Introduction
Periodontitis is a chronic inflammatory disorder of
the periodontium characterized by bacterial infection, as well as
the loss of attached gum and alveolar bone, which is manifested by
destruction of periodontal tissues (1). Periodontal nursing intervention and
standard self-plaque control can effectively reduce the number of
periodontal pathogenic microorganisms in the dental plaque, which
is crucial for reducing disease progression and recurrence
(2). Human periodontal ligament
stem cells (PDLSCs) derived from periodontal tissue are one of the
main functional cells for periodontal tissue regeneration and
repair (3,4). As ideal seed cells for periodontal
tissue engineering, PDLSCs have a high proliferative capacity and
multi-directional differentiation potential, which allows them to
differentiate into osteoblasts to repair the damaged alveolar bone
and periodontal ligament in periodontitis (5,6).
There is a growing body of evidence to suggest that the periodontal
inflammatory microenvironment caused by the increased oral
bacterial burden can affect the bone regenerative ability of PDLSCs
(7,8). Thus, inhibiting the inflammatory
damage and enhancing the osteogenic differentiation ability of
PDLSCs may prove to be a promising approach for the treatment of
periodontitis.
Macrophages exert significant effects on the immune
response during the occurrence, development and resolution of
periodontitis. Due to the plasticity and diversity of macrophages,
naive macrophages (M0) can differentiate into type M1 and type M2
macrophages (9). M1 macrophages
can regulate osteoclast activation and secrete a large number of
pro-inflammatory cytokines involved in the progression of
periodontitis, including TNF-α) IL-1β and IL-6 (10). By contrast, M2 macrophages restrain
pro-inflammatory cytokine production and facilitate tissue repair
and regeneration (11). Vesicles
from lipopolysaccharide (LPS)-stimulated PDLSCs promote the
conversion of macrophages to the M1 type and facilitate the release
of inflammatory factors (12). It
has also been reported that LPS-stimulated PDLSCs can induce the M1
polarization of macrophages (13).
Therefore, the inhibition of M1 polarization of macrophages may be
a key strategy for the treatment of periodontitis.
Wnt7B, as one of the crucial ligands in mammalian
Wnt signaling, exhibits an expression in the process of tooth
development by in situ hybridization (14). There is compelling evidence to
indicate that Wnt7B-mediated enhanced osteoblast activity is one of
the reasons for strengthened bone formation (15). Wnt7B has been demonstrated to
promote the self-renewal and osteogenic differentiation of bone
marrow-derived mesenchymal stem cells and rescue
glucocorticoid-induced bone loss (16,17).
In particular, it has been shown that Wnt7B overexpression in bone
marrow macrophage lineage cells notably disrupts osteoclast
formation and activity, leading to a sharp increase in bone mass
(17). It is widely accepted that
frizzled4 (FZD4), a class FZD G-protein-coupled receptor at the
cell membrane, can be combined with Wnt7B. It should be noted that
the decrease in the expression of FZD4 inhibits the osteogenic
differentiation of cyclic stretch-induced PDLSCs (18). By regulating FZD4 expression,
interferon (IFN)γ-treated macrophages participate in the process of
Crohn's disease (19). Therefore,
the present study aimed to explore whether Wnt7B/FZD4 is involved
in promoting the osteogenic differentiation of PDLSCs and in the
regulation of the polarization of macrophages under inflammatory
conditions.
Materials and methods
Bioinformatics analysis
Wnt7B expression in the periodontitis-affected
gingival tissue of patients (n=3) and healthy gingival tissue of
patients (n=3) was analyzed using the GSE23586 dataset (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi). The
platform is GPL570. The binding between Wnt7B and FZD4 was analyzed
using the STRING database (https://cn.string-db.org/).
Cell culture and treatment
PDLSCs (iCell Bioscience; http://www.icellbioscience.com/cellDetail/74) and
the human peripheral blood monocyte cell line (THP-1; BeNa Culture
Collection) were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS (Thermo Fisher Scientific, Inc.) in a
humidified incubator with 5% CO2 at 37°C. To induce
inflammatory conditions, the PDLSCs were treated with 10 µg/ml LPS
(Sigma-Aldrich; Merck KGaA) for 24 h (20). Osteoblast differentiation was
induced by culturing the PDLSCs in α-MEM medium (VivaCell
Biosciences) containing 10% FBS (Thermo Fisher Scientific, Inc.),
50 µg/ml l-ascorbic acid (Sigma-Aldrich; Merck KGaA), 10 nM
dexamethasone (Sigma-Aldrich; Merck KGaA) and 10 mM
β-glycerophosphate (Sigma-Aldrich; Merck KGaA) for 14 days in a
37°C incubator with 5% CO2 (21). To generate M0 macrophages (M0),
THP-1 cells were treated with 100 ng/ml phorbol 12-myristate
13-acetate (MilliporeSigma) for 48 h.
Transfection
For transfection, the Wnt7B overexpression plasmid
(Ov-Wnt7B), the empty vector plasmid (Ov-NC), FZD4-labeled short
hairpin RNA (shRNA; shRNA-FZD4-1, sense,
5′-GAGTCTGAACTGCAGCAAATT-3′, antisense,
5′-AATTTGCTGCAGTTCAGACTC-3′; shRNA-FZD4-2, sense,
5′-GGTCATGAAGCCATTGAAATG-3′, antisense,
5′-CATTTCAATGGCTTCATGACC-3′) or the nonsense negative control
(shRNA-NC, sense, 5′-CTCGTATTCTCCTGCAGCATG-3′, antisense,
5′-CATGCTGCAGGAGAATACGAG-3′) were produced by Sangon Biotech Co.,
Ltd. When cell confluency reached 70%, transfection experiments
were carried out with the application of Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h at 37°C.
At 48 h following transfection, western blot analysis was employed
to measure the transfection efficiency.
Measurement of alkaline phosphatase
(ALP) activity
Following transfection, the PDLSCs were incubated at
37°C in osteogenic differentiation medium with or without LPS for 7
days. The PDLSCs were then lysed with ice-cold RIPA buffer
(Beyotime Institute of Biotechnology) and centrifuged (600 × g) for
10 min at 4°C to obtain the supernatant. The supernatant was used
to examine ALP activity using the ALP Assay Kit (Beyotime Institute
of Biotechnology). The absorbance was quantified at 405 nm under a
microplate reader (Bio-Rad Laboratories, Inc.).
Alizarin Red S (ARS) staining
The mineralization of the PDLSCs following the
indicated treatments was evaluated by ARS staining when the PDLSCs
were grown in the osteogenic differentiation medium for 2 weeks.
The PDLSCs were fixed with 4% paraformaldehyde for 15 min at 37°C.
Subsequently, 0.2% ARS solution (Beyotime Institute of
Biotechnology) was used to stain the PDLSCs for 10 min at 37°C. The
PDLSCs were rinsed with distilled water and the images were
captured using an inverted light microscope (Olympus
Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the 5×105
cells using TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. The PrimeScript RT
reagent kit (Takara Bio, Inc.) was employed to obtain cDNA
according to the manufacturer's recommendations. The ABI 7500
Real-Time PCR System was employed to conduct qPCR with the
SYBR-Green PCR Master Mix (Takara Bio, Inc.) following the
manufacturer's recommendations. The following thermocycling
conditions were used: Initial denaturation at 95°C for 10 min;
followed by 35 cycles of denaturation at 95°C for 15 sec, annealing
at 60°C for 1 min and extension of 10 min at 65°C. The relative
mRNA expression was computed using the 2−ΔΔCq method
(22). β-actin was used as the
housekeeping control. The experiments were repeated three times
independently. The following primer pairs were used for qPCR: ALP,
forward 5′-CTATCCTGGCTCCGTGCTCC-3′, reverse
5′-GCACCCCAAGACCTGCTTTA-3′; osteocalcin (OCN), forward
5′-CCACCGAGACACCATGAGAG-3′, reverse 5′-CGCCTGGGTCTCTTCACTAC-3′;
runt-related transcription factor 2 (RUNX2), forward
5′-CCACCGAGACCAACAGAGTC-3′, reverse 5′-TCACTGTGCTGAAGAGGCTG-3′;
bone morphogenetic protein-2 (BMP2), forward
5′-AGAATAACTTGCGCACCCCA-3′, reverse 5′-GGACCGAATGTCCGTTCCTT-3′;
TNF-α, forward 5′-CAAGGACAGCAGAGGACCAG-3′, reverse
5′-TCCTTTCCAGGGGAGAGAGG-3′; IL-1β, forward
5′-CAGAAGTACCTGAGCTCGCC-3′, reverse 5′-AGATTCGTAGCTGGATGCCG-3′;
IL-6, forward 5′-CCACCGGGAACGAAAGAGAA-3′, reverse
5′-GAGAAGGCAACTGGACCGAA-3′; β-actin, forward
5′-CTTCGCGGGCGACGAT-3′, reverse 5′-CCACATAGGAATCCTTCTGACC-3′.
Co-immunoprecipitation (Co-IP)
Co-IP assay was conducted adopting the Co-IP kit
(cat. no. 26149; Pierce; Thermo Fisher Scientific, Inc.). Cells
were lysed with ice-cold RIPA buffer (Beyotime Institute of
Biotechnology). The proteins were incubated with anti-Wnt7B (cat.
no. sc-365459, 1–2 µg per 100–500 µg of total protein; Santa Cruz
Biotechnology, Inc.), anti-FZD4 (cat. no. sc-293454, 1–2 µg per
100–500 µg of total protein; Santa Cruz Biotechnology, Inc.) or
anti-IgG (cat. no. sc-515946, 1–2 µg per 100–500 µg of total
protein; Santa Cruz Biotechnology, Inc.) antibody overnight at 4°C.
Subsequently, 20 µl protein A/G agarose beads (Invitrogen; Thermo
Fisher Scientific, Inc.) were added to identify the protein
complexes. Following 600 × g centrifugation at 4°C for 10 min, the
immunoprecipitants were washed three times using PBS and then
boiled with SDS loading buffer. Western blot analysis was used to
analyze the immuno-complexes. IgG was used as a negative
control.
Immunofluorescence staining
Cells on coverslips (1×105 cells) were
fixed with 4% paraformaldehyde for 30 min at room temperature,
followed by permeabilization with 0.1% Triton X-100 for 10 min. The
slides were immersed in 5% bovine serum albumin (BSA; Beyotime
Institute of Biotechnology) and then incubated with cluster of
differentiation 86 (CD86; cat. no. ab239075; 1:100; Abcam) antibody
at 4°C. The following day, the samples were probed with Alexa
Fluor-488 conjugated secondary antibody (cat. no. 4412S, 1:100;
Cell Signaling Technology, Inc.) at room temperature for 1 h.
Following the staining of the nucleus with DAPI (Beyotime Institute
of Biotechnology) for 3 min at room temperature, the coverslips
were viewed under a fluorescence microscope (magnification, 200×;
Olympus Corporation).
Western blot analysis
Cells were lysed with ice-cold RIPA buffer (Beyotime
Institute of Biotechnology). Bradford assays (Beyotime Institute of
Biotechnology) were employed to assess the protein concentration. A
total of 40 µg sample of protein was separated using 10% SDS-PAGE
gels, which were then transferred onto PVDF membranes (EMD
Millipore). The membranes were blocked with 5% BSA for 1 h at room
temperature and then probed with primary antibodies at 4°C
overnight. The blots were then probed with the anti-rabbit
HRP-linked secondary antibody (cat. no. 7074P2; 1:10,000; Cell
Signaling Technology, Inc.) or anti-mouse HRP-linked secondary
antibody (cat. no. 7076P2; 1:10,000; Cell Signaling Technology,
Inc.) at room temperature for 1 h. Western blot bands were detected
by enhanced chemiluminescence reagent (MilliporeSigma). β-actin
expression was measured as a control for quantitative analysis. The
grayscale values of the proteins were calculated using ImageJ
software (version 1.8.0; National Institutes of Health). Anti-Wnt7B
(cat. no. sc-365459; 1:1,000) and anti-FZD4 (cat. no. sc-293454,
1:500 dilution) antibodies were provided by Santa Cruz
Biotechnology (CA, USA). Anti-ALP (cat. no. ab229126, 1:1,500
dilution), anti-OCN (cat. no. ab133612, 1:2,000 dilution),
anti-BMP2 (cat. no. ab214821; 1:1,000) and anti-CD86 (cat. no.
ab239075; 1:1,000) antibodies were purchased from Abcam. Anti-RUNX2
(cat. no. 12556S; 1:1,000), anti-inducible nitric oxide synthase
(iNOS; cat. no. 20609S; 1:1,000) and anti-β-actin (cat. no. 4970T;
1:1,000) antibodies were obtained from Cell Signaling Technology,
Inc.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using GraphPad 8.0 software
(Dotmatics). The difference of Wnt7B expression in the
periodontitis-affected gingival tissue of patients (n=3) and
healthy gingival tissue of patients (n=3) was analyzed using GEO2R
(https://www.ncbi.nlm.nih.gov/geo/geo2r/). The data
were conformed to normal distribution using Shapiro-Wilk test.
Comparisons between two groups were performed using unpaired
Student's t-tests. Multi-group comparisons were made using one-way
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
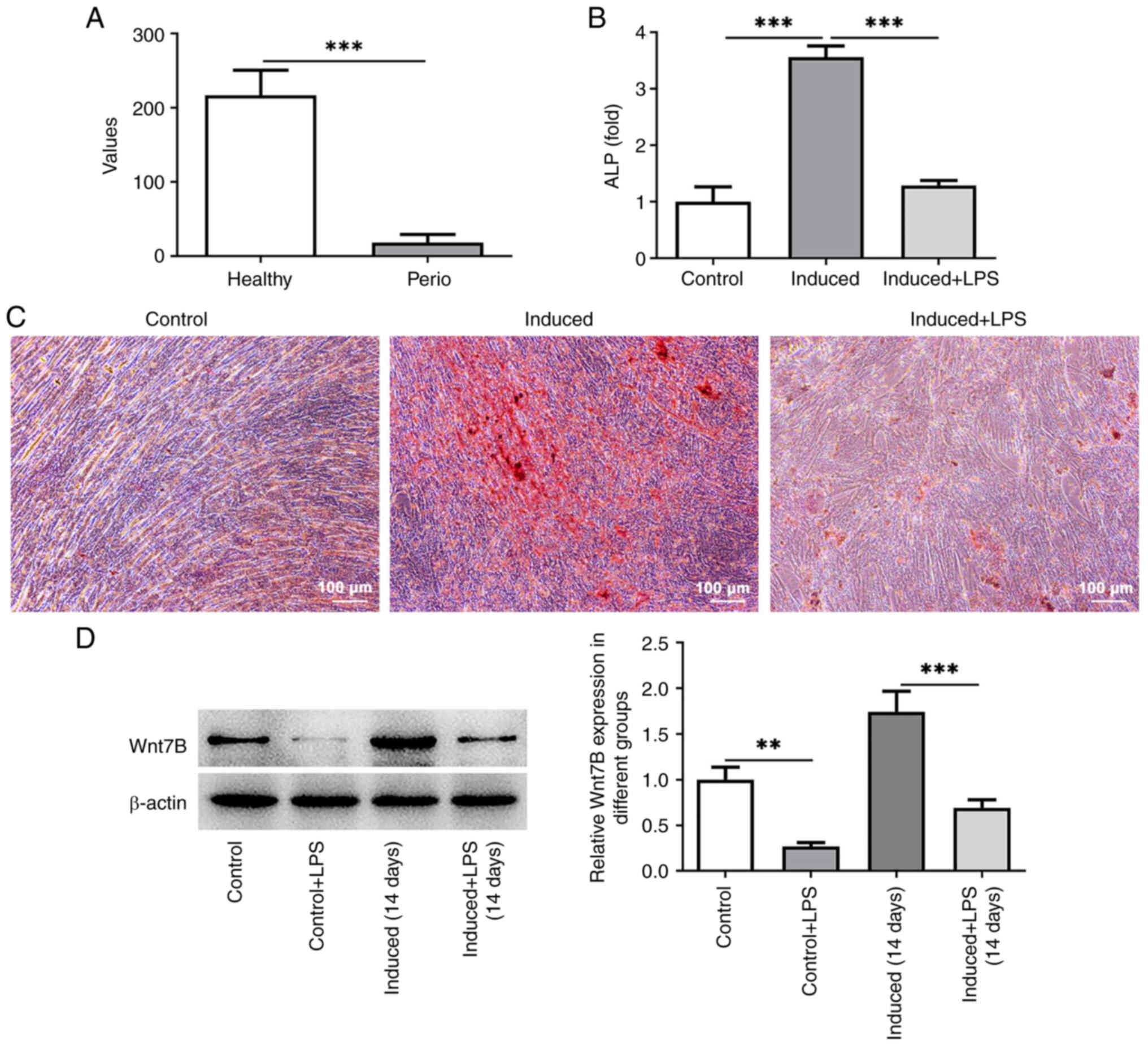
Decreased expression of Wnt7B in the
periodontitis-affected gingival tissue of patients and PDLSCs
stimulated by LPS
The results from the GSE23586 dataset revealed that
Wnt7B expression was notably downregulated in the
periodontitis-affected gingival tissue of patients compared with
that in the healthy gingival tissue (Fig. 1A; P=0.0006). Subsequently, the
osteogenic differentiation of the PDLSCs was induced by culturing
the cells in osteogenic differentiation medium with or without LPS.
A significantly enhanced ALP activity and increased calcium nodule
formation level were observed in the group that was subjected to
induction (induced group) relative to the control group (Fig. 1B and C; P=0.000009). The presence
of LPS decreased the ALP activity and mineralized nodules in the
PDLSCs in comparison to the induced group (Fig. 1B and C; P=0.000019). The results of
western blot analysis then indicated that LPS stimulation
significantly reduced Wnt7B expression in the PDLSCs without
(P=0.001016) or with (P=0.000078) osteogenic differentiation
induction (Fig. 1D). These data
demonstrated the abnormally low level of Wnt7B in
periodontitis-affected gingival tissue and in LPS-stimulated
PDLSCs.
Wnt7B overexpression promotes the
osteogenic differentiation of LPS-stimulated PDLSCs
Wnt7B was overexpressed to elucidate its effects on
the osteogenic differentiation of LPS-stimulated PDLSCs. The PDLSCs
in the Ov-Wnt7B group exhibited a markedly elevated expression of
Wnt7B compared with the Ov-NC group (Fig. 2A; P=0.000002). ALP activity and
calcium nodule formation level were conspicuously elevated in the
Wnt7B-overexpressing PDLSCs (Fig. 2B
and C; P=0.001162). Additionally, mRNA expression of markers
associated with osteogenic differentiation [ALP (P=0.000235), OCN
(P=0.000072), RUNX2 (P=0.004702) and BMP2 (P=0.001784)], which had
been decreased by LPS stimulation, was markedly increased following
the enforced expression of Wnt7B (Fig.
2D). Similar trends were observed in the protein levels of ALP
(P=0.026585), OCN (P=0.001099), RUNX2 (P=0.001031) and BMP2
(P=0.000493) in the PDLSCs exposed to LPS (Fig. 2E). The aforementioned results
indicated that the elevated expression of Wnt7B facilitated the
osteogenic differentiation of LPS-stimulated PDLSCs.
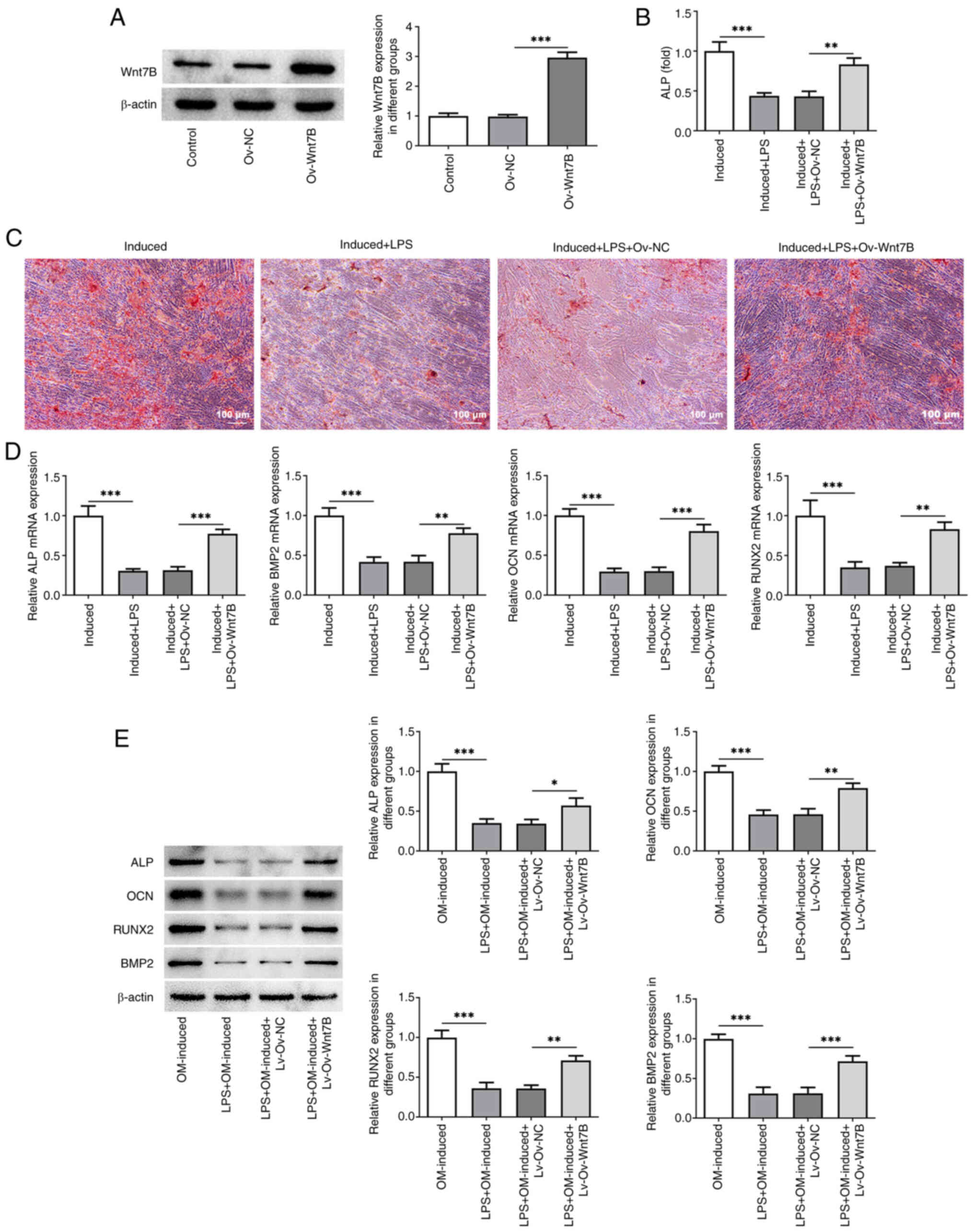 | Figure 2.Wnt7B overexpression promotes the
osteogenic differentiation of LPS-stimulated PDLSCs. (A) Western
blot analysis was used to measure Wnt7B expression in PDLSCs
following transfection. (B) ALP activity in LPS-stimulated PDLSCs
overexpressing Wnt7B was detected using an ALP assay kit. (C) The
mineralization of PDLSCs was evaluated using ARS staining. (D) The
mRNA expression of ALP, OCN, RUNX2 and BMP2 was examined using
reverse transcription-quantitative PCR. (E) Western blot analysis
was used to examine the protein expression of ALP, OCN, RUNX2 and
BMP2 in LPS-stimulated PDLSCs overexpressing Wnt7B. *P<0.05,
**P<0.01, ***P<0.001. LPS, lipopolysaccharide; PDLSCs,
periodontal stem cells; ALP, alkaline phosphatase; ARS, Alizarin
Red S; OCN, osteocalcin; RUNX2, runt-related transcription factor
2; BMP2, bone morphogenetic protein-2. |
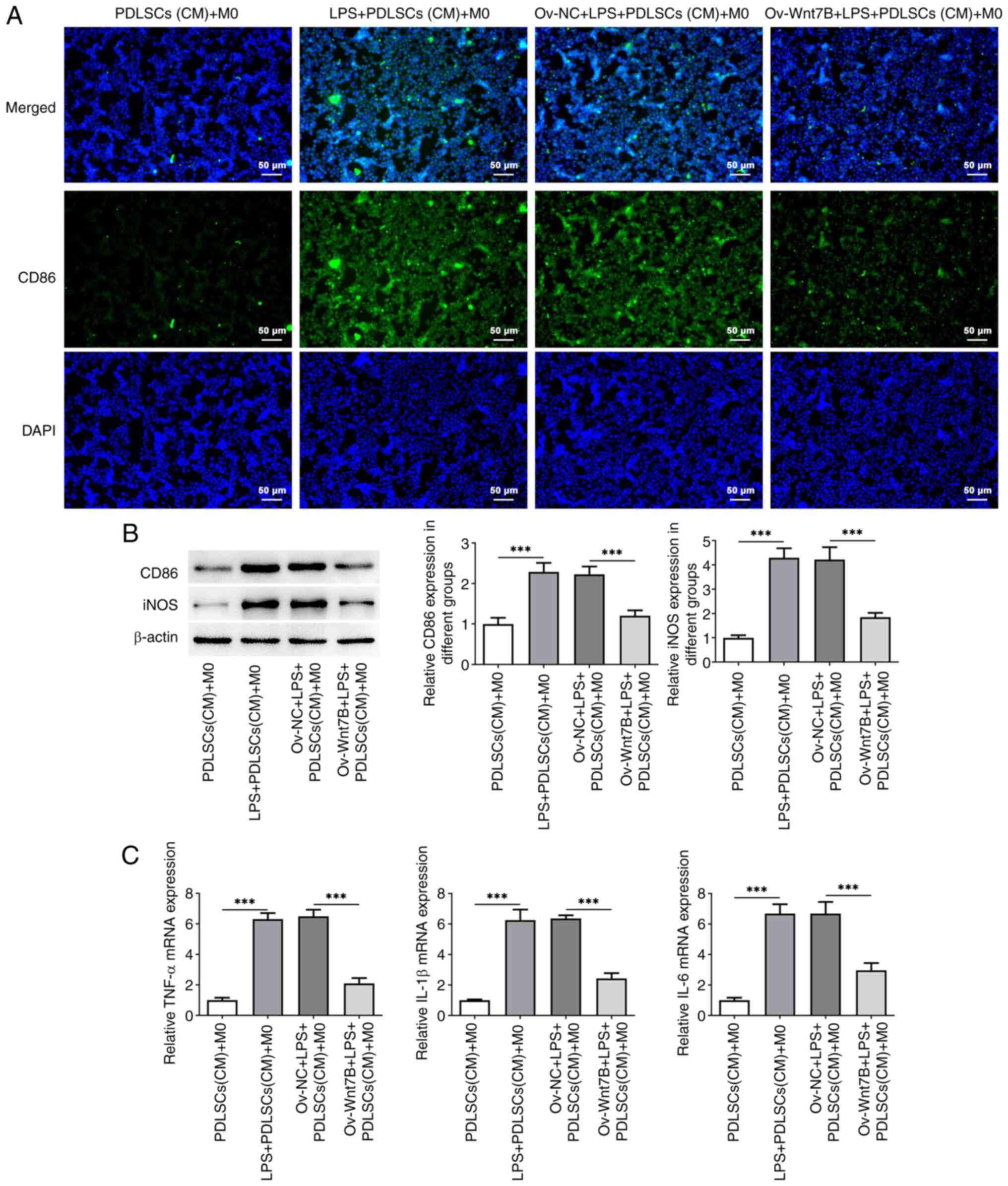
Wnt7B overexpression inhibits the M1
polarization of macrophages in an inflammatory environment
To explore the effects of Wnt7B expression in
inflammatory PDLSCs on the M1 polarization of macrophages,
conditioned medium (CM) from PDLSCs overexpressing Wnt7B was used
for M0 macrophage culture. M1 macrophages highly expresses CD86 and
iNOS and also secrete inflammatory cytokines, such as TNF-α, IL-6
and IL-1β (23). It was found that
CD86 fluorescence intensity was conspicuously enhanced in the M0
macrophages cultured with CM from LPS-stimulated PDLSCs compared
with the PDLSCs (CM) + M0 group, which was reduced following the
overexpression of Wnt7B in LPS-stimulated PDLSCs (Fig. 3A). At the same time, western blot
analysis shown in Fig. 3B
demonstrated that CD86 and iNOS protein expression levels were
significantly increased in the LPS + PDLSCs (CM) + M0 group in
comparison to the PDLSCs (CM) + M0 group (CD86 (P=0.000099), iNOS
(P=0.000010)), which was decreased after transfection with Ov-Wnt7B
[CD86 (P=0.000507), iNOS (P=0.000117)]. In addition, macrophages
cultured in CM from LPS + PDLSCs (CM) exhibited a markedly elevated
mRNA expression of TNF-α (P<0.000001), IL-1β (P=0.000001) and
IL-6 (P=0.000007) (Fig. 3C). By
contrast, Wnt7B overexpression partially counteracted the increase
in the levels of TNF-α (P=0.000002), IL-1β (P=0.000010) and IL-6
(P=0.000154) induced by LPS. Collectively, Wnt7B overexpression
inhibited the M1 polarization of macrophages in an inflammatory
environment.
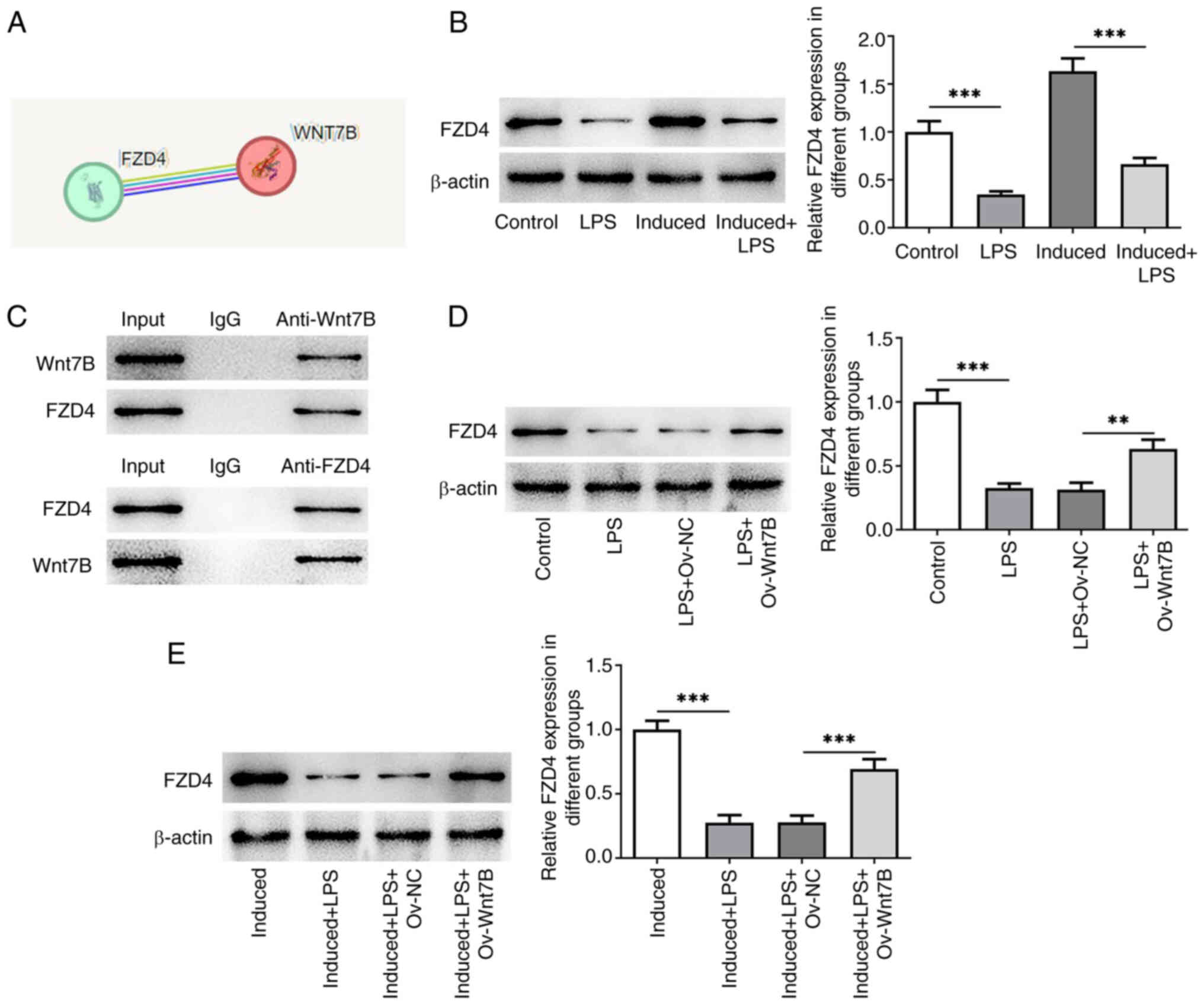
Wnt7B binds to FZD4 and upregulates
FZD4 expression in LPS-stimulated PDLSCs
The STRING database predicted the interaction
between Wnt7B and FZD4 (Fig. 4A).
As illustrated in Fig. 4B, FZD4
expression was downregulated in the LPS-stimulated PDLSCs without
(P=0.000139) or with (P=0.000007) osteogenic differentiation
induction. Furthermore, co-IP assay demonstrated that Wnt7B could
bind to FZD4 (Fig. 4C). In
addition, Wnt7B gain-of-function notably increased FZD4 expression
in PDLSCs exposed to LPS (Fig. 4D;
P=0.001815). Consistently, FZD4 expression was also upregulated
following the overexpression of Wnt7B in PDLSCs cultured in
osteogenic differentiation medium in the presence of LPS (Fig. 4E; P=0.000235). These results
confirmed that Wnt7B could bind to FZD4 and upregulate FZD4
expression in LPS-stimulated PDLSCs.
FZD4 knockdown reverses the beneficial
effects of Wnt7B overexpression on the osteogenic differentiation
of LPS-stimulated PDLSCs
Subsequently, FZD4 was knocked down to perform
rescue assays. As illustrated in Fig.
5A, shRNA-FZD4-1 (P=0.000001) and shRNA-FZD4-2 (P=0.000316)
transfection led to the markedly decreased expression of FZD4 in
PDLSCs. Due to the lower expression of FZD4, the PDLSCs transfected
with shRNA-FZD4-1 were used for follow-up experiments. When
compared with the induced + LPS + Ov-Wnt7B + shRNA-NC group, FZD4
knockdown markedly decreased the osteogenic differentiation
capacity of the PDLSCs, as shown by the inhibited ALP activity
(P=0.013976), the reduced calcium nodule formation level and the
downregulated mRNA and protein expression of ALP (P=0.000855 or
0.001168), OCN (P=0.000403 or 0.041818), RUNX2 (P=0.000892 or
0.000244) and BMP2 (P=0.007455 or 0.000517; Fig. 5B-E). In summary, interfering with
the expression of FZD4 abrogated the beneficial effects of Wnt7B
overexpression on the osteogenic differentiation of LPS-stimulated
PDLSCs.
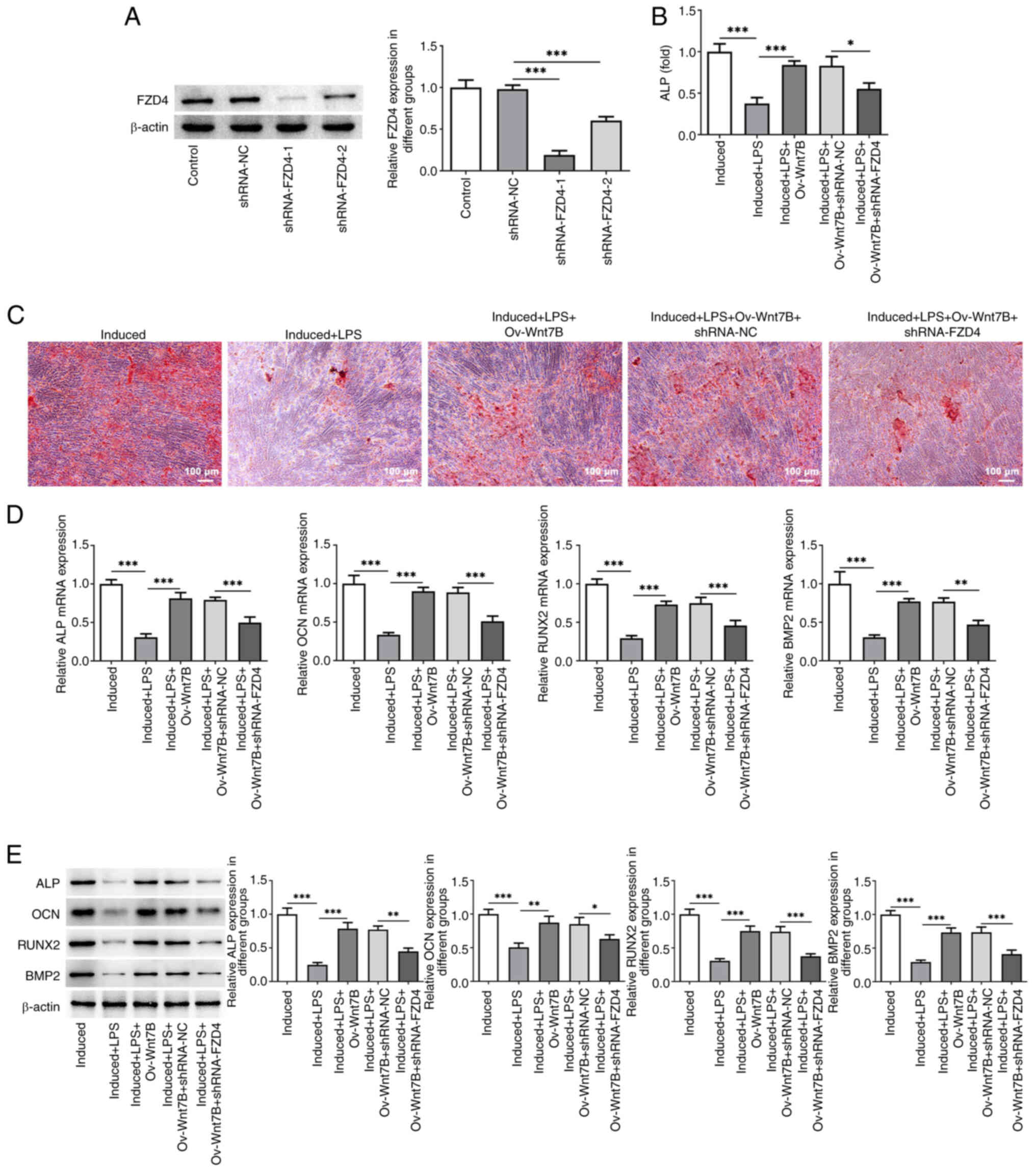 | Figure 5.FZD4 knockdown reverses the
beneficial effects of Wnt7B overexpression on the osteogenic
differentiation of LPS-stimulated PDLSCs. (A) Western blot analysis
was used to examine FZD4 expression in PDLSCs following
transfection. (B) ALP activity in LPS-stimulated PDLSCs
overexpressing Wnt7B and in which FZD4 was knocked down was
detected using the ALP assay kit. (C) The mineralization of PDLSCs
was evaluated using ARS staining. (D) The mRNA expression of ALP,
OCN, RUNX2 and BMP2 was examined using reverse
transcription-quantitative PCR. (E) Western blot analysis was
performed to examine the protein expression of ALP, OCN, RUNX2 and
BMP2 in LPS-stimulated PDLSCs overexpressing Wnt7B and in which was
FZD4 knocked down. *P<0.05, **P<0.01, ***P<0.001. FZD4,
frizzled4; LPS, lipopolysaccharide; PDLSCs, periodontal stem cells;
ALP, alkaline phosphatase; OCN, osteocalcin; RUNX2, runt-related
transcription factor 2; BMP2, bone morphogenetic protein-2; Ov,
overexpression; NC, negative control; sh, short hairpin. |
FZD4 knockdown mitigates the effects
of Wnt7B overexpression on the M1 polarization of macrophages in an
inflammatory environment
As demonstrated in Fig.
6A and B, CD86 (P=0.038984) and iNOS (P=0.016199) expression in
macrophages was markedly increased following the knockdown of FZD4
in LPS-stimulated PDLSCs overexpressing Wnt7B. In addition, the
results of RT-qPCR demonstrated that FZD4 silencing markedly
elevated the mRNA expression of TNF-α (P=0.000023), IL-1β
(P=0.000079) and IL-6 (P=0.000165) relative to that in the Ov-Wnt7B
+ LPS + PDLSCs + shRNA-NC (CM) + M0 group (Fig. 6C). The aforementioned data
suggested that FZD4 knockdown attenuated the effects of Wnt7B
overexpression on the M1 polarization of macrophages in an
inflammatory environment.
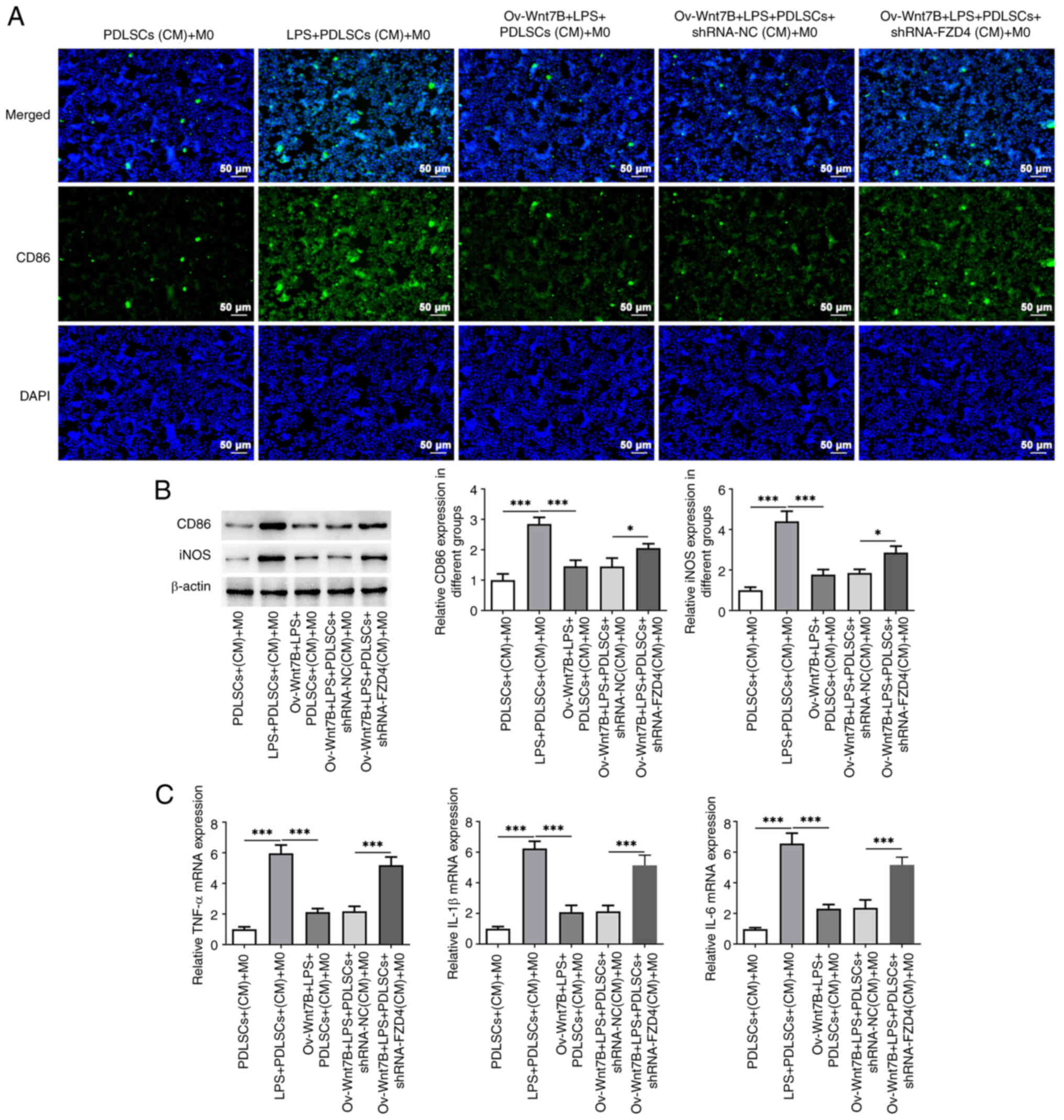 | Figure 6.FZD4 knockdown mitigates the
inhibitory effects of Wnt7B overexpression on the M1 polarization
of macrophages in an inflammatory environment. (A) CD86 expression
in M0 macrophages cultured in conditioned medium from
LPS-stimulated PDLSCs overexpressing Wnt7B and in which FZD4 was
knocked down was detected using immunofluorescence staining. (B)
Western blot analysis was used to examine the expression of CD86
and iNOS. (C) RT-qPCR detected the mRNA expression of TNF-α, IL-1β
and IL-6. *P<0.05, ***P<0.001. FZD4, frizzled4; LPS,
lipopolysaccharide; CD86, cluster of differentiation 86; PDLSCs,
periodontal stem cells; iNOS, inducible nitric oxide synthase; CM,
conditioned medium; Ov, overexpression; NC, negative control; sh,
short hairpin. |
Discussion
Periodontitis is a highly prevalent oral disease
associated with tooth loss caused by periodontal supporting tissue
destruction and accounts for a considerable global public health
burden (24). The present study
suggested that Wnt7B expression was abnormally downregulated in
periodontitis-affected gingival tissue and in LPS-stimulated
PDLSCs. Wnt7B enhanced the osteogenic differentiation of
LPS-stimulated PDLSCs and inhibited the M1 polarization of
macrophages by binding to FZD4.
Wnt proteins are a family of signaling molecules
that activate a context-dependent intracellular signaling to induce
a variety of biological responses by engaging receptors on the cell
membrane (25). In particular, Wnt
signaling has been reported to be involved in both embryonic and
postnatal bone formation (26).
Among the Wnt ligands, Wnt7b has a profound effect on bone
formation and osteoblast differentiation, which has attracted
increasing research attention. Wnt7B is implicated in the
commitment and differentiation of osteoblast lineages by the
rewiring of multiple pathways to enhance osteogenic function
(27,28). Wnt7B is expressed in differentiated
chondrocytes and has been regarded as a key factor secreted by
proliferative chondrocytes to initiate endochondral ossification
(29). Of note, Wnt7B expression
is markedly elevated in the process of osteogenic differentiation
of MC3T3-E1 cells (14). It has
been shown that miR-503-3p downregulation facilitates the
osteogenic differentiation of cyclic strain-treated human
adipose-derived stem cells through the upregulation of Wnt2 and
Wnt7B expression (30). More
importantly, by the targeted inhibition of Wnt7B, miR-342-5p
suppresses the odonto/osteogenic differentiation of human dental
pulp stem cells (31). ALP, a sign
of osteogenic differentiation, is critical for mineralization
during bone formation (32,33).
OCN is a non-collagen protein that is specifically expressed in
bone tissue and levels are indicative of maturity during osteogenic
differentiation (34). In
addition, RUNX2 is a transcription factor that is essential in the
early stages of osteogenic differentiation (35). Consistent with the aforementioned
studies, in the present study a decreased expression of Wnt7B was
found in periodontitis-affected gingival tissue of patients
(GSE23586 dataset) and in PDLSCs subjected to osteogenic
differentiation. Wnt7B gain-of-function accelerated the osteogenic
differentiation of LPS-stimulated PDLSCs, as shown by the elevated
activity of ALP, the increased numbers of mineralized nodules and
the upregulated expression of ALP, OCN, RUNX2 and BMP2. These
results emphasized the critical promoting effects of Wnt7B on
osteogenic differentiation during periodontitis.
Macrophages exhibit a high heterogeneity and
plasticity. In response to local physiological or pathological
conditions, naive macrophages (M0) can differentiate into different
functional phenotypes to participate in the regulation of diseases
(11). An increasing number of
studies have validated that macrophages are associated with the
occurrence, development and resolution of periodontitis (23,36).
Macrophage polarization towards the M1 phenotype in PDLSCs weakens
periodontal regeneration following stem cell transplantation
(23). A previous in vitro
study confirms that M2 macrophages, rather than M1 macrophages,
markedly facilitate the proliferation of mesenchymal stem cells
during periodontal repair (37).
There is emerging evidence to support the hypothesis that vesicles
from LPS-stimulated PDLSCs promote the conversion of macrophages to
the M1 type and facilitate the release of inflammatory factors
(12). AZGP1 aggravates
periodontitis by promoting macrophage M1 polarization under LPS
stimulation to induce high inflammatory activation and low
osteogenic differentiation in PDLSCs (38). Similarly, the present study
demonstrated that CM from LPS-stimulated PDLSCs induced the M1
polarization of macrophages, accompanied by increased levels of
TNF-α, IL-6 and IL-1β. As has been previously reported, macrophage
Wnt7B is crucial for the repair and regeneration of kidney
(39). Wnt7B overexpression in
bone marrow-derived macrophage lineage cells notably disrupts
osteoclast formation and activity, leading to a sharp increase in
bone mass (17). The present study
demonstrated that Wnt7B overexpression in LPS-stimulated PDLSCs
inhibited the M1 polarization of macrophages. Additionally, it
should be considered that other recently introduced compounds have
been demonstrated having a significant influence on oral
environment. The use of postbiotics, lysates and paraprobiotics,
such as paraprobiotic-based toothpaste and mousse, can modify
clinical parameters in periodontal patients, so these products
should also be considered in future research, as adjuvants, to
evaluate their effect on osteogenic differentiation in
lipopolysaccharide-induced human periodontal ligament (40–42).
FZD4, a class FZD G-protein-coupled receptor at the
cell membrane, can be combined with Wnt7B, which is also supported
by the results of the STRING database and Co-IP in the present
study (43). The targeted
activation of FZD4 expression expedites the osteogenic
differentiation of mesenchymal stem cells (44,45).
Notably, human PDLSCs expresses FZD4 and the decrease in FZD4
inhibits the osteogenic differentiation of cyclic stretch-induced
PDLSCs (18). It should be noted
that IFNγ-treated macrophages participate in the process of Crohn's
disease by regulating FZD4 expression (19). In the present study, FZD4 knockdown
reversed the effects of Wnt7B overexpression on the osteogenic
differentiation of LPS-stimulated PDLSCs and the M1 polarization of
macrophages, suggesting that Wnt7B plays an anti-periodontitis role
by binding FZD4.
However, the present study had some limitations. It
only conducted cell experiments and so further in vivo
animal studies will be performed in the future to verify the roles
of Wnt7B and FZD4 in periodontitis. In addition, the downstream
signaling that can be regulated by Wnt7B and FZD4 will be explored
in future studies.
In summary, the present study demonstrated that
Wnt7B expression is abnormally downregulated in
periodontitis-affected gingival tissue and LPS-induced PDLSCs.
Wnt7B plays an anti-periodontitis role by binding FZD4 to
strengthen osteogenic differentiation of LPS-treated PDLSCs and
suppress M1 polarization of macrophages. All these outcomes may
provide a novel therapeutic target related to Wnt7B/FZD4 for the
treatment of periodontitis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HY, YZ, LZ and CW were involved in the
conceptualization and design of the study, acquisition and
interpretation of data. HY, XT and MZ interpreted the data and
wrote the manuscript, which was revised by CW. All authors have
read and approved the final manuscript. HY and CW confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The Ethics Committee of Tianjin Medical University
waived the requirement for ethics approval for using the purchased
human periodontal ligament stem cells.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kwon T, Lamster IB and Levin L: Current
Concepts in the Management of Periodontitis. Int Dent J.
71:462–476. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun J, Tong D, Sun C, Wang X, Zuo Z, Liu
Y, Qi L, Kong L, Luan X and Meng J: Knowledge, attitude, and
practice toward self-control of dental plaque among patients with
periodontal diseases: A cross-sectional study. BMC Oral Health.
23:6282023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Xing Y, Jia L, Ji Y, Zhao B, Wen
Y and Xu X: An in vitro comparative study of multisource derived
human mesenchymal stem cells for bone tissue engineering. Stem
Cells Dev. 27:1634–1645. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomokiyo A, Wada N and Maeda H:
Periodontal ligament stem cells: Regenerative potency in
periodontium. Stem Cells Dev. 28:974–985. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhai Q, Dong Z, Wang W, Li B and Jin Y:
Dental stem cell and dental tissue regeneration. Front Med.
13:152–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao J, Zhang Q, Yang Q, Yu Y, Meng M and
Zou J: Epigenetic regulation of osteogenic differentiation of
periodontal ligament stem cells in periodontitis. Oral Dis.
29:2529–2537. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hasturk H: Inflammation and Periodontal
Regeneration. Dent Clin North Am. 66:39–51. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Deng M, Hao M and Tang J:
Periodontal ligament stem cells in the periodontitis niche:
Inseparable interactions and mechanisms. J Leukoc Biol.
110:565–576. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gibier JB, Swierczewski T, Csanyi M, Hemon
B, Glowacki F, Maboudou P, Van Seuningen I, Cauffiez C, Pottier N,
Aubert S, et al: MUC1 mitigates renal injury and inflammation in
endotoxin-induced acute kidney injury by inhibiting the TLR4-MD2
Axis and reducing pro-inflammatory macrophages infiltration. Shock.
56:629–638. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Locati M, Curtale G and Mantovani A:
Diversity, mechanisms, and significance of macrophage plasticity.
Annu Rev Pathol. 15:123–147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui S, Zhang Z, Cheng C, Tang S, Zhai M,
Li L, Wei F and Ding G: Small extracellular vesicles from
periodontal ligament stem cells primed by lipopolysaccharide
regulate macrophage M1 Polarization via miR-433-3p Targeting
TLR2/TLR4/NF-κB. Inflammation. 46:1849–1858. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang H, Lee MJ, Park SJ and Lee MS:
Lipopolysaccharide-Preconditioned periodontal ligament stem cells
induce M1 polarization of macrophages through extracellular
vesicles. Int J Mol Sci. 19:38432018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sarkar L and Sharpe PT: Expression of Wnt
signalling pathway genes during tooth development. Mech Dev.
85:197–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Tu X, Esen E, Joeng KS, Lin C,
Arbeit JM, Rüegg MA, Hall MN, Ma L and Long F: WNT7B promotes bone
formation in part through mTORC1. PLoS Genet. 10:e10041452014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu F, Wu F, Li F, Liao X, Wang Y, Li X,
Wang C, Shi Y and Ye L: Wnt7b-induced Sox11 functions enhance
self-renewal and osteogenic commitment of bone marrow mesenchymal
stem cells. Stem Cells. 38:1020–1033. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen H, Song F and Long F: WNT7B
overexpression rescues bone loss caused by glucocorticoids in mice.
FASEB J. 35:e216832021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Chang M, Wang B, Liu X, Zhang Z
and Han G: YAP/WNT5A/FZD4 axis regulates osteogenic differentiation
of human periodontal ligament cells under cyclic stretch. J
Periodontal Res. 58:907–918. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Macias-Ceja DC, Coll S, Bauset C,
Seco-Cervera M, Gisbert-Ferrándiz L, Navarro F, Cosin-Roger J,
Calatayud S, Barrachina MD and Ortiz-Masia D: IFNγ-Treated
Macrophages Induce EMT through the WNT Pathway: Relevance in
Crohn's Disease. Biomedicines. 10:10932022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia R, Yi Y, Liu J, Pei D, Hu B, Hao H, Wu
L, Wang Z, Luo X and Lu Y: Cyclic compression emerged dual effects
on the osteogenic and osteoclastic status of LPS-induced
inflammatory human periodontal ligament cells according to loading
force. BMC Oral Health. 20:72020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao Q, Liu S, Zou C and Ai Y: Effect of
LSD1 on osteogenic differentiation of human periodontal ligament
stem cells in periodontitis. Oral Dis. 29:1137–1148. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cavalla F and Hernández M: Polarization
Profiles of T lymphocytes and macrophages responses in
periodontitis. Adv Exp Med Biol. 1373:195–208. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Papapanou PN, Sanz M, Buduneli N, Dietrich
T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani
F, et al: Periodontitis: Consensus report of workgroup 2 of the
2017 World Workshop on the Classification of Periodontal and
Peri-Implant Diseases and Conditions. J Periodontol. 89 (Suppl
1):S173–S182. 2018.PubMed/NCBI
|
|
25
|
van Amerongen R and Nusse R: Towards an
integrated view of Wnt signaling in development. Development.
136:3205–3214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karner CM and Long F: Wnt signaling and
cellular metabolism in osteoblasts. Cell Mol Life Sci.
74:1649–1657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen H, Ji X, Lee WC, Shi Y, Li B, Abel
ED, Jiang D, Huang W and Long F: Increased glycolysis mediates
Wnt7b-induced bone formation. FASEB J. 33:7810–7821. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song D, He G, Song F, Wang Z, Liu X, Liao
L, Ni J, Silva MJ and Long F: Inducible expression of Wnt7b
promotes bone formation in aged mice and enhances fracture healing.
Bone Res. 8:42020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsukamoto S, Kuratani M, Tanaka S, Jimi E,
Oda H and Katagiri T: Wnt7b expressed by hypertrophic chondrocytes
is a stimulatory factor for endochondral ossification that is
regulated by Smad4 activity. Development. 150:dev2017342023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo Y, Ding X, Ji H, Li M, Song H, Li S,
Wang C, Wu H and Du H: MicroRNA-503-3p affects osteogenic
differentiation of human adipose-derived stem cells by regulation
of Wnt2 and Wnt7b under cyclic strain. Stem Cell Res Ther.
11:3182020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng K, Li W, Kang Q, Li Y, Cheng Q and
Xia W: miR-342-5p inhibits odonto/osteogenic differentiation of
human dental pulp stem cells via targeting Wnt7b. Oral Dis.
29:2107–2116. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Genge BR, Sauer GR, Wu LN, McLean FM and
Wuthier RE: Correlation between loss of alkaline phosphatase
activity and accumulation of calcium during matrix vesicle-mediated
mineralization. J Biol Chem. 263:18513–18519. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li S, Wang J, Han Y, Li X, Liu C, Lv Z,
Wang X, Tang X and Wang Z: Carbenoxolone inhibits mechanical
stress-induced osteogenic differentiation of mesenchymal stem cells
by regulating p38 MAPK phosphorylation. Exp Ther Med. 15:2798–2803.
2018.PubMed/NCBI
|
|
34
|
Komori T: Functions of osteocalcin in
bone, pancreas, testis, and muscle. Int J Mol Sci. 21:75132020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Almalki SG and Agrawal DK: Key
transcription factors in the differentiation of mesenchymal stem
cells. Differentiation. 92:41–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang W, Zheng C, Yang J and Li B:
Intersection between macrophages and periodontal pathogens in
periodontitis. J Leukoc Biol. 110:577–583. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen B, Li S, Chang Y, Zhang J, Liu J,
Dong Y and Yan F: Macrophages contribute to periodontal wound
healing mainly in the tissue proliferation stage. J Periodontal
Res. 58:122–130. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang S, Yin Y, Sun Y, Ai D, Xia X, Xu X
and Song J: AZGP1 Aggravates Macrophage M1 polarization and
pyroptosis in periodontitis. J Dent Res. 103:631–641. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin SL, Li B, Rao S, Yeo EJ, Hudson TE,
Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, et al: Macrophage
Wnt7b is critical for kidney repair and regeneration. Proc Natl
Acad Sci USA. 107:4194–4199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Butera A, Maiorani C, Gallo S, Pascadopoli
M, Venugopal A, Marya A and Scribante A: Evaluation of adjuvant
systems in non-surgical peri-implant treatment: A literature
review. Healthcare (Basel). 10:8862022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vale GC and Mayer MPA: Effect of probiotic
Lactobacillus rhamnosus by-products on gingival epithelial cells
challenged with Porphyromonas gingivalis. Arch Oral Biol.
128:1051742021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Butera A, Pascadopoli M, Nardi MG, Ogliari
C, Chiesa A, Preda C, Perego G and Scribante A: Clinical use of
paraprobiotics for pregnant women with periodontitis: Randomized
clinical trial. Dent J (Basel). 12:1162024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu LJ, Lv Z, Xue X, Xing ZY and Zhu F:
Canonical WNT signaling activated by WNT7B Contributes to
L-HBs-Mediated sorafenib resistance in hepatocellular carcinoma by
inhibiting mitophagy. Cancers (Basel). 14:57812022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao H, Dong H, Zheng J, Jiang X, Gong M,
Hu L, He J and Wang Y: LINC01119 negatively regulates osteogenic
differentiation of mesenchymal stem cells via the Wnt pathway by
targeting FZD4. Stem Cell Res Ther. 13:432022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Z, Zhou H, Sun F, Han J and Han Y:
Circ_FBLN1 promotes the proliferation and osteogenic
differentiation of human bone marrow-derived mesenchymal stem cells
by regulating let-7i-5p/FZD4 axis and Wnt/β-catenin pathway. J
Bioenerg Biomembr. 53:561–572. 2021. View Article : Google Scholar : PubMed/NCBI
|