Introduction
Thyroid cancer has the ninth highest cancer
incidence worldwide according to the GLOBOCAN 2020 database of
cancer incidence and mortality by the WHO International Agency for
Research on Cancer (1). Thyroid
cancer is traditionally classified as differentiated thyroid cancer
or anaplastic thyroid cancer (ATC) based on the degree of
differentiation (2). ATC is a rare
but aggressive form of thyroid cancer. Although ATC accounts for
2–5% of all thyroid cancers worldwide, half of all thyroid
cancer-related deaths are attributed to ATC (3,4). Due
to its high proliferation rate and invasive behavior, ATC accounts
for 40–50% of most thyroid cancer-related deaths worldwide
(5). The median survival time of
patients with ATC is 3–5 months, and it is estimated that the
1-year survival rate is between 10 and 20% (6,7). ATC
typically shows a limited response to conventional treatments such
as radioactive iodine and chemotherapy, leaving limited and largely
ineffective treatment options for ATC (8).
In theory, the molecular pathogenesis of ATC
involves the dysregulation of multiple signal transduction pathways
including TP53, TERT promoter, PI3K/AKT/mTOR pathway effectors,
SWI/SNF subunits and histone methyltransferase (9). The abnormal expression of receptor
tyrosine kinases (RTKs), which is associated with gene point
mutations, amplification and protein upregulation, has been
detected in cancer samples including lung, esophageal and thyroid
cancers and contributes to diverse malignant behaviors such as
gain-of-function mutations, genomic amplification, chromosomal
rearrangements and autocrine activation (10). The use of small chemical inhibitors
or monoclonal antibodies such as bevacizumab and cetuximab to
target RTKs has shown effectiveness in cancer treatment, and thus,
comprehending the intricate mechanisms of RTK regulation is of
clinical significance (11,12).
Tyrosine kinase receptor RON, also termed macrophage stimulating
protein receptor, is a typical RTK belonging to the methionine
family and serves a role in regulating the malignant behavior of
specific cancer types through altered RON expression (13,14).
Furthermore, RON-transduced signals are essential components for
the proliferation and survival of thyroid cancer cells (15). A recent study demonstrated that RON
could enhance cholesterol biosynthesis, while upregulating the
glycolytic enzyme hexokinase 2 (HK2), thereby serving a role in
breast cancer (16). In addition,
HK2 serves a pivotal role in influencing the ferroptosis process of
tumor cells (17). However, it
remains unclear whether RON can regulate glycolysis and ferroptosis
processes in ATC, and whether the mechanism involved in influencing
ferroptosis is linked to glycolysis.
Recent research has indicated that RON can promote
the migration and invasion of bladder cancer cells by activating
MAPK/ribosomal s6 kinase (RSK)/cAMP-response element binding
protein (CREB) signaling (18).
Notably, its downstream CREB signaling can affect ferroptosis and
reduce myocardial damage, and can also affect the glycolysis
process in hepatocellular carcinoma cells (19,20).
Additionally, CREB signaling has been reported in breast cancer,
but related mechanisms such as glycolysis and ferroptosis have not
yet been explored (21).
Therefore, the present study aimed to explore RON expression, and
its effect on glycolysis, ferroptosis and chemotherapy sensitivity
of thyroid cancer cells in vitro, and explore its potential
mechanisms.
Materials and methods
Cell culture and establishment of
doxorubicin (Dox)-resistant ATC cells
The KHM-5M human ATC cell line was purchased from
Cellverse Bioscience Technology, Co., Ltd. BHT101, 8305C and ACT-1
cell lines were obtained from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences. The Nthy-ori 3–1
normal human thyroid cell line was obtained from Cellverse
Bioscience Technology Co., Ltd. All cells were cultured in
RPMI-1640 medium (Thermo Fisher Scientific, Inc.) containing 10%
FBS and 1% penicillin/streptomycin solution at 37°C in a humidified
atmosphere of 5% CO2. In addition, 8305C cells were
treated with the p38 MAPK agonist P79350 (50 µM) for 24 h at
37°C.
To establish the Dox-resistant ATC cells, cells were
selected using stepwise increasing concentrations of Dox (0.01–1
µM). Finally, cells were maintained in 1 µM Dox for the resistance
characteristics and referred to as 8305C/Dox cells. At 1 week
before the experiment, the medium with Dox was removed and replaced
with normal medium (22).
Small interfering RNA (siRNA)
transfection
The RON-specific siRNA (si-RON#1:
GGAGUACUAUAGUGUUCAACA; si-RON#2: GGAACAAAUGACUAUUAAAGC), the
corresponding negative control (si-NC: UUCUCCGAACGUGUCACGU), the
HK2-specific pcDNA overexpression vector (Ov-HK2) and the empty
vector (Ov-NC) as a negative control were all provided by Shanghai
GeneChem Co., Ltd. Lipofectamine® 3000 (Wuhan Kehaojia
Biotechnology Co., Ltd.) was used to transfect the aforementioned
recombinants (100 nM) into ATC cells for 48 h at 37°C according to
the recommended instructions. After 48 h transfection, cells were
collected for subsequent experiments.
Western blotting
The proteins, which were isolated from ATC cells
using RIPA lysis buffer (Bio-Rad Laboratories, Inc.), were
quantified using a BCA protein assay kit (Thermo Fisher Scientific,
Inc.) according to the standard protocol. After separation by 8%
SDS-PAGE, the proteins (30 µg) were transferred onto PVDF
membranes. The PVDF membranes were blocked with 5% non-fat milk for
1 h at 25°C, and incubated overnight at 4°C with primary
antibodies, including anti-glutathione peroxidase 4 (GPX4; cat. no.
ab125066; dilution, 1:400; Abcam), anti-ferritin heavy chain 1
(FTH1; cat. no. ab75972; dilution, 1:500; Abcam), anti-solute
carrier family 7 member 11 (SLC7A11; cat. no. ab307601; dilution,
1:400; Abcam), anti-acyl-CoA synthetase long chain family member 4
(ACSL4; cat. no. ab155282; dilution, 1:1,500; Abcam), anti-glucose
transporter 1 (GLUT1; cat. no. ab115730; dilution, 1:400; Abcam),
anti-HK2 (cat. no. ab209847; dilution, 1:1,000; Abcam),
anti-pyruvate kinase M1/2 (PKM2; cat. no. ab85555; dilution,
1:1,500; Abcam), Bcl-2 (cat. no. ab32124; dilution, 1:1,000;
Abcam), Bax (cat. no. ab32503; dilution, 1:1,000; Abcam),
cleaved-caspase3 (cat. no. ab32042; dilution, 1:500; Abcam),
caspase3 (cat. no. ab32351; dilution, 1:500; Abcam), ERK (cat. no.
ab184699; dilution, 1:1,000; Abcam), p-ERK (cat. no. ab201015;
dilution, 1:1,000; Abcam), MAPK (cat. no. ab182453; dilution,
1:1,000; Abcam), p-MAPK (cat. no. ab308038; dilution, 1:1,000;
Abcam), CREB (cat. no. ab32515; dilution, 1:1,000; Abcam), p-CREB
(cat. no. ab32096; dilution, 1:1,000; Abcam) and β-actin (cat. no.
ab8226; dilution, 1:2,500; Abcam) antibodies, followed by
incubation with the HRP-labeled secondary anti-rabbit antibody
(cat. no. ab109489; dilution, 1:1,000; Abcam) at room temperature
for 2 h. The protein bands were visualized using an ECL detection
system (Beyotime Institute of Biotechnology). The density of the
band was determined using ImageJ software (version 1.49; NIH).
Measurement of the extracellular
acidification rate (ECAR)
The ECAR was assessed using the Seahorse XFe 96
Extracellular Flux Analyzer (Seahorse Bioscience; Agilent
Technologies, Inc.) with the Seahorse XF Glycolysis Stress Test Kit
(Seahorse Bioscience; Agilent Technologies, Inc.) according to the
manufacturer's protocols. The data were analyzed by the
aforementioned analyzer and ECAR detection was noted as
mPH/min.
Glucose uptake and lactic acid
concentration
The level of glucose uptake in 8305C cells was
measured using the Glucose Uptake Assay Kit (Colorimetric; cat. no.
ab136955; Abcam). To measure the lactic acid concentration in
culture medium from 8305C cells, the lactic acid assay kit (Nanjing
Jiancheng Bioengineering Institute) was used according to the
manufacturer's instructions.
Ferroptosis analysis
Cells were transfected with si-RON with or without
Ov-HK2 and were seeded into 96-well plates. The total iron level
was detected by the Iron Assay Kit (ScienCell Research
Laboratories, Inc.) in keeping with the recommendation of
manufacturer. Finally, the absorbance of the cells was read at 590
nm by means of a spectrophotometer (Shanghai Mapada Instruments,
Co., Ltd.).
The reactive oxygen species (ROS) levels of the
cells were assessed using 20,70-dichlorofluorescein diacetate
(DCFH-DA) staining. In brief, treated cells were collected and
washed three times with PBS, then cells were incubated in DCFH-DA
(10 mM) at 37°C for 30 min in darkness. The cells were then washed
with PBS three times and the fluorescence images were obtained by a
fluorescence microscope at excitation wavelength of 485 nm and
emission wavelength of 520 nm.
Mitochondrial membrane potential
assay
The mitochondrial membrane potential was examined
using JC-1 (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Briefly, 8305C cells were cultured in
6-well plates (3×105 cells per well). After 24 h of
growth, cells were treated with superparamagnetic iron oxide-serum
at a concentration of 100 µg Fe/ml for 24 h at 37°C. After staining
with JC-1 according to the manufacturer's protocol, the cells were
analyzed by fluorescence microscopy (BD Biosciences). Mitochondrial
integrity was quantified by measuring the fluorescence intensity of
J-aggregates. Green fluorescence represented the monomeric form of
JC-1, appearing in the cytosol after mitochondrial membrane
depolarization. Red emission of the dye represented a
potential-dependent aggregation in the mitochondria, reflecting
ΔΨm. The excitation wavelength of JC-1 is 488 nm, and the
approximate emission wavelength of the monomeric and J-aggregate
forms is 529 and 590 nm, respectively.
Intracellular ATP detection assay
The cells were trypsinized and lysed with the lysis
buffer provided in the commercial kit on ice. The supernatant of
the lysate was collected and incubated with the ENLITEN®
ATP assay system (Promega Corporation) according to the
manufacturer's protocol. The luminescence was detected using a
spectrophotometer (BioTek Synergy H1; Agilent Technologies,
Inc.).
Detection of biochemical factors
The levels of reduced glutathione (GSH) in cell
lysates were examined using a Glutathione Assay Kit (cat. no.
#CS0260; Sigma-Aldrich; Merck KGaA) according to the manufacturer's
instructions. The levels of malondialdehyde (MDA) and
4-hydroxynonenal (4-HNE), and the activities of superoxide
dismutase (SOD) and GSH peroxidase (GSH-Px) were detected using the
MDA assay kit, 4-HNE assay kit, SOD assay kit and GSH-Px assay kit
(all Nanjing Jiancheng Bioengineering Institute), respectively,
according to the manufacturer's instructions.
Flow cytometry
Apoptosis was detected by flow cytometry with
Annexin V/PI double staining. Briefly, 8305C cells and 8305C/Dox
cells were washed with PBS and re-suspended in binding buffer.
Next, FITC-conjugated anti-annexin-V staining antibody (BD
Biosciences) and PI solution were mixed and gently resuspended in
Annexin V Binding buffer. Following incubation of cells, flow
cytometry was performed using a flow cytometer (Becton, Dickinson
and Company). The data were analyzed using FlowJo software (version
10; Tree Star, Inc.).
Statistical analysis
Data are expressed as the mean ± SD of results
derived from three independent experiments performed in triplicate.
The results were analyzed using GraphPad Prism 8.0 software
(Dotmatics). Differences in multiple groups were compared using
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
RON is highly expressed in thyroid
cancer cells
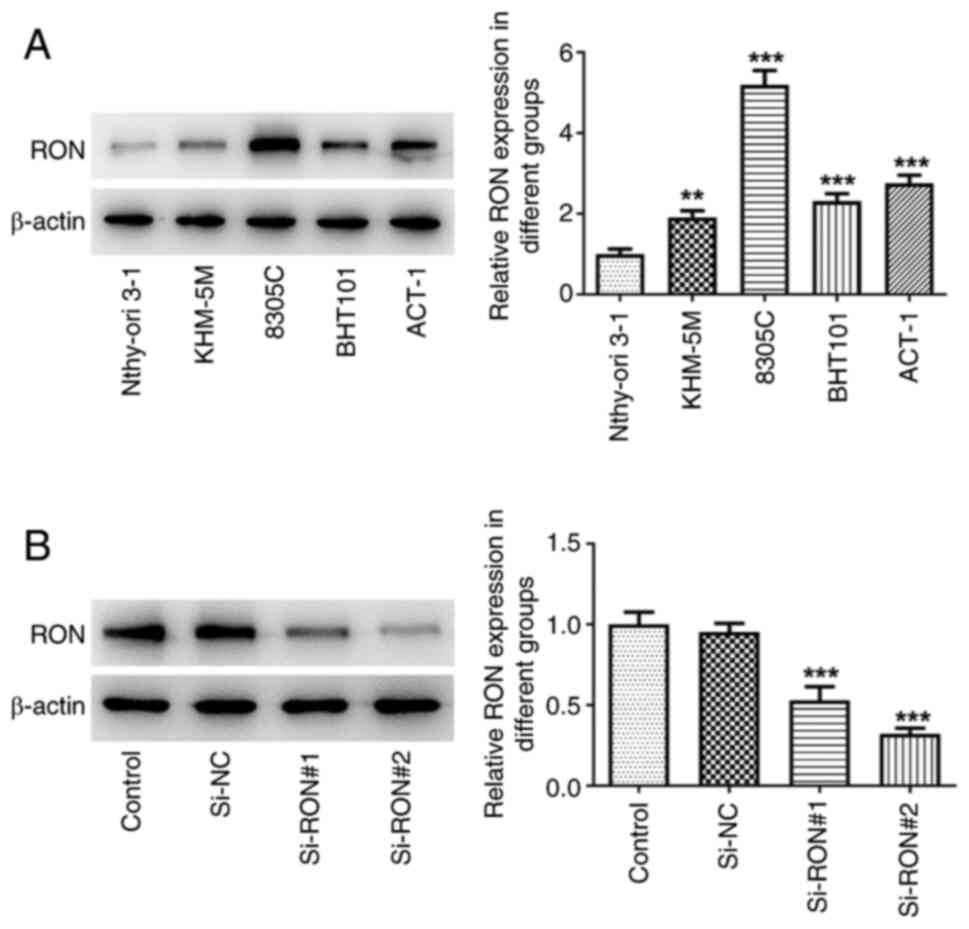
RON expression in KHM-5M, BHT101, 8305C and ACT-1
thyroid cancer cell lines was examined by western blotting. The
results demonstrated that RON expression was higher in thyroid
cancer cells compared with in Nthy-ori 3–1 normal human thyroid
cells, with the most pronounced increase in 8305C cells (Fig. 1A). Therefore, 8305C cells were
selected for the subsequent experiments. Subsequently, in order to
further study the mechanism of RON, transfection was used to
interfere with RON expression (Fig.
1B). si-RON#2 had a more effective knockdown effect and was
thus selected for the following assays (termed si-RON).
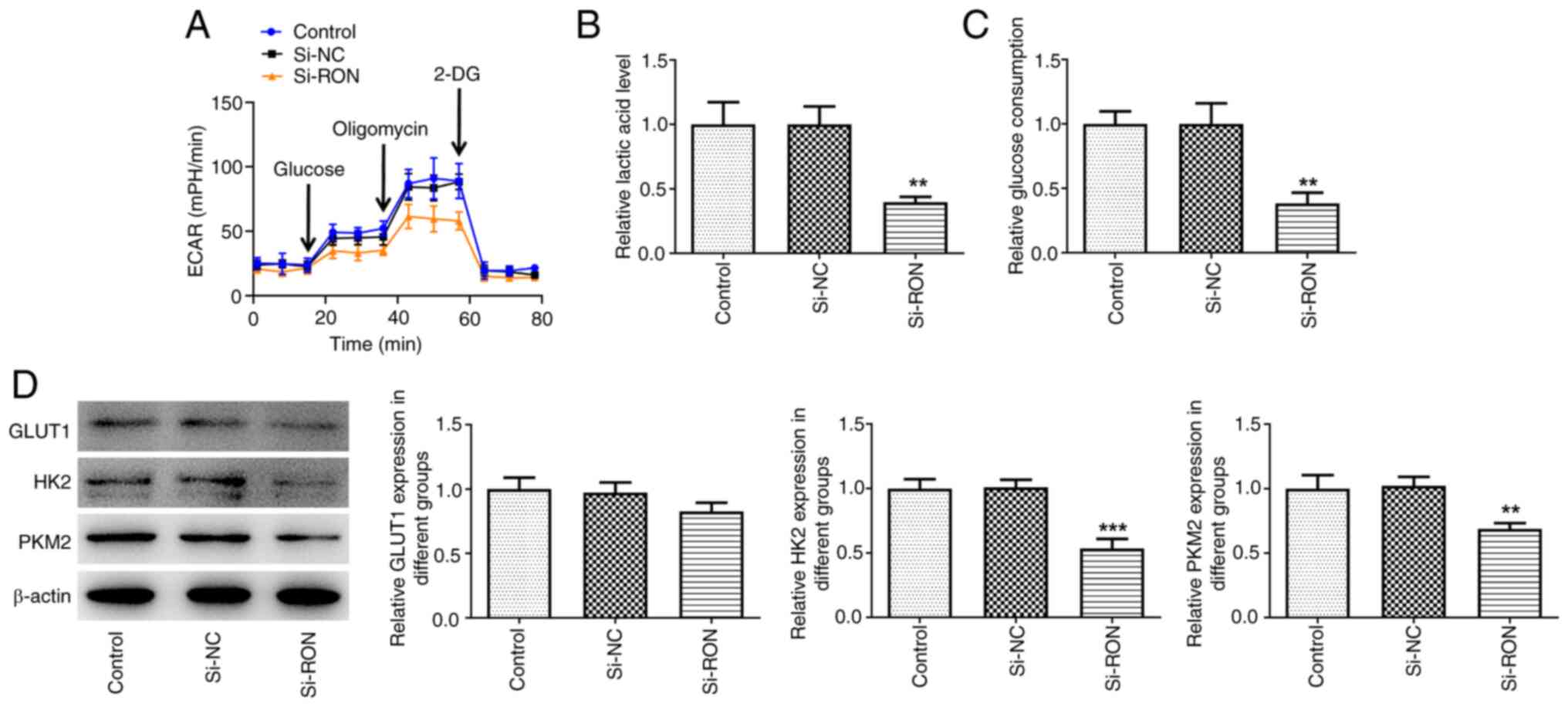
RON interference inhibits glycolysis
in 8305C cells
The effect of RON interference on glycolysis in
8305C cells was studied. The results demonstrated that the levels
of ECAR, lactic acid and glucose in the RON-silenced 8305C cell
culture medium were reduced compared with the negative group
(Fig. 2A-C). Furthermore, western
blotting indicated that the expression levels of HK2 and PKM2 were
decreased in the si-RON 8305C cell group compared with the negative
group (Fig. 2D). Taken together,
these data suggested that RON interference inhibited glycolysis in
thyroid cancer cells.
RON interference increases ferroptosis
levels in 8305C cells
To investigate the role of ROS in si-RON
group-induced ferroptosis, the DCFH-DA probe was used to monitor
intracellular ROS production. After RON interference, total iron,
intracellular ROS, MDA and 4-HNE levels were markedly increased,
and SOD and GSH-Px levels and the levels of the ferroptosis-related
proteins GPX4, FTH1 and SLC7A11 were markedly decreased, while the
protein expression levels of ACSL4 were markedly increased in
thyroid cancer cells compared with the negative group (Fig. 3A-D). These results suggested that
si-RON induced ferroptosis in 8305C cells.
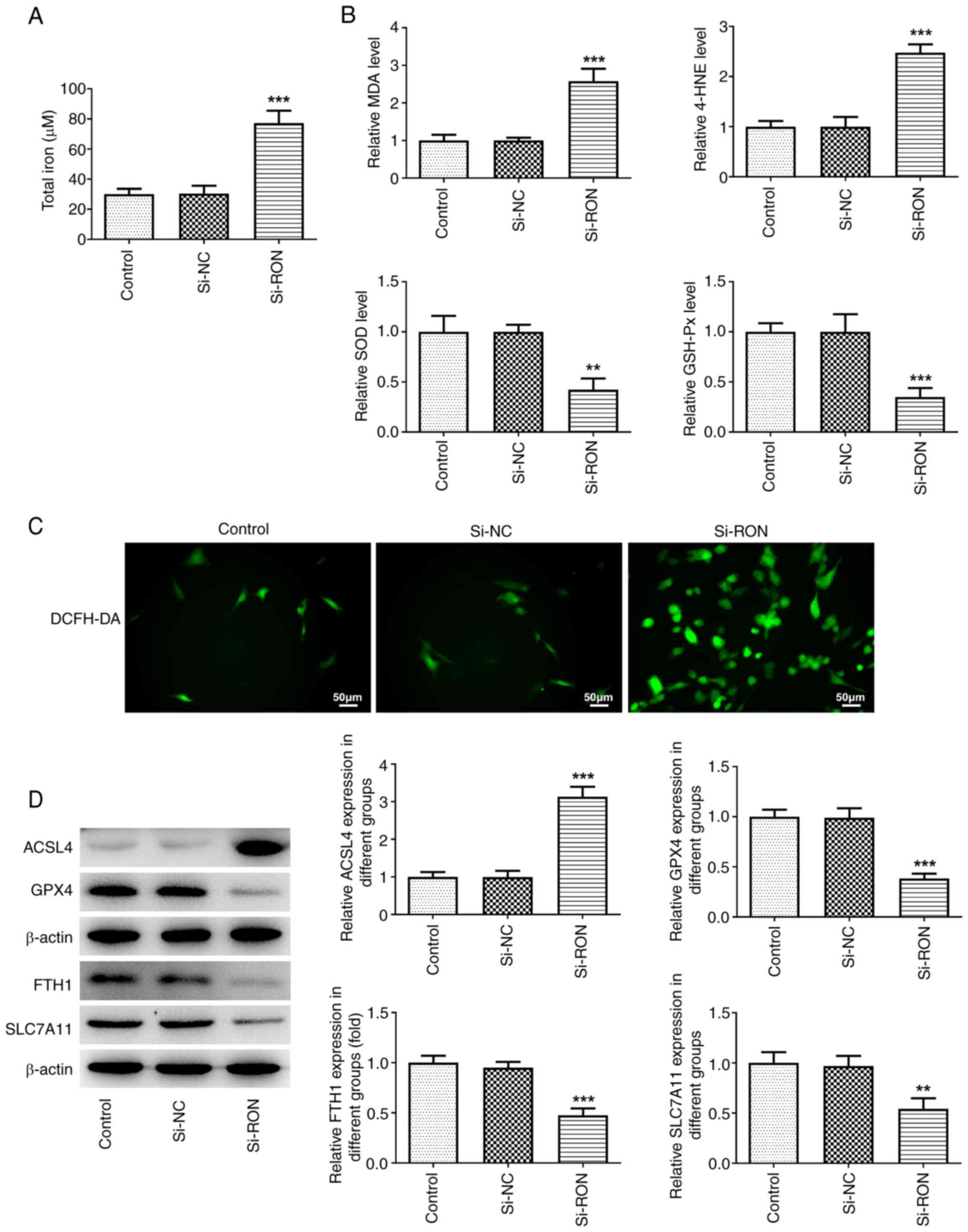 | Figure 3.Effects of interference with RON on
ferroptosis in thyroid cancer cells. (A) Total iron levels; (B)
levels of MDA, 4-HNE, SOD and GSH-Px levels; (C) intracellular ROS
levels; (D) protein levels of ferroptosis-related proteins ACSL4,
GPX4, FTH1, and SLC7A11 were estimated by western blotting.
**P<0.01, ***P<0.001 vs. si-NC. NC, negative control; MDA;
malondialdehyde; 4-HNE, 4-hydroxynonenal; SOD, superoxide
dismutase; GSH-Px, glutathione peroxidase; ROS, reactive oxygen
species; ACSL4, acyl-CoA synthetase long chain family member 4;
GPX4, glutathione peroxidase 4; FTH1, ferritin heavy chain 1;
SLC7A11, solute carrier family 7 member 11. |
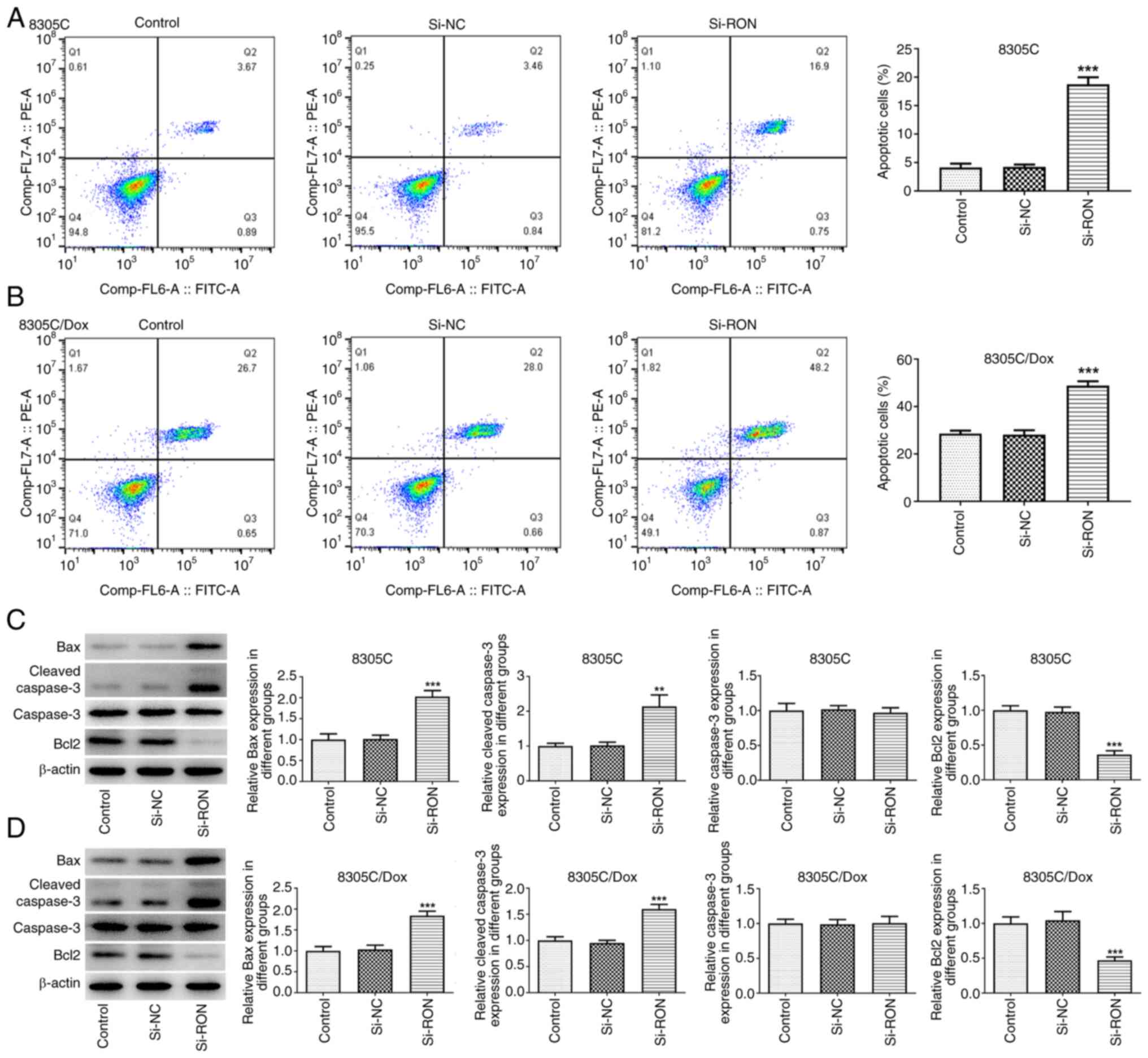
RON interference inhibits Dox
resistance in 8305C cells
The results of flow cytometry demonstrated that,
compared with that of the corresponding control cells, the
apoptosis level of 8305C/Dox cells was markedly increased, and the
percentage of apoptotic cells after RON interference was markedly
higher compared with the negative group (Fig. 4A and B). To evaluate whether RON
interference is involved in the development of Dox resistance in
thyroid cancer cells, western blotting was used to evaluate the
expression changes of apoptosis-related proteins in the 8305C/Dox
cell line following RON interference (Fig. 4C and D). The present data confirmed
that RON silencing increased the sensitivity of 8305C/Dox cells to
apoptotic cell death, as illustrated by a higher percentage of
apoptotic cells, and increased the expression of Bax
(pro-apoptotic) and cleaved caspase3, and reduced the Bcl2 level.
These results indicated that RON interference was associated with
Dox resistance in 8305C cells.
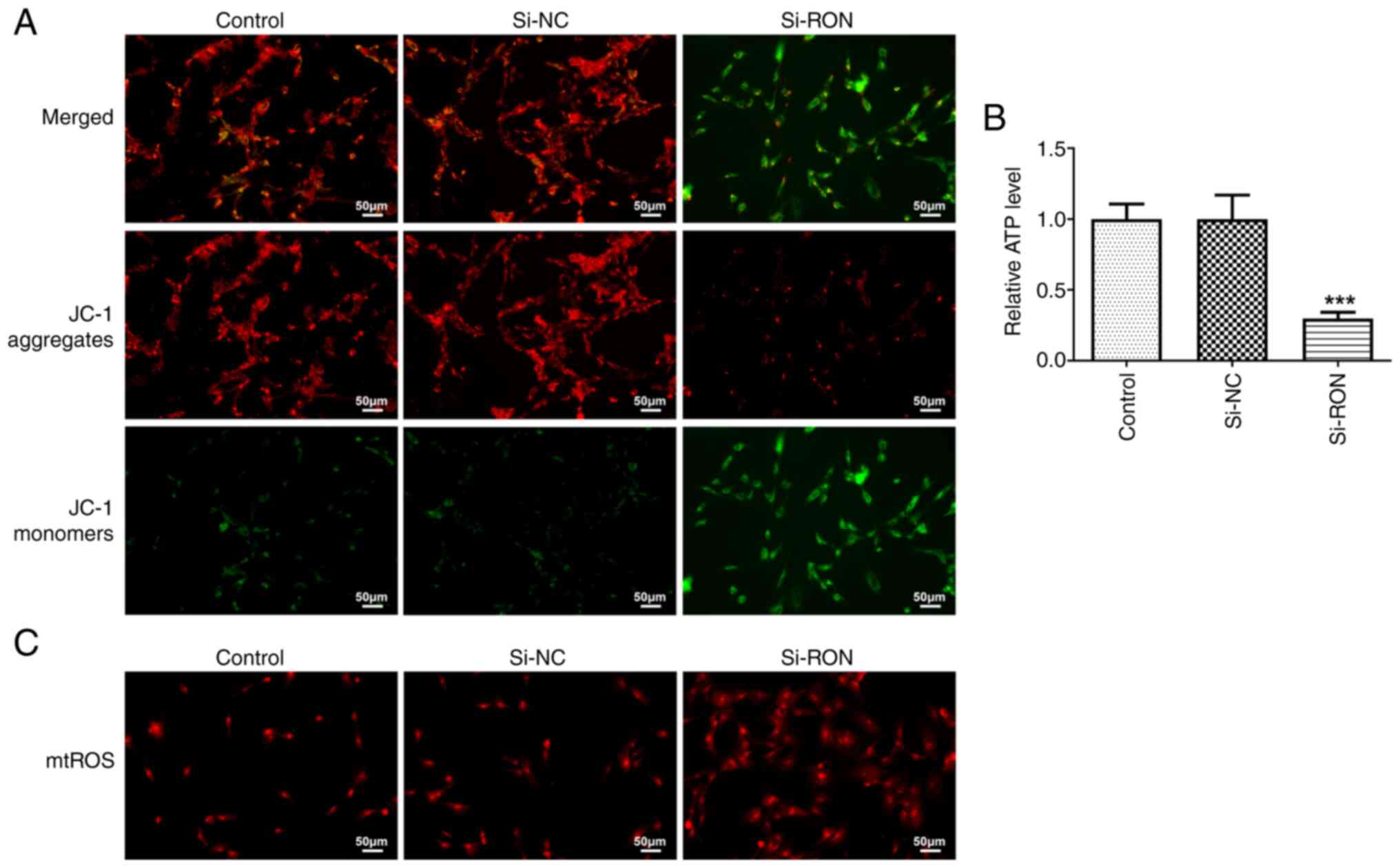
RON interference affects mitochondrial
function in 8305C cells
The mitochondrial membrane potential reflects the
normal structure and function of mitochondria, while depolarization
of the membrane potential suggests that the structure and function
of mitochondria are impaired (23). In the present study, the
mitochondrial membrane potential in thyroid cancer cells was
detected using a JC-1 fluorescence probe. Compared with the si-NC
group, si-RON caused the depolarization of the mitochondrial
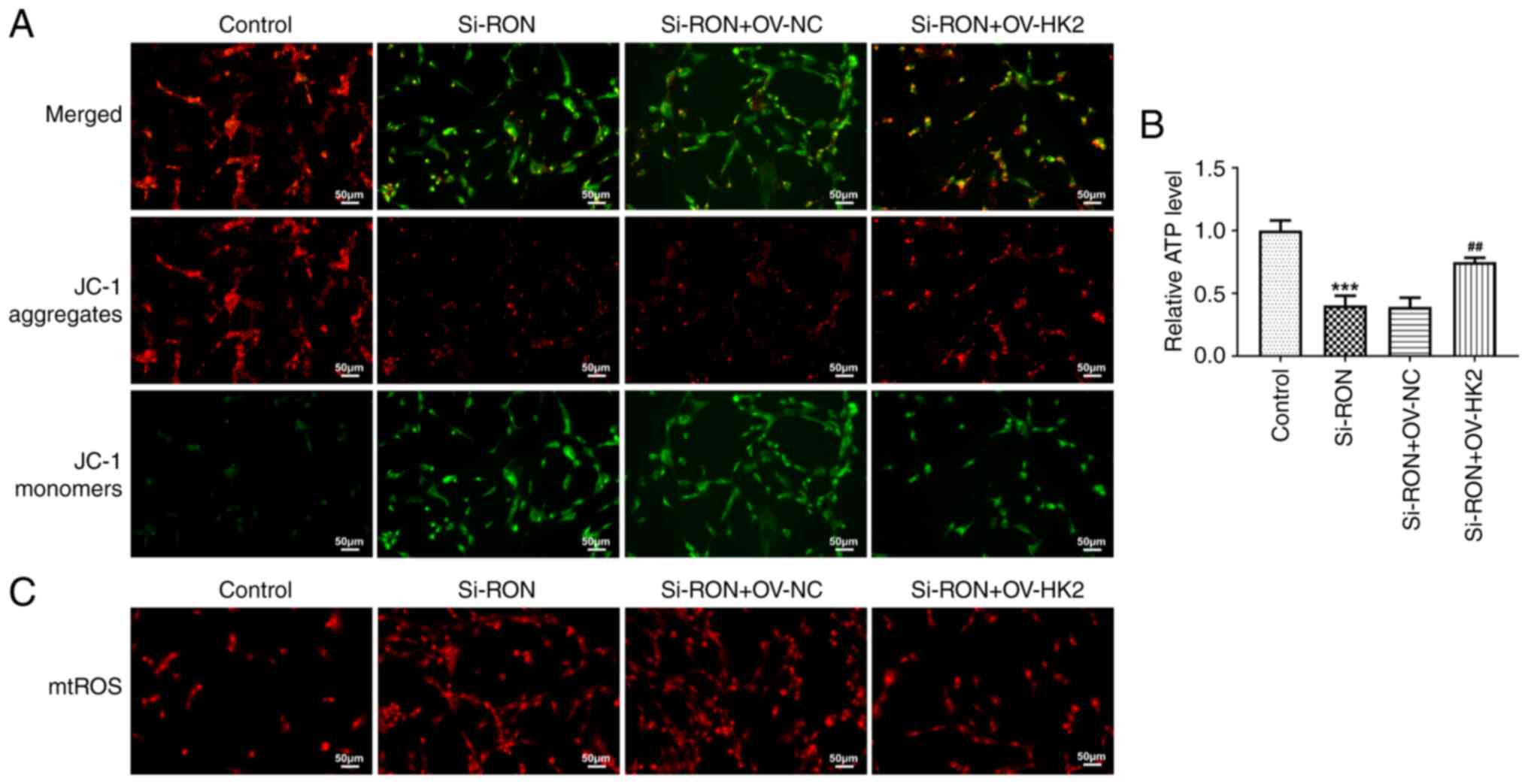
membrane potential in 8305C cells (Fig. 5A). Furthermore, the ATP content was
decreased, while the mitochondrial ROS content of thyroid cancer
cells was increased after RON interference (Fig. 5B and C).
RON interference affects ferroptosis
levels in 8305C cells by inhibiting the glycolysis process
Previous data has shown that RON had a high impact
on HK2 (24), so it was
hypothesized that RON may affect the glycolysis process through
HK2. Therefore, a HK2 overexpression plasmid was constructed to
explore its effect of glycolysis on ferroptosis in thyroid cancer
cells by regulating the expression of HK2 (Fig. 6A). To further explore the effects
of RON on the level of ferroptosis in thyroid cancer cells by
inhibiting the glycolytic process, the DCFH-DA probe was used to
monitor intracellular ROS production. The results demonstrated that
HK2 overexpression reversed the effects of RON interference on
total iron, intracellular ROS, MDA, 4-HNE, SOD and GSH-Px levels,
as well as the expression levels of ferroptosis-related proteins,
including GPX4, FTH1, SLC7A11 and ACSL4 (Fig. 6B-E). These results indicated that
RON interference affected the level of ferroptosis in thyroid
cancer cells by inhibiting the glycolytic process.
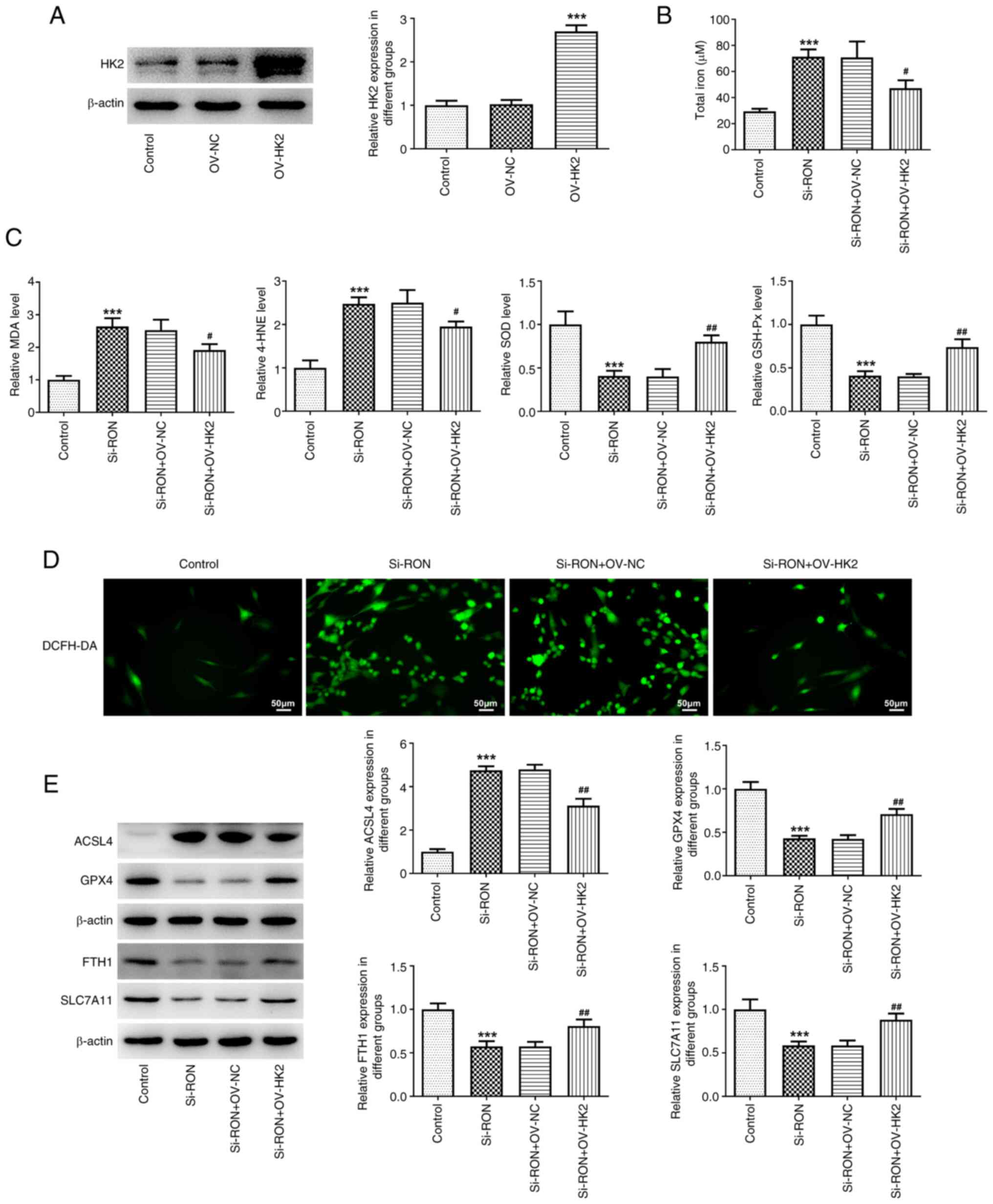 | Figure 6.Effects of interference with RON on
ferroptosis levels in thyroid cancer cells by inhibiting the
glycolysis process. (A) Relative HK2 protein expression after
overexpression. (B) total iron levels; (C) levels of MDA, 4-HNE,
SOD and GSH-Px levels; (D) intracellular ROS levels; (E) protein
levels of ferroptosis-related proteins ACSL4, GPX4, FTH1 and
SLC7A11 were estimated by western blotting. ***P<0.001 vs.
control, #P<0.05, ##P<0.01 vs.
si-RON+OV-NC. NC, negative control; MDA; malondialdehyde; 4-HNE,
4-hydroxynonenal; SOD, superoxide dismutase; GSH-Px, glutathione
peroxidase; ACSL4, acyl-CoA synthetase long chain family member 4;
GPX4, glutathione peroxidase 4; FTH1, ferritin heavy chain 1;
SLC7A11, solute carrier family 7 member 11; HK2, hexokinase 2;
DCFH-DA, 20,70-dichlorofluorescein diacetate. |
RON interference affects chemotherapy
sensitivity of 8305C cells by regulating HK2 in glycolysis
The present study further explored whether RON
interference affects chemotherapy sensitivity in thyroid cancer
cells by regulating HK2 in glycolysis. The results demonstrated
that HK2 overexpression reversed the effects of RON interference on
the apoptosis rate of cells, and the expression levels of the
apoptosis-related proteins Bax, cleaved caspase3 and Bcl2 (Fig. 7A-D). The results indicated that RON
interference affected ferroptosis of 8305C cells by regulating the
expression of HK2 in glycolysis of thyroid cancer cells.
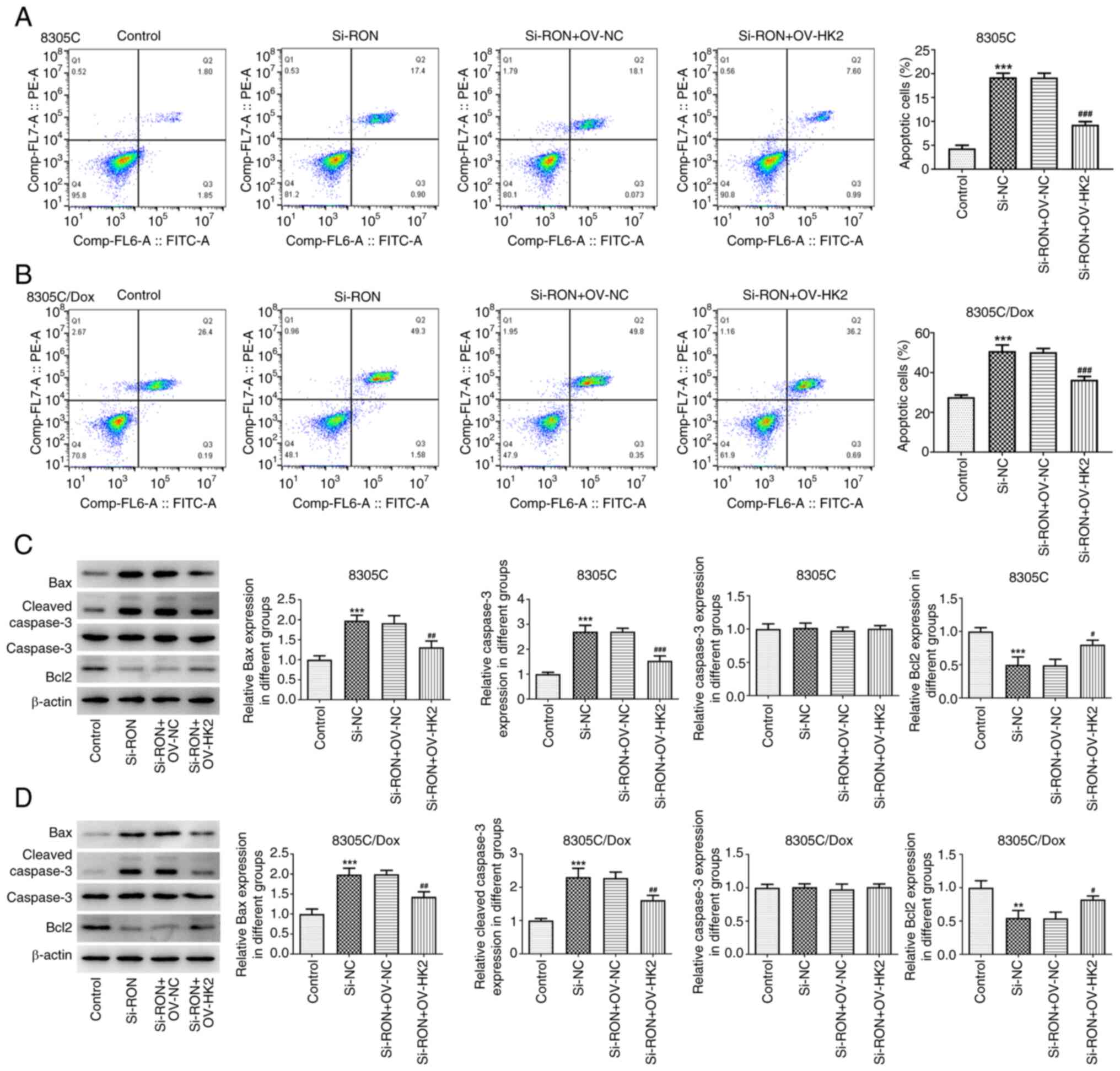 | Figure 7.Effects of interference with RON on
chemotherapy sensitivity of thyroid cancer cells by regulating HK2
in glycolysis. (A) Apoptosis rate of 8305C cells was detected by
flow cytometry; (B) apoptosis rate of 8305C/Dox cells was detected
by flow cytometry; (C) expression of apoptosis-related proteins
Bax, cleaved caspase3, caspase3 and Bcl2 in 8305C cells were
estimated by western blotting; (D) expression of apoptosis-related
proteins Bax, cleaved caspase3, caspase3 and Bcl2 in 8305C/Dox
cells were estimated by western blotting. **P<0.01,
***P<0.001 vs. control, #P<0.05,
##P<0.01, ###P<0.001 vs. si-RON+OV-NC.
NC, negative control; Dox, doxorubicin. |
RON interference affects mitochondrial
function in 8305C cells by regulating HK2 in glycolysis
To further explore the effect of RON interference on
the mitochondrial function of thyroid cancer cells via regulation
of HK2 in glycolysis, the JC-1 fluorescent probe was used to detect
the mitochondrial membrane potential, and then the levels of ATP
and ROS were detected. The results suggested that HK2
overexpression reversed the effects of RON interference on the
depolarization of the mitochondrial membrane potential,
mitochondrial ROS and ATP content (Fig. 8A-C). Thus, RON interference
affected mitochondrial function of thyroid cancer cells by
regulating HK2 in glycolysis.
RON interference affects glycolysis by
regulating MAPK/CREB signaling
To explore whether RON affects glycolysis by
regulating MAPK/CREB signaling in thyroid cancer cells, western
blotting was used to detect the expression levels of proteins
related to the MAPK/CREB signaling pathway. The results
demonstrated that the levels of phosphorylated (p-)p38 MAPK, p-CREB
and p-Erk1/2 in thyroid cancer cells were markedly downregulated
after RON interference (Fig. 9A and
B). Subsequently, to further explore the effect of RON on
glycolysis in thyroid cancer cells via MAPK/CREB signaling, the p38
MAPK agonist P79350 was added to 8305C cells. The results
demonstrated that, compared with the si-RON group, the si-RON +
P79350 group exhibited markedly increased ECAR, lactate and glucose
levels in the culture medium by affecting MAPK/CREB signaling in
8305C cells (Fig. 9C-E). Western
blotting demonstrated that the expression levels of GLUT1, HK2 and
PKM2 were increased in the si-RON + P79350 group (Fig. 9F). These results suggested that RON
interference affected glycolysis via MAPK/CREB signaling.
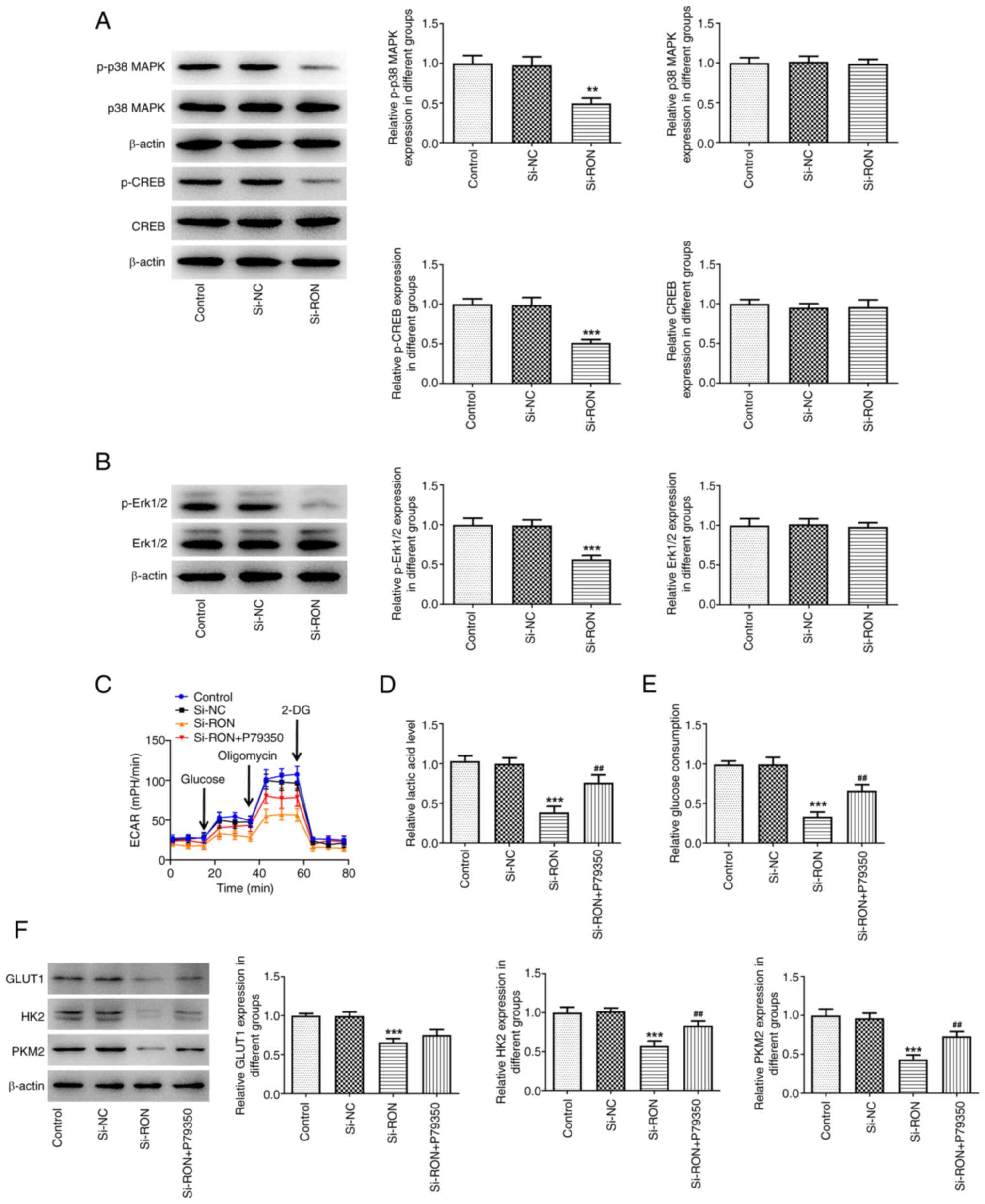 | Figure 9.Effects of interference with RON on
glycolysis through MAPK/CREB signaling. (A) Expression levels of
proteins related to the MAPK/CREB signaling were assessed using
western blotting; (B) expression levels of proteins p-Erk1/2 and
Erk1/2 were assessed using western blotting, ***P<0.001 vs.
si-NC; (C) ECAR assessment; (D) levels of lactic acid; (E) levels
of glucose; (F) protein levels of GLUT1, HK2, PKM2 were estimated
by western blotting. **P<0.01, ***P<0.001 vs. control,
##P<0.01, ###P<0.001 vs. si-RON. p-,
phosphorylated; ECAR, extracellular acidification rate; HK2,
hexokinase 2; GLUT1, glucose transporter 1; PKM2, pyruvate kinase
M1/2; NC, negative control. |
Discussion
ATC is a rare, highly aggressive malignant tumor
(25). There exists no effective
or standard therapy for the treatment of ATC (26). Therefore, it is an urgent issue to
explore the underlying molecular mechanisms involved in the
initiation and progression of ATC as a greater number of novel
candidate targets are needed to improve treatment decisions. The
present data demonstrated that RON expression was markedly higher
in thyroid cancer cell lines compared with the Nthy-ori 3–1 normal
human thyroid cell line. Targeting RON may provide promising
therapeutic approaches for the treatment of patients with ATC.
RON RTK is overexpressed in more than half of human
breast cancers and can upregulate the glycolytic enzyme HK2
(16). The present results are
similar to a previous study where RON interference inhibited
glycolysis in thyroid cancer cells, and RON interference had the
most obvious effect on HK2 in glycolysis (27). Previous research has revealed that
ferroptosis serves a crucial role in ischemic organ damage,
neurodegenerative diseases such as Alzheimer's disease and
Parkinson's disease, and tumor cell death (28). Therefore, the present study
explored the role of RON in the ferroptosis process in thyroid
cancer. The present results demonstrated that RON interference
increased ferroptosis levels in thyroid cancer cells.
Activation of RON RTK can confer resistance to
tamoxifen in breast cancer cell lines, while silencing of RON
receptor signaling can promote apoptosis and gemcitabine
sensitivity in pancreatic cancer (29,30).
Notably, in breast cancer, BMS-777607 (a RON tyrosine kinase
inhibitor) can increase resistance to cytotoxic chemotherapy drugs,
including Dox (31). However, the
mechanism of RON on the chemotherapy drug Dox has not been fully
characterized. The results of the present study revealed that RON
interference increased the sensitivity of 8305C/Dox cells to
apoptotic cell death, with a higher percentage of apoptotic cells,
increased expression of Bax (pro-apoptotic) and cleaved caspase 3,
which indicated that RON interference was associated with Dox
resistance in 8305C cells. Furthermore, iron overload caused
cardiac mitochondrial dysfunction, as indicated by increased
mitochondrial ROS levels and mitochondrial membrane potential
depolarization (32). Thus, the
present study subsequently detected the mitochondrial function in
thyroid cancer cells after RON interference, and the data indicated
that RON interference affected mitochondrial function in thyroid
cancer cells.
Previous result revealed that RON interference had
the most notable effect on HK2 in glycolysis (16). A novel study also demonstrated that
the key glycolysis gene HK2 could affect the ferroptosis process of
tumor cells, and the most direct manifestation of ferroptosis was
abnormal lipid metabolism (33).
Therefore, the present study further explored whether the mechanism
by which RON affects ferroptosis is related to the key glycolysis
gene HK2. The present study demonstrated that RON interference
affected the level of ferroptosis in thyroid cancer cells by
inhibiting the HK2-mediated glycolytic process. In previous
research, higher HK2 expression has been associated with
chemoresistance in ovarian cancer (34). However, whether HK2 functionally
contributes to chemotherapy sensitivity in ATC remains unclear. The
present study explored the effect of RON interference on
chemotherapy sensitivity of thyroid cancer cells by regulating
HK2-mediated glycolysis. The results suggested that RON
interference affected the chemotherapy sensitivity of thyroid
cancer cells by regulating HK2-mediated glycolysis. A recent study
delineated a crucial role of HK2 in governing glycolytic flux and
mitochondrial activity, thereby modulating microglial functions in
maladaptive inflammation in brain diseases (35). The present study also revealed that
RON interference affected mitochondrial function in thyroid cancer
cells by regulating HK2 in glycolysis.
A previous study demonstrated that RON activated the
MAPK/RSK/CREB signaling pathway to enhance C-X-C motif chemokine
receptor 4 expression and promote cell migration and invasion in
bladder cancer (18). Furthermore,
CREB, which is associated with proliferation in thyroid cancer,
serves a role in the proliferation of normal thyroid follicular
cells (36,37). Furthermore, activating mutations in
the MAPK pathway serve an important role in ATC (38). However, there are few studies on
whether RON could regulate glycolysis through MAPK/CREB signaling.
Therefore, the present study evaluated the expression levels of
proteins related to the MAPK/CREB signaling pathway. The results
indicated that RON interference reduced the levels of p-p38 MAPK,
p-CREB and p-Erk1/2 in thyroid cancer cells, and increased ECAR,
lactate and glucose levels in the culture medium by affecting
MAPK/CREB signaling. These findings demonstrated that RON
interference affected glycolysis via MAPK/CREB signaling. Based on
the results of the present study, future endeavors will investigate
whether RON can regulate the ATC process through other mechanisms
affecting glycolysis and ferroptosis, and the mechanisms with which
RON can be administered for patients with ATC. Furthermore, the
present study did not involve animal and clinical studies, which
should be performed in future experiments to confirm the findings
of the present study.
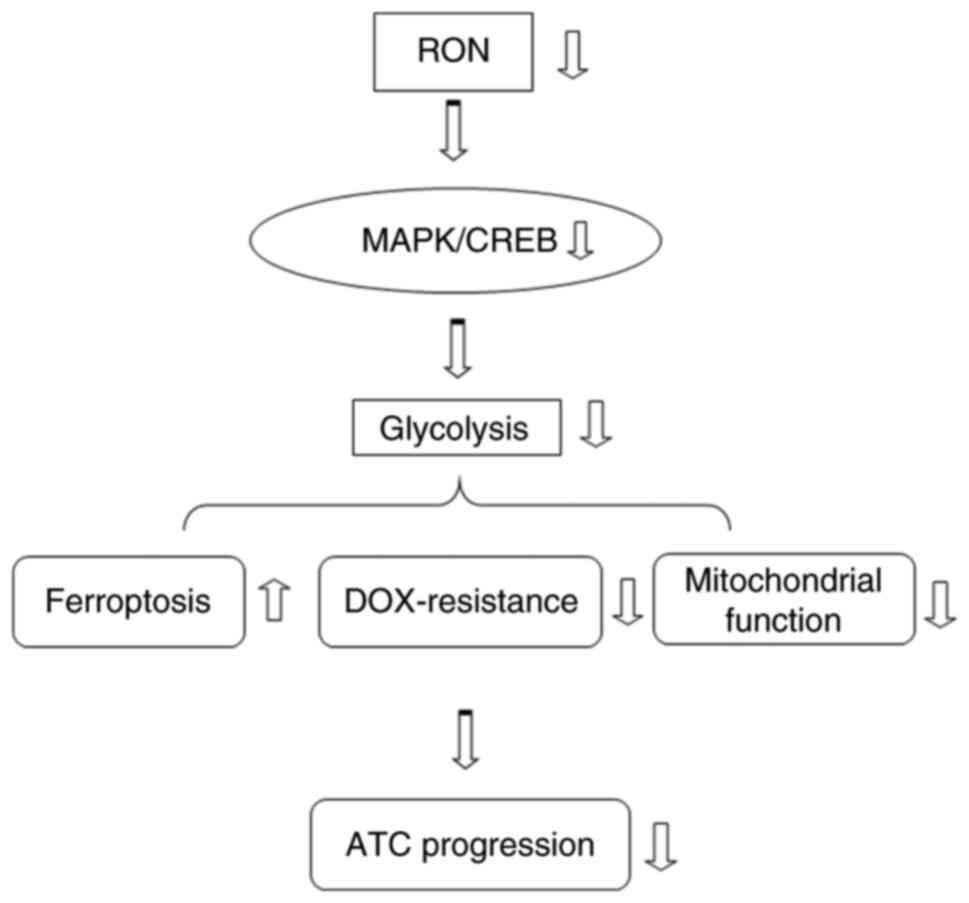
In conclusion, the present observations revealed
that RON silencing modulated glycolysis via the MAPK/CREB signaling
pathway, thereby promoting ferroptosis and chemotherapy sensitivity
of thyroid cancer cells (Fig.
10). RON may serve as a potential therapeutic opportunity for
ATC treatment.
Acknowledgements
Not applicable.
Funding
The present study received funding from the Zhejiang Public
Welfare Technology Applied Research Project (grant no.
LGF18H160014) and College Student's Science and Technology
Innovation Activity Plan of Zhejiang Province (grant no.
2023R465020).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XJ conceived and designed the study, and contributed
to the writing of the manuscript; HZ and XC acquired and
interpreted the data; and YY and DS collected and analyzed the
data. XJ and HZ confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar
|
|
2
|
Ragazzi M, Ciarrocchi A, Sancisi V,
Gandolfi G, Bisagni A and Piana S: Update on anaplastic thyroid
carcinoma: Morphological, molecular, and genetic features of the
most aggressive thyroid cancer. Int J Endocrinol. 2014:7908342014.
View Article : Google Scholar
|
|
3
|
Nagaiah G, Hossain A, Mooney CJ,
Parmentier J and Remick SC: Anaplastic thyroid cancer: A review of
epidemiology, pathogenesis and treatment. J Oncol. 2011:5423582011.
View Article : Google Scholar
|
|
4
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American thyroid association guidelines for management
of patients with anaplastic thyroid cancer. Thyroid. 22:1104–1139.
2012. View Article : Google Scholar
|
|
5
|
Molinaro E, Romei C, Biagini A, Sabini E,
Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini
A, Torregrossa L, et al: Anaplastic thyroid carcinoma: From
clinicopathology to genetics and advanced therapies. Nat Rev
Endocrinol. 13:644–660. 2017. View Article : Google Scholar
|
|
6
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005. View Article : Google Scholar
|
|
7
|
Are C and Shaha AR: Anaplastic thyroid
carcinoma: Biology, pathogenesis, prognostic factors, and treatment
approaches. Ann Surg Oncol. 13:453–464. 2006. View Article : Google Scholar
|
|
8
|
Sasanakietkul T, Murtha TD, Javid M, Korah
R and Carling T: Epigenetic modifications in poorly differentiated
and anaplastic thyroid cancer. Mol Cell Endocrinol. 469:23–37.
2018. View Article : Google Scholar
|
|
9
|
Saini S, Maker AV, Burman KD and Prabhakar
BS: Molecular aberrations and signaling cascades implicated in the
pathogenesis of anaplastic thyroid cancer. Biochim Biophys Acta Rev
Cancer. 1872:1882622019. View Article : Google Scholar
|
|
10
|
Robertson SC, Tynan J and Donoghue DJ: RTK
mutations and human syndromes: When good receptors turn bad. Trends
Genet. 16:3682000. View Article : Google Scholar
|
|
11
|
Adams GP and Weiner LM: Monoclonal
antibody therapy of cancer. Nat Biotechnol. 23:1147–1157. 2005.
View Article : Google Scholar
|
|
12
|
Geng L, Wang XT, Yu J and Yang YL:
Antagonism of cortistatin against cyclosporine-induced apoptosis in
rat myocardial cells and its effect on myocardial apoptosis gene
expression. Eur Rev Med Pharmacol Sci. 22:3207–3213. 2018.
|
|
13
|
Ronsin C, Muscatelli F, Mattei MG and
Breathnach R: A novel putative receptor protein tyrosine kinase of
the met family. Oncogene. 8:1195–1202. 1993.
|
|
14
|
Camp ER, Liu W, Fan F, Yang A, Somcio R
and Ellis LM: RON, a tyrosine kinase receptor involved in tumor
progression and metastasis. Ann Surg Oncol. 12:273–281. 2005.
View Article : Google Scholar
|
|
15
|
Wang MH, Lee W, Luo YL, Weis MT and Yao
HP: Altered expression of the RON receptor tyrosine kinase in
various epithelial cancers and its contribution to tumourigenic
phenotypes in thyroid cancer cells. J Pathol. 213:402–411. 2007.
View Article : Google Scholar
|
|
16
|
Hunt BG, Davis JC, Fox LH, Vicente-Muñoz
S, Lester C, Wells SI and Waltz SE: RON-augmented cholesterol
biosynthesis in breast cancer metastatic progression and
recurrence. Oncogene. 42:1716–1727. 2023. View Article : Google Scholar
|
|
17
|
Zheng XJ, Chen WL, Yi J, Li W, Liu JY, Fu
WQ, Ren LW, Li S, Ge BB, Yang YH, et al: Apolipoprotein C1 promotes
glioblastoma tumorigenesis by reducing KEAP1/NRF2 and CBS-regulated
ferroptosis. Acta Pharmacol Sin. 43:2977–2992. 2022. View Article : Google Scholar
|
|
18
|
Chen J, Wang K, Ye S, Meng X, Jia X, Huang
Y and Ma Q: Tyrosine kinase receptor RON activates MAPK/RSK/CREB
signal pathway to enhance CXCR4 expression and promote cell
migration and invasion in bladder cancer. Aging (Albany NY).
14:7093–7108. 2022. View Article : Google Scholar
|
|
19
|
Ma X, Xu J, Gao N, Tian J and Song T:
Dexmedetomidine attenuates myocardial ischemia-reperfusion injury
via inhibiting ferroptosis by the cAMP/PKA/CREB pathway. Mol Cell
Probes. 68:1018992023. View Article : Google Scholar
|
|
20
|
Sun RF, Zhao CY, Chen S, Yu W, Zhou MM and
Gao CR: Androgen receptor stimulates hexokinase 2 and induces
glycolysis by PKA/CREB signaling in hepatocellular carcinoma. Dig
Dis Sci. 66:802–813. 2021. View Article : Google Scholar
|
|
21
|
Farhat D, Ghayad SE, Icard P, Le Romancer
M, Hussein N and Lincet H: Lipoic acid-induced oxidative stress
abrogates IGF-1R maturation by inhibiting the CREB/furin axis in
breast cancer cell lines. Oncogene. 39:3604–3610. 2020. View Article : Google Scholar
|
|
22
|
Xu Y, Han YF, Ye B, Zhang YL, Dong JD, Zhu
SJ and Chen J: miR-27b-3p is involved in doxorubicin resistance of
human anaplastic thyroid cancer cells via targeting peroxisome
proliferator-activated receptor gamma. Basic Clin Pharmacol
Toxicol. 123:670–677. 2018. View Article : Google Scholar
|
|
23
|
Sakamuru S, Zhao J, Attene-Ramos MS and
Xia M: Mitochondrial membrane potential assay. Methods Mol Biol.
2474:11–19. 2022. View Article : Google Scholar
|
|
24
|
Park JS, Choi HI, Kim DH, Kim CS, Bae EH,
Ma SK and Kim SW: RON receptor tyrosine kinase regulates epithelial
mesenchymal transition and the expression of pro-fibrotic markers
via Src/Smad signaling in HK-2 and NRK49F cells. Int J Mol Sci.
20:54892019. View Article : Google Scholar
|
|
25
|
Bible KC, Kebebew E, Brierley J, Brito JP,
Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T,
Kasperbauer J, et al: 2021 American thyroid association guidelines
for management of patients with anaplastic thyroid cancer. Thyroid.
31:337–386. 2021. View Article : Google Scholar
|
|
26
|
Tang J, Yang Q, Mao C, Xiao D, Liu S, Xiao
L, Zhou L, Wu G and Tao Y: The deubiquitinating enzyme UCHL3
promotes anaplastic thyroid cancer progression and metastasis
through hippo signaling pathway. Cell Death Differ. 30:1247–1259.
2023. View Article : Google Scholar
|
|
27
|
Davis JC and Waltz SE: The MET family of
receptor tyrosine kinases promotes a shift to pro-tumor metabolism.
Genes. 15:9532024. View Article : Google Scholar
|
|
28
|
Qiu Y, Cao Y, Cao W, Jia Y and Lu N: The
application of ferroptosis in diseases. Pharmacol Res.
159:1049192020. View Article : Google Scholar
|
|
29
|
McClaine RJ, Marshall AM, Wagh PK and
Waltz SE: Ron receptor tyrosine kinase activation confers
resistance to tamoxifen in breast cancer cell lines. Neoplasia.
12:650–658. 2010. View Article : Google Scholar
|
|
30
|
Logan-Collins J, Thomas RM, Yu P, Jaquish
D, Mose E, French R, Stuart W, McClaine R, Aronow B, Hoffman RM, et
al: Silencing of RON receptor signaling promotes apoptosis and
gemcitabine sensitivity in pancreatic cancers. Cancer Res.
70:1130–1140. 2010. View Article : Google Scholar
|
|
31
|
Sharma S, Zeng JY, Zhuang CM, Zhou YQ, Yao
HP, Hu X, Zhang R and Wang MH: Small-molecule inhibitor BMS-777607
induces breast cancer cell polyploidy with increased resistance to
cytotoxic chemotherapy agents. Mol Cancer Ther. 12:725–736. 2013.
View Article : Google Scholar
|
|
32
|
Kumfu S, Chattipakorn S, Fucharoen S and
Chattipakorn N: Mitochondrial calcium uniporter blocker prevents
cardiac mitochondrial dysfunction induced by iron overload in
thalassemic mice. Biometals. 25:1167–1175. 2012. View Article : Google Scholar
|
|
33
|
Dai YQ, Bai Y, Gu J and Fan BY:
Stanniocalcin1 knockdown induces ferroptosis and suppresses
glycolysis in prostate cancer via the Nrf2 pathway. Neoplasma.
69:1396–1405. 2022. View Article : Google Scholar
|
|
34
|
Zhang XY, Zhang M, Cong Q, Zhang MX, Zhang
MY, Lu YY and Xu CJ: Hexokinase 2 confers resistance to cisplatin
in ovarian cancer cells by enhancing cisplatin-induced autophagy.
Int J Biochem Cell Biol. 95:9–16. 2018. View Article : Google Scholar
|
|
35
|
Fang J, Luo S and Lu Z: HK2: Gatekeeping
microglial activity by tuning glucose metabolism and mitochondrial
functions. Mol Cell. 83:829–831. 2023. View Article : Google Scholar
|
|
36
|
Siu YT and Jin DY: CREB-a real culprit in
oncogenesis. FEBS J. 274:3224–3232. 2007. View Article : Google Scholar
|
|
37
|
Nguyen LQ, Kopp P, Martinson F, Stanfield
K, Roth SI and Jameson JL: A dominant negative CREB (cAMP response
element-binding protein) isoform inhibits thyrocyte growth,
thyroid-specific gene expression, differentiation, and function.
Mol Endocrinol. 14:1448–1461. 2000. View Article : Google Scholar
|
|
38
|
Scheffel RS, Dora JM and Maia AL: BRAF
mutations in thyroid cancer. Curr Opin Oncol. 34:9–18. 2022.
View Article : Google Scholar
|