Introduction
Osteoarthritis (OA) is a long-term, degenerative
joint disease that presents significant clinical challenges and
imposes considerable financial burdens. Currently, >300 million
individuals worldwide suffer from OA and by 2030, it is expected to
become the leading cause of disability globally (1–3).
The lack of a clear pathophysiology has hindered
efforts to develop effective treatments. OA was once thought to
affect only cartilage; however, it is now characterized as a
low-grade inflammatory disease affecting the entire joint.
Long-term OA can induce changes in the meniscus, subchondral bone
remodeling, osteophyte synthesis, and progressive cartilage
destruction (3–5). These mechanical processes should be
considered alongside the inflammation, such as synovitis, which
plays a major role in the pathogenesis of the disease (2,6).
The complex system referred to as the arthritic
microenvironment comprises a number of cell types, including
fibroblasts and inflammatory cells, such as dendritic cells,
macrophages, and monocytes (7).
Among these, monocytes and macrophages make up most of the cells
observed in the arthritic milieu (8). Cytokines attract specific subsets of
these cells, which influence the course of OA. Note that the
migration of monocytes and macrophages into the joint and their
subsequent attachment to the synovial membrane are important steps
in the development and progression of OA (9). Vascular cell adhesion molecule-1
(VCAM-1) is one of several molecules that control the adhesion of
monocytes/macrophages to the synovium (10). Researchers have determined that the
synovial tissue of OA patients synthesizes more VCAM-1 than does
normal synovial tissue (11).
Thus, one potential strategy to reducing OA-related inflammation is
to moderate the production of VCAM-1 in the synovium (7,12).
Fibrosis plays a crucial role in the etiology of
several degenerative illnesses, including OA; however, the complex
underlying processes have yet to be elucidated. Fibrotic processes
can be triggered or exacerbated by inflammatory responses and
chronic inflammation is frequently associated with the persistent
deposition of extracellular matrix (ECM) and the ensuing tissue
scarring (13). The interaction
between fibrotic tissue and inflammatory reactions is a reciprocal
process, often resulting in a vicious cycle that accelerates OA
progression (14). Connective
tissue growth factor (CTGF), also known as CCN2, is a key factor in
the pathogenic processes driving inflammation and fibrotic tissue
formation in OA (15,16). CTGF expression is higher in the
cartilage from OA patients than in normal cartilage and expression
levels are associated with disease severity (17).
It has been established that CTGF promotes
osteoclastogenesis and enhances the production of proinflammatory
cytokines in OASFs (18–20); however, the effect of monocyte
adherence to the synovium during OA remains poorly understood. The
current study revealed that CTGF and monocyte marker expression
levels were higher in OA patients compared with normal individuals.
It was also determined that CTGF promoted VCAM-1 expression in
OASFs and facilitated the adhesion of monocytes to synovial
fibroblasts via the focal adhesion kinase (FAK) and JNK signaling
cascades. These findings suggested a plausible mechanism to explain
the association between CTGF and OA, while offering a novel
therapeutic target for OA.
Materials and methods
Materials
CTGF recombinant protein was obtained from
PeproTech, Inc. Antibodies against phosphorylated (p-)FAK (cat. no.
3283S) and p-JNK (cat. no. 9251S) were acquired from Cell Signaling
Technology, Inc. Antibodies against VCAM-1 (cat. no. GTX110684),
FAK (cat. no. GTX50489), JNK (cat. no. GTX52360) and β-actin (cat.
no. GTX109639) were acquired from GeneTex International
Corporation. Specific inhibitors of FAK (cat. no. 324878) were
obtained from Calbiochem (Merck KGaA). Specific inhibitors of JNK;
SP600125 (cat. no. BML-EI305-0010) was obtained from Enzo Life
Sciences, Inc. Supplements for cell culture were purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). Small interfering
(si)RNAs targeting FAK and JNK were purchased from GE Healthcare
Dharmacon, Inc. The source of all other reagents was
MilliporeSigma.
Cell cultures
OA synovial tissue specimens were obtained from
patients with OA undergoing knee replacement surgery (n=5) and
patients undergoing arthroscopy after trauma or joint derangement,
who served as normal controls (n=5), at the Department of
Orthopedic Surgery, China Medical University Hospital, Taichung,
Taiwan. The specimens were collected between January 2021 and
January 2022. The ages of OA patients ranged from 65 to 82 years.
The male-to-female ratio was 2:3. Prior to participation, all
patients provided written informed consent, with approval from the
Institutional Review Board (IRB) of China Medical University
Hospital (approval no. CMUH109-REC2-181). OASFs were digested in
Collagenase Type II and then cultured in accordance with the
methods in a previous study (21).
Cells from the human monocyte cell line THP-1 from the American
Type Culture Collection were cultivated in Dulbecco's Modified
Eagle's Medium (DMEM) containing 10% FBS, penicillin, and
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) in a
humidified environment with 5% CO2 at 37°C.
Examination of monocyte adhesion to
OASFs
Monocytes were stimulated with BCECF-AM (Invitrogen,
Thermo Fisher Scientific, Inc.) (10 µM) at 37°C for 1 h. OASFs were
exposed to CTGF for 24 h, and then exposed to the monocytes for 1
h. Nonadherent monocytes were rinsed off and adherent monocytes
were quantified using a fluorescence microscope.
Bioinformatic analysis
Synovial tissue samples from healthy donors and OA
patients were examined in terms of CTGF expression levels and
fibrosis-related variables using the GDS5401 dataset from the
Global Data Services database
(ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS5401).
Anterior cruciate ligament transection
(ACLT)-induced OA model
Male Sprague Dawley (SD) rats (eight-weeks-old;
weighing 300–350 g) were purchased from the National Laboratory
Animal facility (Taipei, Taiwan). The animals were housed in the
animal care facility of China Medical University (Taichung, Taiwan)
and were housed in a specific pathogen-free environment with
controlled temperature (22–24°C) and humidity (45–55%). Rats had ad
libitum access to sterilized rodent chow and autoclaved water
throughout the experiment, and they were maintained on a 12/12-h
light/dark cycle. A total of 12 Rats were used in the
experiment.
Rats in the experiment group (n=6) underwent
anterior cruciate ligament (ACL) transection during arthrotomy in
accordance with the methods outlined in previous studies (22,23).
In brief, the left knee was prepared using a surgically sterile
technique. The ACL fibers were transected with a scalpel, and the
entire medial meniscus was excised through a medial parapatellar
mini-arthrotomy. The joint surface was irrigated with sterile
saline solution, and both the capsule and skin were sutured
following ACL transection and medial meniscectomy. Ampicillin (50
mg/kg body weight) was administered for five days postoperatively.
Rats in the control group (Control; n=6) did not have their ACLs
severed during arthrotomy. All animal experiments were conducted in
strict accordance with a protocol approved by the Institutional
Animal Care and Use Committee of China Medical University (approval
no. CMUIACUC-2021-050-2).
Immunohistochemical (IHC)
staining
Slices were obtained from serial sections of human
synovial tissue. After fixing the synovial tissue in 1%
formaldehyde at 37°C overnight, the formalin-fixed,
paraffin-embedded tissues were sectioned at a thickness of 4 µm and
baked in an oven at 65°C for >2 h. The paraffin sections were
deparaffinized using xylene and washed with gradient alcohol and
then distilled water. The sections were placed in a citrate-based
solution, heated in a microwave oven, and cooled to room
temperature for antigen retrieval. Endogenous peroxidase activity
was blocked using 3% hydrogen peroxide (37°C, 10 min). Then, bovine
serum albumin (BSA; Thermo Fisher Scientific, Inc.) was used to
block and prevent nonspecific staining (37°C, 10 min). All sections
were stained with anti-CTGF (cat. nos. MAB660, R&D, MN, USA) or
anti-CD11b (cat. nos. MAB1699, R&D, MN, USA) antibodies (1:100)
and incubated overnight at 4°C. The staining results were measured
as previously described (19).
After incubation with the primary antibodies, peroxidase-labeled
second antibodies (1:100) (cat. nos. RE7280-K, Leica Biosystems,
Newcastle-upon-Tyne, UK) were applied at room temperature for 60
min to visualize antigens. The slices were rinsed in PBS, stained
with 3,3′-diaminobenzidine; DAB (liquid DAB + substrate, Leica
Biosystems), and counterstained with hematoxylin (37°C, 3 min)
followed drying, and mounting. The staining procedures were carried
out using the Leica Novolink Polymer Detection system (Leica
Biosystems Inc.). The tissue sections were finally observed under
an optical photographic light microscopy (magnifications, ×10 and
×20) and the final staining scores were calculated by summing the
staining intensity and the percentage of positive cells (24–26).
Reverse transcription-quantitative
(RT-q) PCR
3×105 OASF cells were seeded in 6-well
dishes, and the RNA was extracted using a TRIzol kit (Invitrogen,
Thermo Fisher Scientific, Inc.) following the manufacturer's
instructions. The quality and quantity of the RNA were assessed
using a Nanovue Spectrophotometer (GE Healthcare) based on A260
values. cDNA synthesis was performed using an M-MLV RT kit
(Invitrogen; Thermo Fisher Scientific, Inc.) with 1 µg of total RNA
in accordance with the manufacturer's recommendations. RT-qPCR was
performed using the KAPA SYBR® FAST qPCR Kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) (23,24,27).
RT-qPCR assays were carried out in triplicate using a StepOnePlus
sequence detection system. The cycling conditions were as follows:
Initial 10-min polymerase activation at 95°C followed by 40 cycles
of denaturation at 95°C for 15 sec and annealing/extension at 60°C
for 60 sec. The expression levels of VCAM-1 and ICAM-1, relative to
GAPDH, were determined using the ΔCq comparative methods (18). The primer sequences used were as
follows: VCAM-1 forward primer (TTCCAGGGACTTCCTGTCTG) and reverse
primer (TCCGTCTCATTGACTTGCAG); intercellular adhesion molecule-1
(ICAM-1) forward primer (ATGCCCAGACATCTGTGTC) and reverse primer
(GGGGTCTCTATGCCCAACAA); GAPDH forward primer (ACCACAGTCCATGCCATCAC)
and reverse primer (TCCACCACCCTGTTGCTGTA).
Western blot analysis
OASFs were lysed in RIPA lysis buffer (cat. no.
P0013; Beyotime Institute of Biotechnology), and the BCA Protein
Assay Kit (Thermo Fisher Scientific Inc., IL, USA) was used to
quantify the protein in the extracts. SDS-PAGE on 10% gels was used
to resolve the extracted proteins (30 µg), followed by transfer to
PVDF membranes in accordance with the procedures outlined in our
earlier publications (28–31). Briefly, the membranes were blocked
for 1 h in PBST containing 4% non-fat milk at room temperature and
incubated overnight at 4°C with primary antibodies targeting p-FAK
(cat. no. 3283S; 1:1,000; Cell Signaling Technology, Inc.), p-JNK
(cat. no. 9251S; 1:1,000; Cell Signaling Technology, Inc.), VCAM-1
(cat. no. GTX110684; 1:1,000; GeneTex International Corporation.),
FAK (cat. no. GTX50489; 1:1,000; GeneTex International
Corporation.), JNK (cat. no. GTX52360; 1:1,000; GeneTex
International Corporation.), and FAK (cat. no. 324878; 1:1,000;
Calbiochem, Merck KGaA). After washing using a PBST washing buffer,
the blots were incubated for 60 min at 37°C with horseradish
peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG,
cat. no. sc-2357; 1:1,000; goat anti-mouse IgG, cat. no. sc-516102;
1:1,000; Santa Cruz Biotechnology, Inc.). β-actin (cat. no.
GTX109639; 1:5,000; GeneTex International Corporation.) were used
as internal references for total protein. Immunoreactive bands were
detected using an enhanced chemiluminescence reagent (ECL) from
Merck Millipore. The blot membranes were visualized using a
Fujifilm LAS-3000 imaging system (FUJIFILM Wako Pure Chemical
Corporation). After standardization to β-actin, ImageJ v1.48
software (National Institutes of Health) was used to measure the
optical density of the blot.
ELISA
The levels of CTGF in the serum samples of all
subjects were determined using commercial ELISA kits (Human
CTGF/CCN2 DuoSet ELISA kit, DY9190, R&D, MN, USA) according to
the manufacturer's instructions. The plates were read at 450 nm,
and the absorbance values were measured using a Multiskan GO
microplate reader (Thermo Fisher Scientific, Inc.). Calculations
were performed based on the standard curve to determine sample
concentrations.
Statistical analysis
Quantified data were analyzed using GraphPad Prism
8.0 software (Dotmatics). All values are presented as the mean ±
SD). Statistical significance between experiment groups was
evaluated using unpaired Student's t-test. Comparisons involving
more than two groups were conducted using one-way analysis of
variance followed by Bonferroni's post hoc test. Spearman's rank
correlation was used to assess IHC staining scores in human
synovial tissue. P<0.05 was considered to indicate a
statistically significant difference.
Results
CTGF and monocyte expression levels
are higher in tissue from OA patients compared with normal
tissue
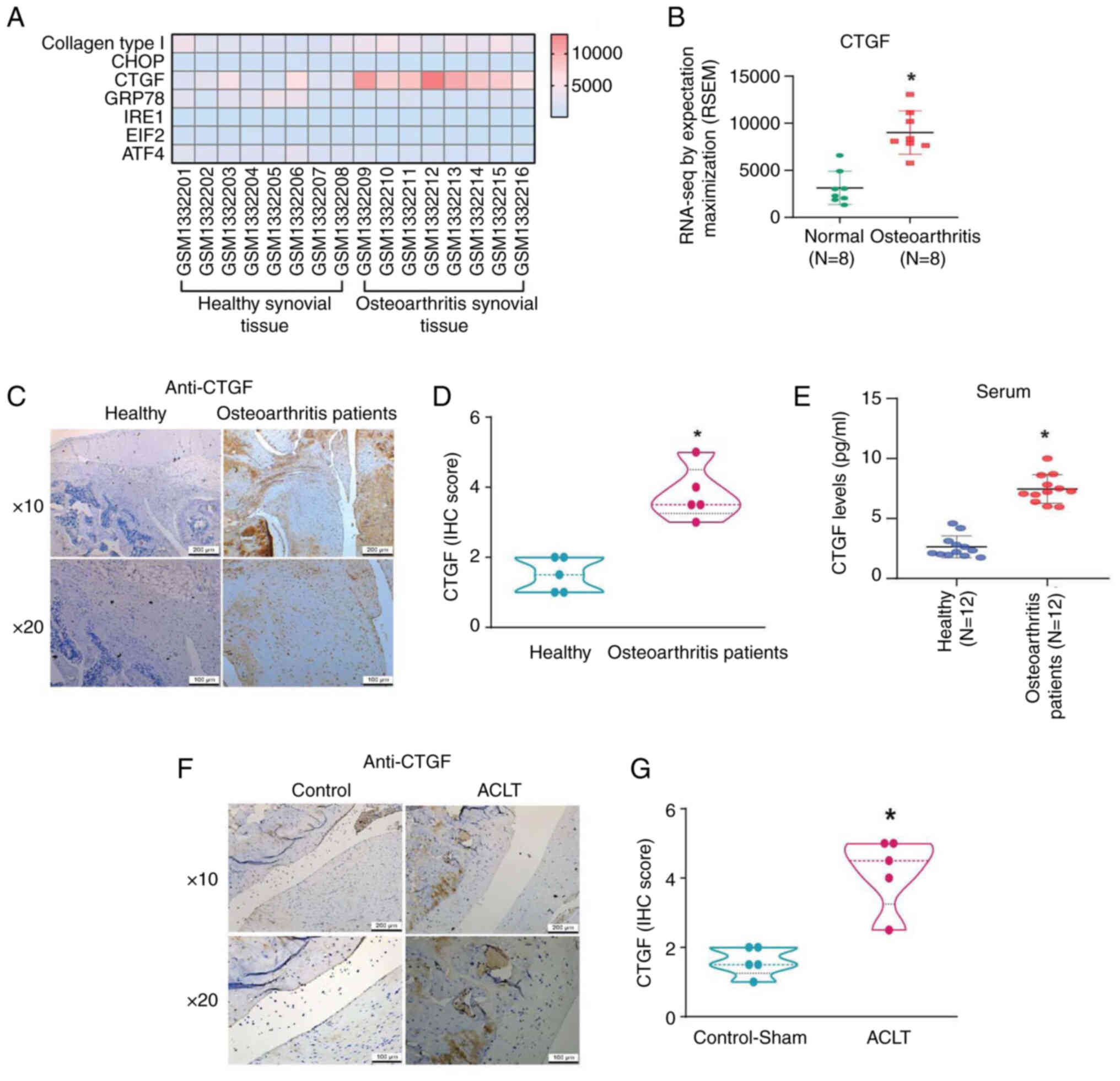
Fibrosis has been shown to play a key role in the
pathogenesis of degenerative disorders, such as OA (14). GDS5401 dataset was used to explore
the expression of fibrosis factors during OA development. Among the
various fibrosis factors, CTGF expression was higher among OA
patients than among normal individuals; however, no difference was
observed between the two groups in terms of type I collagen, C/EBP
homologous protein, glucose-regulated protein 78,
Inositol-requiring enzyme 1 (IRE1), Eukaryotic Initiation Factor 2
and Activating transcription factor 4 (ATF4) expression (Figs. 1A and B and S1). IHC analysis of synovial tissues and
ELISA of serum from clinical samples confirmed that CTGF expression
levels were higher in OA patients than in normal controls (Fig. 1C-E). Moreover, CTGF expression
levels were higher in rats with ACLT-induced OA compared with the
controls (Fig. 1F and G).
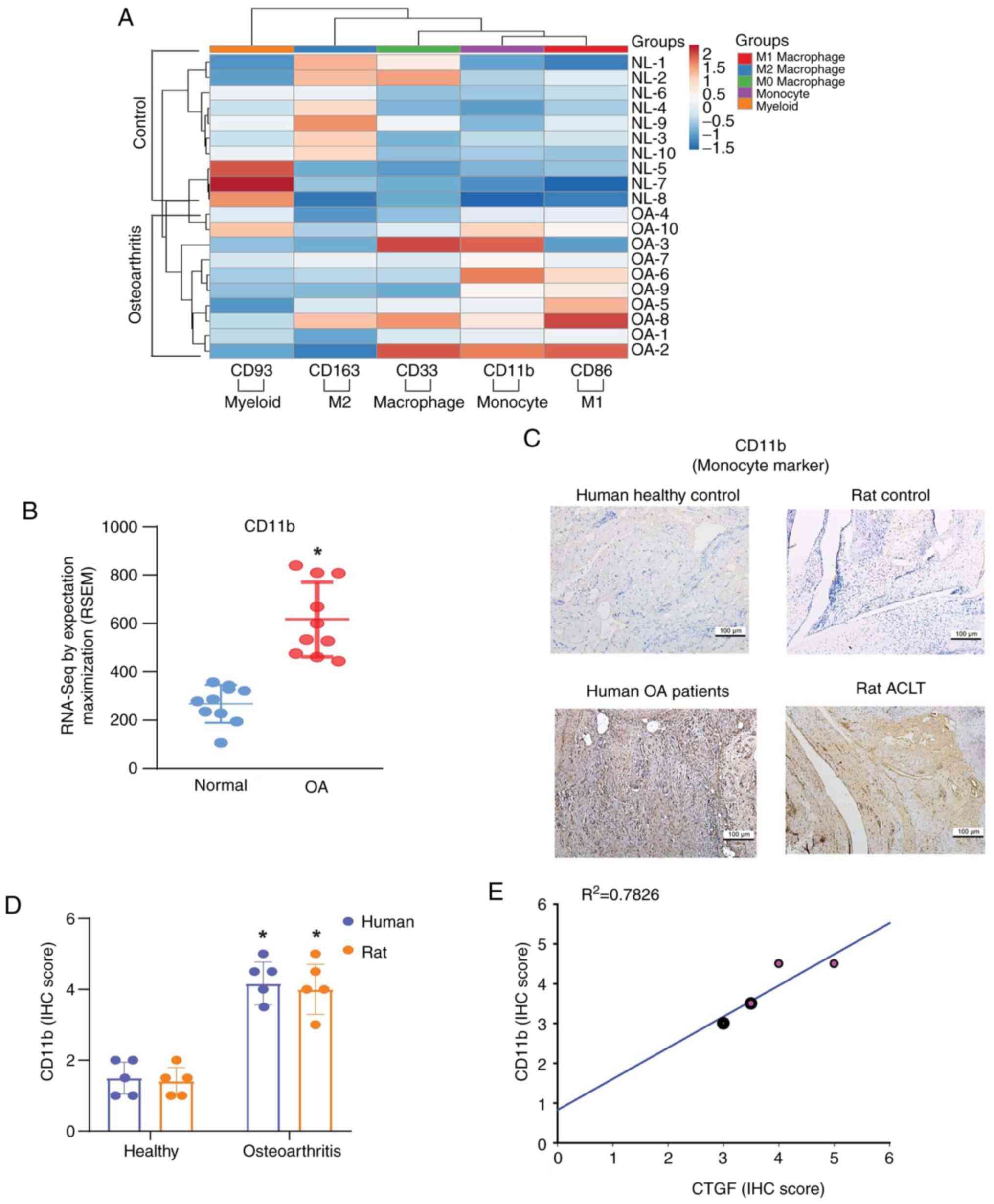
Mononuclear cell migration and adhesion through the synovial
membrane is a crucial step in regulating the progression of OA
(32). Analysis of mononuclear
cell expression in the GDS5401 dataset revealed that the expression
of monocyte marker CD11b was significantly higher in OA patients
than in normal controls (Fig. 2A and
B). Elevated CD11b expression levels were also detected in OA
samples from clinical patients and the ACLT model (Fig. 2C and D). When individual IHC
staining scores were analyzed by GraphPad Prism 8.0 software,
significant positive correlations were found between CTGF and CD11b
IHC staining scores from human synovial tissue (Fig. 2E).
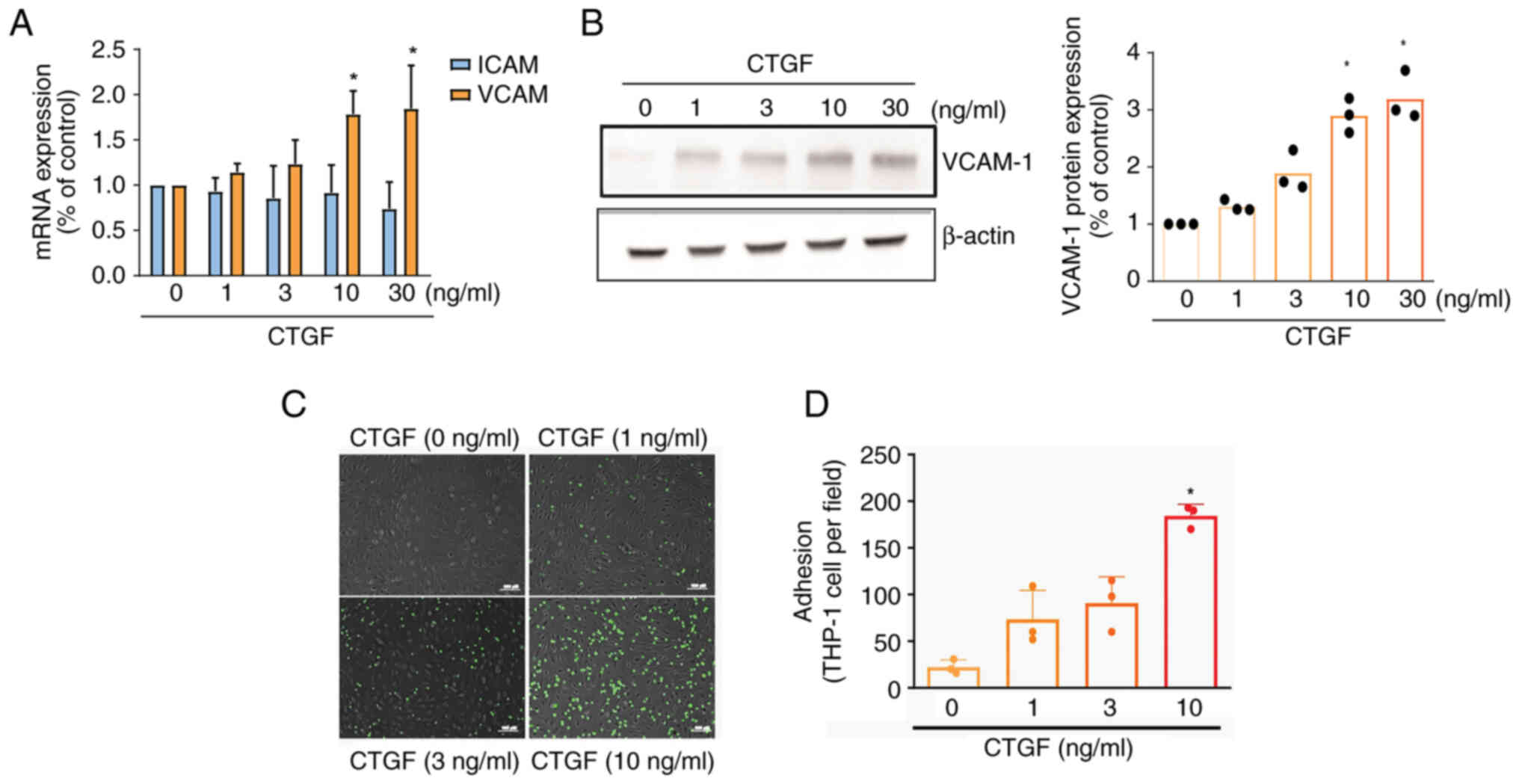
CTGF promotes VCAM-1 synthesis and
monocyte adhesion via the FAK and JNK pathways
ICAM-1 and VCAM-1 are known to control monocyte
adhesion (7). Stimulating OASFs
with CTGF was shown to promote the mRNA expression of VCAM-1, but
not ICAM-1 (Fig. 3A). Western blot
analysis revealed that the synthesis of VCAM-1 protein was
upregulated after treatment with CTGF (Fig. 3B). Stimulating OASFs with CTGF also
enhanced monocyte adhesion in a concentration-dependent manner
(Fig. 3C and D).
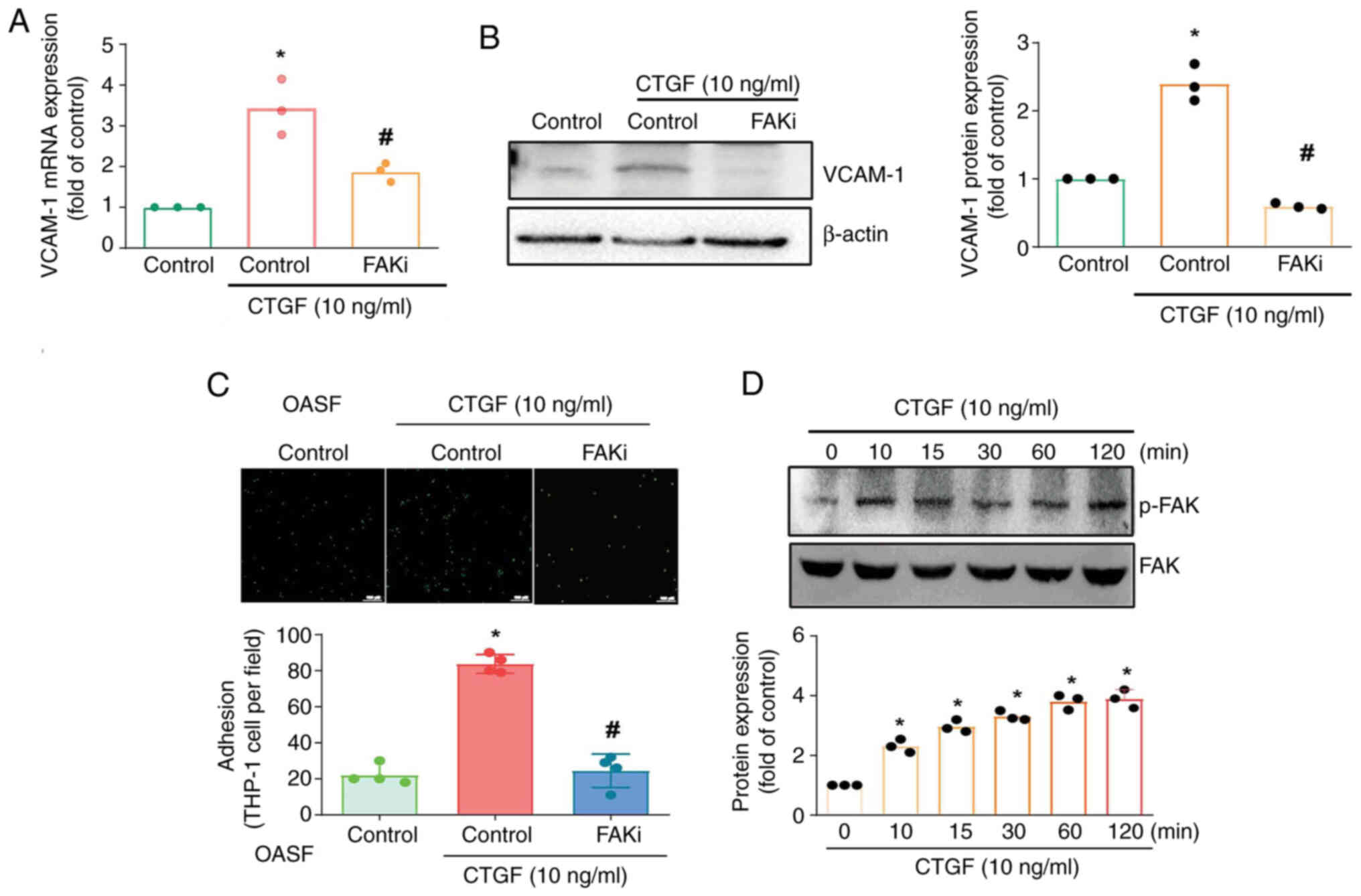
Monocyte adhesion has been linked to FAK activation
(33). In the current study,
treating OASFs with an FAK inhibitor antagonized CTGF-promoted
VCAM-1 mRNA expression and protein synthesis (Fig. 4A and B). The FAK inhibitor also
inhibited CTGF-enhanced monocyte adhesion to OASFs (Fig. 4C). Stimulating OASFs with CTGF was
shown to enhance FAK phosphorylation in a time-dependent manner
(Fig. 4D).
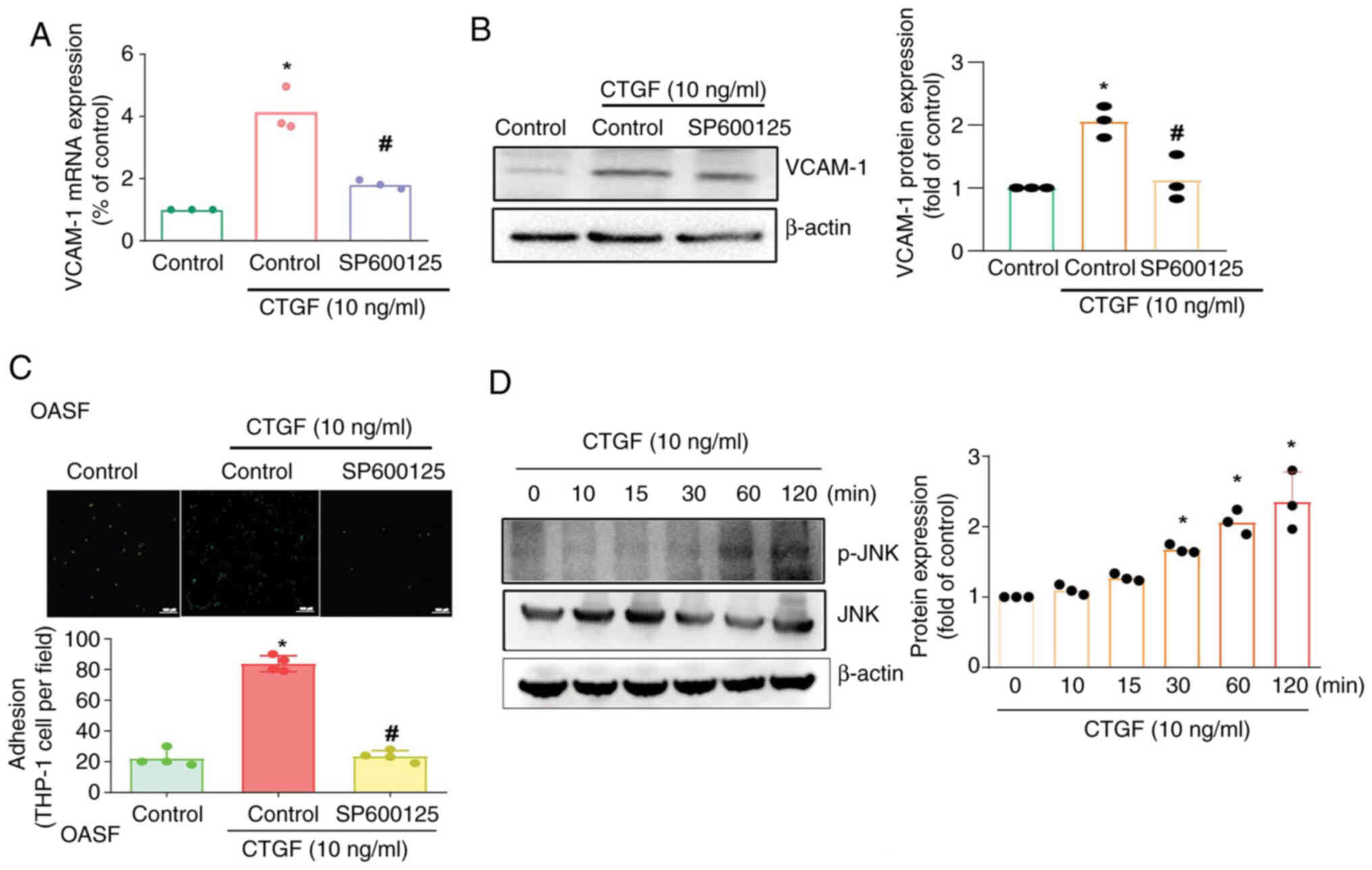
JNK signaling has been shown to regulate monocyte
migration and adhesion in the development of OA (11). In the present study, the JNK
inhibitor SP600125 antagonized CTGF-induced VCAM-1 synthesis and
monocyte adhesion (Fig. 5A-C).
Treating OASFs with CTGF facilitated JNK phosphorylation (Fig. 5D). These findings indicated that
the FAK and JNK pathways mediate CTGF-induced, VCAM-1-regulated
monocyte adhesion.
Discussion
Numerous factors have been implicated in the
development of OA, including weight-bearing activities, joint
damage and advanced age. The pathological characteristics of OA
include bone resorption and remodeling, cartilage degradation, and
inflammation of the synovium. These pathogenic characteristics
affect the progression of OA through similar molecular regulatory
mechanisms (34,35). Inflamed synovial cells generate
large quantities of pro-inflammatory compounds (36), which induce the secretion of
inflammatory mediators by monocytes, macrophages, chondrocytes, and
osteoblasts, resulting in the joint pain, swelling, and redness
typical of OA (37,38). The present study determined that
the fibrosis factor CTGF participated in OA progression by
promoting monocyte adhesion to the synovial membrane. This is a
potentially valuable insight into the connection between fibrosis
and OA.
Fibrosis is usually a result of incomplete healing
of damaged tissue. In cases of normal healing, ECM organization and
baseline levels are nearly restored; however, in cases of fibrosis,
ECM production is excessive and uncontrolled (39). Fibrotic lesions and the excessive
deposition of resilient fibers can cause an imbalance in the
structure and function of the tissue (40). Fibrosis factor CTGF significantly
affects arthritic disease by increasing inflammatory processes
(18). Its elevated levels in OA
cartilage correlates with disease severity (41). CTGF promotes cartilage degradation,
stimulates proinflammatory cytokine IL-6 synthesis (42), enhances monocyte migration through
MCP-1 expression (20) and
influences angiogenesis by modulating VEGF production in
osteoarthritic synovial fibroblasts (18). Analysis of the online GDS5401
dataset revealed that CTGF was the only fibrosis factor that was
higher in OA patients than in normal controls. These findings are
supported by clinical samples and the ACLT-mimic OA model,
indicating that CTGF regulates the progression of OA.
In cases of OA, chemokines secreted by active
synovial fibroblasts, such as CCL2, draw inflammatory monocytes
from the bloodstream into the synovium, where a number develop into
inflammatory macrophages. These macrophages produce inflammatory
factors that further stimulate fibroblasts, creating a sustained
feedback loop of synovial inflammation. In this loop, activated
synovial cells release inflammatory factors that activate
macrophages, driving them to transform into the pro-inflammatory M1
macrophage state (43,44). Analysis of the GDS5401 dataset
suggests that monocytes play a more significant role than other
mononuclear cells, such as myeloid cells, macrophages, M1
macrophages and M2 macrophages, based on elevated expression levels
in OA patients. IHC staining of specimens from clinical patients
and an ACLT-mimic OA rat model revealed similar results. It was
also determined that CTGF facilitates monocyte adhesion to OASFs by
promoting VCAM-1 production.
The FAK and JNK signaling pathways are known to
control various cellular functions, such as bone formation and
metastasis (45). The present
study demonstrated that a FAK inhibitor can suppresses CTGF-induced
VCAM-1 synthesis in OASFs as well as monocyte adhesion (46). Treating OASFs with CTGF facilitated
the phosphorylation of FAK. Similar effects were observed with the
JNK inhibitor SP600125, which moderated CTGF-enhanced VCAM-1
synthesis and monocyte adhesion. These results provided evidence
that the FAK/JNK pathway contributes to CTGF-mediated VCAM-1
production in OASFs and monocyte adhesion.
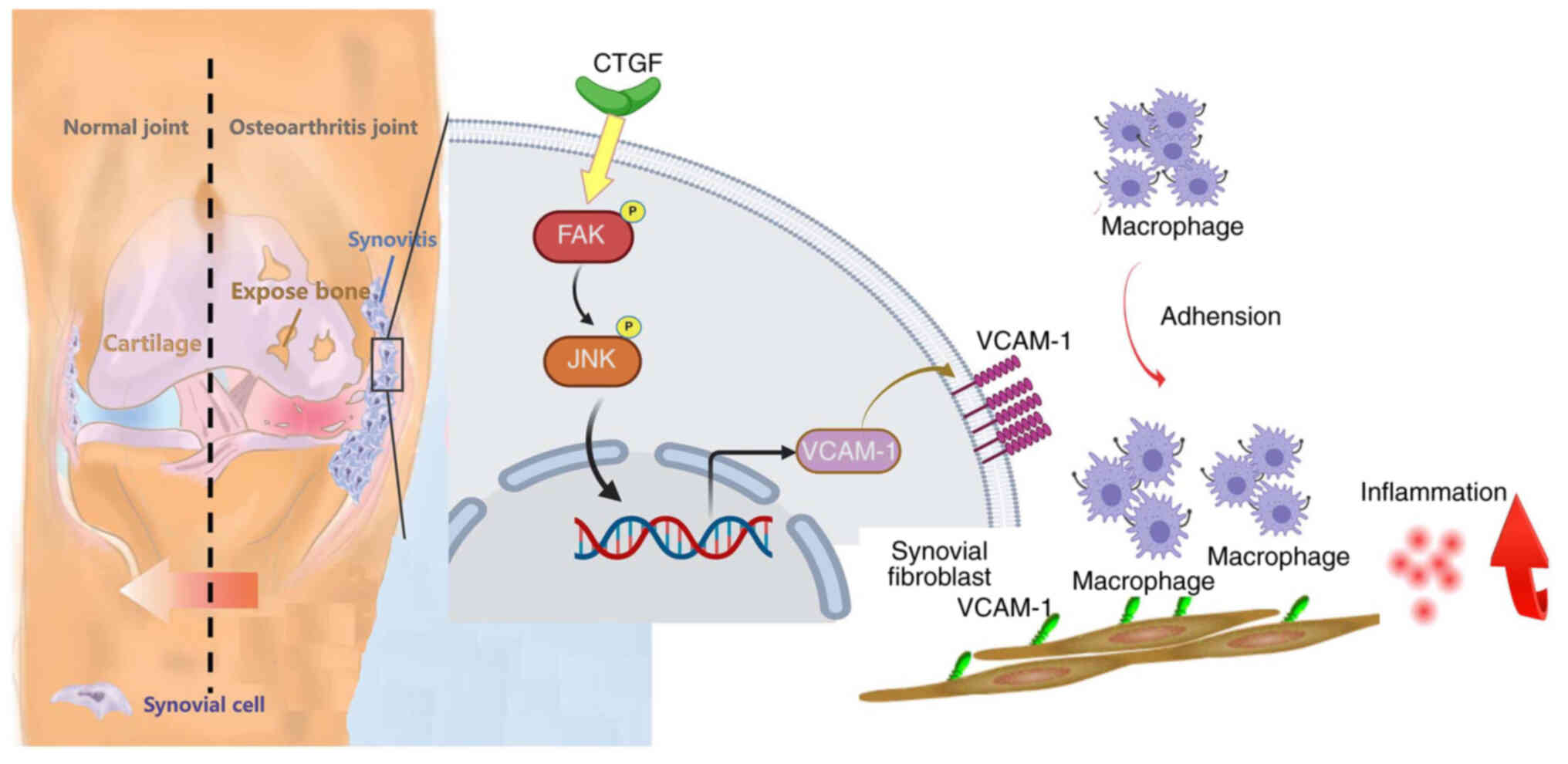
In summary, the present study demonstrated that CTGF
enhances VCAM-1 synthesis in human OASFs, thereby promoting
monocyte adhesion via the FAK and JNK pathways (Fig. 6). Taken together, these findings
suggest that CTGF is a promising novel target for the treatment of
OA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Science and
Technology Council of Taiwan (grant no. MOST
111-2314-B-039-048-MY3); the National Science and Technology
Council, Taiwan (grant nos. NSTC 113-2320-B-039-049-MY3 and NSTC
113-2314-B-715-009), China Medical University (grant nos.
CMU113-ASIA-01 and CMU113-MF-14); China Medical University Hospital
(grant nos. DMR-113-071 and DMR-113-201) and China Medical
University Beigang Hospital (grant no. 111CMUBHR-09).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SCL, YYL, WCC and CHTa conceived the research
project. SCL, YYL, WCC, YYW, YLH and CHTs provided
reagents/materials and conducted data analysis. SCL and CHTa wrote
the manuscript and confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were conducted in strict adherence to
the principles of the Declaration of Helsinki and received approval
from the Institutional Review Board of China Medical University
Hospital (approval no. CMUH109-REC2-181). All patients provided
written informed consent prior to participation in the study. All
animal experiments were conducted in strict accordance with a
protocol approved by the Institutional Animal Care and Use
Committee of China Medical University (approval no.
CMUIACUC-2021-050-2).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y and Ji Q: Macrophage polarization
in osteoarthritis progression: A promising therapeutic target.
Front Cell Dev Biol. 11:12697242023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H, Lin C, Zeng C, Wang Z, Wang H, Lu
J, Liu X, Shao Y, Zhao C, Pan J, et al: Synovial macrophage M1
polarisation exacerbates experimental osteoarthritis partially
through R-spondin-2. Ann Rheum Dis. 77:1524–1534. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Cai D and Bai X: Macrophages
regulate the progression of osteoarthritis. Osteoarthritis
Cartilage. 28:555–561. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hügle T and Geurts J: What drives
osteoarthritis?-synovial versus subchondral bone pathology.
Rheumatology (Oxford). 56:1461–1471. 2016.PubMed/NCBI
|
|
5
|
Martel-Pelletier J, Barr AJ, Cicuttini FM,
Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl
AJ and Pelletier JP: Osteoarthritis. Nat Rev Dis Primers.
2:160722016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mathiessen A and Conaghan PG: Synovitis in
osteoarthritis: Current understanding with therapeutic
implications. Arthritis Res Ther. 19:182017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang JW and Tang CH: The role of
macrophage polarization in rheumatoid arthritis and osteoarthritis:
Pathogenesis and therapeutic strategies. Int Immunopharmacol.
142:1130562024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadomoto S, Izumi K and Mizokami A:
Macrophage polarity and disease control. Int J Mol Sci. 23:1442021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang YH, Tsai CH, Liu SC, Chen HT, Chang
JW, Ko CY, Hsu CJ, Chang TK and Tang CH: miR-150-5p and XIST
interaction controls monocyte adherence: Implications for
osteoarthritis therapy. Front Immunol. 13:10043342022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu TJ, Chang SL, Lin CY, Lai CY, He XY,
Tsai CH, Ko CY, Fong YC, Su CM and Tang CH: IL-17 facilitates
VCAM-1 production and monocyte adhesion in osteoarthritis synovial
fibroblasts by suppressing miR-5701 synthesis. Int J Mol Sci.
23:68042022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen WC, Lin CY, Kuo SJ, Liu SC, Lu YC,
Chen YL, Wang SW and Tang CH: Resistin enhances VCAM-1 expression
and monocyte adhesion in human osteoarthritis synovial fibroblasts
by inhibiting MiR-381 expression through the PKC, p38, and JNK
signaling pathways. Cells. 9:13692020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujita M, Sasada M, Iyoda T and Fukai F:
Involvement of matricellular proteins in cellular senescence:
Potential therapeutic targets for age-related diseases. Int J Mol
Sci. 25:65912024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bolia IK, Mertz K, Faye E, Sheppard J,
Telang S, Bogdanov J, Hasan LK, Haratian A, Evseenko D, Weber AE
and Petrigliano FA: Cross-communication between knee osteoarthritis
and fibrosis: Molecular pathways and key molecules. Open Access J
Sports Med. 13:1–15. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
MacDonald IJ, Huang CC, Liu SC, Lin YY and
Tang CH: Targeting CCN proteins in rheumatoid arthritis and
osteoarthritis. Int J Mol Sci. 22:43402021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jun JI and Lau LF: Taking aim at the
extracellular matrix: CCN proteins as emerging therapeutic targets.
Nat Rev Drug Discov. 10:945–963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Omoto S, Nishida K, Yamaai Y, Shibahara M,
Nishida T, Doi T, Asahara H, Nakanishi T, Inoue H and Takigawa M:
Expression and localization of connective tissue growth factor
(CTGF/Hcs24/CCN2) in osteoarthritic cartilage. Osteoarthritis
Cartilage. 12:771–778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu SC, Chuang SM, Hsu CJ, Tsai CH, Wang
SW and Tang CH: CTGF increases vascular endothelial growth
factor-dependent angiogenesis in human synovial fibroblasts by
increasing miR-210 expression. Cell Death Dis. 5:e14852014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu SC, Hsieh HL, Tsai CH, Fong YC, Ko CY,
Wu HC, Chang SL, Hsu CJ and Tang CH: CCN2 facilitates IL-17
production and osteoclastogenesis in human osteoarthritis synovial
fibroblasts by inhibiting miR-655 expression. J Bone Miner Res.
37:1944–1955. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu SC, Hsu CJ, Fong YC, Chuang SM and
Tang CH: CTGF induces monocyte chemoattractant protein-1 expression
to enhance monocyte migration in human synovial fibroblasts.
Biochim Biophys Acta. 1833:1114–1124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou CH, Tang CH, Chen PC and Liu JF:
Thrombospondin 2 promotes IL-6 production in osteoarthritis
synovial fibroblasts via the PI3K/AKT/NF-kappaB pathway. J Inflamm
Res. 14:5955–5967. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang SL, Lin YY, Liu SC, Tsai YS, Lin SW,
Chen YL, Chen CC, Ko CY, Chen HT, Chen WC and Tang CH: Oral
administration of clostridium butyricum GKB7 ameliorates signs of
osteoarthritis in rats. Cells. 11:21692022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu SC, Tsai CH, Wu TY, Tsai CH, Tsai FJ,
Chung JG, Huang CY, Yang JS, Hsu YM, Yin MC, et al:
Soya-cerebroside reduces IL-1β-induced MMP-1 production in
chondrocytes and inhibits cartilage degradation: Implications for
the treatment of osteoarthritis. Food Agr Immunol. 30:620–632.
2019. View Article : Google Scholar
|
|
24
|
Achudhan D, Liu SC, Lin YY, Lee HP, Wang
SW, Huang WC, Wu YC, Kuo YH and Tang CH: Antcin K inhibits
VEGF-dependent angiogenesis in human rheumatoid arthritis synovial
fibroblasts. J Food Biochem. 46:e140222022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang JW, Liu SC, Lin YY, He XY, Wu YS, Su
CM, Tsai CH, Chen HT, Fong YC, Hu SL, et al: Nesfatin-1 stimulates
CCL2-dependent monocyte migration and M1 macrophage polarization:
Implications For rheumatoid arthritis therapy. Int J Biol Sci.
19:281–293. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou CH, Lin FL, Hou SM and Liu JF: Cyr61
promotes epithelial-mesenchymal transition and tumor metastasis of
osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 signaling pathway. Mol
Cancer. 13:2362014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu JF, Chen PC, Chang TM and Hou CH:
Monocyte chemoattractant protein-1 promotes cancer cell migration
via c-Raf/MAPK/AP-1 pathway and MMP-9 production in osteosarcoma. J
Exp Clin Cancer Res. 39:2542020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee HP, Chen PC, Wang SW, Fong Y, Tsai CH,
Tsai F, Chung JG, Huang CY, Yang JS, Hsu Y, et al: Plumbagin
suppresses endothelial progenitor cell-related angiogenesis in
vitro and in vivo. J Funct Foods. 52:537–544. 2019. View Article : Google Scholar
|
|
29
|
Lee HP, Wang SW, Wu YC, Lin LW, Tsai F,
Yang JS, Li TM and Tang CH: Soya-cerebroside inhibits
VEGF-facilitated angiogenesis in endothelial progenitor cells. Food
Agr Immunol. 31:193–204. 2020. View Article : Google Scholar
|
|
30
|
Liu JF, Chen PC, Chang TM and Hou CH:
Thrombospondin-2 stimulates MMP-9 production and promotes
osteosarcoma metastasis via the PLC, PKC, c-Src and NF-κB
activation. J Cell Mol Med. 24:12826–12839. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu JF, Hou CH, Lin FL, Tsao YT and Hou
SM: Nimbolide induces ROS-regulated apoptosis and inhibits cell
migration in osteosarcoma. Int J Mol Sci. 16:23405–23424. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsieh SL, Yang SY, Lin CY, He XY, Tsai CH,
Fong YC, Lo YS and Tang CH: MCP-1 controls IL-17-promoted monocyte
migration and M1 polarization in osteoarthritis. Int
Immunopharmacol. 132:1120162024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin SL, Yang SY, Tsai CH, Fong YC, Chen
WL, Liu JF, Lin CY and Tang CH: Nerve growth factor promote
VCAM-1-dependent monocyte adhesion and M2 polarization in
osteosarcoma microenvironment: Implications for larotrectinib
therapy. Int J Biol Sci. 20:4114–4127. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eriksen EF: Cellular mechanisms of bone
remodeling. Rev Endocr Metab Disord. 11:219–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng CF, Huang ET, Kuo JT, Liao KY and
Tsai FJ: Report of clinical bone age assessment using deep learning
for an Asian population in Taiwan. BioMedicine (Taipei). 11:50–58.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abramoff B and Caldera FE: Osteoarthritis:
Pathology, diagnosis, and treatment options. Med Clin North Am.
104:293–311. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mukherjee A and Das B: The role of
inflammatory mediators and matrix metalloproteinases (MMPs) in the
progression of osteoarthritis. Biomater Biosyst.
13:1000902024.PubMed/NCBI
|
|
38
|
Kondo N, Kuroda T and Kobayashi D:
Cytokine networks in the pathogenesis of rheumatoid arthritis. Int
J Mol Sci. 22:109222021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duffield JS, Lupher M, Thannickal VJ and
Wynn TA: Host responses in tissue repair and fibrosis. Annu Rev
Pathol. 8:241–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Davidson EN, Vitters EL, Mooren FM, Oliver
N, Berg WB and van der Kraan PM: Connective tissue growth
factor/CCN2 overexpression in mouse synovial lining results in
transient fibrosis and cartilage damage. Arthritis Rheum.
54:1653–1661. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu SC, Hsu CJ, Chen HT, Tsou HK, Chuang
SM and Tang CH: CTGF increases IL-6 expression in human synovial
fibroblasts through integrin-dependent signaling pathway. PLoS One.
7:e510972012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haubruck P, Pinto M, Moradi B, Little CB
and Gentek R: Monocytes, macrophages, and their potential niches in
synovial joints-therapeutic targets in post-traumatic
osteoarthritis? Front Immunol. 12:7637022021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu M and Ji Y: Immunoregulation of
synovial macrophages for the treatment of osteoarthritis. Open Life
Sci. 18:202205672023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsai HC, Tzeng HE, Huang CY, Huang YL,
Tsai CH, Wang SW, Wang PC, Chang AC, Fong YC and Tang CH: WISP-1
positively regulates angiogenesis by controlling VEGF-A expression
in human osteosarcoma. Cell Death Dis. 8:e27502017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tsai SY, Huang YL, Yang WH and Tang CH:
Hepatocyte growth factor-induced BMP-2 expression is mediated by
c-Met receptor, FAK, JNK, Runx2, and p300 pathways in human
osteoblasts. Int Immunopharmacol. 13:156–162. 2012. View Article : Google Scholar : PubMed/NCBI
|