Introduction
Estrogen-related receptor (ERR) has a similar
structure to that of estrogen receptor and is an orphan nuclear
receptor whose endogenous ligand is unknown (1). ERR has a high homology with the
DNA-binding domain of the estrogen receptor and binds to the
estrogen-responsive element on the promoter. However, it has low
homology with the ligand-binding domain and estrogen does not act
as a ligand for ERR (1,2).
ERRα, ERRβ and ERRγ are the three subtypes of ERR.
ERRα and ERRγ are expressed in tissues with active energy
metabolism, such as the heart, kidneys, skeletal muscle and adipose
tissues (3,4). In addition, it has been reported that
ERRα and ERRγ regulate intracellular metabolic functions, such as
oxidative phosphorylation in mitochondria (5–7). It
has further been reported that ERRβ is expressed in the placenta
and villous tissue and involved in placenta formation (8).
Estrogen regulates a variety of physiological and
disease processes, including reproduction, bone remodeling and
breast cancer, among others. It has been revealed that ERR shares
target genes and regulatory proteins with estrogen receptor
(9). Furthermore, ERR actively
influences estrogen responses and it has been suggested that
pharmacologically modulating ERR activity may be useful for the
prevention and treatment of various symptoms related to women's
health (9).
The skin is an estrogen-sensitive organ and skin
fibroblasts produce extracellular matrix components, such as
collagen, hyaluronic acid and elastin (10–12).
These components are also related to skin antiaging and wrinkles
and sagging of the skin are likely to occur in menopausal women
owing to the decreased secretion of estrogen (10). Furthermore, skin fibroblasts
express estrogen receptors α and β and are susceptible to estrogen
(13). As ERR interacts with
estrogen signaling (14) and is
expressed in the skin, it may also play an important role in skin
antiaging.
In normal human skin, ERRα and ERRβ are expressed in
epidermal keratinocytes (15,16)
and ERRγ is expressed in keratinocytes and fibroblasts (17). However, their functions remain
unknown.
The present study analyzed the function of ERRα in
human skin fibroblasts by silencing its gene expression. It
performed microarray and pathway analyses and reverse transcription
quantitative (RT-q) PCR. Cell proliferation and apoptosis-positive
cells were examined and the cell cycle was analyzed using flow
cytometry. The present study is the first to report the function of
ERRα in human skin fibroblasts, to the best of the authors'
knowledge.
Materials and methods
Cell culture
Human normal adult skin fibroblasts (TIG113;
JCRB0539) and human neonatal foreskin fibroblasts (NFF; KF-4009,
passage 2, http://www.kurabo.co.jp/bio/celltissue/skin/03/)
were obtained from the Health Science Research Resources Bank
(Japan) and KURABO, respectively. The cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; FUJIFILM Wako Pure
Chemical Corporation) with 10% fetal bovine serum (Sigma-Aldrich;
Merck KGaA), 100 units/ml penicillin and 100 µg/ml streptomycin
(FUJIFILM Wako Pure Chemical Corporation). All culture experiments
were conducted at 37°C in a humidified incubator supplemented with
5% CO2.
Small interfering (si)RNA
transfection
The expression of the human ERRα gene was silenced
using transient transfection of ERRα siRNA (siERRα; cat. no.
sc-44706; Santa Cruz Biotechnology, Inc.), which was performed
using DharmaFECT 1 transfection reagent (Horizon Discovery Ltd.),
according to the manufacturer's instructions. TIG113 cells were
incubated with 50 nM siRNA at 37°C for 24–72 h before use in
subsequent assays. As a negative control, TIG113 cells were
transfected with Silencer Negative Control #1 siRNA (siNC; cat. no.
4390843; Thermo Fisher Scientific, Inc.).
Microarray analysis and
Wikipathways
TIG113 cells were seeded in a 100-mm cell culture
dish, cultured until 80% confluence as described in the Cell
culture section and then transfected with siERRα or siNC. After
48 h, total RNA was extracted using the RNeasy Mini kit (Qiagen
GmbH), according to the manufacturer's instructions.
The RNA (1 µg) was used to produce biotin-labeled
complementary RNA (cRNA). The labeled and fragmented cRNA was
subsequently hybridized to the SurePrint G3 Human Gene Expression
microarray (8×60 K ver. 3; Agilent Technologies Inc.). Labeling,
hybridization, image scanning and data analysis were performed at
Macrogen Japan and the Research Institute of Bio-System Informatics
(Iwate, Japan). The TIG113 microarray datasets are available at
http://www.ncbi.nlm.nih.gov/geo under
accession code GSE245234 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE245234).
The ratio of gene expression change in cells treated with siERRα to
that in cells treated with siNC was expressed as fold change. Genes
with 1.5-fold or greater upregulation or downregulation (n=3)
following siRNA transfection in TIG113 cells were subjected to
biological pathway enrichment analyses using Wikipathways (version
number 20201210; http://www.wikipathways.org/).
Reverse transcription-quantitative
(RT-q) PCR
TIG113 cells were seeded in 6-well plates and
cultured as described in the Cell culture section until they
reached 80% confluence and were then transfected with siERRα or
siNC. After incubating at 37°C for 48 h, cells were washed twice
with PBS. Total RNA was extracted from the TIG113 cells using the
RNeasy mini kit (Qiagen KK) according to the manufacturer's
instructions. cDNA was reverse-transcribed from total RNA using the
PrimeScript RT Master Mix (Takara Bio, Inc.) according to the
manufacturer's instructions. Levels of each mRNAs were quantified
by qPCR using TB Green Premix Ex Taq II (Tli RNaseH Plus; Takara
Bio, Inc.). The thermocycling conditions were as follows: 30 sec at
95°C, followed by 40 cycles of 5 sec at 95°C and 30 sec at 60°C.
Transcription levels were normalized to those of GAPDH cDNA.
The primer sequences were as follows (5′-3′): ERRα, forward
GGCCCTTGCCAATTCAGA and reverse GGCCTCGTGCAGAGCTTCT (18); ERRβ, forward
GTCTCATACCTACTGGTGGC and reverse AGGTCACAGAGAGTGGTCAG (19); ERRγ, forward
CAGACGCCAGTGGGAGCTA and reverse TGGCGAGTCAAGTCCGTTCT (19); CDKN1C, forward
GCGGCGATCAAGAAGCTGTC and reverse CCGGTTGCTGCTACATGAAC (20); peroxisome proliferator-activated
receptor gamma, coactivator 1 alpha (PGC-1α), forward
AGCCTCTTTGCCCAGATCTT and reverse GGCAATCCGTCTTCATCCAC (21) caspase 3 (CASP3), forward
GCGGTTGTAGAAGAGTTTCGTG and reverse CTCACGGCCTGGGATTTCAA (22); Fas cell surface death receptor
(FAS), forward CAATTCTGCCATAAGCCCTGTC and reverse
GTCCTTCATCACACAATCTACATCTTC (23);
cell division cycle 25C (CDC25C), forward
GCAGAAGTGGCCTATATCGCT and reverse TTCCACCTGCTTCAGTCTTGG (24); cyclin E2 (CCNE2), forward
TCAAGACGAAGTAGCCGTTTAC and reverse TGACATCCTGGGTAGTTTTCCTC
(25); cyclin B1 (CCNB1),
forward AATAAGGCGAAGATCAACATGGC and reverse TTTGTTACCAATGTCCCCAAGAG
(26); and GAPDH
(NM_001256799.3), forward TGAGAACGGGAAGCTTGTCA and reverse
TCTCCATGGTGGTGAAGACG. The GAPDH primers were designed using
the Primer 3 Plus interface
(https://www.bioinformatics.nl/cgi-bin/primer3
plus/primer3plus.cgi). PCR specificity was assessed using melting
curve analysis. All samples were analyzed in duplicate and relative
gene expression was calculated using the 2−ΔΔCq method
(27). Three independent
experiments were performed.
Western blotting
TIG113 cells were seeded in 30-mm plates and
cultured as described in the Cell culture section until they
reached 80% confluence and then transfected with siERRα or siNC.
After incubating at 37°C for 72 h, cells were washed twice with
PBS. TIG113 cell lysates were prepared with RIPA lysis buffer
(Santa Cruz Biotechnology, Inc.). The protein concentrations were
determined using a Takara BCA Protein Assay kit (Takara Bio, Inc.).
Total protein (20 µg/lane) was separated by SDS-polyacrylamide gel
electrophoresis on 12% (w/v) polyacrylamide gels and was
electroblotted onto Hybond nitrocellulose membranes (Cytiva).
Subsequently, blocking was performed with 3% non-fat milk powder at
room temperature for 2 h. Blots were probed with anti-ERRα antibody
(cat. no. 13826; Cell Signaling Technology, Inc.; 1:300) or
anti-β-actin antibody (cat. no. 81115-1-RR; Proteintech Group,
Inc.; 1:1,000) at 4°C overnight, followed by incubation with
horseradish peroxidase-conjugated anti-rabbit IgG (cat. no. ab6721;
Abcam; 1:2,000) at room temperature for 1.5 h. The signal was
detected using ImmunoStar Zeta (FUJIFILM Wako Pure Chemical
Corporation), according to the manufacturer's protocol. Luminescent
images were analyzed using a LumiCube (Liponics).
Cell proliferation assay
A total of 4,000 TIG113 cells were seeded in a
96-well plate, cultured as described in the Cell culture
section for 24 h and then transfected with siRNA. TIG113 cell
proliferation was analyzed with a Cell Counting Kit-8 (CCK-8;
Dojindo Laboratories, Inc.) following the manufacturer's protocol.
Briefly, the CCK-8 reagent was added to the cells for 1.5 h at
37°C. Absorbance was measured on a Benchmark microplate reader
(Bio-Rad Laboratories, Inc.) at a wavelength of 450 nm. The number
of cells treated with siNC for 0 h was defined as 100% and the
increase rate at each time point was expressed as a percentage.
Scratch wound healing assay
TIG113 cells were seeded in triplicate 6-well plates
and cultured as described in the Cell culture section until
they reached 80% confluence and then transfected with siERRα or
siNC in DMEM containing 10% fetal bovine serum. After 24 h, the
surface of the dishes was scratched linearly with a 200-µl pipette
tip and the cells were incubated in serum-free DMEM for 48 h at
37°C. Images were captured using a phase-contrast and an inverted
microscope (CK40; Olympus Corporation; magnification, ×40) equipped
with an Anyty digital microscope camera (3R-DKMCO4; 3R solution).
The wound area for each treatment was calculated by averaging three
individual measurements at 0 and 48 h using ImageJ software
(ver.1.53; National Institutes of Health). Cell migration was
expressed as the percentage of the scratch area filled by migrating
cells 48 h post-scratch: migration (%)=(scratch area at 0 h-scratch
area at 48 h/scratch area at 0 h) ×100.
Apoptosis detection
A total of 4,000 TIG113 cells were seeded in a
96-well plate, cultured as described in the Cell culture
section for 24 h and then transfected with siERRα or siNC. After 72
h, apoptosis was detected using the Poly Caspase Assay Kit Green
FLICA (ImmunoChemistry Technologies, LLC). Relative fluorescent
units were measured using a Tecan Infinite 200 Pro Microplate
reader (excitation, 530 nm; emission, 590 nm; Tecan Group,
Ltd.).
Cell cycle analysis
Cell cycle analysis was performed as in our previous
study (28). Briefly, TIG113 cells
were seeded in a 100-mm cell culture dish and cultured as described
in the Cell culture section until they reached 80%
confluence. Cells were transfected with siERRα or siNC and cultured
for 72 h prior to DNA staining. Cells were washed in PBS,
resuspended in propidium iodide (PI)/RNase Staining Buffer (BD
Biosciences) and incubated for 15 min at 25°C. PI fluorescence
(FL3) was measured using an FC500 flow cytometer (Beckman Coulter,
Inc.). Data were analyzed using the MultiCycle AV software (Phoenix
Flow Systems).
Type I collagen and hyaluronan
quantification in the medium
TIG113 cells were seeded in a 30-mm cell culture
dish, cultured as described in the Cell culture section
until they reached 80% confluence and were then transfected with
siERRα or siNC. After 72 h, the supernatant was collected and
filtered through a sterile filter (0.2 µm). Type I collagen and
hyaluronan secreted into the medium were quantified using a human
collagen type I enzyme-linked immunosorbent assay (ELISA) kit (cat.
no. EC1-E105; ACEL, Inc.) and Hyaluronan Quantification Kit (cat.
no. HA-KIT; Iwai Chemicals Company, Co., Ltd.), respectively,
following the manufacturer's instructions.
Statistical analysis
The results are expressed as mean ± standard
deviation. Statistically significant differences were determined
using Welch's t-test or Kruskal-Wallis analysis with the Steel
post-hoc test between two groups and multiple groups, respectively,
using Bell Curve for Excel ver. 4.04 (Social Survey Research
Information Co., Ltd.). Wikipathways that were significant were
determined by Fisher's Exact Test. Furthermore, p.adjust was
calculated by performing multiple testing corrections using the
Benjamini-Hochberg method. Values with p.adjust <0.05 were
considered statistically significant. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of ERRs and ERRα
silencing
To investigate the function of ERRα in human skin
fibroblasts, ERRα expression in TIG113 cells was suppressed using
siRNA targeting ERRα. The ERRα siRNA (siERRα)
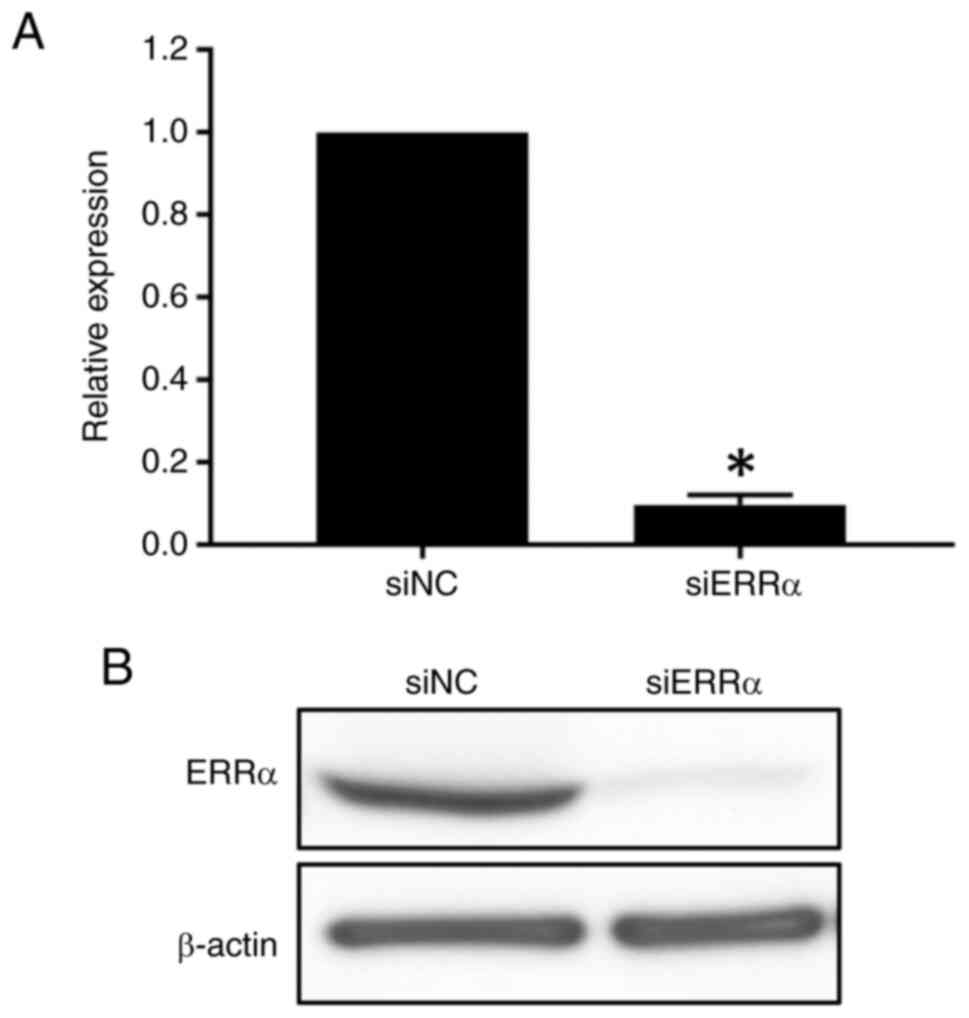
significantly decreased ERRα expression at the mRNA level
compared with a nontargeting control (siNC; P<0.05; Fig. 1A). Furthermore, western blot
analysis revealed that the ERRα expression was reduced in
siERRα-treated TIG113 cells (Fig.
1B). Moreover, microarray analysis revealed that the high
expression of ERRα compared with that of estrogen receptors
α and β suggested the importance of ERRα in skin fibroblasts
(Table SI). In addition, when the
expression of ERRα, ERRβ and ERRγ in TIG113 cells was
analyzed using RT-qPCR, the relative expression level of
ERRβ was only 1.3% that of ERRα, and ERRγ was
not notably expressed (P<0.05; Fig. S1A). The same experiments using
human NFFs yielded similar results (P<0.05; Fig. S1B).
Pathways enrichment analysis
Microarray analysis found 580 upregulated and 738
downregulated genes (Table SII)
that had a fold change of 1.5-fold upon ERRα knockdown
(n=3). Using the genes whose relative expression changed by
1.5-fold or more, biological pathway analyses in TIG113 cells were
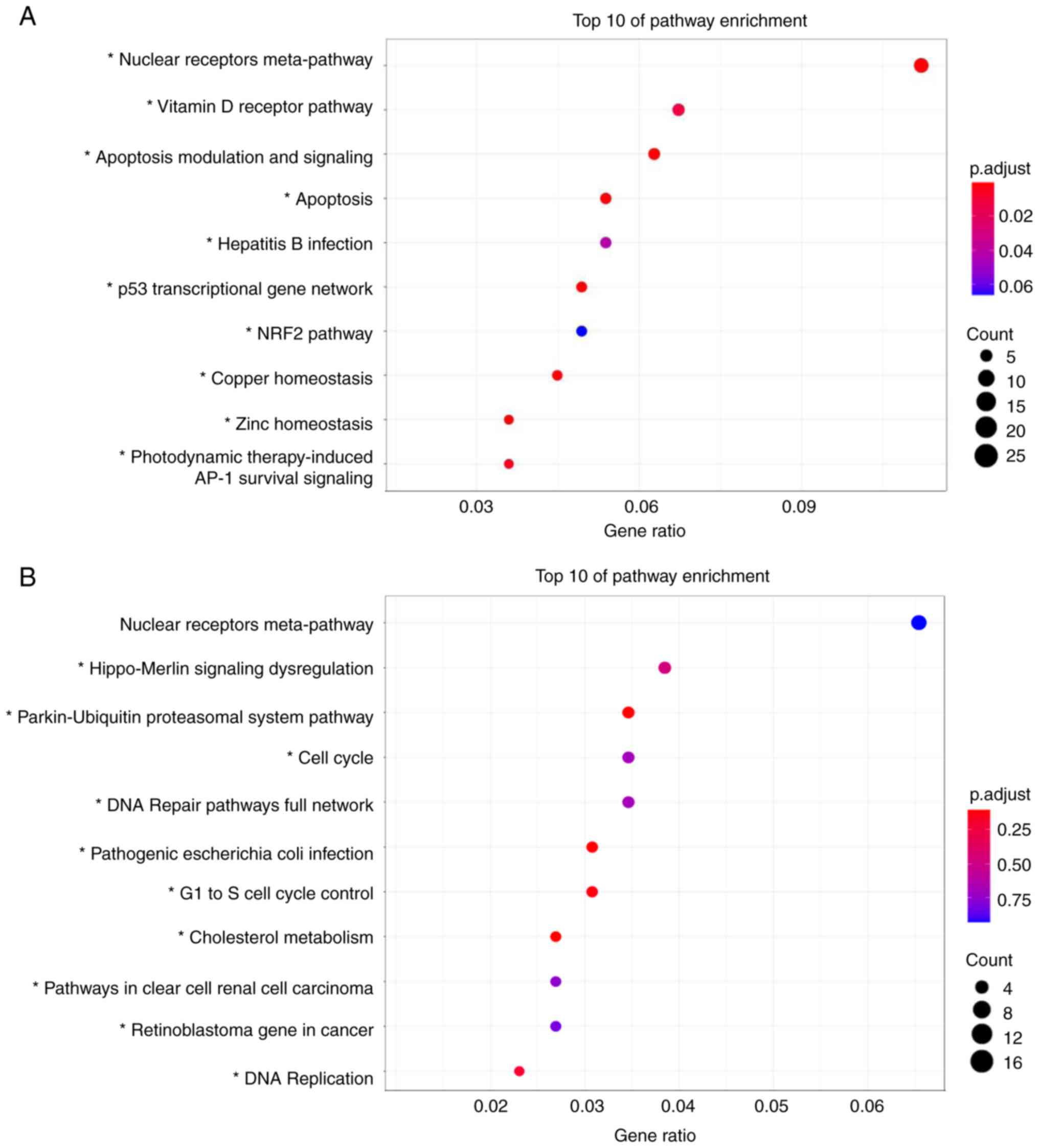
performed using Wikipathways. The top 10 upregulated or
downregulated pathways detected using Wikipathways are shown in
Fig. 2A and B, respectively. The
‘Nuclear Receptors Meta-Pathway’ ranked the highest in upregulated
and downregulated pathways, but no significant difference was
observed in downregulated pathways. The upregulated pathways were
mainly apoptosis-related pathways, such as ‘Apoptosis Modulation
and Signaling’ and ‘p53 transcriptional gene network’ (Fig. 2A). Furthermore, the downregulated
pathways were related to cell cycles such as ‘Cell Cycle’,
‘G1 to S cell cycle control’, and ‘DNA Replication’
(Fig. 2B). Microarray analysis
revealed that the expression of 25 genes belonging to the ‘Nuclear
Receptors Meta-Pathway’, such as CDKN1C and PGC-1α
and 12 apoptosis-related genes, such as CASP3 and
FAS, were upregulated. A total of nine cell cycle-related
genes, including CDC25C, CCNE2 and CCNB1 were
downregulated (Table I). RT-qPCR
validated the aforementioned findings (Fig. 3).
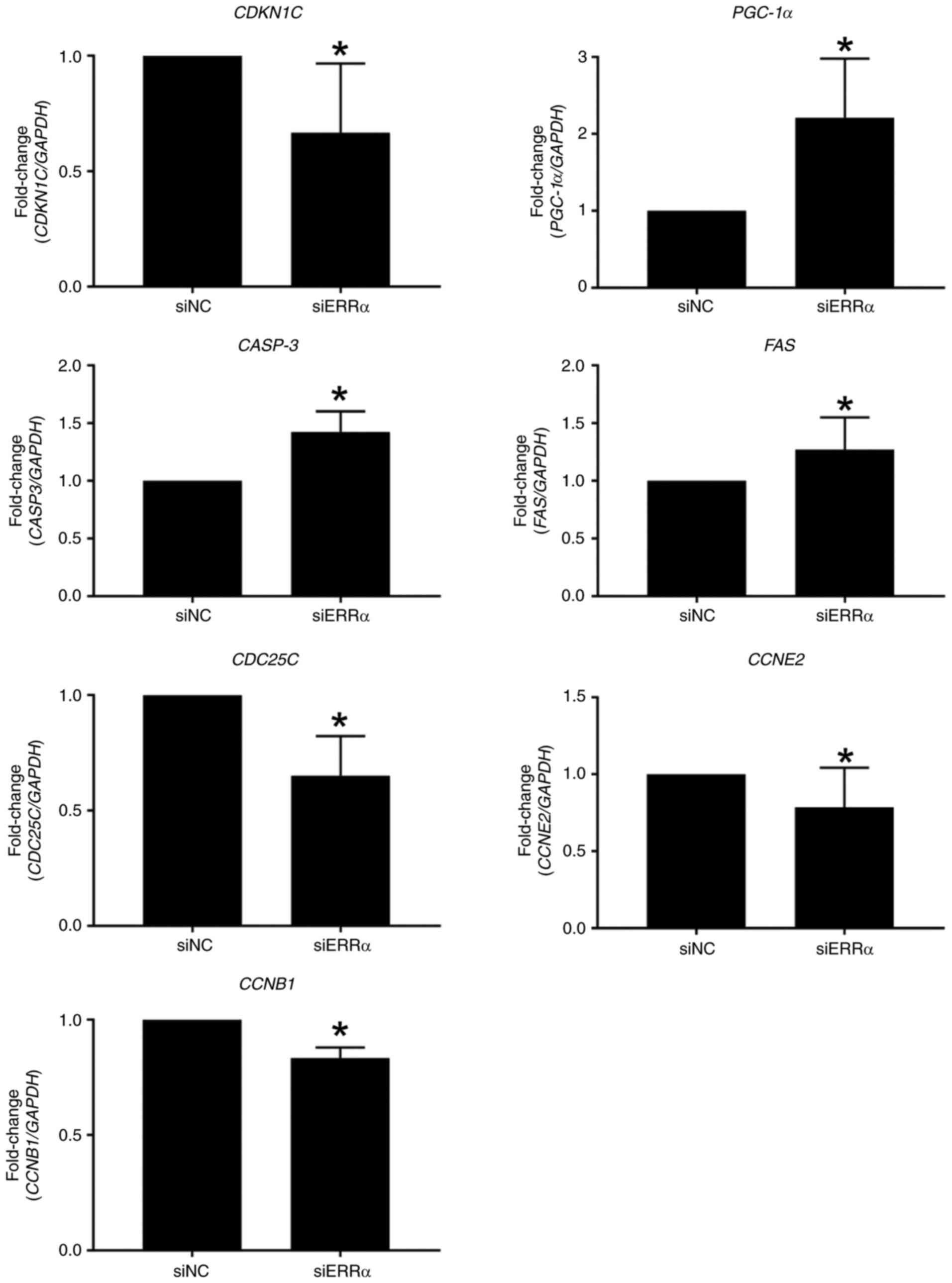 | Figure 3.Validation of gene expression in
TIG113 cells. TIG113 cells were transfected with siERRα or siNC and
cultured for 48 h. The mRNA levels of each gene were quantified
using reverse transcription-quantitative PCR. Relative expression
was normalized to that of GAPDH. Data represent the mean ±
standard deviation of three independent experiments.
*P<0.05 vs. siNC. ERRα, estrogen-related receptor
α; si, small interfering; NC, negative control; CDKN1C,
cyclin-dependent kinase inhibitor 1C; PGC-1α, peroxisome
proliferator-activated receptor gamma, coactivator 1 α;
CASP3, caspase 3; FAS, Fas cell surface death
receptor; CDC25C, cell division cycle 25C; CCNE2,
cyclin E2; CCNB1, cyclin B1. |
 | Table I.Fold change of selected gene
expression by ERRα silencing. |
Table I.
Fold change of selected gene
expression by ERRα silencing.
| A, Nuclear
receptors meta-pathway (upregulation) |
|---|
|
|---|
| Gene symbol | Gene name | Fold change |
|---|
| TGFB2 | Transforming growth
factor, β 2 | 3.65±0.45 |
| SLC2A14 | Solute carrier
family 2 (facilitated glucose transporter), member 14 | 3.05±0.31 |
| SLC2A3 | Solute carrier
family 2 (facilitated glucose transporter), member 3 | 2.53±0.22 |
| ABCC3 | ATP-binding
cassette, sub-family C (CFTR/MRP), member 3 | 2.37±0.30 |
| RGS2 | Regulator of
G-protein signaling 2 | 2.77±0.58 |
| CDKN1C | Cyclin-dependent
kinase inhibitor 1C (p57, Kip2) | 2.21±0.19 |
| PGC-1α | Peroxisome
proliferator-activated receptor gamma, coactivator 1 alpha | 2.39±0.22 |
| CYP3A5 | Cytochrome P450,
family 3, subfamily A, polypeptide 5 | 2.30±0.22 |
| ESR1 | Estrogen receptor
1 | 2.05±0.19 |
| EPHA2 | EPH receptor
A2 | 2.10±0.20 |
| CYP3A7 | Cytochrome P450,
family 3, subfamily A, polypeptide 7 | 2.41±0.41 |
| SLC7A11 | Solute carrier
family 7 (anionic amino acid transporter light chain, xc-system),
member 11 | 1.88±0.16 |
| SLC6A6 | Solute carrier
family 6 (neurotransmitter transporter), member 6 | 1.94±0.07 |
| CYP1B1 | Cytochrome P450,
family 1, subfamily B, polypeptide 1 | 1.73±0.09 |
| SPRY1 | Sprouty homolog 1,
antagonist of FGF signaling (Drosophila) | 1.86±0.40 |
| PDK4 | Pyruvate
dehydrogenase kinase, isozyme 4 | 2.01±0.34 |
| GCLC | Glutamate-cysteine
ligase, catalytic subunit | 1.71±0.06 |
| JUNB | Jun B
proto-oncogene | 1.92±0.27 |
| HBEGF | Heparin-binding
EGF-like growth factor | 1.61±0.07 |
| SLC7A5 | Solute carrier
family 7 (amino acid transporter light chain, l system), member
5 | 1.75±0.06 |
| SLC39A8 | Solute carrier
family 39 (zinc transporter), member 8 | 1.76±0.12 |
| PPARA | Peroxisome
proliferator-activated receptor alpha | 1.71±0.12 |
| LRRC8A | leucine rich repeat
containing 8 family, member A | 1.56±0.06 |
|
PPP1R14C | Protein phosphatase
1, regulatory (inhibitor) subunit 14C | 1.70±0.10 |
| ABCC2 | ATP-binding
cassette, sub-family C (CFTR/MRP), member 2 | 2.11±0.78 |
|
| B, Apoptosis
(upregulation) |
|
| Gene
symbol | Gene
name | Fold
change |
|
| IRF7 | Interferon
regulatory factor 7 | 2.97±0.64 |
| TNFSF10 | Tumor necrosis
factor (ligand) superfamily, member 10 | 2.40±0.88 |
| PMAIP1 |
Phorbol-12-myristate-13-acetate-induced
protein 1 | 2.28±0.20 |
| BCL2L11 | Bcl2-like 11
(apoptosis facilitator) | 2.24±0.28 |
| APAF1 | Apoptotic peptidase
activating factor 1 | 2.15±0.13 |
| BBC3 | Bcl2 binding
component 3 | 2.10±0.11 |
| CASP1 | Caspase 1,
apoptosis-related cysteine peptidase | 2.02±0.13 |
| CASP3 | Caspase 3,
apoptosis-related cysteine peptidase | 1.81±0.15 |
| CASP4 | Caspase 4,
apoptosis-related cysteine peptidase | 1.72±0.07 |
|
TNFRSF21 | Tumor necrosis
factor receptor superfamily, member 21 | 1.68±0.16 |
| HRK | Harakiri,
Bcl2interacting protein | 1.59±0.08 |
| FAS | Fas cell surface
death receptor | 1.53±0.04 |
|
| C, Cell cycle
(downregulation) |
|
| Gene
symbol | Gene
name | Fold
change |
|
| CDC25C | Cell division cycle
25C | −2.21±0.55 |
| E2F1 | E2F transcription
factor 1 | −1.79±0.27 |
| PKMYT1 | Protein kinase,
membrane associated tyrosine/threonine 1 | −1.76±0.20 |
| RBL1 | Retinoblastoma-like
1 | −1.75±0.17 |
| ORC5 | Origin recognition
complex, subunit 5 | −1.72±0.02 |
| MCM6 | Minichromosome
maintenance complex component 6 | −1.72±0.10 |
| CCNE2 | Cyclin E2 | −1.63±0.10 |
| CCNB1 | Cyclin B1 | −1.58±0.09 |
| PCNA | Proliferating cell
nuclear antigen | −1.56±0.05 |
Reduction of cell proliferation and
induction of TIG113 apoptosis cells by ERRα silencing
As silencing of ERRα downregulates cell
cycle-related genes and upregulates apoptosis-related genes in
fibroblasts, cell proliferation and apoptosis analyses were
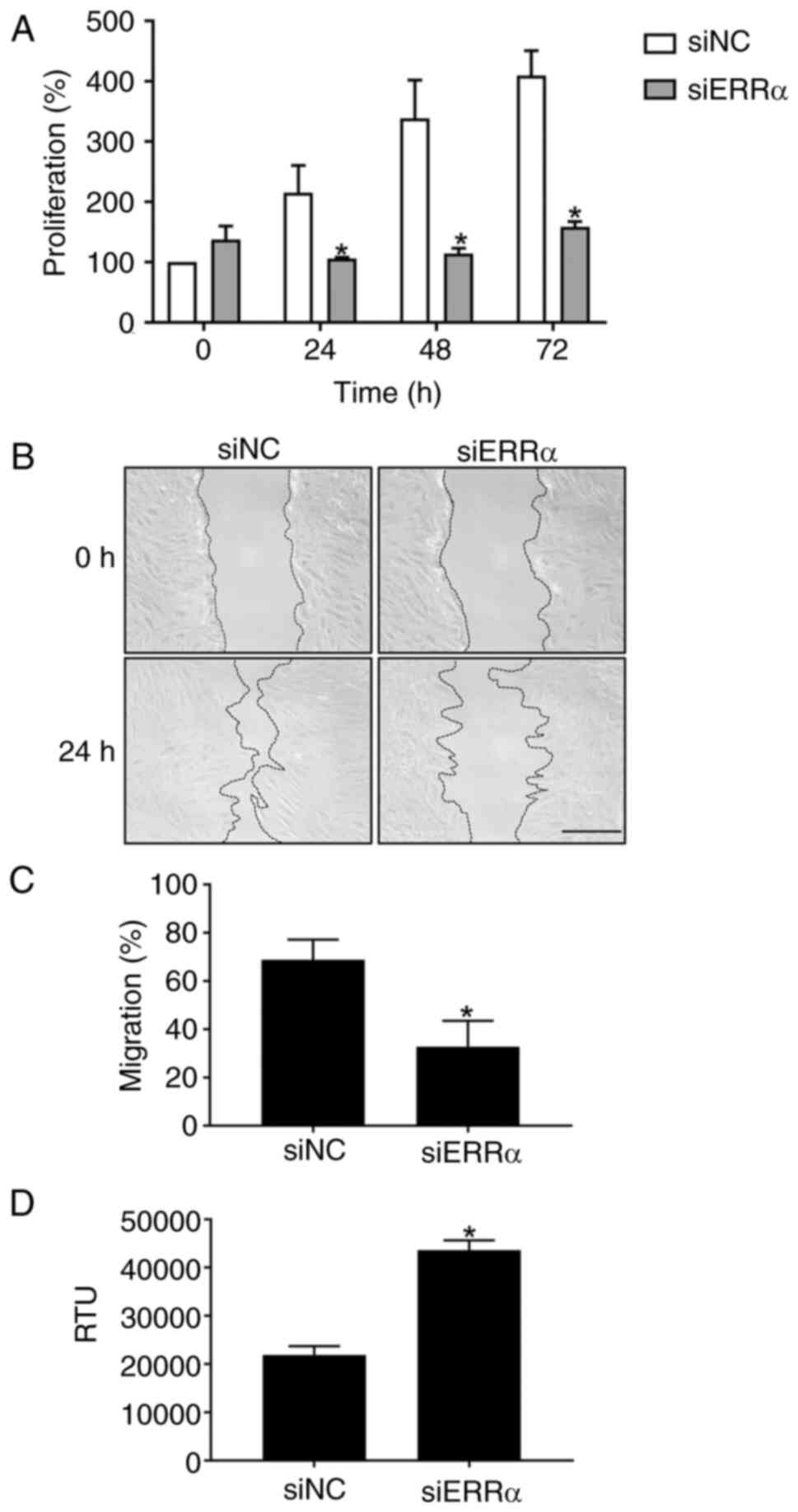
performed. ERRα was silenced in TIG113 cells and cell
proliferation was evaluated every 24 h. Cells continued to
proliferate for up to 72 h in siNC. By contrast, in
ERRα-silenced TIG113 cells, cell proliferation was
significantly reduced after 24 h and the difference in cell
proliferation increased after 48 and 72 h, suggesting that cell
proliferation was suppressed in ERRα-silenced TIG113
(Fig. 4A). Furthermore, in the
scratch wound healing assay, the migration percentage of TIG113
cells treated with siNC was 68.9%, whereas it decreased to 32.8%
with siERRa (Fig. 4B and C).
As silencing of ERRα increased the expression of
apoptosis-related genes (Table I
and Fig. 3), whether apoptosis was
induced was examined. The activity of poly caspase, an apoptosis
induction-related enzyme, increased ~2-fold 72 h after transfection
with siERRα (Fig. 4D). These
results suggest that apoptosis was induced in ERRα-silenced
TIG113 cells.
Silencing ERRα causes cell cycle
arrest in TIG113 cells
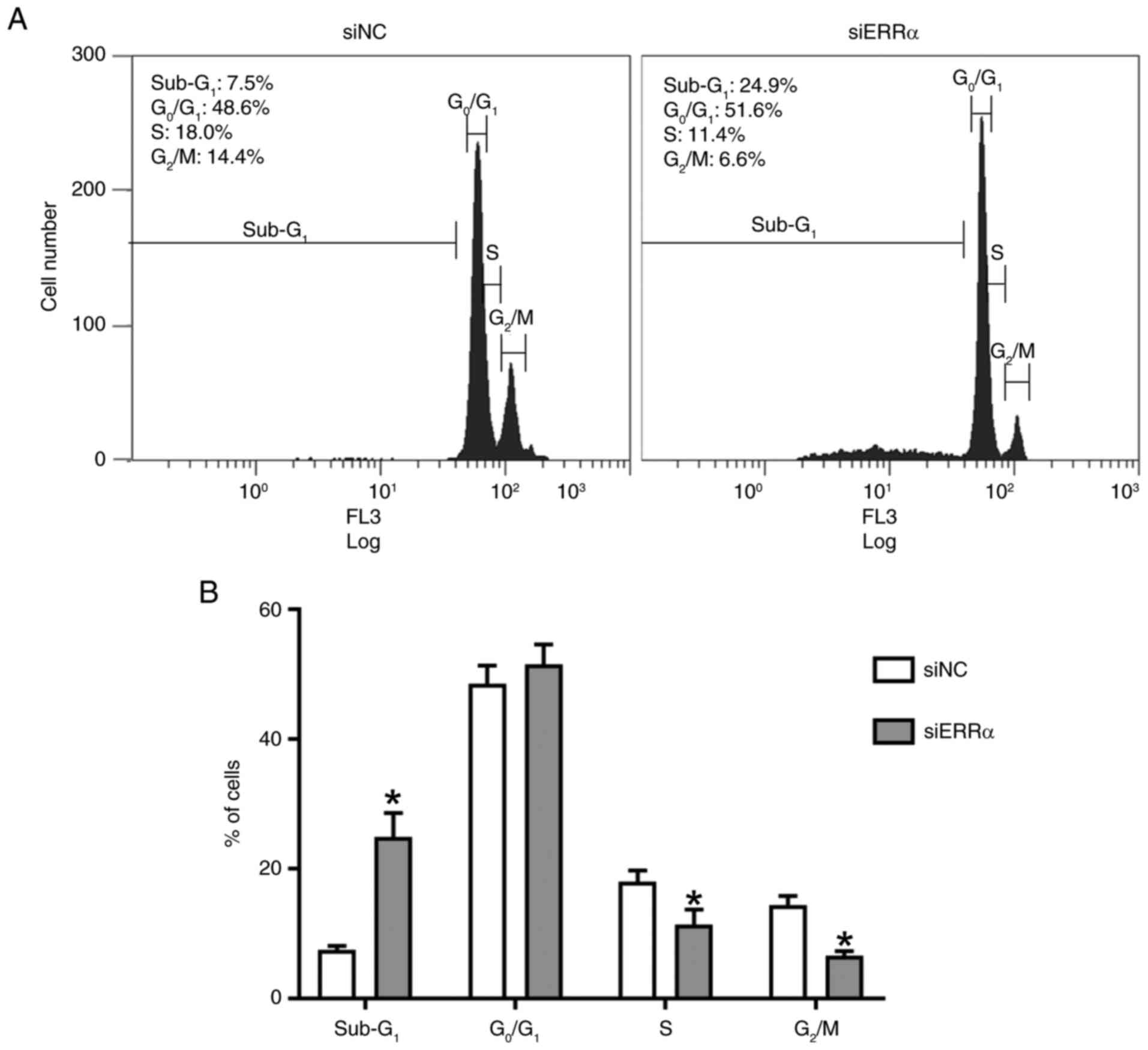
TIG113 cells were treated with siERRα for 72 h prior
to cell cycle analysis. The siERRα treatment significantly
increased the proportion of Sub-G1 phase cells and
decreased the proportion of S and G2/M phase cells
(Fig. 5A and B).
Quantification of type I collagen and
hyaluronan
ELISA revealed that the amount of type I collagen
produced by TIG113 cells was significantly decreased after
transfection with siERRα for 72 h (Fig. S2A). Similarly, the amount of
hyaluronic acid was significantly decreased after transfection with
siERRα (Fig. S2B).
Discussion
ERRα is expressed in skin tissue, but its function
is unknown. In the present study, ERRα was silenced by siRNA
in human skin fibroblasts and its function was analyzed. ERRα, ERRβ
and ERRγ are expressed in keratinocytes of the skin epidermis and
it has been reported that only ERRγ is expressed in fibroblasts
(15–17). However, in the present study, the
expression levels of ERRα in siNC-treated TIG113 cells in
microarrays was higher than that of ERRβ and γ. As the
present study mainly aimed to clarify the function of ERRα in
TIG113 cells, the comparison of the expression of ERRα, β and γ, as
well as the estrogen receptor, was only a supplementary analysis
and thus absolute quantitative expression analysis was not
performed. However, detailed analysis using absolute quantification
is required to compare the expression levels of ERRs and estrogen
receptors α and β in the future. Furthermore, because only a few
studies reported expression of ERRs in skin tissues and cells,
further analysis with more specimens is required.
Silencing of ERRα decreased the expression of
cell cycle-related genes such as CDC25C, CCNE2 and
CCNB1. CDC25C is known to control the transition from the
G1 phase to the S phase and the transition from the
G2 phase to the M phase (29). In addition, cyclin E binds to
cyclin-dependent kinase 2 in the G1 phase to form a
complex that is required for the cell cycle transition from the
G1 phase to the S phase where DNA replication is
initiated (30) and CCNB1 is a
regulatory protein involved in mitosis (31). Furthermore, silencing of ERRα
increases the expression of CDKN1C, a known cell cycle
inhibitor (32). Expression of
these genes related to the cell cycle was decreased and cell
proliferation was suppressed in ERRα-silenced TIG113, suggesting
that a normal cell cycle did not occur. Cell cycle analysis showed
that siERRα knockdown decreased the number of cells in the S and
G2/M phases. ERRα regulates CDC25C and
CCNB1 in gastric cancer cells, suggesting that it also
regulates these genes in fibroblasts (33).
Silencing of ERRα enhanced apoptosis and the
expression of apoptosis induction-related genes such as
CASP3 and FAS. Furthermore, Sub-G1 phase
cells were increased in siERRα-treated TIG113 cells. An increase in
the Sub-G1 phase was observed in apoptotic cells
(34), suggesting that apoptosis
was induced by siERR treatment. Caspases are a family of proteases
that play central roles in numerous processes, including cell death
and inflammation and CASP3 is an important mediator of
apoptosis (35). FAS is a
type I transmembrane protein and apoptosis is induced upon binding
of the Fas ligand (36,37). The results of the present study
suggested that increased expression of these apoptosis-related
genes induce cell death in ERRα-silenced TIG113 cells. The
p53 gene encodes a protein that has the function of
regulating suppression of the cell growth cycle such as DNA repair,
cell growth arrest and apoptosis (38). It has recently been reported that
ERRα and p53 protein directly bind to regulate colon cancer growth
through regulation of mitochondrial biogenesis and that knockdown
of ERRα suppresses p53 gene expression and impairs
mitochondrial biogenesis (39).
Although no change was observed in the expression level of
p53 in this study (data not shown), it is possible that
silencing of ERRα abolished its interaction with p53 and
reduced mitochondrial biogenesis. ERRα contributes to the
proliferation of some cancer cells and knockdown of ERRα reduces
cell proliferation and induces apoptosis (40–42),
consistent with the results of the present study. This suggested
that ERRα also contributes to cell proliferation in normal skin
fibroblasts.
The PGC-1 family includes PGC-1α, PGC-1β and
PGC-1-related coactivators, which regulate mitochondrial biogenesis
(43). PGC-1α induces ERRα
expression and interacts with ERRα (44) and the ERRα/PGC-1α axis is known to
decrease with aging, accelerating osteoporosis, kidney dysfunction,
sarcopenia and neurodegeneration (7). Furthermore, the expression of PGC-1α
is enhanced in the myocardium of ERRα-null mice (45), consistent with the findings of the
present study. Although the mechanism is not clear, it is possible
that the silencing of ERRα in fibroblasts compensates for the
enhancement of PGC-1α expression, or that ERRα regulates the
expression of PGC-1α.
Thus, knocking down ERRα altered various genes,
leading to cell cycle modifications and the induction of apoptosis.
However, the present study was unable to identify any genes
directly regulated by ERRα. Future research should focus on
identifying the direct targets of ERRα.
In the skin, fibroblasts secrete components that
contribute to skin antiaging, such as type I collagen and
hyaluronan. It was hypothesized that the decrease in cell
proliferation was due to a decrease in these components.
Furthermore, when TIG113 cells were treated with siERRα, the amount
of type I collagen and hyaluronan secreted into the culture
supernatant decreased. These results suggested that ERRα may also
be an important factor for skin antiaging.
ERRα is an orphan nuclear receptor that can be
activated by exogenous agonists such as phytoestrogens such as
genistein and daidzein (46),
which exhibit estrogenic activity and are found in plants. These
compounds share target genes with estrogen receptors and
phytoestrogens may activate the ERR pathway, potentially
contributing to skin fibroblast proliferation. As estrogen is not
an ERR ligand and does not activate ERR, the present study did not
investigate the activation of ERRα by estrogen treatment. However,
given that various phytoestrogens may act as ligands for ERRα,
future research should explore these possibilities to uncover new
activators of ERRα.
ERR is expressed in skin tissue, but its function is
unknown. The present study found that suppression of ERRα
expression using siRNA suppresses cell proliferation and induces
apoptosis. As a reduction in skin fibroblasts accelerates skin
aging, the discovery of new exogenous ligands for ERRα and
activation of ERRα may lead to the development of new skin
antiaging treatments.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was partly supported by the Japan Society for
the Promotion of Science KAKENHI (grant nos. 20K02402 and
23K02038).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The TIG113 microarray
datasets generated in the present study may be found in the Gene
Expression Omnibus under accession number GSE245234 or at the
following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE245234.
Authors' contributions
NN designed the study. NN, TN, MN, CH and KH
performed the experiments and analyzed the data. NN, MN and KH
confirm the authenticity of all the raw data. NN and CH wrote the
original manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CASP3
|
caspase 3
|
|
CDC25C
|
cell division cycle 25C
|
|
ERR
|
Estrogen-related receptor
|
|
FAS
|
Fas cell surface death receptor
|
|
PGC-1α
|
peroxisome proliferator-activated
receptor gamma, coactivator 1 α
|
References
|
1
|
Giguère V, Yang N, Segui P and Evans RM:
Identification of a new class of steroid hormone receptors. Nature.
331:91–94. 1988. View
Article : Google Scholar
|
|
2
|
Horard B and Vanacker JM: Estrogen
receptor-related receptors: Orphan receptors desperately seeking a
ligand. J Mol Endocrinol. 31:349–357. 2003. View Article : Google Scholar
|
|
3
|
Huss JM, Imahashi K, Dufour CR, Weinheimer
CJ, Courtois M, Kovacs A, Giguère V, Murphy E and Kelly DP: The
nuclear receptor ERRalpha is required for the bioenergetic and
functional adaptation to cardiac pressure overload. Cell Metab.
6:25–37. 2007. View Article : Google Scholar
|
|
4
|
Deblois G and Giguère V: Functional and
physiological genomics of estrogen-related receptors (ERRs) in
health and disease. Biochim Biophys Acta. 1812:1032–1040. 2011.
View Article : Google Scholar
|
|
5
|
Fan W, He N, Lin CS, Wei Z, Hah N,
Waizenegger W, He MX, Liddle C, Yu RT, Atkins AR, et al: ERRγ
promotes angiogenesis, mitochondrial biogenesis, and oxidative
remodeling in PGC1α/β-deficient muscle. Cell Rep. 22:2521–2529.
2018. View Article : Google Scholar
|
|
6
|
Schreiber SN, Emter R, Hock MB, Knutti D,
Cardenas J, Podvinec M, Oakeley EJ and Kralli A: The
estrogen-related receptor alpha (ERRalpha) functions in PPARgamma
coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis.
Proc Natl Acad Sci USA. 101:6472–6477. 2004. View Article : Google Scholar
|
|
7
|
Vernier M and Giguère V: Aging, senescence
and mitochondria: The PGC-1/ERR axis. J Mol Endocrinol. 66:R1–R14.
2021. View Article : Google Scholar
|
|
8
|
Luo J, Sladek R, Bader JA, Matthyssen A,
Rossant J and Giguère V: Placental abnormalities in mouse embryos
lacking the orphan nuclear receptor ERR-beta. Nature. 388:778–782.
1997. View Article : Google Scholar
|
|
9
|
Giguère V: To ERR in the estrogen pathway.
Trends Endocrinol Metab. 13:220–225. 2002. View Article : Google Scholar
|
|
10
|
Brincat MP, Baron YM and Galea R:
Estrogens and the skin. Climacteric. 8:110–123. 2005. View Article : Google Scholar
|
|
11
|
Stern R and Maibach HI: Hyaluronan in
skin: Aspects of aging and its pharmacologic modulation. Clin
Dermatol. 26:106–122. 2008. View Article : Google Scholar
|
|
12
|
Thornton MJ: Estrogens and aging skin.
Dermatoendocrinol. 5:264–270. 2013. View Article : Google Scholar
|
|
13
|
Haczynski J, Tarkowski R, Jarzabek K,
Slomczynska M, Wolczynski S, Magoffin DA, Jakowicki JA and Jakimiuk
AK: Human cultured skin fibroblasts express estrogen receptor alpha
and beta. Int J Mol Med. 10:149–153. 2002.
|
|
14
|
Saito K and Cui H: Emerging roles of
estrogen-related receptors in the brain: Potential interactions
with estrogen signaling. Int J Mol Sci. 19:10912018. View Article : Google Scholar
|
|
15
|
Bertil E, Bolzinger MA, André V, Rousselle
P and Damour O: Expression of oestrogen-related receptor alpha in
human epidermis. Exp Dermatol. 17:208–213. 2008. View Article : Google Scholar
|
|
16
|
Krahn-Bertil E, Dos Santos M, Damour O,
Andre V and Bolzinger MA: Expression of estrogen-related receptor
beta (ERRbeta) in human skin. Eur J Dermatol. 20:719–723. 2010.
|
|
17
|
Krahn-Bertil E, Bolzinger MA, Andre V,
Orly I, Kanitakis J, Rousselle P and Damour O: Expression of
estrogen-related receptor gamma (ERRgamma) in human skin. Eur J
Dermatol. 18:427–432. 2008.
|
|
18
|
Liu D, Zhang Z, Gladwell W and Teng CT:
Estrogen stimulates estrogen-related receptor alpha gene expression
through conserved hormone response elements. Endocrinology.
144:4894–4904. 2003. View Article : Google Scholar
|
|
19
|
Fujimoto J, Nakagawa Y, Toyoki H,
Sakaguchi H, Sato E and Tamaya T: Estrogen-related receptor
expression in placenta throughout gestation. J Steroid Biochem Mol
Biol. 94:67–69. 2005. View Article : Google Scholar
|
|
20
|
Hoffmann MJ, Florl AR, Seifert HH and
Schulz WA: Multiple mechanisms downregulate CDKN1C in human bladder
cancer. Int J Cancer. 114:406–413. 2005. View Article : Google Scholar
|
|
21
|
Thamizhanambi TP, Rameshkumar A, Ramya R,
Krishnan R, Dineshkumar T and Nandhini G: Analysis of metabolic
regulators PGC1-α and PGC1-β in oral squamous cell carcinoma with
and without hyperglycemia. Asian Pac J Cancer Prev. 23:2797–2803.
2022. View Article : Google Scholar
|
|
22
|
Jafari N, Zargar SJ, Yassa N and Delnavazi
MR: Induction of apoptosis and cell cycle arrest by Dorema glabrum
root extracts in a gastric adenocarcinoma (AGS) cell line. Asian
Pac J Cancer Prev. 17:5189–5193. 2016.
|
|
23
|
Piri-Gharaghie T, Ghajari G, Hassanpoor M,
Jegargoshe-Shirin N, Soosanirad M, Khayati S, Farhadi-Biregani A
and Mirzaei A: Investigation of antibacterial and anticancer
effects of novel niosomal formulated Persian Gulf Sea cucumber
extracts. Heliyon. 9:e141492023. View Article : Google Scholar
|
|
24
|
Zhang W, Shang X, Yang F, Han W, Xia H, Lu
N, Liu Y and Wang X: CDC25C as a predictive biomarker for immune
checkpoint inhibitors in patients with lung adenocarcinoma. Front
Oncol. 12:8677882022. View Article : Google Scholar
|
|
25
|
Chen M, Wu R, Li G, Liu C, Tan L, Xiao K,
Ye Y and Qin Z: Motor neuron and pancreas homeobox 1/HLXB9 promotes
sustained proliferation in bladder cancer by upregulating CCNE1/2.
J Exp Clin Cancer Res. 37:1542018. View Article : Google Scholar
|
|
26
|
Du Y, Zheng Y, Yu K, Zhan C and Qiao T:
Genome-wide analyses of lung cancer after single high-dose
radiation at five time points (2, 6, 12, 24, and 48 h). Front
Genet. 14:11262362023. View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Nanashima N, Horie K, Chiba M, Nakano M,
Maeda H and Nakamura T: Anthocyanin-rich blackcurrant extract
inhibits proliferation of the MCF10A healthy human breast
epithelial cell line through induction of G0/G1 arrest and
apoptosis. Mol Med Rep. 16:6134–6141. 2017. View Article : Google Scholar
|
|
29
|
Perdiguero E and Nebreda AR: Regulation of
Cdc25C activity during the meiotic G2/M transition. Cell Cycle.
3:733–737. 2004. View Article : Google Scholar
|
|
30
|
Fagundes R and Teixeira LK: Cyclin E/CDK2:
DNA replication, replication stress and genomic instability. Front
Cell Dev Biol. 9:7748452021. View Article : Google Scholar
|
|
31
|
Hayward D, Alfonso-Pérez T and Gruneberg
U: Orchestration of the spindle assembly checkpoint by CDK1-cyclin
B1. FEBS Lett. 593:2889–2907. 2019. View Article : Google Scholar
|
|
32
|
Matsuoka S, Edwards MC, Bai C, Parker S,
Zhang P, Baldini A, Harper JW and Elledge SJ: p57KIP2, a
structurally distinct member of the p21CIP1 Cdk inhibitor family,
is a candidate tumor suppressor gene. Genes Dev. 9:650–662. 1995.
View Article : Google Scholar
|
|
33
|
Li FN, Zhang QY, Li O, Liu SL, Yang ZY,
Pan LJ, Zhao C, Gong W, Shu YJ and Dong P: ESRRA promotes gastric
cancer development by regulating the CDC25C/CDK1/CyclinB1 pathway
via DSN1. Int J Biol Sci. 17:1909–1924. 2021. View Article : Google Scholar
|
|
34
|
Bedner E, Li X, Gorczyca W, Melamed MR and
Darzynkiewicz Z: Analysis of apoptosis by laser scanning cytometry.
Cytometry. 35:181–195. 1999. View Article : Google Scholar
|
|
35
|
Orlinick JR and Chao MV: TNF-related
ligands and their receptors. Cell Signal. 10:543–551. 1998.
View Article : Google Scholar
|
|
36
|
Nagata S and Golstein P: The Fas death
factor. Science. 267:1449–1456. 1995. View Article : Google Scholar
|
|
37
|
Akagi T, Yoshino T and Kondo E: The Fas
antigen and Fas-mediated apoptosis in B-cell differentiation. Leuk
Lymphoma. 28:483–489. 1998. View Article : Google Scholar
|
|
38
|
Götz C and Montenarh M: p53: DNA damage,
DNA repair, and apoptosis. Rev Physiol Biochem Pharmacol.
127:65–95. 1996. View Article : Google Scholar
|
|
39
|
De Vitto H, Ryu J, Calderon-Aparicio A,
Monts J, Dey R, Chakraborty A, Lee MH, Bode AM and Dong Z:
Estrogen-related receptor alpha directly binds to p53 and
cooperatively controls colon cancer growth through the regulation
of mitochondrial biogenesis and function. Cancer Metab. 8:282020.
View Article : Google Scholar
|
|
40
|
Matsushima H, Mori T, Ito F, Yamamoto T,
Akiyama M, Kokabu T, Yoriki K, Umemura S, Akashi K and Kitawaki J:
Anti-tumor effect of estrogen-related receptor alpha knockdown on
uterine endometrial cancer. Oncotarget. 7:34131–34148. 2016.
View Article : Google Scholar
|
|
41
|
Sun P, Mao X, Gao M, Huang MM, Chen LL,
Ruan GY, Huang WY, Braicu EI and Sehouli J: Novel endocrine
therapeutic strategy in endometrial carcinoma targeting
estrogen-related receptor α by XCT790 and siRNA. Cancer Manag Res.
10:2521–2535. 2018. View Article : Google Scholar
|
|
42
|
Zhang J, Guan X, Liang N and Li S:
Estrogen-related receptor alpha triggers the proliferation and
migration of human non-small cell lung cancer via interleukin-6.
Cell Biochem Funct. 36:255–262. 2018. View Article : Google Scholar
|
|
43
|
Scarpulla RC: Metabolic control of
mitochondrial biogenesis through the PGC-1 family regulatory
network. Biochim Biophys Acta. 1813:1269–1278. 2011. View Article : Google Scholar
|
|
44
|
Schreiber SN, Knutti D, Brogli K, Uhlmann
T and Kralli A: The transcriptional coactivator PGC-1 regulates the
expression and activity of the orphan nuclear receptor
estrogen-related receptor alpha (ERRalpha). J Biol Chem.
278:9013–9018. 2003. View Article : Google Scholar
|
|
45
|
Dufour CR, Wilson BJ, Huss JM, Kelly DP,
Alaynick WA, Downes M, Evans RM, Blanchette M and Giguère V:
Genome-wide orchestration of cardiac functions by the orphan
nuclear receptors ERRalpha and gamma. Cell Metab. 5:345–356. 2007.
View Article : Google Scholar
|
|
46
|
Suetsugi M, Su L, Karlsberg K, Yuan YC and
Chen S: Flavone and isoflavone phytoestrogens are agonists of
estrogen-related receptors. Mol Cancer Res. 1:981–991. 2003.
|