Introduction
Gastric cancer (GC) is the fifth most commonly
diagnosed cancer and the third leading cause of cancer-related
deaths globally (1). Recent
advancements in surgical techniques, chemotherapy, adjuvant
radiotherapy, and molecular targeted therapies have significantly
improved the treatment of GC, achieving a survival rate of over 95%
for early-stage cases (2).
However, early diagnosis of GC remains challenging, and the overall
survival rate for patients with recurrent or metastatic GC remains
poor (2–4). Numerous studies have demonstrated
that gastric cancer stem cells (GCSCs) are crucial to the
aggressive nature of GC, including its progression, metastasis,
recurrence, and resistance to treatment (5,6).
GCSCs are known to overexpress specific stem cell markers such as
CD133, CD44, aldehyde dehydrogenase 1 (ALDH1), NANOG, SOX2, and
OCT4, which are linked to poor prognosis and aggressive biological
behavior in GC (5). Thus,
identifying and targeting upstream molecular mechanisms that
regulate these GCSC markers is essential for improving current GC
treatment strategies.
Cyclophilin A (CypA) stands out as the most
prevalent member within the immunophilin family, which has
peptidyl-prolyl cis-trans isomerase activity (7). While primarily localized in the
cytoplasm, CypA can also be discharged into the extracellular
environment in response to inflammatory triggers. Once outside the
cell, secreted CypA engages with CD147, a transmembrane protein
from the immunoglobulin superfamily, fostering intercellular
connections and eliciting intracellular responses (8). CypA orchestrates a range of
biological processes, including protein folding and trafficking,
activation of immune cells, and modulation of cell signaling
pathways (7,9). Additionally, it plays a pathological
role in various human diseases, encompassing viral infections,
inflammatory conditions, autoimmune disorders, and cancer (10,11).
Increasing evidence underscores CypA's overexpression across
diverse tumor types, where it fuels cancer cell survival,
proliferation, migration, and invasion (10,12).
The interplay between CypA and CD147 triggers several oncogenic
signaling cascades, notably the phosphoinositide 3-kinase
(PI3K)/AKT and mitogen-activated protein kinase
(MAPK)/extracellular regulated kinase (ERK) pathways, ultimately
fostering cancer cell growth, metastasis, drug resistance, and
tumor recurrence (10).
Consequently, CypA and CD147 emerge as pivotal targets for cancer
therapy.
Our recent findings reveal the potential of natural
CypA inhibitors, such as cyclosporin A (CsA) and 23-demethyl
8,13-deoxynargenicin (C9), to suppress the growth of GCSCs
(13). Both CsA and C9 effectively
halted the proliferation of GCSCs derived from MKN45 cells, both
in vitro and in vivo, by arresting cell cycle at the
G0/G1 phase and triggering caspase-driven apoptosis. These
inhibitors also downregulated the expression of crucial GCSC
markers by modulating the MAPK and AKT signaling pathways mediated
by CypA/CD147. Furthermore, recent experiments demonstrated that
CsA and C9 can impede the expansion of cancer stem cells in
non-small cell lung cancer (NSCLC) by interfering with the
interaction between the epidermal growth factor receptor (EGFR) and
CypA/CD147 (14). Hence, our
results indicate that CypA inhibitors like CsA and C9 show promise
as anticancer agents targeting cancer stem cells, including GCSCs.
However, the direct involvement of CypA in GCSCs remains
unexplored. To further elucidate whether CypA could serve as a
therapeutic target for eradicating GCSCs, we examined its
functional impact on GCSC proliferation and metastatic potential
using CypA-specific small interfering RNA (siRNA).
Materials and methods
Reagents and antibodies
Accutase (cat. no. A6964), heparin (cat. no. H3149),
gelatin (cat. no. G2500), laminin (cat. no. L2020), and
extracellular matrix (ECM) gel (cat. no. E1270) were sourced from
Sigma-Aldrich. Basic fibroblast growth factor (bFGF, cat. no.
CYT-218) and epidermal growth factor (EGF, cat. no. CYT-217) were
acquired from Prospecbio. Trypsin (cat. no. SH30042.01), RPMI-1640
(cat. no. SH30027.01), DME/F-12 (cat. no. SH30023.01), and
antibiotics (cat. no. SV30079.01) were obtained from HyClone. B-27
supplement (cat. no. 17504-044) and fetal bovine serum (FBS, cat.
no. A56708-01) were sourced from Gibco. Antibodies for vimentin
(cat. no. A11952), E-cadherin (cat. no. A11492), and N-cadherin
(cat. no. A0433) were acquired from ABclonal. Antibodies for
β-actin (cat. no. 4967), CD44 (cat. no. 37259), CD133 (cat. no.
64326), ALDH1A1 (cat. no. 12035), SOX2 (cat. no. 3579), NANOG (cat.
no. 3580), OCT4 (cat. no. 2750), CypA (cat. no. 2175), CD147 (cat.
no. 13287), STAT3 (cat. no. 9139), phospho-STAT3 (cat. no. 9145),
AKT (cat. no. 9272), phospho-AKT (cat. no. 4060), ERK1/2 (cat. no.
9102), and phospho-ERK1/2 (cat. no. 9101) were all sourced from
Cell Signaling Technology.
Cell culturing
The AGS human gastric cancer cell line (KCLB no.
21739), sourced from the Korean Cell Line Bank (Seoul, South
Korea), was maintained under specific conditions as follows: For
adherent cell growth, RPMI-1640 medium containing 10% FBS and 1%
antibiotics was utilized. Subculturing of adherent cells was
performed using trypsin. For tumorsphere cell propagation, DME/F-12
medium containing 20 ng/ml bFGF, 20 ng/ml EGF, B-27 supplement, 5
µg/ml heparin, and 1% antibiotics was employed. Tumorsphere cells
were subcultured by dissociating them with Accutase (13,15).
The cells were incubated at a constant temperature of 37°C in a
humidified environment with 5% CO2.
CypA-directed RNA interference
CypA-specific siRNA (siCypA) was synthesized by
Bioneer (Daejeon, South Korea). The sequences for siCypA were
designed as follows: the sense sequence was
5′-GCUCGCAGUAUCCUAGAAU-3′ and the antisense sequence was
5′-AUUCUAGGAUACUGCGAGC-3′ (16).
Non-targeting siRNA control was procured from Santa Cruz
Biotechnology. To introduce the siRNAs into AGS GCSCs, dissociated
AGS tumorsphere cells were seeded in culture plates using
serum-free medium and transfected with siRNAs (100 nM) using
Lipofectamine™ 2000 (Invitrogen). Confirmation of CypA
knockdown was carried out through Western blotting.
Cell proliferation assay
AGS GCSCs, either non-silenced or CypA-silenced,
were plated at a quantity of 3×103 cells per well in
96-well plates, using serum-free medium supplemented with bFGF and
EGF, and cultured for 72 h. Tumorspheres with a diameter greater
than 60 µm were detected and enumerated using an optical
microscope. To assess cell proliferation, the
CellTiter-Glo® 2.0 Cell Viability Assay kit (Promega)
was utilized. Luminescence signals were measured with a microplate
reader (13).
Limiting dilution assay
AGS GCSCs, either non-silenced or CypA-silenced,
were seeded at varying densities ranging from 5 to 200 cells per
well in 96-well plates with serum-free medium containing bFGF and
EGF. Following a 7-day incubation period, the presence and quantity
of tumorspheres exceeding 60 µm in diameter in each well were
examined using light microscopy (Olympus). To determine the rate of
tumorsphere formation, data analysis was conducted using Extreme
Limiting Dilution Analysis (ELDA) tool, accessible at http://bioinf.wehi.edu.au/software/elda/. This
analysis was performed on November 16, 2023 (14).
Cell cycle and apoptosis analysis
AGS GCSCs, with or without CypA silencing, were
cultured in 60-mm culture dishes at a quantity of 2×105
cells per well in serum-free medium containing bFGF and EGF for 72
h. Following incubation, cells were collected and stained in
accordance with the manufacturer's instructions, using either
Muse® Cell Cycle (cat. no. MCH100106) or Annexin V &
Dead Cell reagent (cat. no. MCH100105) (Luminex). Subsequently,
cell cycle distribution and the proportion of apoptotic cells were
assessed using the Muse® Cell Analyzer, operated with
MuseSoft_V1.8.0.3 software (13).
Wound closure assay
Wounds were created using the ibidi culture insert
system (ibidi GmbH). After placing a culture insert into each well
of a laminin-coated 24-well plate, non-silenced or CypA-silenced
AGS GCSCs (15×104 cells/70 µl) were inoculated into each
insert using serum-free medium containing bFGF and EGF. After a
24-h incubation for cell attachment, the culture inserts were
removed. Afterwards, cell migration was monitored by light
microscopy for 0, 2, 4, and 6 h, and the gap area was measured
(16).
Invasion analysis
Cell invasion was evaluated utilizing a Transwell
system equipped with polycarbonate membrane inserts with an 8.0 µm
pore size (SPL Life Sciences, Pocheon, South Korea). Gelatin (1
mg/ml) and ECM gel (3 mg/ml) coatings were applied to the bottom
and upper surfaces of the filter, respectively. AGS GCSCs, either
non-silenced or CypA-silenced, were seeded at a quantity of
5×104 cells per well in the filter chamber using
serum-free culture medium containing bFGF and EGF and allowed to
culture for 24 h. Following staining with hematoxylin and eosin,
cells that had invaded were observed and quantified using an
optical microscope (16).
Western blot
Equal quantities of cell extracts were subjected to
sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed
by transfer to polyvinylidene difluoride membranes. The membranes
were next incubated with 5% skim milk for blocking before being
immunolabeled with primary antibodies, with dilutions ranging from
1:2,000 to 1:10,000. Detection of the immunolabeling was carried
out using an enhanced chemiluminescence kit (DoGenBio, Seoul, South
Korea), following incubation with secondary antibodies conjugated
to horseradish peroxidase at a dilution of 1:3,000. Band
intensities were quantified utilizing ImageJ 1.5 tool. Levels of
expression were measured by comparing the ratio of each target
protein relative to β-actin (13).
CAM assay
The CAM assay serves as a widely adopted in
vivo model, valued for its speed, simplicity, and inherent
immunodeficiency, particularly in assessing angiogenesis,
tumorigenesis, toxicology, and drug delivery (17,18).
In this study, non-silenced or CypA-silenced AGS GCSCs were
utilized, with a total of 2×106 cells per egg, mixed
with ECM gel at a ratio of 40 µl per egg, and then transplanted
into the CAM of fertilized eggs. Following transplantation, the
eggs underwent incubation in a humidified environment for 7 days.
At the end of this incubation period, the tumors that developed in
the CAM were collected, and their weight and diameter were recorded
(13).
Statistical evaluation
The data are presented as the average ± standard
deviation derived from a minimum of three separate experiments.
Statistical evaluation was conducted using one-way ANOVA followed
by Tukey's post-hoc test, employing SPSS version 9.0 software. A
P-value below 0.05 denotes statistical relevance.
Results
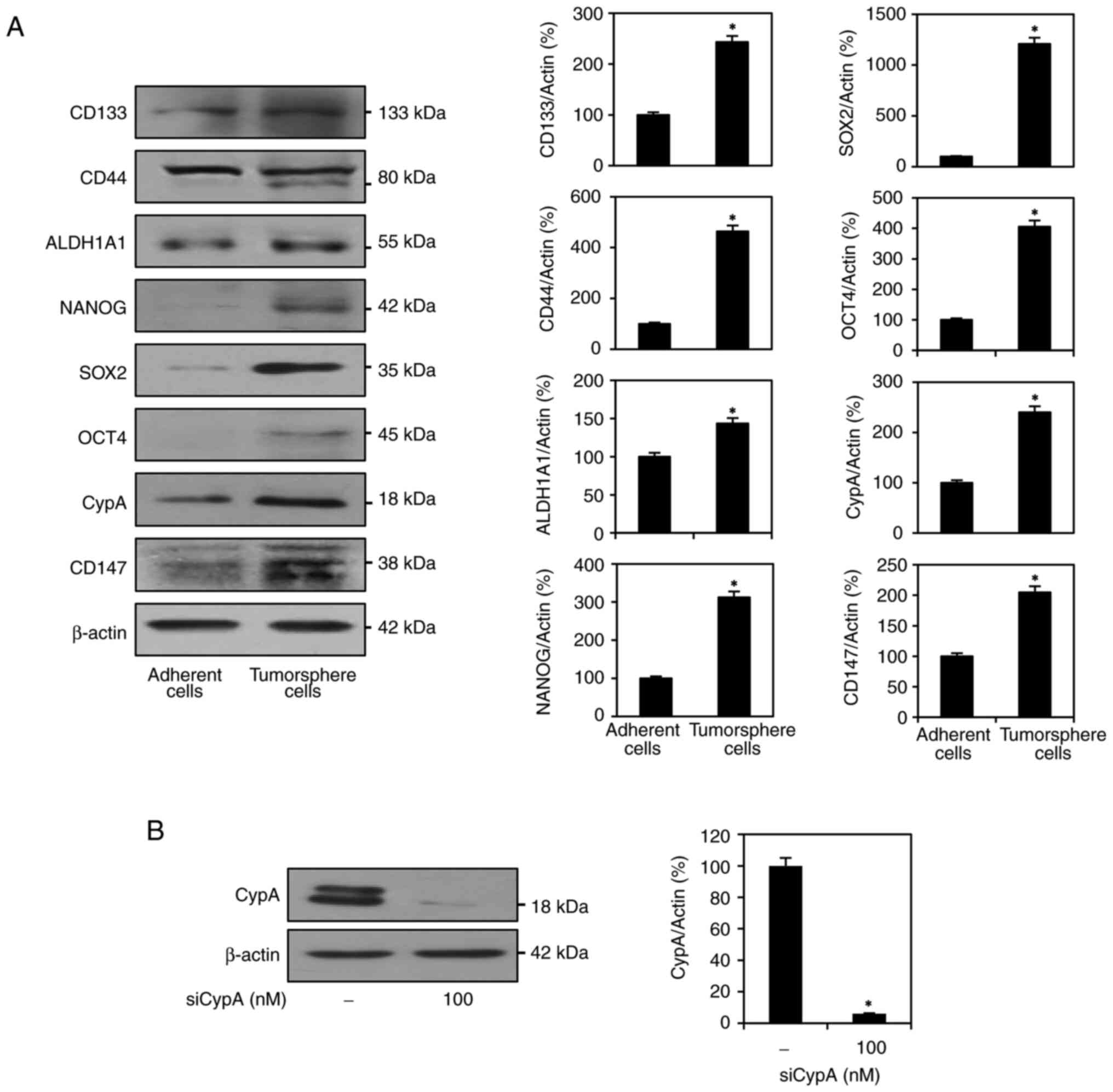
Genetic knockdown of CypA in AGS GCSCs
by RNA interference
We have previously demonstrated that GCSCs can be
selectively enriched from GC cell lines as tumorspheres under
serum-free medium supplemented with bFGF and EGF (13,15).
The stem-like characteristics of GC cells are accompanied by
upregulation of stemness-related factors. AGS tumorsphere cells
grown in these cancer stem cell culture conditions significantly
overexpressed key GCSC markers, such as CD44, CD133, ALDH1A1,
NANOG, SOX2, and OCT4, compared to AGS adherent cells cultured in
10% serum-supplemented conditions (Fig. 1A). These data indicate that AGS
tumorsphere cells have stem-like properties, and the serum-free
spheroid culture can enhance the expansion of the AGS-derived GCSC
population. Furthermore, in AGS tumorsphere cells, the levels of
CypA and CD147 expression were higher than in AGS adherent cells,
indicating that the CypA/CD147 pathway might be crucial for the
maintenance of GCSCs (Fig. 1A).
Therefore, all experiments in this study using AGS GCSCs were
performed under serum-free tumorsphere culture conditions selective
for cancer stem cells.
To investigate the role of CypA in GCSCs, we
performed genetic knockdown of CypA (gene name: peptidylprolyl
isomerase A, PPIA) using RNA interference and then analyzed
phenotypic changes. AGS-derived GCSCs were transfected with either
siRNA targeting CypA (siCypA) or a non-targeting siRNA control. As
depicted in Fig. 1B, the
significant reduction of CypA expression by siCypA was confirmed by
Western blotting.
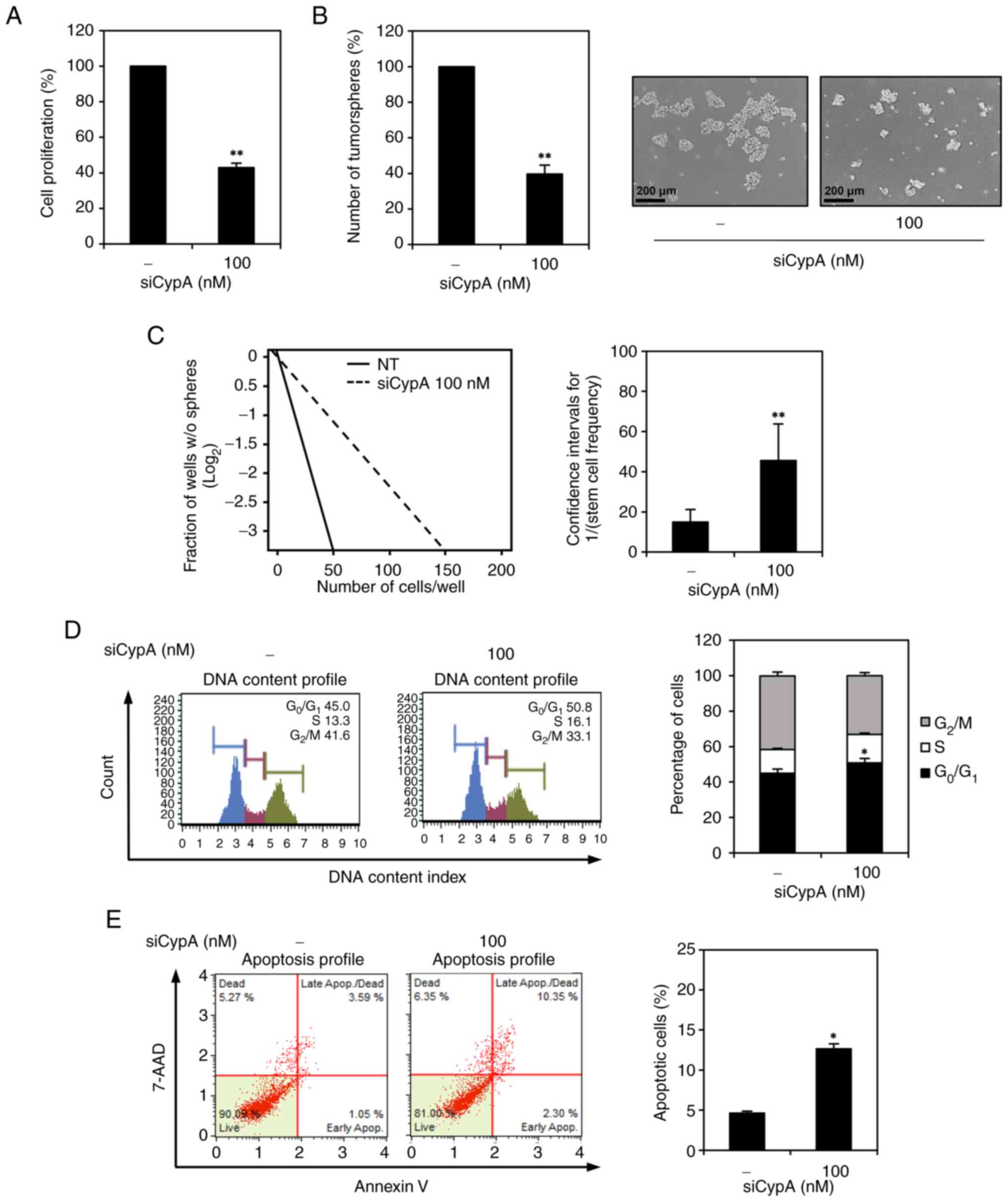
CypA knockdown inhibits proliferation
and tumorsphere formation capability of AGS GCSCs
Initially, we evaluated the influence of CypA
knockdown on the proliferation of GCSCs derived from AGS cells
through an ATP-monitoring luminescence assay. CypA knockdown
demonstrated a notable inhibitory effect on AGS GCSC proliferation,
as illustrated in Fig. 2A.
Tumorsphere formation capability stands as a distinctive trait of
cancer stem cells (5,6). Furthermore, silencing CypA resulted
in a decrease in both the quantity and size of tumorspheres
generated by AGS GCSCs, as depicted in Fig. 2B. The impact of CypA knockdown on
the tumorsphere-forming ability of AGS GCSCs was further evaluated
by limiting dilution assay (LDA). CypA-silenced AGS GCSCs exhibited
a 3-fold lower frequency of tumorsphere formation than non-silenced
control cells (Fig. 2C).
Next, we investigated whether inhibition of AGS GCSC
proliferation by CypA knockdown was associated with regulation of
cell cycle and apoptosis using flow cytometry. CypA knockdown
increased G0/G1 and S phase cell populations and decreased G2/M
phase cell populations in comparison to control cells (Fig. 2D). Additionally, reducing CypA
levels led to an elevated percentage of apoptotic cells relative to
the control group, as indicated in Fig. 2E. These findings underscore that
silencing CypA not only restrains the proliferation and
tumorsphere-forming capacity of GCSCs derived from AGS cells but
also arrests the cell cycle at G0/G1 and S phases, along with
inducing apoptosis. Consequently, these results suggest a favorable
role for CypA in the proliferation and sustenance of GCSCs.
CypA knockdown inhibits migration and
invasion of AGS GCSCs
We further assessed whether CypA knockdown affects
key metastatic functions such as migration and invasion of
AGS-derived GCSCs. As shown in the results from the wound closure
assay, CypA-silenced AGS GCSCs showed reduced migratory capacity
compared to non-silenced control cells (Fig. 3A). In addition, Transwell invasion
assay revealed that CypA knockdown markedly inhibited the
invasiveness of AGS GCSCs (Fig.
3B).
The high metastatic capacity of GCSCs is closely
associated with the promotion of epithelial-mesenchymal transition
(EMT) (5,19–21).
We investigated whether CypA silencing affects the expression of
key EMT-related proteins in AGS GCSCs. Western blotting results
showed that CypA knockdown increased the levels of the epithelial
cell marker E-cadherin, while decreasing the levels of mesenchymal
cell markers, including vimentin and N-cadherin (Fig. 3C). This indicates that CypA
silencing downregulates EMT. Therefore, these results suggest that
CypA may upregulate the metastatic ability of GCSCs by promoting
EMT.
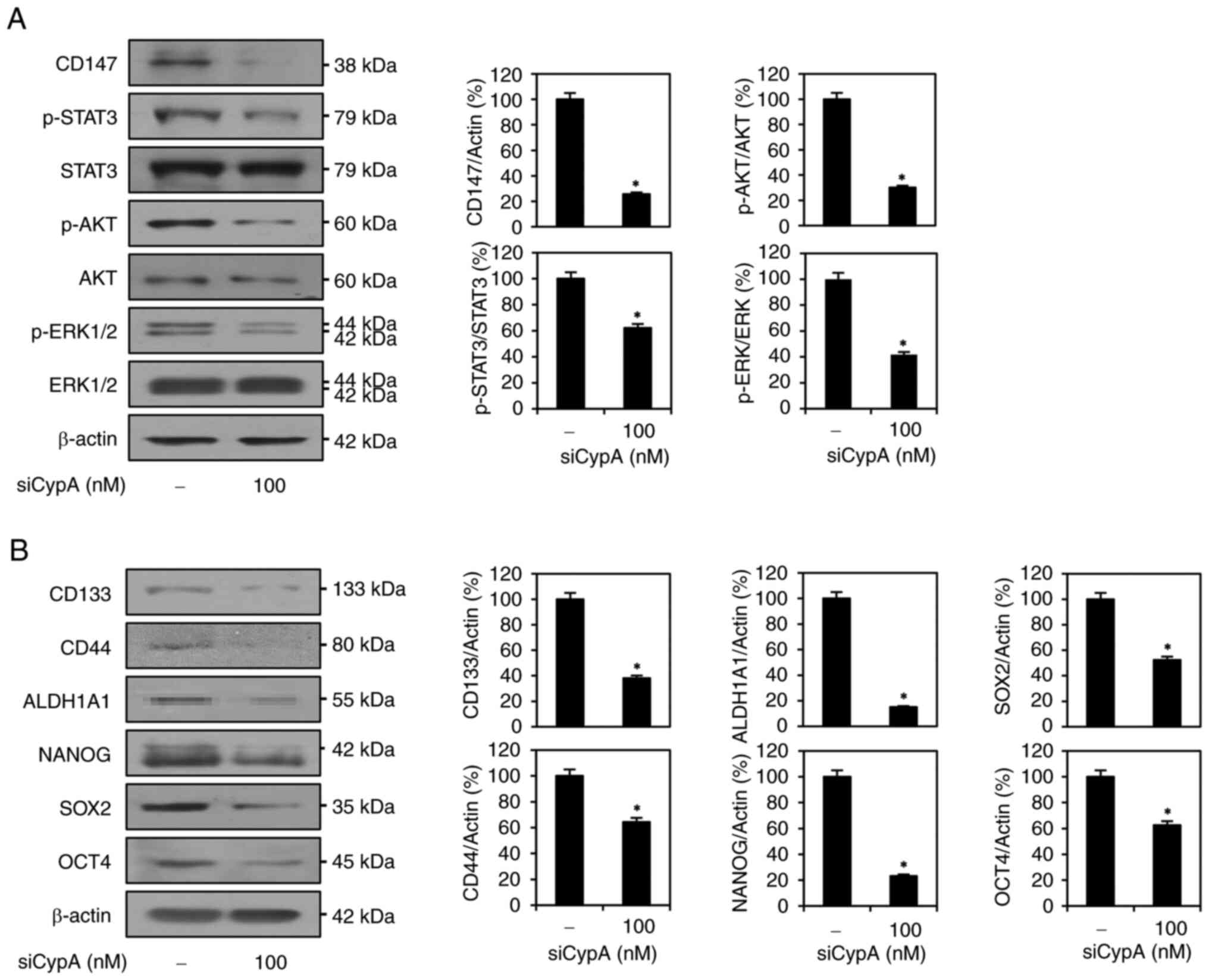
CypA knockdown attenuates the
CD147/STAT3/AKT/ERK pathway in AGS GCSCs
Accumulating evidence has shown that CypA/CD147
interaction promotes cancer cell proliferation, metastasis,
stemness, and resistance to therapies via the activation of key
downstream oncogenic signaling pathways (10,22–24).
Thus, we examined whether CypA silencing affects the expression
levels of CD147 and downstream signaling effectors mediated by the
CypA/CD147 axis, including ERK1/2, AKT, and signal transducer and
activator of transcription 3 (STAT3), in AGS-derived GCSCs. As a
result, CD147 expression was significantly suppressed by CypA
knockdown (Fig. 4A). Moreover,
CypA silencing markedly inhibited the levels of phosphorylated
STAT3, AKT, and ERK1/2 relative to their total protein levels
(Fig. 4A). These results indicate
that CypA knockdown downregulates CypA/CD147-mediated downstream
signaling pathways by reducing CD147 expression in AGS GCSCs.
Next, we evaluated the impact of CypA knockdown on
the expression of major stem cell regulators. As shown in Fig. 4B, CypA knockdown effectively
suppressed the expression of key stem cell surface markers such as
CD133 and CD44, the specific cytoplasmic stem cell marker ALDH1A1,
and master transcriptional regulators of stem cells such as OCT4,
SOX2, and NANOG in AGS GCSCs. Taken together, these data imply that
CypA may enhance the stem-like features of GC by upregulating
stemness-related signaling and regulators through interaction with
CD147.
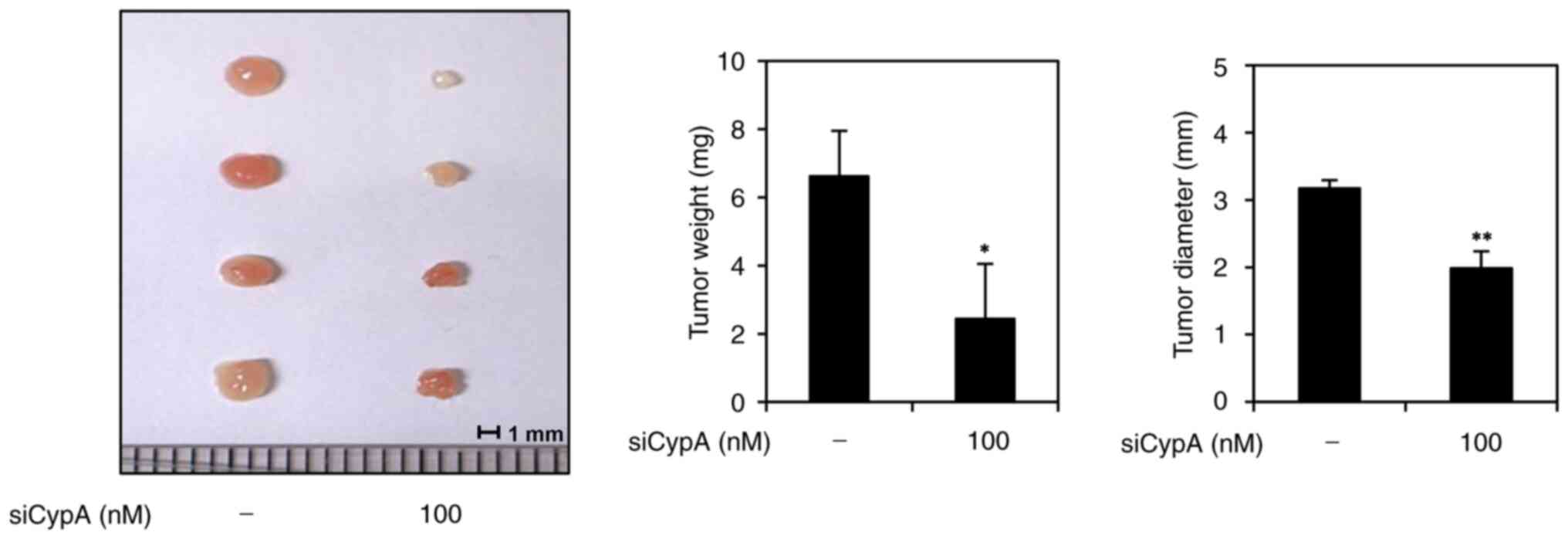
CypA knockdown suppresses tumorigenic
ability of AGS GCSCs
To analyze the impact of CypA knockdown on the in
vivo tumor-forming potential of AGS-derived GCSCs, we performed
a chick embryo chorioallantoic membrane (CAM) assay. Non-silenced
control cells or CypA-silenced cells were mixed with ECM gel,
implanted on the CAM surface, and cultured for 7 days. The weight
and size of the developed tumor were then calculated. In the
control group, tumors averaged 6.6 mg in weight and 3.2 mm in
diameter, whereas tumors in the CypA knockdown group averaged 2.4
mg in weight and 2.0 mm in diameter (Fig. 5). These findings indicate that CypA
silencing significantly suppressed in vivo tumor growth
derived by AGS GCSCs. Therefore, CypA may play a crucial role in
sustaining the tumorigenic ability of GCSCs.
Discussion
Increasing evidence indicates that CypA and its cell
membrane receptor CD147 are frequently upregulated in numerous
cancer types, including GC. Their interaction triggers
intracellular signaling cascades that drive cancer cell
proliferation, metastasis, resistance to chemotherapy, and
acquisition of stem-like properties (10,13,14).
Furthermore, analysis of data from The Cancer Genome Atlas (TCGA)
revealed a correlation between higher cancer stages and increased
expression levels of CypA and CD147, indicating a link between
CypA/CD147 overexpression and unfavorable outcomes in cancer
patients (6). Recent
investigations have highlighted the significance of the CypA/CD147
axis in sustaining and expanding cancer stem cell populations. This
axis orchestrates the activation of key signaling pathways
associated with stemness, such as STAT3, PI3K/AKT, MAPK, NF-κB,
Notch, and Wnt/β-catenin, in various cancer types, including breast
cancer, glioma, liver cancer, pancreatic cancer, and colon cancer
(10,13,14,22).
Consequently, targeting the CypA/CD147 axis emerges as a promising
therapeutic approach in cancer management.
More recently, we demonstrated that the natural CypA
inhibitors, CsA and C9, effectively suppressed the stem-like
properties of GC and NSCLC cells (13,14).
Both CsA and C9 impeded the growth of GCSCs and NSCLC stem cells
in vitro and in vivo through the induction of cell
cycle arrest and intrinsic apoptosis. These CypA inhibitors
modulated CypA/CD147-mediated MAPK and AKT signaling in GCSCs and
disrupted the crosstalk between EGFR and CypA/CD147 in NSCLC stem
cells, leading to a reduction in the expression of key stem cell
markers. Accordingly, our findings indicate that the CypA
inhibitors CsA and C9 could serve as potential anticancer agents by
targeting cancer stem cells, potentially improving clinical
treatment outcomes. However, despite the demonstrated antitumor
effects of CypA inhibitors on GCSCs and NSCLC stem cells, the
specific role of CypA in these cancer stem cells has not yet been
directly investigated.
EMT is the process where cells develop invasive
mesenchymal characteristics by losing epithelial cell adhesion,
reorganizing cytoskeletal structures, and remodeling the ECM,
thereby enhancing their ability to invade (19,25–27).
Recent research has shown that abnormal activation of EMT in
various tumors, including GC, plays a crucial role in tumor
advancement, invasion, metastasis, and development (19,28,29).
In particular, the CypA/CD147 axis has been recognized as a crucial
promoter of tumor cell proliferation and metastasis by regulating
the expression of EMT-related markers through the activation of
essential signaling pathways, such as PI3K/AKT, MAPK, NF-κB, STAT3,
Notch, Wnt/β-catenin, and transforming growth factor-β
(TGF-β)/Smad3, across various cancer types (10,19,28–34).
In colon cancer cells, CypA knockdown has been demonstrated to
impede cell migration and invasion by suppressing EMT,
characterized by increased E-cadherin and decreased N-cadherin and
Snail expression (35). Similarly,
in liver cancer cells, CD147 has been linked to EMT by mediating
TGF-β1 signaling, thereby promoting invasion through the elevated
levels of EMT transcription factors Slug and Snail (30). However, whether the CypA/CD147 axis
enhances the metastatic potential of GCSCs by promoting EMT remains
unclear.
In this investigation, we unveiled the functional
significance of CypA in the proliferation and metastasis of GCSCs
for the first time, utilizing CypA-specific siRNA. Knocking down
CypA resulted in the inhibition of both proliferation and
tumorsphere-forming capability of GCSCs derived from AGS cells.
This inhibition was attributed to the promotion of apoptosis and
the arrest of the cell cycle in the G0/G1 and S phases.
Furthermore, CypA depletion resulted in an increase in the
expression level of E-cadherin, coupled with a decrease in vimentin
and N-cadherin. Consequently, CypA suppression hindered the
migration and invasion of AGS GCSCs by downregulating the process
of EMT. Moreover, CypA knockdown exerted a strong downregulation
effect on key stemness markers, such as CD44, CD133, ALDH1A1, OCT4,
NANOG, and SOX2, which are associated with the aggressive
properties of GCSCs. This downregulation was achieved via the
inactivation of the CD147/STAT3/AKT/ERK pathway. Additionally, CypA
silencing attenuated the tumor-forming potential of AGS GCSCs in a
CAM model. Notably, we also tested a different sequence of siCypA
(siCypA_2) in AGS GCSCs to further validate CypA's role in
regulating their proliferation and metastatic potential. The in
vitro experiments showed consistent results between the two
siRNAs (Fig. S1, Fig. S2, Fig. S3). Nevertheless, future additional
in vivo animal model studies are needed to strengthen these
findings. In summary, our findings suggest that CypA contributes
positively to the proliferation and metastasis of GCSCs through the
upregulation of CD147/STAT3/AKT/ERK signaling and facilitation of
EMT. Thus, targeting the CypA/CD147 axis holds promise in improving
the treatment outcomes of GC patients by suppressing the growth and
metastasis of GCSCs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MSIT) (grant no. RS-2024-00343772).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HJJ and HJC designed and conceptualized the
experiments. HJC performed the experiments and data analysis. HJC
wrote the original draft, and HJJ revised the manuscript. HJJ was
responsible for project administration and funding acquisition. HJJ
and HJC confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bilici A: Treatment options in patients
with metastatic gastric cancer: Current status and future
perspectives. World J Gastroenterol. 20:3905–3915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li T, He Y, Zhong Q, Yu J and Chen X:
Advances in treatment models of advanced gastric cancer. Technol
Cancer Res Treat. 21:153303382210903532022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsieh HL, Yu MC, Cheng LC, Yeh TS and Tsai
MM: Molecular mechanism of therapeutic approaches for human gastric
cancer stem cells. World J Stem Cells. 14:76–91. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Feng F and Zhou YN: Stem cells in
gastric cancer. World J Gastroenterol. 21:112–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue C, Sowden MP and Berk BC:
Extracellular and intracellular cyclophilin A, native and
post-translationally modified, show diverse and specific
pathological roles in diseases. Arterioscler Thromb Vasc Biol.
38:986–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yurchenko V, Constant S, Eisenmesser E and
Bukrinsky M: Cyclophilin-CD147 interactions: A new target for
anti-inflammatory therapeutics. Clin Exp Immunol. 160:305–317.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Obchoei S, Wongkhan S, Wongkham C, Li M,
Yao Q and Chen C: Cyclophilin A: Potential functions and
therapeutic target for human cancer. Med Sci Monit. 15:RA221–RA232.
2009.PubMed/NCBI
|
|
10
|
Han JM and Jung HJ: Cyclophilin A/CD147
interaction: A promising target for anticancer therapy. Int J Mol
Sci. 23:93412022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao Y, Luo D, Peng K and Zeng Y:
Cyclophilin A: A key player for etiological agent infection. Appl
Microbiol Biotechnol. 105:1365–1377. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chu MY, Huang HC, Li EM and Xu LY: CypA: A
potential target of tumor radiotherapy and/or chemotherapy. Curr
Med Chem. 28:3787–3802. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho HJ and Jung HJ: Cyclophilin A
inhibitors suppress proliferation and induce apoptosis of MKN45
gastric cancer stem-like cells by regulating CypA/CD147-mediated
signaling pathway. Int J Mol Sci. 24:47342023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han JM, Kim SM, Kim HL, Cho HJ and Jung
HJ: Natural cyclophilin A inhibitors suppress the growth of cancer
stem cells in non-small cell lung cancer by disrupting crosstalk
between CypA/CD147 and EGFR. Int J Mol Sci. 24:94372023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi YS, Cho HJ and Jung HJ: Atorvastatin
inhibits the proliferation of MKN45-derived gastric cancer stem
cells in a mevalonate pathway-independent manner. Korean J Physiol
Pharmacol. 26:367–375. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han JM, Sohng JK, Lee WH, Oh TJ and Jung
HJ: Identification of cyclophilin A as a potential anticancer
target of novel nargenicin A1 analog in AGS gastric cancer cells.
Int J Mol Sci. 22:24732021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nawara HM, Afify SM, Hassan G, Zahra MH,
Atallah MN, Seno A and Seno M: An assay for cancer stem
cell-induced angiogenesis on chick chorioallantoic membrane. Cell
Biol Int. 45:749–756. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palumbo C, Sisi F and Checchi M: CAM
model: Intriguing natural bioreactor for sustainable research and
reliable/versatile testing. Biology (Basel). 12:12192023.PubMed/NCBI
|
|
19
|
Wang SS, Jiang J, Liang XH and Tang YL:
Links between cancer stem cells and epithelial-mesenchymal
transition. Onco Targets Ther. 8:2973–2980. 2015.PubMed/NCBI
|
|
20
|
Peng Z, Wang CX, Fang EH, Wang GB and Tong
Q: Role of epithelial-mesenchymal transition in gastric cancer
initiation and progression. World J Gastroenterol. 20:5403–5410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia P and Xu XY: Epithelial-mesenchymal
transition and gastric cancer stem cell. Tumour Biol.
39:10104283176983732017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng Y, Fan XY, Yang LJ, Xu BQ, He D, Xu
Z, Wu D, Wang B, Cui HY, Wang SJ, et al: Detachment activated
CyPA/CD147 induces cancer stem cell potential in non-stem breast
cancer cells. Front Cell Dev Biol. 8:5438562020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galoczova M, Coates P and Vojtesek B:
STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett.
23:122018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dubrovska A, Kim S, Salamone RJ, Walker
JR, Maira SM, García-Echeverría C, Schultz PG and Reddy VA: The
role of PTEN/Akt/PI3K signaling in the maintenance and viability of
prostate cancer stem-like cell populations. Proc Natl Acad Sci USA.
106:268–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanabe S, Quader S, Cabral H and Ono R:
Interplay of EMT and csc in cancer and the potential therapeutic
strategies. Front Pharmacol. 11:9042020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia M, Wang Y, Guo Y, Yu P, Sun Y, Song Y
and Zhao L: Nitidine chloride suppresses epithelial-mesenchymal
transition and stem cell-like properties in glioblastoma by
regulating JAK2/STAT3 signaling. Cancer Med. 10:3113–3128. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, Li
J and Zhang Q: Resveratrol reverses doxorubicin resistance by
inhibiting epithelial-mesenchymal transition (EMT) through
modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin
Cancer Res. 36:192017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Becerril-Rico J, Alvarado-Ortiz E,
Toledo-Guzmán ME, Pelayo R and Ortiz-Sánchez E: The cross talk
between gastric cancer stem cells and the immune microenvironment:
A tumor-promoting factor. Stem Cell Res Ther. 12:4982021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y and Weinberg RA:
Epithelial-to-mesenchymal transition in cancer: Complexity and
opportunities. Front Med. 12:361–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ru NY, Wu J, Chen ZN and Bian H:
HAb18G/CD147 is involved in TGF-β-induced epithelial-mesenchymal
transition and hepatocellular carcinoma invasion. Cell Biol Int.
39:44–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Tang Z, Jiang X, Wang T, Zhao L, Xu
Z and Liu K: Cyclophilin A/CD147 signaling induces the
epithelial-to-mesenchymal transition and renal fibrosis in chronic
allograft dysfunction by regulating p38 MAPK signaling. Ren Fail.
44:1585–1594. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sadrkhanloo M, Entezari M, Orouei S,
Ghollasi M, Fathi N, Rezaei S, Hejazi ES, Kakavand A, Saebfar H,
Hashemi M, et al: STAT3-EMT axis in tumors: Modulation of cancer
metastasis, stemness and therapy response. Pharmacol Res.
182:1063112022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mirzaei S, Saghari S, Bassiri F, Raesi R,
Zarrabi A, Hushmandi K, Sethi G and Tergaonkar V: NF-κB as a
regulator of cancer metastasis and therapy response: A focus on
epithelial-mesenchymal transition. J Cell Physiol. 237:2770–2795.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu H, Ma J, Li Z, Ding Z, Chen W, Peng Y,
Tao Z, Chen L, Luo M, Wang C, et al: CyPA interacts with SERPINH1
to promote extracellular matrix production and inhibit
epithelial-mesenchymal transition of trophoblast via enhancing
TGF-β/Smad3 pathway in preeclampsia. Mol Cell Endocrinol.
548:1116142022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamamoto T, Takakura H, Mitamura K and
Taga A: Cyclophilin a knokdown inhibits cell migration and invasion
through the suppression of epithelial-mesenchymal transition in
colorectal cancer cells. Biochem Biophys Res Commun. 526:55–61.
2020. View Article : Google Scholar : PubMed/NCBI
|