Introduction
Vitiligo is a common pigmentary disorder
characterized by acquired localized or generalized skin
depigmentation due to melanocyte dysfunction or loss (1). It presents a notable treatment
challenge due to its propensity for relapse and resistance to
treatment, and can be a mental and financial burden on patients and
their families (2). The worldwide
prevalence of vitiligo is estimated to be ~2% (3), with an increasing incidence in recent
years, which has been suggested to be due to a fast-paced lifestyle
and heightened psychological stress (4,5).
Vitiligo can affect any area of the body, particularly exposed
areas, and may lead to feelings of shame, anxiety and depression,
with an impact on quality of life comparable to that of conditions
such as psoriasis and eczema (2,6).
Current western medicine therapies for vitiligo include oral or
topical glucocorticoids, vitamin D3 analogs, immunosuppressants,
surgical interventions and antioxidants (7). While these treatments demonstrate
notable short-term efficacy, their long-term effectiveness is
limited, often resulting in relapses and adverse reactions
(8). This highlights the potential
for research and development in traditional Chinese medicine, which
is valued for its safety, affordability and minimal toxicity
(9).
The etiology of vitiligo is unclear, with heredity,
autoimmunity and environmental factors recognized as major
contributors (10). Clinically,
vitiligo manifests as depigmented patches on the skin or mucous
membranes, with melanocytes as the primary targets of pathogenic
mechanisms (11). Emerging
evidence implicates oxidative stress in melanocyte depletion and
dysfunction (7), suggesting that
antioxidants from traditional Chinese medicine may aid in the
management of vitiligo.
Purslane, known scientifically as Portulaca
oleracea (L.), is an annual succulent herb, and its dried
aerial parts are frequently used in the management of various
dermatological conditions, including vitiligo, psoriasis,
dermatitis and pruritus (12,13).
The plant name has been verified using the World Flora Online
database (www.worldfloraonline.org). Purslane has a rich
historical background in the treatment of skin disorders, and is
mentioned in ancient Chinese herbal texts such as the Compendium of
Materia Medica, New Revision of Materia Medica, Dietary Materia
Medica and Southern Yunnan Materia Medica. Pharmacological studies
have identified numerous bioactive constituents in purslane, such
as alkaloids, flavonoids, catecholamines and polysaccharides, that
exhibit diverse pharmacological properties, including
anti-inflammatory, antioxidant, analgesic, antibacterial and
immunomodulatory effects (12,14).
Despite the extensive use of purslane in the
management of vitiligo, the pharmacological mechanisms underlying
its efficacy remain inadequately elucidated. Therefore, the present
study utilized network pharmacology, molecular docking and in
vitro experiments to comprehensively analyze the key active
compounds of purslane, their targets and associated pathways in the
context of vitiligo treatment. The present study aims to establish
a theoretical basis for understanding the therapeutic mechanisms of
purslane in the treatment of vitiligo, and to provide new
perspectives for clinical research into this natural product.
Materials and methods
Network pharmacology
Collection and screening of active ingredients of
purslane
The Traditional Chinese Medicine Systems
Pharmacology Database (TCMSP; http://tcmspw.com/tcmsp.php/) was utilized to compile
all bioactive compounds of purslane (15). Active ingredients meeting the
criteria of oral bioavailability ≥30%, drug-likeness ≥0.1,
molecular weight ≤500, hydrogen bond donors ≤5, hydrogen bond
acceptors ≤10 and fat-water partition coefficient ≤5 were
identified as the principal bioactive constituents of purslane
(16).
Screening of potential targets for the
main active components of purslane
The primary bioactive compounds identified from the
TCMSP database were cross-referenced with the PubChem database
(https://pubchem.ncbi.nlm.nih.gov) to
identify known protein targets and map protein names to their
respective gene names (17). In
addition, the Swiss Target Prediction database (http://www.swisstargetprediction.ch/)
was used to predict potential targets of the main bioactive
constituents, with P>0 for target prediction (18). The potential targets obtained from
the PubChem and Swiss Target Prediction databases were integrated,
de-duplicated and regarded as the potential targets of the primary
bioactive compounds of purslane.
Screening of potential targets for
vitiligo
The GeneCards database (https://www.genecards.org/) (19) and DisGeNET database (https://www.disgenet.org/) (20) were searched using the keyword
‘vitiligo’ to identify disease-related targets. The targets
identified from the two databases were consolidated to obtain the
potential targets associated with vitiligo.
Screening of potential targets of
purslane in the treatment of vitiligo
The potential targets of the primary bioactive
compounds of purslane were compared with the potential targets
associated with vitiligo and entered into the Microscopic Letter
platform (http://www.bioinformatics.com.cn/) for analysis. An
intersection map was created to identify the potential targets of
purslane for the management of vitiligo (21).
Construction of a purslane active
ingredient-target network
The bioactive constituents of purslane and their
potential targets for the treatment of vitiligo were imported into
Cytoscape version 3.7.2 software (22) to generate a drug
component-potential target network for purslane in the context of
vitiligo treatment. In this network, each node represents an active
ingredient or a potential target, while each edge denotes the
interaction between the bioactive compound and the potential
target. Subsequently, the Cytoscape plug-in CytoNCA version 2.1.6
was used to analyze the topological properties of the network
(23). Metrics such as betweenness
centrality (BC), closeness centrality (CC), and degree centrality
(DC) were calculated to identify the primary bioactive compounds of
purslane that may be useful for vitiligo treatment. In network
pharmacology, the calculation of BC, CC and DC values is primarily
employed to identify key nodes within the network, assess the
efficiency of information transfer among nodes and uncover the
network's core regulatory mechanisms. This analytical approach
facilitates drug target prediction, enhances the design of
multi-target drugs and improves the overall comprehensiveness of
network analysis, thereby providing a robust theoretical foundation
for drug discovery and disease treatment (23).
Construction of a protein-protein
interaction (PPI) network
The potential targets of purslane for the treatment
of vitiligo were uploaded to the STRING database (https://string-db.org) (24) to generate a PPI network. The
analysis was performed by choosing ‘Multiple proteins’ to integrate
the overlapping genes of purslane and vitiligo, designating
‘Homo sapiens’ as the organism, configuring the ‘Minimum
required interaction score’ to medium confidence (0.400), and
maintaining default settings for other parameter options to produce
the PPI network. The resulting data were imported into Cytoscape
version 3.7.2 software for visualization of the PPI network.
Subsequently, the topological properties of the network were
analyzed using the Cytohubba 0.1 plug-in in Cytoscape. Key metrics,
including Degree, Edge Percolated Component (EPC), Maximum
Neighborhood Component (MNC), Maximal Clique Centrality (MCC),
Betweenness and Closeness, were calculated to identify potential
key targets of purslane for the treatment of vitiligo (25).
Gene Ontology (GO) enrichment analysis
and Kyoto Genes and Genomes (KEGG) pathway enrichment analysis of
potential targets
The potential target genes associated with the
purslane treatment of vitiligo were analyzed for GO enrichment and
KEGG pathway enrichment using the DAVID database (https://david.ncifcrf.gov) (26). The analysis was performed by
inputting the overlapping gene list between purslane and vitiligo,
using ‘OFFICIAL_GENE_SYMBOL’ as the identifier, ‘Homo
sapiens’ as the species and ‘Gene list’ as the list type, and
submitting the list for annotation. P<0.002 was applied as the
cutoff for GO annotation and KEGG pathway enrichment analysis
(27).
Molecular docking
Molecular docking of the key constituents of
purslane with the key targets was performed. The 3-dimensional (3D)
crystal structures of the target proteins were obtained from the
RCSB Protein Data Bank (PDB) database (https://www.rcsb.org) to act as the receptors. Using
PyMol version 2.2.0 software, the target proteins were dehydrated,
non-relevant ligands were removed, and the results were saved in
standard PDB format (28). The 3D
structures of the compounds were acquired from the TCMSP database
and saved in mol2 format. OpenBabel version 2.4.1 (http://openbabel.org) was used to convert the 3D
structure file format to a PDB format file (29).
AutoDockTools version 1.5.7 was used to add hydrogen
atoms to the target proteins and calculate their charges. Then
AutoDockTools version 1.5.7 Vina software was applied to dock the
receptor protein with the ligand and calculate the binding energy
(30). Following this, PyMol
version 2.2.0 was used to visualize the docking outcomes.
Experimental verification
B16F10 cell culture
B16F10 mouse melanoma cells (cat. no. CX0109) were
acquired from Wuhan Boster Biological Technology, Ltd. The cells
were verified to be free of Mycoplasma contamination. In
addition, the B16F10 cells were identified by STR analysis as a
subline of B16-F0 cells. The cells were cultured in RPMI-1640
complete medium (cat. no. C11875500BT; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (cat. no.
FSP500; Shanghai ExCell Biology, Inc.) in a CO2
incubator at 37°C with 5% CO2. The culture medium was
replenished every 2 days (31).
Purslane total flavonoids (PTF) dosage
screening
B16F10 cells in the logarithmic growth phase were
plated in 96-well plates at a density of 5×103 cells per
well. Subsequently, 100 µl PTF medium containing different
concentrations of PTF (0, 10, 20, 40, 80, 160, 320, 640 and 1,280
µg/ml) was added to each well. The medium was prepared by
dissolving PTF (purity ≥80%; MF2023115; Mufan Biotechnology Co.,
Ltd.) in dimethyl sulfoxide (DMSO; Beijing Solarbio Science &
Technology Co., Ltd.) and diluting the solution to the desired
concentration in the medium before use, ensuring the final
concentration of DMSO was <0.2%. Each group comprised 6
replicate wells and cultured in an incubator at 37°C with 5%
CO2 for 24 h. After incubation, 10% Cell Counting Kit-8
(CCK-8) reagent [cat. no. C6005; UElandy (Suzhou) Co., Ltd.] was
added to each well. Following incubation for 1–2 h, the absorbance
(A) value at 450 nm was measured using a Synergy 2 microplate
reader (BioTek; Agilent Technologies, Inc.). Subsequently, the cell
viability was calculated using the following formula: Cell
viability
(%)=(Acontrol-Ablank)/(Adrug-Ablank)
(32).
Evaluation of
1,1-diphenyl-2-trinitrophenylhydrazide (DPPH) free radical
scavenging ability
PTF solution of various concentrations (10, 20, 40,
80, 160, 320, 640 and 1,280 µg/ml) was mixed with 1.0 ml of 200 µM
DPPH (cat. no. D273092; Shanghai Aladdin Biochemical Technology
Co., Ltd.) in ethanol. The ethanolic DPPH solution was used as a
control, and the reaction between PTF and DPPH was carried out at
37°C for 60 min, followed by colorimetric measurement at a
wavelength of 517 nm. The DPPH radical scavenging activity was
calculated using the following formula: DPPH
(%)=1-[(Ablank-Asample)/Ablank]
×100 (33).
Effect of PTF on oxidative stress
injury in B16F10 melanocytes
Cells in the logarithmic growth phase were harvested
and counted after digestion with 0.25% trypsin solution (C25200056;
Gibco; Thermo Fisher Scientific, Inc.) and the cells were
centrifuged at 400 × g for 5 min using a high-speed refrigerated
centrifuge (D3024R; Dalong Xingchuang Experimental Instrument
(Beijing) Co., Ltd,). The cell concentration was adjusted to
5×103 cells/100 µl, and 100 µl cell suspension was
seeded into each well of a 96-well plate. The cells were then
cultured for 24 h at 37°C in a 5% CO2 incubator, after
which the culture medium was removed. The control group was
incubated with RPMI-1640 medium alone, while the drug intervention
groups were exposed to RPMI-1640 medium supplemented with varying
concentrations of PTF (40, 80, 160, 320 and 640 µg/ml) cultured in
an incubator at 37°C with 5% CO2 for 2, 4 and 6 h.
Following this, 500 µM hydrogen peroxide
(H2O2; cat. no. 20230726; Tianjin Damao
Chemical Reagent Co., Ltd.) was added to each well and the plate
was incubated cultured in an incubator at 37°C with 5%
CO2 for an additional 24 h (34). Subsequently, 10% CCK-8 reagent was
added to each well to assess cell viability using the
aforementioned protocol.
Detection of oxidative stress levels
in cells
B16F10 cells were plated in 12-well plates at a
density of 5×105 cells per well. The cells were treated
with PTF (40, 80 and 160 µg/ml) or extract of Ginkgo biloba
(EGB; 10:1 purification, water extract; cat. no. G195711-25g;
Shanghai Aladdin Biochemical Technology Co., Ltd.) for 2 h,
followed by 500 µM hydrogen peroxide for 24 h as described above.
In addition, in certain wells, the B16F10 cells were pretreated
with the 20 µmol/l PI3K inhibitor LY294002 for 2 h (cat. no.
S1737-1mg; Beyotime Institute of Biotechnology) prior to the 160
µg/ml PTF and H2O2 treatments. The medium was
aspirated, and the DCFH-DA probe from a Reactive Oxygen Species
(ROS) Detection kit (cat. no. S0033S; Beyotime Institute of
Biotechnology) was applied at 1:1,000 dilution. The cells were then
incubated with the probe for 20 min under standard culture
conditions (37°C, 5% CO2), rinsed with PBS and
visualized using a 488-nm excitation long-wave microscope (35). The superoxide dismutase (SOD) and
catalase (CAT) activity and melanin content in the cells were
measured using specific kits, and the results were determined using
a microplate reader. The kits were as follows: Total SOD Activity
Detection Kit, WST-8 method (cat. no. S0101S; Beyotime Institute of
Biotechnology), Catalase Detection Kit (cat. no. S0051; Beyotime
Institute of Biotechnology) and Mouse Melanin (MC) ELISA Research
Kit (cat. no. MM-0870M2; Shanghai Enzyme-linked Biotechnology Co.,
Ltd.) (36).
Western blotting
B16F10 cells from each experimental group were
harvested, homogenized with RIPA Lysis and Extraction Buffer (cat.
no. 89900; Thermo Fisher Scientific, Inc.) with
phenylmethylsulfonyl fluoride (100 mM; cat. no. P0100-1ml; Beijing
Solarbio Science & Technology Co., Ltd.), and then subjected to
lysis on ice for 30 min. The supernatant was collected for
subsequent analysis. The protein concentration was quantified using
a BCA Protein Concentration Assay Kit (500X dilution; cat. no.
P0012, Beyotime Institute of Biotechnology). After mixing with
buffer, protein denaturation was carried out in a water bath at
100°C for 10 min. The precast gel (4–12% Bis-Tris prefabricated
glue; cat. no. WBT41212BOX; Thermo Fisher Scientific, Inc.) was
secured in an electrophoresis chamber, and electrophoresis buffer
was added to the reservoir. The protein samples and marker (20–120
kDa marker, cat. no. M00521, GenScript Biotech Corporation; 10–250
kDa marker, cat. no. MF028-plus-01, Mei5 Biotechnology Co., Ltd.)
were loaded into the wells at a total protein content of 40
µg/lane. The gel was subjected to electrophoresis at a constant
voltage of 180 V until the bromophenol blue dye front reached the
bottom of the gel, typically 40 min. Subsequently, the cells were
transferred to a PVDF membrane (cat. no. ISEQ15150; Merck KGaA),
which was then blocked with 5% skimmed milk powder for 2 h at room
temperature. After washing with Tris-buffered saline with 0.1%
Tween 20, the PVDF membranes were incubated with primary antibodies
targeting β-actin (1:30,000; cat. no. 66009-1-Ig; Wuhan Sanying
Biotechnology), GAPDH (1:30,000; cat. no. 60004-1-Ig; Wuhan Sanying
Biotechnology), AKT (1:2,000; cat. no. 10176-2-AP; Wuhan Sanying
Biotechnology), phosphorylated (p-)AKT (1:1,000; cat. no. 4060;
Cell Signaling Technology, Inc.), Bax (1:8,000; cat. no,
50599-2-Ig; Wuhan Sanying Biotechnology) and Bcl-2 (1:2,000; cat.
no, 26593-1-AP; Wuhan Sanying Biotechnology) overnight at 4°C. The
following day, HRP was used to label sheep anti-rabbit or sheep
anti-mouse secondary antibody (1:10,000; cat. nos. BA1054 or
BA1051; Wuhan Boster Biological Technology, Ltd.) was applied and
the membrane was incubated for 2 h at room temperature on a shaker.
ECL reagent (cat. no. KF8003, Affinity Biosciences) was then used
to treat the PVDF membrane, which was subjected to automatic
exposure. If the bands were not clearly visible or did not appear,
manual exposure was performed using chemiluminescence imaging
system for color development (model SH-523; Hangzhou Shenhua
Technology Co., Ltd.). Image J software (version 1.8.0, National
Institutes of Health) was used to analyze the gray values of
protein bands.
Immunofluorescence detection
B16F10 cells were cultured in 24-well plates. After
drug treatment, the culture medium was removed, and the cells were
fixed with 4% paraformaldehyde at 25°C for 30 min and permeabilized
with 5% Triton X-100 (cat. no. ST795; Beyotime Institute of
Biotechnology). The cells were subsequently incubated at 37°C for
30 min and blocked with 10% goat serum at room temperature for 1 h
(cat. no. AR1009; Boster Biological Technology). Primary antibodies
against melan-A (1:100; cat no. SC56-02; HUABIO) were added and the
samples were incubated overnight at 4°C. Melan-A is the most used
indicator to detect the presence and delineate the distribution of
melanocytes in skin pathology (37). The next day, sheep anti-rabbit
secondary antibody (BA1032; Boster Biological Technology Co. Ltd.)
was applied in the dark. Following 1 h incubation at room
temperature, the cells were stained with
4′,6-diamidino-2′-phenylindole (C1002; Beyotime Institute of
Biotechnology) in the dark for 5 min to visualize the nuclei. The
stained samples were then observed under a fluorescence
microscope.
Statistical analysis
Statistical analysis and graphical representation
were performed using GraphPad Prism 9.0 software (Dotmatics). Data
are presented as the mean ± standard error of the mean. All
experiments were performed in triplicate at least to ensure the
reproducibility of the experimental results. Statistical analysis
was performed using one-way analysis of variance followed by
Dunnett's or Tukey's post hoc tests to assess differences among
multiple groups. When Tukey's test was performed, only some
significant differences are shown for clarity. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Major active components and targets of
purslane
In total, 54 chemical components of purslane were
extracted from the TCMSP database. Following screening, 10
candidate compounds were identified as the principal active
constituents. These were kaempferol, hesperetin, luteolin,
isobetanidin, isobetanin_qt, quercetin, arachidonic acid,
cycloartenol, β-carotene and β-sitosterol (Table I).
 | Table I.Main active components of
purslane. |
Table I.
Main active components of
purslane.
| Compound | Molecular
weight | Drug likeness | Oral
bioavailability, % | MolID |
|---|
| Kaempferol | 286.25 | 0.24 | 41.88 | MOL000422 |
| Hesperetin | 302.3 | 0.27 | 47.74 | MOL005100 |
| Luteolin | 286.25 | 0.25 | 36.16 | MOL000006 |
| Isobetanidin | 388.36 | 0.52 | 59.73 | MOL006657 |
| Isobetanin_qt | 388.36 | 0.52 | 30.16 | MOL006662 |
| Quercetin | 302.25 | 0.28 | 46.43 | MOL000098 |
| Arachidonic
acid | 304.52 | 0.2 | 45.57 | MOL001439 |
| Cycloartenol | 426.8 | 0.78 | 38.69 | MOL003578 |
| β-carotene | 536.96 | 0.58 | 37.18 | MOL002773 |
| β-sitosterol | 414.79 | 0.75 | 36.91 | MOL000358 |
Analysis using the Swiss Target Prediction database
platform led to the identification of a total of 563 targets with
P>0. Among these, 92 targets were associated with hesperetin,
100 with arachidonic acid, 44 with β-sitosterol, 1 with β-carotene,
25 with cycloartenol, 2 with isobetanidin, 12 with kaempferol, 100
with luteolin and 100 with quercetin. Through the integration of
all targets obtained from the database screening process and the
elimination of duplicate or invalid targets, a total of 255 targets
associated with the primary active components of purslane were
obtained (Table SI).
Main component-target gene-signaling
pathway network of purslane for vitiligo treatment
A total of 1,736 vitiligo-related targets were
identified through searches in the DisGeNet and GeneCards
databases. Following the elimination of duplicate targets, 1,487
unique vitiligo targets were obtained. By intersecting the targets
of the active components in purslane with the known disease-related
targets of vitiligo, 54 common targets were identified as potential
targets for purslane in the treatment of vitiligo (Fig. 1A). Subsequently, a network
illustrating the relationships between the active components of
purslane and the associated target genes for the treatment of
vitiligo was constructed, comprising 54 potential targets and seven
active components (Fig. 1B). This
network comprised 64 nodes and 137 edges, with an average BC of
118.59, CC of 0.355 and DC of 4.28. Notably, the seven active
components kaempferol, hesperetin, luteolin, quercetin, arachidonic
acid, cycloartenol and b-sitosterol had BC, CC and DC values
exceeding the network average (Fig.
1C-E), suggesting their potential as key active components of
purslane in the treatment of vitiligo and offering valuable
insights for future studies. Moreover, a PPI network was generated,
comprising 49 nodes and 230 edges. Cytohubba analysis highlighted
six key targets, namely AKT1, tumor protein p53 (TP53), peroxisome
proliferator-activated receptor γ (PPARG), estrogen receptor 1
(ESR1), prostaglandin-endoperoxidase synthase 2 (PTGS2) and
mitogen-activated protein kinase 1 (MAPK1), which are suggested to
be important as potential targets of purslane in the treatment of
vitiligo (Fig. 1F-I).
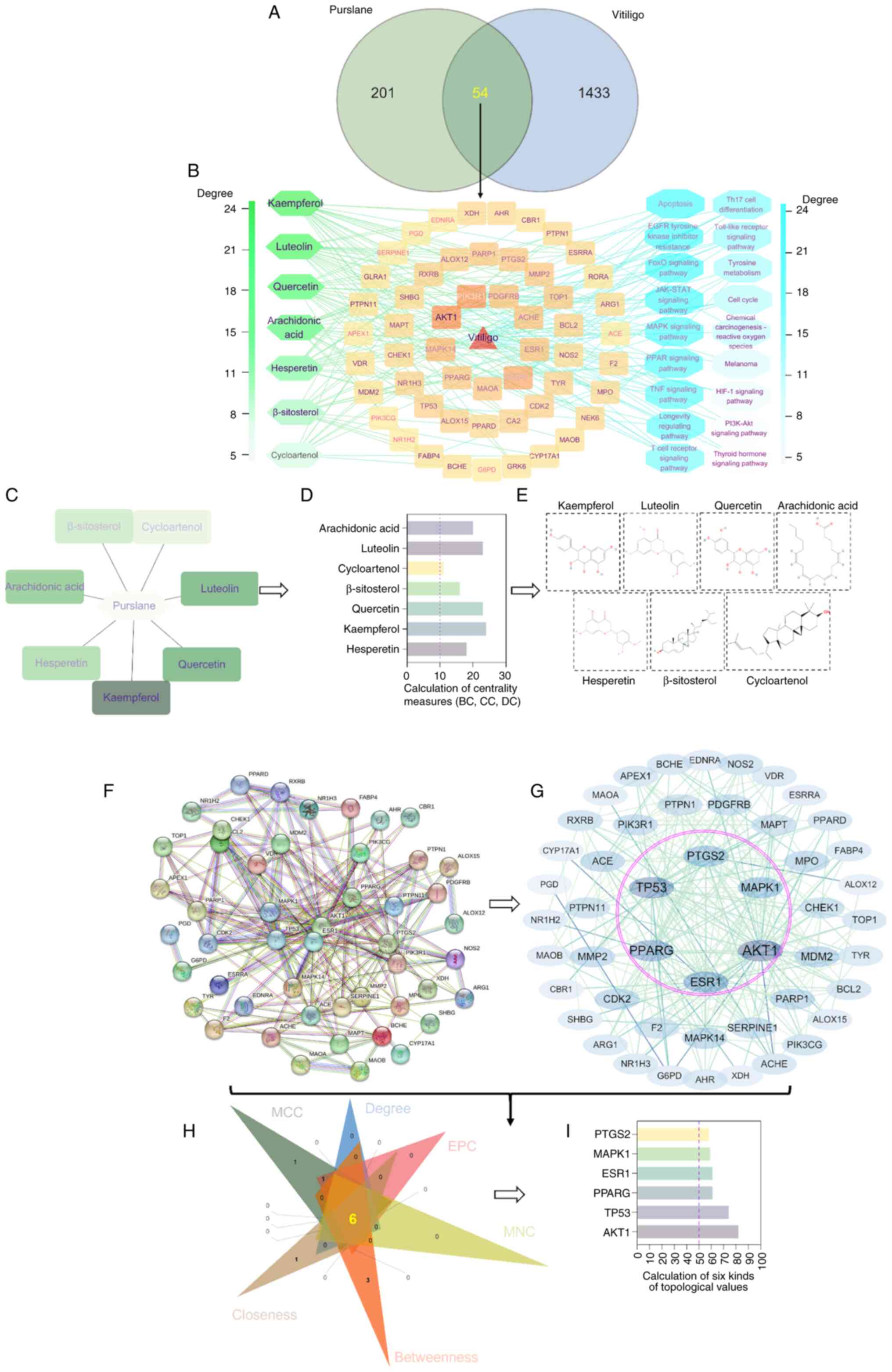 | Figure 1.Pharmacological screening of the
target network associated with the use of purslane for vitiligo
treatment. (A) Venn diagram illustrating intersecting targets
associated with purslane and vitiligo. (B) Network diagram of
purslane active components, their gene targets and target pathways.
(C) Core active components of purslane. (D) Utilization of the
CytoNCA plugin in Cytoscape to select and quantify the core
ingredients of purslane. (E) Chemical structures of the active
components of purslane. Screening of the key targets of purslane in
vitiligo treatment was performed. (F) Protein-protein interaction
network comprising key targets of purslane in vitiligo treatment
and (G) Cytohubba analysis. Nodes represent targets, and edges
represent interactions between targets. Nodes with larger font and
darker color indicate higher degree values, signifying a greater
impact on vitiligo. (H) Six algorithms, namely degree, MCC, EPC,
MNC, betweenness and closeness, were utilized to identify core
targets. (I) Quantification of the core targets of purslane in the
treatment of vitiligo. MCC, maximal clique centrality; EPC, edge
percolated component; MNC, maximum neighborhood component; PTGS2,
prostaglandin-endoperoxidase synthase 2; MAPK1, mitogen-activated
protein kinase 1; ESR1, estrogen receptor 1; PPARG, peroxisome
proliferator-activated receptor γ; TP53, tumor protein p53. |
GO functional enrichment analysis indicated that the
use of purslane in vitiligo treatment may potentially influence
‘DNA binding’ and various cellular functions. This is potentially
mediated via the effects of arachidonic acid, kaempferol, luteolin
and quercetin on key genes, namely AKT1, ESR1, PPARG, PTGS2, MAPK1
and TP53, by modulating the processes ‘cellular response to
hypoxia’, ‘enzyme binding’ and ‘zinc ion binding’, by affecting the
cellular components ‘cytosol’ and ‘nucleus’, as well as ‘response
to xenobiotic stimulus’ (Fig.
2A).
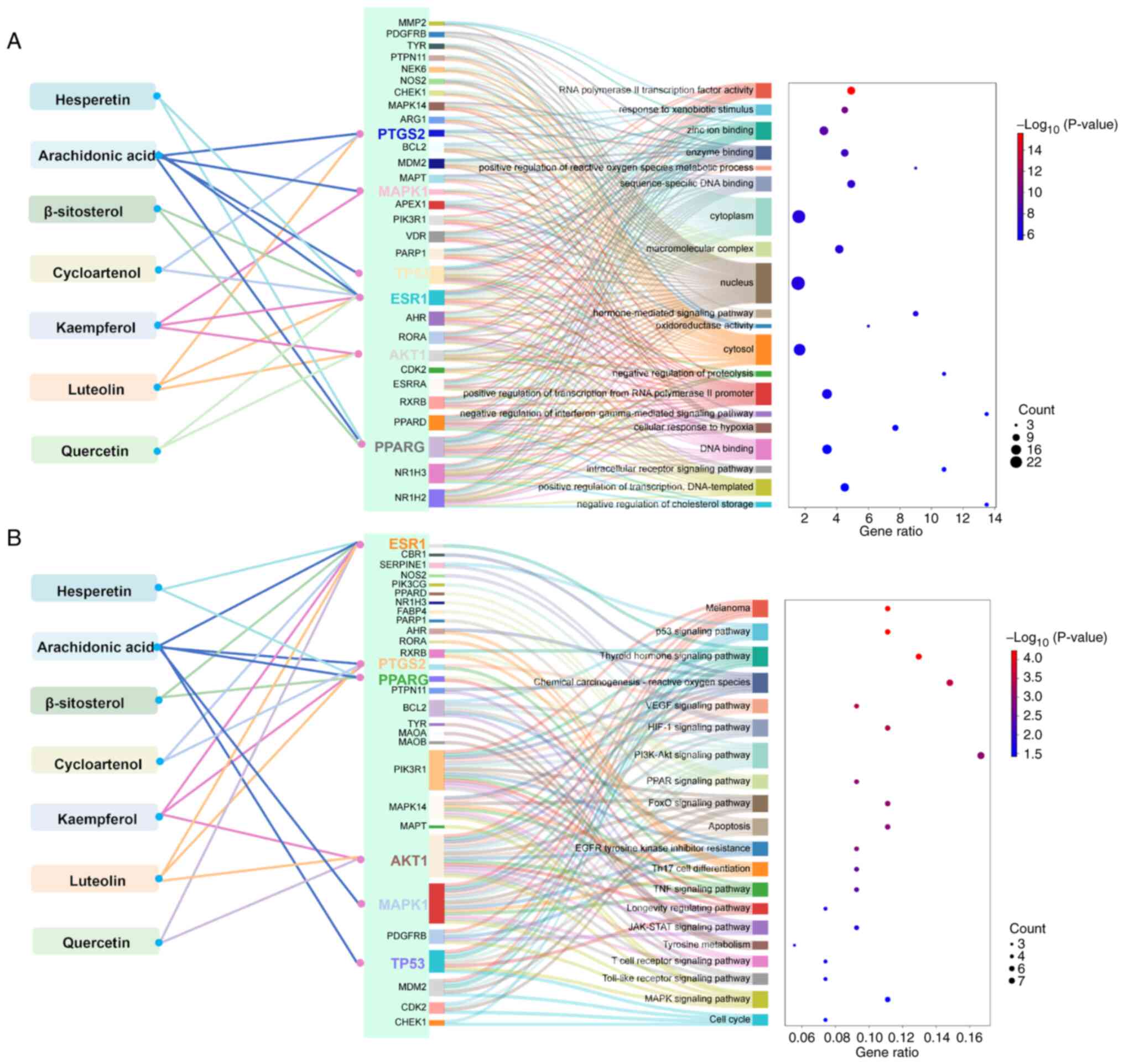 | Figure 2.Target enrichment analysis of
purslane in vitiligo treatment. (A) GO enrichment analysis of core
targets of purslane components in vitiligo treatment. Core
components are on the left, core targets in the middle and GO terms
on the right. (B) KEGG enrichment analysis of core targets of
purslane components in vitiligo treatment: Core components are on
the left, core targets in the middle and KEGG pathways on the
right. In both panels, red and green colors indicate distinct
P-value thresholds, and dot size reflects the number of genes
associated with each term. GO, Gene Ontology; KEGG, Kyoto
Encyclopedia of Genes and Genomes; PTGS2,
prostaglandin-endoperoxidase synthase 2; MAPK1, mitogen-activated
protein kinase 1; ESR1, estrogen receptor 1; PPARG, peroxisome
proliferator-activated receptor γ; TP53, tumor protein p53. |
KEGG pathway enrichment analysis revealed that the
targeting of ESR1 and PPARG by hesperetin could potentially
influence the ‘thyroid hormone signaling pathway’, ‘PPAR signaling
pathway’ and ‘longevity regulating pathway’ in the context of
vitiligo treatment. Additionally, arachidonic acid was suggested to
impact ‘melanoma’ and ‘VEGF signaling pathway’ by targeting ESR1,
PTGS2, PPARG, MAPK1 and TP53. Furthermore, kaempferol and luteolin
were indicated to act on ESR1, PTGS2 and AKT targets, demonstrating
therapeutic effects on vitiligo through modulation of the ‘PI3K-Akt
signaling pathway’, ‘p53 signaling pathway’ and ‘PPAR signaling
pathway’ (Fig. 2B).
Overall, the screening process led to the
identification of 7 principal components, namely kaempferol,
hesperetin, luteolin, quercetin, arachidonic acid, cycloartenol and
β-sitosterol, from 54 potential ingredients, in addition to 6 key
targets, namely AKT1, TP53, PPARG, ESR1, PTGS2 and MAPK1, from 54
potential targets, and 8 pathways from 20 potential pathway for
purslane-based vitiligo treatment.
Molecular docking of purslane core
components with core targets
Utilizing the findings from the PPI network and the
purslane component-target gene network analyses, AKT1 (7nh5), TP53
(1ycq), PPARG (1fm6), ESR1 (1hcq), PTGS2 (P35354) and MAPK1
(P28482) were selected as candidate macromolecules for further
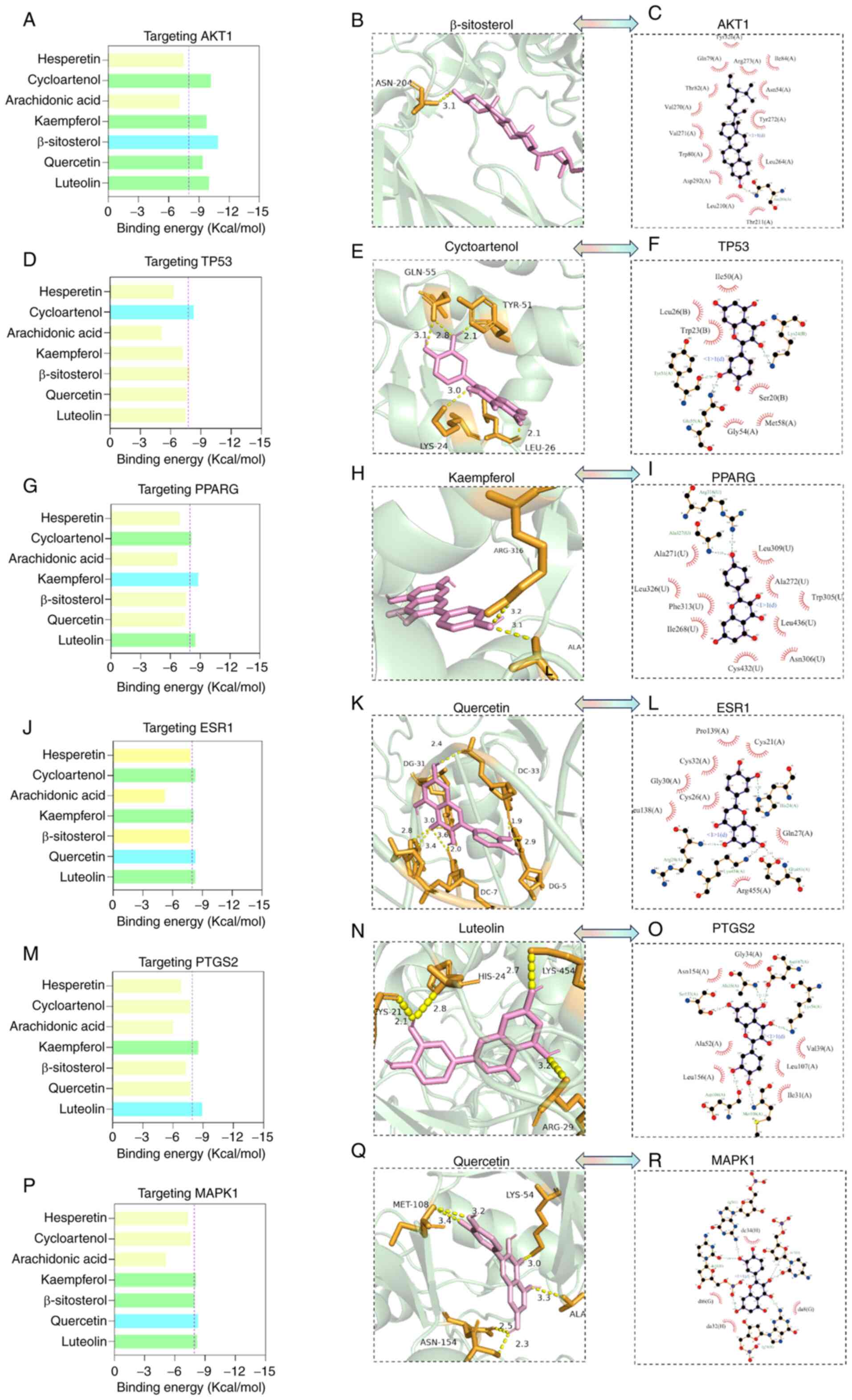
investigation. The binding energy of these proteins with the
corresponding active compounds was evaluated. The analysis
suggested that b-sitosterol could form a hydrogen bond with the
Asn-204 residue of AKT1 with a binding energy of −10.9 kcal/mol
(Fig. 3A and B). In addition, it
suggested that cycloartenol could form hydrogen bonds with the
Gln-55, Tyr-51, Lys-24 and Leu-26 residues of TP53 with a binding
energy of −7.9 kcal/mol (Fig. 3D and
E); kaempferol could form hydrogen bonds with the Arg-316 and
Ala-327 residues in PPARG with a binding energy of −8.8 kcal/mol
(Fig. 3G and H); and luteolin
could form hydrogen bonds with the Cys-21, His-24, Lys-454 and
Arg-29 residues of PTGS2 with a binding energy of −8.5 kcal/mol
(Fig. 3M and N). In addition,
quercetin was predicted to form hydrogen bonds with the
diacylglycerol (DG)-31, dicarboxylic acid (DC)-7, DG-5 and DC-33
residues of ESR1 with a binding energy of −8.3 kcal/mol (Fig. 3J and K), and with the Met-108,
Lys-54, Ala-35 and Asn-154 residues of MAPK1 with a binding energy
of −7.8 kcal/mol (Fig. 3P and Q).
Hydrogen bonding and hydrophobic interactions between the purslane
core components and the selected targets are presented in (Fig. 3C, F, I, L, O and R). These findings
suggest that flavonoids are the predominant components of purslane
that interact with vitiligo-associated targets.
Total flavonoids of purslane alleviate
oxidative stress impairment in B16F10 cells
To investigate the protective effects of PTF against
oxidative stress, in vitro experiments were conducted.
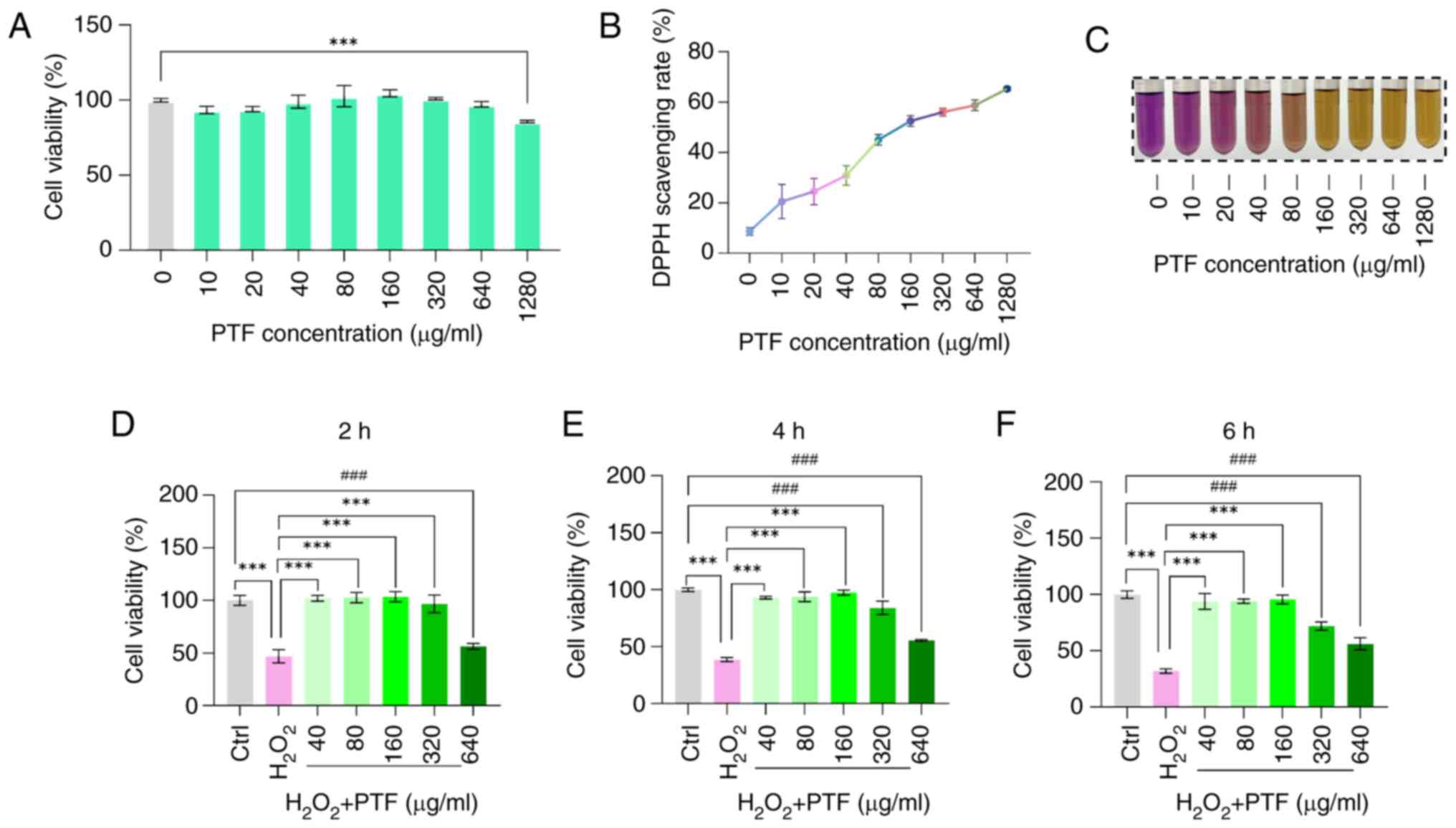
Initially, a CCK-8 assay was utilized to determine the optimal
concentration of PTF. Results indicated that interventions of
10–640 µg/ml had no significant impact on B16F10 cell viability.
However, an increase in concentration to 1,280 µg/ml resulted in a
significant reduction in B16F10 cell viability and notable
cytotoxicity (Fig. 4A).
The DPPH assay was then employed to evaluate the
antioxidant effect of PTF. This assay assesses the ability of
antioxidants to scavenge DPPH free radicals by monitoring changes
in absorbance. The results revealed that antioxidant effect
increased as the PTF concentration increased (Fig. 4B and C).
To investigate the antioxidative effect of PTF on
H2O2-induced oxidative stress in B16F10
cells, the cells were treated with H2O2 (500
µM) to establish an oxidative stress model. The cells were
pretreated with various concentrations of PTF (40, 80, 160, 320 and
640 µg/ml) prior to treatment with H2O2 for
24 h. Results revealed that following H2O2
treatment, the viability of the B16F10 cells significantly
decreased compared with that of the control cells. However,
preincubation with PTF at concentrations of 40, 80 and 160 µg/ml
for 2, 4 and 6 h significantly increased cell viability compared
with that of the model group. By contrast, the cell survival rate
decreased significantly in cells preincubated with 320 and 640
µg/ml PTF for 4 and 6 h compared with that in the control group
(Fig. 4D-F). Consequently, PTF
concentrations of 40, 80 and 160 µg/ml were selected for use in
subsequent experiments, in which they were referred to as the low,
medium and high PTF, respectively.
To examine the protective effect of PTF against
H2O2-induced oxidative damage, the B16F10
cells were incubated with various concentrations of PTF or 100 µg
EBG for 2 h prior to treatment with 500 µM
H2O2 for 24 h, and melan-A fluorescence
levels, melanin content, SOD and CAT activity and ROS levels were
then measured. The results demonstrate that the melan-A
fluorescence intensity and melanin content were significantly
reduced in the H2O2 model group compared with
that in the control group. Conversely, the melan-A fluorescence
intensity and melanin content were significantly elevated in the
EGB and various PTF dose groups compared with those in the model
group (Fig. 5A, C and D). The ROS
level in the H2O2 model group was
significantly elevated compared with that in the control group,
whereas the CAT and SOD levels were significantly reduced.
Following intervention with different doses of PTF and EGB, the ROS
levels were significantly decreased compared with those in the
H2O2 model group, with no significant
difference observed among the low, medium and high PTF
concentration groups. In addition, significant increases in the
activity levels of CAT in the medium and high PTF and EGB groups,
and of SOD in all PTF groups were observed compared with those in
the model group. Furthermore, as the PTF concentration increased,
the activity levels of CAT and SOD also increased. While ROS levels
decreased and CAT activity levels increased in the EGB intervention
group compared with those in the model group, the difference in SOD
activity between these two groups was not statistically significant
(Fig. 5B and E-G). These results
suggest that flavonoids from purslane have the potential to
ameliorate oxidative stress damage in vitiligo.
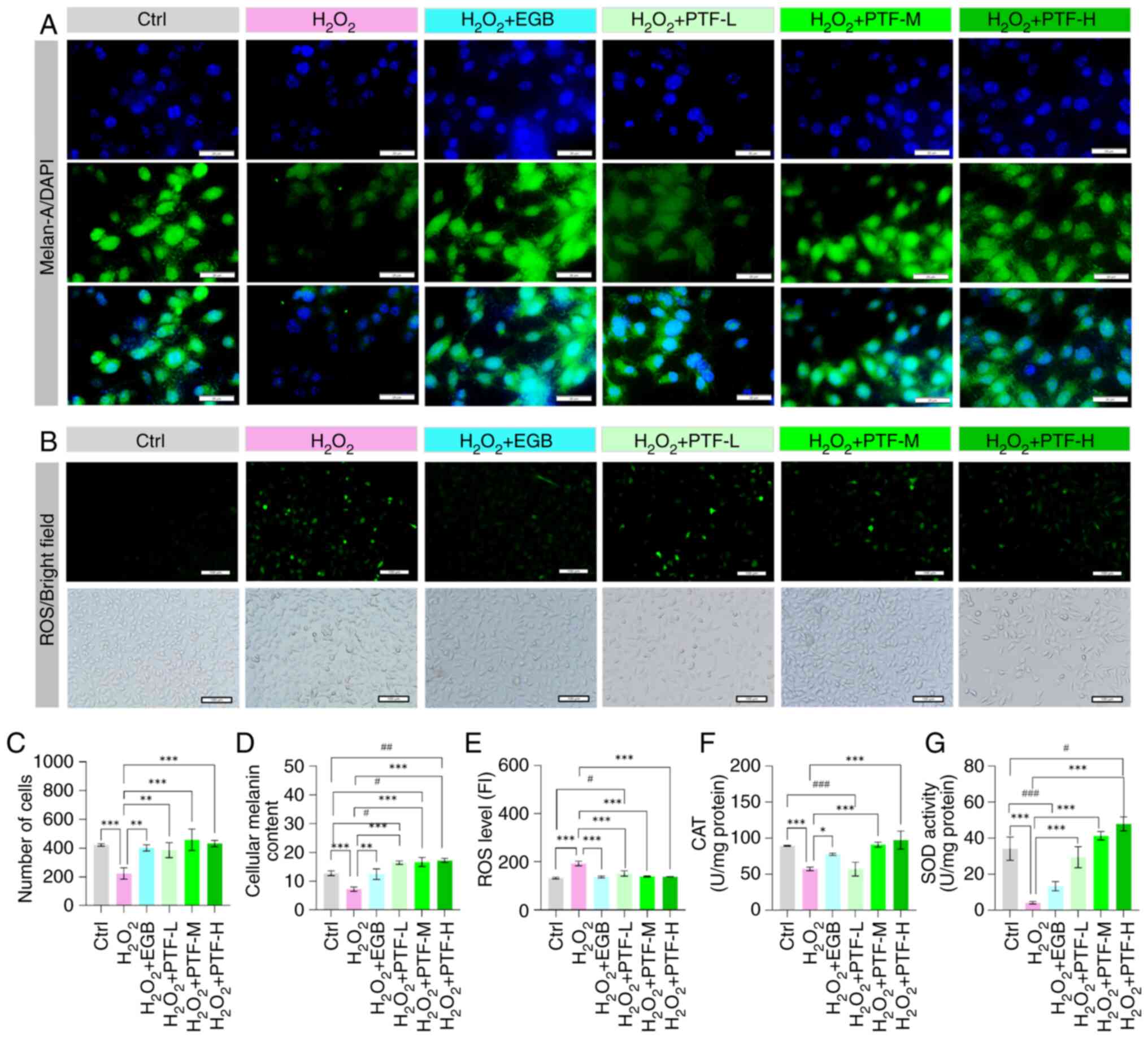 | Figure 5.Protective effect of PTF against
H2O2 -induced oxidative damage in B16F10
cells. (A) Effect of different treatments on melan-A protein
expression in B16F10 cells (scale bar, 20 µm). Green signals
represent the staining of melan-A with a melanocyte-specific
antibody, and blue signals represent DAPI. (B) Effects of different
treatments on the ROS content of B16F10 cells (scale bar, 100 µm).
Quantification of (C) the numbers of cells positive for melan-A and
(D) melanin content in B16F10 cells. Quantification of the (E) ROS
content, (F) CAT activity and (G) SOD activity in the B16F10 cells.
All data are presented as the mean ± standard error of the mean.
#P<0.05, ##P<0.01 and
###P<0.001 vs. the Ctrl group; *P<0.05,
**P<0.01 and ***P<0.001 vs. the H2O2
group. PTF, purslane total flavonoids; H2O2,
hydrogen peroxide; Ctrl, control; DAPI,
4′,6-diamidino-2′-phenylindole; ROS, reactive oxygen species; CAT,
catalase; SOD, superoxide dismutase; EGB, extract of Ginkgo
biloba; L, low; M, medium; H, high; FI, fluorescence
intensity. |
PTF ameliorates oxidative stress
impairment in B16F10 cells via the PI3K/AKT signaling axis
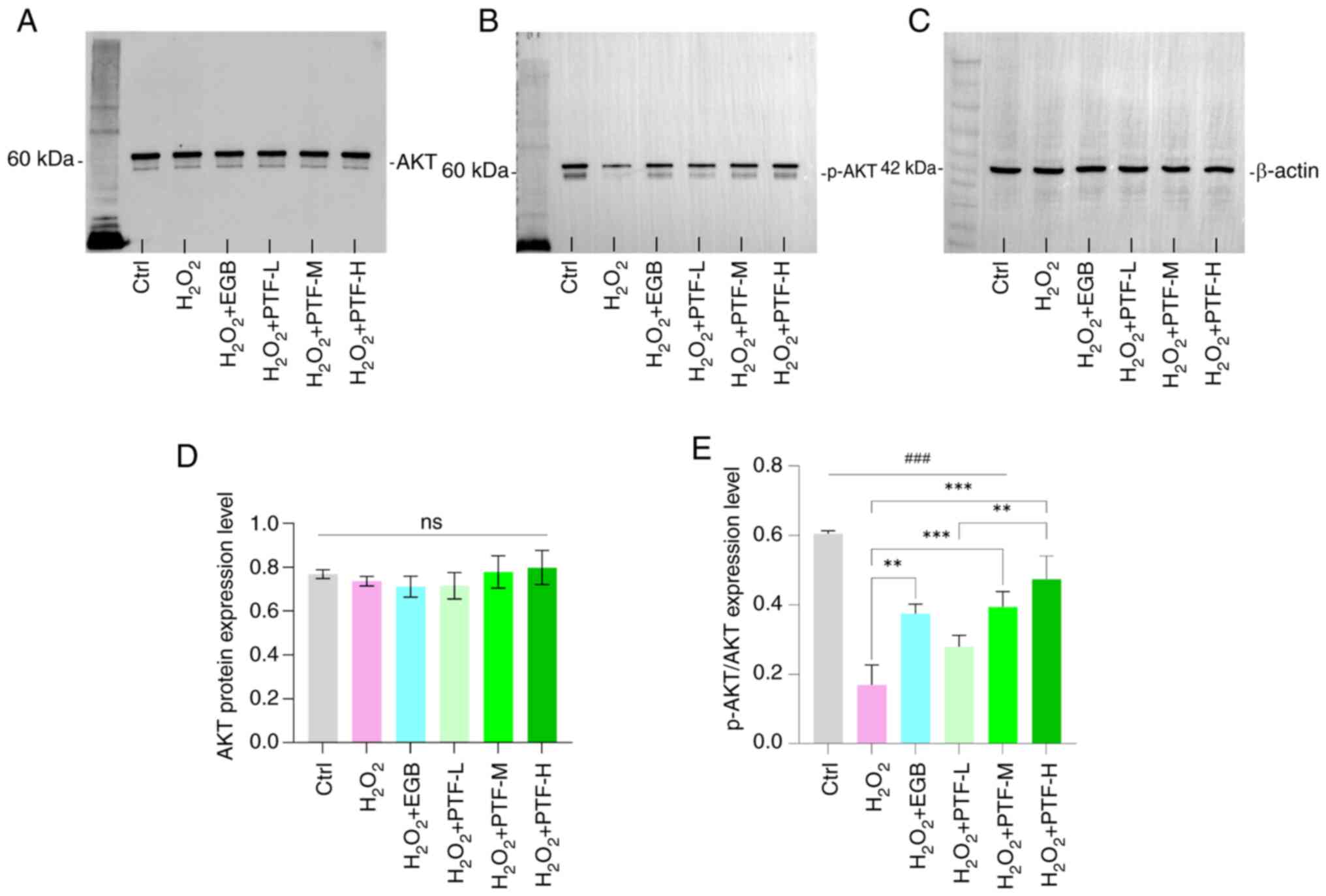
Western blot analysis demonstrated a significant
reduction in the level of p-AKT/AKT protein in the
H2O2 group compared with that in the control
group. By contrast, the medium and high PTF groups and the EGB
group exhibited elevated p-AKT/AKT protein levels compared with
those in the H2O2 group. In particular, the
high PTF group exhibited significantly higher p-AKT/AKT levels
compared with those in the low PTF group. However, compared to the
control group, the model group, the EGB intervention group and all
PTF dose groups showed significant reductions (Fig. 6). No significant differences were
observed in total AKT protein levels among the groups.
To investigate the involvement of the PI3K-AKT
signaling pathway in the protective effect of PTF against
H2O2-induced injury in B16F10 cells, the PI3K
inhibitor LY294002 was used. Compared to the
H2O2+PTF-H group, the LY294002 pretreatment
group showed no significant difference in the expression level of
Bcl-2, but a significant increase in Bax expression levels was
observed (Fig. 7E-I). Furthermore,
the PI3K inhibitor attenuated the protective effect of PTF against
H2O2-induced oxidative damage in B16F10
cells. The ROS levels in the control and high PTF groups were
significantly lower compared with those in the model group, whereas
the SOD and CAT activities in the control and high PTF groups were
significantly higher compared with those in the model group. The
PI3K inhibitor significantly attenuated the changes in SOD and CAT
activities compared with those in the high PTF group (Fig. 7A-D). These results suggest that
PI3K/AKT signaling plays a key role in the protective effect of
purslane on melanocytes subjected to oxidative stress.
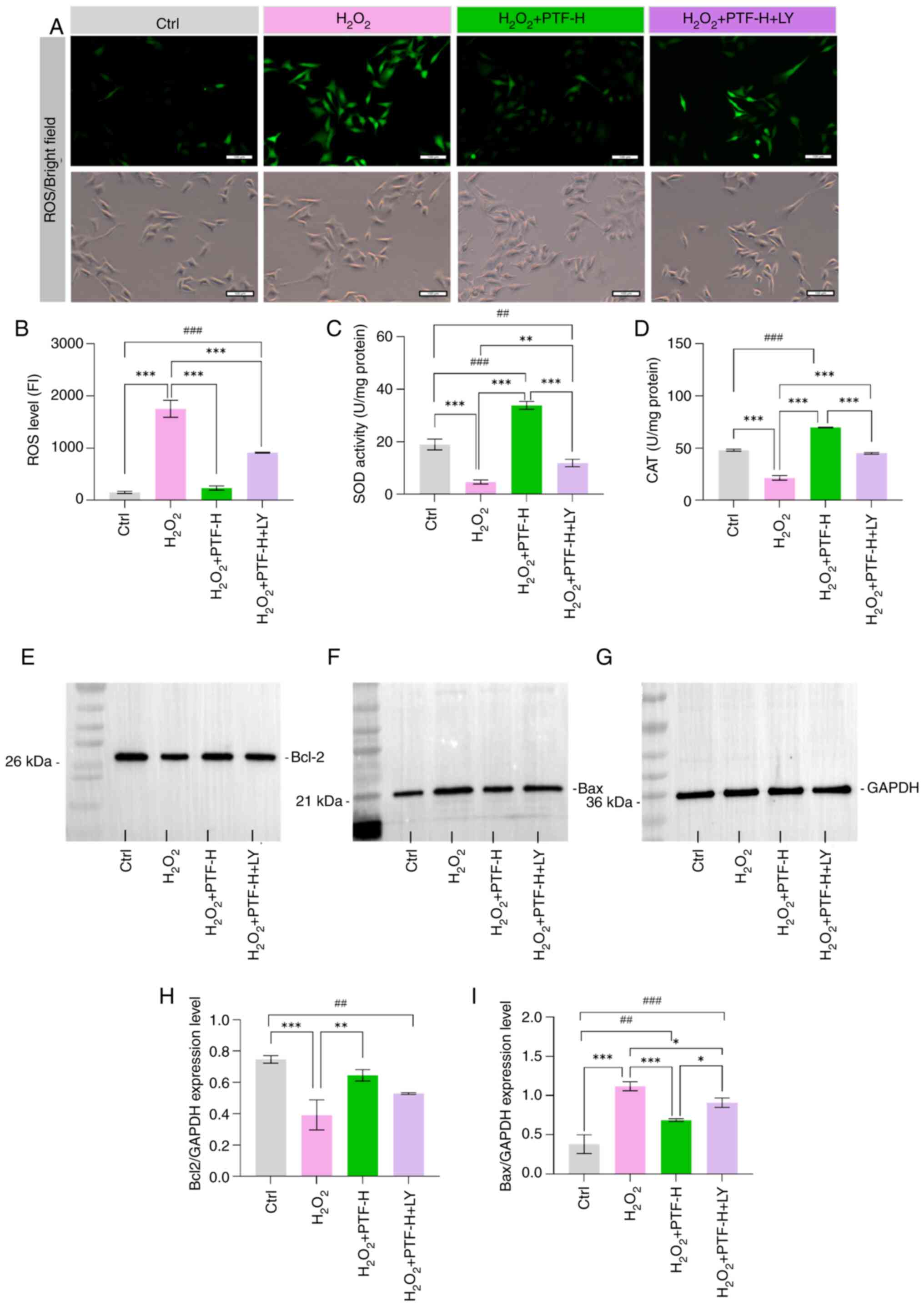 | Figure 7.PI3K/AKT inhibitor attenuates the
protective effect of PTF against H2O2-induced
B16F10 cell injury. (A) Effects of different treatments on the ROS
content of B16F10 cells (scale bar, 100 µm). Quantitative analysis
of (B) ROS levels, (C) SOD activity and (D) CAT activity in the
B16F10 cells. Western blot analysis of (E) Bcl-2, (F) Bax and (G)
GAPDH internal control. Quantitative analysis of (H) Bcl-2 and (I)
Bax protein expression. All data are presented as the mean ±
standard error of the mean. ##P<0.01 and
###P<0.001 vs. the Ctrl group; *P<0.05,
**P<0.01 and ***P<0.001 vs. the H2O2
group. PTF, purslane total flavonoids; H2O2,
hydrogen peroxide; Ctrl, control; ROS, reactive oxygen species;
SOD, superoxide dismutase; CAT, catalase; LY, LY294002; FI,
fluorescence intensity. |
Discussion
Purslane has a rich history of use in the treatment
of various skin conditions, including vitiligo. However, the
precise pharmacological mechanisms through which purslane
ameliorates vitiligo remain elusive. In the present study,
utilizing network pharmacology and molecular docking technology,
seven core components, six core targets and one key signaling
pathway of purslane in the treatment of vitiligo were identified.
The key targets and pathway of the core components of purslane in
the treatment of vitiligo were validated using a cell model. The
findings indicate that the total flavonoids extracted from purslane
exhibit therapeutic potential in vitiligo by mitigating oxidative
stress-induced damage in melanocytes via the PI3K/AKT signaling
pathway.
Vitiligo has been established to be an autoimmune
disease, with known associations with various autoimmune conditions
and hormone levels (8). Through
network pharmacology analysis, the present study identified ESR1
and PTGS2 as potential regulators of vitiligo symptoms, which may
act by modulating the molecular function of the ‘hormone-mediated
signaling pathway’. ESR1, a member of the NR3 subfamily of the
nuclear hormone receptor family, plays a role in the proliferation,
differentiation and homeostasis of melanocytes (38). It has been reported that elevated
levels of estrogen in the serum are associated with increased skin
pigmentation, and estrogen therapy is successful in the treatment
of vitiligo (39). PTGS enzymes
such as PTGS2, also known as cyclooxygenases, are essential for
prostaglandin biosynthesis and function as dioxygenases and
peroxidases. Studies have demonstrated that PTGS2 expression
promotes the proliferation of keratinocytes following acute UV
irradiation, leading to increased epidermal melanocyte and melanin
production. This finding underscores an elevated functional
activity of the melanocytes. (40,41).
Collectively, these findings highlight the intricate relationship
between hormone levels and skin pigmentation.
Patients with vitiligo typically present with
hypopigmented macules and melanocyte dysfunction in the skin and
mucosa (7). The pathogenesis of
this condition remains incompletely understood, with proposed
mechanisms encompassing genetic factors, melanocyte
self-destruction, immune responses, oxidative stress and
neurohumoral influences (42).
Notably, the oxidative stress theory holds a central position in
the pathogenesis of vitiligo. Elevated levels of ROS have been
reported in the blood of patients with vitiligo compared with those
in healthy individuals, with even higher levels observed in
patients with active vitiligo relative to that in patients with
stable vitiligo (43). These
observations underscore the importance of oxidative stress in the
pathophysiology of vitiligo. In the present study, network
pharmacology analysis suggested that PTF may exert therapeutic
effects on vitiligo by modulating the biological processes
associated with ‘cellular response to hypoxia’, ‘oxidoreductase
activity’ and ‘positive regulation of reactive oxygen species
metabolic process’ through targeting AKT, TP53 and PPARG.
The loss of melanocytes is a key pathological
feature of vitiligo, as they are the primary target cells in this
condition. A recent study implicated genetic defects in melanocyte
death, with ROS being pivotal in the pathogenesis of vitiligo
(44). Oxidative stress disrupts
the redox balance of cells, leading to the excessive production and
inadequate clearance of ROS. Elevated ROS levels contribute to
melanocyte destruction and cause structural and functional
impairments to DNA, lipids, proteins and metabolites (45). In the present study, an oxidative
damage model using B16F10 cells was used to evaluate the
therapeutic potential of PTF in vitiligo and elucidate its
underlying mechanism. The results demonstrated that PTF treatment
significantly decreased H2O2-induced
cytotoxicity in B16F10 cells, reduced ROS levels and increased the
activity of SOD and CAT. Thus, PTF exhibits diverse biological
functions, including cellular protection, that may be attributed to
its robust antioxidant properties. Previous studies have
highlighted the ability of PTF to ameliorate liver tissue injury
and lipid peroxidation in non-alcoholic fatty liver disease models
through the modulation of various markers including alanine
aminotransferase, aspartate aminotransferase, triglycerides,
fasting blood glucose, acetyl-CoA carboxylase, IL-6 and
malondialdehyde, and by increasing carnitine palmitoyltransferase-1
and IL-10 levels and SOD activity (46). Collectively, these findings suggest
that the therapeutic efficacy of purslane in vitiligo may be
associated with the protective effects of total flavonoids against
oxidative stress-induced damage in melanocytes.
In the present study, three flavonoids present in
purslane, namely luteolin, kaempferol and quercetin, were indicated
to play a pivotal role in the therapeutic management of vitiligo.
Molecular docking studies revealed robust binding interactions
between these flavonoids and the vitiligo therapeutic targets AKT1,
MAPK1 and PPARG. AKT1 is a key signaling molecule involved in cell
growth, division and apoptosis inhibition, which has been reported
to phosphorylate β-catenin, leading to the activation of
microphthalmia-associated transcription factor (MITF) and
tyrosinases, thereby facilitating melanin synthesis (47). Melanocytes express three PPAR
isoforms, namely PPARα, PPARβ and PPARγ, and activators of PPARα
and PPARγ have been demonstrated to inhibit melanocyte
proliferation and enhance melanin secretion in a dose-dependent
manner (48). Moreover, the MAPK
signaling pathway is directly implicated in the regulation of the
expression and activation of MITF, a key regulator of melanin
biosynthesis (49). These findings
suggest that the flavonoids luteolin, kaempferol and quercetin from
purslane may mitigate melanocyte damage and apoptosis through
modulation of AKT, MAPK1 and PPARG signaling pathways in
melanocytes.
In the KEGG pathway enrichment analysis, ‘PI3K-Akt
signaling pathway’ was identified as being pivotal in the treatment
of vitiligo with PTF. Previous studies have indicated that in skin
tissue, activation of this signaling pathway contributes to the
maintenance of skin homeostasis (50) and protects melanocytes against
apoptosis induced by oxidative stress (51). Notably, patients with vitiligo have
been found to exhibit impaired activation of the PI3K/AKT pathway
and reduced Nrf2 and phosphorylated PI3K levels in lesional skin
cells, leading to the accumulation of oxidative species that
heighten susceptibility to apoptosis and promote melanocyte death
(43). However, purslane has been
reported to protect hepatocytes through its anti-inflammatory and
anti-oxidative properties, mediated by the PI3K/AKT/mTOR pathway
(52). In addition, purslane
extract has been shown to exert antioxidant and hypoglycemic
effects via the PI3K/AKT pathway (53). In the present study, treatment with
PTF was shown to activate the PI3K/AKT signaling pathway, as
evidenced by significantly elevated levels of p-AKT in PTF-treated
groups compared with the model group. Pre-treatment with the PI3K
inhibitor LY294002 prior to PTF and H2O2
reduced the expression levels of Bcl-2 and increased the expression
levels of Bax, indicating inhibition of the PI3K/AKT pathway by
LY294002. In addition, the protective effects of PTF on B16F10
cells were markedly attenuated. These results underscore that
PI3K/AKT signaling contributes to the protective effect of purslane
against oxidative stress in melanocytes cells in vitiligo.
The present study has certain limitations; notably,
the protective effect of each specific component of purslane
against H2O2-induced oxidative damage in
B16F10 cells was not verified. Furthermore, additional in
vivo and clinical investigations are necessary to further
corroborate the efficacy of purslane and its individual components
in the treatment of skin lesions.
In summary, the present study used network
pharmacology, molecular docking and in vitro assays to
indicate that the total flavonoids of purslane may help to treat
vitiligo via the mitigation of oxidative stress-induced damage in
melanocytes through the PI3K/AKT signaling axis.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Jinqiang
Zhang and Professor Tao Zhou (both Resource Institute for Chinese
and Ethnic Materia Medica of Guizhou University of Traditional
Chinese Medicine, Guiyang, China) who provided assistance during
the experimental process.
Funding
This study was supported by the National Natural Science
Foundation of China (grant/award no. 82160908).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CW conceived and designed the study. XZ established
the oxidative stress models and analyzed the cells using
immunofluorescence and western blotting. LM detected the oxidative
stress indicators. SL and XR collected and organized the data. XZ
wrote the manuscript. CW and XZ confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PTF
|
purslane total flavones
|
|
DPPH
|
1,1-diphenyl-2-trinitrophenylhydrazine
|
|
ROS
|
reactive oxygen species
|
|
SOD
|
superoxide dismutase
|
|
DCFH-DA
|
2′,7′-dichlorofluorescein
diacetate
|
|
DAPI
|
4′,6-diamidino-2′-phenylindole
|
|
PPI
|
protein-protein interaction
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
References
|
1
|
Ezzedine K, Eleftheriadou V, Whitton M and
van Geel N: Vitiligo. Lancet. 386:74–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ezzedine K, Sheth V, Rodrigues M,
Eleftheriadou V, Harris JE, Hamzavi IH and Pandya AG; Vitiligo
Working Group, : Vitiligo is not a cosmetic disease. J Am Acad
Dermatol. 73:883–885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kruger C and Schallreuter KU: A review of
the worldwide prevalence of vitiligo in children/adolescents and
adults. Int J Dermatol. 51:1206–1212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bergqvist C and Ezzedine K: Vitiligo: A
review. Dermatology. 236:571–592. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kussainova A, Kassym L, Akhmetova A,
Glushkova N, Sabirov U, Adilgozhina S, Tuleutayeva R and Semenova
Y: Vitiligo and anxiety: A systematic review and meta-analysis.
PLoS One. 15:e02414452020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ongenae K, Dierckxsens L, Brochez L, van
Geel N and Naeyaert JM: Quality of life and stigmatization profile
in a cohort of vitiligo patients and effect of the use of
camouflage. Dermatology. 210:279–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frisoli ML, Essien K and Harris JE:
Vitiligo: Mechanisms of pathogenesis and treatment. Annu Rev
Immunol. 38:621–648. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khaitan BK and Sindhuja T: Autoimmunity in
vitiligo: Therapeutic implications and opportunities. Autoimmun
Rev. 21:1029322022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Y, Liang A, Fan X, Hu L, Hao F and Li
Y: Safety research in traditional Chinese medicine: Methods,
applications, and outlook. Engineering. 5:76–82. 2019. View Article : Google Scholar
|
|
10
|
Abdel-Malek ZA, Jordan C, Ho T, Upadhyay
PR, Fleischer A and Hamzavi I: The enigma and challenges of
vitiligo pathophysiology and treatment. Pigment Cell Melanoma Res.
33:778–787. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodrigues M, Ezzedine K, Hamzavi I, Pandya
AG and Harris JE: New discoveries in the pathogenesis and
classification of vitiligo. J Am Acad Dermatol. 77:1–13. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li K, Xia T, Jiang Y, Wang N, Lai L, Xu S,
Yue X and Xin H: A review on ethnopharmacology, phytochemistry,
pharmacology and potential uses of Portulaca oleracea L. J
Ethnopharmacol. 319:1172112024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lv WJ, Huang JY, Li SP, Gong XP, Sun JB,
Mao W and Guo SN: Portulaca oleracea L. extracts alleviate
2,4-dinitrochlorobenzene-induced atopic dermatitis in mice. Front
Nutr. 9:9869432022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou YX, Xin HL, Rahman K, Wang SJ, Peng C
and Zhang H: Portulaca oleracea L.: A review of
phytochemistry and pharmacological effects. Biomed Res Int.
2015:9256312015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ru J, Li P, Wang J, Zhou W, Li B, Huang C,
Li P, Guo Z, Tao W, Yang Y, et al: TCMSP: A database of systems
pharmacology for drug discovery from herbal medicines. J
Cheminformatics. 6:132014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang MQ and Wilkinson B: Drug discovery
beyond the ‘rule-of-five’. Curr Opin Biotech. 18:478–488. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Xiao J, Suzek TO, Zhang J, Wang J
and Bryant SH: PubChem: A public information system for analyzing
bioactivities of small molecules. Nucleic Acids Res. 37:W623–W633.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gfeller D, Michielin O and Zoete V:
Shaping the interaction landscape of bioactive molecules.
Bioinformatics. 29:3073–3079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stelzer G, Rosen N, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The GeneCards suite: From gene data mining to disease genome
sequence analyses. Curr Protoc Bioinformatics. 54:1.30.1–1.30.33.
2016.PubMed/NCBI
|
|
20
|
Bauer-Mehren A, Rautschka M, Sanz F and
Furlong LI: DisGeNET: A Cytoscape plugin to visualize, integrate,
search and analyze gene-disease networks. Bioinformatics.
26:2924–2926. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bardou P, Mariette J, Escudie F, Djemiel C
and Klopp C: jvenn: An interactive Venn diagram viewer. Bmc
Bioinformatics. 15:2932014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Y, Li M, Wang J, Pan Y and Wu FX:
CytoNCA: A cytoscape plugin for centrality analysis and evaluation
of protein interaction networks. Biosystems. 127:67–72. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: cytoHubba: Identifying hub objects and sub-networks from
complex interactome. Bmc Syst Biol. 8 (Suppl 4):S112014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dennis GJ, Sherman BT, Hosack DA, Yang J,
Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rigsby RE and Parker AB: Using the PyMOL
application to reinforce visual understanding of protein structure.
Biochem Mol Biol Edu. 44:433–437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Boyle NM, Morley C and Hutchison GR:
Pybel: A Python wrapper for the OpenBabel cheminformatics toolkit.
Chem Cent J. 2:52008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hseu YC, Chen XZ, Vudhya GY, Yen HR,
Chuang JY and Yang HL: The Skin-whitening effects of ectoine via
the suppression of alpha-MSH-stimulated melanogenesis and the
activation of antioxidant Nrf2 pathways in UVA-irradiated
keratinocytes. Antioxidants (Basel). 9:632020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan M, Chen L, Wang W, Qin D, Jia C, Liu
C, Wang H, Zhu J, Guo Y, Zhou Y, et al: Emodin inhibits the
proliferation and migration of B16F10 cells and induces their
apoptosis. Transl Cancer Res. 9:6198–6205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baliyan S, Mukherjee R, Priyadarshini A,
Vibhuti A, Gupta A, Pandey RP and Chang CM: Determination of
antioxidants by DPPH radical scavenging activity and quantitative
phytochemical analysis of ficus religiosa. Molecules. 27:13262022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiong J, Yang J, Yan K and Guo J:
Ginsenoside Rk1 protects human melanocytes from
H2O2-induced oxidative injury via regulation
of the PI3K/AKT/Nrf2/HO-1 pathway. Mol Med Rep. 24:8212021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hseu YC, Vudhya GY, Wang LW, Zhang YZ,
Chen XZ, Huang PJ, Yen HR and Yang HL: The in vitro and in vivo
depigmenting activity of pterostilbene through induction of
autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in
keratinocytes via Nrf2-mediated antioxidant pathways. Redox Biol.
44:1020072021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Wang S, Jin G, Gao K, Wang S, Zhang
X, Zhou K, Cai Y, Zhou X and Zhao Z: Network pharmacology-based
study on the mechanism of ShenKang injection in diabetic kidney
disease through Keap1/Nrf2/Ho-1 signaling pathway. Phytomedicine.
118:1549152023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Busam KJ and Jungbluth AA: Melan-A, a new
melanocytic differentiation marker. Adv Anat Pathol. 6:12–18. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin SY, Park HH, Li GZ, Lee HJ, Hong MS,
Park HJ, Park HK, Seo JC, Yim SV, Chung JH and Lee MH: Association
of estrogen receptor 1 intron 1 C/T polymorphism in Korean vitiligo
patients. J Dermatol Sci. 35:181–186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin SY, Park HH, Li GZ, Lee HJ, Hong MS,
Park HJ, Park HK, Seo JC, Yim SV, Chung JH and Lee MH: Association
of estrogen receptor 1 intron 1 C/T polymorphism in Korean vitiligo
patients. J Dermatol Sci. 35:181–186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li M, Gao Y, Li C, Liu L, Li K, Gao L,
Wang G, Zhang Z and Gao T: Association of COX2 functional
polymorphisms and the risk of vitiligo in Chinese populations. J
Dermatol Sci. 53:176–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tripp CS, Blomme EA, Chinn KS, Hardy MM,
LaCelle P and Pentland AP: Epidermal COX-2 induction following
ultraviolet irradiation: Suggested mechanism for the role of COX-2
inhibition in photoprotection. J Invest Dermatol. 121:853–861.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
LeWitt TM and Kundu RV: Vitiligo. JAMA
Dermatol. 157:11362021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim H, Park CS and Lee AY: Reduced Nrf2
activation in PI3K phosphorylation-impaired vitiliginous
keratinocytes increases susceptibility to ROS-generating
chemical-induced apoptosis. Environ Toxicol. 32:2481–2491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xuan Y, Yang Y, Xiang L and Zhang C: The
role of oxidative stress in the pathogenesis of vitiligo: A culprit
for melanocyte death. Oxid Med Cell Longev. 2022:84984722022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y, Li S and Li C: Perspectives of new
advances in the pathogenesis of vitiligo: From oxidative stress to
autoimmunity. Med Sci Monitor. 25:1017–1023. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Farkhondeh T, Samarghandian S,
Azimi-Nezhad M and Hozeifi S: The hepato-protective effects of
Portulaca oleracea L. extract: Review. Curr Drug Discov
Technol. 16:122–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Niu C, Yin L and Aisa HA: Novel
furocoumarin derivatives stimulate melanogenesis in B16 melanoma
cells by Up-regulation of MITF and TYR family via Akt/GSK3β/β
-catenin signaling pathways. Int J Mol Sci. 19:7462018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang HY, Chung E, Lee M, Cho Y and Kang
WH: Expression and function of peroxisome proliferator-activated
receptors in human melanocytes. Brit J Dermatol. 150:462–428. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shin S, Ko J, Kim M, Song N and Park K:
Morin induces melanogenesis via activation of MAPK signaling
pathways in B16F10 mouse melanoma cells. Molecules. 26:21502021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Teng Y, Fan Y, Ma J, Lu W, Liu N, Chen Y,
Pan W and Tao X: The PI3K/Akt pathway: Emerging roles in skin
homeostasis and a group of Non-malignant skin disorders. Cells.
10:12192021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Denat L, Kadekaro AL, Marrot L, Leachman
SA and Abdel-Malek ZA: Melanocytes as instigators and victims of
oxidative stress. J Invest Dermatol. 134:1512–1518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guoyin Z, Hao P, Min L, Wei G, Zhe C and
Changquan L: Antihepatocarcinoma Effect of Portulaca
oleracea L. in Mice by PI3K/Akt/mTOR and Nrf2/HO-1/NF-κ B
pathway. Evid-Based Compl Alt. 2017:82313582017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee JH, Park JE and Han JS: Portulaca
oleracea L. extract reduces hyperglycemia via PI3k/Akt and AMPK
pathways in the skeletal muscles of C57BL/Ksj-db/db mice. J
Ethnopharmacol. 260:1129732020. View Article : Google Scholar : PubMed/NCBI
|