Introduction
The skin is the largest organ in the body and plays
a crucial role in protecting the internal organs from various
external threats (1). It is
continuously exposed to various factors, including pollutants,
smoking, diet, heat and ultraviolet radiation (UVR) (2). It is primarily composed of two main
layers: the epidermis and the dermis, each with distinct structural
and physiological characteristics (3). The epidermis, which is the outermost
layer, is directly exposed to the environment and functions
primarily as a protective barrier against external agents (4). It is primarily composed of
keratinocytes, constituting 90–95% of skin cells, which are
essential for maintaining skin hydration and barrier function
(5).
Skin aging is generally categorized into
chronological aging and UVR-induced photoaging (6). Photoaging is the most significant
contributor to skin damage. Long-term exposure to UVR leads to
wrinkles, uneven pigmentation, skin dryness and decreased dermal
and epidermal thickness (7). UVR
can be classified into three types based on wavelength: UVA
(320–400 nm), UVB (280–320 nm) and UVC (100–280 nm) (8). Among them, the majority of UVC and
some UVB are absorbed by the ozone layer (9). The rest of UVB penetrates the skin
epidermis, while UVA invades the dermis, breaking down the
extracellular matrix (10).
Epidermal cells exposed to UVB exhibit increased levels of reactive
oxygen species (ROS). Under physiological conditions, ROS function
as essential second messengers in various cellular processes,
including cell signaling and immune responses. However, excessive
production of ROS induces oxidative stress and DNA damage,
contributing to the development of pathological conditions, as well
as premature aging and photoaging (11,12).
Maintaining a balance between ROS production and
antioxidant defense mechanisms is crucial for cellular health.
Cells possess an antioxidant defense system in the cytoplasm and
cellular organelles, comprising enzymatic and non-enzymatic
antioxidants (13). Enzymatic
antioxidants include superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GPx) and glutathione reductase (GR).
Non-enzymatic antioxidants include glutathione, coenzyme Q10 and
vitamins C and E. Among them, SOD, CAT and GPx are crucial players
for the first line antioxidant system (14). Nuclear factor erythroid 2-related
factor 2 (Nrf2) serves as the transcriptional master regulator of a
multitude of antioxidant enzymes involved in the detoxification and
elimination of oxidative stress genes, such as SOD and CAT
(15).
Natural killer (NK) cells, constituting 10–15% of
human peripheral blood lymphocytes (16), possess the unique ability to
eliminate target cells without the need for major
histocompatibility complex restriction. NK cells secrete a diverse
array of cytokines that are pivotal in eradicating pathogens and
infected cells, as well as in modulating the immune response
(17). The cytokines produced by
NK cells include interleukin (IL)-1β, IL-6, IL-10, IL-12, tumor
necrosis factor α, transforming growth factor β, interferon gamma
interferon γ, IL-15 and IL-18 (18). Consequently, NK cell-conditioned
medium (NK-CdM), enriched with bioactive cytokines, has
demonstrated promising potential across various applications,
including immunotherapy, cancer treatment, antiviral, antibacterial
and antifungal activities, as well as wound healing (19). Furthermore, previous studies have
highlighted the wrinkle-preventive effects of NK-CdM on the dermis
of UVB-exposed skin (20).
However, the effect of NK-CdM on the epidermal skin barrier
following UVB exposure remains unexplored to date. Therefore, the
present study aimed to investigate the effects of NK-CdM on the
epidermal skin barrier.
Materials and methods
Culture of NK cells and conditioned
media preparation
NK-CdM was manufactured with some modifications
referring to the conditions described previously (21). Peripheral blood mononuclear cells
(PBMCs) were collected from healthy donors (n=3; 2 males and 1
female; average age=36.6) via lymph apheresis for 2–4 batch
productions in February 2019 at Seoul National University Hospital
(Seoul, Korea; Institutional Review Board approval no.
H-1811-023-985). CD3+ T cells in PBMCs were depleted via a magnetic
cell sorting system. NK cells present in the CD3-depleted PBMCs
were enriched and expanded using irradiated feeder cells and
culture medium for ~3 weeks. Feeder cells included genetically
engineered T cell lines and the culture medium was Cellgro SCGM
medium (CellGenix GmbH) containing human plasma and IL-2. When NK
cell cultivation was completed, NK-CdM was harvested using a
continuous centrifugation system at 400 × g for 3 min at 4°C to
remove NK cells. NK cell concentration at harvesting NK-CDM was
0.5×106−3×106 cells/ml. NK-CdM collection and
production were performed at GC Cell. The characteristics of NK-CdM
following cultivation are summarized in Table I.
 | Table I.Characterization of the NK-CdM
following cultivation. |
Table I.
Characterization of the NK-CdM
following cultivation.
| Characteristic | Value |
|---|
| Cell viability at
harvest, % | 93 |
| Cell density at
harvest, ×106 cell/ml | 2.45 |
| Population doubling
level | 12.98 |
| Cytotoxicity
(E:T=10:1, Specific lysis), % | 75.9 |
| Identity, % |
|
|
CD3−CD56+ | 97.9 |
|
CD56+CD16+ | 95.1 |
| Purity, % |
|
|
CD3+ | 0.0 |
|
CD14+ | 0.0 |
|
CD19+ | 0.0 |
Cell culture and treatment
The human keratinocyte cell line, HaCaT (CLS; cat.
no. 300493; Cell Lines Service GmbH), was maintained in Dulbecco's
Modified Eagle's Medium (DMEM; Welgene, Inc.) containing 10% fetal
bovine serum (FBS; Hyclone; Cytiva) and 1% penicillin/streptomycin
at 37°C with 5% CO2. The cells were pretreated with 1, 3
or 10% NK-CdM. Following incubation for 3 or 6 h, the culture
medium was replaced with 0.5 ml of Dulbecco's phosphate-buffered
saline (DPBS; Welgene, Inc.). Subsequently, the cells were exposed
to UVB (30 mJ/cm2) and then treated with NK-CdM.
Cell viability assay
Cytotoxicity of NK-CdM was investigated using a
WST-8 assay in HaCaT cells. Cells were seeded into 96-well plates
at a density of 5,000 cells per well. Cells were treated with 0, 1,
3, 10, 30 or 100% NK-CdM for 24 h; 0, 5, 10, 15, 20, 25 or 30%
NK-CdM for 24 h; or exposed to 30 mJ/cm2 UVB followed by
treatment with 0, 1, 3 or 10% NK-CdM and incubation for 24 h.
Additionally, the cells were pretreated with 1, 3 or 10% NK-CdM for
6 h before being exposed to UVB. Cell viability was analyzed using
a WST-8 assay at 450 nm using a microplate spectrophotometer
(SpectraMax 340; Molecular Devices, LLC).
Intracellular ROS measurement
Intracellular ROS levels were measured using the
Cellular ROS Detection Assay Kit (cat. no. ab1183851; Abcam).
Fluorescence images were observed using a fluorescence microscope
(DMi8; Leica Microsystems GmbH) and fluorescence absorbance was
measured at 485 and 535 nm using a spectrophotometer (SpectraMax
340; Molecular Devices, LLC).
Reverse transcription-quantitative
(RT-q) PCR
HaCaT cells were seeded into 6-well plates at a
density of 3×104 cells per well. The cells were treated
with 0, 1, 3 or 10% NK-CdM for 3 h; or exposed to 30
mJ/cm2 UVB followed by treatment with 0, 1, 3 or 10%
NK-CdM and incubation for 1 or 3 h. Total RNA from HaCaT cells was
extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Additionally, cDNA was synthesized from 1 µg of purified RNA via
reverse transcription with oligo-dT primers using a PrimeScript RT
Master Mix (Takara Bio, Inc.) according to the manufacturer's
instructions. Using qPCR PreMIX SYBR Green (Enzynomics), qPCR was
performed on a CFX96 thermocycler (Bio-Rad Laboratories, Inc.). The
thermocycling program was as follows: 95°C for 10 min, followed by
40 cycles at 95°C for 10 sec, 60°C for 15 sec and 72°C for 15 sec.
At least three separate biological replicates were conducted for
the RT-qPCR. Analysis of relative gene expression data using
real-time quantitative PCR and the 2−ΔΔCq method
(22), and then normalized to
β-actin expression. Table II
contains a list of the primers used in the qPCR.
 | Table II.Primer sequences used for
quantification of gene expression. |
Table II.
Primer sequences used for
quantification of gene expression.
| Gene | Primer sequence
(5′→ 3′) |
|
|---|
| Human superoxide
dismutase 1 | Forward |
CGACAGAAGGAAAGTAATG |
|
| Reverse |
TGGATAGAGGATTAAAGTGAG |
| Human catalase | Forward |
CGTGCTGAATGAGGAACAGA |
|
| Reverse |
AGTCAGGGTGGACCTCAGTG |
| Human
filaggrin | Forward |
AGGCTCCTTCAGGCTACATTC |
|
| Reverse |
CAGGAGAGTAGACATCTTTTGGCA |
| Human
involucrin | Forward |
TGCCTGAGCAAGAATGTGAG |
|
| Reverse |
AGCTGCTGATCCCTTTGTGT |
| Human hyaluronan
synthase 1 | Forward |
CAAGATTCTTCAGTCTGGAC |
|
| Reverse |
TAAGAACGAGGAGAAAGCAG |
| Human hyaluronan
synthase 2 | Forward |
ATTACCCAGTCCTGGCTTCG |
|
| Reverse |
CCTGTGGAAGACTCAGCAGAA |
| Human hyaluronan
synthase 3 | Forward |
CTTAAGGGTTGCTTGCTTGC |
|
| Reverse |
GTTCGTGGGAGATGAAGGAA |
| Human
aquaporin3 | Forward |
AGACAGCCCCTTCAGGATTT |
|
| Reverse |
TCCCTTGCCCTGAATATCTG |
| Human elongation of
very long chain fatty acids 1 | Forward |
AATGGGCTCTTTCCATGCCA |
|
| Reverse |
GGGAGATGTGCAGTGAGACC |
| Human elongation of
very long chain fatty acids 5 | Forward |
TGTGATGAACTGGGTCCCCTG |
|
| Reverse |
CCAGAGGTATGGACGCATGG |
| Human elongation of
very long chain fatty acids 6 | Forward |
CCTGTCAGCAAATTCTGGGC |
|
| Reverse |
ATGTGGTGATACCAGTGCAGG |
| Human ceramide
synthase2 | Forward |
ATCGTCTTCGCCATTGTTTT |
|
| Reverse |
GGCAGGATAGAGCTCCAGTG |
| Human ceramide
synthase3 | Forward |
CCAGGCTGAAGAAATTCCAG |
|
| Reverse |
AACGCAATTCCAGCAACAGT |
| Human
serine-palmitoyl transferase 2 | Forward |
AGCCGCCAAAGTCCTTGAG |
|
| Reverse |
CTTGTCCAGGTTTCCAATTTCC |
| Human sphingomyelin
synthase 2 | Forward |
CACCCAGTGGCTGTTTCTGA |
|
| Reverse |
TGCATTCCAGGCACAGGTAGA |
| Human acid
sphingomyelinase | Forward |
TGGCTCTATGAAGCGATGG |
|
| Reverse |
AGGCCGATGTAGGTAGTTGC |
| Human peroxisome
proliferator-activated receptor-α | Forward |
CCATCGGCGAGGATAGTTCTG |
|
| Reverse |
TCTACATTCGATGTTCAATGCTCCA |
| Human peroxisome
proliferator-activated receptor-γ | Forward |
TGGAATTAGATGACAGCGACTTGG |
|
| Reverse |
CTGGAGCAGCTTGGCAAACA |
| Human β-actin | Forward |
AGCGAGCATCCCCCAAAGTT |
|
| Reverse |
GGGCACGAAGGCTCATCATT |
Western blotting
HaCaT cells were seeded into 6-well plates at a
density of 3×104 cells per well. HaCaT cells were
pretreated with NK-CdM and NAC (10 mM) for 3 h and then exposed to
UVB (30 mJ/cm2) irradiation for 3 h or 24 h at 37°C with
5% CO2. Additionally, cells were pretreated with 3%
NK-CdM for 3 h, exposed to 30 mJ/cm2 UVB, and
subsequently treated with 3% NK-CdM, followed by incubation for 1
or 24 h. RIPA lysis buffer (Thermo Fisher Scientific, Inc.) was
used to extract the HaCaT cellular proteins and the Bradford
reagent (Millipore Sigma) was used to measure the protein
concentration. Then, 15 µg of the protein samples were separated on
a 10% sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gel and
transferred onto a nitrocellulose membrane (Cytiva). After that,
the membranes were blocked with 5% skimmed milk in Tris-buffered
saline containing 0.1% Tween-20 (TBS-T) at room temperature for 1 h
and then incubated overnight at 4°C with primary antibodies listed
in Table III. Following washes,
the membranes were incubated with horseradish peroxidase
(HRP)-conjugated anti-mouse or anti-rabbit secondary antibodies
(Vector Laboratories, Ltd.). Immunodetection was performed using an
Amersham ECL kit (Cytiva) according to the manufacturer's
instructions. Protein bands were visualized using a ChemiDoc MP
Imaging System (Bio-Rad Laboratories, Inc.) and ImageJ v1.8.0
(National Institutes of Health) was used for analysis, normalizing
all target proteins to β-actin.
 | Table III.Antibodies used for western blot
analysis. |
Table III.
Antibodies used for western blot
analysis.
| Antibodies | Dilution | Catalogue
number | Supplier |
|---|
| Anti-superoxide
dismutase 1 | 1:3,000 | sc-101523 | Santa Cruz
Biotechnology, Inc. |
| Anti-catalase | 1:3,000 | sc-271803 | Santa Cruz
Biotechnology, Inc. |
| Anti-nuclear factor
erythroid 2-related factor 2 | 1:3,000 | sc-81342 | Santa Cruz
Biotechnology, Inc. |
| Anti-lamin B | 1:5,000 | 13435 | Cell Signaling
Technology, Inc. |
| Anti-filaggrin | 1:5,000 | PA5-116911 | Thermo Fisher
Scientific, Inc. |
| Anti-filaggrin | 1:100 | GTX37695 | GeneTex |
|
Anti-involucrin | 1:5,000 | ab53112 | Abcam |
| Anti-hyaluronan
synthase1 | 1:5,000 | ab198846 | Abcam |
| Anti-hyaluronan
synthase2 | 1:5,000 | sc-365263 | Santa Cruz
Biotechnology, Inc. |
| Anti-hyaluronan
synthase3 | 1:5,000 | sc-365322 | Santa Cruz
Biotechnology, Inc. |
|
Anti-Aquaporin3 | 1:5,000 | PA5-78811 | Thermo Fisher
Scientific, Inc. |
| Anti-matrix
metalloproteinase 9 | 1:5,000 | ab38898 | Abcam |
| Anti-p-p38 | 1:5,000 | 4511 | Cell Signaling
Technology, Inc. |
| Anti-p38 | 1:5,000 | 9212 | Cell Signaling
Technology, Inc. |
| Anti-p-c-Jun
N-terminal kinases | 1:5,000 | 9251 | Cell Signaling
Technology, Inc. |
| Anti-c-Jun
N-terminal kinases | 1:5,000 | 9252 | Cell Signaling
Technology, Inc. |
|
Anti-p-extracellular signal-regulated
kinase | 1:5,000 | 9101 | Cell Signaling
Technology, Inc. |
| Anti-extracellular
signal-regulated kinase | 1:5,000 | 9102 | Cell Signaling
Technology, Inc. |
| Anti-p-Jun
Proto-Oncogene | 1:5,000 | #3270 | Cell Signaling
Technology, Inc. |
| Anti-Jun
Proto-Oncogene | 1:5,000 | #9165 | Cell Signaling
Technology, Inc. |
| Anti-p-cellular
oncogene fos | 1:5,000 | #5348 | Cell Signaling
Technology, Inc. |
| Anti-cellular
oncogene fos | 1:5,000 | #2250 | Cell Signaling
Technology, Inc. |
| Anti-ceramide
synthases 3 | 1:5,000 | PA1-12923 | Thermo Fisher
Scientific, Inc. |
|
Anti-serine-palmitoyl transferase | 1:5,000 | ab307432 | Abcam |
| Anti-acid
sphingomyelinase | 1:5,000 | PA5-77047 | Thermo Fisher
Scientific, Inc. |
| Anti-β-actin | 1:10,000 | sc-47778 | Santa Cruz
Biotechnology, Inc. |
SOD activity measurement
SOD activity was assessed using a colorimetric assay
kit (Biomax Ltd.) following the manufacturer's instructions. Cells
were treated with 3% NK-CdM or NAC (N-Acetyl cysteine; antioxidant)
for 6 h. Following UVB irradiation, the cells were homogenized in
cold lysis buffer and absorbance was measured at 450 nm to evaluate
SOD activity using a microplate spectrophotometer (SpectraMax 340;
Molecular Devices, LLC).
Reconstructed human skin model
Neoderm-ED, a reconstructed human skin model, was
purchased from Tego Science. Neoderm-ED was removed from the
agar-containing medium and placed into 12-well plates to
equilibrate at 37°C (5% CO2) for 24 h. Neoderm-ED was
pretreated with NK-CdM or D-panthenol (DPA) for 24 h before being
exposed to UVB irradiation (30 mJ/cm2) for 48 h.
Histological observation and
immunohistochemistry (IHC)
Neoderm-ED was fixed in 10% formalin at room
temperature for 24 h, dehydrated in ethanol, cleared with xylene,
embedded in paraffin, sectioned into 3-µm-thick slices, and stained
with hematoxylin and eosin (H&E). The skin model was subjected
to antigen retrieval with Tris-EDTA at 4°C for 15 min, followed by
treatment with BLOXALL blocking solution (Vector Laboratories,
Ltd.) at room temperature for 10 min to quench endogenous
peroxidase activity. The slides were then incubated with 2.5%
normal horse serum (Vector Laboratories, Ltd.) and Filaggrin
antibodies (Table III) overnight
at 4°C. Following this, the slides were incubated with HRP using
the ImmPRESS® Excel Amplified Polymer Staining Kit
(Vector Laboratories, Ltd.) and staining was developed using the
3,3′-diaminobenzidine (DAB) chromogenic substrate kit (Vector
Laboratories, Ltd.). Finally, the slides were counterstained with
hematoxylin at room temperature for <1 min to identify nuclei.
The slides were cleaned, dried and then mounted with
PermountTM mounting medium (Thermo Fisher Scientific,
Inc.). The stained slides were observed under a light microscope
(DM750; Leica Microsystems GmbH). A slide scanner (Panoramic MIDI;
3DHISTECH Ltd.) was used to capture images of all the stained
tissue slides at a magnification of 200×.
Enzyme-linked immunosorbent assay
(ELISA)
HaCaT cells were seeded into 6-well plates at a
density of 500,000 cells per well. The Cells were pretreated with
3% NK-CdM and 1% DPA, positive control, for 9 h, followed by UVB
(30 mJ/cm2) irradiation for 24 h. Following incubation,
samples were taken and centrifuged at 3,000 × g for 10 min at 4°C.
The supernatants were then analyzed for HA using an ELISA. The
procedure was performed using an ELISA kit (cat. no. DHYAL0;
R&D Systems) following the provided instructions.
Statistical analyses
Data were obtained from at least three independent
experiments and presented as mean ± standard deviation (SD).
Statistical analyses were performed using unpaired one-way analysis
of variance followed by a Bonferroni post hoc test on GraphPad
Prism 9.0 (Dotmatics). The Bonferroni post hoc test was used among
the groups. Data are likely to be sampled from a normally
distributed population, as determined using Shapiro-Wilk test
(P<0.05). The results were considered significant as follows:
#P<0.05, ##P<0.01,
###P<0.001 and ####P<0.0001 vs. the
control group (untreated group). *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs. the UVB-irradiated group.
P<0.05 was considered to indicate a statistically significant
difference.
Results
NK-CdM protects against UVB-induced
cytotoxicity by reducing ROS production
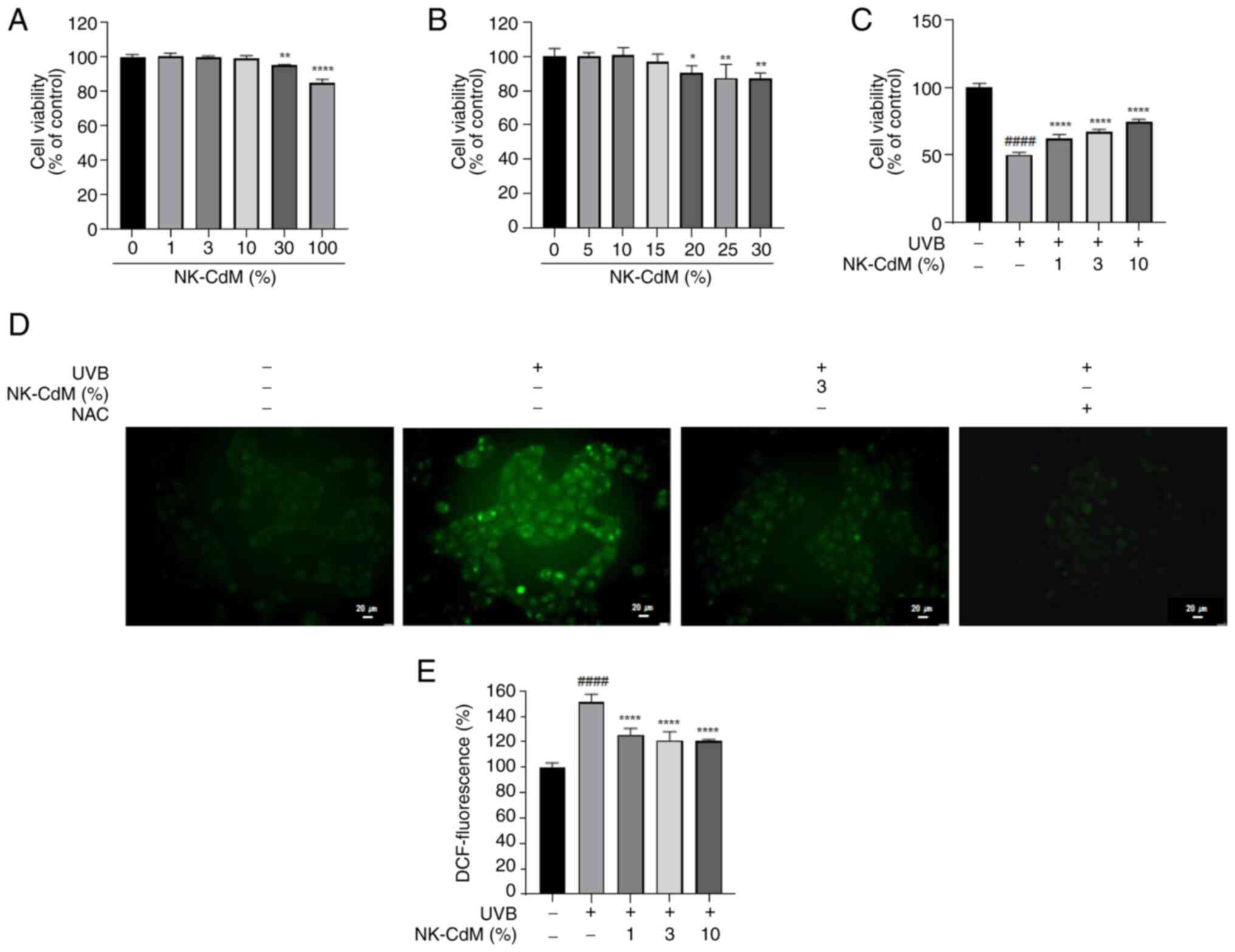
The viability of HaCaT cells was assessed using the
WST-8 assay. A viability threshold of ~80% was established to
indicate non-toxic conditions. NK-CdM did not exhibit any toxicity
at concentrations up to 10%, but cell viability decreased by 15% at
a concentration of 100% (Fig. 1A).
A more detailed assessment of NK-CdM concentrations ~30% revealed
that cell viability showed a slight decrease at 15%, though it was
not statistically significant. However, cell viability decreased
markedly at 20% NK-CdM (Fig. 1B).
Therefore, 10% was selected as the maximum concentration for
subsequent experiments with NK-CdM. To evaluate the protective
effects of NK-CdM against UVB-induced damage, HaCaT cells were
pretreated with NK-CdM at concentrations of 1, 3 and 10% prior to
UVB exposure at a dose of 30 mJ/cm2. Cell viability
increased by 12% (UVB + NK-CdM 1% group), 17% (UVB + NK-CdM 3%
group) and 24% (UVB + NK-CdM 10% group) compared with the UVB-only
group (Fig. 1C). To determine
whether the antioxidant properties of NK-CdM contribute to its
protective effects against UVB, intracellular and extracellular ROS
production was assessed using the H2DCFDA assay. Furthermore,
DCF-DA staining was conducted to measure the ROS levels in the
cells. As shown in Fig. 1D, UVB
irradiation markedly increased ROS accumulation in the cytoplasm
and mitochondria, while pretreatment with NK-CdM alleviated the ROS
levels in the cells. In addition, UVB markedly increased ROS
production compared with the control, whereas pretreatment with 3%
NK-CdM reduced ROS production by 30% relative to the UVB-only group
(Fig. 1D and E).
NK-CdM alleviates UVB-induced
oxidative stress
Next, the present study investigated the role of
NK-CdM in modulating the antioxidant defense system. The mRNA and
protein expression levels of SOD1 and CAT were analyzed. As shown
in Fig. 2A and B, UVB irradiation
decreased the expression of these proteins; however, treatment with
NK-CdM reversed this effect compared with UVB-treated cells.
Similarly, NK-CdM treatment alleviated the UVB-induced reduction in
SOD activity (Fig. 2C). Nrf2, a
key transcription factor that regulates SOD 1 and CAT genes, was
markedly downregulated at both mRNA and protein levels in
UVB-irradiated cells. However, NK-CdM attenuated this effect
(Fig. 2D and E). Moreover, NK-CdM
prevented the UVB-induced decrease in Nrf2 localization to the
nucleus (Fig. 2F).
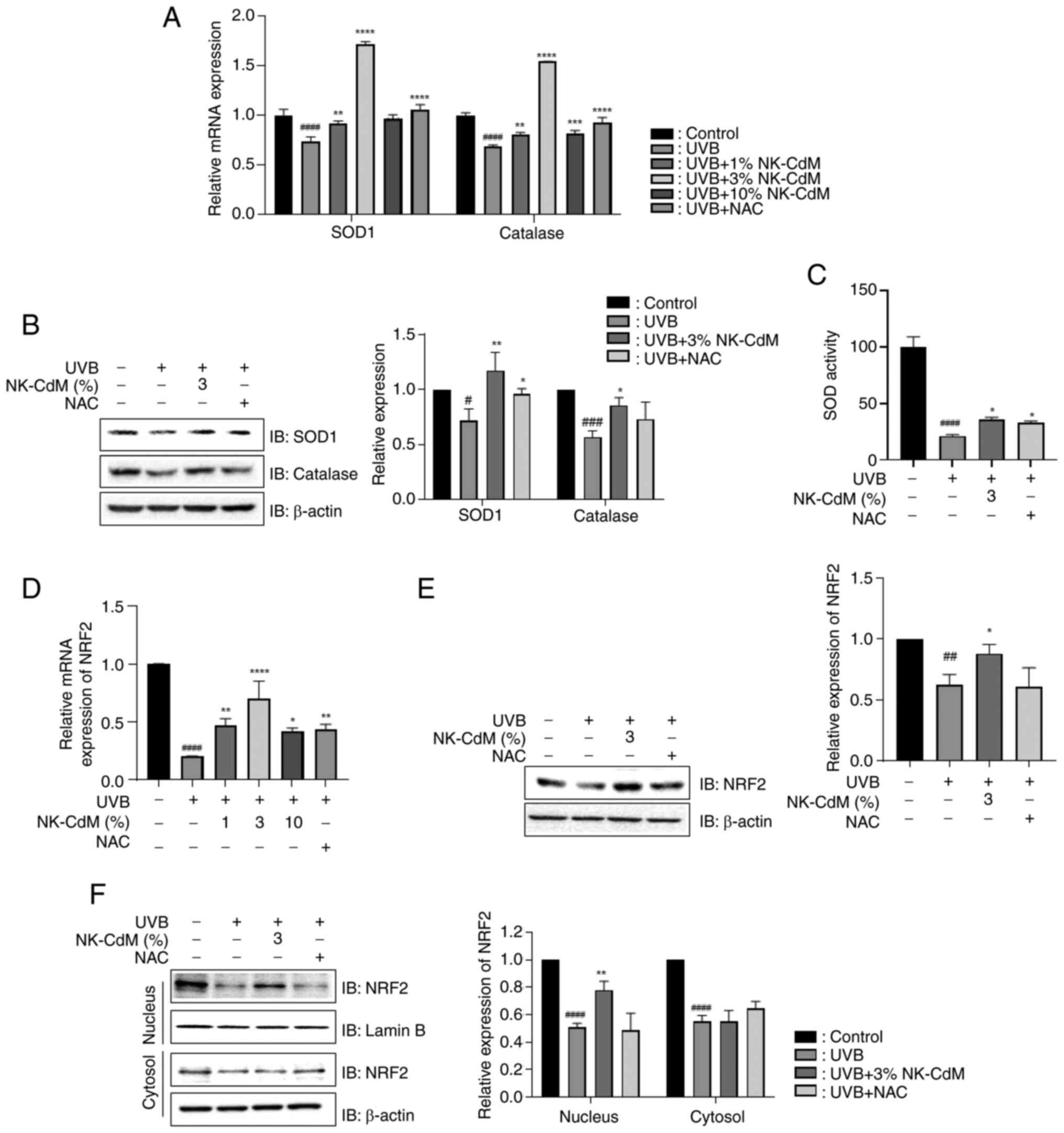 | Figure 2.Effect of NK-CdM on UVB-induced
oxidative stress in UVB-stimulated HaCaT cells. The mRNA and
protein levels in HaCaT cells pretreated with NK-CdM and NAC (10
mM) for 3 h and exposed to UVB (30 mJ/cm2) irradiation
for 3 and 24 h. The (A) mRNA and (B) protein levels of antioxidant
enzymes, including SOD1 and CAT. (C) Effect of NK-CdM on SOD
activity. The (D) mRNA and (E) protein levels of Nrf2, a key
regulator of intracellular antioxidants. (F) Protein expression of
Nrf2 in cytoplasm and nucleus fractions. The results are expressed
as the mean ± standard deviation of three independent experiment.
#P<0.05, ##P<0.01,
###P<0.001 and ####P<0.0001 vs. the
control group (untreated group). *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs. the UVB-irradiated group.
NK-CdM, NK cell-conditioned medium; UVB, ultraviolet B; SOD1,
superoxide dismutase 1; CAT, catalase; Nrf2, nuclear factor
erythroid 2-related factor 2. |
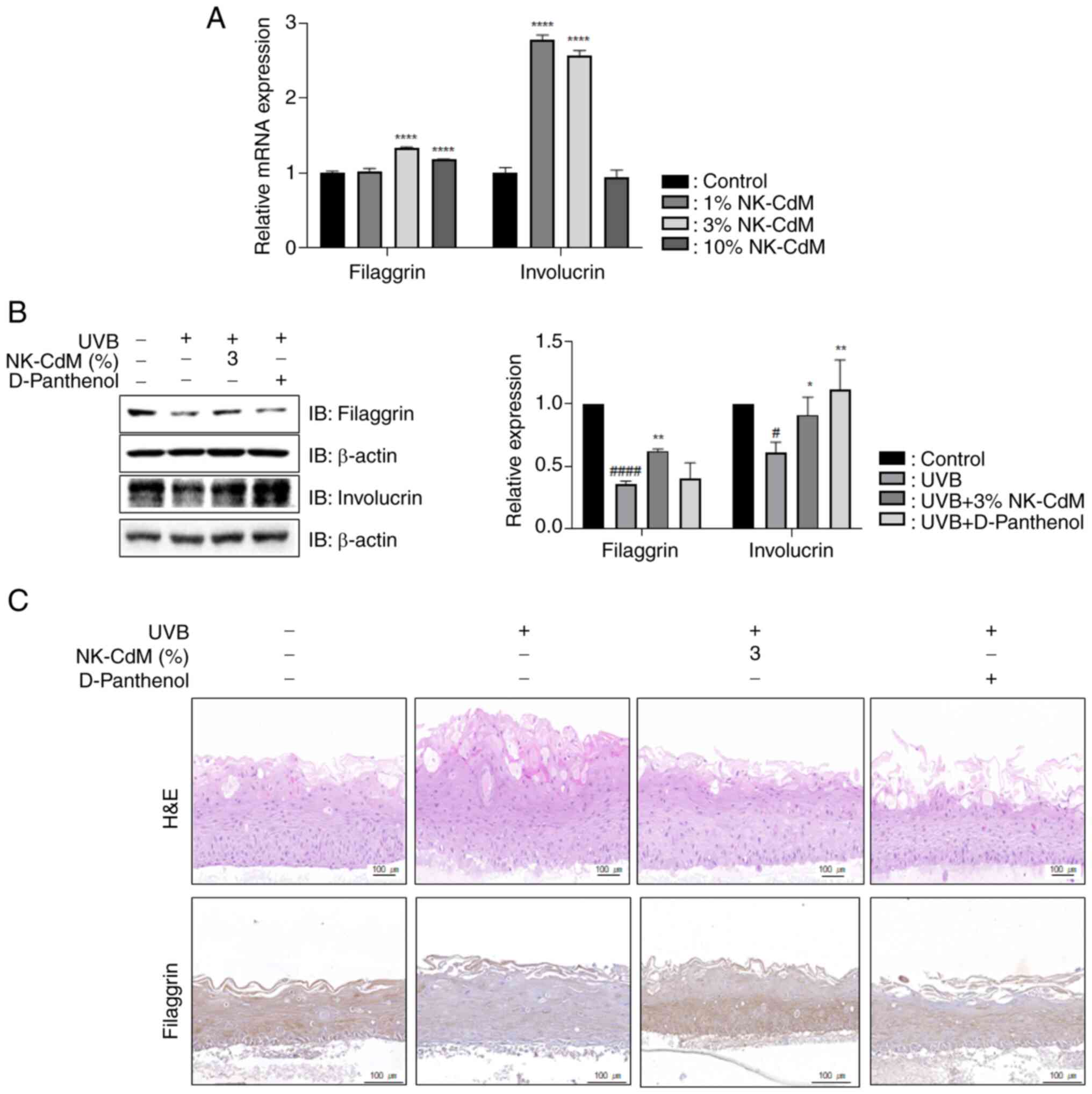
NK-CdM protects the skin barrier by
increasing filaggrin (FLG) and involucrin (IVL) expression
To determine the role of NK-CdM in skin barrier
function, the present study measured the mRNA expression levels of
both FLG and IVL following NK-CdM treatment. NK-CdM increased the
expression levels of FLG and IVL (Fig.
3A). As shown in Fig. 3B, the
levels of FLG and IVL decreased in the UVB-only group compared with
the non-irradiated group. However, the expression levels of FLG and
IVL were upregulated in the NK-CdM-treated group compared with the
UVB-only group. Additionally, in a three-dimensional (3D)
artificial skin model, NK-CdM markedly reduced UVB-induced
epidermal hyperplasia (Fig. 3C)
and restored FLG expression that had been decreased by UVB
irradiation. These results suggest that NK-CdM exerts an
anti-photoaging effect by enhancing skin barrier function.
NK-CdM attenuates the UVB-induced
reduction in the levels of hyaluronan synthase (HAS)1, HAS2, HAS3,
aquaporin 3 (AQP3) and hyaluronan (HA)
As shown in Fig.
4A, HA production markedly increased by 43% in the UVB + NK-CdM
group compared with the UVB-only group. NK-CdM also reversed the
UVB-induced reduction in HA production. To further investigate the
skin hydration efficacy of NK-CdM, the mRNA levels of HAS1, HAS2,
HAS3 and AQP3 were examined. Treatment with 1 and 3% NK-CdM
markedly increased the expression levels of HAS1, HAS2, HAS3 and
AQP3 (Fig. 4B). Moreover, NK-CdM
effectively mitigated UVB-induced reduction in these proteins
(Fig. 4C).
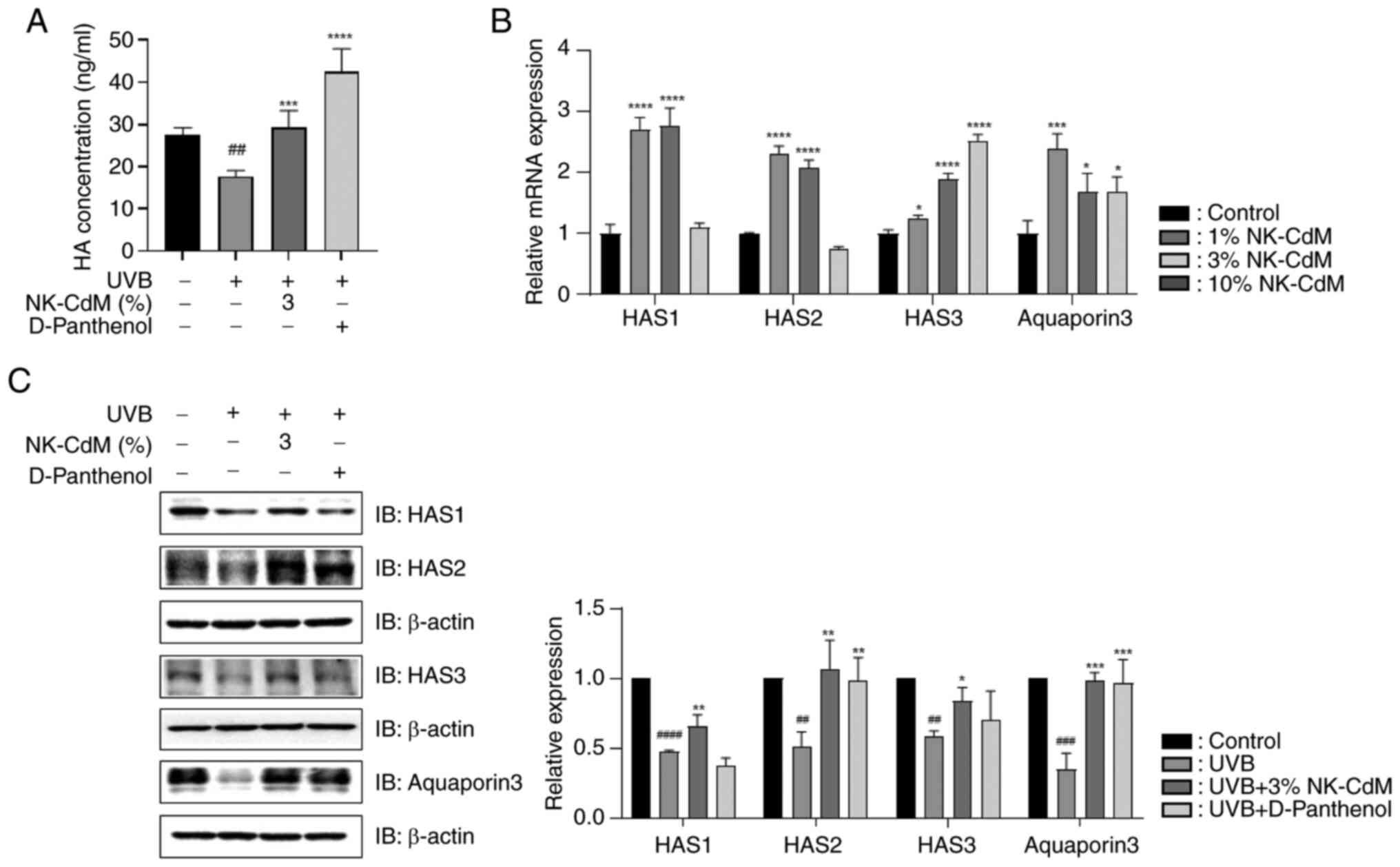 | Figure 4.Effect of NK-CdM on skin hydration in
UVB-stimulated HaCaT cells. (A) HA expression levels were analyzed
using ELISA in HaCaT cells. (B) Direct effect of NK-CdM on the mRNA
levels of HAS1, HAS2, HAS3 and AQP3. (C) Protein levels of HAS1,
HAS2, HAS3 and AQP3 in UVB-stimulated HaCaT cells. The results are
expressed as the mean ± standard deviation of three independent
experiment. ##P<0.01, ###P<0.001 and
####P<0.0001 vs. the control group (untreated group).
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs. the
UVB-irradiated group. The western blot results in Figure 4C are images taken from different
gels. NK-CdM, NK cell-conditioned medium; UVB, ultraviolet B;
ELISA, enzyme-linked immunosorbent assay; HAS, hyaluronan synthase;
AQP3, aquaporin 3. |
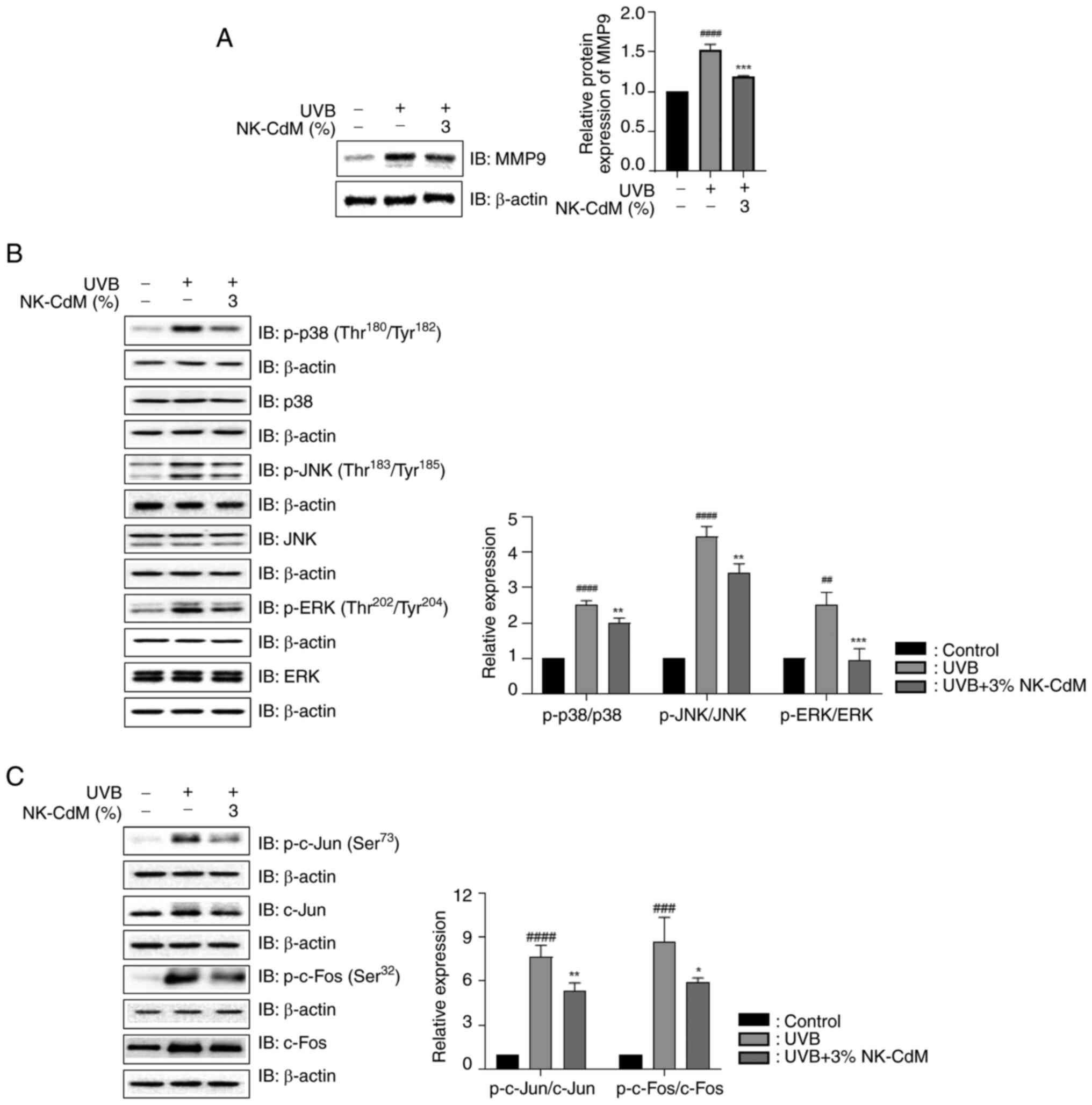
NK-CdM inhibits UVB-induced matrix
metalloproteinase-9 (MMP-9) expression and mitogen-activated
protein kinase (MAPK) phosphorylation
The effect of NK-CdM treatment on MMP-9 expression
following UVB irradiation was examined. NK-CdM markedly reduced the
UVB-induced MMP-9 production (Fig.
5A). The results revealed that UVB exposure stimulated overall
MAPK signaling molecules, but NK-CdM suppressed the phosphorylation
of extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinases (JNK) and p38 (Fig. 5B).
In addition, UVB-induced phosphorylation of the AP-1 subunits
(c-Fos and c-Jun) was markedly suppressed by NK-CdM treatment
(Fig. 5C).
NK-CdM increases the mRNA levels of
enzymes involved in ceramide (CER) synthesis
To examine the effect of NK-CdM on the expression of
enzymes involved in the synthesis of CERs containing long-chain
fatty acids (FAs), the mRNA levels of ELOVL isozyme, CER synthase
(CerS) isozymes and serine-palmitoyl transferase 2 (SPT2) were
assessed. NK-CdM treatment significantly upregulated the mRNA
expression of ELOVL1 at a concentration of 1%, ELOVL5 at 3%, and
ELOVL6 at 10% (Fig. 6A).
Similarly, MK-CdM increased the mRNA expression levels of CerS2,
CerS3 and SPT2 across these concentrations (Fig. 6B). Further assessment of CER
production in the stratum corneum (SC) was conducted by measuring
the expression of sphingomyelin synthase (SMS) and acid
sphingomyelinase (ASM). NK-CdM increased the mRNA expression levels
of SMS2 and ASM (Fig. 6C). The
effect of NK-CdM on PPAR-α was assessed and it was found that
NK-CdM upregulated PPAR-α expression (Fig. 6D). Additionally, to evaluate the
role of NK-CdM in UVB-induced lipid synthesis dysfunction, the mRNA
expression levels of ELOVLs, CerSs, SPT2, SMS and ASM were
examined. NK-CdM reversed the UVB-mediated downregulation of these
genes (Fig. 6E-G). NK-CdM
treatment restored the UVB-induced downregulation of CerS3, SPT and
ASM protein expression (Fig.
6H).
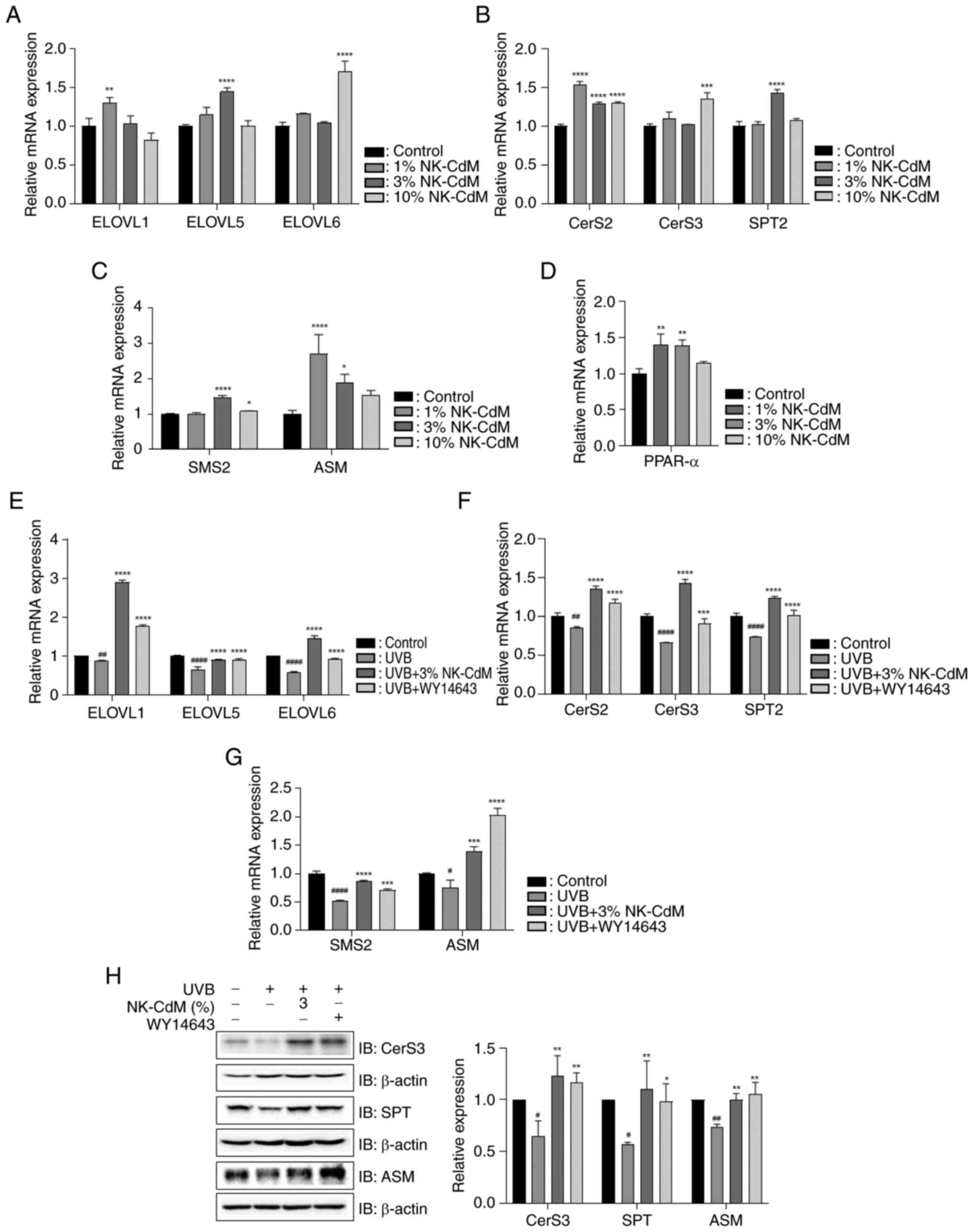 | Figure 6.Effect of NK-CdM on skin lipid
synthesis in UVB-stimulated HaCaT cells. Direct effect of NK-CdM on
the mRNA levels of (A) ELOVL1, ELOVL5 and ELOVL6, (B) CerS2, CerS3
and SPT2, (C) SMS2 and ASM and (D) PPAR-α. The mRNA levels of (E)
ELOVL1, ELOVL5 and ELOVL6, (F) CerS2, CerS3 and SPT2 and (G) SMS2
and ASM in HaCaT cells pretreated with 3% NK-CdM and WY14643 (1 µM)
for 3 h, followed by UVB (30 mJ/cm2) irradiation for 1
h. (H) The protein levels of CerS3, SPT and ASM were examined using
western blotting (images taken from different gels). The results
are expressed as the mean ± standard deviation of three independent
experiment. #P<0.05, ##P<0.01 and
####P<0.0001 vs. the control group (untreated group).
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs. the
UVB-irradiated group. NK-CdM, NK cell-conditioned medium; UVB,
ultraviolet B; ELOVL, elongation of very long chain fatty acids;
CerS, ceramide synthases; SPT, serine-palmitoyl transferase; SMS,
sphingomyelin synthase; ASM, acid sphingomyelinase. |
Discussion
With the increase in life expectancy, there is a
growing concern for health and beauty, which has produced
significant interest in the role of immune cells in skin health.
The skin serves as a physical and chemical barrier and prevents
moisture loss. Despite the unavoidable skin damage and aging caused
by external factors, including UVR, cigarette smoke and other
environmental pollutants, efforts are being made to mitigate these
effects by enhancing the function of skin cells. In the present
study, the effects of NK-CdM on UVB-induced skin photoaging were
evaluated, demonstrating that NK-CdM effectively promotes the
recovery of the skin barrier damaged by UVB exposure.
HA is naturally present in the epidermis, where it
binds to the extracellular space via CD44 and may play a role in
maintaining epidermal barrier function and hydration (23). HA synthesis is mediated by three
types of HA synthases (HAS1, HAS2 and HAS3), which are localized to
the inner plasma membrane (24).
AQPs are membrane proteins that function as channels for water
transport (25). Among them, AQP3
is the most abundant in the epidermis and is crucial for skin
hydration, as it transports both water and glycerol (26). Herein, NK-CdM preserved the mRNA
and protein expression levels of HA1, HAS2, HAS3 and AQP3 under UVB
irradiation conditions. In addition, NK-CdM prevented the reduction
in HA production caused by UVB irradiation, indicating its crucial
role in maintaining hydration homeostasis.
The free radical-oxidative stress theory of skin
photoaging, extensively proposed by Sohal (27), suggests that oxidative stress plays
a significant role in extrinsic skin aging, with ROS being the
major contributor (28,29). NK-CdM markedly suppressed ROS
production, demonstrating its antioxidant properties. UVB-induced
ROS activates MAPKs, culminating in the transcriptional regulation
of MMPs and resulting in the degradation of collagen and elastin
(30). In addition, AP-1, a
heterodimer composed of proteins belonging to c-Fos and c-Jun,
inhibits transforming growth factor β signaling, causing a
reduction in collagen synthesis, subsequently leading to photoaging
(31). NK-CdM suppressed
UVB-induced MMP9 expression, MAPK expression and AP-1 activation.
Therefore, it can be inferred that NK-CdM exhibits positive effects
against UVB-induced photoaging.
Epidermal differentiation is the process in which
keratinocytes in the epidermis undergo maturation (32). This process is crucial for
maintaining skin health with several functions, including barrier,
regulation of water balance, protection from UV radiation, immune
response, regeneration and wound healing (33). The SC is the final product of the
terminal differentiation of keratinocytes in the epidermis
(34). In particular, FLG and IVL
are essential proteins involved in the formation and functioning of
the skin barrier, serving as important structural components in the
SC (35). FLG primarily aids the
organization and structural integrity of the SC and, upon
proteolytic processing, produces free amino acids that contribute
to natural moisturizing factors, helping to maintain skin hydration
and pH balance (36). Conversely,
involucrin provides mechanical strength and resilience to the skin
(37). DPA promotes epidermal
differentiation during the wound-healing process and increases skin
hydration (38), thereby improving
miniaturization and reducing transepidermal water loss, acting as
an effective moisturizer. In HaCaT cells, NK-CdM demonstrated a
more pronounced effect than DPA in inhibiting the reduction of FLG
expression caused by UVB exposure. Furthermore, in the artificial
skin model, IHC staining for FLG revealed that NK-CdM maintained
FLG expression at a level comparable to that of DPA under
UVB-exposed conditions. These findings suggested that NK-CdM
exhibits superior efficacy in protecting the skin barrier.
In the skin, cutaneous lipids contribute to the
formation of an optimal epidermal barrier (39). CERs, which constitute ~50% of the
intercellular lipid content, are particularly important (40). Alterations in the epidermal CERs
content have been observed in certain inflammatory skin diseases,
including atopic dermatitis (AD) (41). In AD skin lesions, the levels of
CERs containing long-chain FAs (22–26 carbons in length) are
markedly reduced, while CERs with short-chain FAs (<20 carbons
in length) are elevated. This imbalance compromises the integrity
of the skin barrier, leading to impaired function, increased water
loss and decreased resistance to external irritants (42).
ELOVL elongases, particularly ELOVL-1, ELOVL-5 and
ELOVL-6, are critical in the biosynthesis of CERs, as they elongate
FAs to produce very long-chain fatty acids (VLCFAs) (43). These VLCFAs are essential
components of CERs, which are crucial for maintaining the integrity
and functionality of the skin barrier (44). The initial step in de novo
sphingolipid synthesis, involving the condensation of serine and
palmitoyl-CoA, is catalyzed by SPT (45). Ceramide synthases (CerS) 1 and 2
are key enzymes in the biosynthesis of CERs. Subsequently, SMS
synthesizes sphingomyelin, while ASM hydrolyzes it between the SC
and the stratum granulosum, ultimately producing CER in the SC,
which forms a strong and cohesive lipid barrier (46,47).
NK-CdM increases the expression levels of ELOVL-1, ELOVL-5 and
ELOVL-6, as well as CerS-2 and CerS-3. It has been reported that
the expression of PPAR-α is upregulated during epidermal
differentiation. Activation of PPAR-α subsequently enhances the
synthesis of cholesterol and CERs in keratinocytes (48,49).
NK-CdM also increased the expression of PPAR-α, suggesting that
NK-CdM not only stimulates epidermal differentiation but also
promotes CER synthesis. Moreover, NK-CdM demonstrated a protective
effect against the expression of CER synthesis-related factors
induced by UVB, similar to the PPAR-α agonist WY14643.
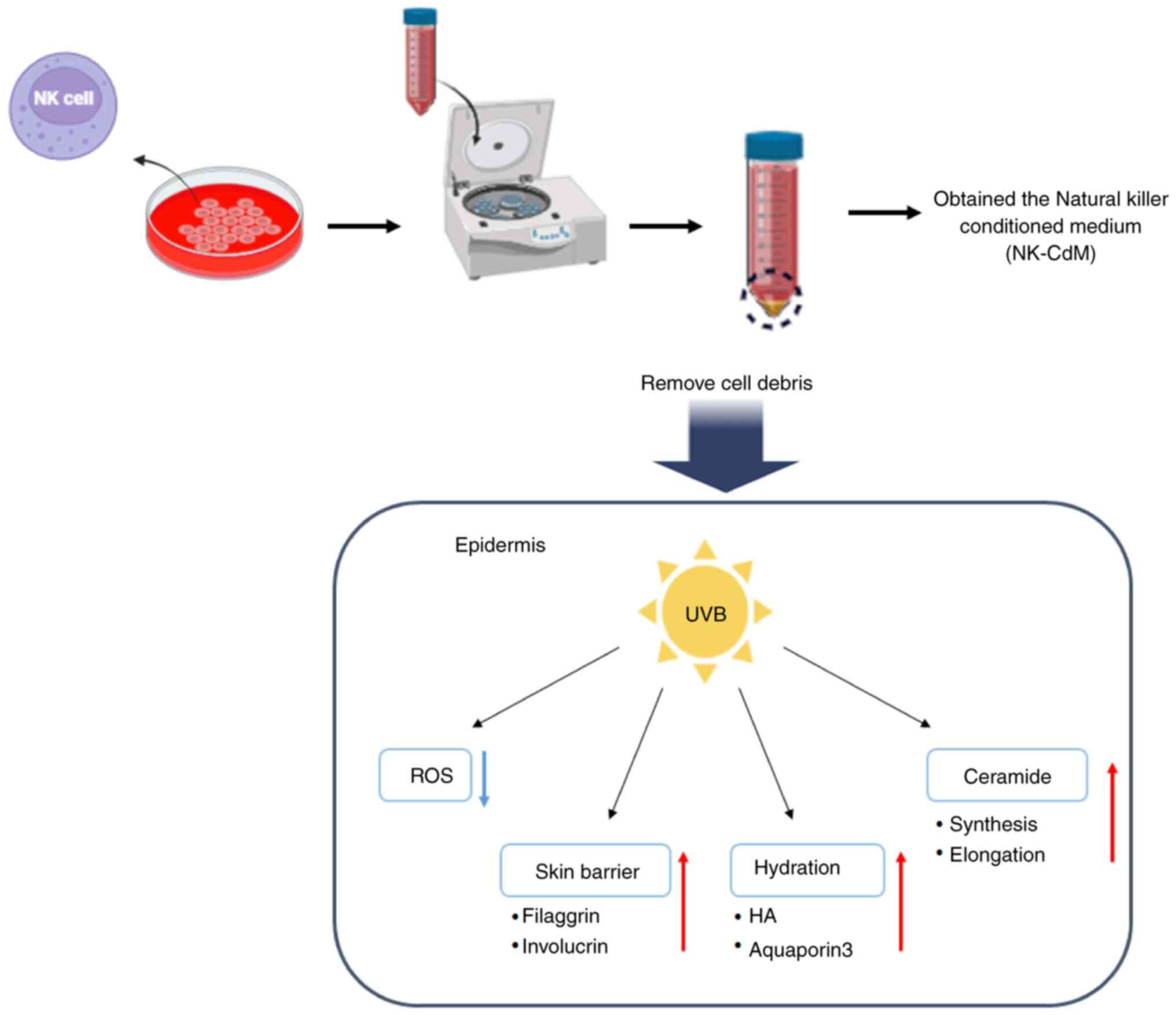
The results of the present study demonstrated that
NK-CdM effectively maintains and protects skin barrier homeostasis
in HaCaT cells and 3D artificial skin exposed to UVB radiation
(Fig. 7). However, there are major
challenges that need to be addressed in future research. First, a
larger, more diverse cohort is needed to improve the
reproducibility and generalizability of the findings. In the
future, the sample size will be increased to enhance the
reproducibility and generalizability of the research findings.
Second, there is currently no information on which specific
cytokines within NK-CdM improve skin barrier damage caused by
photoaging. Third, the study is limited by the inability to confirm
the effects of NK-CdM on photoaging in a mouse model. Future
research will aim to investigate the effects of NK-CdM in a mouse
photoaging model. As a result, the effects of the designated NK-CdM
medium cannot be scientifically substantiated at this time.
Accordingly, future research will focus on identifying which
cytokines included in NK-CdM are effective against photoaging.
In conclusion, these findings underscored the
potential of NK-CdM for both cosmetic and therapeutic applications
in mitigating UVB-induced skin aging.
Acknowledgements
Not applicable.
Funding
This research was supported by Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Education (grant no. RS-2024-00349603).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JOL was responsible for the study conception and
design, acquisition of data, analysis and interpretation of data,
as well as drafting the manuscript or critically revising it for
important intellectual content. JML was responsible for
methodology, investigation, formal analysis, writing the manuscript
and conduct of the experiments. YK and AYP were responsible for
investigation, methodology and formal analysis. DY, SYK and JH were
responsible for conducting the experiments and analysing the data.
SH, HN, HS, KJ and MI were responsible for revising the manuscript
and analysing the data. BJK was responsible for conceptualization,
methodology, research and project administration. JOL and BK
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walker M: Human skin through the ages. Int
J Pharm. 622:1218502022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zegarska B, Pietkun K, Zegarski W, Bolibok
P, Wiśniewski M, Roszek K, Czarnecka J and Nowacki M: Air
pollution, UV irradiation and skin carcinogenesis: What we know,
where we stand and what is likely to happen in the future? Postepy
Dermatol Alergol. 34:6–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lotfollahi Z: The anatomy, physiology and
function of all skin layers and the impact of ageing on the skin.
Wound Pract Res. 32:6–10. 2024.
|
|
4
|
Baroni A, Buommino E, De Gregorio V,
Ruocco E, Ruocco V and Wolf R: Structure and function of the
epidermis related to barrier properties. Clin Dermatol. 30:257–262.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wikramanayake TC, Stojadinovic O and
Tomic-Canic M: Epidermal differentiation in barrier maintenance and
wound healing. Adv Wound Care (New Rochelle). 3:272–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo
Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and
chronological skin aging. Arch Dermatol. 138:1462–1470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ansary TM, Hossain MR, Kamiya K, Komine M
and Ohtsuki M: Inflammatory molecules associated with ultraviolet
radiation-mediated skin aging. Int J Mol Sci. 22:39742021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brenner M and Hearing VJ: The protective
role of melanin against UV damage in human skin. Photochem
Photobiol. 84:539–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D'Orazio J, Jarrett S, Amaro-Ortiz A and
Scott T: UV radiation and the skin. Int J Mol Sci. 14:12222–12248.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang X, Yang T, Yu D, Xiong H and Zhang S:
Current insights and future perspectives of ultraviolet radiation
(UV) exposure: Friends and foes to the skin and beyond the skin.
Environ Int. 185:1085352024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rastogi RP, Richa Kumar A, Tyagi MB and
Sinha RP: Molecular mechanisms of ultraviolet radiation-induced DNA
damage and repair. J Nucleic Acids. 2010:5929802010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kozlov AV, Javadov S and Sommer N:
Cellular ROS and antioxidants: Physiological and pathological role.
Antioxidants (Basel). 13:6022024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ngo V and Duennwald ML: Nrf2 and oxidative
stress: A general overview of mechanisms and implications in human
disease. Antioxidants (Basel). 11:23452022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lubos E, Loscalzo J and Handy DE:
Glutathione peroxidase-1 in health and disease: From molecular
mechanisms to therapeutic opportunities. Antioxid Redox Signal.
15:1957–1997. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hammad M, Raftari M, Cesário R, Salma R,
Godoy P, Emami SN and Haghdoost S: Roles of oxidative stress and
Nrf2 signaling in pathogenic and non-pathogenic cells: A possible
general mechanism of resistance to therapy. Antioxidants (Basel).
12:13712023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campbell KS and Hasegawa J: Natural killer
cell biology: An update and future directions. J Allergy Clin
Immunol. 132:536–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lodoen MB and Lanier LL: Natural killer
cells as an initial defense against pathogens. Curr Opin Immunol.
18:391–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paul S and Lal G: The molecular mechanism
of natural killer cells function and its importance in cancer
immunotherapy. Front Immunol. 8:11242017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim DS, Kim DJ, Kim HP, Hwang SH and Kang
JH: Potential of natural killer cell enriched conditioned media for
skin care and anti-aging. J Cosmet Dermatol Sci Appl. 11:123–139.
2021.
|
|
20
|
Lee SE, Kwon TR, Kim JH, Lee BC, Oh CT, Im
M, Hwang YK, Paik SH, Han S, Kim JY and Kim BJ: Anti-photoaging and
anti-oxidative activities of natural killer cell conditioned medium
following UV-B irradiation of human dermal fibroblasts and a
reconstructed skin model. Int J Mol Med. 44:1641–1652.
2019.PubMed/NCBI
|
|
21
|
Min B, Choi H, Her JH, Jung MY, Kim HJ,
Jung MY, Lee EK, Cho SY, Hwang YK and Shin EC: Optimization of
large-scale expansion and cryopreservation of human natural killer
cells for anti-tumor therapy. Immune Netw. 18:e312018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang MC, Yumnam S and Kim SY: Oral intake
of collagen peptide attenuates ultraviolet B irradiation-induced
skin dehydration in vivo by regulating hyaluronic acid synthesis.
Int J Mol Sci. 19:35512018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vigetti D, Genasetti A, Karousou E, Viola
M, Clerici M, Bartolini B, Moretto P, De Luca G, Hascall VC and
Passi A: Modulation of hyaluronan synthase activity in cellular
membrane fractions. J Biol Chem. 284:30684–30694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agarwal S and Gupta A: Aquaporins: The
renal water channels. Indian J Nephrol. 18:95–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boury-Jamot M, Sougrat R, Tailhardat M, Le
Varlet B, Bonté F, Dumas M and Verbavatz JM: Expression and
function of aquaporins in human skin: Is aquaporin-3 just a
glycerol transporter? Biochim Biophys Acta. 1758:1034–1042. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sohal RS: Role of oxidative stress and
protein oxidation in the aging process. Free Radic Biol Med.
33:37–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Yang C and Jiang G: Research
progress on skin photoaging and oxidative stress. Postepy Dermatol
Alergol. 38:931–936. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Papaccio F, Arino A, Caputo S and Bellei
B: Focus on the contribution of oxidative stress in skin aging.
Antioxidants (Basel). 11:11212022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JM, Kim SY, Noh EM, Song HK, Lee GS,
Kwon KB and Lee YR: Reversine inhibits MMP-1 and MMP-3 expressions
by suppressing of ROS/MAPK/AP-1 activation in UV-stimulated human
keratinocytes and dermal fibroblasts. Exp Dermatol. 27:298–301.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Silbiger S, Lei J and Neugarten J:
Estradiol suppresses type I collagen synthesis in mesangial cells
via activation of activator protein-1. Kidney Int. 55:1268–1276.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moltrasio C, Romagnuolo M and Marzano AV:
Epigenetic mechanisms of epidermal differentiation. Int J Mol Sci.
23:48742022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meyer W and Seegers U: Basics of skin
structure and function in elasmobranchs: A review. J Fish Biol.
80:1940–1967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haftek M, Oji V, Feldmeyer L, Hohl D,
Hadj-Rabia S and Abdayem R: The fate of epidermal tight junctions
in the stratum corneum: Their involvement in the regulation of
desquamation and phenotypic expression of certain skin conditions.
Int J Mol Sci. 23:74862022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Del Rosso JQ: Repair and maintenance of
the epidermal barrier in patients diagnosed with atopic dermatitis:
An evaluation of the components of a body wash-moisturizer skin
care regimen directed at management of atopic skin. J Clin Aesthet
Dermatol. 4:45–55. 2011.PubMed/NCBI
|
|
36
|
McAleer MA, Jakasa I, Raj N, O'Donnell
CPF, Lane ME, Rawlings AV, Voegeli R, McLean WHI, Kezic S and
Irvine AD: Early-life regional and temporal variation in
filaggrin-derived natural moisturizing factor, filaggrin-processing
enzyme activity, corneocyte phenotypes and plasmin activity:
Implications for atopic dermatitis. Br J Dermatol. 179:431–441.
2018.PubMed/NCBI
|
|
37
|
Guneri D, Voegeli R, Gurgul SJ, Munday MR,
Lane ME and Rawlings AV: A new approach to assess the effect of
photodamage on corneocyte envelope maturity using combined
hydrophobicity and mechanical fragility assays. Int J Cosmet Sci.
Mar 23–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Camargo FB Jr, Gaspar LR and Maia Campos
PM: Skin moisturizing effects of panthenol-based formulations. J
Cosmet Sci. 62:361–370. 2011.PubMed/NCBI
|
|
39
|
Knox S and O'Boyle NM: Skin lipids in
health and disease: A review. Chem Phys Lipids. 236:1050552021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kono T, Miyachi Y and Kawashima M:
Clinical significance of the water retention and barrier
function-improving capabilities of ceramide-containing
formulations: A qualitative review. J Dermatol. 48:1807–1816. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Upadhyay PR, Seminario-Vidal L, Abe B,
Ghobadi C and Sims JT: Cytokines and epidermal lipid abnormalities
in atopic dermatitis: A systematic review. Cells. 12:27932023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Janssens M, van Smeden J, Gooris GS, Bras
W, Portale G, Caspers PJ, Vreeken RJ, Hankemeier T, Kezic S,
Wolterbeek R, et al: Increase in short-chain ceramides correlates
with an altered lipid organization and decreased barrier function
in atopic eczema patients. J Lipid Res. 53:2755–2766. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X, Yu H, Gao R, Liu M and Xie W: A
comprehensive review of the family of very-long-chain fatty acid
elongases: Structure, function, and implications in physiology and
pathology. Eur J Med Res. 28:5322023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Uchida Y: The role of fatty acid
elongation in epidermal structure and function. Dermatoendocrinol.
3:65–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hanada K: Serine palmitoyltransferase, a
key enzyme of sphingolipid metabolism. Biochim Biophys Acta.
1632:16–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jenkins RW, Canals D and Hannun YA: Roles
and regulation of secretory and lysosomal acid sphingomyelinase.
Cell Signal. 21:836–846. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huh YE, Chiang MSR, Locascio JJ, Liao Z,
Liu G, Choudhury K, Kuras YI, Tuncali I, Videnovic A, Hunt AL, et
al: β-Glucocerebrosidase activity in GBA-linked Parkinson disease:
The type of mutation matters. Neurology. 95:e685–e696. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chon SH, Tannahill R, Yao X, Southall MD
and Pappas A: Keratinocyte differentiation and upregulation of
ceramide synthesis induced by an oat lipid extract via the
activation of PPAR pathways. Exp Dermatol. 24:290–295. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rivier M, Castiel I, Safonova I, Ailhaud G
and Michel S: Peroxisome proliferator-activated receptor-alpha
enhances lipid metabolism in a skin equivalent model. J Invest
Dermatol. 114:681–687. 2000. View Article : Google Scholar : PubMed/NCBI
|