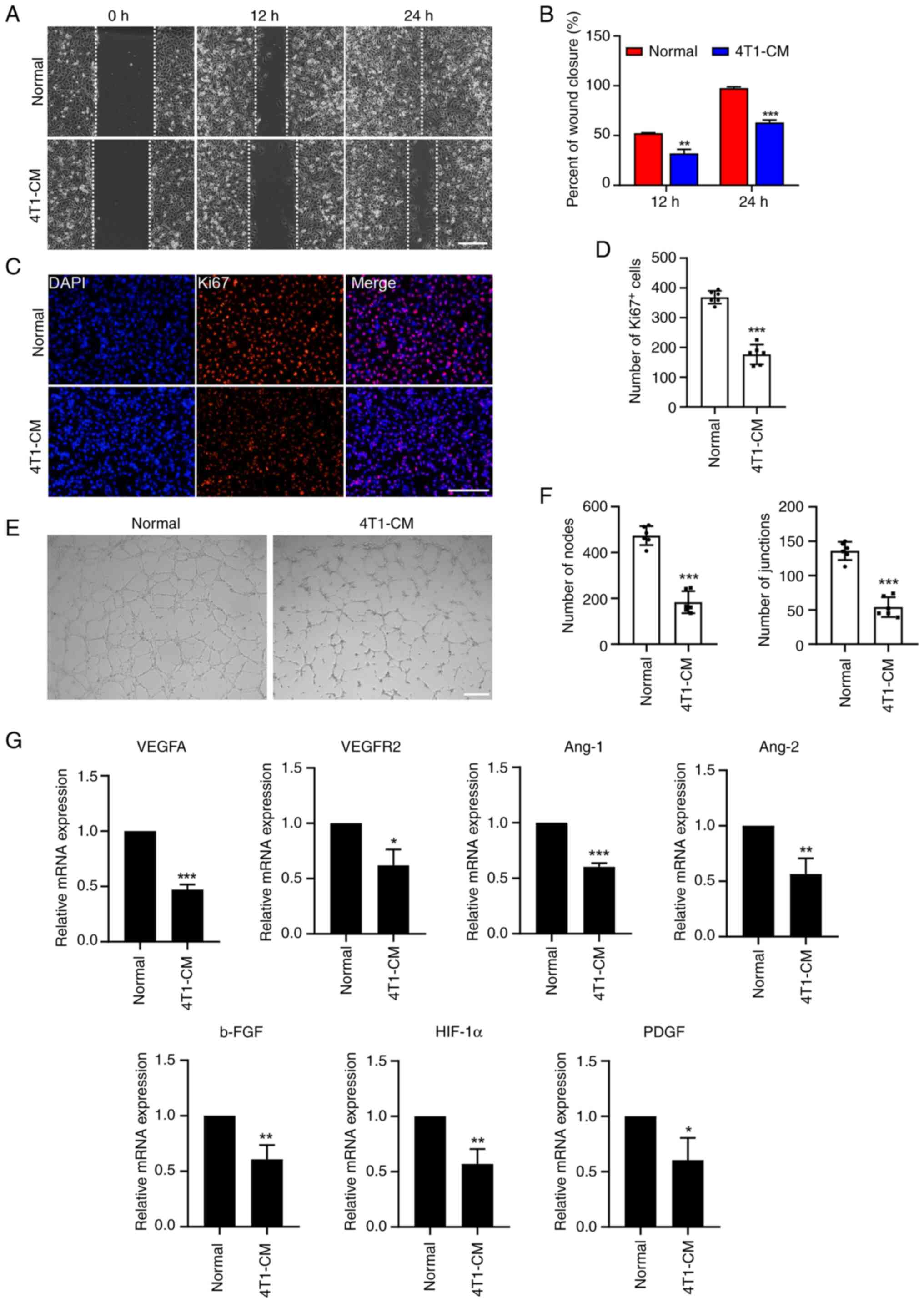
was collected as CM. Then, a wound healing assay was performed in
HUVECs and the results showed that 4T1-CM led to decreased cell
motility in HUVECs (Fig. 3A and
B). Similarly, the Ki-67 immunofluorescence assay showed that
4T1-CM reduced the number of proliferating endothelial cells
(Fig. 3C and D). In addition,
Matrigel was used to detect the tube formation of endothelial
cells. By counting nodes and junctions, it was found that 4T1-CM
treatment markedly reduced HUVEC tube formation (Fig. 3E and F). Furthermore, RT-qPCR
revealed that the expression of angiogenesis-related genes (VEGFA,
VEGFR2, Ang-1, Ang-2, b-FGF, HIF-1α and PDGF) was significantly
downregulated in HUVECs following 4T1-CM treatment (Fig. 3G). These results indicated that
hPMSC-EVs can indirectly reduce the neovasculature of 4T1
tumors.
hPMSC-EVs reduced 4T1 cell migration
and colony formation
Tumor metastasis occurs when tumor cells migrate
from one site to another and stably proliferate. The migration and
colony formation abilities of tumor cells indicate a metastatic
phenotype, which is closely related to a poor prognosis. The
results of the present study revealed that, according to the wound
healing assay, 4T1 cell migration was significantly suppressed by
EV treatment (Fig. 4A and B).
Consistent results were obtained in the Transwell assay, showing a
decrease in the migration of GFP-labeled 4T1 cells (Fig. 4C and D). The colony formation assay
also revealed decreased colony formation after EV treatment
(Fig. 4E and F). These findings
suggested that hPMSC-derived EVs may suppress the migration of 4T1
breast cancer cells.
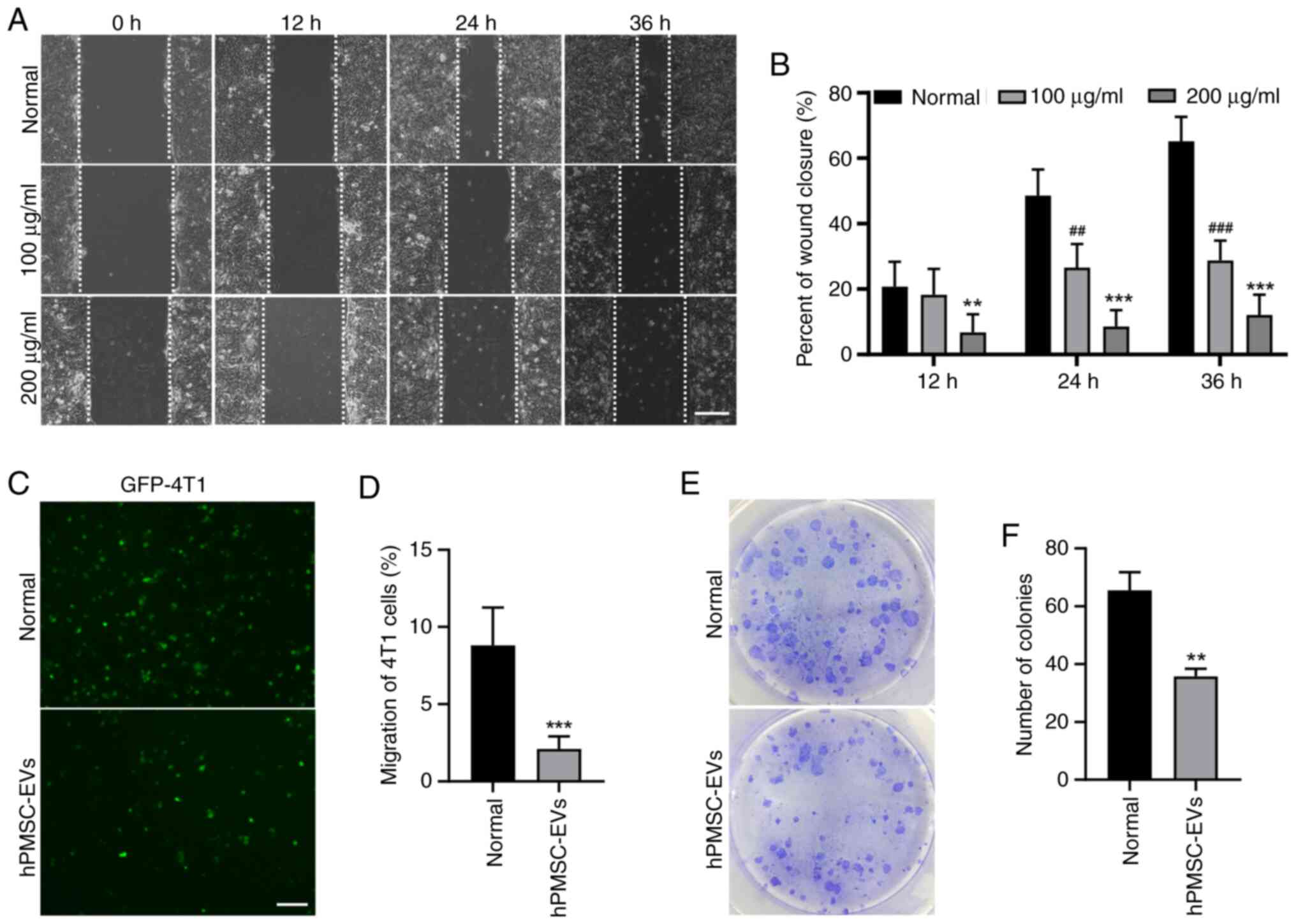 | Figure 4.hPMSC-EVs inhibited 4T1 cell
migration and colony formation in vitro. (A) Representative
images of the wound healing assay of 4T1 cells treated with 100/200
µg/ml EVs. Scale bar, 100 µm. (B) The wound healing area per field
was reduced following EV treatment. n=3, ##P<0.01,
###P<0.001, 100 µg/ml EVs vs. normal group,
**P<0.01, ***P<0.001, 200 µg/ml EVs vs. normal group. (C)
Transwell assays showed that 4T1 cell migration was reduced after
treatment with 100 µg/ml EV. Scale bar, 200 µm. (D) Number of
migratory cells calculated using ImageJ software. ***P<0.001. (E
and F) The effects of EVs on the independent viability of 4T1
cells, as shown by colony formation experiments. n=3, **P<0.01.
hPMSC-EVs, human placenta MSC-derived extracellular vesicles; EVs,
extracellular vesicles. |
hPMSC-EVs suppress tumor growth
As the aforementioned assays revealed that
hPMSCs-EVs could inhibit the growth and migration of 4T1 cells
in vitro, the present study next performed in vivo
studies for further verification by BLI. A murine model of breast
cancer was established by subcutaneously transplanting
106 Fluc/GFP-labeled 4T1 cells into BALB/c mice. BLI
showed that the number of tumor cells was reduced and that tumor
progression was inhibited by the local administration of
hPMSC-derived EVs (Fig. 5A and B).
The mice were sacrificed 14 days following hPMSC-EV treatment and
the tumors were harvested. Morphologically, the tumors in the EV
treatment group were smaller than those in the control group
(Fig. 5C). This finding was
verified by further statistical analysis of tumor volume and weight
(Fig. 5D and E). Furthermore,
immunofluorescence staining of the Ki67 protein, a classical tumor
cell proliferation marker, revealed a significant decrease in the
number of cells in the proliferating state and the quantification
analysis also showed an identical trend (Fig. 5F and G). No toxicity of hPMSCs-EVs
was observed and all animals reached endpoints of the present
study.
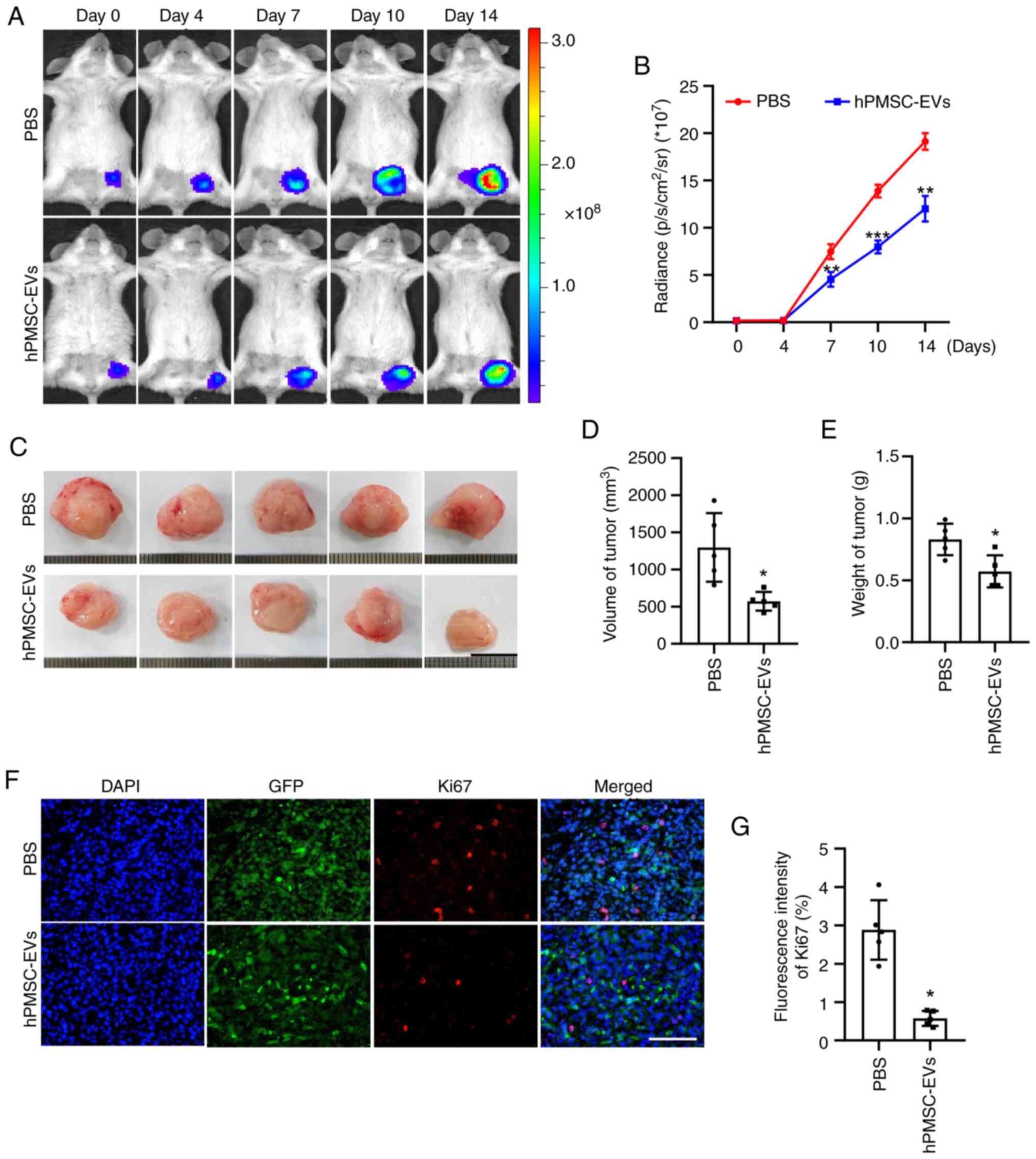 | Figure 5.Inhibitory effect of hPMSC-EVs on
tumor growth in vivo. (A) Firefly luciferase (Fluc) imaging
of tumor growth. Representative animals were injected with
1×106 4T1 cells (Fluc/GFP). (B) Quantitative analysis of
the Fluc signal, n=3. (C) Representative images of tumors isolated
from sacrificed mice after 14 days of EV treatment. Scale bar, 1
cm. Quantitative analysis of tumor (D) volume and (E) weight, n=5.
(F) Representative image of Ki-67 immunofluorescence staining in
4T1 cells. Scale bar, 100 µm. (G) Quantitative analysis of Ki-67
expression in 4T1 cells. n=5, PBS, *P<0.05, **P<0.01,
***P<0.001 vs. PBS. hPMSC-EVs, human placenta MSC-derived
extracellular vesicles; EVs, extracellular vesicles. |
hPMSCs-EVs suppress angiogenesis in
4T1 breast tumors
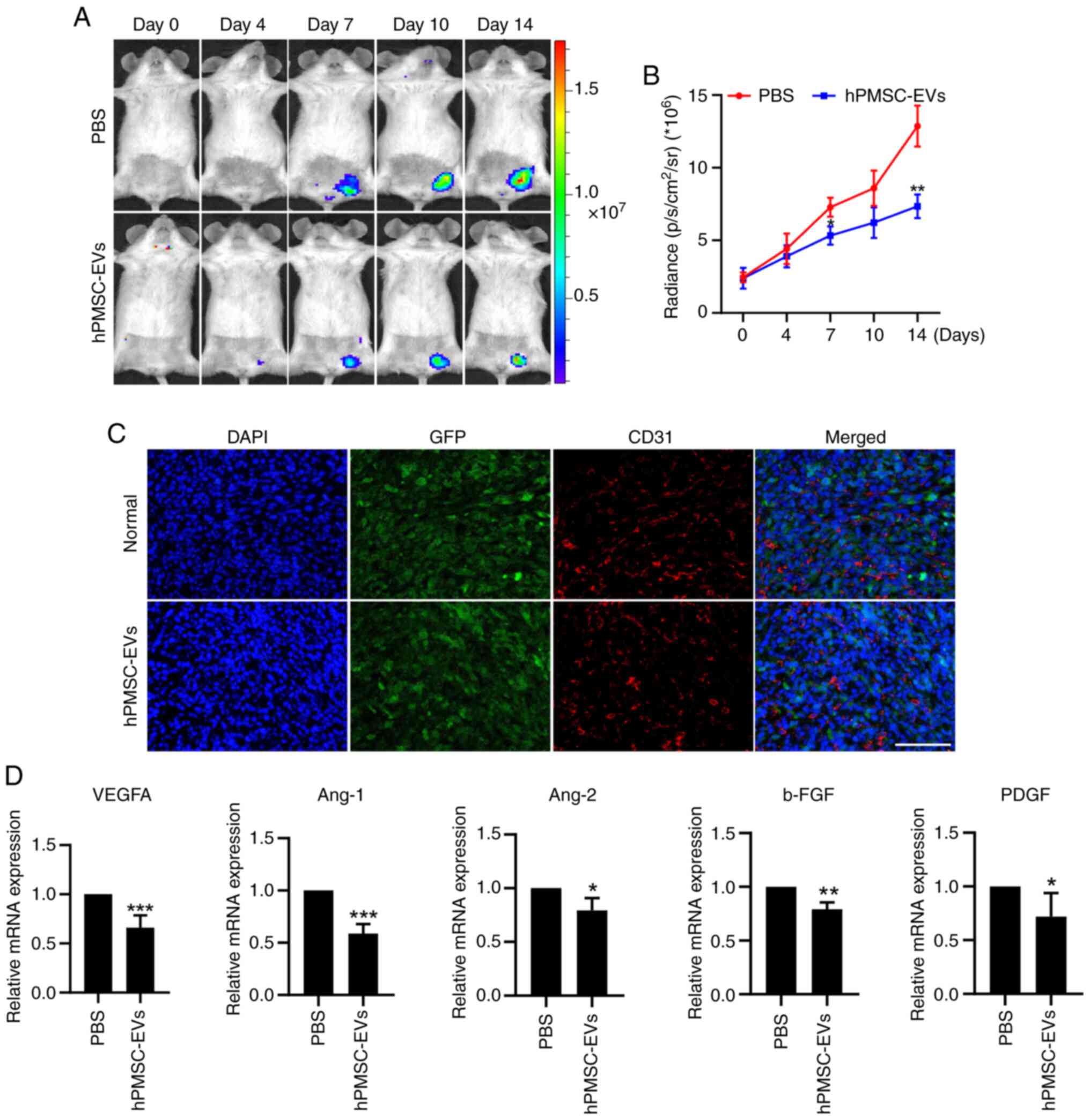
VEGFR2-Fluc transgenic mice were subjected to
angiogenesis observation via BLI at tumor sites. After hPMSC-EVs
were administered, there was a dramatic decrease in VEGFR2
expression (Fig. 6A and B). CD31
immunofluorescence staining was performed after the mice were
sacrificed and the tumors were removed, showing that EV treatment
led to a reduction in the number of new blood vessels at the tumor
sites (Fig. 6C). Then, RNA was
extracted from tumor tissues and RT-qPCR revealed that the
expression of the angiogenesis-related genes, VEGFA, Ang-1, Ang-2,
b-FGF, and PDGF, was significantly downregulated at tumor sites
(Fig. 6D). These results indicated
that MSC-EVs suppressed angiogenesis in 4T1 breast tumors.
Discussion
MSC-EVs have been identified as crucial paracrine
regulators in a number of physiological and pathological processes
(5). With a strong tendency to
migrate to injury and tumor sites in vivo, MSC-EVs hold
great promise as cell-free therapeutic approaches for a variety of
medical conditions (27,28). However, the effects of MSC-EVs on
tumor development and progression remain controversial (6,29).
Some studies have shown proliferative effects, while others have
demonstrated inhibitory effects of MSC-EVs on tumors (30,31).
This difference in function could be attributed to the different
tissue origins of MSCs and tumor types. The placenta is a
neonatal-related proliferative tissue, usually discarded after
fetal birth, in which MSCs are abundant. Placenta-derived MSCs are
more proliferative, with an extended cell cycle and greater
differentiation potential. Notably, compared with that of other
MSCs, the wider antitumor effect of MSCs is hypothesized to be
associated with the ability of the placenta to filter out harmful
substances in the bloodstream of the mother (31,32).
Therefore, placenta-derived MSC-EVs can act as candidates for
antitumor therapies (7,33,34).
The present study revealed that human
placenta-derived MSC-EVs had strong antitumor effects on 4T1 breast
cancer models both in vitro and in vivo. hPMSC-EVs
decreased the proliferation, migration and colony formation of
cultured 4T1 cells but did not induce cell apoptosis. The
angiogenic potential of HUVECs was also weakened following exposure
to CM from 4T1 cells pretreated with hPMSC-EVs. In 4T1
tumor-bearing mouse models, systemic administration of hPMSC-EVs
resulted in impaired tumor growth, decreased vascularization and
downregulated expression of angiogenesis-related genes. These
findings indicated that hPMSC-EVs are antitumorigenic and may be
good candidates for therapeutic alternatives for the treatment of
breast cancer (35).
The tumor neovasculature is crucial for maintaining
the survival and growth of solid tumors (35). MSC-EVs have been reported to
modulate tumor vascularization in a positive or negative manner,
similar to their bidirectional effect on tumor progression
(36). The present study revealed
that hPMSC-EVs inhibited tumor neovasculature, which may indicate
that EVs, which contain abundant active cargoes, modify the
function of 4T1 cells and lead to decreased release of
proangiogenic factors. The major active components in hPMSC-EVs
responsible for the antitumor effects need to be elucidated. On the
one hand, the delayed tumor progression observed in the present
study was attributed to multiple factors, including the reduced
proliferation of 4T1 breast cancer cells, the weakened migration
and tube formation ability of HUVECs and decreased angiogenesis.
This pathological phenomenon, as a direct or indirect result of
hPMSC-EV effects on 4T1 cells or breast cancer cells, is associated
with several mechanisms. However, the inducers that lead to the
occurrence of the aforementioned pathological phenomenon may be
numerous and not completely identical. The results of the present
study supported the diversity and complexity of the biological
components of hPMSC-EVs. The cargo carried by EVs, including DNA,
RNA, miRNA and other molecules, is abundant. Therefore, by
combining the multiple medical effects of hPMSC-EVs on 4T1-based
breast cancer cells and the complexity of the active factors
contained in EVs, the anticancer effect of hPMSC-EVs may be exerted
through the synergetic interaction of numerous active factors. As
mentioned in the introduction, the potential applications of MSCs
in cancer treatment are an area of active research and the outcomes
and mechanisms involved are complex and can vary depending on
several factors, including the type of cancer, the microenvironment
and the specific characteristics of the MSCs used. Thus, the
interaction between EVs and the microenvironment of the cancer nest
may be one of the major factors that boosts the anticancer effect.
In conclusion, multiple factors may be involved in the anticancer
effect of hPMSC-EVs, the synergetic interaction of which commonly
suppresses breast cancer progression. Furthermore, compared with
the absence of apoptosis, female menstruation is a highly important
angiogenic process in organs related to pregnancy and this
regulation could also be extended to stromal cells and cell
products, which seems to support the notable antiangiogenic effect
of hPMSC-EVs, as observed in the present study.
The present study, is the first to report, to the
best of the authors' knowledge, that non-modified hPMSC-EVs acted
as antitumor agents for 4T1-derived breast cancer and to reveal
several mechanisms related to their actions. 4T1 represents a
triple-negative, highly malignant murine breast cancer cell line
that is normally used for standardized animal models of human
breast cancer. MCF-7, MDA-231 and other human-derived breast cancer
cell lines that need an immune suppressor environment to grow into
xenograft tumors, may lead to unreliable results because MSC-EVs
are also deeply involved in the process of immune regulation by
nature, while the 4T1 cell line can avoid these concerns, as it can
grow in immunocompetent rodents.
In summary, the present study explored the effects
of hPMSC-EVs on 4T1 breast cancer cells and revealed that tumor
cell growth could be suppressed both in vitro and in
vivo by inhibiting cellular proliferation and tumor
angiogenesis. This significant finding indicated that if
reproducible in humans, hPMSC-EVs could be clinically exploited for
their antitumor potential and could represent a promising strategy
for the treatment of breast cancer.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was partially supported by the Tianjin Natural
Science Foundation (grant nos. 22JCZXJC00170 and 21JCZDJC00070),
Open Funding from the Nankai University Eye Institute (grant no.
NKYKD202203) and the Tianjin Key Medical Discipline (Specialty)
Construction Project (grant no. TJYXZDXK-043A).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
HW and ZL were responsible for conceptualization.
MZ, HL, JZ and QZ were responsible for the acquisition of data. MZ,
HL and LZ were responsible for formal analysis. HW and ZJ were
responsible for methodology. ZL and LZ were responsible for project
administration and supervision. ZH and ZCH were responsible for
validation and visualization. MZ and HL wrote the original draft of
the manuscript and ZL and LZ reviewed and edited the manuscript.
MZ, HL and ZL confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee for the Use of Animals of
Nankai University approved the experimental protocols for studies
of EVs for cancer therapy (approval no. 20210022; date of approval:
February 23, 2021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests. AmCellGene Co., Ltd. had no commercial interest in the
present study.
References
|
1
|
Al-Awsi GRL, Alsaikhan F, Margiana R,
Ahmad I, Patra I, Najm MAA, Yasin G, Rasulova I, Hammid AT, Kzar
HH, et al: Shining the light on mesenchymal stem cell-derived
exosomes in breast cancer. Stem Cell Res Ther. 14:212023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wan W, Miao Y, Niu Y, Zhu K, Ma Y, Pan M,
Ma B and Wei Q: Human umbilical cord mesenchymal stem cells
conditioned medium exerts anti-tumor effects on KGN cells in a cell
density-dependent manner through activation of the Hippo pathway.
Stem Cell Res Ther. 14:462023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bae J, Liu L, Moore C, Hsu E, Zhang A, Ren
Z, Sun Z, Wang X, Zhu J, Shen J, et al: IL-2 delivery by engineered
mesenchymal stem cells re-invigorates CD8+ T cells to
overcome immunotherapy resistance in cancer. Nat Cell Biol.
24:1754–1765. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen F, Zhong X, Dai Q, Li K, Zhang W,
Wang J, Zhao Y, Shen J, Xiao Z, Xing H and Li J: Human umbilical
cord MSC delivered-soluble TRAIL inhibits the proliferation and
promotes apoptosis of B-ALL cell in vitro and in vivo.
Pharmaceuticals (Basel). 15:13912022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou X, Zhang W, Liu Y, Zhang L and Li Z:
Chapter 5-Mesenchymal stem cells: A promising weapon for cancer
therapy. Zhang L, Han Z, Wang J, Li Z and Huang Q: Mesenchymal Stem
Cells. Academic Press; pp. 119–141. 2023, View Article : Google Scholar
|
|
6
|
Wang J, Ma Y, Long Y and Chen Y:
Extracellular vesicle derived from mesenchymal stem cells have
bidirectional effects on the development of lung cancer. Front
Oncol. 12:9148322022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eiro N, Fraile M, Fernández-Francos S,
Sánchez R, Costa LA and Vizoso FJ: Importance of the origin of
mesenchymal (stem) stromal cells in cancer biology: ‘Alliance’ or
‘war’ in intercellular signals. Cell Biosci. 11:1092021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Z, Xie W, Luo T, Hu Z, Hua J, Zhou J,
Yang T, Wang W, Song Z, Yu X, et al: Exosomes from TNF-α
preconditioned human umbilical cord mesenchymal stromal cells
inhibit the autophagy of acinar cells of severe acute pancreatitis
via shuttling bioactive metabolites. Cell Mol Life Sci. 80:2572023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee MW, Ryu S, Kim DS, Lee JW, Sung KW,
Koo HH and Yoo KH: Mesenchymal stem cells in suppression or
progression of hematologic malignancy: Current status and
challenges. Leukemia. 33:597–611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia C, Wang T, Cheng H, Dong Y, Weng Q,
Sun G, Zhou P, Wang K, Liu X, Geng Y, et al: Mesenchymal stem cells
suppress leukemia via macrophage-mediated functional restoration of
bone marrow microenvironment. Leukemia. 34:2375–2383. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li R, Wang C, Zhou M, Liu Y, Chen S, Chai
Z, Huang H, Zhang K, Han Z, Hua G, et al: Heparan sulfate
proteoglycan-mediated internalization of extracellular vesicles
ameliorates liver fibrosis by targeting hepatic stellate cells.
Extracellul Vesicle. 1:1000182022. View Article : Google Scholar
|
|
12
|
Zhao J, Lin H and Huang K: Mesenchymal
stem cell-derived extracellular vesicles transmitting
MicroRNA-34a-5p suppress tumorigenesis of colorectal cancer through
c-MYC/DNMT3a/PTEN axis. Mol Neurobiol. 59:47–60. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You B, Jin C, Zhang J, Xu M, Xu W, Sun Z
and Qian H: MSC-derived extracellular vesicle-delivered L-PGDS
inhibit gastric cancer progression by suppressing cancer cell
stemness and STAT3 phosphorylation. Stem Cells Int.
2022:96682392022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang S, Wang L, Gu L, Wang Z, Wang Y, Wang
J and Zhang Y: Mesenchymal stem cell-derived extracellular vesicles
alleviate cervical cancer by delivering microRNA-331-3p to reduce
LIM zinc finger domain containing 2 methylation in tumor cells. Hum
Mol Genet. 31:3829–3845. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su W, Wang L, Zhou M, Liu Z, Hu S, Tong L,
Liu Y, Fan Y, Kong D, Zheng Y, et al: Human embryonic stem
cell-derived endothelial cells as cellular delivery vehicles for
treatment of metastatic breast cancer. Cell Transplant.
22:2079–2090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arnold M, Morgan E, Rumgay H, Mafra A,
Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S
and Soerjomataram I: Current and future burden of breast cancer:
Global statistics for 2020 and 2040. Breast. 66:15–23. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z and Han ZC: Introduction of perinatal
tissue-derived stem cells. Han ZC, Takahashi TA, Han Z and Li Z:
Perinatal Stem Cells: Biology, Manufacturing and Translational
Medicine. Singapore: Springer Singapore; pp. 1–7. 2019
|
|
18
|
Zhang K, Li R, Chen X, Yan H, Li H, Zhao
X, Huang H, Chen S, Liu Y, Wang K, et al: Renal endothelial
cell-targeted extracellular vesicles protect the kidney from
ischemic injury. Adv Sci (Weinh). 10:e22046262023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hezam K, Wang C, Fu E, Zhou M, Liu Y, Wang
H, Zhu L, Han Z, Han ZC, Chang Y and Li Z: Superior protective
effects of PGE2 priming mesenchymal stem cells against LPS-induced
acute lung injury (ALI) through macrophage immunomodulation. Stem
Cell Res Ther. 14:482023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia P, Zhao X, Liu Y, Liu M, Zhang Q, Chen
S, Huang H, Jia Y, Chang Y, Chen S, et al: The RGD-modified
self-assembling D-form peptide hydrogel enhances the therapeutic
effects of mesenchymal stem cells (MSC) for hindlimb ischemia by
promoting angiogenesis. Chem Eng J. 450:1380042022. View Article : Google Scholar
|
|
21
|
Li H, Huang H, Chen X, Chen S, Yu L, Wang
C, Liu Y, Zhang K, Wu L, Han ZC, et al: The delivery of
hsa-miR-11401 by extracellular vesicles can relieve
doxorubicin-induced mesenchymal stem cell apoptosis. Stem Cell Res
Ther. 12:772021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang K, Zhao X, Chen X, Wei Y, Du W, Wang
Y, Liu L, Zhao W, Han Z, Kong D, et al: Enhanced therapeutic
effects of mesenchymal stem cell-derived exosomes with an
injectable hydrogel for hindlimb ischemia treatment. ACS Appl Mater
Interfaces. 10:30081–30091. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Hezam K, Fu E, Pan K, Liu Y and Li
Z: In vivo tracking of mesenchymal stem cell dynamics and
therapeutics in LPS-induced acute lung injury models. Exp Cell Res.
437:1140132024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . The National Academies Collection: Reports funded by
National Institutes of Health. Guide for the Care and Use of
Laboratory Animals. 8th edition. Washington (DC): National
Academies Press (US). Copyright © 2011, National Academy of
Sciences. 2011, PubMed/NCBI
|
|
25
|
Zhou M, Wang L, Su W, Tong L, Liu Y, Fan
Y, Luo N, Zheng Y, Zhao H, Xiang R and Li Z: Assessment of
therapeutic efficacy of liposomal nanoparticles mediated gene
delivery by molecular imaging for cancer therapy. J Biomed
Nanotechnol. 8:742–750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moradi-Chaleshtori M, Bandehpour M,
Heidari N, Mohammadi-Yeganeh S and Mahmoud Hashemi S:
Exosome-mediated miR-33 transfer induces M1 polarization in mouse
macrophages and exerts antitumor effect in 4T1 breast cancer cell
line. Int Immunopharmacol. 90:1071982021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang M, Li J, Wang D, Xin Y and Liu Z: The
effects of mesenchymal stem cells on the chemotherapy of colorectal
cancer. Biomed Pharmacother. 160:1143732023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong IS, Lee HY and Kang KS: Mesenchymal
stem cells and cancer: Friends or enemies? Mutat Res. 768:98–106.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rahimi Tesiye M, Abrishami Kia Z and
Rajabi-Maham H: Mesenchymal stem cells and prostate cancer: A
concise review of therapeutic potentials and biological aspects.
Stem Cell Res. 63:1028642022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Shen M, Wu L, Yang H, Yao Y, Yang
Q, Du J, Liu L, Li Y and Bai Y: Stromal cells in the tumor
microenvironment: Accomplices of tumor progression? Cell Death Dis.
14:5872023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li T, Zhang C, Ding Y, Zhai W, Liu K, Bu
F, Tu T, Sun L, Zhu W, Zhou F, et al: Umbilical cord-derived
mesenchymal stem cells promote proliferation and migration in MCF-7
and MDA-MB-231 breast cancer cells through activation of the ERK
pathway. Oncol Rep. 34:1469–1477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du L, Tao X and Shen X: Human umbilical
cord mesenchymal stem cell-derived exosomes inhibit migration and
invasion of breast cancer cells via miR-21-5p/ZNF367 pathway.
Breast Cancer. 28:829–837. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bailey AJM, Tieu A, Gupta M, Slobodian M,
Shorr R, Ramsay T, Rodriguez RA, Fergusson DA, Lalu MM and Allan
DS: Mesenchymal stromal cell-derived extracellular vesicles in
preclinical animal models of tumor growth: Systematic review and
meta-analysis. Stem Cell Rev Rep. 18:993–1006. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aravindhan S, Ejam SS, Lafta MH, Markov A,
Yumashev AV and Ahmadi M: Mesenchymal stem cells and cancer
therapy: Insights into targeting the tumour vasculature. Cancer
Cell Int. 21:1582021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo T, von der Ohe J and Hass R:
MSC-Derived extracellular vesicles in tumors and therapy. Cancers
(Basel). 13:52122021. View Article : Google Scholar : PubMed/NCBI
|