Introduction
Oxidative stress due to the imbalance between
reactive oxygen species (ROS) generation and the capacity of
antioxidant defense systems is a contributing factor or common
mechanism in numerous diseases such as cancer, cardiovascular,
neurological, respiratory, kidney and gastrointestinal mucosal
diseases (1–3). ROS are generated under both
physiological and pathological conditions including mitochondrial
respiration, immune cell activation, inflammation, infection,
ischemia, aging, exposure to toxic exogenous substances (such as
heavy metals, cigarette smoke and environmental pollutants),
alcohol, radiations and certain drugs (1). Low and moderate levels of
intracellular ROS, mainly derived from electron leakage in the
electron transport chain during cellular respiration, exert
beneficial effects on certain physiological activities, serving as
essential signaling molecules (1).
However, high ROS levels during pathological or stressful
conditions can cause oxidative stress and damage important
biomolecules, including proteins, lipids, carbohydrates and nucleic
acids (1). Furthermore, cellular
structures and functions can be irreversibly impaired due to a
persistent redox imbalance, ultimately leading to cell death and
the aforementioned diseases (1–4).
Mammalian cells exhibit various antioxidant defense systems,
involving enzymatic and non-enzymatic mechanisms, to maintain
normal ROS levels, preventing adverse effects of free radicals and
oxidative stress (3).
Endogenous enzymatic antioxidants include direct
(first line) antioxidant enzymes and indirect detoxifying
(conjugating) enzymes. Direct antioxidant enzymes, such as
superoxide dismutase (SOD), catalase (CAT) and glutathione
peroxidase (GPx), directly inactivate ROS to prevent reactions
initiated by ROS (3,4). Indirect antioxidant enzymes, such as
glutathione S-transferase isozymes, NADP(H):quinine oxidoreductase
(NQO1) and heme oxygenase (HMOX or HO-1), maintain redox
homeostasis through reduction/conjugation reactions, thus
performing a detoxifying role (5–7).
These enzymes also promote the excretion of secondary oxidized
reactive metabolites, such as epoxides, peroxides, quinones and
aldehydes (3–6). Moreover, endogenous non-enzymatic
antioxidants, such as ferritin, glutathione, thioredoxin and
melatonin, also inhibit ROS formation (8). Additionally, several exogenous
antioxidants are derived primarily from the diet or nutritional
supplements, including vitamin C and E, carotenoids, certain
minerals and polyphenols, and are crucial for individuals with
malnutrition or malabsorption (2,3).
The gastrointestinal tract is susceptible to
ROS-related injury because of its accessibility to the external
environment. Oxidative stress is also implicated in numerous
gastrointestinal mucosal diseases such as gastritis, colonic
inflammation, inflammatory bowel disease (IBD) and cancer (2). Therefore, incorporating antioxidants
into treatment regimens is a viable approach to alleviate
gastrointestinal tissue damage and these oxidative stress-related
diseases (2).
Protein hydrolysates, composed of different proteins
and bioactive peptides hydrolyzed from intact proteins, exhibit
potential health benefits and antioxidative, antithrombotic,
antihypertensive, antimicrobial, anticancer and immunomodulatory
properties (9–13). Protein hydrolysates from fish have
been reported to reduce H2O2-induced
oxidative stress, enhancing cellular tolerance to subsequent
apoptosis (11). Soluble protein
hydrolysate (SPH), also termed ProGo, which is produced by Hofseth
BioCare ASA, contains various potential bioactive peptides derived
from the hydrolysis of fresh salmon protein through a proprietary
process and non-genetically modified organism protease enzymes
(13). Previous studies have
reported health benefits of SPH, including antioxidant effects
(10–13). Therefore, the present study aimed
to investigate the protective effect of soluble protein hydrolysate
(SPH) against H2O2-induced intestinal
oxidative stress injury and explore the underlying mechanisms using
in vivo and in vitro experiments.
Materials and methods
Cell culture
Human intestinal epithelial cells (HIEC-6) were
purchased from the American Type Culture Collection and cultured in
Opti-MEM Reduced Serum Medium supplemented with 10% fetal bovine
serum (FBS), 20 mM HEPES, 10 mM GlutaMAX, 10 ng/ml epidermal growth
factor and 1% penicillin-streptomycin, all of which were obtained
from Gibco (Thermo Fisher Scientific, Inc.). HIEC-6 cells were
seeded in 96-well plates at 70% confluency and cultured in a 5%
CO2 incubator at 37°C for 24 h. After which, the cells
were cultured with SPH (Hofseth BioCare ASA) at increasing
concentrations (0, 50, 100, 200, 400, 800 and 1,600 µg/ml) at 37°C
for 48 h. SPH powder was first diluted to 10 mg/ml with
phosphate-buffered saline (PBS), and then added into the complete
culture medium to achieve final concentrations of 50, 100, 200,
400, 800 and 1,600 µg/ml as required. SPH cytotoxicity at different
concentrations in HIEC-6 cells was assessed based on lactate
dehydrogenase (LDH) levels in the culture medium using an LDH
assay. Accordingly, 50 and 100 µg/ml SPH concentrations were
selected for subsequent experiments. Cells in the control group, 50
and 100 µg/ml SPH groups were cultured in complete culture medium
containing 0, 50 and 100 µg/ml SPH, respectively and incubated at
37°C for 4 days (8 wells/group). Subsequently, half of the cells in
these three groups (4 wells/group) were treated with 0.88 mM
H2O2 and further cultured at 37°C for 48 h.
The other half of the cells were cultured at 37°C for 48 h without
treatment. The protective role of SPH against
H2O2-induced cell death was determined based
on the LDH concentration released into the culture medium.
LDH assay
A total of 50 µl supernatant was collected from each
well of a 96-well plate and transferred to another plate in
triplicate. After which, 50 µl 2% NP40 (Thermo Fisher Scientific,
Inc.) was added to each well, followed by incubation at room
temperature with shaking for 15 min. Thereafter, 100 µl of LDH
cytotoxicity detection kit (Takara Bio Inc.) solution was added
into each well. Following incubation at room temperature for 15
min, optical density (OD) was measured at 492 nm with a filter at
620 nm using an i3× multimode microplate reader (Molecular Devices,
LLC.). In the LDH assay, higher OD values indicated greater cell
death.
Animal model
Animal experiments were conducted in an animal
facility at Stanford University using a protocol approved by The
Stanford University School of Medicine Institutional Animal Care
and Use Committee (Stanford, USA; approval no. A3213-01) in
accordance with NIH guidelines of the Animal Welfare Act, and the
Guide for the Care and Use of Laboratory Animals (14). Male and female friend virus B NIH
Jackson mice used for breeding were purchased from The Jackson
Laboratory and bred in a barrier-controlled, specific pathogen-free
environment overseen by The Administrative Panel on Laboratory
Animal Care at Stanford University. The temperature and relative
humidity of the environment were maintained at 20±2°C and 55±5%,
respectively, with a 12 h light-dark cycle.
Briefly, 56 mice (21 days old and 10–14 g) were
randomly divided into six groups: i) Control [n=10; 5M (male)/5F
(female)]; ii) 0.3% H2O2 group (n=10; 5M/5F);
iii) 0.6% H2O2 group (n=10; 5M/5F); iv) 0.3%
H2O2+SPH group (n=10; 5M/5F); v) 0.6%
H2O2+SPH group (n=10; 5M/5F); and vi) SPH
group (n=6; 3M/3F; Fig. 1). All
the mice had ad libitum access to high-pressure sterilized
food. For SPH intervention, 5% (w/v) SPH was administered to the
mice via drinking water for 14 days immediately after weaning,
whereas the other mice had ad libitum access to normal
filtered drinking water. Subsequently, the mice underwent a
survival surgical procedure involving an intestinal injection. Mice
were anesthetized with 3 and 1.5% isoflurane-mixed gas for
induction and maintenance, respectively. The surgical site was
cleaned with 70% alcohol and a 1.0-cm midline abdominal incision
was made. After exposing the small intestine,
H2O2 solution at different concentrations was
injected into the intestinal lumen adjacent to the ileocecal
region, and the abdominal wall was sutured. The mice were then
placed in clean cages to collect fecal samples. After 24 h, the
mice were euthanized in a CO2 chamber equipped with an
automatic air flow-regulator at a fill rate of 50% displacement of
the chamber volume/min with CO2, and the small
intestines were harvested. Mice in the control group were
administered PBS instead of H2O2.
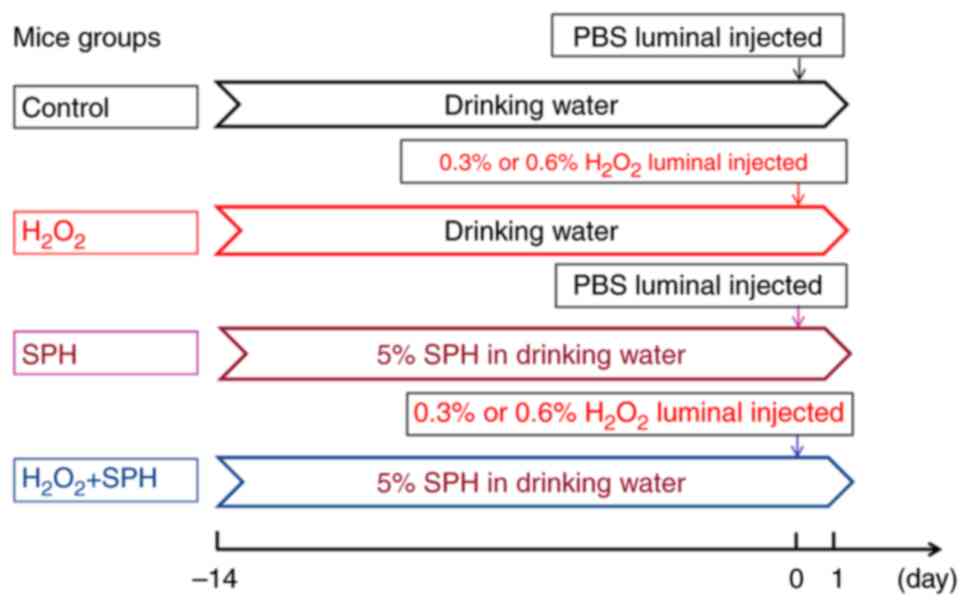 | Figure 1.Establishment of an acute
H2O2-induced intestinal injury model in mice.
Mice requiring SPH intervention were administered 5% (w/v) SPH
through drinking water for 14 days. Acute oxidative stress-induced
intestinal injury was induced by injecting 0.3%
H2O2 or 0.6% H2O2 into
the lumen of the small intestine. The control group intervention
solution contained PBS instead of H2O2. In
the control, 0.3% H2O2, SPH + 0.3%
H2O2, 0.6% H2O2, SPH +
0.3% H2O2 and SPH groups there were 10, 10,
10, 10, 10 and six mice, respectively. SPH, soluble protein
hydrolysate; PBS, phosphate-buffered saline. |
Notably, H2O2 at
concentrations of 0.3 and 0.6% were used based on the mortality of
mice treated with various concentrations of
H2O2 in preliminary experiments, in which the
intervention method was the same as that aforementioned. When the
concentration of H2O2 ≥1.0%, the mortality
rate of the mice increased (Fig.
S1).
Histopathological examination and
histological grading
Intestinal tissues from the mice were fixed in 4%
paraformaldehyde solution overnight at 20–25°C, embedded in
paraffin and sliced into 8-µm sections. After which, the sections
were dewaxed, hydrated and stained using a hematoxylin and eosin
(H&E) staining kit (ScyTek Laboratories, Inc.) following the
manufacturer's instructions. The H&E-stained tissue sections
were observed under a microscope (BX53; Olympus Corporation) and
scored by two blinded observers according to the following
histopathological scoring criteria (15): 0, normal; 1, slight epithelial
separation; 2, moderate submucosal separation and local villus
necrosis; 3, severe submucosal separation and villus necrosis; and
4, transmural intestinal necrosis. Each observer captured a
high-magnification field of view of the typical lesion site on each
pathological slide for scoring and capturing images. The scores
from the two observers were summarized and statistically
analyzed.
Modified enzyme-linked immunosorbent
assay (ELISA)
The insoluble protein cytokeratin-8 (K8) is the
primary component of intermediate filaments in monolayer epithelial
cells such as enterocytes and cannot be detected using the
traditional ELISA method for soluble proteins (16). Intestinal epithelial damage due to
various pathological conditions may significantly increase K8
levels in feces (13,16). Therefore, fecal K8 level can serve
as an indicator of the extent of intestinal epithelial damage.
Fecal samples collected from the mice were diluted at a
mass-to-volume ratio of 1:5, and K8 levels were measured using the
rat anti-mouse K8 antibody Troma-1 (cat. no. MABT329M;
MilliporeSigma) following the modified ELISA protocol (16) and a SpectraMax® i3×
microplate reader (Molecular Devices, LLC.). The final K8
concentration was determined using a standard curve.
Immunofluorescence staining
Immunofluorescence staining for K8 in the gut of
mice in the control group was conducted to confirm its abundance
and epithelial specific expression. The intestinal tissue sections
were prepared as aforementioned for H&E staining, blocked with
a PBS solution containing 10% FBS (cat. no. A5256701; Gibco; Thermo
Fisher Scientific, Inc.), and incubated at 20–25°C for 30 min.
After which, the sections were incubated with the K8 primary
antibody (1:500; cat. no. MABT329M; MilliporeSigma) at 4°C
overnight. Thereafter, the sections were exposed to a fluorescein
5-isothiocyanate-conjugated goat anti-rat secondary antibody
(1:500; cat. no. F6258; Sigma-Aldrich; Merck KGaA) for 60 min at
20–25°C. Following nuclei staining with DAPI (Sigma-Aldrich; Merck
KGaA) for 5 min at 20–25°C, the sections were sealed, observed
under a fluorescence microscope (BX53; Olympus Corporation), and
images were captured at 200× magnification.
Assessment of oxidative stress-related
gene expression
The expression of 84 genes related to antioxidant
and ROS metabolism in the intestinal tissues of mice was
determined. The RNeasy UCP Micro kit (cat. no. 73934), Reverse
Transcriptase Mix (cat. no. RT32-010), RT2 SYBR Green Fluor qPCR
Mastermix (cat. no. 330512) and RT2 Profiler™ PCR Array
Mouse Oxidative Stress and Antioxidant Defense (including the
forward and reverse primers for 84 related genes; cat. no. 330231)
were purchased from Qiagen GmbH and used following the
manufacturer's instructions. The thermocycling protocol for PCR
amplification was 95°C for 10 min, followed by 40 cycles of 95°C
for 15 sec and 60°C for 1 min, using an iCycler PCR system (Bio-Rad
Laboratories, Inc.). Gene expressions were compared using the
2−ΔΔCq method (17)
with normalization to the mean expression of the housekeeping
genes, including Actb, B2m, Gapdh, Hprt and Rpl13a.
Subsequently, the fold change for genes with different expressions
between the H2O2 + SPH and
H2O2 groups was also calculated as follows:
The fold change of a gene (triplicate)=2−ΔΔCq of the
H2O2+SPH group/2−ΔΔCq of the
H2O2 group.
Statistical analysis
Normally distributed quantitative data conforming to
the homogeneity of variance were presented as the mean±standard
deviation. Statistical analysis was conducted using unpaired
Student's t-test to analyze the difference between two groups or
one-way analysis of variance followed by Tukey test to analyze the
difference among multiple groups using GraphPad Prism 5.0 software
(Dotmatics), or Welch's corrected t-test or the Kruskal-Wallis test
followed by Dunn test was used. P<0.05 was considered to
indicate a statistically significant difference.
Results
SPH exerts a protective effect against
oxidative stress injury in HIEC-6 cells in vitro
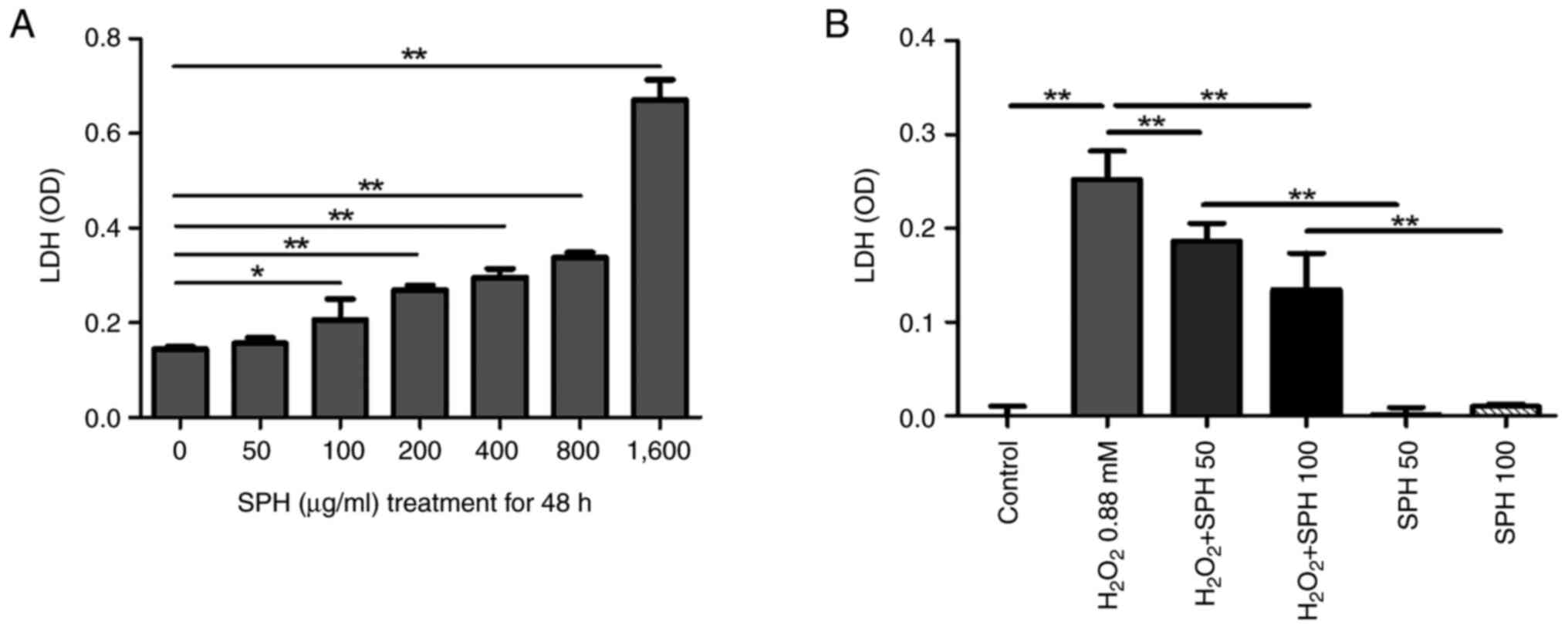
LDH assay revealed a dose-dependent cytotoxic effect
of SPH on HIEC-6 cells, with significantly increased cytotoxicity
at concentrations >1,600 µg/ml (Fig. 2A). However, SPH at appropriate
doses, such as 50 µg/ml and 100 µg/ml, exerted a protective effect
against H2O2-induced oxidative stress injury
in HIEC-6 cells (Fig. 2B).
SPH treatment diminishes
H2O2-induced gut injury in mice, and the
underlying protective mechanism is not related to excess nutrient
supply
H2O2, a potent inducer of
oxidative stress (4), was used for
establishing an in vivo animal model of intestinal injury.
H2O2 at concentrations of 0.3 and 0.6% were
used based on the mortality of mice treated with various
concentrations of H2O2 in preliminary
experiments (Fig. S1). Notably,
H2O2 concentrations ≥1.0% resulted in severe
intestinal damage and fatality in mice.
As shown by Fig.
3A, K8 is abundant and specifically expressed within epithelial
cells in the gut lining, and will release to the lumen mixing with
feces when the gut is damaged, which is used as a quantitative
measurement for assessing the degree of gut injury (13,16).
Fecal K8 levels (Fig. 3B), H&E
staining and histological grading of small intestine tissues
(Fig. 4A and B) revealed
significant intestinal tissue injury and elevated fecal K8 levels
in mice receiving intraluminal injections of 0.3 and 0.6%
H2O2 compared with those in the control
group, with more severe effects observed in the 0.6%
H2O2 group (all P<0.01), which was in a
dose-dependent manner. Compared with the 0.3%
H2O2 group, prophylactic SPH treatment
alleviated the gut injury, manifested as a reduced histological
grading score (P<0.05; Fig. 4B)
in the SPH + 0.3% H2O2 group, although the
histological grading score was still higher when compared with the
control group (P<0.01). Furthermore, there was no significant
difference in fecal K8 level between the SPH + 0.3%
H2O2 group and the control group. However,
SPH treatment showed only mild mitigation in the 0.6%
H2O2-induced gut injury in the mice. Notably,
there were no significant differences in histological grading score
and fecal K8 level when comparing the SPH + 0.6%
H2O2 group with the 0.6%
H2O2 group, whereas there were significant
differences when comparing the SPH + 0.6%
H2O2 group with the control group
(P<0.01).
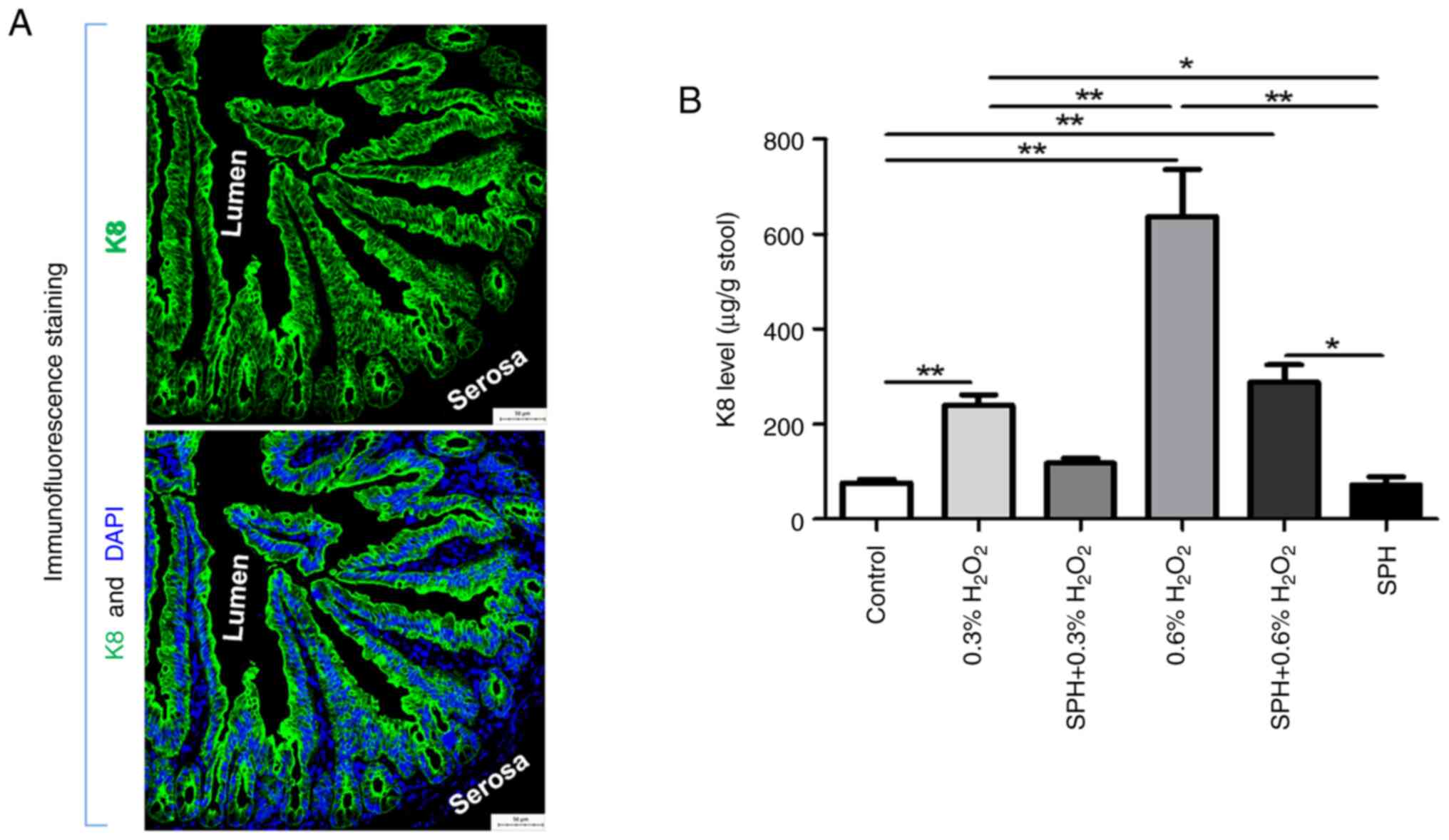 | Figure 3.SPH reduced the levels of stool K8 in
H2O2-induced intestinal injury in mice. (A)
Immunofluorescence staining of K8 in the gut of normal mice
(magnification, ×200; scale bar, 50 µm). (B) Stool K8 levels
measured by ELISA in mice treated with H2O2
and SPH. In the control, 0.3% H2O2, SPH +
0.3% H2O2, 0.6% H2O2,
SPH + 0.3% H2O2 and SPH groups there were 10,
10, 10, 10, 10 and six mice, respectively. *P<0.05 and
**P<0.01. SPH, soluble protein hydrolysate; K8,
cytokeratin-8. |
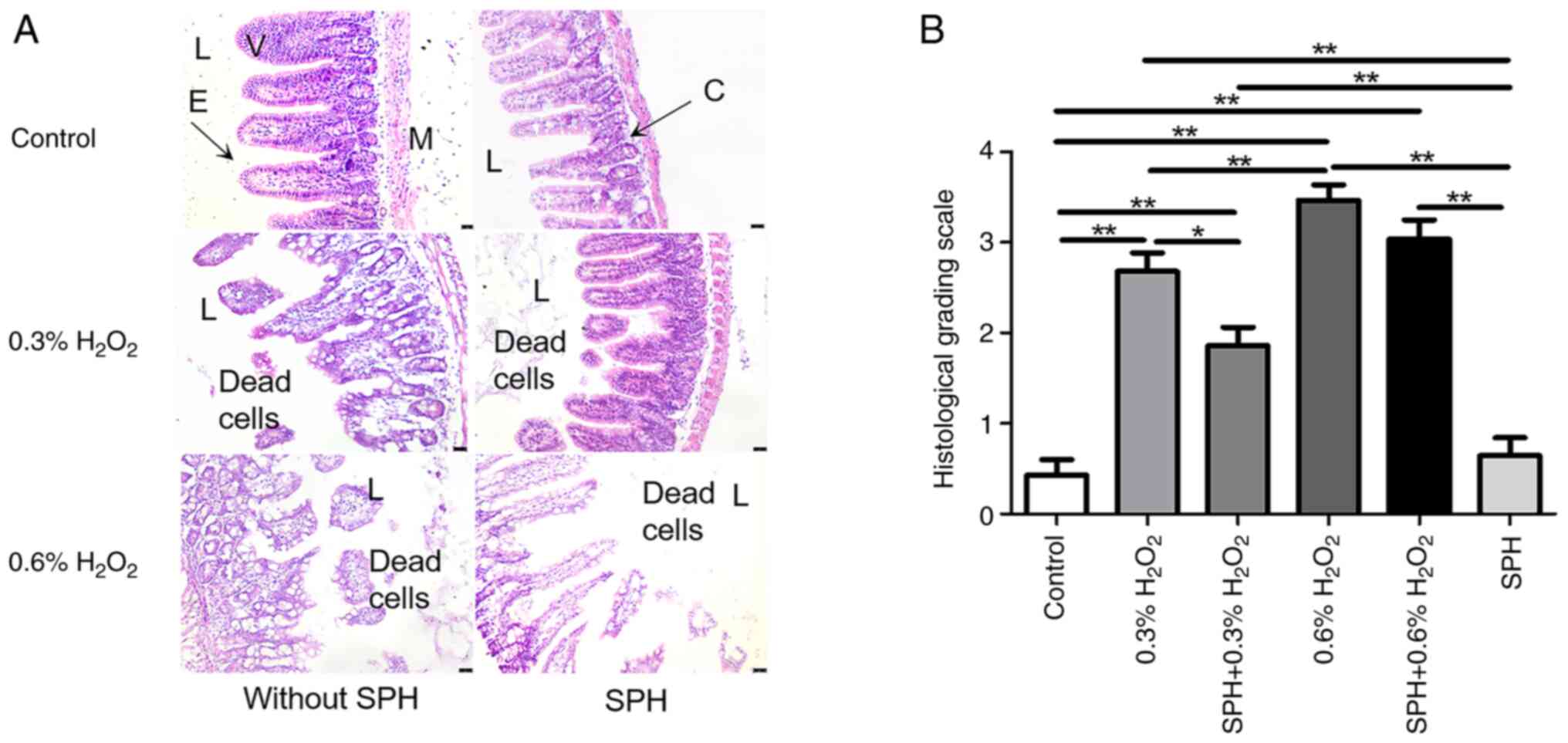 | Figure 4.SPH reduced
H2O2-induced intestinal injury in mice. (A)
Histology images with H&E staining of small intestine tissue
(magnification, ×200; scale bar, 25 µm). (B) Histology grading
scale on intestinal injury. In the control, 0.3%
H2O2, SPH + 0.3% H2O2,
0.6% H2O2, SPH + 0.3%
H2O2 and SPH groups there were 10, 10, 10,
10, 10 and six mice, respectively. *P<0.05 and **P<0.01. SPH,
soluble protein hydrolysate; V, villus; E, epithelial cells; M,
muscle layer; L, lumen; C, crypt. |
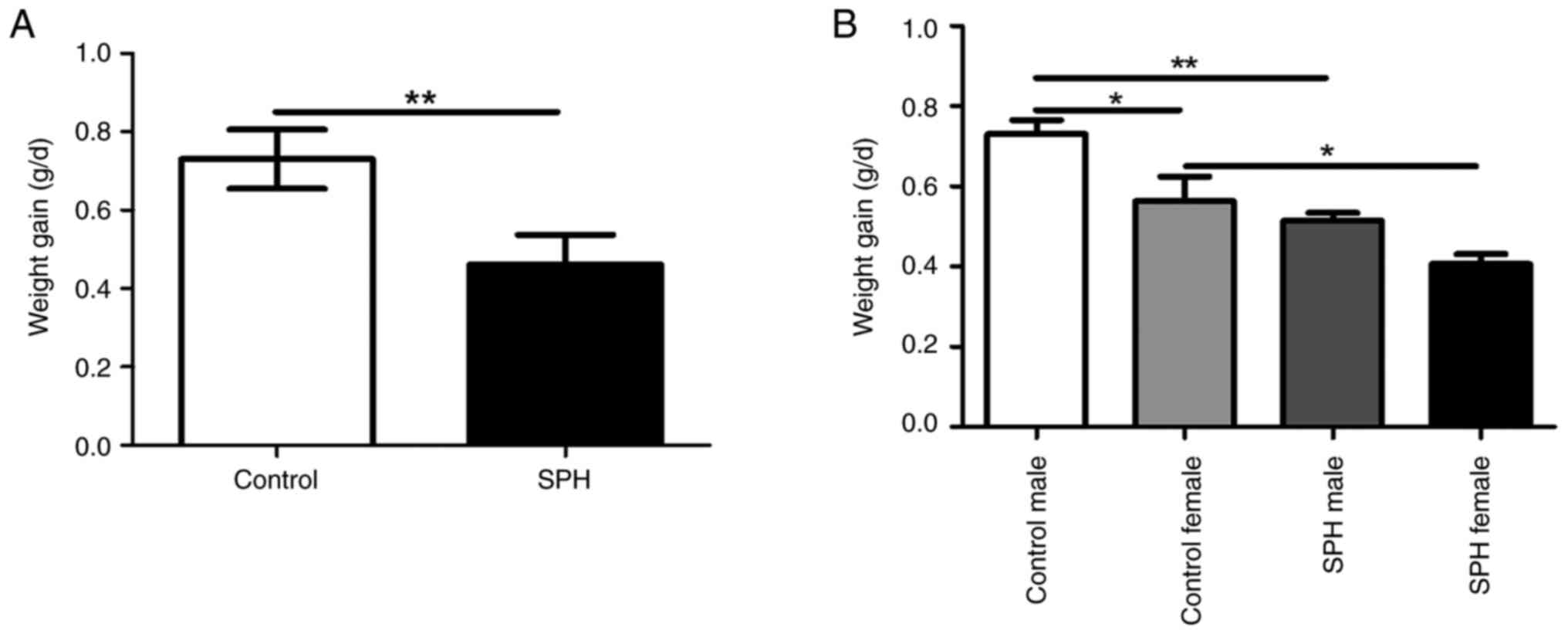
As no accelerated body growth was observed in either
SPH-treated group, the protective mechanism of SPH is unlikely to
be related to an excess nutrient supply (Fig. 5A). Notably, SPH-treated male and
female mice gained less weight compared with the control group
(P<0.01 and P<0.05). Furthermore, the additional weight gain
in female mice was lower than that in male mice in the control
group (P<0.05, Fig. 5B).
Although there was no significant difference in the weight gain
between the male and female mice in the SPH group, the same trend
as the control group was observed.
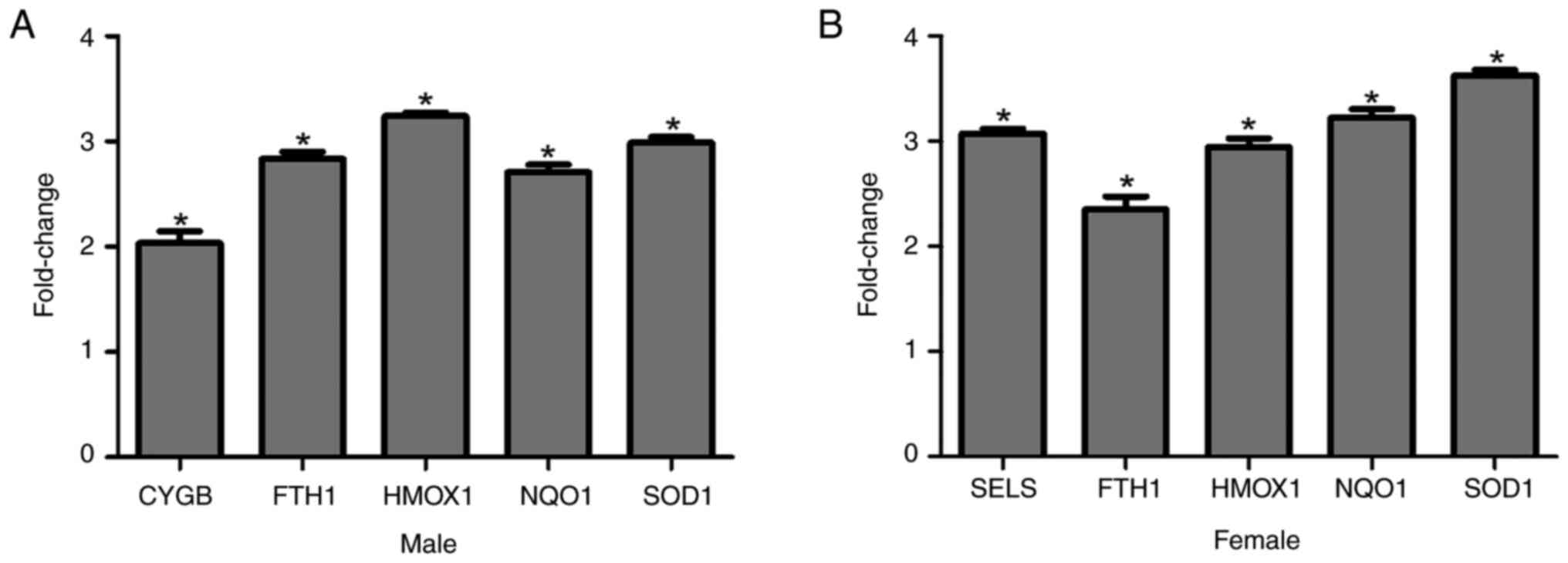
Treatment with SPH upregulates five
oxidative stress-related genes in mice with 0.3%
H2O2-induced intestinal injury
Compared with the mice treated with 0.3%
H2O2 without SPH treatment, the expression of
5 of the 84 oxidative stress-related genes differed significantly
in male and female mice pretreated with SPH (Fig. 6A and B). However, the intestinal
tissue samples from male and female mice treated with 0.6%
H2O2 without SPH treatment did not exhibit
adequate housekeeping gene signals to validate gene expression
changes. Notably, the upregulation of five genes was not entirely
consistent between male and female mice. However, four genes
(Fth1, Hmox1, Nqo1, and Sod1) were commonly
upregulated.
Discussion
The present study demonstrated that suitable SPH
doses exerted protective effects against
H2O2-induced oxidative stress in HIEC-6 cells
in vitro. Furthermore, prophylactic SPH treatment also
alleviated oxidative stress (0.3%
H2O2)-induced gut injury in a mouse model by
potentially upregulating four antioxidant-protective genes:
Fth1, Hmox1, Nqo1 and Sod1.
Iron is a trace element that is essential for normal
cell growth, proliferation and physiological homeostasis (18). Iron metabolism facilitates dynamic
interactions between oxidative stress and antioxidants in numerous
pathophysiological processes such as inflammation and cell death
(18). An imbalance in iron
homeostasis, such as iron deficiency or overload, can disrupt this
equilibrium. Iron imbalance can be restored to a physiological
state by supplementing iron or chelating iron, as the human body
exhibits highly regulated mechanisms to control intracellular iron
levels (18). Ferritin, an iron
storage protein consisting of heavy (H) and light (L) chains,
serves a key role in maintaining the balance between intracellular
iron and redox as a non-enzymatic antioxidant, which protects
against oxygen free radical-mediated damage (18,19).
Fth1 encodes the H chain of ferritin, which is responsible
for iron oxidation and can sequester free iron from dietary sources
into a soluble and nontoxic form (19). Therefore, it is crucial for iron
homeostasis and the antioxidant system. The synthesis of both
ferritin chains increases under oxidative stress, and the
overexpression of ferritin H or L subunits can decrease ROS
accumulation (19). The gene assay
results in the present study indicated Fth1 upregulation
both under oxidative stress and in response to diets with
antioxidant properties, such as SPH.
HO-1, an inducible enzyme, is the initial and
rate-limiting enzyme in heme degradation and catalyzes the
conversion of heme to equimolar biliverdin, ferrous ions and carbon
monoxide (5). Both HO-1 and its
degradation products exhibit cytoprotective properties and play
crucial roles in modulating various biological processes and
maintaining cellular homeostasis (5). Hmox1 expression, which is
typically low in most cells under normal physiological conditions,
can be upregulated by its substrate heme, and in response to
various pathological conditions, such as oxidative stress, heat
shock, ultraviolet radiation and ischemia/reperfusion injury
(3,5). Regulation of HO-1 levels is a
significant protective factor against numerous oxidative
stress-related gastrointestinal, cardiovascular and renal diseases
(20–23). Furthermore, HO-1 deficiency
promotes the development of neonatal necrotizing enterocolitis
(NEC)-like intestinal injury, whereas the induction of intestinal
HO-1 enhances the Treg/Teff ratio, reduces intestinal injury and
decreases the incidence of NEC in a neonatal mouse model of
intestinal inflammation (24,25).
The results of the present study demonstrated that SPH upregulated
Hmox1 expression.
A functional link also exists between FHC and HO-1
(26,27). Iron released from heme by HO-1 is
essential for inducing ferritin synthesis, which is important for
the expression and protective effects of HO-1 (26). In the present study, the induction
of HO-1 expression accompanied induced FHC synthesis in endothelial
cells exposed to H2O2-induced oxidative
stress. Conversely, HO-1 inhibition reduced FHC expression.
Furthermore, the protective effect of HO-1 requires FHC
co-expression to mitigate the pro-oxidative effect of labile iron
produced during heme breakdown (27). Notably, without FHC, HO-1 could
only partially exert its protective effect against oxidative stress
(27).
Nqo1 encodes the
NAD(P)H:quinoneoxidoreductase (NQO1). NQO1 is extensively
distributed in numerous organs, especially in the gastrointestinal
tract, liver and kidney, and can be induced by various chemical
substances, such as polycyclic aromatic hydrocarbons,
hydroquinones, acrylates and broccoli (28,29).
NQO1 is also a phase 2 detoxification enzyme that plays a vital
role in detoxification metabolism and the prevention of oxidative
damage (3,28,29).
SOD, an enzymatic antioxidant with a vital role in
the first line of defense system, can catalyze
O2·− to H2O2 conversion
and promote the diffusion of superoxide to maintain the redox
balance in oxidative stress-induced pathology (3). A previous study reported that
proteolysates and antioxidant peptides isolated from monkfish
muscle could protect HepG2 cells from
H2O2-induced oxidative damage by activating
intracellular antioxidant enzymes such as SOD, CAT and GPx and
decreasing ROS and malondialdehyde (MDA) levels in a
concentration-dependent manner (30). Therefore, these may serve as
powerful antioxidants in the development of nutraceuticals and the
treatment of diseases associated with oxidative stress in the
future.
Selenoprotein S (SELS), a carrier protein that
transports selenium, regulates various essential life processes,
such as oxidative stress, glucose metabolism, inflammation,
lipogenesis, endoplasmic reticulum (ER) stress and the
ER-associated degradation pathway (31–33).
SELS exhibits peroxidase activity that breaks down the substrate
H2O2 into H2O, and SELS
overexpression can notably enhance cell viability and SOD activity
while reducing MDA production after treatment with
H2O2 (31–33).
Cytoglobin (CYGB), a heme-containing protein, also exhibits
peroxidase activity against H2O2 and lipid
hydroperoxides. It can also protect cells and organs from oxidative
stress by binding to oxygen, carbon monoxide and nitric oxide
(34,35). The findings of the present study
revealed that there were differences in SELS and CYGB levels
between SPH-treated male and female mice, warranting further
investigation.
Nuclear factor erythroid 2-related factor 2 (NRF2),
an intracellular transcription factor, regulates the expression of
multiple cytoprotective genes encoding antioxidant and
detoxification enzymes such as ferritin, HO-1, NAD(P)H
dehydrogenase quinone 1 (NQO-1) and SOD and modulates the cellular
redox balance (5,36). Under normal conditions, NRF2 is
ubiquitinated by its interaction with kelch-like ECH-associated
protein 1 (KEAP1) and is quickly degraded in the cytoplasm. Under
stressful conditions, NRF2 is phosphorylated and dissociated from
the NRF2:KEAP1 complex and translocated to the nucleus following
the initiation of the NRF2-dependent cellular defense mechanism by
electrophiles and oxidants, where it binds to the conserved
antioxidant response element sequence and activates the
transcription of cytoprotective genes (5,8,29,36).
Therefore, NRF2 activation could be a potential pharmacological
target for developing antioxidative therapies, serving as a novel
option for the treatment of gastrointestinal oxidative
stress-related diseases such as peptic ulcers, types of
gastrointestinal cancer and IBD (2).
In summary, in the present study prophylactic SPH
treatment alleviated gastrointestinal injury by upregulating the
expression of oxidative protective genes in 0.3%
H2O2-treated mice, which is consistent with
the results of a previous study performed with a mice colitis model
(13). Although SPH also mitigated
0.6% H2O2-induced gastrointestinal injury in
mice to some extent, structural damage to the intestinal tissue
remained severe. This severity resulted in inadequate housekeeping
gene signals in samples from male and female mice without SPH
treatment, rendering fold-change calculations impossible. Despite
being a functional food, SPH did not accelerate body growth in the
treated mice. Notably, female mice gained less weight per day on
average. Similar results have been reported in mice and humans
(37). Furthermore, the
upregulation of protective genes also differed between male and
female mice. Unfortunately, there is a lack of comparable studies,
especially regarding the regulation of antioxidative genes and the
difference between male and female mice, although the
anti-oxidative effect of protein hydrolysate has been reported.
Therefore, the mechanism of action of SPH requires further
investigation in the future and will be the focus of future
endeavors.
The present study also had certain limitations.
First, the effects of SPH on the protein levels and activity of
antioxidants, such as FTH1, HMOX1, NQO-1 and SOD were not
comprehensively studied. Notably, future studies should assess the
effects of SPH on the protein levels and activity of these
antioxidants, and should determine which components of SPH have
bio-activity. Second, SPH contains thousands of peptides, and has
been fractionated using dialysis into nine fractions based on
molecular weight. In the present study, the metabolic effects
showed that bioactivity only existed in the smallest molecular
weight fraction (200–2,000 Dalton) and the largest molecular weight
fraction (10,000–20,000 Dalton). There are 1,674 peptides in the
<2,000 Dalton fraction, the structures of which have been
identified. The largest fraction contains 9 peptides; however, the
structures are currently unknown. Furthermore, the components of
SPH that are responsible for the observed effects have not been
completely identified and will be the focus of research in the
future. Third, K8 expression is typically abundant and specifically
localized in the intestinal epithelial cells. Although K8
immunofluorescence staining was carried out only on the control
mice, it is hypothesized that K8 staining would be positive for all
intact and damaged epithelial cells, and K8 expression would be
high, even when intestinal epithelial cells are damaged. In
addition, animal models established using
H2O2 do not completely and truly simulate and
reproduce oxidative stress-associated diseases induced by several
complex pathological conditions. Therefore, the results of the
present study may not be directly applicable to clinical
practice.
In conclusion, prophylactic SPH treatment can
upregulate specific oxidative protective genes in the intestinal
epithelial cells and exert an antioxidative effect on oxidative
stress-induced gastrointestinal injury. Future research will focus
on the mechanism of action and specific clinical applications of
SPH in the treatment of gastrointestinal, inflammatory and
oxidative stress-related diseases.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Partial financial support was obtained for the present study
from Hofseth Biocare ASA.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GT, BF and KS contributed to the study design. JW,
JL and GT performed the experiments and analyzed data. JW drafted
the manuscript, and GT, BF and KS edited and revised it. BF
provided information regarding the SPH components, and analyzed and
interpretated the data. GT and KS were responsible for project
management. All the authors read and approved the final version of
the manuscript. JW, JL, BF and GT confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
All animal experiments were approved by The
Stanford University School of Medicine Institutional Animal Care
and Use Committee (Stanford, USA; approval no. A3213-01) in
accordance with NIH guidelines of the Animal Welfare Act, and the
Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The author BF is affiliated with the company
Hofseth BioCare ASA, who provided the drug SPH used in this study.
The other authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CAT
|
catalase
|
|
CYGB
|
cytoglobin
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
ER
|
endoplasmic reticulum
|
|
FBS
|
fetal bovine serum
|
|
FTH1
|
ferritin heavy polypeptide-1
|
|
FHC
|
the heavy chain of ferritin
|
|
GPx
|
glutathione peroxidase
|
|
HMOX1/HO-1
|
heme oxygenase-1
|
|
IBD
|
inflammatory bowel disease
|
|
K8
|
cytokeratin-8
|
|
KEAP1
|
kelch-like ECH-associated protein
1
|
|
LDH
|
lactate dehydrogenase
|
|
MDA
|
malondialdehyde
|
|
NQO-1
|
NAD(P)H dehydrogenase quinone 1
|
|
NEC
|
necrotizing enterocolitis
|
|
NRF2
|
nuclear factor erythroid 2-related
factor 2
|
|
PBS
|
phosphate-buffered saline
|
|
SPH
|
soluble protein hydrolysate
|
|
ROS
|
reactive oxygen species
|
|
SOD
|
superoxide dismutase
|
|
SELS
|
selenoprotein S
|
References
|
1
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017:84167632017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhattacharyya A, Chattopadhyay R, Mitra S
and Crowe SE: Oxidative stress: An essential factor in the
pathogenesis of gastrointestinal mucosal diseases. Physiol Rev.
94:329–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajendran P, Nandakumar N, Rengarajan T,
Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J and
Nishigaki I: Antioxidants and human diseases. Clin Chim Acta.
436:332–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development,
oxidative stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li
W, Zhou ML and Wang XL: Astaxanthin activates nuclear factor
erythroid-related factor 2 and the antioxidant responsive element
(Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in
rats and attenuates early brain injury. Mar Drugs. 12:6125–6141.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Talalay P, Dinkova-Kostova AT and
Holtzclaw WD: Importance of phase 2 gene regulation in protection
against electrophile and reactive oxygen toxicity and
carcinogenesis. Adv Enzyme Regul. 43:121–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mirończuk-Chodakowska I, Witkowska AM and
Zujko ME: Endogenous non-enzymatic antioxidants in the human body.
Adv Med Sci. 63:68–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiewiet MBG, Faas MM and de Vos P:
Immunomodulatory protein hydrolysates and their application.
Nutrients. 10:9042018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parolini C, Vik R, Busnelli M, Bjørndal B,
Holm S, Brattelid T, Manzini S, Ganzetti GS, Dellera F, Halvorsen
B, et al: A salmon protein hydrolysate exerts lipid-independent
anti-atherosclerotic activity in ApoE-deficient mice. PLoS One.
9:e975982014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Espe M, Holen E, He J, Provan F, Chen L,
Øysæd KB and Seliussen J: Hydrolyzed fish proteins reduced
activation of caspase-3 in H2O2 induced oxidative stressed liver
cells isolated from atlantic salmon (salmo salar). Springerplus.
4:6582015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Idowu AT, Benjakul S, Sinthusamran S,
Sookchoo P and Kishimura H: Protein hydrolysate from salmon frames:
Production, characteristics and antioxidative activity. J Food
Biochem. 43:e127342019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei J, Tao G, Xu B, Wang K, Liu J, Chen
CH, Dunn JCY, Currie C, Framroze B and Sylvester KG: Soluble
protein hydrolysate ameliorates gastrointestinal inflammation and
injury in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in
mice. Biomolecules. 12:12872022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
Edition. National Academies Press; Washington, DC: 2011
|
|
15
|
McElroy SJ, Castle SL, Bernard JK,
Almohazey D, Hunter CJ, Bell BA, Al Alam D, Wang L, Ford HR and
Frey MR: The ErbB4 ligand neuregulin-4 protects against
experimental necrotizing enterocolitis. Am J Pathol. 184:2768–2778.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang K, Tao G, Sun Z, Wei J, Liu J, Taylor
J, Gibson M, Mostaghimi M, Good M and Sylvester KG: Fecal keratin 8
is a noninvasive and specific marker for intestinal injury in
necrotizing enterocolitis. J Immunol Res. 2023:53566462023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imam MU, Zhang S, Ma J, Wang H and Wang F:
Antioxidants mediate both iron homeostasis and oxidative stress.
Nutrients. 9:6712017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orino K, Lehman L, Tsuji Y, Ayaki H, Torti
SV and Torti FM: Ferritin and the response to oxidative stress.
Biochem J. 357:241–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CY and Chau LY: Heme oxygenase-1 in
cardiovascular diseases: Molecular mechanisms and clinical
perspectives. Chang Gung Med J. 33:13–24. 2010.PubMed/NCBI
|
|
21
|
Correa-Costa M, Amano MT and Câmara NO:
Cytoprotection behind heme oxygenase-1 in renal diseases. World J
Nephrol. 1:4–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naito Y, Takagi T, Uchiyama K and
Yoshikawa T: Heme oxygenase-1: A novel therapeutic target for
gastrointestinal diseases. J Clin Biochem Nutr. 48:126–133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang M, Xue J, Sharma V and Habtezion A:
Protective role of hemeoxygenase-1 in gastrointestinal diseases.
Cell Mol Life Sci. 72:1161–1173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schulz S, Wong RJ, Jang KY, Kalish F,
Chisholm KM, Zhao H, Vreman HJ, Sylvester KG and Stevenson DK: Heme
oxygenase-1 deficiency promotes the development of necrotizing
enterocolitis-like intestinal injury in a newborn mouse model. Am J
Physiol Gastrointest Liver Physiol. 304:G991–G1001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schulz S, Chisholm KM, Zhao H, Kalish F,
Yang Y, Wong RJ and Stevenson DK: Heme oxygenase-1 confers
protection and alters T-cell populations in a mouse model of
neonatal intestinal inflammation. Pediatr Res. 77:640–648. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eisenstein RS, Garcia-Mayol D, Pettingell
W and Munro HN: Regulation of ferritin and heme oxygenase synthesis
in rat fibroblasts by different forms of iron. Proc Natl Acad Sci
USA. 88:688–692. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng HT, Yen CJ, Chang CC, Huang KT, Chen
KH, Zhang RY, Lee PY, Miaw SC, Huang JW, Chiang CK, et al: Ferritin
heavy chain mediates the protective effect of heme oxygenase-1
against oxidative stress. Biochim Biophys Acta. 1850:2506–2517.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Tang X, Zhou B, Zhou Z, Xu N and
Wang Y: A ROS-mediated mitochondrial pathway and Nrf2
pathway activation are involved in BDE-47 induced apoptosis in
Neuro-2a cells. Chemosphere. 184:679–686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu YR, Ma H, Zou ZY, He K, Xiao YB, Wang
Y, Feng M, Ye XL and Li XG: Activation of Akt and
JNK/Nrf2/NQO1 pathway contributes to the protective
effect of coptisine against AAPH-induced oxidative stress. Biomed
Pharmacother. 85:313–322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu XM, Wang YM, Zhao YQ, Chi CF and Wang
B: Antioxidant peptides from the protein hydrolysate of monkfish
(LOPHIUS LITULON) muscle: Purification, identification, and
cytoprotective function on HepG2 cells damage by
H2O2. Mar Drugs. 18:1532020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Li H, Men LL, Huang RC, Zhou HC,
Xing Q, Yao JJ, Shi CH and Du JL: Effects of selenoprotein S on
oxidative injury in human endothelial cells. J Transl Med.
11:2872013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye Y, Fu F, Li X, Yang J and Liu H:
Selenoprotein S is highly expressed in the blood vessels and
prevents vascular smooth muscle cells from apoptosis. J Cell
Biochem. 117:106–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Chen M, Yang Z, Wang W, Lin H and Xu
S: Selenoprotein S silencing triggers mouse hepatoma cells
apoptosis and necrosis involving in intracellular calcium imbalance
and ROS-mPTP-ATP. Biochim Biophys Acta Gen Subj. 1862:2113–2123.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okina Y, Sato-Matsubara M, Matsubara T,
Daikoku A, Longato L, Rombouts K, Thanh Thuy LT, Ichikawa H,
Minamiyama Y, Kadota M, et al: TGF-β1-driven reduction of
cytoglobin leads to oxidative DNA damage in stellate cells during
non-alcoholic steatohepatitis. J Hepatol. 73:882–895. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lv Y, Wang Q, Diao Y and Xu R: Cytoglobin:
A novel potential gene medicine for fibrosis and cancer therapy.
Curr Gene Ther. 8:287–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu C and Xiao JH: The Keap1-Nrf2 system: A
mediator between oxidative stress and aging. Oxid Med Cell Longev.
2021:66354602021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chevrier G, Mitchell PL, Rioux LE, Hasan
F, Jin T, Roblet CR, Doyen A, Pilon G, St-Pierre P, Lavigne C, et
al: Low-molecular-weight peptides from salmon protein prevent
obesity-linked glucose intolerance, inflammation, and dyslipidemia
in LDLR-/-/ApoB100/100 mice. J Nutr. 145:1415–1422. 2015.
View Article : Google Scholar : PubMed/NCBI
|