Introduction
In recent years, the global population has aged
rapidly (1). The incidence of
entrapment neuropathy increases with age; thus, its prevalence is
expected to increase in an aging society (2). In clinical practice, treatments for
entrapment neuropathy do not always yield satisfactory outcomes
(3). Therefore, it is necessary to
consider ways to improve the outcomes of entrapment neuropathy.
Treatment outcomes for entrapment neuropathy are worse in elderly
patients than young patients (4).
In carpal tunnel syndrome, elderly patients showed less improvement
in numbness and distal latency after carpal tunnel release than
young patients (5,6). These outcomes suggest that there are
differences in the condition of the peripheral nerves between young
and elderly patients. Previous studies reported chronic macrophage
infiltration of peripheral nerves and elevated inflammatory
cytokine expression in the peripheral nerve (7), and disorganization and degeneration
of the myelin sheath in age-related peripheral nerves (8). The pathophysiology of axon
regeneration, which may be a therapeutic target for peripheral
neuropathy, remains to be elucidated.
Repressor element-1 silencing transcription factor
(REST) is a transcriptional regulator that regulates the expression
of various nerve-specific genes. In the central nervous system, the
expression of REST increases with aging and it protects nerves
against apoptosis and oxidative stress (9,10).
The expression of REST is decreased in neurodegenerative diseases
such as Parkinson's disease and Alzheimer's disease (10). On the other hand, REST inhibits
axon regeneration (11). REST has
multiple roles, including neuroprotection and neurotoxicity, and a
previous study identified it as a critical regulator in neural
survival (12). A previous study
by our group reported that axon regenerative capacity is poor in
aging mice compared to young mice using a mouse model of peripheral
nerve injury (13). Furthermore,
we found that the expression of REST increases in peripheral nerves
with aging in the peripheral nervous system (14).
Based on these findings, we hypothesized that REST
plays a major role in the reduction of peripheral nerve axon
regenerative capacity with aging. Furthermore, as the expression of
REST increases with aging and given its functions in regulating
neuronal gene expression and inhibiting axon regeneration, we
hypothesized that identifying and regulating molecules controlled
by REST in peripheral nerves could improve the decline in axon
regenerative capacity associated with aging.
In this study, animal models and REST-regulated
cells were used to investigate the mechanism of REST-mediated axon
regeneration in peripheral nerves in vivo and in
vitro.
Materials and methods
Animals
The present study was approved by the Animal Care
Committee of Juntendo University, Tokyo, Japan (registration no.
1555; approval no. 2023202, date of approval: December 11,
2023).
Ten male C57BL/6J mice (Young group: 10-week-old
mice, n=5; Aged group: 70-week-old mice, n=5) for
immunofluorescence staining; 10 male C57BL/6J mice (Young group:
8-week-old mice, n=5; Aged group: 78-week-old mice, n=5) for qPCR
and western blotting; and 6 male aged C57BL/6J mice for treatment
of GP130 receptor agonist-1 (Ga1, Selleck, Tokyo, Japan) analysis
(78-week-old, treated with vehicle, n=3; treated with Ga1, n=3)
were purchased from JAPAN SLC, Inc. Mice were housed at five
animals/cage in a sterile environment controlled at a temperature
of 22± 2°C, humidity of 40–60%, and 12-h light and dark cycle, and
were given water and CRF-1 gamma-ray-irradiated (15 kGy) (Oriental
Yeast Co., Ltd.) ad libitum. Ten mice for immunofluorescence
staining, and 10 mice for qPCR and western blotting were monitored
only once when they were carried out, then they were sacrificed
immediately. Six mice treated with vehicle and Ga1 were monitored 5
times, when they were carried out, before and after treatment, 1 h
after treatment and 24 h after treatment. Humane endpoints were
defined as a loss of 20% of body weight, difficulty breathing,
coughing, wheezing, severe diarrhea, vomiting, flaccid or spastic
paralysis, convulsions, coupled with body temperature significantly
below normal. No mice were reached humane endpoints. All mice were
anaesthetized using 5% isoflurane and sacrificed by cervical
dislocation and used for each experiment. Death was confirmed when
breathing and heart rate had stopped.
There are some reports that low estrogen affects
peripheral neuropathy. Because estrogen decreases with age, males
with less estrogen fluctuations and susceptibility to estrogen were
used in this study.
Cell culture
Mouse embryonic fibroblast cell line NIH3T3 (Cell
Line Service) was cultured in a humidified incubator with 5%
CO2 at 37°C. The culture medium was Dulbecco's modified
Eagle's medium/Ham's F-12 (DMEM/F-12) (Sigma-Aldrich) supplemented
with 10% fetal bovine serum and penicillin (100 U/ml).
Construct REST expression-regulated
cells
Using cultured cell lines, REST expression-regulated
cells were constructed. The lentiviral vector used to overexpress
REST in our study was constructed by VectorBuilder Inc. The vector
ID is VB900006-3284rup. Meanwhile, the mock plasmid acted as the
negative control. They were propagated in Escherichia coli DH5α.
All plasmid DNA used for transfection was isolated using
QIAGEN® Plasmid Maxi kit from propagated Escherichia
coli. To make REST-overexpressed (REST-OE) cells, cells were
transfected with isolated REST plasmid using Lipofectamine 3000
(Thermo Fisher Scientific) according to the manufacturer's
instructions. To make REST-low expressed (siREST) cells, cells were
transfected with REST-targeting siRNA (Sigma-Aldrich,
SASI_Mm01_00196017) and control siRNA (Sigma-Aldrich,
SIC001-10NMOL) using Lipofectamine RNAimax (Thermo Fisher
Scientific) according to the manufacturer's instructions.
REST-targeting siRNA can be purchased by registration and logging
in to website of Sigma-Aldrich at https://www.sigmaaldrich.com/JP/ja/login?redirect=%2FJP%2Fja,
and entering ‘REST’ into the gene search box and choosing the mouse
at https://www.sigmaaldrich.com/JP/ja/semi-configurators/sirna?activeLink=selectAssays.
Control siRNA can be purchased at https://www.sigmaaldrich.com/JP/ja/product/sigma/sic001.
Histochemical assessment
The harvested sciatic nerve (SN) and dorsal root
ganglia (DRG) were fixed in 4% paraformaldehyde at room temperature
for 72 h and paraffin blocks were prepared. Immunofluorescence
staining was performed to assess the expression of REST and growth
associated protein 43 (GAP43). Tissue sections were prepared by
cutting the harvested SN and DRG at a thickness of 3 µm. Samples
were deparaffinized and autoclaved at 121°C for 10 min for antigen
retrieval. After treatment with True ViewTM (SP-8400,
Vector, CA, USA) to suppress autofluorescence, samples were blocked
using 2% bovine serum albumin (A2153, Sigma Aldrich, MO, USA) in
PBS containing 0.05% Tween 20 for 30 min. Samples were then reacted
with antibodies against the target proteins at 4°C for 15 h. After
washing with Tris-buffered saline with Tween 20 (TBST), a goat
anti-mouse IgG antibody labeled with Alexa Fluor 488 (A11001,
Thermo Fisher Scientific) was used as a secondary antibody, and a
rabbit IgG monoclonal antibody as a negative control. The intensity
of fluorescence in each section was quantified in the photon
counting mode using a fluorescence imaging microscope (Leica,
TCSSP5). The antibodies used in the present study were against
REST, a transcription factor that regulates the expression of
nerve-specific proteins, and GAP43, a neuronal protein known for
its important role in axonal outgrowth. Primary antibodies were as
follows: rabbit polyclonal anti-REST (1:100, 22242-1-AP,
ProteinTech, IL, USA), and rabbit polyclonal anti-GAP43 (1:100,
16971-1-AP, ProteinTech).
In the photon counting mode, fluorescence intensity
(gray value) was measured at 10 randomly selected sites from the
perikaryon in a region of interest set in a fluorescence-emitting
area, and mean fluorescence intensity was calculated. Fluorescence
intensity measured using each antibody was compared between the
Young and Aged groups.
Western blotting
For western blotting, protein was extracted by
1×radio immunoprecipitation assay buffer. Equal amounts of proteins
from the SN and DRG in animal models, and REST expression-regulated
cells were fractionated by sodium dodecyl sulfate polyacrylamide
gel electrophoresis and transferred onto a poly vinylidene
di-fluoride (PVDF) membrane by Trans-Blot Turbo (BIORAD).
Non-specific sites were blocked with PVDF Blocking Reagent (Toyobo
Co., Ltd.) for 1 h at room temperature following which the membrane
was washed with TBST three times, for 10 min each. The blot was
then incubated overnight at 4°C with appropriate primary antibody
in solution 1 (Toyobo Co., Ltd.) according to the supplier's
specific instructions. Primary antibodies were as follows: rabbit
polyclonal anti-REST (1:1,000, 22242-1-AP; ProteinTech), rabbit
polyclonal anti-GAP43 (1:1,000, 16971-1-AP; ProteinTech), rabbit
polyclonal anti-glycoprotein 130 (GP130, 1:1,000, #3732; Cell
Signaling), anti-signal transducer and activator of transcription 3
(STAT3, 1:1,000, #9132; Cell Signaling), anti-phosphorylated STAT3
(pSTAT3, 1:1,000, #9131; Cell Signaling), and mouse monoclonal
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:2,000,
sc-32233; Santa Cruz). The blots were washed with TBST and
incubated with appropriate secondary antibody for 2 h at room
temperature. After washing, Amersham Imager 680 (GE Healthcare Life
SciencesA) was applied, and blot images were captured using a gel
documentation system. Relative optical density of protein bands was
analyzed using gel software image lab 3.0. The membranes were
stripped and reprobed with GAPDH as a loading control.
Quantitative PCR
Total RNA was isolated from the SN, DRG in animal
models and REST expression-regulated cells by using RNeasy Microkit
(Qiagen) in accordance with the manufacturer's instructions.
Complementary DNA was synthesized using PrimeScript™ RT reagent Kit
(Takara). Next, qPCR was performed with SYBR Green real-time PCR
assay (Thermo Fisher Scientific) according to the ΔΔCq method. The
expression levels of targets were normalized to GAPDH. Used primers
are listed in Table I.
 | Table I.Primer sequences used for RT-qPCR in
the present study. |
Table I.
Primer sequences used for RT-qPCR in
the present study.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (3′-5′) | Seq ID |
|---|
| Rest |
ACCTGCAGCAAGTGCAACTA |
CCGCATGTGTCGCGTTAGA | XM_036164926.1 |
| Gap43 |
AAGGCAGGGGAAGATACCAC |
TTGTTCAATCTTTTGGTCCTCAT | NM_008083.2 |
| Il-6 |
CCACTTCACAAGTCGGAGGCTTA |
TGCAAGTGCATCATCGTTGTTC | NM_001314054.1 |
| Il-6
receptor |
GCCGGATCCACCTGCCAACCTT |
GGGCCACCGGGAGCAGCAACAC | X53802.1 |
| Gp130 |
TCCCATGGGCAGGAATATAG |
CCATTGGCTTCAGAAAGAGG | NM_010560.3 |
| Jak1 |
CATGGTGGAAGAGTTTGTGGA |
CAGCTGTTTGGCAACCTTGAA | NM_146145.2 |
| Stat3 |
AGGAGTCTAACAACGGCAGC |
ACAGGATTGATGCCCAAGCA | AY299489.1 |
| Gapdh |
TGTGTCCGTCGTGGATCTG |
TTGCTGTTGAAGTCGCAGG | GU214026.1 |
Analysis of REST and GAP43 expression
in young and aged mice
Young mice (n=5) and aged mice (n=5) were sacrificed
by cervical dislocation on the day that SN and DRG were harvested.
The expression of REST and GAP43 in SN and DRG was compared between
the young and aged mice by immunofluorescence staining, qPCR, and
western blotting.
Analysis of molecules expression of
JAK1/STAT3 pathway in REST expression-regulated cells
The expression of interleukin 6 (IL6), IL6 receptor,
GP130, janus kinase 1 (JAK1), and STAT3, which are components of
the JAK1/STAT3 pathway involved in regulating GAP43 expression
(15), was evaluated by qPCR in
REST-regulated cells. The expression of GP130 was evaluated by
western blot additionally. As the expression of GAP43 is promoted
when STAT3 is phosphorylated by JAK1, we evaluated STAT3 and pSTAT3
by western blotting (16).
Analysis of GAP43 expression in
REST-OE treated with Ga1
Since expression of JAK1/STAT3 pathway was
investigated and GP130 was found to be important, we treated
REST-OE cells with Ga1 to investigate the effect on axonal
regeneration markers. The stock solution of Ga1 was prepared by
transferring 5 mg to dimethyl sulfoxide (DMSO) at a concentration
of 10 mM. Further dilutions were made fresh in cell culture medium.
The final concentration of DMSO was 0.1% and Ga1 was 10 µM. After
48 h culture, REST-OE cells were incubated with 10 µM Ga1 for an
additional 30 min at 37°C. After being treated by Ga1, the
expression of REST, GP130, and GAP43 in REST-OE was evaluated by
western blotting and qPCR. DMSO was used as the vehicle
control.
Analysis of GAP43 expression in SN of
Aged mice treated with Ga1
Six 78-week-old mice were used and divided into a
DMSO group (n=3) and Ga1 group (n=3). DMSO was used as the vehicle
control. The Ga1 group mice received an intraperitoneal injection
of Ga1 dissolved by DMSO at a dose of 10 mg/kg. Since 10 mg/kg Ga1
was used to mice to evaluate the nervous system in the previous
study reported by Alam et al (17), a dose of 10 mg/kg was administered
to mice in this study. Only one dose was given during the entire
experimental period. The mice were sacrificed at 24 h after
treatment and SN was harvested to confirm the effect of Ga1 on the
expression of axon regeneration marker GAP43. The expression of
REST and GAP43 in SN was evaluated by western blotting and
qPCR.
Date analysis and statistics
Data are presented as the mean ± standard deviation
and were analyzed for significant differences using unpaired
Student's t-test, significance was defined as P<0.05 (Prism 7;
GraphPad Software).
Results
Expression of REST and GAP43 in the
sciatic nerve and dorsal root ganglia
To investigate the difference in the expression of
REST and GAP43 in young (Young group) and aged (Aged group) mice,
the expression of REST and GAP43 in the SN and DRG were quantified
by immunofluorescence staining, western blotting, and qPCR.
Immunofluorescence staining revealed a significant increase in the
fluorescence intensity of REST in the Aged group compared to the
Young group in the SN (Young group 132.3±14.5; Aged group
189.4±12.1, P=0.0003) (Fig. 1A)
and DRG (Young group 115.9±25.5; Aged group 174.7±41.1, P=0.026)
(Fig. 1B). Furthermore, a
significant decrease in the fluorescence intensity of GAP43 in the
Aged group compared to the Young group was found in the SN (Young
group 192.3±16.3; Aged group 119.2±9.0, P<0.0001) (Fig. 1C) and DRG (Young group 168.5±16.3;
Aged group 89.0±45.0, P=0.005) (Fig.
1D).
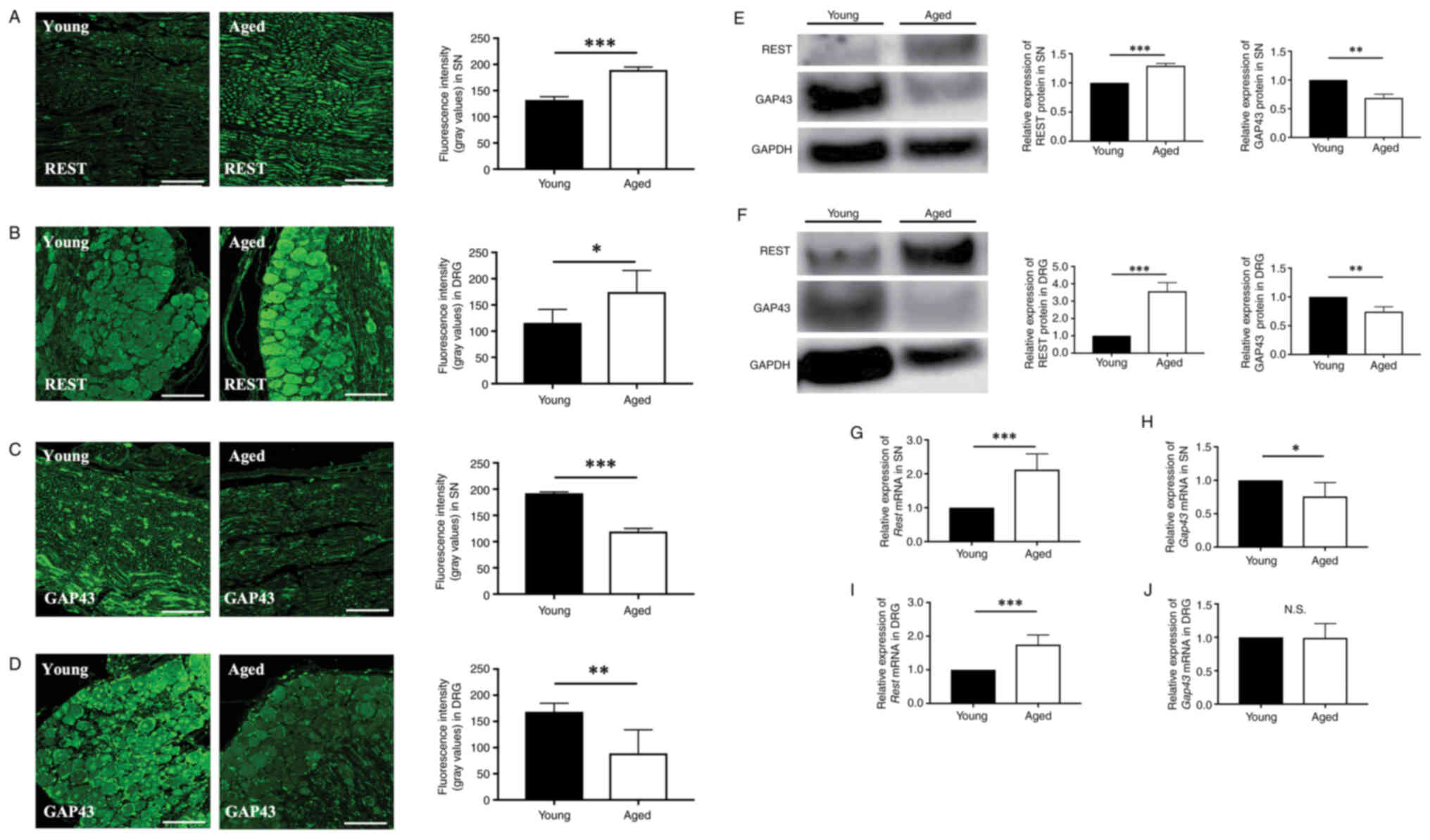 | Figure 1.Expression of REST and GAP43 in the
SN and DRG of young and aged mice are evaluated by
immunofluorescence staining, western blotting and RT-qPCR. Aged
mice (70- or 78-week-old, white bars) compared with young mice (10-
or 8-week-old, black bars). Histochemical assessment of the
expression of REST in the (A) SN and (B) DRG of young and aged mice
by immunofluorescence staining (scale bar, 100 µm). Histochemical
assessment of the expression of GAP43 in the (C) SN and (D) DRG of
young and aged mice by immunofluorescence staining (scale bar, 100
µm). Western blotting analysis of the expression of REST and GAP43
in the (E) SN and (F) DRG. RT-qPCR analysis of the expression of
(G) REST and (H) GAP43 in the SN. RT-qPCR analysis of the
expression of (I) REST and (J) GAP43 in the DRG. Data are expressed
as mean ± standard deviation (n=5 mice per group). *P<0.05,
**P<0.01 and ***P<0.001. REST, repressor element-1 silencing
transcription factor; GAP43, growth-associated protein 43; SN,
sciatic nerve; DRG, dorsal root ganglia; RT-qPCR, reverse
transcription-quantitative PCR. |
Western blotting revealed a significant increase in
the expression of REST in the Aged group compared to Young group in
the SN (1.29±0.04-fold, P=0.002) (Fig.
1E) and DRG (3.57±0.52-fold, P=0.009) (Fig. 1F). On the other hand, a significant
decrease was observed in the expression of GAP43 in the Aged group
compared to the Young group in the SN (0.68±0.07-fold, P=0.026)
(Fig. 1E) and DRG (0.74±0.09-fold,
P=0.012) (Fig. 1F).
qPCR revealed a significant increase in the
expression of REST in the Aged group compared to the Young group in
the SN (2.12±0.46-fold, P=0.006) (Fig.
1G) and DRG (1.75±0.29-fold, P=0.009) (Fig. 1H). A significant decrease was found
in the expression of GAP43 in the Aged group compared to the Young
group in the SN (0.75±0.21-fold, P=0.031) (Fig. 1I). No significant difference
between the expression of GAP43 in the Aged group and the Young
group was observed in the DRG (0.99±0.21-fold, P=0.935) (Fig. 1J). GAP43 is known to be strictly
regulated in terms of its expression at the mRNA level and protein
level, by regulating mRNA stability post-transcriptionally and by
post-translational modification including phosphorylation and
palmitoylation (18). Future study
may clarify whether GAP43 is degraded by post-translational
modification. In addition, GAP43 mRNA and protein may be regulated
by different mechanisms that are tissue-dependent, such as in the
SN and DRG.
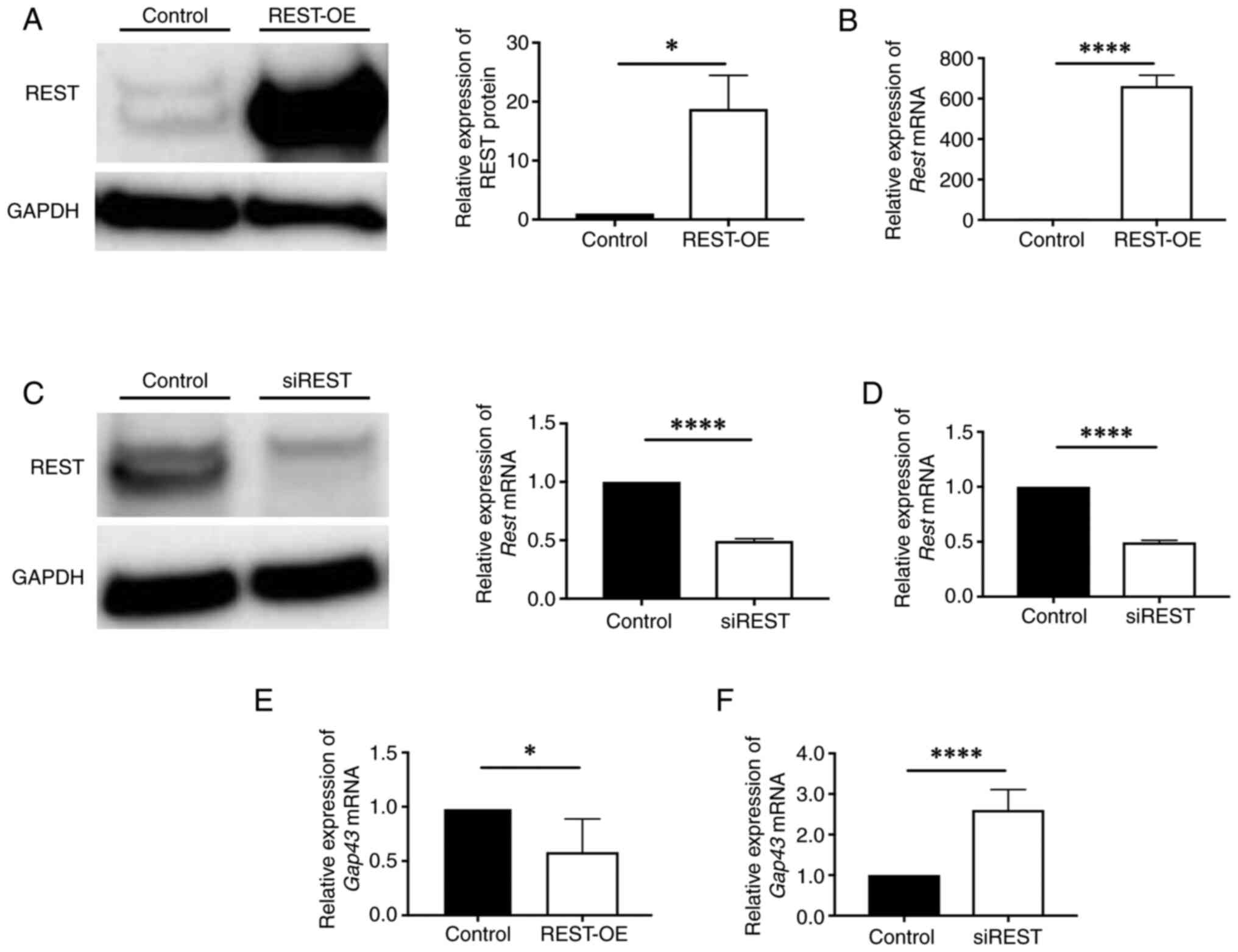
The expression of REST and GAP43 in
REST-regulated cells
To determine whether REST plays a role in GAP43
expression, REST plasmid and siRNA were used to construct REST-OE
cells and siREST cells using NIH3T3. Western blotting and qPCR
revealed a significant increase in the expression of REST in
REST-OE compared to the Control (protein: 18.8±5.5-fold, P=0.038;
mRNA: 662.6±53.6-fold, P<0.0001) (Fig. 2A and B) and a significant decrease
in the expression of REST in siREST compared to the Control
(protein: 0.63±0.25-fold, P=0.026; mRNA: 0.33±0.18-fold,
P<0.0001) (Fig. 2C and D).
Therefore, REST-OE cells and siREST cells were used for the
following studies. Interestingly, qPCR revealed a significant
decrease in the expression of GAP43 in REST-OE and a significant
increase in the expression of GAP43 in siREST compared to the
Control (REST-OE: 0.58±0.31-fold, P=0.016; siREST: 2.60±0.50-fold,
P<0.0001) (Fig. 2E and F). This
relationship between REST and GAP43 supports the findings in the
animal models.
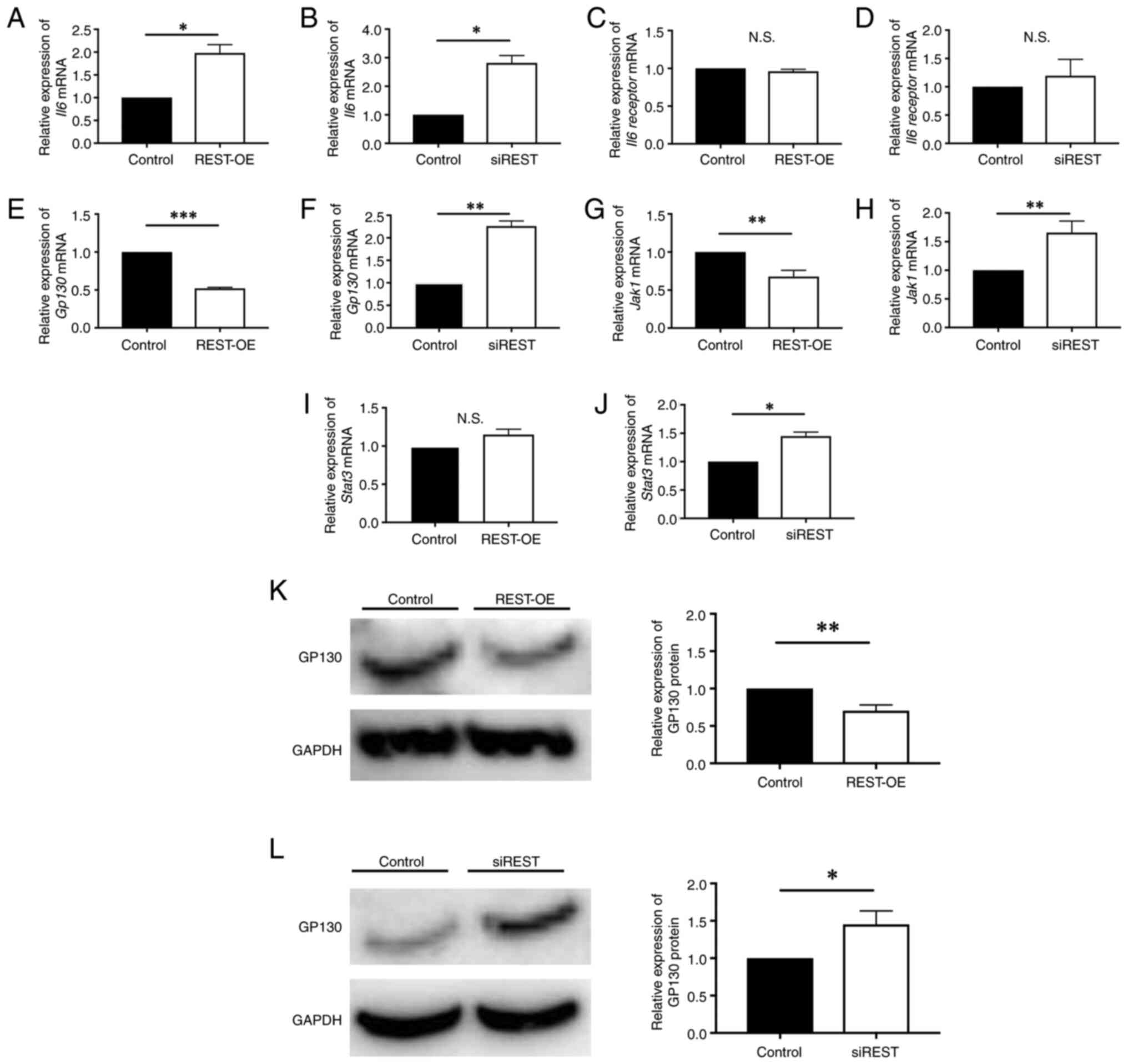
The expression of molecules of
JAK1/STAT3 pathway in REST-regulated cells
To determine the role of REST in GAP43 expression,
the involvement of molecules in the JAK1/STAT3 pathway was
investigated. The expression levels of IL-6, IL-6 receptor, GP130,
JAK1, and STAT3 were investigated using qPCR. qPCR revealed a
significant increase in IL-6 expression in REST-OE and siREST
compared to the Control (REST-OE: 1.98±0.18-fold, P=0.017; siREST:
2.81±0.26-fold, P=0.010) (Fig. 3A and
B). There was no significant difference in IL-6 receptor
expression between REST-OE and the Control (0.96±0.02-fold,
P=0.184) (Fig. 3C), or between
siREST and Control (1.20±0.29-fold, P=0.442) (Fig. 3D). A significant decrease in GP130
expression was observed in REST-OE, whereas a significant increase
was observed in siREST compared to the Control (REST-OE:
0.52±0.01-fold, P=0.004; siREST: 2.26±0.11-fold, P=0.004) (Fig. 3E and F). JAK1 expression
significantly decreased in REST-OE and significantly increased in
siREST compared to the Control (REST-OE: 0.68±0.08-fold, P=0.003;
siREST: 1.66±0.20-fold, P=0.005) (Fig.
3G and H). No significant difference in STAT3 expression was
observed between REST-OE and Control (1.14±0.02-fold, P=0.095)
(Fig. 3I), whereas a significant
increase was noted in siREST compared to the Control
(1.51±0.04-fold, P=0.012) (Fig.
3J). Since GP130 was considered to be an important molecule,
GP130 expression was also evaluated by western blotting. As a
result, a significant decrease in GP130 expression was observed in
REST-OE, whereas a significant increase was observed in siREST
compared to the Control (REST-OE: 0.69±0.04-fold, P=0.003; siREST:
1.48±0.17-fold, P=0.013) (Fig. 3K and
L).
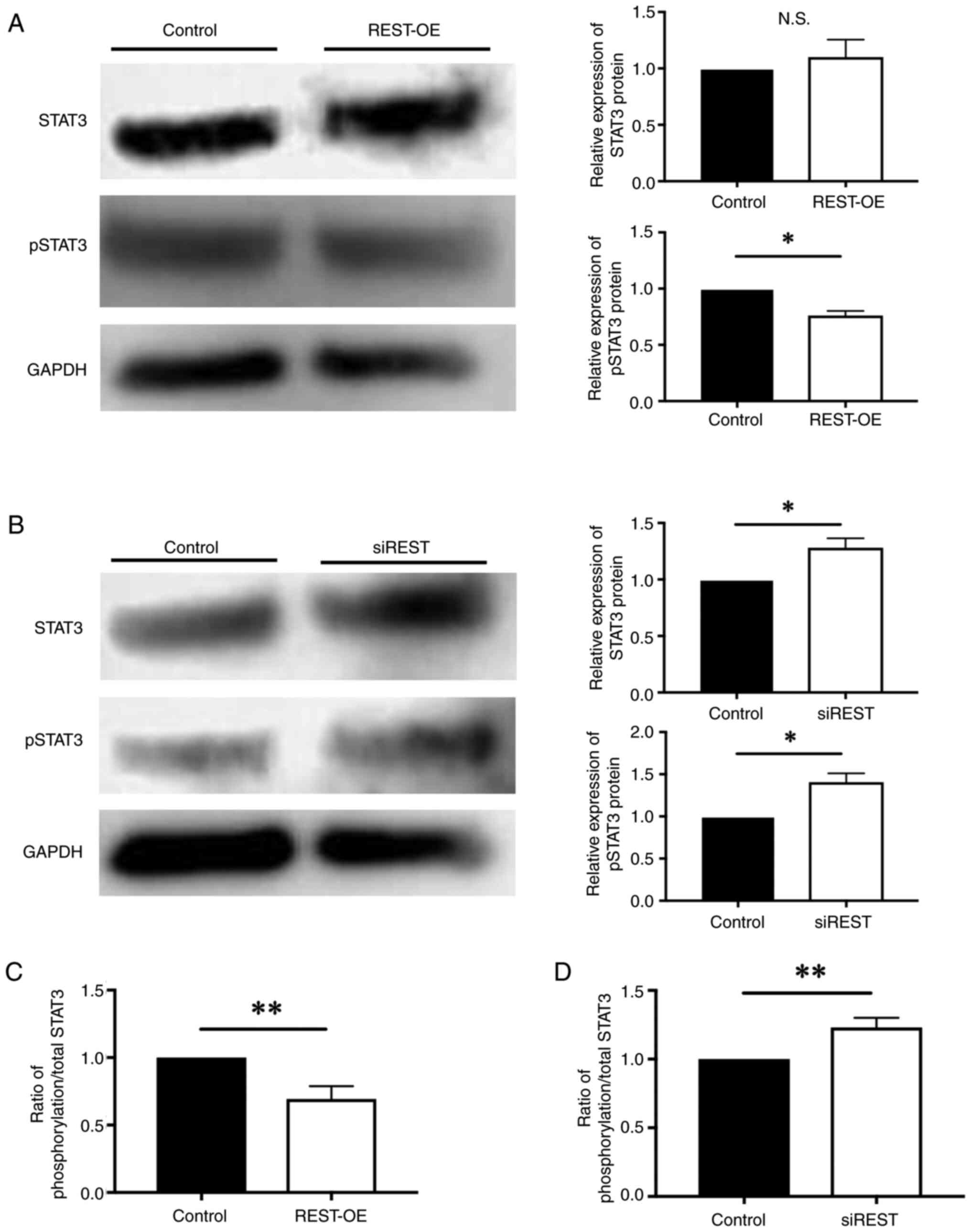
The expression of GAP43 is promoted when STAT3 is
phosphorylated by JAK1 (16).
Therefore, the expression of STAT3 and pSTAT3 protein was evaluated
by western blotting. Western blotting revealed no significant
difference between the expression of STAT3 in REST-OE and Control,
however a significant decrease in the expression of pSTAT3 in
REST-OE compared to the Control (STAT3: 1.10 ± 0.15-fold, P=0.459,
pSTAT3: 0.76±0.04-fold, P=0.015) (Fig.
4A). Moreover, a significant increase in the expression of
STAT3 and pSTAT3 was observed in siREST compared to the Control
(STAT3: 1.28±0.08-fold; P=0.043, pSTAT3: 1.33±0.07-fold, P=0.033)
(Fig. 4B). Furthermore, the ratio
of phosphorylation/total STAT3 protein was significantly decreased
in REST-OE compared to the Control (0.69±0.09-fold; P=0.005)
(Fig. 4C), whereas the ratio of
that was significantly increased in siREST compared to the Control
(1.21±0.07-fold; P=0.005) (Fig.
4D). These findings suggest that the expression of STAT3 does
not change, but the activation of STAT3 is low in REST-OE.
In summary, despite similar changes of IL6 and IL6
receptor in REST-OE and siREST, the expression of GP130, JAK1, and
phosphorylation of STAT3 were decreased in REST-OE, whereas the
expression of GP130, JAK1, and phosphorylation of STAT3 were
increased in siREST. These findings suggest that REST may regulate
the expression of GAP43 by the JAK1/STAT3 pathway via the
expression of GP130 (Fig. 5).
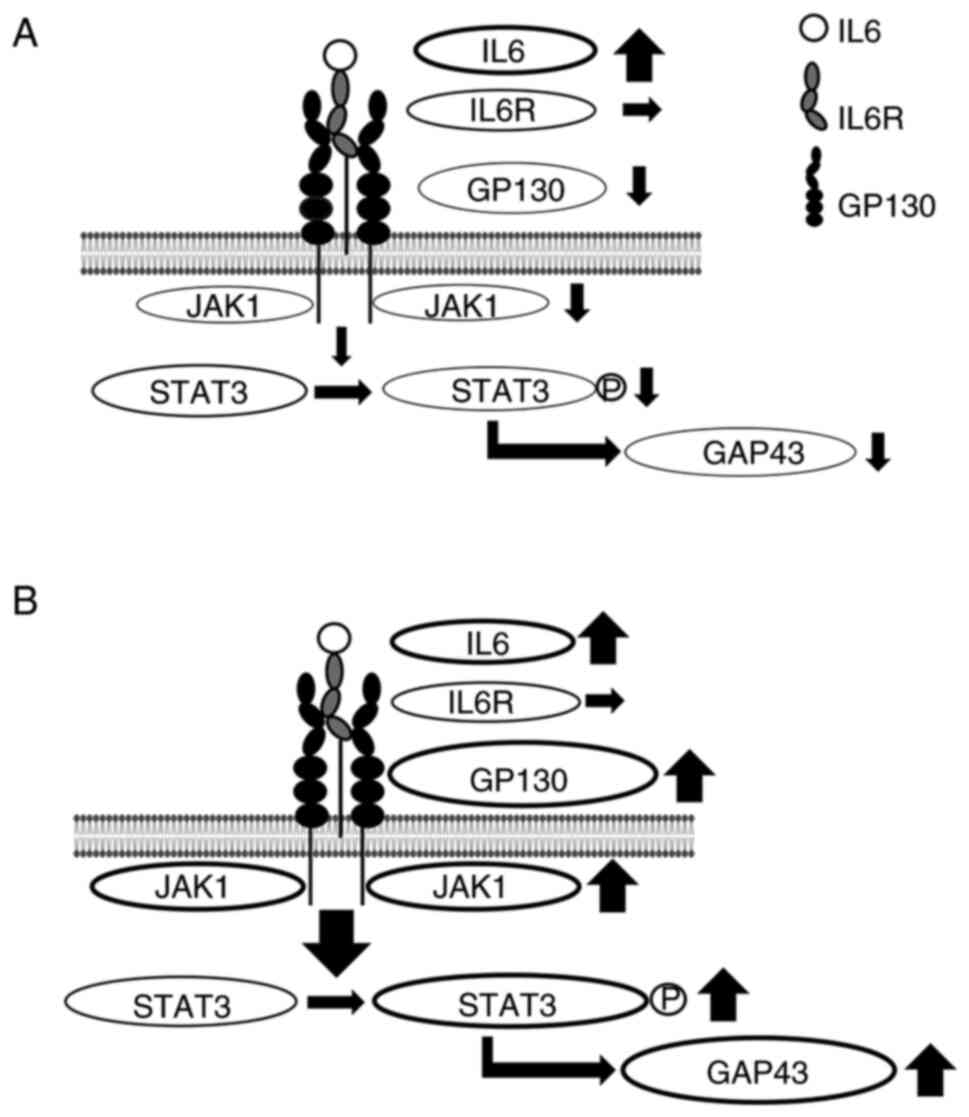 | Figure 5.Summary of the expression of
molecules of the JAK1/STAT3 pathway in REST-regulated cells.
Despite similar changes of IL6 and IL6 receptor in REST-OE and
siREST, the expression of GP130, JAK1 and pSTAT3 was increased in
REST-OE and the expression of GP130, JAK1 and pSTAT3 was decreased
in siREST. These findings suggest that REST regulates the
expression of GAP43 by the JAK1/STAT3 pathway via the expression of
GP130. (A) Summary of the expression of molecules in REST-OE. In
REST-OE, the expression of IL6 was significantly increased, there
was no significant difference in the expression of IL6 receptor and
the expression of GP130, JAK1 and pSTAT3 were significantly
decreased compared with the control. (B) Summary of the expression
of molecules in siREST. In siREST, the expression of IL6 was
significantly increased, there was no significant difference in the
expression of IL6 receptor, and the expression of GP130, JAK1 and
pSTAT3 was significantly increased compared with the control.
STAT3, signal transducer and activator of transcription 3; REST,
repressor element-1 silencing transcription factor; REST-OE,
REST-overexpressed; siREST, REST-low expressed; p, phosphorylated;
GP130, glycoprotein 130; p, phosphorylated; IL, interleukin; IL6R,
IL6 receptor; GAP43, growth-associated protein 43. |
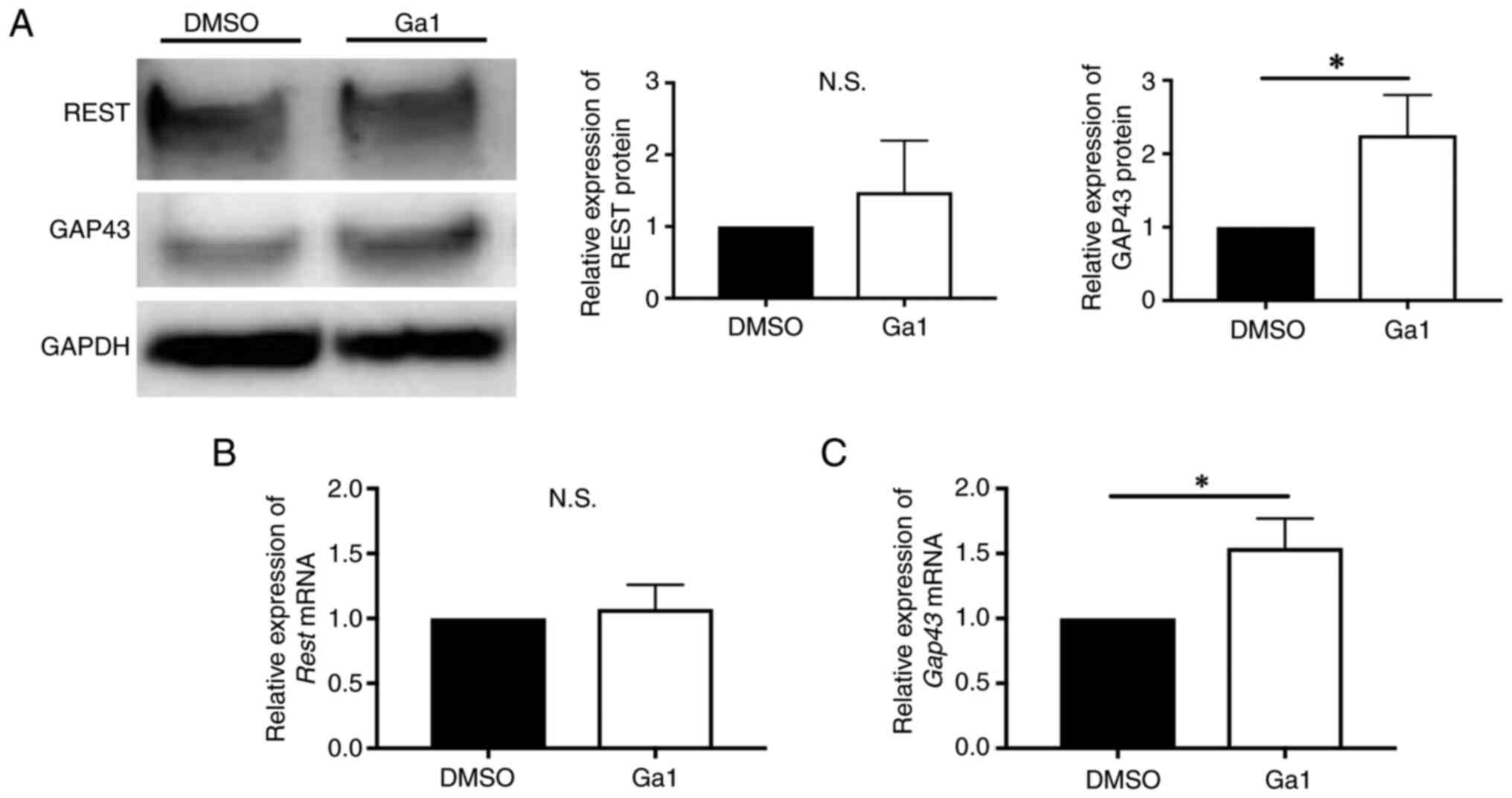
The expression of GAP43 in REST-OE
cells cultured with GP130 agonist
As REST possibly regulates the expression of GAP43
by the JAK1/STAT3 pathway via the expression of GP130, we predicted
that regulation of GP130-related molecular expression may change
the expression of GAP43 in REST-OE cells. To investigate this
hypothesis, we used Ga1, a GP130 agonist. The expression of REST,
GP130, and GAP43 was investigated by western blotting and qPCR.
Western blotting and qPCR revealed no significant difference
between the expression of REST in Ga1 and DMSO control (protein:
1.01±0.03-fold, P=0.54; mRNA: 1.05±0.33-fold, P=0.85) (Fig. 6A and B), no significant difference
between the expression of GP130 in Ga1 and a DMSO control (protein:
0.95±0.07-fold, P=0.28; mRNA: 0.92±0.13-fold, P=0.34) (Fig. 6A and C). Interestingly, a
significant increase was observed in the expression of GAP43 in Ga1
compared to a DMSO control (protein: 1.41±0.10-fold, P=0.018; mRNA:
1.66±0.26-fold, P=0.040) (Fig. 6A and
D). Thus, Ga1 enhanced GAP43 expression without changes of REST
and GP130 expression.
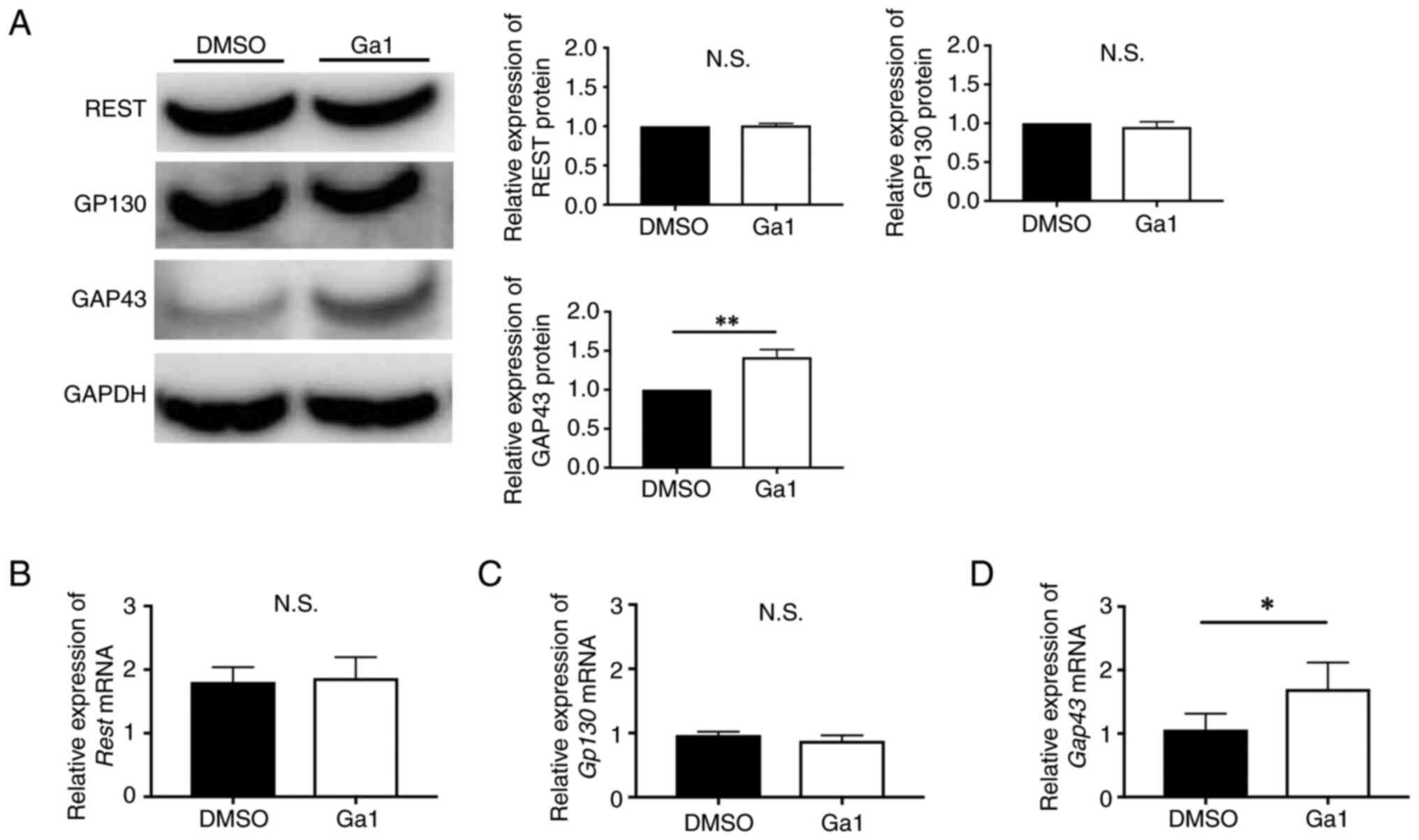 | Figure 6.Expression of REST, GP130 and GAP43
in REST-OE cultured with Ga1. REST-OE cultured with Ga1 (white
bars) compared with REST-OE cultured with DMSO (black bars). Graphs
show quantification of relative protein and protein and mRNA
abundance. (A) Western blotting analysis of REST, GP130 and GAP43.
RT-qPCR analysis of (B) REST, (C) GP130 and (D) GAP43. Data are
presented as mean ± standard deviation. *P<0.05 and **P<0.01.
REST, repressor element-1 silencing transcription factor; REST-OE,
REST-overexpressed; Ga1, GP130 receptor agonist-1; GP130,
glycoprotein 130; DMSO, dimethyl sulfoxide; GAP43,
growth-associated protein 43; RT-qPCR, reverse
transcription-quantitative PCR; N.S., not significant. |
The expression of GAP43 in mice
treated with Ga1
Ga1 enhanced GAP43 expression in REST-OE cells;
therefore, we dosed Ga1 into aged mice that had high REST
expression to investigate its influence in vivo. The
expression of REST and GAP43 was investigated by western blotting
and qPCR. Western blotting and qPCR revealed no significant
difference between the expression of REST in Ga1 and a DMSO control
(protein: 1.48±0.72-fold, P=0.54; mRNA: 1.07±0.19-fold, P=0.55)
(Fig. 7A and B). Interestingly, a
significant increase was observed in the expression of GAP43 in Ga1
compared to a DMSO control (protein: 2.25±0.55-fold, P=0.016, mRNA:
1.54±0.22-fold; P=0.013) (Fig. 7A and
C). Our findings suggest that Ga1 enhanced GAP43 expression
in vitro and in vivo.
Discussion
Our findings suggest that the JAK1/STAT3 pathway is
involved in the pathology of the reduction in peripheral nerve axon
regenerative capacity with aging. Then, our in vitro
findings using REST-OE cells and siREST cells suggest that
regulating GP130 expression is important in addressing the
reduction of peripheral nerve axon regenerative capacity that
occurs with aging. Furthermore, our finding that treatment with the
GP130 agonist Ga1 enhanced the expression of the axon regeneration
marker GAP43 suggests that GP130 is an important molecule for
peripheral nerve axon regeneration.
Previous studies reported that the JAK1/STAT3
pathway is activated by cytokines binding to GP130 (19,20).
Quarta et al (21) reported
that suppression of JAK1/STAT3 pathway activity in GP130 knockout
mice resulted in decreased axon regeneration and delayed functional
recovery after nerve injury. Furthermore, inhibition of leukocyte
migration inhibitory factor, which is a ligand for GP130,
suppressed axon regeneration after nerve injury, whereas
administration of ciliary neurotrophic factor, which is another
ligand for GP130, promoted axon regeneration (22,23).
In the present study, expression of GP130 and JAK1, and
phosphorylation of STAT3 in the JAK1/STAT3 pathway in vitro
were decreased in REST-OE cells and increased in siREST cells.
Furthermore, REST-OE cells in aged mice treated with Ga1 promoted
axon regeneration. Our findings support the findings of previous
studies that reported that GP130 is a key protein and potential
therapeutic target for treating poor axon regenerative capacity
with aging.
Known axon regeneration markers include superior
cervical ganglion (SCG10), small proline-rich protein 1A (SPRR1A),
and GAP43 (24–26). SCG10 is a marker that expresses in
microtubules of regenerating axons (24,27).
SPRR1A is an axon regenerative marker which is increased in neurons
after nerve injury (25). The
protein GAP43 is a marker that can assess axon regeneration in the
distal axon terminals of motor and sensory nerves (28–30).
Therefore, in the present study, to assess the axon regenerative
capacity with aging, we analyzed the expression of GAP43, which we
hypothesized would be able to assess axon regeneration at the
distal end of axons. There are some reports on intracellular
signaling pathways involved in peripheral nerve axon regeneration
(15,31–34).
Among these pathways, the PI3K/AKT pathway and JAK1/STAT3 pathway
are involved in GAP43 expression (35). Furthermore, it has been reported
that PI3K inhibitors do not inhibit axon regeneration, whereas JAK
inhibitors inhibit axon regeneration (35). Based on these findings, GAP43, as a
marker of axon regeneration, and the JAK1/STAT3 pathway, as an
intracellular signaling pathway, were analyzed to assess the
pathology of the reduction in axon regenerative capacity associated
with aging in the present study.
REST inhibits axon regeneration by suppressing the
expression of L1 cell adhesion molecule, which is an adhesion
factor that promotes axon regeneration, and suppressing Elk-1,
which is a transcription factor that promotes axon regeneration, by
inhibiting its phosphorylation (11,36,37).
Gervasi et al (38)
reported that inhibiting carboxy-terminal domain small phosphatase
1, which stabilizes REST by dephosphorylation, increases
brain-derived neurotrophic factor expression and promotes axon
regeneration. Thus, several studies have reported a mechanism of
axon regeneration inhibition by REST. However, the effects of REST
on the expression of molecules involved in the JAK1/STAT3 pathway
remain to be elucidated.
The present study investigated the effects of REST
on the intracellular signaling pathways associated with axon
regeneration in peripheral nerves. We found that REST inhibits axon
regeneration by suppressing the activity of the JAK1/STAT3 pathway
via GP130. Our findings suggest the importance of GP130 for
understanding the pathology of the reduction in axon regenerative
capacity associated with aging. It is known that the function of
motor and sensory nerves is declined in elderly (39). Furthermore, the improvement of the
function of motor and sensory nerves can be led by the enhancement
of axon regeneration (40). The
results of this study suggested that GP130 could be a potential
therapeutic target with problems of peripheral nerve systems in
elderly.
Our study has some major limitations that must be
taken into account when interpreting our results. Firstly, the
REST-regulated cells were constructed by fibroblasts, which are
non-neuronal cells. Gene expression patterns vary between cell
lines (41). However, there have
been no reports of differences in gene expression patterns between
nervous system cells and fibroblasts, although basic research of
nervous systems using fibroblasts has been reported (42,43).
This suggests that NIH3T3 is suitable for experiments analyzing the
expression of nervous system genes; thus, this cell line was used
in the present study. Secondly, REST is a transcriptional regulator
that protects neural homeostasis by regulating the expression of
various nervous system genes and has multiple functions (10). In this study we focused on only
axon regeneration; however, further studies are needed to
comprehensively assess axon regeneration in vivo. The
function of transcription factors is being investigated for the
treatment of various diseases (44,45).
Cao et al (44) reported
that PTEN, which is a multifunctional cancer transcriptional
repressor, had a cell survival function and that increased PTEN
expression promoted apoptosis and suppressed cancer. Thirdly, the
experiments of GP130 knockdown were not conducted in this study. It
has been reported that axonal regeneration after nerve injury is
reduced in GP130 knockout mice (20). Moreover, it has also been reported
that axonal regeneration is reduced when the GP130 ligand is
knocked out (21,22). Based on these reports, it is well
known that GP130 is necessary for axonal regeneration. In this
study, REST-OE cells were used to investigate the molecules
expression of JAK1/STAT3 pathway involved in regulating GAP43
expression. Then, it was revealed that GP130 expression was 48%
decreased in REST-OE compared to Control in this study. In previous
reports of gene knockdown experiments, experiments were conducted
with 30 to 50% reduction in expression of target gene using siRNA,
and with 46% reduction in expression of target gene using shRNA
(46–48). In other words, the 48% reduction in
GP130 in REST-OE in this study is considered to equivalent to the
gene knockdown state. Therefore, it is considered that the
experiments using REST-OE mimic the experiments of GP130 knockdown
and can evaluate the effect on axonal regeneration marker GAP43.
According to above reason, the experiments of GP130 knockdown were
not conducted in this study. Fourthly, mice treated with Ga1 in
this study did not evaluate using DRG. However, several previous
studies have been reported that have evaluated axonal regeneration
without using DRG, but only using SN (49,50).
Therefore, the evaluation of the SN would be sufficient for
evaluating axonal regeneration. Furthermore, we evaluated the
expression of GAP43 in the SN rather than the DRG since GAP43 is a
protein that is expressed in the distal axon terminals (28).
In conclusion, we found that reduced JAK1/STAT3
pathway activity, caused by decreased GP130 expression due to REST,
is a key factor in the reduction of axon regenerative capacity with
aging and represents a potential therapeutic target. This study may
improve axon regenerative capacity by aging.
Acknowledgements
Not applicable.
Funding
This work was supported by Japan Society for the Promotion of
Science KAKENHI (grant no. 22K09342) and the Nakatomi Foundation
(grant no. NF-2022-R12).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KN, DK and YU conceptualized the study. SK, TNK and
YY designed the methodology. SK, TS, NI and KK conducted the
investigation. SK, KN, NH and MI analyzed and interpreted the data.
SK and KN prepared the original draft, while SK, KN and MI reviewed
and edited the manuscript. KN secured funding. SK, KN, DK and NH
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
Committee of Juntendo University (Tokyo, Japan; registration no.
1555; approval no. 2023202).
Patient consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheng X, Yang Y, Schwebel DC, Liu Z, Li L,
Cheng P, Ning P and Hu G: Population ageing and mortality during
1990–2017: A global decomposition analysis. PLoS Med.
17:e10031382020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tadjerbashi K, Åkesson A and Atroshi I:
Incidence of referred carpal tunnel syndrome and carpal tunnel
release surgery in the general population: Increase over time and
regional variations. J Orthop Surg (Hong Kong).
27:23094990198255722019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyd KU, Gan BS, Ross DC, Richards RS,
Roth JH and MacDermid JC: Outcomes in carpal tunnel syndrome:
Symptom severity, conservative management and progression to
surgery. Clin Invest Med. 28:254–260. 2005.PubMed/NCBI
|
|
4
|
Yamamoto K, Shishido T, Masaoka T, Katori
Y and Tanaka S: Postoperative clinical results in cubital tunnel
syndrome. Orthopedics. 29:347–353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hobby JL, Venkatesh R and Motkur P: The
effect of age and gender upon symptoms and surgical outcomes in
carpal tunnel syndrome. J Hand Surg Br. 30:599–604. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilgis EF, Burke FD, Dubin NH, Sinha S and
Bradley MJ: A prospective assessment of carpal tunnel surgery with
respect to age. J Hand Surg Br. 31:401–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Büttner R, Schulz A, Reuter M, Akula AK,
Mindos T, Carlstedt A, Riecken LB, Baader SL, Bauer R and Morrison
H: Inflammaging impairs peripheral nerve maintenance and
regeneration. Aging Cell. 17:e128332018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing Y, Samuvel DJ, Stevens SM, Dubno JR,
Schulte BA and Lang H: Age-related changes of myelin basic protein
in mouse and human auditory nerve. PLoS One. 7:e345002012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen
Y, Yang TH, Kim HM, Drake D, Liu XS, et al: REST and stress
resistance in ageing and Alzheimer's disease. Nature. 507:448–454.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mampay M and Sheridan GK: REST: An
epigenetic regulator of neuronal stress responses in the young and
ageing brain. Front Neuroendocrinol. 53:1007442019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noro T, Shah SH, Yin Y, Kawaguchi R,
Yokota S, Chang KC, Madaan A, Sun C, Coppola G, Geschwind D, et al:
Elk-1 regulates retinal ganglion cell axon regeneration after
injury. Sci Rep. 12:174462022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin L, Liu Y, Wu Y, Huang Y and Zhang D:
REST is not resting: REST/NRSF in health and disease. Biomolecules.
13:14772023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaneko A, Naito K, Nakamura S, Miyahara K,
Goto K, Obata H, Nagura N, Sugiyama Y, Kaneko K and Ishijima M:
Influence of aging on the peripheral nerve repair process using an
artificial nerve conduit. Exp Ther Med. 21:1682021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goto K, Naito K, Nakamura S, Nagura N,
Sugiyama Y, Obata H, Kaneko A and Kaneko K: Protective mechanism
against age-associated changes in the peripheral nerves. Life Sci.
253:1177442020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chung D, Shum A and Caraveo G: GAP-43 and
BASP1 in axon regeneration: Implications for the treatment of
neurodegenerative diseases. Front Cell Dev Biol. 8:5675372020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Wang Z, Li B, Xia Z, Wang X, Xiu
Y, Zhang Z, Chen C, Song H, Li W, et al: The inhibition of
miR-17-5p promotes cortical neuron neurite growth via STAT3/GAP-43
pathway. Mol Biol Rep. 47:1795–1802. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alam MP, Bilousova T, Spilman P, Vadivel
K, Bai D, Elias CJ, Evseenko D and John V: A small molecule mimetic
of the humanin peptide as a candidate for modulating NMDA-induced
neurotoxicity. ACS Chem Neurosci. 9:462–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holahan MR: A Shift from a pivotal to
supporting role for the growth-associated protein (GAP-43) in the
coordination of axonal structural and functional plasticity. Front
Cell Neurosci. 11:2662017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heinrich PC, Behrmann I, Müller-Newen G,
Schaper F and Graeve L: Interleukin-6-type cytokine signalling
through the gp130/Jak/STAT pathway. Biochem J. 334:297–314. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McFarlane A, Bellón JS, Meyer T, Pohler E,
Piehler J and Moraga I: Differential functional coupling in
Gp130-JAK complexes expands the plasticity of the interleukin-6
signaling axis. bioRxiv. doi: 10.1101/2023.05.24.542077.
2023.PubMed/NCBI
|
|
21
|
Quarta S, Baeumer BE, Scherbakov N,
Andratsch M, Rose-John S, Dechant G, Bandtlow CE and Kress M:
Peripheral nerve regeneration and NGF-dependent neurite outgrowth
of adult sensory neurons converge on STAT3 phosphorylation
downstream of neuropoietic cytokine receptor gp130. J Neurosci.
34:13222–13233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cafferty WB, Gardiner NJ, Gavazzi I,
Powell J, McMahon SB, Heath JK, Munson J, Cohen J and Thompson SW:
Leukemia inhibitory factor determines the growth status of injured
adult sensory neurons. J Neurosci. 21:7161–7170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith PD, Sun F, Park KK, Cai B, Wang C,
Kuwako K, Martinez-Carrasco I, Connolly L and He Z: SOCS3 deletion
promotes optic nerve regeneration in vivo. Neuron. 64:617–623.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin JE, Geisler S and DiAntonio A:
Dynamic regulation of SCG10 in regenerating axons after injury. Exp
Neurol. 252:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bonilla IE, Tanabe K and Strittmatter SM:
Small proline-rich repeat protein 1A is expressed by axotomized
neurons and promotes axonal outgrowth. J Neurosci. 22:1303–1315.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jacobson RD, Virág I and Skene JH: A
protein associated with axon growth, GAP-43, is widely distributed
and developmentally regulated in rat CNS. J Neurosci. 6:1843–1855.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grenningloh G, Soehrman S, Bondallaz P,
Ruchti E and Cadas H: Role of the microtubule destabilizing
proteins SCG10 and stathmin in neuronal growth. J Neurobiol.
58:60–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iino S, Taguchi K, Maekawa S and Nojyo Y:
Motor, sensory and autonomic nerve terminals containing NAP-22
immunoreactivity in the rat muscle. Brain Res. 1002:142–150. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laux T, Fukami K, Thelen M, Golub T, Frey
D and Caroni P: GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at
plasmalemmal rafts, and regulate cell cortex actin dynamics through
a common mechanism. J Cell Biol. 149:1455–1472. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goslin K, Schreyer DJ, Skene JH and Banker
G: Changes in the distribution of GAP-43 during the development of
neuronal polarity. J Neurosci. 10:588–602. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmitt AM, Shi J, Wolf AM, Lu CC, King LA
and Zou Y: Wnt-Ryk signalling mediates medial-lateral retinotectal
topographic mapping. Nature. 439:31–37. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christie KJ and Zochodne D: Peripheral
axon regrowth: New molecular approaches. Neuroscience. 240:310–324.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elsaeidi F, Bemben MA, Zhao XF and Goldman
D: Jak/Stat signaling stimulates zebrafish optic nerve regeneration
and overcomes the inhibitory actions of Socs3 and Sfpq. J Neurosci.
34:2632–2644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wood MD and Mackinnon SE: Pathways
regulating modality-specific axonal regeneration in peripheral
nerve. Exp Neurol. 265:171–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu RY and Snider WD: Different signaling
pathways mediate regenerative versus developmental sensory axon
growth. J Neurosci. 21:Rc1642001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mikulak J, Negrini S, Klajn A,
D'Alessandro R, Mavilio D and Meldolesi J: Dual REST-dependence of
L1CAM: From gene expression to alternative splicing governed by
Nova2 in neural cells. J Neurochem. 120:699–709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lietz M, Bach K and Thiel G: Biological
activity of RE-1 silencing transcription factor (REST) towards
distinct transcriptional activators. Eur J Neurosci. 14:1303–1312.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gervasi NM, Dimtchev A, Clark DM, Dingle
M, Pisarchik AV and Nesti LJ: C-terminal domain small phosphatase 1
(CTDSP1) regulates growth factor expression and axonal regeneration
in peripheral nerve tissue. Sci Rep. 11:144622021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma CH, Omura T, Cobos EJ, Latrémolière A,
Ghasemlou N, Brenner GJ, van Veen E, Barrett L, Sawada T, Gao F, et
al: Accelerating axonal growth promotes motor recovery after
peripheral nerve injury in mice. J Clin Invest. 11:4332–4347. 2011.
View Article : Google Scholar
|
|
40
|
Werner RA, Franzblau A, D'Arcy HJ, Evanoff
BA and Tong HC: Differential aging of median and ulnar sensory
nerve parameters. Muscle Nerve. 1:60–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koch CM, Andrews RM, Flicek P, Dillon SC,
Karaöz U, Clelland GK, Wilcox S, Beare DM, Fowler JC, Couttet P, et
al: The landscape of histone modifications across 1% of the human
genome in five human cell lines. Genome Res. 17:691–707. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou H, Welcher AA and Shooter EM:
BDNF/NT4-5 receptor TrkB and cadherin participate in cell-cell
adhesion. J Neurosci Res. 49:281–291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mallei A, Rabin SJ and Mocchetti I:
Autocrine regulation of nerve growth factor expression by Trk
receptors. J Neurochem. 90:1085–1093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao LQ, Chen XL, Wang Q, Huang XH, Zhen
MC, Zhang LJ, Li W and Bi J: Upregulation of PTEN involved in
rosiglitazone-induced apoptosis in human hepatocellular carcinoma
cells. Acta Pharmacol Sin. 28:879–887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Song JX, Malampati S, Zeng Y, Durairajan
SSK, Yang CB, Tong BC, Iyaswamy A, Shang WB, Sreenivasmurthy SG,
Zhu Z, et al: A small molecule transcription factor EB activator
ameliorates beta-amyloid precursor protein and Tau pathology in
Alzheimer's disease models. Aging Cell. 19:e130692020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu B, Battaglia DM, Foster TP and Nichols
CD: Serotonin 5-HT2A receptor activity mediates adipocyte
differentiation through control of adipogenic gene expression. Sci
Rep. 11:197142021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang XD, Wang Q, Chen XL, Huang JF, Yin Y,
Da P and Wu H: Trop2 inhibition suppresses the proliferation and
invasion of laryngeal carcinoma cells via the extracellular
signal-regulated kinase/mitogen-activated protein kinase pathway.
Mol Med Rep. 12:865–870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tan C, Han LI, Zou L, Luo C, Liu A, Sheng
X and Xi D: Expression of P2X7R in breast cancer tissue and the
induction of apoptosis by the gene-specific shRNA in MCF-7 cells.
Exp Ther Med. 10:1472–1478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pola R, Aprahamian TR, Bosch-Marcé M,
Curry C, Gaetani E, Flex A, Smith RC, Isner JM and Losordo DW:
Age-dependent VEGF expression and intraneural neovascularization
during regeneration of peripheral nerves. Neurobiol Aging.
10:1361–1368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Girouard MP, Bueno M, Julian V, Drake S,
Byrne AB and Fournier AE: The molecular interplay between axon
degeneration and regeneration. Dev Neurobiol. 78:978–990. 2018.
View Article : Google Scholar : PubMed/NCBI
|