Introduction
Chronic low back pain due to intervertebral disc
(IVD) degeneration (IVDD) is a ubiquitous disease that seriously
affects the quality of life of patients and causes heavy social and
economic burdens (1). Around 619
million individuals globally had low back pain in 2020 (accounting
for nearly 10% of the world population) and this number is
estimated to reach 843 million by 2050 (2). The prevalence of IVDD in the elderly
is high at 90% (3). IVDD is mainly
caused by the accumulation of interleukin (IL)-1 beta (IL-1β), loss
of proteoglycans and diffusion of cytokines into the extracellular
matrix, leading to activation of MMPs, eventually contributing to
the pathologic apoptosis of nucleus pulposus (NP) cells (4,5).
Currently, clinical treatments for IVDD are mainly conservative
treatment and surgery, which can relieve symptoms and reduce pain,
but cannot reverse IVDD (6).
Therefore, seeking therapeutic measures to delay IVDD progression
or even reverse the IVDD process has become a hot topic in current
research.
Mesenchymal stem cell (MSC) therapy for IVDD has
received extensive attention (7).
IVD cannot provide adequate nutrition for transplanted MSCs because
of the lack of vascular structures (8). In addition, the death of transplanted
MSCs releases deleterious substances, thereby exacerbating IVDD
(9). Exosomes produced by MSCs
through paracrine secretion retain the potential of MSCs and act as
carriers to deliver their contents (metabolites, amino acids,
lipids, proteins and nucleic acids) to target cells to modulate
their activity. Exosomes have a diameter of 30–200 nm (10). Xiao et al (11) revealed that bone mesenchymal stem
cell (BMSC)-derived exosomes weaken NP cell autophagy by regulating
the protein kinase B/mammalian target of rapamycin pathway.
Additionally, BMSC-derived exosomes can improve IVDD by modulating
macrophage polarization through the delivery of hypermethylated
lncRNAs in colorectal adenocarcinoma (12). Due to their low immunogenicity,
good compatibility and avoidance of the adverse effects of MSC
therapy, MSC-derived exosomes are considered emerging therapeutic
methods for IVDD (13,14). Studies have demonstrated that the
clinical efficacy of exosomes can be improved by altering their
composition. For instance, Netrin1-enriched exosomes from
genetically modified adipose-derived stem cells protect muscles,
reduce inflammation, improve collateral artery remodeling and
enhance angiogenesis, thereby improving diabetic limb ischemia
(15). In addition, exosomes
derived from BMSCs modified with miR-340-3p inhibited ferroptosis
to facilitate the recovery of the injured rat uterus (16). Therefore, exosomes modified with
specific genes may provide additional strategies for the treatment
of IVDD.
Heme oxygenase 1 (HO-1) protein is encoded by HMOX1,
which catalyzes the degradation of heme and production of
bilirubin, carbon monoxide and ferrous ions (Fe2+)
(17). HO-1 is associated with
various diseases including osteoarthritis (18), diabetes (19) and cancer (20). In addition, MSC-derived exosomes
have been revealed to weaken the process of diverse diseases, such
as delayed neurocognitive recovery (21), spinal cord injury (22) and acute liver injury (23) by mediating HO-1 expression. In
addition, HO-1 exerts a protective role in the process of IVDD
(24,25). However, whether HO-1-enriched
BMSC-derived exosomes play a protective role in IVDD remains
unclear.
Although both BMSC-derived exosomes and HO-1 have
been demonstrated to exert protective effects against IVDD
progression, the efficacy of HO-1-modified BMSC-derived exosomes
remains unclear. Thus, the present study aimed to investigate the
effects of exosomes derived from HO-1-overexpressing BMSCs on the
apoptosis and senescence of IL-1β-stimulated NP cells, offering
evidence to support the clinical utilization of HO-1-modified
BMSC-derived exosomes in IVDD.
Materials and methods
Incubation of BMSCs, NP and 293T
cells
Mouse BMSCs [CP-M131 (https://www.procell.com.cn/view/2537.html), Procell
Life Science & Technology Co., Ltd.] and NP cells [CP-M146
(https://www.procell.com.cn/view/2549.html), Procell
Life Science & Technology Co., Ltd.], two non-immortalized
populations, were cultured in Dulbecco's modified Eagle's medium
(DMEM; MilliporeSigma) supplemented with 10% fetal bovine serum
(Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Procell Life Science & Technology Co., Ltd.) at 37°C with
additional 5% CO2. BMSCs and NP cells from passages 2–5
were used. 293T cells (cat. no. CL-0005 Procell Life Science &
Technology Co., Ltd.) were also cultured in DMEM with 10% FBS and
1% penicillin/streptomycin in an incubator at 37°C and 5%
CO2. Ethical approval was waived because the present
study did not involve human or animal subjects.
Multilineage differentiation of
BMSCs
The potential of mouse BMSCs for multilineage
differentiation was analyzed using osteogenic, lipogenic and
chondrogenic differentiation assays. Mouse BMSCs were incubated for
2 or 3 weeks in an osteogenic/adipogenic/chondrogenic-induced
differentiation medium (Procell Life Science & Technology Co.,
Ltd.). Finally, mineralized nodules were characterized by alizarin
red staining (ARS), lipid droplets were visualized by oil red O
(ORO) staining and acidic polysaccharides were characterized by
alizarin blue (ALB) staining. For ARS, the incubated BMSCs were
fixed in 4% paraformaldehyde (PFA) for 15 min at room temperature.
After washing with phosphate-buffered saline (PBS), the cells were
stained with ARS solution (40 mM; cat. no. S0141; Cyagen
Biosciences, Inc.) for 5 min at room temperature (26). For ORO staining, BMSCs were fixed
with 4% PFA for 20 min at room temperature. After soaking in 70%
ethanol (cat. no. ml094574; Mlbio Biosciences, Inc.) for 30 sec at
room temperature, the cells were stained with ORO working solution
(0.5 ml; cat. no. MUXMX-90031, Cyagen Biosciences, Inc.) for 30 min
at room temperature and stopped by washing with PBS (27). For ALB staining, BMSCs were
stabilized with 4% PFA for 15 min at room temperature and stained
with BCIP/NBT working solution (0.5 ml; cat. no. C3206; Beyotime
Institute of Biotechnology) away from light for 15 min at 37°C
(28). Images of all stained
sections were captured successfully using an inverted microscope
(Olympus Corporation).
Identification of BMSCs by flow
cytometry
BMSCs were re-suspended and aliquoted into EP tubes
(100 µl/tube). BMSCs were incubated with CD29 (5 µl; cat. no.
E-AB-F1309D, Wuhan Elabscience Biotechnology Co., Ltd.), CD73 (5
µl; cat. no. E-AB-F1089D, Wuhan Elabscience Biotechnology Co.,
Ltd.), CD90 (5 µl; cat. no. E-AB-F1283D, Wuhan Elabscience
Biotechnology Co., Ltd.), CD34 (5 µl; cat. no. E-AB-F1284D, Wuhan
Elabscience Biotechnology Co., Ltd.) and CD45 (5 µl; cat. no.
E-AB-F1136D, Wuhan Elabscience Biotechnology Co., Ltd.) antibodies
for 30 min at 4°C in the dark. BMSCs were treated with a mouse
anti-immunoglobulin G antibody in a separate tube as a negative
control. Following incubation, cells were detected with a
FACSCalibur flow cytometer (BD Biosciences) and the data were
analyzed with FlowJo software version 10.8.1 (FlowJo LLC
Biosciences).
Establishment of BMSCs with stable
HMOX1 overexpression
The full-length HMOX1 cDNA was inserted into the
pHBLV-CMV-MCS-GFP vector (Hanbio Biotechnology Co., Ltd.) to
construct the pHBLV-CMV-MCS-GFP-HMOX1 vector. For lentiviral
packaging, a second-generation transduction system was used. 293T
cells were co-transfected with pHBLV-CMV-MCS-GFP-HMOX1,
pHBLV-CMV-MCS-GFP), pSPAX2 (cat. no. 12260; Addgene, Inc.), and
pMD2G (cat. no. 12259; Addgene, Inc.) plasmids at 2:2:1 ratio (10
µg : 10 µg : 5 µg, respectively) using the Simple-fect reagent
(Signaling Dawn Biotech) following the manufacturer's instructions.
After 48 h, lentivirus particles from the medium were collected by
centrifugation (800 × g, 10 min, 4°C) and filtered via a 0.45-µm
filter, and BMSCs were plated at a density of 1×105
cells in 3.5-mm dishes with negative lentiviral particles or
HMOX1-overexpressing lentiviral particles (multiplicity of
infection=20). At 24 h later, BMSCs were selected with 1 µg/ml
puromycin for 3 days prior to subsequent experiments.
Isolation and identification of
exosomes from BMSCs
Exosomes isolated from BMSCs with and without HO-1
overexpression were named Exo and Exo-HO-1, respectively. Briefly,
the medium was changed to serum-free DMEM and the cells were
incubated for 24 h at 37°C. To remove dead cells and cell debris,
cell supernatants were centrifuged for 10 min (300 × g), 10 min
(2,000 × g), and 30 min (10,000 × g) at 4°C (29). The supernatants were subsequently
centrifuged (200,000 × g, 70 min, 4°C) twice to obtain exosomes.
The morphology of the precipitated exosomes was observed using
transmission electron microscopy (TEM; JEOL, Ltd.). Determination
of exosome-specific markers (TGS101 and CD63) and the endoplasmic
reticulum marker cainexin was performed by western blotting without
loading control blots in accordance with published references
(30–32). The size distribution of Exo and
Exo-HO-1 was determined by nanoparticle tracking analysis (NTA)
using a ZetaView PMX 110 (Particle Metrix).
Exosome labeling and tracking
The exosomes were labeled with green fluorescent
PKH67 (cat. no. 40781; Shanghai Yeasen Biotechnology Co., Ltd.).
Briefly, the amount of exosomal protein was determined using the
bicinchoninic acid (BCA) protein assay kit (CoWin Biosciences).
Dilution buffer was used to prepare the PKH6 dye working solution
at a concentration of 100 µM. The exosomes (20 µg) were added 50 µl
of the dye working solution and vortexed for 1 min at room
temperature. After 10 min of incubation at room temperature, the
exosomes were washed and centrifuged (12,000 × g, 2 min, 4°C) to
remove excess PKH67. Labeled exosomes were co-cultured with NP
cells for 24 h at 37°C and then fixed with 4% paraformaldehyde for
20 min at room temperature. After staining with
4′,6-diamidino-2-phenylindole, the uptake of labeled exosomes by NP
cells was observed using a confocal microscope (Leica Microsystems
GmbH).
NP cell treatment
To simulate the pathological environment of IVDD
in vitro, healthy NP cells (2×105 cells/well)
reaching 70–80% were treated with different concentrations of IL-1β
(0, 5, 10 and 20 ng/ml; Shanghai Yeasen Biotechnology Co., Ltd.)
for 24 h and a concentration of 10 ng/ml IL-1β was utilized to
stimulate NP cells for different times (0, 12, 24 and 48 h) at 37°C
(33,34). To screen for the optimal
therapeutic dose of Exo-HO-1, the cells (2×105
cells/well) were incubated with different concentrations of
Exo-HO-1 (0, 10, 20 and 40 µg) in the absence or presence of IL-1β
(10 ng/ml) for 24 h at 37°C. NP cells were incubated in the
presence of both IL-1β (10 ng/ml) and Exo-HO-1 (20 µg) for
different times (0, 12, 24, 48 and 72 h) at 37°C (35). NP cells were stimulated with 10
ng/ml of IL-1β and treated with Exo-HO-1 at 37°C in the meantime
and the medium was replaced with fresh medium containing Exo-HO-1
without IL-1β 24 h later. After incubation, the NP cells were used
for experimental analysis.
Cell apoptosis analysis
Briefly, different groups of NP cells were harvested
using 0.25% trypsin (Procell Life Science & Technology Co.,
Ltd.), washed twice with PBS and centrifuged (300 × g) for 5 min at
4°C. The cells were resuspended in 1X binding buffer. Cell
apoptosis was determined using an apoptosis kit (Beijing Solarbio
Science & Technology Co., Ltd.), according to the
manufacturer's instructions. Cells (~5×105) were
incubated with 5 µl of Annexin V/FITC in a flow tube (5 ml) for 5
min under light protection at room temperature. Finally, 5 µl of
propidium iodide solution was added and incubated for 5 min at room
temperature. A FACSCalibur flow cytometer was used to analyze the
cells immediately. The collected data were analyzed using FlowJo
software version 10.8.1 (FlowJo LLC Biosciences). The apoptosis
rate is the sum of early apoptotic cells and the percentage of late
apoptotic cells.
Cell viability analysis
After incubation of NP cells with the different
treatments, 10 µl of the Cell Counting Kit-8 (CCK-8; Beijing
Solarbio Science & Technology Co., Ltd.) reagent was added into
each well. After incubation for 2 h, the absorbance was measured at
450 nm using a plate reader (Thermo Fisher Scientific, Inc.).
Cell cycle analysis
The collected NP cells were fixed in 70% ethanol (1
ml) at 4°C overnight. After washing and centrifugation (1,000 × g;
3 min, 4°C), the NP cells were incubated with a mixture of staining
solution containing the staining buffer (500 µl), propidium iodide
staining solution (X20; 25 µl) and RNase A (20×; 10 µl) at room
temperature for 30 min in the dark. These reagents were included in
the Cell Cycle and Apoptosis Detection Kit (cat. no. C1052;
Beyotime Institute of Biotechnology). The stained cells were
analyzed using flow cytometry (BD Biosciences) at an excitation
wavelength of 488 nm and an emission wavelength of 605 nm.
Subcellular fractionation
Nuclear and cytoplasmic components were separated
using a Nuclear and Cytoplasmic Protein Extraction Kit (cat. no.
P0028; Beyotime Institute of Biotechnology). In brief, NP cells
were digested with cytoplasmic protein extraction reagent A
supplemented with PMSF on ice for 15 min. Subsequently, 10 µl of
the reagent B was added and vortexed vigorously for 5 sec, followed
by incubation on ice for 1 min. Centrifugation was performed for 5
min (4°C; 12,000 × g) after another vortex for 5 sec and the
obtained supernatants were cytoplasmic proteins. After aspirating
the supernatant, 50 µl of PMSF-supplemented nuclear protein
extraction reagent was added to the precipitate. After vigorous
vortex for 20 sec, the samples were placed on ice for 30 min, with
high-speed vortex every 1–2 min for 20 sec. Supernatants harvested
by centrifugation (4°C; 12,000 × g) were cytoplasmic proteins.
Western blotting
Radioimmunoprecipitation analysis lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.) was used to
extract proteins from NP cells and exosomes. For protein
quantification, a BCA protein assay kit (CoWin Biosciences) was
used according to the manufacturer's instructions. The extracted
protein samples (30 µg) were electrophoresed by 8–12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto polyvinylidene difluoride membranes (MilliporeSigma). After
blocking with a rapid blocking solution (Beyotime Institute of
Biotechnology) at 4°C overnight, the membranes were incubated with
appropriate primary antibodies including anti-HO-1 (cat. no.
GTX637432; 1:2,000; GeneTex, Inc.), anti-TGS101 (cat. no. A01233-2,
0.25 µg/ml; Boster Bio), anti-CD63 (FNab01490; 1:1,000; Fine
Biotech Co., Ltd, Wuhan, China) and anti-Cainexin (cat. no.
GTX109669; 1:5,000; GeneTex, Inc.), Bcl-2 (cat. no. FNab00839;
1:1,000; Wuhan Fine Biotech Co., Ltd.), Bax (cat. no. FNab00810;
1:2,000; Wuhan Fine Biotech Co., Ltd.), cleaved caspase 3 (cat. no.
MBS9410752; 1:1,000; MyBioSource, Inc.), anti-p65 (cat. no. PB9324;
0.5 µg/ml; Boster Bio), anti-p-p65 (cat. no. A00284S468-2; 1:2,000;
Boster Bio), anti-GAPDH (cat. no. H00227; 1:5,000; Boster Bio) and
anti-Histone H3 (cat. no. M12477-9; 1:1,000; Boster Bio)
antibodies. After incubation with goat anti-rabbit IgG-HRP
secondary antibody (cat. no. GTX213110-01; 1:5,000; GeneTex, Inc.)
for 2 h at room temperature, an enhanced chemiluminescence reagent
(cat. no. P0018; Beyotime Institute of Biotechnology) was used to
visualize the results. Band intensity was quantified using the
ImageJ software (version v1.48; National Institutes of Health).
Senescence-associated β-galactosidase
(SA-β-gal) staining
SA-β-gal-positive NP cells were quantified using the
SA-β-gal staining kit (cat. no. C0602; Beyotime Institute of
Biotechnology). After incubation, NP cells in 6-well plates were
fixed for 15 min using a fixation solution (1 ml) included in the
kit. After rinsing twice with PBS, the cells were stained with the
SA-β-gal staining solution (1 ml) overnight at 37°C. Following
rinsing, SA-β-gal-positive NP cells were observed under an inverted
microscope (Olympus Corporation).
Statistical analysis
A minimum of three biological replicates were used
in all the experiments. Statistical significance was assessed using
the GraphPad Prism 8 software (GraphPad; Dotmatics). Continuous
data are expressed as means ± standard errors of the mean.
Normality of the data was assessed using the Shapiro-Wilk test.
Differences between two groups were analyzed using unpaired t-tests
and one-way analysis of variance followed by Tukey's post hoc test
was performed for multiple-group differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
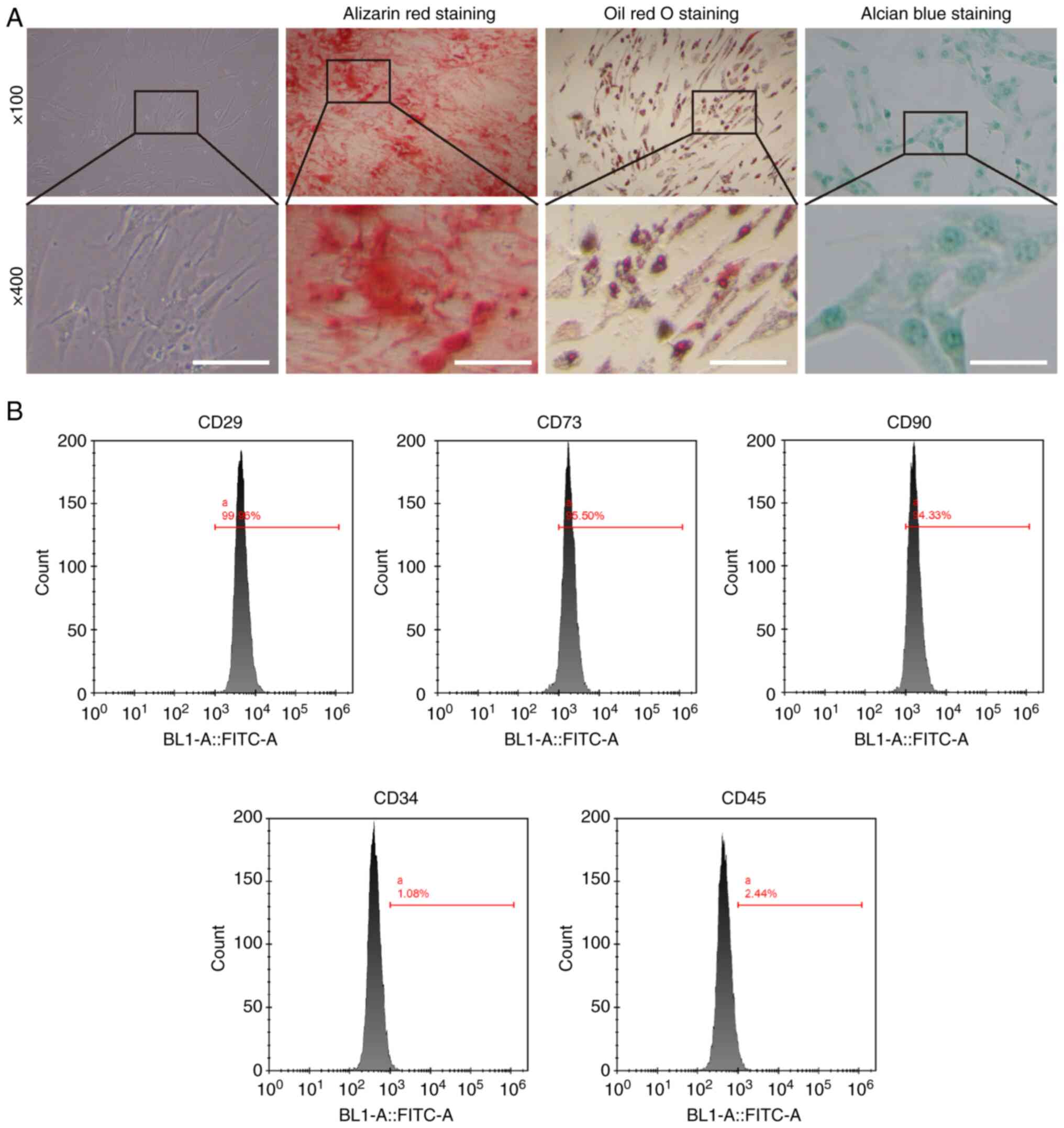
Incubation and characterization of
BMSCs
To elucidate the mechanism underlying the protective
effect of BMSCs against NP cells during IVDD, the present study
first determined the multilineage differentiation capacity of the
purchased BMSCs. Growth and morphological changes in cultured
primary BMSCs were observed using an inverted microscope. BMSCs
were distributed uniformly and showed a spindle-like morphology
(Fig. 1A). After 14 days of
incubation with the osteogenic culture medium for BMSCs, ARS showed
distinct mineralized nodule formation (Fig. 1A). After induction with lipogenic
medium, ORO staining showed evident fat droplet formation,
indicating the differentiation of BMSCs into adipocytes (Fig. 1A). Alcian blue staining showed a
marked accumulation of glycosaminoglycans following incubation in
chondrogenic medium (Fig. 1A).
Flow cytometry for stem cell surface antigens showed that they were
highly positive surface markers CD29 (99.96%), CD73 (99.50%) and
CD90 (94.33%), accompanied by negative surface markers CD34 (1.08%)
and CD45 (2.44%). The multidirectional differentiation potential
and highly positive surface markers suggested high purity of the
cultured BMSCs.
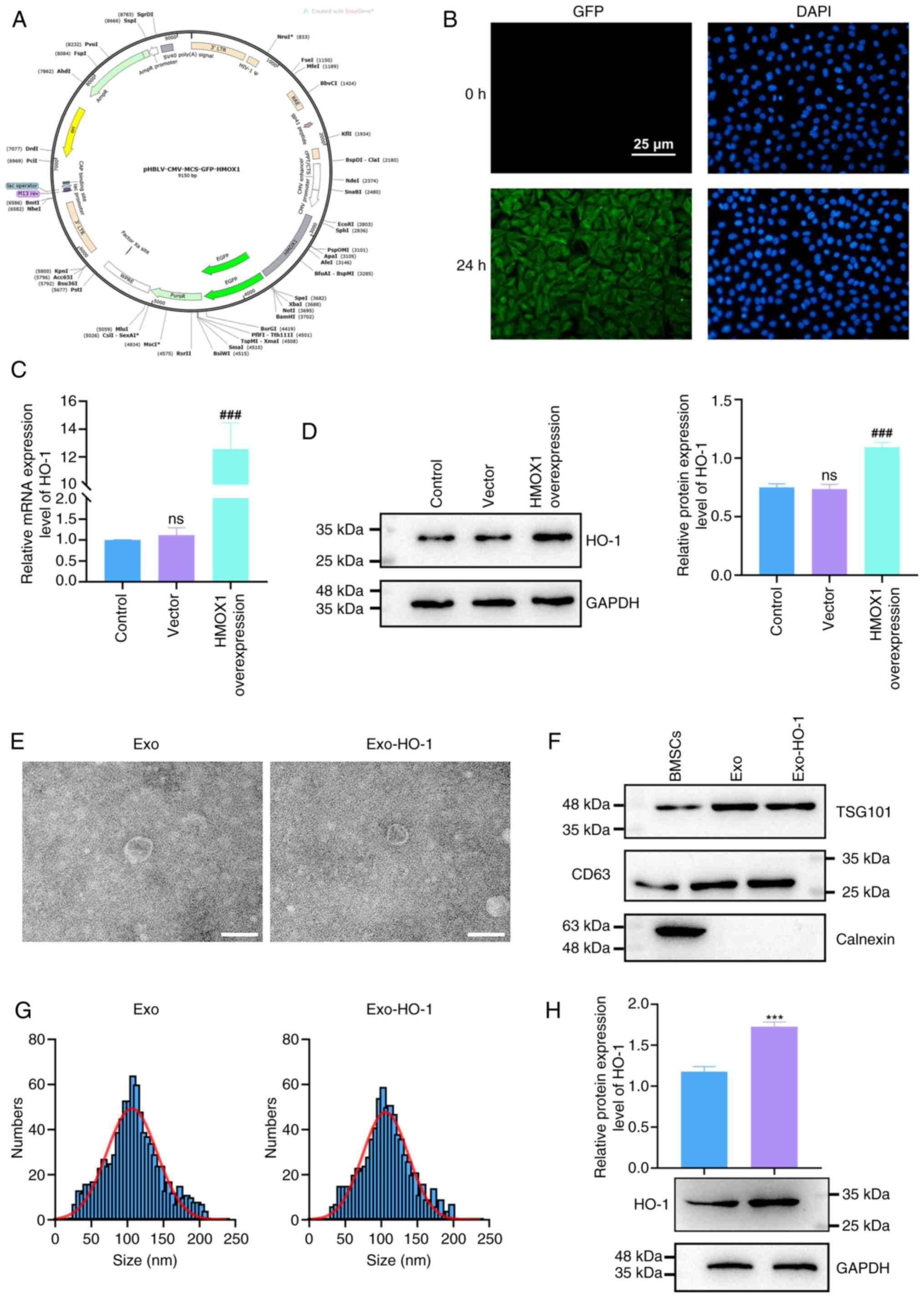
Characterization of Exo and
Exo-HO-1
HO-1 protein encoded by the HMOX1 gene possesses a
protective effect on NP cells in IVDD (36,37).
To characterize the effect of HMOX1-overexpressing BMSC-derived
exosomes on IVDD, HMOX1 overexpression plasmids were transfected
into BMSCs. A schematic representation of the HMOX1 overexpression
plasmid is shown in Fig. 2A. The
green fluorescence exhibited by BMSCs after transfection suggested
the successful expression of the plasmid DNA (Fig. 2B). HMOX1 mRNA and HO-1 protein
levels were markedly upregulated following transfection, as
confirmed by RT-qPCR and western blotting, respectively (Fig. 2C and D). Next, exosomes were
extracted from the BMSCs transfected with the vector or HMOX1 by
ultracentrifugation and termed Exo and Exo-HO-1, respectively. The
diameters of both Exo and Exo-HO-1 with cup or spherical shapes
were observed to be ~100 nm using TEM (Fig. 2E). The exosome marker proteins
tumor susceptibility gene 101 (TSG101) and CD63 were more strongly
expressed in Exo and Exo-HO-1 cells than in BMSCs, whereas calnexin
(an endoplasmic reticulum protein) was not detected in Exo or
Exo-HO-1 cells, indicating that the isolated exosomes were not
contaminated with cellular components (Fig. 2F). NTA showed that these particles,
which were ~114 nm in diameter, were the most abundant, with most
of the particles <200 nm (Fig.
2G). A significant upregulation of HO-1 protein was observed in
Exo-HO-1 compared with that in Exo, indicating that Exo-HO-1
carried greater amounts of HO-1 protein (Fig. 2H). The aforementioned analyses
showed that Exo and Exo-HO-1 were successfully isolated.
Analysis of optimal conditions for
IL-1β incubation and Exo-HO-1 treatment
To investigate the effects of IL-1β treatment on the
apoptosis of NP cells, different IL-1β concentrations (0, 5, 10 and
20 ng/ml) were used to stimulate the cells for 24 h. As shown in
Fig. 3A, IL-1β (10 and 20 ng/ml)
stimulation urged NP cell apoptosis markedly. Next, the NP cells
were stimulated with 10 ng/ml for different durations. Apoptosis of
the NP cells increased progressively with the prolongation of IL-1β
stimulation (Fig. 3B). The
apoptosis of NP cells was shifted to a later stage when the
concentration of IL-1β reached 20 ng/ml or when 10 ng/ml of IL-1β
was treated for 48 h. Consequently, NP cells were stimulated with
10 ng/ml of IL-1β for 24 h to induce IVDD cell models in
vitro. To evaluate the effects of Exo-HO-1 on NP cell
viability, we treated the cells with different doses of Exo-HO-1
(0, 10, 20 and 40 µg) for 24 h in the presence or absence of IL-1β.
The results revealed that Exo-HO-1 treatment did not affect the
viability of healthy NP cells, whereas Exo-HO-1 treatment improved
the viability of NP cells under IL-1β stimulation once the
concentration reached 20 µg (Fig. 3C
and D). Subsequently, the viability of NP cells treated with
Exo-HO-1 (20 µg) at different times (0, 12, 24, 48 and 72 h) was
explored. Incubation of IL-1β-induced NP cells with Exo-HO-1 for
≥24 h could improve their activity, so IL-1β-stimulated NP cells
treated with Exo-HO-1 (20 µg) for 48 h were used for subsequent
analysis (Fig. 3E).
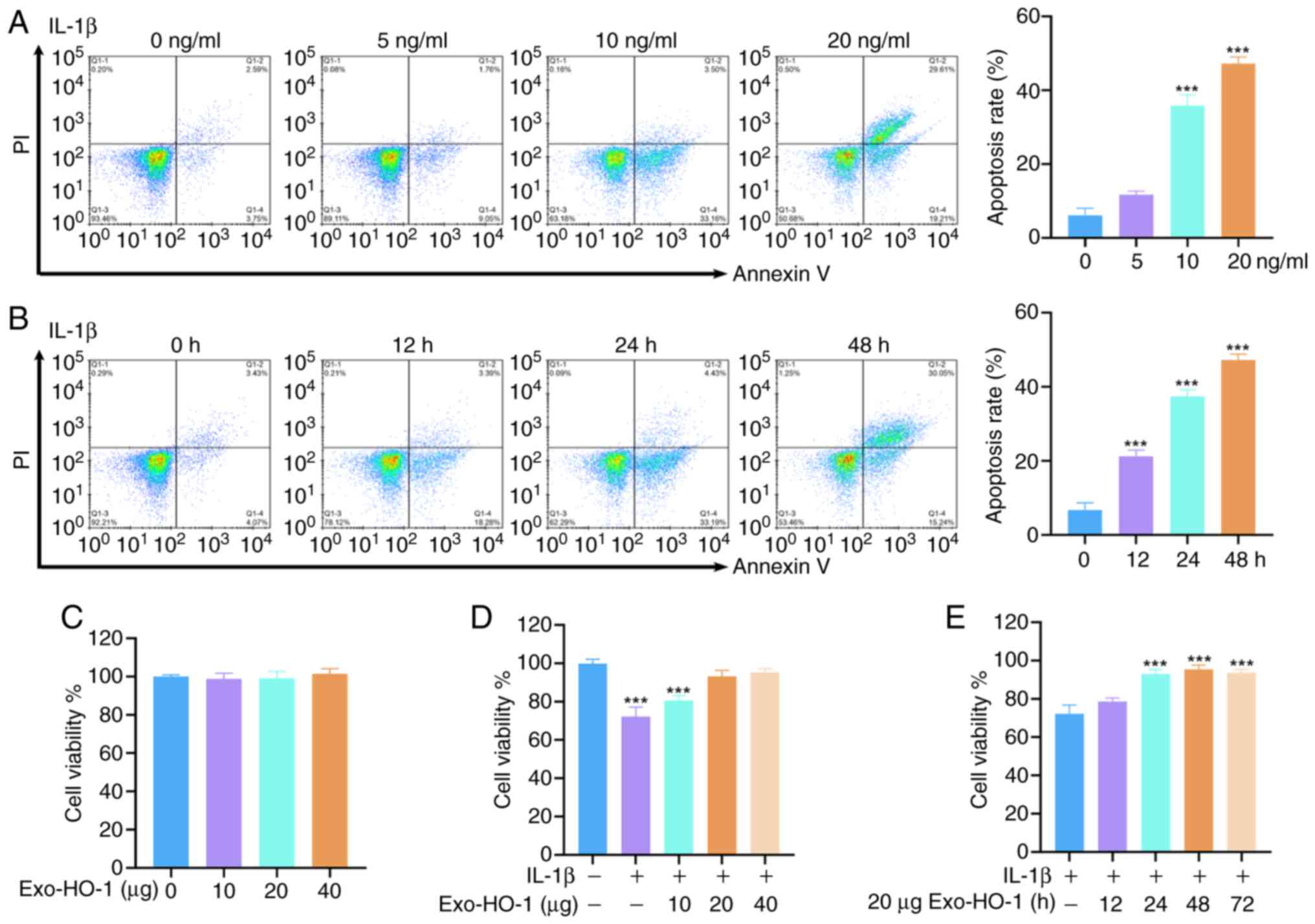 | Figure 3.Determination of optimal conditions
for IL-1β incubation and Exo-HO-1 treatment. (A and B) NP cells
were stimulated with different concentrations of IL-1β (0, 5, 10
and 20 ng/ml) for 24 h or IL-1β (10 ng/ml) for different times (0,
12, 14 and 48 h). The apoptosis of NP cells was determined by flow
cytometry. (C and D) NP cells were treated with different doses of
Exo-HO-1 (0, 10, 20 and 40 µg) for 24 h in the presence or absence
of IL-1β. The viability of NP cells was detected using Cell
Counting Kit-8 (CCK-8) assays. (E) The viability of
IL-1β-stimulated NP cells treated with Exo-HO-1 (20 µg) for
different times (0, 12, 14, 48 and 72 h) was determined by CCK-8
assays. ***P<0.001. IL, interleukin; Exo, exosomes; HO-1, heme
oxygenase 1; NP, nucleus pulposus. |
Exo-HO-1 protects NP cells against
IL-1β-urged apoptosis
Based on these results, PKH67-labeled Exo or
Exo-HO-1 were co-incubated with NP cells to observe the uptake of
exosomes by NP cells. As shown in Fig.
4A, PKH67-labeled Exo or Exo-HO-1 were taken up by NP cells
(Fig. 4A). In addition, NP cells
co-cultured with Exo-HO-1 showed overtly higher HO-1 protein levels
than those incubated with Exo (Fig.
4B). Flow cytometry was used to evaluate NP cell apoptosis.
IL-1β stimulation resulted in marked apoptosis of NP cells,
accompanied with a notable reduction in Bcl-2 protein levels and a
prominent elevation in Bax and cleaved caspase 3 protein levels.
Exo treatment had no effect on NP cell apoptosis under IL-1β
stimulation. However, Exo-HO-1 treatment attenuated IL-1β-urged NP
cell apoptosis, with synchronized alterations in
apoptosis-associated proteins (Fig. 4C
and D). Collectively, these results indicated that Exo-HO-1
mitigated NP cell apoptosis under IL-1β stimulation.
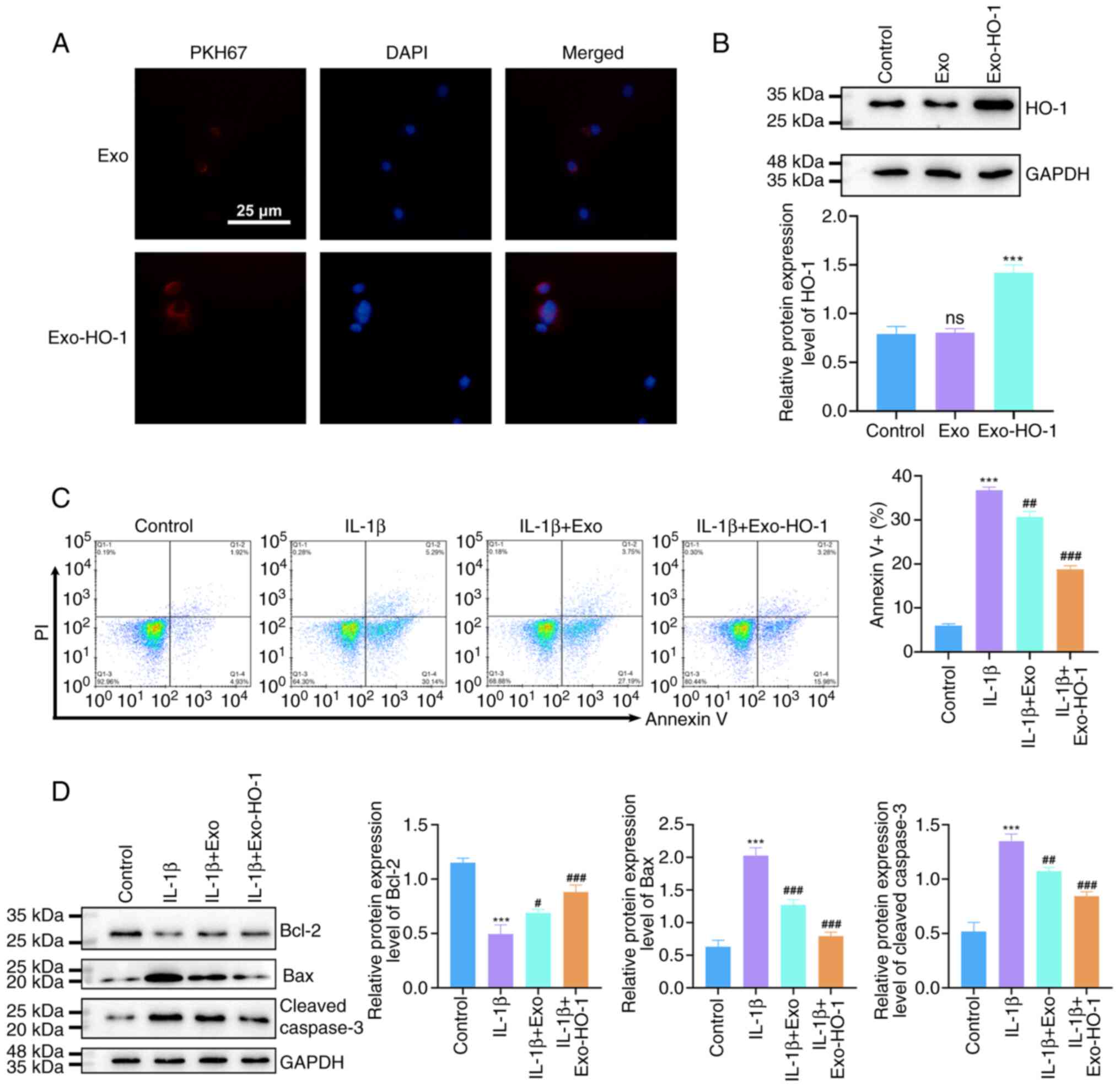 | Figure 4.Exo-HO-1 ameliorated IL-1β-induced
apoptosis in NP cells. (A) Internalization of PKH26-labeled Exo or
Exo-HO-1 by NP cells was examined by confocal microscopy after 24 h
of co-incubation (scale bar, 25 µm). (B) Protein levels of HO-1 in
NP cells co-cultured with phosphate-buffered saline, Exo, or
Exo-HO-1 were detected by western blotting. (C) Flow cytometry
analysis of the apoptosis of NP cells in different groups (control,
IL-1β, IL-1β + Exo and IL-1β + Exo-HO-1). (D) Western blotting
detected apoptosis-associated proteins Bcl-2, Bax and Cleaved
caspase 3 in NP cells with different treatments. ns: not
significant. ***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. IL-1β; ns,
not significant. Exo, exosomes; HO-1, heme oxygenase 1; IL,
interleukin; NP, nucleus pulposus. |
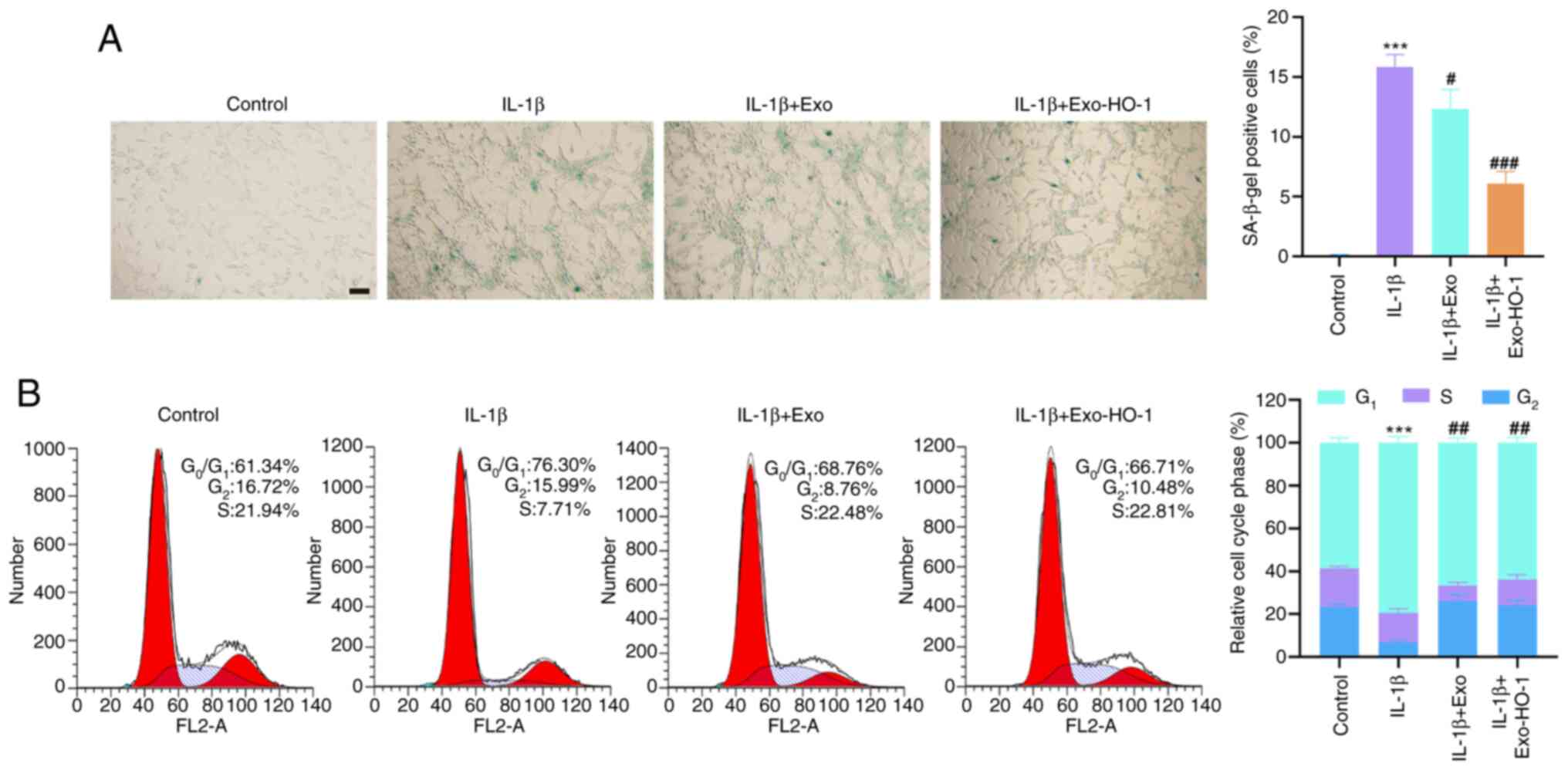
Exo-HO-1 impairs IL-1β-prompted NP
cell senescence
Subsequently, the effects of Exo-HO-1 on NP cell
senescence under IL-1β stimulation were further investigated. The
number of SA-β-gal-positive NP cells was markedly elevated after
IL-1β stimulation. Although treatment with both Exo and Exo-HO-1
reduced the number of SA-β-gal-positive NP cells in response to
IL-1β stimulation, the effect of Exo-HO-1 was stronger than that of
Exo (Fig. 5A). Senescence arrest
occurs mainly in the G0/G1 phase of the cell
cycle. Therefore, the aforementioned results were further validated
using cell cycle assays. IL-1β stimulation caused NP cells to
senesce and arrest in the G0/G1 phase,
although this effect was substantially attenuated upon treatment
with Exo-HO-1 but not with Exo (Fig.
5B). Together, these outcomes indicated that IL-1β-induced NP
cell senescence could be alleviated by Exo-HO-1.
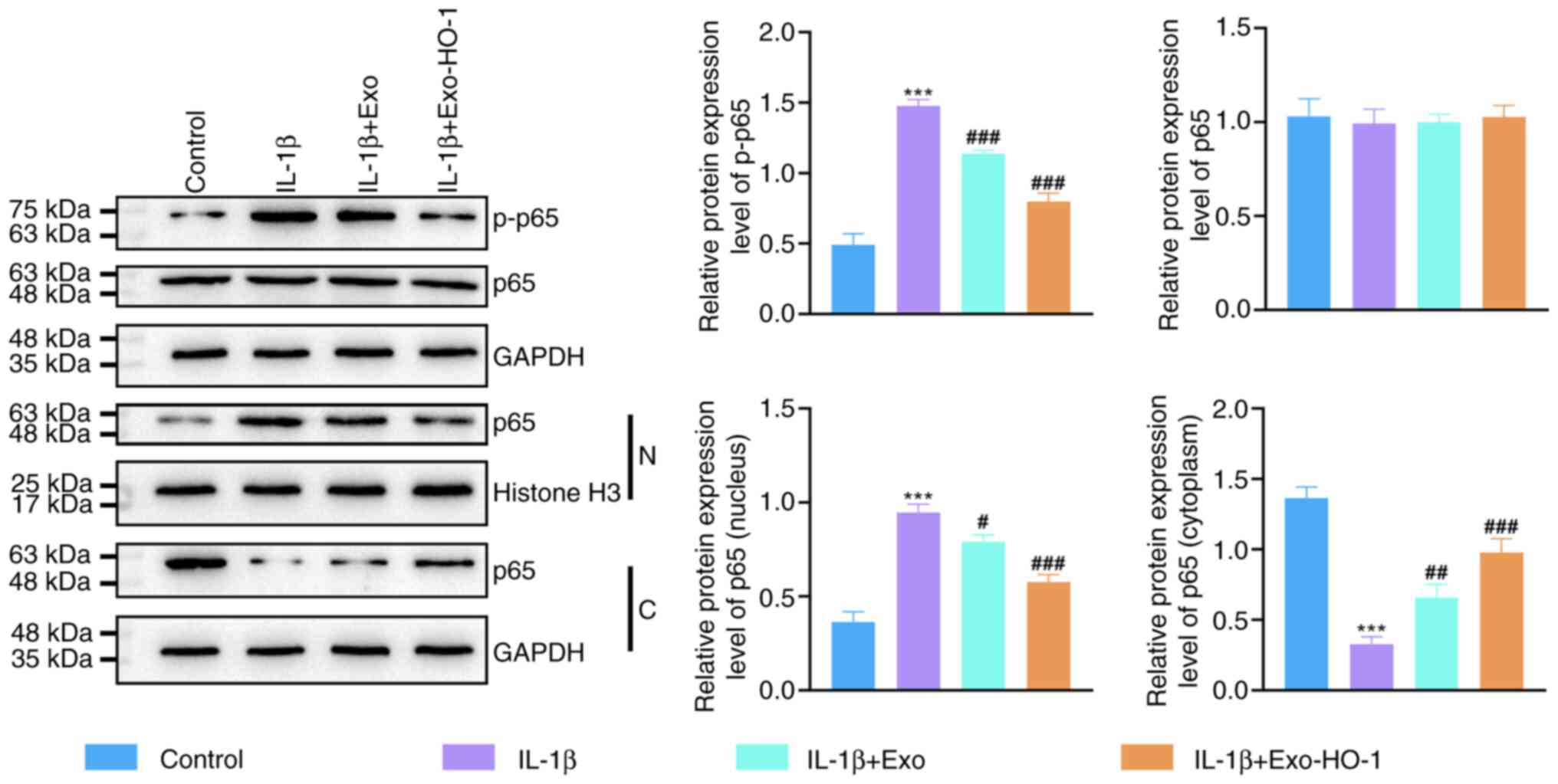
Exo-HO-1 suppresses the NF-κB
signaling in IL-1β-stimulated NP cells
Considering that NF-κB exerts a vital action in the
progression of IVDD (38), the
present study further analyzed whether Exo-HO-1 works by mediating
the NF-κB signaling. IL-1β promoted the phosphorylation of p65
protein, although Exo-HO-1 and Exo decreased the phosphorylation of
p65 protein and Exo-HO-1 had a stronger effect than Exo (Fig. 6). NF-κB p65 was predominantly
distributed in the cytoplasm of control cells, whereas IL-1β
stimulation boosted the translocation of NF-κB p65 from the
cytoplasm to the nucleus. However, Exo and Exo-HO-1 suppressed the
nuclear translocation of NF-κB p65. Activation of the NF-κB
signaling mediated by IL-1β stimulation was restrained in NP cells
after Exo-HO-1 treatment.
Discussion
Exosomes applied alone or loaded with specific genes
or drugs are important tools for exchanging information between
IVDD (39). Some studies have
reported that BMSC-derived exosomes ameliorate IVDD (40) and a few studies have reported on
the function of genetically modified BMSC-derived exosomes in IVDD.
BMSC-derived exosomes and HO-1 have both been demonstrated to exert
protective effects against IVDD progression, but the innovation of
the present study was to reveal that BMSC-derived exosomes modified
with HO-1 attenuated IL-1β-induced NP cell apoptosis and
senescence, suggesting that HO-1-modified BMSC-derived exosomes may
be a promising strategy for IVDD treatment.
The inflammatory response is a key pathological
mechanism in the development of IVDD and the overexpression of
pro-inflammatory cytokines can disrupt extracellular matrix
homeostasis in IVD and enable it to maintain degenerative and
catabolic states (41). IL-1β, as
an important pro-inflammatory factor involved in cell
differentiation and apoptosis through the NF-κB signaling pathway,
is frequently used to induce degeneration of NPCs for establishing
IVDD cell models in vitro (42,43).
The present study observed that IL-1β (10 and 20 ng/ml) stimulation
markedly drove NP cell apoptosis. The apoptosis of NP cells was
shifted to a later stage when the concentration of IL-1β reached 20
ng/ml or 10 ng/ml. IL-1β was treated for 48 h, so NP cells with
were stimulated 10 ng/ml of IL-1β for 24 h to induce IVDD cell
models in vitro.
Currently, BMSC-derived exosomes have become the
focus as a potential alternative to BMSCs in cell therapy including
IVDD. Hu et al have reported that BMSC-derived exosomes
repress compression-mediated oxidative stress in NP cells, thus
alleviating apoptosis (44).
BMSC-derived exosomes modulate the Keap1/Nrf2 axis, thereby
restoring the antioxidant response in degenerative NP cells
(40). HO-1, which exhibits
anti-inflammatory, antioxidant and anti-apoptotic properties, is a
protein downstream of Nrf2 (25).
Furthermore, cyanidin-3-glucoside and dimethyl fumarate weaken the
dysfunction of NP cells via the Nrf2/HO-1 pathway during IVDD
(24,45). Another report has indicated that
HO-1 expression is low in IVDD samples (46) and HO-1 overexpression can protect
NP cell apoptosis under IL-1β stimulation (46,47).
However, the effects of BMSC-derived exosomes loaded with HO-1 on
NP cell apoptosis and senescence are unclear.
The present study characterized BMSCs by
multilineage differentiation and detection of surface marker
molecules. Furthermore, exosomes isolated from BMSCs and BMSCs
overexpressing HO-1 showed little differences in size and
morphology. In addition, Exo-HO-1 treatment at a low dose (10 µg)
could improve the viability of NP cells under IL-1β stimulation.
Incubation of IL-1β-induced NP cells with Exo-HO-1 for ≥24 h
clearly increased their activity, so IL-1β-stimulated NP cells were
treated with Exo-HO-1 (10 µg) for 24 h and used for function
analysis. Functionally, Exo-HO-1 treatment markedly reduced
IL-1β-induced NP cell apoptosis, accompanied with a simultaneous
alteration in Bcl-2, cleaved caspase 3 and Bax levels. In addition,
both Exo and Exo-HO-1 treatment weakened IL-1β-prompted senescence
in NP cells and Exo-HO-1 had a stronger effect than Exo. These
results highlighted the important role of Exo-HO-1 in apoptosis and
senescence of NP cells.
NF-κB signaling is a target for pro-inflammatory
factors, such as tumor necrosis factor-alpha, IL-1β and IL-8,
triggering a cytokine storm and playing a central role in the
inflammatory response (48). NF-κB
proteins are inactivated in the cytoplasm by binding to the
repressor protein IkB to form a trimeric complex (49). However, the NF-κB dimer exposes
nuclear localization sequences and rapidly passes from the
cytoplasm into the nucleus when upstream signaling factors bind to
receptors on the surface of the cell membrane, followed by binding
to specific sequences on nuclear DNA to promote the transcription
of relevant genes (49). The NF-κB
signaling pathway is closely related to the process of IVDD and
blocking the NF-κB signaling pathway markedly delays IVDD (50,51).
In the present study, Exo-HO-1 and Exo treatment decreased the
phosphorylation of the p65 protein in IL-1β-induced NP cells and
Exo-HO-1 had a stronger effect than Exo. However, Exo and Exo-HO-1
repressed the nuclear translocation of NF-κB p65, suggesting that
IL-1β-urged activation of the NF-κB signaling was restrained in NP
cells after Exo-HO-1 treatment. Unfortunately, the present study
did not use animal models of disc degeneration to validate the
therapeutic effect of Exos or Exo-HO-1 on disc recovery, which will
be the direction of future research.
In conclusion, Exo-HO-1 delivered HO-1 to
IL-Iβ-induced NP cells and repressed the nuclear translocation of
p65, resulting in ameliorating NP cell apoptosis and senescence.
The protective effect of Exo-HO-1 may depend on HO-1. Therefore,
the use of BMSCs-derived exosomes as HO-1 carriers may be a
promising approach for IVDD treatment. The present study offers a
promising therapeutic strategy for IVDD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82072496).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HZ, DZ and XZM conceived the study and designed the
experiments. HW, YLL, WYD and GPF contributed to data collection,
performed data analysis and interpreted the results. HZ and DZ
wrote the manuscript. XZM contributed to critical revision of this
article. HW, YLL, WYD and GPF confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohd Isa IL, Teoh SL, Mohd Nor NH and
Mokhtar SA: Discogenic low back Pain: Anatomy, pathophysiology and
treatments of intervertebral disc degeneration. Int J Mol Sci.
24:2082022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
The Lancet Rheumatology, . The global
epidemic of low back pain. Lancet Rheumatol. 5:e3052023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Safiri S, Kolahi AA, Cross M, Hill C,
Smith E, Carson-Chahhoud K, Mansournia MA, Almasi-Hashiani A,
Ashrafi-Asgarabad A, Kaufman J, et al: Prevalence, deaths, and
Disability-adjusted life years due to musculoskeletal disorders for
195 countries and territories 1990–2017. Arthritis Rheumatol.
73:702–714. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohnishi T, Iwasaki N and Sudo H: Causes of
and molecular targets for the treatment of intervertebral disc
degeneration: A review. Cells. 11:3942022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zehra U, Tryfonidou M, Iatridis JC,
Illien-Jünger S, Mwale F and Samartzis D: Mechanisms and clinical
implications of intervertebral disc calcification. Nat Rev
Rheumatol. 18:352–362. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohd Isa IL, Mokhtar SA, Abbah SA, Fauzi
MB, Devitt A and Pandit A: Intervertebral disc degeneration:
Biomaterials and tissue engineering strategies toward precision
medicine. Adv Healthc Mater. 11:e21025302022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munda M and Velnar T: Stem cell therapy
for degenerative disc disease: Bridging the gap between preclinical
promise and clinical potential. Biomol Biomed. 24:210–218. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hajiesmailpoor A, Mohamadi O, Farzanegan
G, Emami P and Ghorbani M: Overview of stem cell therapy in
intervertebral disc disease: Clinical perspective. Curr Stem Cell
Res Ther. 18:595–607. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan X, Yuan W, Ding L, Shi M, Luo L, Wan
Y, Oh J, Zhou Y, Bian L and Deng DY: Cell-adaptable dynamic
hydrogel reinforced with stem cells improves the functional repair
of spinal cord injury by alleviating neuroinflammation.
Biomaterials. 279:1211902021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao Q, Zhao Z, Teng Y, Wu L, Wang J, Xu
H, Chen S and Zhou Q: BMSC-Derived exosomes alleviate
intervertebral disc degeneration by modulating AKT/mTOR-Mediated
autophagy of nucleus pulposus cells. Stem Cells Int.
2022:98964442022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li W, Xu Y and Chen W: Bone mesenchymal
stem cells deliver exogenous lncRNA CAHM via exosomes to regulate
macrophage polarization and ameliorate intervertebral disc
degeneration. Exp Cell Res. 421:1134082022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhujel B, Shin HE, Choi DJ and Han I:
Mesenchymal stem Cell-derived exosomes and intervertebral disc
regeneration: Review. Int J Mol Sci. 23:73062022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DiStefano TJ, Vaso K, Danias G, Chionuma
HN, Weiser JR and Iatridis JC: Extracellular vesicles as an
emerging treatment option for intervertebral disc degeneration:
Therapeutic potential, translational pathways, and regulatory
considerations. Adv Healthc Mater. 11:21005962022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Y, Hu J, Cui C, Peng Z, Yang S, Lei
J, Li B, Yang X, Qin J, Yin M, et al: Netrin1-enriched exosomes
from genetically modified ADSCs as a novel treatment for diabetic
limb ischemia. Adv Healthc Mater. 14:e24035212025. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao B, Zhu Y, Liu M, Chen M, Huang C, Xu
D, Wang F, Sun S, Huang J, Sun N, et al: Correction:
Mir-340-3p-modified bone marrow mesenchymal stem cell-derived
exosomes inhibit ferroptosis through METTL3-mediated m6A
modification of HMOX1 to promote recovery of injured rat uterus.
Stem Cell Res Ther. 15:3572024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gozzelino R, Jeney V and Soares MP:
Mechanisms of cell protection by heme oxygenase-1. Annu Rev
Pharmacol Toxicol. 50:323–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, Zhang Y, Hou M, Liu H, Yang H,
Chen X, Liu T, He F and Zhu X: Melatonin prevents cartilage
degradation in Early-stage osteoarthritis through activation of
miR-146a/NRF2/HO-1 axis. J Bone Miner Res. 37:1056–1072. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng X, Wang S, Sun Z, Dong H, Yu H, Huang
M and Gao X: Ferroptosis enhanced diabetic renal tubular injury via
HIF-1α/HO-1 pathway in db/db mice. Front Endocrinol (Lausanne).
12:6263902021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei R, Zhao Y, Wang J, Yang X, Li S, Wang
Y, Yang X, Fei J, Hao X, Zhao Y, et al: Tagitinin C induces
ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal
cancer cells. Int J Biol Sci. 17:2703–2717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Huang J, Zhang Z, Zhang R, Sun Q,
Zhang Z, Liu Y and Ma B: Mesenchymal stem Cell-derived exosomes
ameliorate delayed neurocognitive recovery in aged mice by
inhibiting hippocampus ferroptosis via activating SIRT1/Nrf2/HO-1
signaling pathway. Oxid Med Cell Longev. 2022:35932942022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo Y, He YZ, Wang YF, Xu YX and Yang L:
Adipose-derived mesenchymal stem cell exosomes ameliorate spinal
cord injury in rats by activating the Nrf2/HO-1 pathway and
regulating microglial polarization. Folia Neuropathol. 61:326–335.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kao YH, Chang CY, Lin YC, Chen PH, Lee PH,
Chang HR, Chang WY, Chang YC, Wun SF and Sun CK: Mesenchymal stem
Cell-derived exosomes mitigate acute murine liver injury via Ets-1
and heme oxygenase-1 Up-regulation. Curr Stem Cell Res Ther.
19:906–918. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bai X, Lian Y, Hu C, Yang S, Pei B, Yao M,
Zhu X, Shang L and Li Z: Cyanidin-3-glucoside protects against high
glucose-induced injury in human nucleus pulposus cells by
regulating the Nrf2/HO-1 signaling. J Appl Toxicol. 42:1137–1145.
2022. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang CY, Hu XC, Zhang GZ, Liu MQ, Chen HW
and Kang XW: Role of Nrf2 and HO-1 in intervertebral disc
degeneration. Connect Tissue Res. 63:559–576. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lei H, He M, He X, Li G, Wang Y, Gao Y,
Yan G, Wang Q, Li T, Liu G, et al: METTL3 induces bone marrow
mesenchymal stem cells osteogenic differentiation and migration
through facilitating M1 macrophage differentiation. Am J Transl
Res. 13:4376–4388. 2021.PubMed/NCBI
|
|
27
|
Wang Y, Hang K, Ying L, Wu J, Wu X, Zhang
W, Li L, Wang Z, Bai J and Gao X: LAMP2A regulates the balance of
mesenchymal stem cell adipo-osteogenesis via the
Wnt/β-catenin/GSK3β signaling pathway. J Mol Med (Berl).
101:783–799. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Q, Fu Y, Cui CP, Ding Y, Deng Z, Ning
C, Hu F, Qiu C, Yu B, Zhou X, et al: OTUB1 promotes osteoblastic
bone formation through stabilizing FGFR2. Signal Transduct Target
Ther. 8:1422023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao J, Ding Y, He R, Huang K, Liu L,
Jiang C, Liu Z, Wang Y, Yan X, Cao F, et al: Dose-effect
relationship and molecular mechanism by which BMSC-derived exosomes
promote peripheral nerve regeneration after crush injury. Stem Cell
Res Ther. 11:3602020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song L, Tang S, Han X, Jiang Z, Dong L,
Liu C, Liang X, Dong J, Qiu C, Wang Y, et al: KIBRA controls
exosome secretion via inhibiting the proteasomal degradation of
Rab27a. Nat Commun. 10:16392019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang YT, Huang YY, Zheng L, Qin SH, Xu XP,
An TX, Xu Y, Wu YS, Hu XM, Ping BH, et al: Comparison of isolation
methods of exosomes and exosomal RNA from cell culture medium and
serum. Int J Mol Med. 40:834–844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Q, Wang H, Zhao X, Han C, Liu C, Li
Z, Du T, Sui Y, Zhang X, Zhang J, et al: Transcriptome sequencing
of circular RNA reveals the involvement of hsa-SCMH1_0001 in the
pathogenesis of Parkinson's disease. CNS Neurosci Ther.
30:e144352024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian Y, Yuan W, Fujita N, Wang J, Wang H,
Shapiro IM and Risbud MV: Inflammatory cytokines associated with
degenerative disc disease control aggrecanase-1 (ADAMTS-4)
expression in nucleus pulposus cells through MAPK and NF-κB. Am J
Pathol. 182:2310–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi C, Wu H, Du D, Im HJ, Zhang Y, Hu B,
Chen H, Wang X, Liu Y, Cao P, et al: Nicotinamide
phosphoribosyltransferase inhibitor APO866 prevents IL-1β-Induced
human nucleus pulposus cell degeneration via autophagy. Cell
Physiol Biochem. 49:2463–2482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li M, Li R, Yang S, Yang D, Gao X, Sun J,
Ding W and Ma L: Exosomes derived from bone marrow mesenchymal stem
cells prevent acidic pH-induced damage in human nucleus pulposus
cells. Med Sci Monit. 26:e9229282020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang C, Lu Z, Lyu C, Zhang S and Wang D:
Andrographolide inhibits static mechanical Pressure-Induced
intervertebral disc degeneration via the MAPK/Nrf2/HO-1 pathway.
Drug Des Devel Ther. 17:535–550. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao B, Cai Y, Wan L, Deng J, Zhao L, Wang
W and Han Z: BACH1 promotes intervertebral disc degeneration by
regulating HMOX1/GPX4 to mediate oxidative stress, ferroptosis, and
lipid metabolism in nucleus pulposus cells. J Gene Med.
25:e34882023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang GZ, Liu MQ, Chen HW, Wu ZL, Gao YC,
Ma ZJ, He XG and Kang XW: NF-κB signalling pathways in nucleus
pulposus cell function and intervertebral disc degeneration. Cell
Prolif. 54:e130572021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu YC, Zhang XB, Lin MQ, Zhou HY, Cong MX,
Chen XY, Zhang RH, Yu DC, Gao XD and Guo TW: Nanoscale treatment of
intervertebral disc degeneration: mesenchymal stem cell exosome
transplantation. Curr Stem Cell Res Ther. 18:163–173. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu G, Lu X, Liu S, Zhang Y, Xu S, Ma X,
Xia X, Lu F, Zou F, Wang H, et al: MSC-Derived exosomes ameliorate
intervertebral disc degeneration by regulating the Keap1/Nrf2 axis.
Stem Cell Rev Rep. 19:2465–2480. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee S, Moon CS, Sul D, Lee J, Bae M, Hong
Y, Lee M, Choi S, Derby R, Kim BJ, et al: Comparison of growth
factor and cytokine expression in patients with degenerated disc
disease and herniated nucleus pulposus. Clin Biochem. 42:1504–1511.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Liu H, Meng Z and Zhang W:
Mechanism of KMT2D-mediated epigenetic modification in
IL-1β-induced nucleus pulposus cell degeneration. Histol
Histopathol. 188132024.PubMed/NCBI
|
|
43
|
Xia J, Jia D and Wu J: Protective effects
of alpinetin against interleukin-1β-exposed nucleus pulposus cells:
Involvement of the TLR4/MyD88 pathway in a cellular model of
intervertebral disc degeneration. Toxicol Appl Pharmacol.
492:1171102024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu Y, Tao R, Wang L, Chen L, Lin Z, Panayi
AC, Xue H, Li H, Xiong L and Liu G: Exosomes derived from bone
mesenchymal stem cells alleviate Compression-Induced nucleus
pulposus cell apoptosis by inhibiting oxidative stress. Oxid Med
Cell Longev. 2021:23100252021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang R, Luo D, Li Z and Han H: Dimethyl
fumarate ameliorates nucleus pulposus cell dysfunction through
activating the Nrf2/HO-1 pathway in intervertebral disc
degeneration. Comput Math Methods Med. 2021:60217632021.PubMed/NCBI
|
|
46
|
Zhu C, Jiang W, Cheng Q, Hu Z and Hao J:
Hemeoxygenase-1 suppresses IL-1β-induced apoptosis through the
NF-κB pathway in human degenerative nucleus pulposus cells. Cell
Physiol Biochem. 46:644–653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zou L, Lei H, Shen J, Liu X, Zhang X, Wu
L, Hao J, Jiang W and Hu Z: HO-1 induced autophagy protects against
IL-1 β-mediated apoptosis in human nucleus pulposus cells by
inhibiting NF-κB. Aging (Albany NY). 12:2440–2452. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bacher S, Meier-Soelch J, Kracht M and
Schmitz ML: Regulation of transcription factor NF-κB in its natural
habitat: The nucleus. Cells. 10:7532021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liao Y, Tan RZ, Li JC, Liu TT, Zhong X,
Yan Y, Yang JK, Lin X, Fan JM and Wang L: Isoliquiritigenin
attenuates UUO-Induced renal inflammation and fibrosis by
inhibiting Mincle/Syk/NF-Kappa B signaling pathway. Drug Des Devel
Ther. 14:1455–1468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhongyi S, Sai Z, Chao L and Jiwei T:
Effects of nuclear factor kappa B signaling pathway in human
intervertebral disc degeneration. Spine (Phila Pa 1976). 40:224–32.
2015. View Article : Google Scholar : PubMed/NCBI
|