Cardiovascular diseases, including cardiac
arrhythmias, atherosclerosis and heart failure (HF), are diseases
of the circulatory system and are a leading cause of morbidity and
mortality worldwide (1). According
to the World Health Organization, cardiovascular disease accounts
for 31% of the deaths worldwide (2). Despite the numerous therapeutic
interventional methods, cardiovascular diseases account for
approximately one-third of all deaths worldwide (3). Cardiovascular diseases aggravate
healthcare costs and are a significant economic burden (4). Currently, the present understanding
of the mechanisms and risk factors underlying the pathogenesis of
cardiovascular diseases is limited, although there is increasing
interest in the role of trace elements in cardiovascular diseases
(5). Therefore, it is crucial to
elucidate the composition of trace elements in cardiovascular
diseases relative to physiological levels and provide valuable
insights into treatment strategies for cardiovascular diseases.
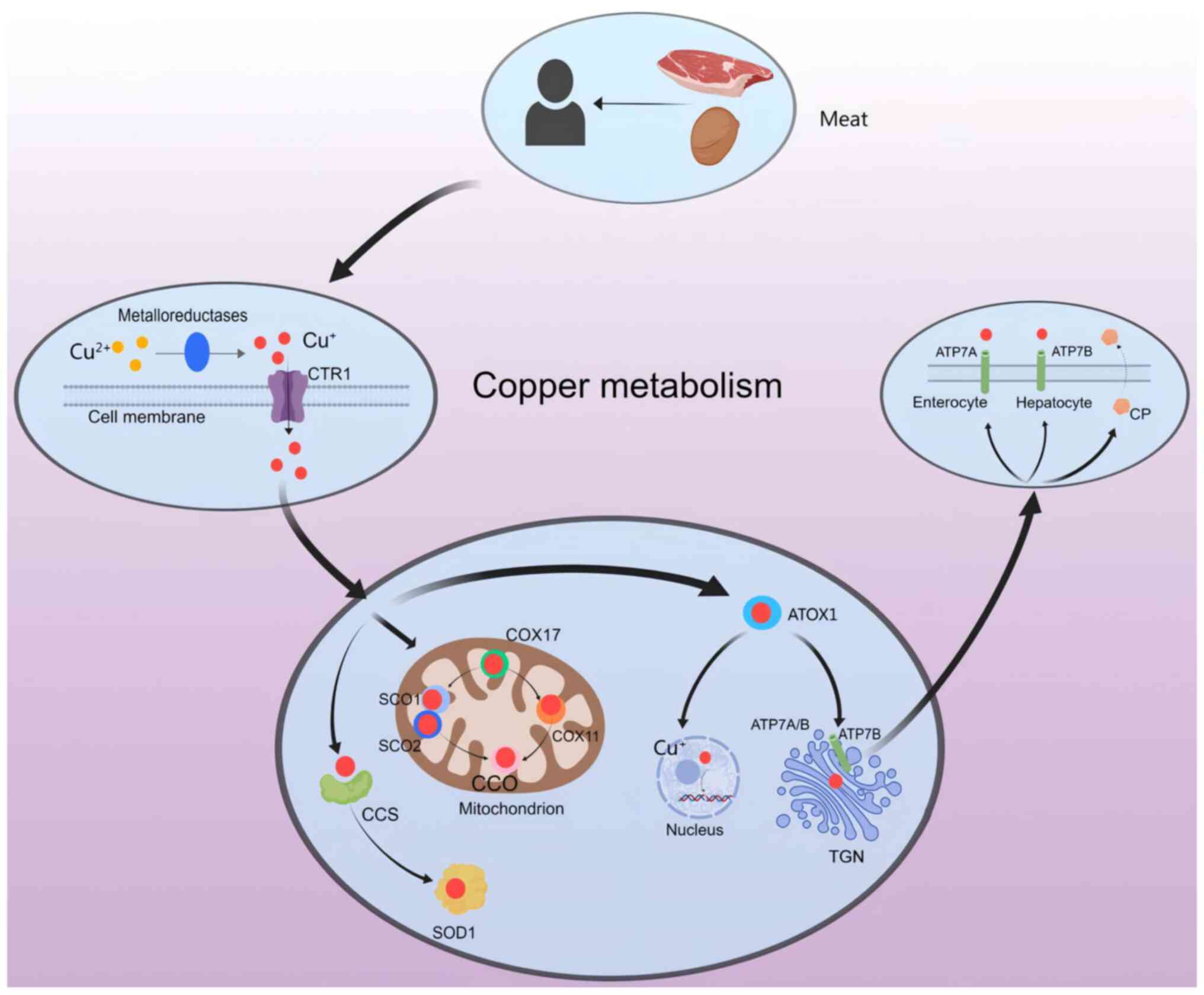
Copper is primarily obtained from the consumption of
vegetables, shellfish, meat, seeds and nuts (6), and plays a crucial role as a trace
element in maintaining physiological functioning (7). There are two ionic forms of copper
found in the body, Cu+ (cuprous ion, reduced form) and
Cu2+ (copper ion, oxidized form) (8), both of which are involved in
regulating enzymatic cellular functions (9). Generally, Cu2+ is
converted to Cu+ by reductase enzymes such as duodenal
cytochrome b (DCYTB) and six-layer epithelial antigen (Fig. 1) (10). Once taken up by copper transporter
1 (CTR1), Cu2+ is transported to different organelles in
the cytoplasm and is metabolized by a copper chaperone for
cytochrome c oxidase [CCO; COX (cytochrome c oxidase assembly
homolog (COX)] 17 (11) and
antioxidant-1 (ATOX1) (12).
Copper acts as a catalyst for numerous physiological
processes, including energy metabolism, mitochondrial respiration
and antioxidant activity (13).
Copper is present in the body in its ionic form (14). When the homeostatic balance of
copper ion levels is disrupted, such as through excess copper ions,
this imbalance can trigger cellular toxicity and induce cell death
via various pathways (15).
Dysregulation of copper ions can disturb lipid metabolism,
resulting in oxidative stress, mitochondrial damage and endothelial
cell dysfunction (14), and induce
atherosclerosis and other cardiovascular diseases such as
arrhythmia and cardiomyopathy (15). While there is a considerable body
of literature describing the initial causes of copper
dysregulation, there is a dearth of comprehensive exploration into
the underlying pathological consequences (16). The primary treatment for
dysregulated copper ion levels in cardiovascular diseases involves
copper chelating agents; however, alternative methods such as
copper ion carriers may also be used but are constrained by
technical limitations that necessitate further research and
improvement (17).
The present review aims to elucidate the mechanisms
by which copper ions are involved in cardiovascular diseases,
summarize the impact of copper ion abnormalities on cardiovascular
diseases and potential therapeutic approaches, and investigate
whether modulating copper ion levels can ameliorate cardiovascular
diseases. This review offers innovative perspectives for managing
cardiovascular diseases via the regulation of copper levels and
paves the way for novel research directions. The inclusion criteria
for the present study included: i) Research hypotheses and methods
were similar to the research content of the present article to
ensure the accuracy of the narrative; ii) the exact date that the
research was conducted or published; iii) clear regulations on
sample size; iv) clear criteria for patient selection, case
diagnosis and staging; v) clear measures for intervention and
control; vi) can provide OR (odds ratio) [relative risk (RR), rate
difference and hazard ratio (HR)] and its 95% confidence interval,
or can be converted into OR (RR, rate difference and HR) and its
95% confidence interval; and vii) if it is measurement data, the
mean, standard deviation and sample size should be provided. The
literature exclusion criteria were as follows: i) Duplicate
reports; ii) research design flaws or poor quality; iii) incomplete
data or unclear outcome effects; iv) the statistical method was
incorrect and could not be corrected; v) OR was not provided or
could not be converted into OR (RR, rate difference, HR) and its
95% confidence interval; vi) the measurement data could not provide
mean and standard deviation; and vii) inaccurate animal
experiments.
Copper, a trace element essential for life, plays a
crucial role in various physiological functions such as
respiration, connective tissue formation, wound repair, nutrient
energy metabolism and catecholamine synthesis (18). Additionally, copper serves as an
important regulator of numerous enzymes involved in physiological
processes including neuromodulation and angiogenesis. Dyla et
al (19) demonstrated the key
role of P-type Wilson ATPase in preventing copper deficiency or
toxicity by facilitating the transfer of copper from the liver to
the secretory pathway. Maintaining copper homeostasis relies on
copper transport proteins; dysregulation and subsequent copper
toxicity can occur if this process is disrupted (20). Recent studies have revealed that
copper toxicity significantly impacts normal cardiac function
(21) and contributes to
pathologies including myocardial ischemia/reperfusion (I/R) injury
(22), HF (23), atherosclerosis (24) and arrhythmias (25).
The physiological processes of copper metabolism are
multifaceted but can be broadly categorized into three main stages:
Absorption, transportation and excretion (Fig. 1). The most effective form of copper
absorption occurs within the intestinal epithelial cells, where
dietary copper is digested and absorbed as Cu2+ mediated
by divalent metal transport protein 1 (26). While dietary copper typically
exists in the form of Cu2+, only Cu+ can be
absorbed and utilized by the body (27). Therefore, in various cell types,
Cu2+ often requires reduction to Cu+ through
the action of reductases such as DCYTB, and uptake via a
high-affinity mechanism involving CTR1 (28). COX17 facilitates the transport of
copper ions to specific proteins such as cytochrome C oxidase (SCO)
1, SCO2 and COX11 to activate enzyme activity within the
respiratory chain (29). Chaperone
protein copper chaperone for superoxide dismutase (CCS) facilitates
the transport of copper ions to superoxide dismutase 1 (SOD1)
(28), and ATOX1 plays a crucial
role in transporting copper ions to the nucleus for binding with
transcription factors to regulate gene expression while also
transferring them from the trans-Golgi network (TGN) to ATPase
α-peptide (ATP7A) and ATPase β-peptide (ATP7B) (30). ATP7A facilitates the efflux of
Cu+ from the intestinal epithelium into the circulation
whereas ATP7B stores excess Cu+ in intracellular
vesicles to maintain normal homeostasis (31). Copper bound with ceruloplasmin (CP)
or albumin can be transported within specific organelles or
secreted from cells and transferred to the liver via the
bloodstream (32). In hepatocytes,
ATP7B plays a crucial role in facilitating the p62-mediated release
of copper from intracellular stores into the cytoplasm, thereby
enabling the excretion of surplus copper through its incorporation
into bile (33).
Apoptosis induced by copper ions is a crucial step
in several cardiovascular diseases, involving oxidative stress,
copper-mitochondrial crosstalk and vascular homeostasis (34). These mechanisms can contribute to
the development of atherosclerosis, myocardial injury and coronary
heart disease (35). Oxidative
stress is associated with excess copper ions, while copper ion
deficiency affects the association between copper and the
mitochondria, as well as copper and vascular regulation (36).
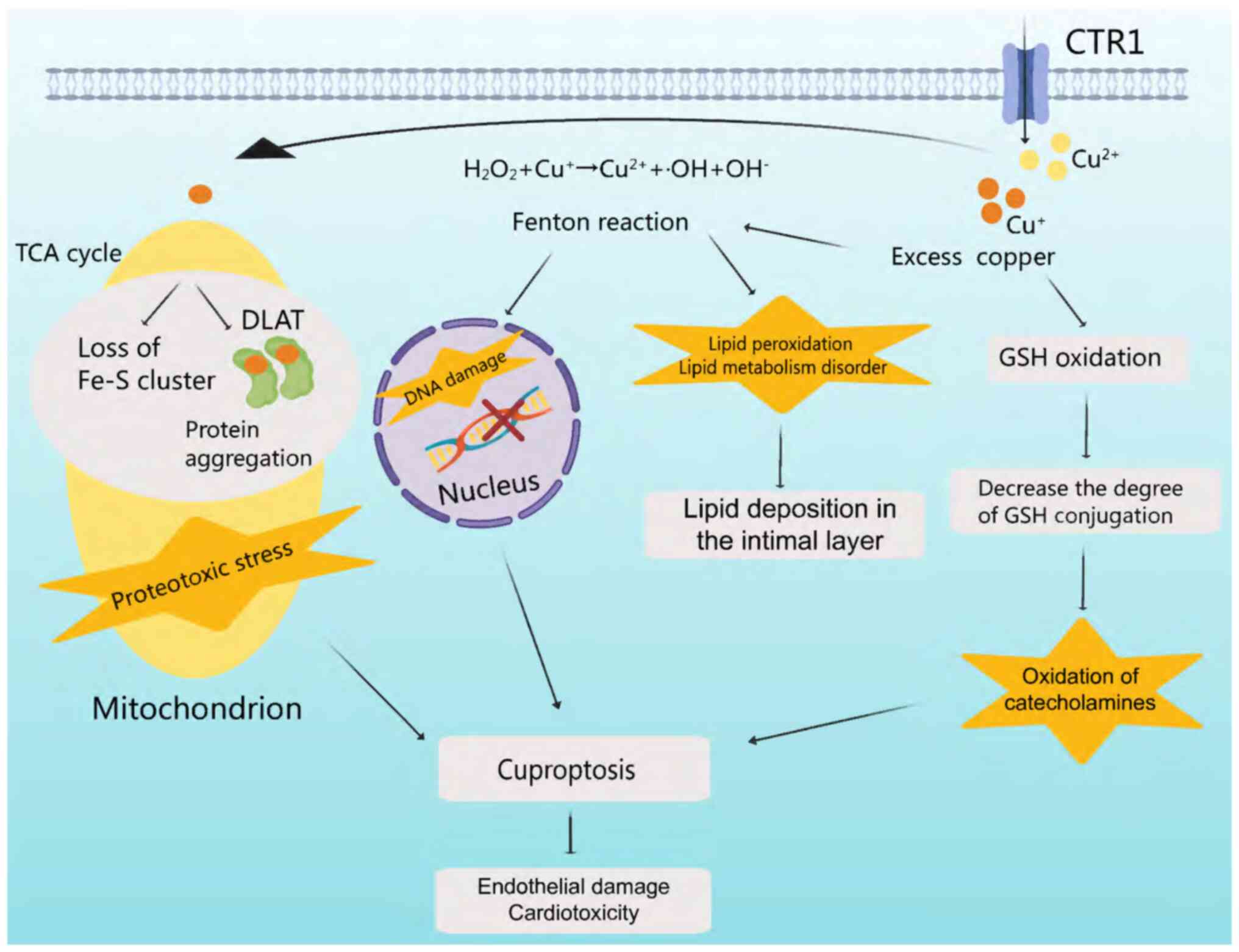
Cells meticulously maintain a delicate balance
between oxidation and antioxidant capacity (37). Disruption of oxidative homeostasis
in the cardiovascular system can lead to oxidative stress, causing
cellular damage and cardiovascular disease (38). Copper ions undergo cycles of
oxidation and reduction, generating hydroxyl radicals which can
cause DNA damage and lipid peroxidation (39). Excess copper promotes glutathione
(GSH) oxidation, leading to catecholamine oxidation (39). Copper-mediated Fenton reactions
induce oxidative stress, which disrupts lipid metabolism and
induces DNA fragmentation (14).
Direct binding of copper ions to fatty acylated components in the
tricarboxylic acid (TCA) cycle results in protein aggregation and
dysregulation (40), blocking the
TCA cycle and inducing proteotoxic stress and cell death (Fig. 2) (41).
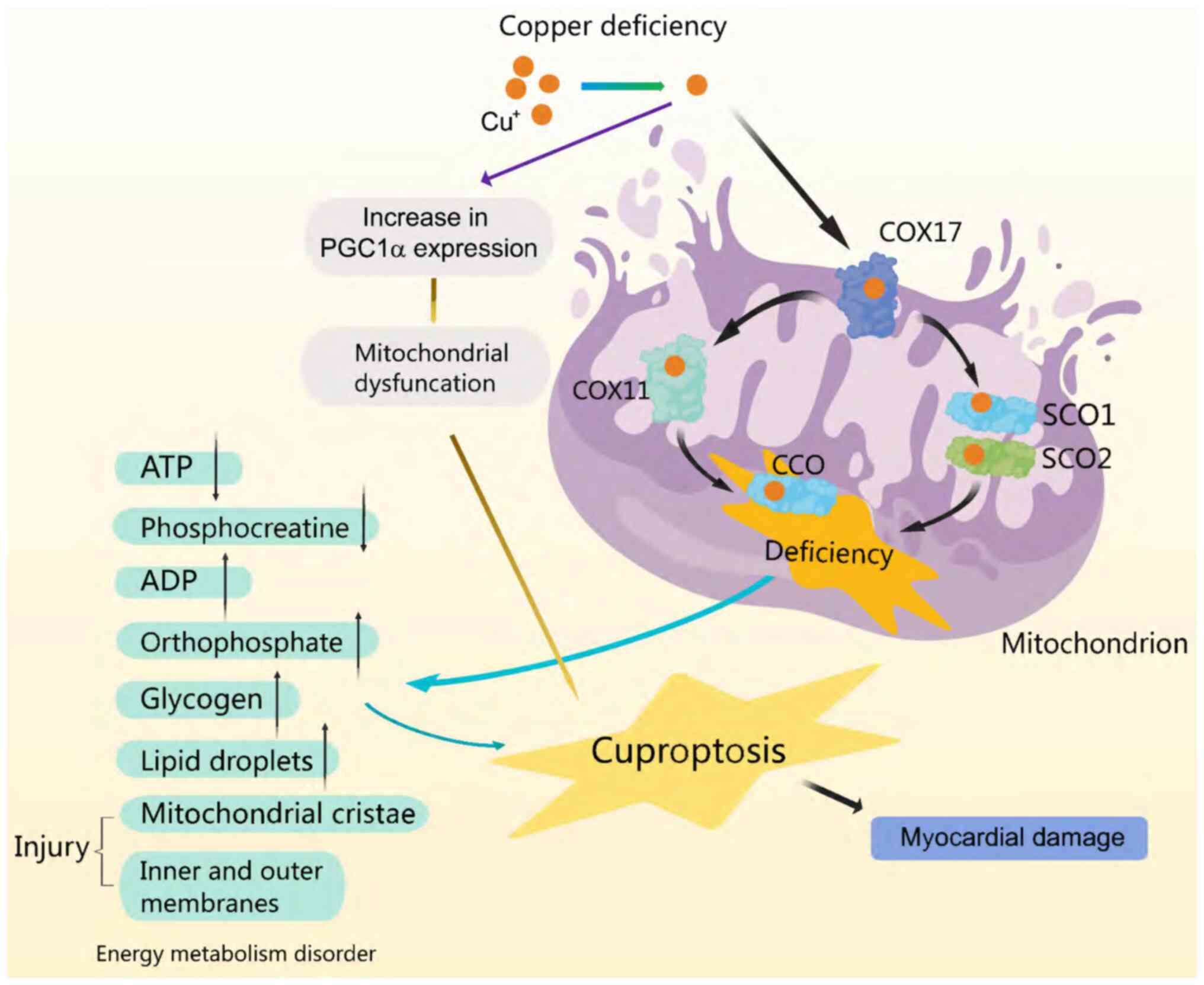
Mitochondria coordinate cellular metabolic processes
and serve as a comprehensive source of metabolism and energy
(42). Micronutrients are
essential for proper mitochondrial function, particularly in the
cardiac muscle cells (43). In the
latter scenario, it is crucial for the activation of Cu+
enzyme function within the respiratory chain and for ensuring the
physiological function of CCO (44). Copper deficiency results in reduced
transport via COX17 to SCO1/SCO2 and COX11, leading to diminished
CCO synthesis (45). In addition,
mitochondrial dysfunction occurs due to copper deficiency via an
increase in the expression of other mitochondria-associated protein
molecules (46). Specifically,
increased expression of peroxisome proliferator-activated
receptor-γ coactivator-1α protein, a key regulator of mitochondrial
biosynthesis, disrupts the mitochondrial structure and leads to
dysfunctional proliferation, which is involved in the development
of certain cardiac diseases, such as heart failure and
atherosclerosis (45). CCO
activity and expression, leading to cardiomyocyte fibre stiffening,
ultimately contribute to fatal cardiovascular diseases (44). In addition, exacerbation of the
reduction in ATP and observed phosphocreatine levels, is
accompanied by an increase in ADP and orthophosphate levels, in
both cardiac tissue and other organs (30). Changes in the structure of the
cristae and mitochondrial membranes are concomitant with these
alterations, eventually leading to mitochondrial rupture, which
impairs energy metabolism and induces myocardial injury (46) (Fig.
3).
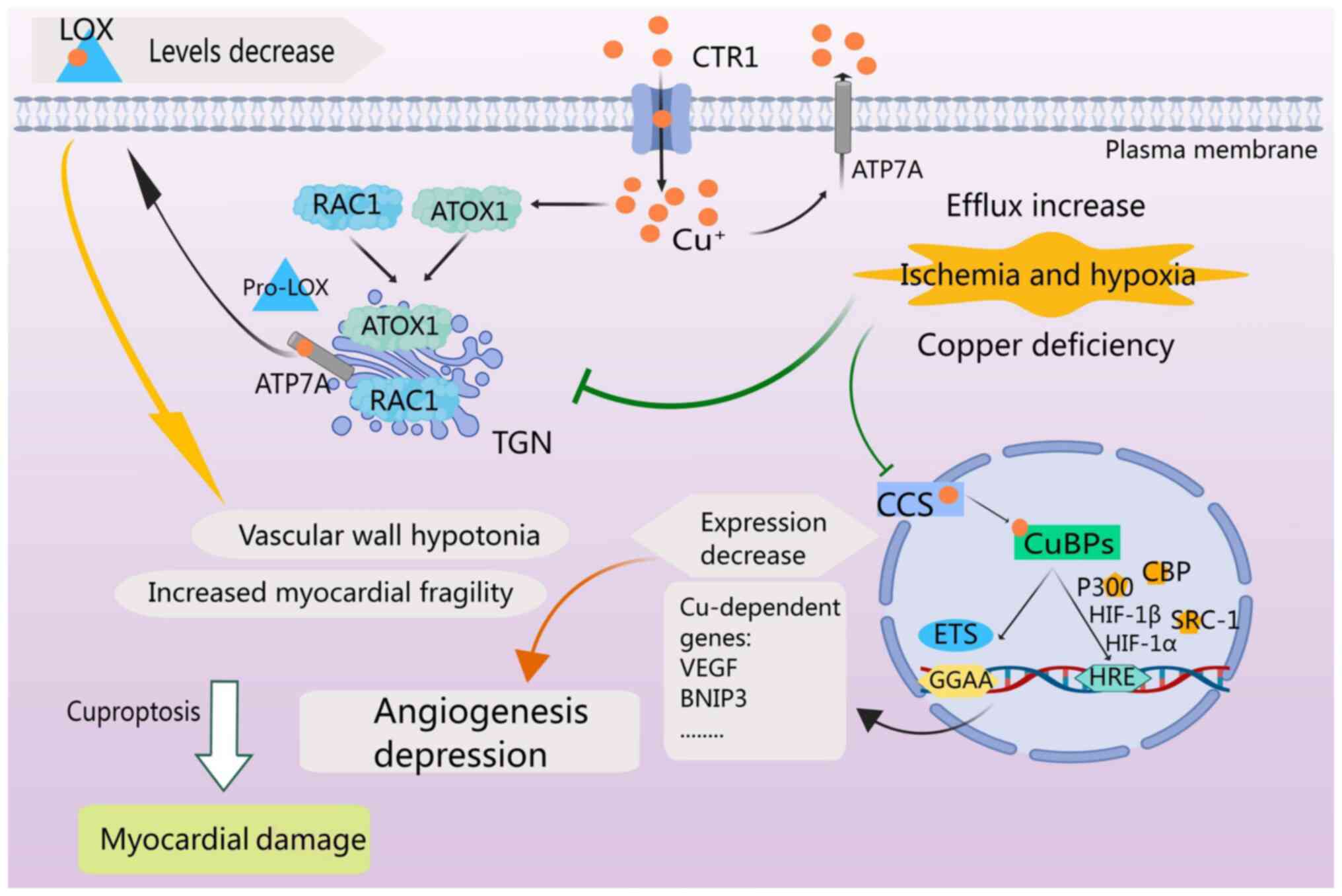
Hypoxia-inducible factor 1 (HIF-1) is the primary
transcription factor regulating angiogenesis (47). Following ischemic injury, the
copper concentration in the heart gradually decreases and is
positively associated with HIF-1-mediated angiogenesis and the
expression of angiogenesis and glycolysis-associated genes
(48). HIF-1α, a crucial subunit
of HIF-1, plays a major role in regulating HIF-1 activity during a
myocardial infarction (49).
Copper plays a role in multiple aspects of HIF-1 regulation,
including the stabilization of HIF-1α, formation of transcriptional
complexes and binding to hypoxia-responsive element (HRE) sequences
of target genes (50). Copper
transport into the nucleus is facilitated by CCS and subsequently
mediated by the copper-binding protein (CuBP) (49). The ‘GGAA’ core motif is critical
for binding to copper-dependent gene sites (49). Additionally, p300, also termed
CREB-binding protein, and steroid receptor coactivator-1 act as
cofactors to form the HIF-1 transcriptional complex 38 (49). Copper also plays a role in
mediating the interaction between HIF-1 and HRE to initiate the
expression of copper-dependent genes such as VEGF (49). For vascular maturation,
lysyloxidase (LOX) is critical, and copper can modulate LOX
production through ATOX1, ATP7A and Ras-related C3 botulinum toxin
substrate 1 (RAC1) (51).
Increased copper efflux is observed during ischemia and under
hypoxic conditions (50).
Inhibition of these mechanisms by copper efflux results in reduced
vascular wall tone, increased myocardial fragility, reduced
angiogenesis and ultimately myocardial injury (Fig. 4) (52).
A summary of the effects of excess copper on
cardiovascular diseases is provided in Table I. When excess copper is ingested,
oxidative stress caused by excessive copper can lead to various
problems, including lipid metabolism disorder, thus inducing
(14) cardiovascular diseases such
as arrhythmia, atherosclerosis and HF.
When a patient experiences an arrhythmia, it may
indicate the presence of an abnormal heart rhythm, such as
premature beats, atrial fibrillation, ventricular fibrillation or
ventricular tachycardia (53).
Elevated serum copper levels are often observed in these patients
due to the release of copper-containing enzymes after myocardial
injury or because sympathetic nerves promote the release of copper
from liver stores into the bloodstream during stress (54). Hsiao et al (54) demonstrated that copper disrupts the
cardiac rhythm of zebrafish larval embryos. A trial with
Mediterranean mussels showed that high Cu2+
concentrations led to valve closure and a decreased heart rate in
mussels (55). Bobbio et al
(56) found a high prevalence of
ventricular fibrillation and tachycardia in patients with
arrhythmia, which was associated with myocardial copper
accumulation.
Atherosclerosis, inflammation and the accumulation
of lipids in the inner layer of blood vessel walls lead to vascular
blockage and contributes to the development of coronary heart
disease, cerebrovascular disease and peripheral arterial vascular
disease, and is associated with significant clinical morbidity and
mortality rates (57). Extensive
research has demonstrated a close association between elevated
serum copper levels and atherosclerosis (58). Copper serves as a cofactor for
numerous enzymes and participates in normal physiological
processes. However, excessive levels can exert toxic effects,
causing cellular damage or even death (58). A previous study revealed a positive
association between copper levels and the progression of
atherosclerosis (59). Another
study involving patients with acute myocardial infarction have
supported these findings by showing significantly higher serum
copper levels in those with acute myocardial infarction compared
with non-acute cases (60). Copper
plays a role in the oxidative modification of low density
lipoprotein (LDL) and influences their susceptibility to oxidation;
increased copper levels promote LDL oxidation and stimulate the
production of oxidized lipoproteins, thus promoting the formation
of atherosclerotic plaques (61).
Furthermore, increased copper levels can increase reactive oxygen
species (ROS) production and activate the NF-κB signalling pathway,
exacerbating inflammatory changes within the vascular wall and
promoting atherosclerosis (25). A
study involving mice also found that the release of free copper
ions induced neointimal thickening and contributed to the
development of atherosclerotic lesions in damaged rat carotid
arteries (62).
HF is characterized by impaired pumping function of
the heart and inadequate cardiac output, which cannot meet the
metabolic demands of bodily tissues due to multiple causes. It
represents the terminal stage in the progression of various
cardiovascular diseases (63).
Mitochondrial energy supply, inflammation levels and intracellular
oxidative stress are key mechanisms in the pathogenesis of HF
(64). Of particular importance,
copper plays a regulatory role in several biological processes
associated with HF (65). A
meta-analysis (66) incorporating
1,504 patients revealed a significant association between elevated
serum copper levels and HF. Similarly, an animal model of
diabetes-induced HF showed altered expression levels of copper
transporter proteins and disturbed copper metabolism in rat
cardiomyocytes (67). Fluctuations
in copper homeostasis during HF may disrupt mitochondrial function
and exacerbate oxidative stress (66). Elevated copper levels have been
demonstrated to decrease the activity of antioxidant enzymes such
as catalase and GSH peroxidase in rat tissues, leading to DNA
damage via peroxygen-derived free radicals (68). In primary cardiac cells,
Cu2+ has been shown to increase interleukin-6 release
and activate MAP kinase (69),
contributing to cardiac inflammation and hypertrophy (59). Copper-induced oxidative stress also
promotes inflammation through ROS production, resulting in lipid,
protein and DNA damage (70).
Mutations in sarcomeres in hypertrophic
cardiomyopathy (HCM) lead to cardiac fibrosis, which affects
contractility (71). Typical
histopathological features of the disease include myocyte
disorganization and changes in myocardial fibrosis (72). A study on patients with HCM
revealed abnormal copper accumulation, and the use of the
Cu2+ selective chelator tretinoin hydrochloride was
found to slow or reverse disease progression in HCM (73). Accumulation of ROS plays a crucial
role in the pathogenesis of cardiomyopathy, leading to myocardial
fibrosis, ventricular remodelling and direct damage to
cardiomyocytes (74). Excess
copper ions can catalyse the formation of destructive hydroxyl
radicals via the Fenton reaction, resulting in oxidative stress and
inflammatory responses that cause structural and morphological
changes in cardiac tissue (75).
In addition to oxidative stress, excessive copper ion accumulation
alters energy metabolism patterns within cardiomyocytes by inducing
abnormal aggregation of thioctylated proteins and loss of
iron-sulfur cluster proteins in the respiratory chain complex
through direct binding to thioctylated proteins in the
mitochondrial TCA cycle (76).
Excess copper can lead to the development of aortic
aneurysms, which are characterized by abnormal dilation of the
aortic wall and compression of the surrounding organs (77). The aetiology of aortic aneurysms is
complex, with a previous a study suggesting possible links to
inflammation, copper toxicity and endothelial cell damage (78). In cases of pathological
inflammation in aortic aneurysms, tissue copper levels are
significantly elevated (79).
Excess copper disrupts the balance between NO production and
degradation, which plays a crucial role in regulating vascular tone
and endothelial function (79).
Elevated copper levels upregulate inducible NO synthase expression,
leading to excessive NO production; peroxynitrite is then formed as
a potent oxidant that can cause further oxidative damage (80). Furthermore, copper interacts with
atherosclerotic risk factors such as homocysteine, resulting in
increased hydrogen peroxidation and oxidative stress (81). Please refer to Table I for details (82–92).
Not only does an excess of copper lead to
cardiovascular disease, but copper deficiency may also result in
alterations to cardiac morphology, swelling of the mitochondria,
and fragmentation and enlargement of myocytes (93). During a copper-deficient state, the
copper deficiency leads to mitochondrial dysfunction and ROS
accumulation, which in-turn lead to cardiovascular diseases
including myocardial I/R injury (94). Nutritional surveys conducted in the
West as far back as the 1990s revealed a significant decline in
dietary copper content, with half of adults consuming less than the
amount recommended by the European Community and the United Kingdom
(95). Additionally, at least a
quarter of adults were found to consume less than the average
estimated requirement published by the United States and Canada,
which suggests that diseases such as Alzheimer's disease, ischemic
heart disease and osteoporosis are linked to a low intake of copper
(96). Data from one study
summarized information from >60 medical publications
incorporating >2,500 patients suffering from cardiovascular,
musculoskeletal and neurological disorders due to copper
malnutrition. Of these patients, >1,000 benefited from copper
supplementation (97). Saari
(98) demonstrated that dietary
copper deficiency resulted in various cardiovascular defects with
systemic effects including hypertension, increased inflammation,
anaemia decreased blood clotting and possibly atherosclerosis,
which also has effects on specific organs or tissues such as
diminished structural integrity of the heart and blood vessels,
impaired energy use by the heart, decreased contractility of the
heart, altered ability for blood vessels to control their diameter
growth and structural-functional changes in circulating blood
cells.
Copper deficiency hinders the function of
mitochondria and energy production, which leads to impaired
mitochondrial respiration and ECG abnormalities in copper-deficient
hearts (99). For example,
myocardial dysfunction has been linked to mitochondrial dysfunction
caused by copper deficiency-induced expression of molecules related
to mitochondria (100). Oxidative
stress (101), inflammation,
endothelial dysfunction and impaired lipid metabolism (102) may be associated with the
mechanism behind copper deficiency-induced atherosclerosis. Enzyme
function is also compromised by copper deficiency, including that
of copper-zinc SOD (Cu-Zn SOD), resulting in a weakened antioxidant
defence system and increased vulnerability to oxidative stress,
contributing to the development of atherosclerosis (103). Furthermore, copper deficiency
impairs the activity of certain Cu/Zn-oxides, which may lead to
increased accumulation of ROS and oxidative stress, which further
promotes inflammation and atherosclerosis (104). Other research has indicated that
copper deficiency inhibits the expression of adhesion molecules,
while activating endothelial cells through inducing leukocyte
adhesion (105). Cholesterol
levels are a significant risk factor for atherosclerosis, as
demonstrated by a study by Habas and Shang (106), which found that copper deficiency
elevated cholesterol levels and impacted lipid metabolism thereby
influencing the development of atherosclerosis (107). Additionally, Jeney et al
(108) suggested that reduced NO
levels due to SOD1, itself induced by copper deficiency, may
contribute to atherosclerosis by hindering vasodilation.
The mechanism of myocardial I/R injury caused by
copper deficiency may be attributed to the upregulation of
inflammatory factors such as interleukins and free radicals due to
the excessive accumulation of ROS (109). By contrast, relatively low levels
of copper may exacerbate the inflammatory response during I/R
injury (22). Copper
supplementation may mitigate tissue damage during this process
(109). An early study indicated
that copper deficiency decreases CCO activity in the heart
(110). Thus, copper deficiency
in cardiomyocytes significantly reduces the expression of copper
chaperones and the enzymatic activity of CCO, leading to a decrease
in left ventricular (LV) copper content and impaired LV contractile
function in dilated cardiomyopathy (111). Copper deficiency also induces
cardiac hypertrophy (112).
However, a direct reduction in the size of certain hypertrophic
cardiomyocytes and replication of other size-reduced hypertrophic
cardiomyocytes contribute significantly to the regression of
copper-deficient cardiac hypertrophy, resulting in the
normalization of the size and number of cardiomyocytes in the heart
(113). It has also been
demonstrated that copper deficiency decreases vascular elasticity
and increases platelet aggregation, thereby increasing the risk of
ischemic vascular disease (114).
Furthermore, a nutritional study has shown that prolonged periods
of dietary copper deficiency can lead to elevated cholesterol,
blood pressure, homocysteine, isoprostane and uric acid levels;
adversely affect arteries and the cardiac rhythm; decrease
dehydroepiandrosterone levels; impair glucose tolerance and
paraoxonase activity; and promote thrombosis and oxidative damage
(115).
Despite high levels of research into the
understanding of the role of copper physiologically and
pathophysiologically, there remain uncertainties regarding the
accurate measurement of copper levels and the long-term effects of
copper exposure on cardiovascular health (118). The World Health Organization has
recommended a daily intake of 0.9 mg/day for adults weighing 70 kg
and has specified a safe upper limit (119). The U.S. Environmental Protection
Agency (EPA) has not formally established an oral reference dose
for copper or a maximum acceptable dose for inclusion in its
Integrated Risk Information System database (120). Toscano et al (121) demonstrated that Wistar rats
exposed to copper for 30 days showed a significant increase in
blood pressure and myocardial contractility. Another study
emphasized the potential adverse effects of copper exposure on
myocardial contractility at recommended daily doses, tolerable
maximum intake doses, and twice the tolerable maximum intake level,
but the validity of these doses requires verification through
extensive experimentation (122).
Serum copper concentration is influenced not only by food and
environmental intake, but also by absorption, excretion and
storage, due to human variability. Therefore, it is challenging to
estimate the individual benefits or losses from serum copper
concentration or determine an optimal level, and researching these
issues remains a challenge (123).
Currently, treatments based on copper metabolism in
cardiovascular disease can encompass a wide range of options,
including copper chelating agents [such as triethylenetetramine
(TETA), tetrathiomolybdate (TTM) and disodium ethylene diamine
tetraacetic acid (EDTA)], small-molecule inhibitors of copper
chaperone proteins (such as DCAL50), copper ion carriers and
natural antidote agents (Table
II).
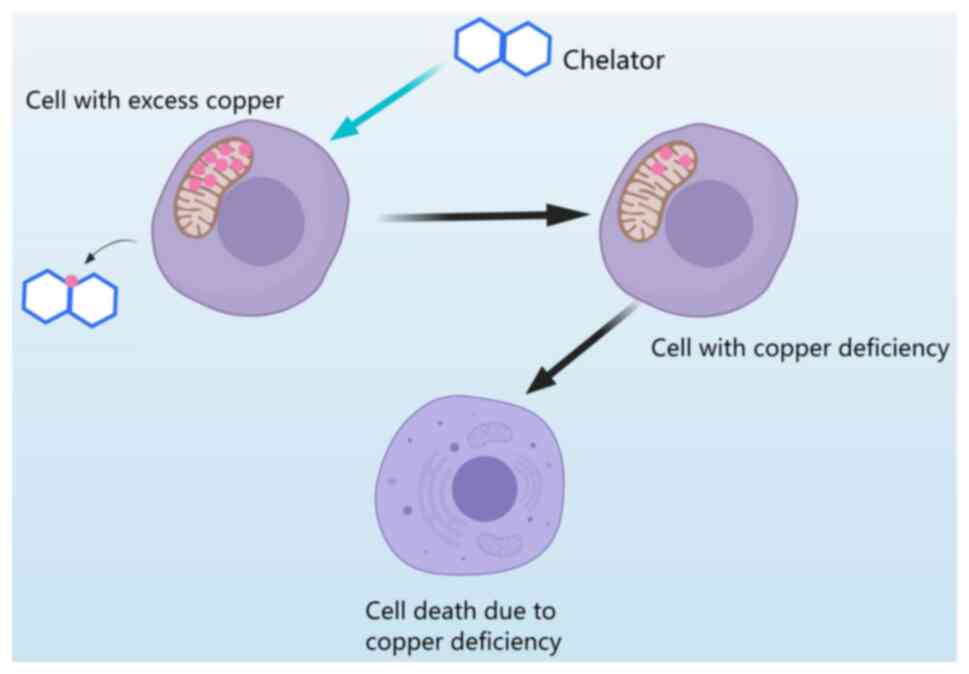
Copper chelators are compounds that bind to copper
ions to remove toxic copper from cells and prevent its accumulation
(124). However, excessive
chelation of copper by these compounds can lead to cellular copper
deficiency and cell death (9)
(Fig. 5). TETA is a chelator that
specifically binds to Cu2+ ions and has been widely used
for the treatment of Wilson's disease (125). It may improve myocardial function
in diabetic patients by restoring mitochondrial CCO, CCS and SOD1
activity (67). Additionally, it
inhibits the elevation of serum copper levels and effectively
mitigates the increase in CP activity following myocardial ischemia
(126). TTM is a small
hydrophilic compound with high specificity as a copper chelator. It
is commonly used to treat Wilson's disease and exhibits a
favourable safety profile in this regard (127). Wilson's disease is typically
characterized by excessive accumulation of copper in the liver
(25). TTM chelates bioavailable
copper by forming a TTM-Cu-protein triple complex (128). A study has found that TTM
specifically forms a TTM-Cu-ATX1 complex with the intracellular
chaperone ATX1 to inhibit copper transport to the TGN and its
downstream incorporation into copper proteins (127). Another study revealed that TTM
inhibits atherosclerosis in ApoE-deficient mice by reducing
bioavailable copper and vascular inflammation (129). In addition to TETA and TTM, EDTA
also acts as a metal chelator for various metals including copper
(130). A clinical trial
demonstrated that EDTA disodium-based infusion reduced recurrent
cardiovascular events in type 1 and type 2 diabetic patients with a
prior myocardial infarction (131). Trientine diHClide is another
common copper chelator used for treating Wilson's disease (132), which can selectively bind
Cu2+ ions to improve mitochondrial function in patients
with hypertrophic cardiomyopathy (73), as well as restore mitochondrial
function and normalize myocardial expression and enzymatic activity
of proteins involved in energy metabolism among diabetic patients
(133).
The disadvantages of the use of metal chelators need
to be considered. They have been shown to redistribute heavy metals
from other tissues to the brain, increasing brain neurotoxicity and
leading to the loss of essential metals, such as zinc, or even
hepatotoxicity due to serious side effects due to hepatotoxicity
(134). Therefore, when using
metal chelators for diseases characterized by excess Cu intake, one
must consider carefully their potential risk of neurotoxicity or
loss of essential metals.
In addition to broad-spectrum metal ion chelators,
drugs that specifically regulate the concentration and distribution
of intracellular copper ions have greater potential. DCAL50, an
inhibitor of copper chaperones, blocks intracellular copper ion
transport and can bind to the copper chaperone proteins ATOX1 and
CCS, thereby specifically inhibiting the proliferation of cancer
cells without affecting normal cellular function (135). This mechanism may be attributed
to the interference with copper ion transport leading to increased
ROS levels, mitochondrial dysfunction and reduced ATP production,
ultimately inhibiting Cu/Zn SOD1 activity (136). Inhibitors of copper chaperones
address the concern of other copper chelators in that they
non-selectively bind other metal cations and produce toxic side
effects (11). Further research
into these inhibitors may offer valuable insights for drug
development for the management of cardiovascular diseases.
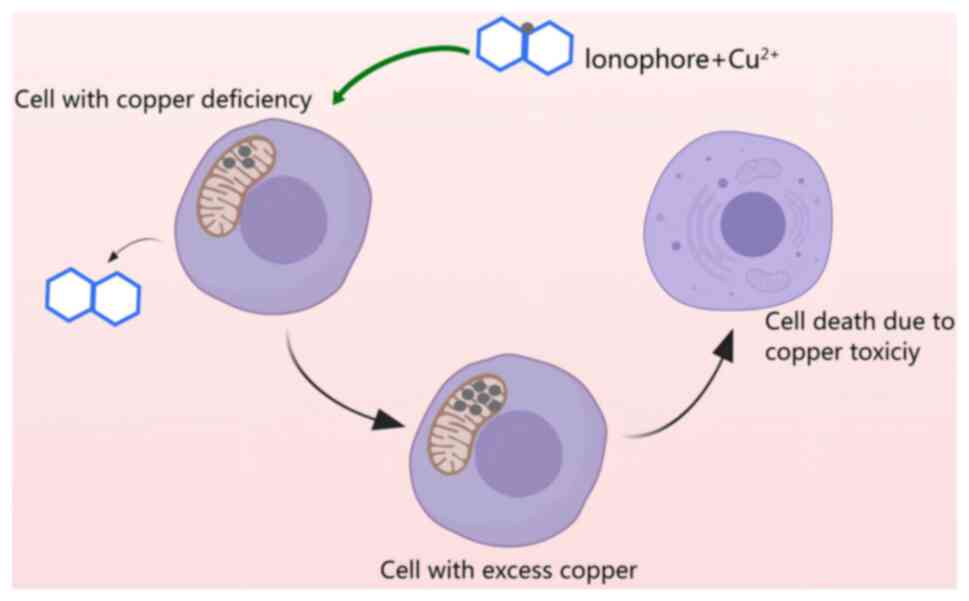
At present, the methodology for addressing copper
deficiency involves the use of copper ion carriers, which are small
molecules that form complexes with copper and facilitate its
transport across the cell membrane and into the mitochondria
(137). The entry of copper ion
carriers into the cell leads to copper accumulation, which in-turn,
triggers oxidative stress and induces cell death (Fig. 6) (138). Common copper ion carriers include
elesclomol and disulfiram. Elesclomol is widely recognized to
induce copper overdose and cell death (139), by acting as a copper ion carrier
with a hydrophilic pore in its centre, to which Cu2+
ions can bind easily, facilitating copper entry into cells, thus
increasing the intracellular copper concentration (140), and this, in-turn increases ROS
levels, triggering oxidative stress (141) and ultimately cell death.
Disulfiram is another known copper ion carrier that helps transport
copper into cells; it has shown value in the treatment of
alcoholism (138). Both these
copper ion carriers have shown value in cancer treatment, but their
ability to treat cardiovascular disease requires further study
(11). It is important to note
that copper ion carriers do not have a well-defined understanding
of its specificity for copper ions and exhibit a range of
functions, several of which remain incompletely understood
(137). Additionally, they are
not easy to manipulate during transport and inappropriate copper
transport may lead to tissue-specific issues (25), thus, further study is required to
improve the function of these carriers. There are natural
antidotes, derived from herbs and their derivatives, that target
multiple proteins and exhibit fewer side effects and toxicity,
while showing higher stability than synthetic chelating agents. For
example, turmeric, by itself and its derivatives are highly
effective in the treatment of cardiovascular diseases (142). Please refer to Table II for details (143–152).
Copper ion levels in cells must be maintained in a
state of relative equilibrium, as both excessive and deficient
amounts can significantly impact health (176). The present review provides an
overview of the fundamental role of copper and its metabolic
pathways in physiology. It discusses the mechanisms underlying
cardiovascular diseases resulting from copper excess and
deficiency, particularly focusing on the pathogenic mechanisms
related to oxidative stress and mitochondrial metabolism disorders.
Furthermore, it explores potential therapeutic approaches for
managing copper-related cardiovascular diseases, such as using
copper chelating agents and ion carriers, while also highlighting
the limitations of these methods. The balance between normal
cellular oxidation and antioxidants must be maintained, as excess
copper levels can induce oxidative stress, leading to cell death
and cardiovascular disease (14).
Therefore, special attention should be paid to this during
treatment. For instance, Jomova et al (177) demonstrated that an excess of
copper ions led to the aggregation of fatty proteins and abnormal
expression of Fe-S cluster proteins, resulting in protein toxic
stress and cell death. In addition to oxidative stress and
mitochondrial metabolic disorders causing cell death, abnormal
copper levels can induce cell death through ROS, ER and
inflammatory responses (178). An
association has been established between oxidative stress and
inflammation, which inevitably plays a crucial role in the
pathogenesis of cardiovascular diseases such as atherosclerosis,
stroke and HF (41). The presence
of copper ions in cells serves a dual function; clinical studies
have yielded conflicting results regarding the relationship between
copper ion levels and the development of cardiovascular disease
(76). Thus, further in-depth
research is necessary for future validation. It is worth delving
into its pathogenic mechanism and the regulatory mechanisms both
physiologically and pathophysiologically (179). While copper chelating agents and
carriers may treat diseases effectively, their disadvantages are
evident. For example, chelating agents may induce toxicity by
binding with other metal ions while off-target effects make copper
regulation using copper ion carriers difficult, thus additional
studies on how to regulate copper levels are required (180). Furthermore, different organs have
distinct copper ion requirements, further complicating the issue of
tight copper regulation (181).
Investigating the optimal concentration of copper ions in different
organs can offer valuable guidance for the optimal treatment of
copper ions. Future development of ion regulators should focus on
organ/cell specificity and targeting. Recently, Liu et al
(182) developed a
multifunctional nanocomposite material capable of precisely
delivering drugs for treating atherosclerosis. While this method
addresses the issue of copper deficiency, further research is
required to determine if it can also address excessive copper ion
levels. Although various studies on copper-induced cell death have
shown promising results, there still remain several challenges,
such as how the aggregation of fatty acylated proteins triggers
cell death, optimizing the performance of copper regulators and
reducing their side effects, determining the mode of death induced
by copper overload and deficiency, gaining a comprehensive
understanding of the roles of copper in the mitochondria, and
whether copper ion carriers can selectively deliver drugs to
hypertrophic myocardial tissues through drug delivery systems
(183). Further investigation
into these questions will improve the understanding of the
relationship between copper-induced cell death and heart disease,
leading to the development of innovative therapeutic strategies for
heart disease.
Not applicable.
This work was supported by the General Medical Research Projects
from The Science and Technology Bureau of Xi'an City. (grant nos.
2024JH-YLYB-0274 and SZY-NLTL-2024-002).
Not applicable.
YW wrote the manuscript, conceived the topic of
review and collected the data; LF conceived the study and collected
the data; AX and MZ collected and analyzed the data needed in the
article; XM assisted in the collection of data and edited the
manuscript; and JZ participated in drafting the initial draft. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Tang C, Zhou K, Wu D and Zhu H:
Nanoparticles as a novel platform for cardiovascular disease
diagnosis and therapy. Int J Nanomedicine. 19:8831–8846. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ayob R, Vally M, Khan R and Orchard A:
Disparities in patients' understanding of cardiovascular disease
management. Cardiovasc J Afr. 34:1–7. 2024.PubMed/NCBI
|
|
3
|
(WHO) WHO, . Cardiovascular diseases
(CVDs) 2023. 2017.
|
|
4
|
Frumuzachi O, Babotă M, Tanase C and Mocan
A: A systematic review of randomized controlled trials on the
health effects of chocolate enriched/fortified/supplemented with
functional components. Food Funct. 15:6883–6899. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding D, Lawson KD, Kolbe-Alexander TL,
Finkelstein EA, Katzmarzyk PT, van Mechelen W and Pratt M; Lancet
Physical Activity Series 2 Executive Committee, : The economic
burden of physical inactivity: A global analysis of major
non-communicable diseases. Lancet. 388:1311–1324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kazi DS, Katznelson E, Liu CL, Al-Roub NM,
Chaudhary RS, Young DE, McNichol M, Mickley LJ, Kramer DB, Cascio
WE, et al: Climate change and cardiovascular health: A systematic
review. JAMA Cardiol. 9:748–757. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hundley WG: Fifty years of cardiovascular
magnetic resonance: Continuing evolution toward the ‘One-Stop Shop’
for cardiovascular diagnosis. Circulation. 149:1859–1861. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng X, Zhou L, Zeng Q, Zhu H and Luo J:
High serum copper as a risk factor of all-cause and cause-specific
mortality among US adults, NHANES 2011–2014. Front Cardiovasc Med.
11:13409682024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazur T, Malik M and Bieńko DC: The impact
of chelating compounds on Cu2+,
Fe2+/3+, and Zn2+ ions in
Alzheimer's disease treatment. J Inorg Biochem. 257:1126012024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Einhorn V, Haase H and Maares M:
Interaction and competition for intestinal absorption by zinc,
iron, copper, and manganese at the intestinal mucus layer. J Trace
Elem Med Biol. 84:1274592024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue Q, Kang R, Klionsky DJ, Tang D, Liu J
and Chen X: Copper metabolism in cell death and autophagy.
Autophagy. 19:2175–2195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsymbal SA, Refeld AG and Kuchur OA: The
p53 tumor suppressor and copper metabolism: An unrevealed but
important link. Mol Biol (Mosk). 56:1057–1071. 2022.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Jiang Y, Shi H, Peng Y, Fan X and
Li C: The molecular mechanisms of copper metabolism and its roles
in human diseases. Pflugers Arch. 472:1415–1429. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruiz LM, Libedinsky A and Elorza AA: Role
of copper on mitochondrial function and metabolism. Front Mol
Biosci. 8:7112272021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kerkadi A, Raïq H, Prince MS, Bader L,
Soltani A and Agouni A: A cross-sectional analysis of zinc and
copper levels and their relationship to cardiovascular disease risk
markers in Qatar biobank participants. Front Cardiovasc Med.
10:13055882024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Weichenthal S, Kwong JC, Burnett
RT, Hatzopoulou M, Jerrett M, van Donkelaar A, Bai L, Martin RV,
Copes R, et al: A Population-based cohort study of respiratory
disease and long-term exposure to Iron and copper in fine
particulate air pollution and their combined impact on reactive
oxygen species generation in human lungs. Environ Sci Technol.
55:3807–3818. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rashidmayvan M, Mansoori A, Aghasizadeh M,
Dianati M, Barati S, Sahranavard T, Darroudi S, Ahari RK, Esmaily
H, Ferns G, et al: Prediction of cardiovascular disease risk by
serum zinc and copper concentrations and anthropometric
measurements. J Trace Elem Med Biol. 83:1273852024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lan L, Feng Z, Liu X and Zhang B: The
roles of essential trace elements in T cell biology. J Cell Mol
Med. 28:e183902024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dyla M, Kjærgaard M, Poulsen H and Nissen
P: Structure and mechanism of P-type ATPase Ion pumps. Annu Rev
Biochem. 89:583–603. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu C, Liu Y, Zhang Y and Gao L: The role
of a cuproptosis-related prognostic signature in colon cancer tumor
microenvironment and immune responses. Front Genet. 13:9281052022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shan J, Geng R, Zhang Y, Wei J, Liu J and
Bai J: Identification of cuproptosis-related subtypes,
establishment of a prognostic model and tumor immune landscape in
endometrial carcinoma. Comput Biol Med. 149:1059882022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Wang D, Wu C, Wang B, He S, Wang
H, Liang G and Zhang Y: MMP 9-instructed assembly of bFGF
nanofibers in ischemic myocardium to promote heart repair.
Theranostics. 12:7237–7249. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Xu X, Zhang T, Xu L, Tao H, Liu Y,
Zhang Y and Meng X: Fatty acid metabolism disorders and potential
therapeutic traditional Chinese medicines in cardiovascular
diseases. Phytother Res. 37:4976–4998. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mazaheri-Tehrani S, Haghighatpanah MA,
Abhari AP, Fakhrolmobasheri M, Shekarian A and Kieliszek M: Dynamic
changes of serum trace elements following cardiac surgery: A
systematic review and meta-analysis. J Trace Elem Med Biol.
81:1273312023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teschke R and Eickhoff A: Wilson disease:
Copper-mediated Cuproptosis, Iron-related Ferroptosis, and clinical
highlights, with comprehensive and critical analysis update. Int J
Mol Sci. 25:47532024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang L, Garrick MD, Garrick LM, Zhao L
and Collins JF: Divalentmetal transporter 1 (Dmt1) mediates copper
transport in the duodenum of iron-deficient rats and when
overexpressed in iron-deprived HEK-293 cells. J Nutr.
143:1927–1933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui X and Wang Y, Liu H, Shi M, Wang J and
Wang Y: The molecular mechanisms of defective copper metabolism in
diabetic cardiomyopathy. Oxid Med Cell Longev. 2022:54183762022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nývltová E, Dietz JV, Seravalli J,
Khalimonchuk O and Barrientos A: Coordination of metal center
biogenesis in human cytochrome c oxidase. Nat Commun. 13:36152022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pagnotta S, Tramutola A, Barone E, Di
Domenico F, Pittalà V, Salerno L, Folgiero V, Caforio M, Locatelli
F, Petrini S, et al: CAPE and its synthetic derivative VP961
restore BACH1/NRF2 axis in Down syndrome. Free Radic Biol Med.
183:1–13. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matson Dzebo M, Ariöz C and
Wittung-Stafshede P: Extended functional repertoire for human
copper chaperones. Biomol Concepts. 7:29–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tadini-Buoninsegni F and Smeazzetto S:
Mechanisms of charge transfer in human copper ATPases ATP7A and
ATP7B. IUBMB Life. 69:218–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pierson H, Muchenditsi A, Kim BE, Ralle M,
Zachos N, Huster D and Lutsenko S: The function of ATPase copper
transporter ATP7B in intestine. Gastroenterology. 154:168–180.e5.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ovchinnikova EV, Garbuz MM, Ovchinnikova
AA and Kumeiko VV: Epidemiology of Wilson's disease and pathogenic
variants of the ATP7B gene leading to diversified protein
disfunctions. Int J Mol Sci. 25:24022024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang L, Yang P, Lip GYH and Ren J: Copper
homeostasis and cuproptosis in cardiovascular disease therapeutics.
Trends Pharmacol Sci. 44:573–585. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Ling J, Hu Q, Fang C, Mei K, Wu Y,
Huang J, Ling Q, Chen Y, Yu P, et al: Association of serum copper
(Cu) with cardiovascular mortality and all-cause mortality in a
general population: A prospective cohort study. BMC Public Health.
23:21382023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dascalu AM, Anghelache A, Stana D, Costea
AC, Nicolae VA, Tanasescu D, Costea DO, Tribus LC, Zgura A, Serban
D, et al: Serum levels of copper and zinc in diabetic retinopathy:
Potential new therapeutic targets (Review). Exp Ther Med.
23:3242022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lutsenko S, Roy S and Tsvetkov P:
Mammalian copper homeostasis: Physiologic roles and molecular
mechanisms. Physiol Rev. Aug 22–2024.doi:
10.1152/physrev.00011.2024 (Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Forman HJ and Zhang H: Targeting oxidative
stress in disease: Promise and limitations of antioxidant therapy.
Nat Rev Drug Discov. 20:689–709. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Husain N and Mahmood R: Copper(II)
generates ROS and RNS, impairs antioxidant system and damages
membrane and DNA in human blood cells. Environ Sci Pollut Res Int.
26:20654–20668. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jomova K, Alomar SY, Alwasel SH,
Nepovimova E, Kuca K and Valko M: Several lines of antioxidant
defense against oxidative stress: Antioxidant enzymes,
nanomaterials with multiple enzyme-mimicking activities, and
low-molecular-weight antioxidants. Arch Toxicol. 98:1323–1367.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun D, Sun X, Zhang X, Wu J, Shi X, Sun J,
Luo C, He Z and Zhang S: Emerging chemodynamic nanotherapeutics for
cancer treatment. Adv Healthc Mater. 16:e24008092024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang R, Zhu L, Huang Y, Chen J and Tang
Q: Mitochondria: Fundamental characteristics, challenges, and
impact on aging. Biogerontology. Aug 28–2024.doi:
10.1007/s10522-024-10132-8 (Epub ahead of print). View Article : Google Scholar
|
|
43
|
Zhu SY, Liu J and Yu C: Research progress
on mitochondrial copper homeostasis imbalance and fibrosis
diseases. Sheng Li Xue Bao. 76:597–604. 2024.(In Chinese).
PubMed/NCBI
|
|
44
|
Bomer N, Pavez-Giani MG, Grote Beverborg
N, Cleland JGF, van Veldhuisen DJ and van der Meer P: Micronutrient
deficiencies in heart failure: Mitochondrial dysfunction as a
common pathophysiological mechanism? J Intern Med. 291:713–731.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Swaminathan AB and Gohil VM: The role of
COA6 in the mitochondrial copper delivery pathway to cytochrome c
oxidase. Biomolecules. 12:1252022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qian L, Zhu Y, Deng C, Liang Z, Chen J,
Chen Y, Wang X, Liu Y, Tian Y and Yang Y: Peroxisome
proliferator-activated receptor gamma coactivator-1 (PGC-1) family
in physiological and pathophysiological process and diseases.
Signal Transduct Target Ther. 9:502024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xiao Y, Wang T, Song X, Yang D, Chu Q and
Kang YJ: Copper promotion of myocardial regeneration. Exp Biol Med
(Maywood). 245:911–921. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wan JJ, Yi J, Wang FY, Zhang C and Dai AG:
Expression and regulation of HIF-1a in hypoxic pulmonary
hypertension: Focus on pathological mechanism and Pharmacological
Treatment. Int J Med Sci. 21:45–60. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li D, Li D, Wang Z, Li J, Shahzad KA, Wang
Y and Tan F: Signaling pathways activated and regulated by stem
cell-derived exosome therapy. Cell Biosci. 14:1052024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Himoto T, Fujita K, Nomura T, Tani J,
Miyoshi H, Morishita A, Yoneyama H, Kubota S, Haba R, Suzuki Y and
Masaki T: Roles of copper in Hepatocarcinogenesis via the
activation of Hypoxia-inducible factor-1α. Biol Trace Elem Res.
174:58–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Martínez-González J, Varona S, Cañes L,
Galán M, Briones AM, Cachofeiro V and Rodríguez C: Emerging roles
of Lysyl oxidases in the cardiovascular system: New concepts and
therapeutic challenges. Biomolecules. 9:6102019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ashino T, Kohno T, Sudhahar V, Ash D,
Ushio-Fukai M and Fukai T: Copper transporter ATP7A interacts with
IQGAP1, a Rac1 binding scaffolding protein: Role in PDGF-induced
VSMC migration and vascular remodeling. Am J Physiol Cell Physiol.
315:C850–C862. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Viskin S, Chorin E, Schwartz AL, Kukla P
and Rosso R: Arrhythmogenic effects of cardiac memory. Circulation.
146:1170–1181. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hsiao CD, Wu HH, Malhotra N, Liu YC, Wu
YH, Lin YN, Saputra F, Santoso F and Chen KH: Expression and
purification of recombinant GHK Tripeptides are able to protect
against acute cardiotoxicity from exposure to waterborne-copper in
Zebrafish. Biomolecules. 10:12022020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shen H and Nugegoda D: Real-time automated
behavioural monitoring of mussels during contaminant exposures
using an improved microcontroller-based device. Sci Total Environ.
806:1505672022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bobbio E, Forsgard N, Oldfors A,
Szamlewski P, Bollano E, Andersson B, Lingbrant M, Bergh N, Karason
K and Polte CL: Cardiac arrest in Wilson's disease after curative
liver transplantation: A life-threatening complication of
myocardial copper excess? ESC Heart Fail. 6:228–231. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bagheri B, Akbari N, Tabiban S, Habibi V
and Mokhberi V: Serum level of copper in patients with coronary
artery disease. Niger Med J. 56:39–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kciuk M, Gielecińska A, Kałuzińska-Kołat
Ż, Yahya EB and Kontek R: Ferroptosis and cuproptosis:
Metal-dependent cell death pathways activated in response to
classical chemotherapy-Significance for cancer treatment? Biochim
Biophys Acta Rev Cancer. 1879:1891242024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen Z, Li YY and Liu X: Copper
homeostasis and copper-induced cell death: Novel targeting for
intervention in the pathogenesis of vascular aging. Biomed
Pharmacother. 169:1158392023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Begum S, Sultana I, Faysal MR, Alam S,
Tasnim J, Akter T, Hossain MS, Banu M, Jenea AT, Hasan M, et al:
Study of changes in serum copper level in patients with acute
myocardial infarction. Mymensingh Med J. 32:39–43. 2023.PubMed/NCBI
|
|
61
|
El-Hajjar L, Hindieh J, Andraos R,
El-Sabban M and Daher J: Myeloperoxidase-oxidized LDL activates
human aortic endothelial cells through the LOX-1 scavenger
receptor. Int J Mol Sci. 23:28372022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gao L and Zhang A: Copper-instigated
modulatory cell mortality mechanisms and progress in oncological
treatment investigations. Front Immunol. 14:12360632023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He Y, Huang W, Zhang C, Chen L, Xu R, Li
N, Wang F, Han L, Yang M and Zhang D: Energy metabolism disorders
and potential therapeutic drugs in heart failure. Acta Pharm Sin B.
11:1098–1116. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xing L, Liu Y, Wang J, Tian P and Liu P:
High-density lipoprotein and heart failure. Rev Cardiovasc Med.
24:3212023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yuan HJ, Xue YT and Liu Y: Cuproptosis,
the novel therapeutic mechanism for heart failure: A narrative
review. Cardiovasc Diagn Ther. 12:681–692. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Huang L, Shen R, Huang L, Yu J and Rong H:
Association between serum copper and heart failure: A
meta-analysis. Asia Pac J Clin Nutr. 28:761–769. 2019.PubMed/NCBI
|
|
67
|
Zhang S, Liu H, Amarsingh GV, Cheung CCH,
Wu D, Narayanan U, Zhang L and Cooper GJS: Restoration of
myocellular copper-trafficking proteins and mitochondrial copper
enzymes repairs cardiac function in rats with diabetes-evoked heart
failure. Metallomics. 12:259–272. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu Y and Miao J: An emerging role of
defective copper metabolism in heart disease. Nutrients.
4:7002019.
|
|
69
|
Qi W, Liu L, Zeng Q, Zhou Z, Chen D, He B,
Gong S, Gao L, Wang X, Xiong J, et al: Contribution of cuproptosis
and Cu metabolism-associated genes to chronic obstructive pulmonary
disease. J Cell Mol Med. 27:4034–4044. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bost M, Houdart S, Oberli M, Kalonji E,
Huneau JF and Margaritis I: Dietary copper and healthy: Current
evidence and unresolved issues. J Trace Elem Med Biol. 35:107–115.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gelpi Acevedo LM, Salinas AL, Polanco JS,
Nizami H, Marsh D, Patel M, Parikh K and Jain R and Jain R: A
narrative review of the pathophysiology and treatment of
hypertrophic cardiomyopathy. South Med J. 115:926–929. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Butzner M, Aronitz E, Cameron H, Tantakoun
K, Shreay S and Drudge C: An evidence review and gap analysis for
obstructive hypertrophic cardiomyopathy. BMC Cardiovasc Disord.
24:4162024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Reid A, Miller C, Farrant JP, Polturi R,
Clark D, Ray S, Cooper G and Schmitt M: Copper chelation in
patients with hypertrophic cardiomyopathy. Open Heart.
9:e0018032022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cinato M, Andersson L, Miljanovic A,
Laudette M, Kunduzova O, Borén J and Levin MC: Role of perilipins
in oxidative Stress-implications for cardiovascular disease.
Antioxidants (Basel). 13:2092024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ali SA, Bommaraju S, Patwa J, Khare P,
Rachamalla M, Niyogi S and Datusalia AK: Melatonin attenuates
extracellular matrix accumulation and cardiac injury manifested by
copper. Biol Trace Elem Res. 201:4456–4471. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Van Den Heuvel LJF, Peeters S, Meester
JAN, Coucke PJ and Loeys BL: An exploration of alternative
therapeutic targets for aortic disease in Marfan syndrome. Drug
Discov Today. 29:1040232024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu B, Yang H, Song YS, Sorenson CM and
Sheibani N: Thrombospondin-1 in vascular development, vascular
function, and vascular disease. Semin Cell Dev Biol. 155:32–44.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tsui KH, Hsiao JH, Lin LT, Tsang YL, Shao
AN, Kuo CH, Chang R, Wen ZH and Li CJ: The Cross-communication of
Cuproptosis and regulated cell death in human pathophysiology. Int
J Biol Sci. 20:218–230. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li Y, Qi P, Song SY and Wang Y, Wang H,
Cao P, Liu Y and Wang Y: Elucidating cuproptosis in metabolic
dysfunction-associated steatotic liver disease. Biomed
Pharmacother. 174:1165852024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Rucklidge JJ, Eggleston MJF, Darling KA,
Stevens AJ, Kennedy MA and Frampton CM: Can we predict treatment
response in children with ADHD to a vitamin-mineral supplement? An
investigation into pre-treatment nutrient serum levels, MTHFR
status, clinical correlates and demographic variables. Prog
Neuropsychopharmacol Biol Psychiatry. 89:181–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kunutsor SK, Dey RS and Laukkanen JA:
Circulating serum copper is associated with atherosclerotic
cardiovascular disease, but not venous thromboembolism: A
prospective cohort study. Pulse (Basel). 9:109–115. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhu C, Wang B, Xiao L, Guo Y, Zhou Y, Cao
L, Yang S and Chen W: Mean platelet volume mediated the
relationships between heavy metals exposure and atherosclerotic
cardiovascular disease risk: A community-based study. Eur J Prev
Cardiol. 27:830–839. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Alexanian I, Parissis J, Farmakis D,
Athanaselis S, Pappas L, Gavrielatos G, Mihas C, Paraskevaidis I,
Sideris A, Kremastinos D, et al: Clinical and echocardiographic
correlates of serum copper and zinc in acute and chronic heart
failure. Clin Res Cardiol. 103:938–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Malek F, Jiresova E, Dohnalova A,
Koprivova H and Spacek R: Serum copper as a marker of inflammation
in prediction of short term outcome in high risk patients with
chronic heart failure. Int J Cardiol. 113:e51–e53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Nyström-Rosander C, Frisk P, Edvinsson M,
Hjelm E, Thelin S, Friman G and Ilbäck NG: Thoracic aortic aneurysm
patients with Chlamydophila pneumoniae infection showed a shift in
trace element levels in serum and diseased aortic tissue. J Trace
Elem Med Biol. 23:100–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Koksal C, Ercan M, Bozkurt AK,
Cortelekoglu T and Konukoglu D: Abdominal aortic aneurysm or aortic
occlusive disease: Role of trace element imbalance. Angiology.
58:191–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Qin Z, Konaniah ES, Neltner B, Nemenoff
RA, Hui DY and Weintraub NL: Participation of ATP7A in macrophage
mediated oxidation of LDL. J Lipid Res. 51:1471–1477. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ploplis VA, Cornelissen I, Sandoval-Cooper
MJ, Weeks L, Noria FA and Castellino FJ: Remodeling of the vessel
wall after copper-induced injury is highly attenuated in mice with
a total deficiency of plasminogen activator inhibitor-1. Am J
Pathol. 158:107–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bini G, Santini G and Chelazzi G:
Pre-exposure to cadmium or zinc alters the heart rate response of
the crayfish Procambarus clarkii towards copper. Bull
Environ Contam Toxicol. 95:12–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li Q, Liao J, Lei C, Shi J, Zhang H, Han
Q, Guo J, Hu L, Li Y, Pan J and Tang Z: Metabolomics analysis
reveals the effect of copper on autophagy in myocardia of pigs.
Ecotoxicol Environ Saf. 213:1120402021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li S, Zhao H, Wang Y, Shao Y, Wang B, Wang
Y and Xing M: Regulation of autophagy factors by oxidative stress
and cardiac enzymes imbalance during arsenic or/and copper induced
cardiotoxicity in Gallus gallus. Ecotoxicol Environ Saf.
148:125–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zheng L, Han P, Liu J, Li R, Yin W, Wang
T, Zhang W and Kang YJ: Role of copper in regression of cardiac
hypertrophy. Pharmacol Ther. 148:66–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Charkiewicz AE: Is copper still safe for
us? What do we know and what are the latest literature statements?
Curr Issues Mol Biol. 46:8441–8463. 2024.PubMed/NCBI
|
|
95
|
Milanković V, Tasić T, Leskovac A,
Petrović S, Mitić M, Lazarević-Pašti T, Novković M and Potkonjak N:
Metals on the Menu-analyzing the presence, importance, and
consequences. Foods. 13:18902024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Klevay LM: Is the Western diet adequate in
copper? J Trace Elem Med Biol. 25:204–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Klevay LM: Lack of a recommended dietary
allowance for copper may be hazardous to your health. J Am Coll
Nutr. 17:322–326. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Saari JT: Copper deficiency and
cardiovascular disease: Role of peroxidation, glycation, and
nitration. Can J Physiol Pharmacol. 78:848–855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yamane R, Tanaka M, Kikugawa N, Yasui H,
Takei K, Harada M and Kaneda S: Mesh-like vascular changes in
copper deficiency-induced rat cardiomyopathy. J Toxicol Pathol.
34:127–133. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu ZY, Liu ZY, Lin LC, Song K, Tu B,
Zhang Y, Yang JJ, Zhao JY and Tao H: Redox homeostasis in cardiac
fibrosis: Focus on metal ion metabolism. Redox Biol. 71:1031092024.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ramani PK and Parayil Sankaran B: Menkes
disease. 2023 Nov 14. StatPearls [Internet] Treasure Island (FL):
StatPearls Publishing; Jan. 2024
|
|
102
|
Parsanathan R: Copper's dual role:
Unravelling the link between copper homeostasis, cuproptosis, and
cardiovascular diseases. Hypertens Res. 47:1440–1442. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang S, Li Y, Zhou L, Wang X, Liu L and Wu
M: Copper homeostasis and cuproptosis in atherosclerosis:
Metabolism, mechanisms and potential therapeutic strategies. Cell
Death Discov. 10:252024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Al-Bayati MA, Jamil DA and Al-Aubaidy HA:
Cardiovascular effects of copper deficiency on activity of
superoxide dismutase in diabetic nephropathy. N Am J Med Sci.
7:41–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang Z, Jin D, Zhou S, Dong N, Ji Y, An P,
Wang J, Luo Y and Luo J: Regulatory roles of copper metabolism and
cuproptosis in human cancers. Front Oncol. 13:11234202023.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Habas K and Shang L: Alterations in
intercellular adhesion molecule 1 (ICAM-1) and vascular cell
adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue
Cell. 54:139–143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen L, Min J and Wang F: Copper
homeostasis and cuproptosis in health and disease. Signal Transduct
Target Ther. 7:3782022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jeney V, Itoh S, Wendt M, Gradek Q,
Ushio-Fukai M, Harrison DG and Fukai T: Role of antioxidant-1 in
extracellular superoxide dismutase function and expression. Circ
Res. 96:723–729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tural K, Ozden O, Bilgi Z, Kubat E,
Ermutlu CS, Merhan O, Findik Guvendi K and Kucuker SA: The
protective effect of betanin and copper on heart and lung in
end-organ ischemia reperfusion injury. Bratisl Lek Listy.
121:211–217. 2020.PubMed/NCBI
|
|
110
|
Srinivasan S and Avadhani NG: Cytochrome c
oxidase dysfunction in oxidative stress. Free Radic Biol Med.
53:1252–1263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Johnson WT and Newman SM Jr: Hearts in
adult offspring of copper-deficient dams exhibit decreased
cytochrome c oxidase activity, increased mitochondrial hydrogen
peroxide generation and enhanced formation of intracellular
residual bodies. J Nutr Biochem. 18:97–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Medeiros DM and Wildman RE: Newer findings
on a unified perspective of copper restriction and cardiomyopathy.
Proc Soc Exp Biol Med. 215:299–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhou Z, Johnson WT and Kang YJ: Regression
of copper-deficient heart hypertrophy: Reduction in the size of
hypertrophic cardiomyocytes. J Nutr Biochem. 20:621–628. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Gu J, Huang W, Duanmu Z, Zhuang R and Yang
X: Cuproptosis and copper deficiency in ischemic vascular injury
and repair. Apoptosis. 29:1007–1018. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Klevay LM: IHD from copper deficiency: A
unified theory. Nutr Res Rev. 29:172–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Klevay LM and Viestenz KE: Abnormal
electrocardiograms in rats deficient in copper. Am J Physiol.
240:H185–H189. 1981.PubMed/NCBI
|
|
117
|
Viestenz KE and Klevay LM: A randomized
trial of copper therapy in rats with electrocardiographic
abnormalities due to copper deficiency. Am J Clin Nutr. 35:258–266.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Bevan R and Levy L: Biomonitoring for
workplace exposure to copper and its compounds is currently not
interpretable. Int J Hyg Environ Health. 258:1143582024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
WHO. World Health Organization, . Copper
in Drinking-Water. Background document for development of WHO
Guidelines for Drinking-water Quality. 2004.
|
|
120
|
Taylor AA, Tsuji JS, McArdle ME, Adams WJ
and Goodfellow WL Jr: Recommended reference values for risk
assessment of oral exposure to copper. Risk Anal. 43:211–218. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Toscano CM, Filetti FM, Almenara CCP,
Fioresi M and Vassallo DV: Copper exposure for 30 days at a daily
dose twice the recommended increases blood pressure and cardiac
contractility. Life Sci. 300:1205792022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Filetti FM, Schereider IRG, Wiggers GA,
Miguel M, Vassallo DV and Simões MR: Cardiovascular harmful effects
of recommended daily doses (13 µg/kg/day), tolerable upper intake
doses (0.14 mg/kg/day) and twice the tolerable doses (0.28
mg/kg/day) of copper. Cardiovasc Toxicol. 23:218–229. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Abbasi H, Khoshdooz S, Abbasi MM, Pasand M
and Eslamian G: Shining a light on trace elements: A systematic
review and Meta-analysis of serum concentrations in febrile
seizure. Biol Trace Elem Res. May 8–2024.doi:
10.1007/s12011-024-04221-5 (Epub ahead of print). View Article : Google Scholar
|
|
124
|
Gucký A and Hamuľaková S: Targeting
biometals in Alzheimer's disease with metal chelating agents
including coumarin derivatives. CNS Drugs. 38:507–532. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kannan S, Gillespie SW, Picking WL,
Picking WD, Lorson CL and Singh K: Inhibitors against DNA
polymerase I family of enzymes: Novel targets and opportunities.
Biology (Basel). 13:2042024.PubMed/NCBI
|
|
126
|
Yang D, Wang T, Liu J, Wang H and Kang YJ:
Reverse regulation of hepatic ceruloplasmin production in rat model
of myocardial ischemia. J Trace Elem Med Biol. 64:1266862021.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zou Y, Wu S, Xu X, Tan X, Yang S, Chen T,
Zhang J, Li S, Li W and Wang F: Cope with copper: From molecular
mechanisms of cuproptosis to copper-related kidney diseases. Int
Immunopharmacol. 133:1120752024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gromadzka G, Grycan M and Przybyłkowski
AM: Monitoring of copper in wilson disease. Diagnostics (Basel).
13:18302023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wei H, Zhang WJ, McMillen TS, Leboeuf RC
and Frei B: Copper chelation by tetrathiomolybdate inhibits
vascular inflammation and atherosclerotic lesion development in
apolipoprotein E-deficient mice. Atherosclerosis. 223:306–313.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ferrero ME: Neuron protection by EDTA may
explain the successful outcomes of toxic metal chelation therapy in
neurodegenerative diseases. Biomedicines. 10:24762022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fulgenzi A and Ferrero ME: EDTA chelation
therapy for the treatment of neurotoxicity. Int J Mol Sci.
20:10192019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Litwin T, Antos A, Bembenek J and Cz
Onkowska A: Neurological deterioration in Wilson's disease-types,
etiology, course, and management. Discov Med. 36:646–654. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ramli FF, Hashim SAS, Raman B, Mahmod M
and Kamisah Y: Role of Trientine in hypertrophic cardiomyopathy: A
review of mechanistic angles. Pharmaceuticals (Basel). 15:11452022.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Amadi CN, Offor SJ, Frazzoli C and
Orisakwe OE: Natural antidotes and management of metal toxicity.
Environ Sci Pollut Res Int. 26:18032–18052. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Karginova O, Weekley CM, Raoul A, Alsayed
A, Wu T, Lee SS, He C and Olopade OI: Inhibition of copper
transport induces apoptosis in Triple-negative breast cancer cells
and suppresses tumor angiogenesis. Mol Cancer Ther. 18:873–885.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Leitch JM, Jensen LT, Bouldin SD, Outten
CE, Hart PJ and Culotta VC: Activation of Cu, Zn-superoxide
dismutase in the absence of oxygen and the copper chaperone CCS. J
Biol Chem. 284:21863–21871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yang Y, Feng Q, Luan Y, Liu H, Jiao Y, Hao
H, Yu B, Luan Y and Ren K: Exploring cuproptosis as a mechanism and
potential intervention target in cardiovascular diseases. Front
Pharmacol. 14:12292972023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hinshaw DC and Shevde LA: The Tumor
microenvironment innately modulates cancer progression. Cancer Res.
79:4557–4566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Tardito S, Bassanetti I, Bignardi C,
Elviri L, Tegoni M, Mucchino C, Bussolati O, Franchi-Gazzola R and
Marchiò L: Copper binding agents acting as copper ionophores lead
to caspase inhibition and paraptotic cell death in human cancer
cells. J Am Chem Soc. 133:6235–6242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Krasnovskaya O, Naumov A, Guk D, Gorelkin
P, Erofeev A, Beloglazkina E and Majouga A: Copper coordination
compounds as biologically active agents. Int J Mol Sci.
21:39652020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zheng P, Zhou C, Lu L, Liu B and Ding Y:
Elesclomol: A copper ionophore targeting mitochondrial metabolism
for cancer therapy. J Exp Clin Cancer Res. 41:2712022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhang HA and Kitts DD: Turmeric and its
bioactive constituents trigger cell signaling mechanisms that
protect against diabetes and cardiovascular diseases. Mol Cell
Biochem. 476:3785–3814. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Banfi G, Salvagno GL and Lippi G: The role
of ethylenediamine tetraacetic acid (EDTA) as in vitro
anticoagulant for diagnostic purposes. Clin Chem Lab Med.
45:565–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Alvarez HM, Xue Y, Robinson CD,
Canalizo-Hernández MA, Marvin RG, Kelly RA, Mondragón A,
Penner-Hahn JE and O'Halloran TV: Tetrathiomolybdate inhibits
copper trafficking proteins through metal cluster formation.
Science. 327:331–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhang S, Liu H, Amarsingh GV, Cheung CC,
Hogl S, Narayanan U, Zhang L, McHarg S, Xu J, Gong D, et al:
Diabetic cardiomyopathy is associated with defective myocellular
copper regulation and both defects are rectified by divalent copper
chelation. Cardiovasc Diabetol. 13:1002014. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang L, Ward ML, Phillips AR, Zhang S,
Kennedy J, Barry B, Cannell MB and Cooper GJ: Protection of the
heart by treatment with a divalent-copper-selective chelator
reveals a novel mechanism underlying cardiomyopathy in diabetic
rats. Cardiovasc Diabetol. 12:1232013. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Lu J, Gong D, Choong SY, Xu H, Chan YK,
Chen X, Fitzpatrick S, Glyn-Jones S, Zhang S, Nakamura T, et al:
Copper(II)-selective chelation improves function and antioxidant
defences in cardiovascular tissues of rats as a model of diabetes:
Comparisons between triethylenetetramine and three less
copper-selective transition-metal-targeted treatments.
Diabetologia. 53:1217–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Gong D, Lu J, Chen X, Choong SY, Zhang S,
Chan YK, Glyn-Jones S, Gamble GD, Phillips AR and Cooper GJ:
Molecular changes evoked by triethylenetetramine treatment in the
extracellular matrix of the heart and aorta in diabetic rats. Mol
Pharmacol. 70:2045–2051. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Pan Q, Bao LW and Merajver SD:
Tetrathiomolybdate inhibits angiogenesis and metastasis through
suppression of the NFkappaB signaling cascade. Mol Cancer Res.
1:701–706. 2003.PubMed/NCBI
|
|
150
|
Ouyang P, Gottlieb SH, Culotta VL and
Navas-Acien A: EDTA chelation therapy to reduce cardiovascular
events in persons with diabetes. Curr Cardiol Rep. 17:962015.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Lamas GA, Goertz C, Boineau R, Mark DB,
Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J and Lee KL; TACT
Investigators, : Effect of disodium EDTA chelation regimen on
cardiovascular events in patients with previous myocardial
infarction: The TACT randomized trial. JAMA. 309:1241–1250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ujueta F, Arenas IA, Escolar E, Diaz D,
Boineau R, Mark DB, Golden P, Lindblad L, Kim H, Lee KL and Lamas
GA: The effect of EDTA-based chelation on patients with diabetes
and peripheral artery disease in the Trial to Assess Chelation
Therapy (TACT). J Diabetes Complications. 33:490–494. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Jomova K, Makova M, Alomar SY, Alwasel SH,
Nepovimova E, Kuca K, Rhodes CJ and Valko M: Essential metals in
health and disease. Chem Biol Interact. 367:1101732022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Stiles LI, Ferrao K and Mehta KJ: Role of
zinc in health and disease. Clin Exp Med. 24:382024. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Pajarillo EAB, Lee E and Kang DK: Trace
metals and animal health: Interplay of the gut microbiota with
iron, manganese, zinc, and copper. Anim Nutr. 7:750–761. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Qiu Y, Li C, Huang Y, Wu C, Li F, Zhang X
and Xia D: Exploring the causal associations of micronutrients on
Urate levels and the risk of gout: A Mendelian randomization study.
Clin Nutr. 43:1001–1012. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Salehifar E, Shokrzadeh M, Ghaemian A,
Aliakbari S and Saeedi Saravi SS: The study of Cu and Zn serum
levels in idiopathic dilated cardiomyopathy (IDCMP) patients and
its comparison with healthy volunteers. Biol Trace Elem Res.
125:97–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wacewicz M, Socha K, Soroczyńska J,
Niczyporuk M, Aleksiejczuk P, Ostrowska J and Borawska MH:
Concentration of selenium, zinc, copper, Cu/Zn ratio, total
antioxidant status and c-reactive protein in the serum of patients
with psoriasis treated by narrow-band ultraviolet B phototherapy: A
case-control study. J Trace Elem Med Biol. 44:109–114. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ghaemian A, Salehifar E, Jalalian R,
Ghasemi F, Azizi S, Masoumi S, Shiraj H, Mohammadpour RA and
Bagheri GA: Zinc and copper levels in severe heart failure and the
effects of atrial fibrillation on the zinc and copper status. Biol
Trace Elem Res. 143:1239–1246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Luo Z, Yin F, Wang X and Kong L: Progress
in approved drugs from natural product resources. Chin J Nat Med.
22:195–211. 2024.PubMed/NCBI
|
|
161
|
Han Y, Zhu J, Yang L, Nilsson-Payant BE,
Hurtado R, Lacko LA, Sun X, Gade AR, Higgins CA, Sisso WJ, et al:
SARS-CoV-2 infection induces ferroptosis of sinoatrial node
pacemaker cells. Circ Res. 130:963–977. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Del Re DP, Amgalan D, Linkermann A, Liu Q
and Kitsis RN: Fundamental mechanisms of regulated cell death and
implications for heart disease. Physiol Rev. 99:1765–1817. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Corradini E, Buzzetti E and Pietrangelo A:
Genetic iron overload disorders. Mol Angles Med.
75:1008962020.PubMed/NCBI
|
|
165
|
Müller T, Dewitz C, Schmitz J, Schröder
AS, Bräsen JH, Stockwell BR, Murphy JM, Kunzendorf U and Krautwald
S: Necroptosis and ferroptosis are alternative cell death pathways
that operate in acute kidney failure. Cell Mol Life Sci.
74:3631–3645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhao Y, Li Y, Zhang R, Wang F, Wang T and
Jiao Y: The role of Erastin in Ferroptosis and its prospects in
cancer therapy. Onco Targets Ther. 13:5429–5441. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Kunutsor SK, Voutilainen A, Kurl S and
Laukkanen JA: Serum copper-to-zinc ratio is associated with heart
failure and improves risk prediction in middle-aged and older
Caucasian men: A prospective study. Nutr Metab Cardiovasc Dis.
32:1924–1935. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Wang D, Tian Z, Zhang P, Zhen L, Meng Q,
Sun B, Xu X, Jia T and Li S: The molecular mechanisms of
cuproptosis and its relevance to cardiovascular disease. Biomed
Pharmacother. 163:1148302023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Majewski M, Ognik K and Juśkiewicz J:
Copper nanoparticles enhance vascular contraction induced by
prostaglandin F2-alpha and decrease the blood plasma cu-zn ratio in
wistar rats. J Elem. 24:911–922. 2019.
|
|
170
|
Tousson E and El-Gharbawy DM: Impact of
Saussurea lappa root extract against copper oxide nanoparticles
induced oxidative stress and toxicity in rat cardiac tissues.
Environ Toxicol. 38:415–421. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Huo S, Wang Q, Shi W, Peng L, Jiang Y, Zhu
M, Guo J, Peng D, Wang M, Men L, et al: ATF3/SPI1/SLC31A1 signaling
promotes cuproptosis induced by advanced glycosylation end products
in diabetic myocardial injury. Int J Mol Sci. 24:16672023.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Piavchenko G, Alekseev A, Stelmashchuk O,
Seryogina E, Zherebtsov E, Kuznetsova E, Dunaev A, Volkov Y and
Kuznetsov S: A complex morphofunctional approach for zinc toxicity
evaluation in rats. Heliyon. 6:e037682020. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Karagulova G, Yue Y, Moreyra A, Boutjdir M
and Korichneva I: Protective role of intracellular zinc in
myocardial ischemia/reperfusion is associated with preservation of
protein kinase C isoforms. J Pharmacol Exp Ther. 321:517–525. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Yang HJ, Kong B, Shuai W, Zhang JJ and
Huang H: Shensong Yangxin attenuates metabolic syndrome-induced
atrial fibrillation via inhibition of ferroportin-mediated
intracellular iron overload. Phytomedicine. 101:1540862022.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Fang J, Kong B, Shuai W, Xiao Z, Dai C,
Qin T, Gong Y, Zhu J, Liu Q and Huang H: Ferroportin-mediated
ferroptosis involved in new-onset atrial fibrillation with
LPS-induced endotoxemia. Eur J Pharmacol. 913:1746222021.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Kitala K, Tanski D, Godlewski J,
Krajewska-Włodarczyk M, Gromadziński L and Majewski M: Copper and
zinc particles as regulators of cardiovascular system function-a
review. Nutrients. 15:30402023. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Jomova K, Vondrakova D, Lawson M and Valko
M: Metals, oxidative stress and neurodegenerative disorders. Mol
Cell Biochem. 345:91–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Li JW, Mao YM, Chen SL, Ye R, Fei YR, Li
Y, Tong SY, Yang HW and He YB: The interplay between metal ions and
immune cells in glioma: Pathways to immune escape. Discov Oncol.
15:3482024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Yuan S, Chen S, Xi Z and Liu Y:
Copper-finger protein of Sp1: The molecular basis of copper
sensing. Metallomics. 9:1169–1175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Su TA, Bruemmer KJ and Chang CJ: Caged
luciferins for bioluminescent activity-based sensing. Curr Opin
Biotechnol. 60:198–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Guo B, Yang F, Zhang L, Zhao Q, Wang W,
Yin L, Chen D, Wang M, Han S, Xiao H and Xing N: Cuproptosis
induced by ROS responsive nanoparticles with elesclomol and copper
combined with αPD-L1 for enhanced cancer immunotherapy. Adv Mater.
35:e22122672023. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Liu S, Zhao Y, Shen M, Hao Y, Wu X, Yao Y,
Li Y and Yang Q: Hyaluronic acid targeted and pH-responsive
multifunctional nanoparticles for chemo-photothermal synergistic
therapy of atherosclerosis. J Mater Chem B. 10:562–570. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Li Y, Yang J, Zhang Q, Xu S, Sun W, Ge S,
Xu X, Jager MJ, Jia R, Zhang J and Fan X: Copper ionophore
elesclomol selectively targets GNAQ/11-mutant uveal melanoma.
Oncogene. 41:3539–3553. 2022. View Article : Google Scholar : PubMed/NCBI
|