Introduction
Atherosclerosis (AS) is a type of chronic metabolic
disease characterized by the deposition of lipid plaques on the
inner wall of large and medium-sized arteries, leading to arterial
hardening and other secondary pathological changes, which has high
incidence and mortality rates worldwide (1). The pathogenesis of AS is complex and
involves various pathological processes, including abnormal
endothelial cell function, lipid deposition, vascular smooth muscle
cell (VSMC) proliferation and migration, and inflammatory reactions
(1,2). At present, the clinical treatment of
AS mainly includes lifestyle interventions (3), lipid-lowering therapy (4) and anti-thrombotic treatment (5). Notably, these therapies mainly rely
on frequent systemic injections, which lead to unexpected side
effects. For example, lipid-lowering statins can cause muscle pain
and liver toxicity in some patients, whereas anti-thrombotic drugs
such as aspirin may lead to gastrointestinal bleeding (6,7).
Therefore, there is a need to develop new drugs with high delivery
specificity and fewer off-target effects.
NF-κB is a dimer composed of two protein subunits,
p50 and p65, which is expressed in almost all animal cells and
serves as a convergence point for multiple signaling pathways,
playing a crucial role in the occurrence and development of
inflammation (8). NF-κB can induce
the excessive or sustained expression of cytokines, such as TNF-α
and IL-6, adhesion factors and chemotactic factors, promoting
inflammatory reactions in diseases (9,10).
It has previously been shown that the upregulation of NF-κB is
closely related to the occurrence of cardiovascular diseases
(11). In addition, inflammation
induced by oxidized low-density lipoprotein (ox-LDL) is an
important event in the progression of AS (12). Multiple studies have shown that
ox-LDL can promote NF-κB activation, further accelerating the
occurrence and development of AS (13,14).
Sirtuin 1 (SIRT1) is a member of the NAD-dependent deacetylase
family, which can affect inflammatory reactions by regulating the
deacetylation of transcription factors, proteins and histones
(15). Research has shown that
SIRT1 inhibits inflammatory reactions through interaction with the
RelA/p65 subunit of NF-κB (16). A
recent study has shown that dihydromyricetin suppresses the
polarization of proinflammatory cells-M1 macrophages and alleviates
the progression of AS by regulating the SIRT1/NF-κB signaling
pathway (17). These previous
findings suggest that activating SIRT1, blocking NF-κB signaling
and inhibiting inflammatory reactions during the progression of AS
may be a key strategy for its treatment.
Mesenchymal stem cell (MSC)-based therapies have
been shown to exert strong anti-inflammatory and immune-regulatory
effects in various pathological processes of cardiovascular
diseases (18). Notably, the
paracrine function of MSCs serves an important role in
cardiovascular disease progression. Exosomes (exos) are
membrane-derived nanosized vesicles (size range, 30–200 nm), which
serve as the main form of paracrine secretion of MSCs. They contain
bioactive substances, such as nucleic acids, proteins and lipids,
mediating intercellular communication and serving important roles
in physiological and pathological processes (19). Increasing evidence has revealed
that MSC-derived exos (MSC-exos) have beneficial effects in AS.
Zhang et al (20) showed
that MSC-exos can reduce plaque formation in ApoE-knockout mice
with AS through FENDRR. Ma et al (21) demonstrated that MSC-exos can
promote M2 macrophage polarization and reduce macrophage
infiltration to alleviate AS by delivering microRNA
(miRNA/miR)-21a-5p to macrophages. However, the mechanism by which
MSC-exos exert protective effects through regulation of the
SIRT1/NF-κB signaling pathway and the inhibition of inflammation in
AS remains unclear.
It has been reported that pretreatment with
cytokines, drugs, hypoxia or physical factors can improve the
transplantation efficacy of MSC-exos, enhance the biological
functions of MSCs and prepare them with the desired characteristics
(22). For example, hypoxic
preconditioning can increase the secretion of anti-inflammatory
cytokines from MSCs (23), whereas
apelin pretreatment can enhance the cardioprotective effects of
MSCs (24). Baicalin (Ba) is one
of the most abundant active ingredients extracted from the dried
roots of Scutellaria baicalensis, a Chinese herbal medicine;
it has various pharmacological effects, including
anti-inflammatory, antimicrobial, antifibrotic, antioxidant and
anticancer activities (25–27).
Notably, several studies have revealed the potential of Ba in the
prevention and treatment of AS. Ba has been shown to target high
mobility group box 1 and to upregulate miR-126-5p, which can
inhibit the proliferation and migration of ox-LDL-VSMCs, thus
alleviating AS (28). Furthermore,
Ba alleviates oxidative stress and inflammation by deactivating the
NF-κB and p38 MAPK signaling pathways, thus relieving AS (29). However, the benefits of exos
derived from Ba-preconditioned MSCs (Ba-exos) in AS are still
unclear.
The current study aimed to investigate the potential
role of Ba-exos in alleviating the progression of AS using both
in vivo and in vitro models. Furthermore, the key
mechanisms by which Ba-exos alleviate AS progression through
regulation of the SIRT1/NF-κB signaling pathway and the inhibition
of inflammation were investigated further. The current study
elucidated the potential advantages of Ba-exos in the progression
of AS, and provides an effective and promising approach to optimize
the treatment process of MSC-exos in AS.
Materials and methods
Cell culture
Mouse aortic VSMCs (cat. no. CP-M076) and mouse
adipose-derived MSCs (cat. no. CP-M138) were purchased from Wuhan
Pricella Biotechnology Co., Ltd. The use of primary cells was
approved by the Experimental Animal Ethics Review Committee of the
Guangdong Work Injury Rehabilitation Hospital (approval no.
GDWIRH2023044; Guangzhou, China). The cells were cultured in
RMPI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 0.1 mg/ml streptomycin. The cells were maintained in
a CO2 incubator at 37°C with 5% CO2. The
culture medium was replaced every day and subculturing was
performed when cells reached ~85% confluence. In addition, the
medium of MSCs was supplemented with 1 µ M Ba (MedChemExpress) and
incubated at 37°C for 48 h for the subsequent isolation of
exos.
Collection and purification of
MSC-exos
When MSCs reached 80% confluence, they were cultured
in serum-free medium for 24 h to collect the cell supernatant. The
exos were collected using differential centrifugation. Firstly, the
collected cell supernatant was centrifuged at 300 × g for 10 min at
4°C, followed by centrifugation at 2,000 × g for 10 min at 4°C, and
then at 10,000 × g for 30 min at 4°C to obtain the supernatant. The
supernatant was filtered through a 0.22-µm filter and transferred
to an ultracentrifuge tube. The precipitate was collected by
centrifugation at 12,000 × g for 70 min at 4°C and resuspended in
PBS. The resuspended precipitate was then centrifuged again at
12,000 × g for 70 min at 4°C, and the obtained precipitate was
resuspended in an appropriate amount of PBS and stored at −80°C for
further study.
Identification and characterization of
exos
A total of 10 µl exo sample was added to an equal
volume of 2.5% glutaraldehyde and incubated at room temperature for
fixation on a transmission electron microscope (TEM) grid for 2
min. The samples were then embedded in epoxy resin at 60°C for 24 h
and sectioned into 70-nm ultrathin slices. Subsequently, 3%
phosphotungstic acid was added and the grid was stained at room
temperature for 2 min. The grid was then washed with distilled
water and images were captured under a TEM (TM4000plusII; Hitachi,
Ltd.). In addition, 5 µl exo sample was diluted with PBS to a
1,000-fold dilution for particle size analysis using a nanoparticle
tracking analyzer (NTA; NanoSight Pro; Malvern Panalytical, Ltd.).
The expression levels of the specific marker proteins TSG101
(1:2,000; cat. no. 28283-1-AP), CD9 (1:1,000; cat. no. 20597-1-AP)
and CD63 (1:1,000; cat. no. 32151-1-AP) all from Proteintech Group,
Inc.) in exos were detected by western blotting; the membranes were
incubated with these primary antibodies at 4°C overnight.
VSMC grouping and intervention
methods
Third-generation VSMCs with good growth status were
cultured in a 6-well plate (1×105 cells/well) containing
DMEM (Thermo Fisher Scientific, Inc.) supplemented with 100 mg/l
ox-LDL (cat. no. 20605ES05; Shanghai Yeasen Biotechnology Co.,
Ltd.) and incubated for 24 h; this was designated as the ox-LDL
group. Cells cultured in DMEM without ox-LDL served as the control
group. After 24 h of ox-LDL induction, 50 µg MSC-exos or 50 µg
Ba-exos were added to the ox-LDL + MSC-exos group and the ox-LDL +
Ba-exos group, respectively (30).
In addition, small interfering RNA (si)-negative control (NC)- or
si-SIRT1-transfected Ba-exos were co-cultured with VSMCs for 48 h,
which were designated as the Ba-exos + si-NC group and the Ba-exos
+ si-SIRT1 group, respectively. For transfection, a total of
1×106 cells were seeded into a cell culture plate and
transfected with 50 nM siRNA using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 48 h, strictly following the manufacturer's
protocol. The transfection efficiency was evaluated by reverse
transcription-quantitative PCR (RT-qPCR) analysis 48 h
post-transfection. The siRNA sequences that were used were as
follows: si-NC sense, 5′-CAGGAGGUUACGCGCAAGUUC-3′ and antisense,
5′-ACUUGAGCGUCCAAACCUGAU-3′; si-SIRT1 sense,
5′-UGAACAAAAGUAUAUGGACCU-3′ and antisense,
55′-GUCCAUAUACUUUUGUUCAGC-3′.
Cell counting kit-8 (CCK-8) assay
VSMCs in logarithmic growth phase were digested with
0.25% trypsin, centrifuged at 100 × g for 5 min at 4°C, resuspended
and counted. The cells were then adjusted to a concentration of
103 cells/l and were seeded into a 96-well plate at 200
µl/well. The cells were treated according to the aforementioned
grouping strategy. After 48 h, 10 µl CCK-8 solution (cat. no.
C0041; Beyotime Institute of Biotechnology) was added to each well,
and the cells were incubated for 2 h. The absorbance of each group
of cells was measured using an automatic microplate reader (Biotek
SynergyH4; Biotek; Agilent Technologies, Inc.).
Transwell assay
VSMCs in logarithmic growth phase were adjusted to a
concentration of 1×105 cells/ml. Subsequently, 150 µl
cell suspension was seeded in the upper chamber of a 24-well plate
with a Transwell insert (pore size, 8 µm). The lower chamber was
filled with 10% serum-containing medium. After incubation at 37°C
for 24 h, the cells in the upper chamber were gently removed using
a sterile cotton swab. The cells that had migrated to the lower
chamber were fixed in prepared 90% methanol solution for 30 min and
stained with crystal violet for 30 min at room temperature. After
air-drying, the cells were counted under a high-power light
microscope (BX53; Olympus Corporation).
Immunofluorescence
VSMCs cultured in confocal cell culture dishes were
fixed at room temperature with 4% paraformaldehyde for 20 min.
After discarding the solution, the cells were treated with 0.2%
Triton-100 solution at room temperature for 15 min to permeabilize
the membrane. Subsequently, the cells were blocked with 5% bovine
serum albumin (MedChemExpress) solution at room temperature for 30
min and were incubated overnight at 4°C with primary antibodies
against monocyte chemoattractant protein 1 (MCP-1; 1:1,000, cat no.
26161-1-AP; Wuhan Sanying Biotechnology), vascular cell adhesion
molecule 1 (VCAM-1; 1:1,000, cat. no. 11444-1-AP; Wuhan Sanying
Biotechnology), SIRT1 (1:1,000, cat. no. ab189494; Abcam), p65
(1:1,000, cat. no. ab32536; Abcam) and PKH67 (cat. no. C3635S;
Beyotime Institute of Biotechnology). Subsequently, the cells were
incubated at room temperature for 2 h with Goat Anti-Rabbit IgG
H&L (Alexa Fluor® 488) (1:1,000, cat. no. ab150077;
Abcam) secondary antibodies. The cells were then incubated at room
temperature with DAPI (cat. no. C1005; Beyotime Institute of
Biotechnology) diluted solution for 10 min. Finally, 1 ml PBS was
added, and the cells were observed and images were captured using a
laser confocal microscope (BX53; Olympus Corporation).
RT-qPCR
Total RNA was extracted from VSMCs using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
The concentration and purity of RNA were determined by UV analysis.
The RNA was reverse transcribed into cDNA using a RT kit (cat. no.
RR037A; Takara Bio, Inc.) at 37°C for 15 min and 85°C for 5 sec.
qPCR was performed according to the instructions of the SYBR Green
kit (cat. no. FP205; Tiangen Biotech Co., Ltd.) under the following
thermocycling conditions: Pre-denaturation at 95°C for 5 min,
followed by 40 cycles of denaturation at 95°C for 20 sec, annealing
at 60°C for 30 sec and extension at 72°C for 10 sec. The expression
levels of the target genes were calculated using the
2−ΔΔCq method (31),
with GAPDH used as the control gene. The primer sequences are shown
in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ |
|---|
| MCP-1 |
CCACTCACCTGCTGCTACTCA |
TCCTTCTTGGGGTCAGCACA |
| IL-6 |
AGCCAGAGTCCTTCAGAGAGAT |
AGCCACTCCTTCTGTGACTCC |
| VCAM-1 |
AGTCCGTTCTGACCATGGAGC |
GGGGGCCACTGAATTGAATCT |
| ICAM-1 |
CACATTCACGGTGCTGGCTA |
GGGTGTCGAGCTTTGGGATG |
| SIRT1 |
AGCAGGTTGCAGGAATCCAA |
CACCTAGGGCACCGAGGAAC |
| IL-1β |
ACCTGTGTCTTTCCCGTGGA |
GGAACGTCACACACCAGCAG |
| TNF-α |
GCCACCACGCTCTTCTGTC |
CTCCAGCTGCTCCTCCACT |
| GAPDH |
AAATCAAGTGGGGCGATGCT |
AACATGGGGGCATCAGCAGA |
Animal model of AS
A total of 20 male C57BL/6 mice (age, 6–8 weeks;
weight, 18–22 g) were purchased from RayBiotech Life. The mice were
maintained at a temperature of ~25°C, 55% humidity and under a 12-h
light/dark cycle, with free access to food and water. After 1 week
of acclimation, the mice were divided into a normal diet group and
a high-fat diet (composition: 60% kcal from fat, 20% kcal from
carbohydrates and 20% kcal from protein) group. The total feeding
period was 12 weeks. After 12 weeks, the mice were divided into
four groups: Control group, the high-fat diet (model) group, the
model + MSC-exos group, and the model + Ba-exos group (n=5
mice/group). The model + MSC-exos group and the model + Ba-exos
group, which were injected with 150 µg MSC-exos or Ba-exos twice a
week, respectively. The control group mice were injected with
saline twice a week. A high-fat diet was continued during the
entire 16-week high-fat feeding period. Subsequently, 100 µl blood
was collected from the tail vein of mice immediately before
sacrifice. The collected blood was allowed to clot at room
temperature for 30 min, followed by centrifugation at 3,000 × g for
15 min at 4°C to separate the serum. The resulting serum was stored
at −80°C until further analysis. Mouse serum was used for the
detection of blood glucose, total cholesterol, LDL-cholesterol
(LDL-C), high-density lipoprotein-cholesterol (HDL-C),
triglycerides and uric acid levels using an automatic biochemical
analyzer (BS-240VET; Shenzhen Mindray Bio-Medical Electronics Co.,
Ltd.). Subsequently, mice were euthanized by cervical dislocation,
and the chest was opened for dissection. The main arteries were
isolated and rinsed. The aortic arch segment was fixed in 4%
paraformaldehyde at 4°C for 24 h, then embedded in paraffin at 60°C
for 2 h and cut into 4-µm sections. For Oil Red O staining, the
sections were stained with 0.5% Oil Red O solution (cat. no.
G1015-100ML; Wuhan Servicebio Technology Co., Ltd.) for 15 min at
room temperature, then counterstained with hematoxylin for 1 min.
For Masson's trichrome staining (cat. no. G1006; Wuhan Servicebio
Technology Co., Ltd.), the sections were stained with Weigert's
iron hematoxylin for 10 min, differentiated with
phosphomolybdic-phosphotungstic acid for 5 min and stained with
aniline blue for 5 min, all at room temperature (25°C). Images of
the stained sections were captured using a light microscope (BX53;
Olympus Corporation). The animal experiment was approved by the
Experimental Animal Ethics Review Committee of the Guangdong Work
Injury Rehabilitation Hospital (approval no. GDWIRH2023043).
Western blotting
Total proteins were extracted from VSMCs and the
arterial tissues derived from mice with high-fat diet-induced AS
using lysis buffer (cat. no. C500005; Sangon Biotech Co., Ltd.).
Exosomal proteins were extracted using lysis buffer (cat. no.
C500005; Sangon Biotech Co., Ltd.) to lyse the exosomes, followed
by centrifugation at 13,680 × g at 4°C for 5 min to remove
insoluble debris. After quantifying the protein concentration in
the supernatant using the BCA protein assay kit (cat. no. P0009;
Beyotime Institute of Biotechnology), total protein was mixed with
loading buffer and incubated at 100°C for 5 min. Depending on the
molecular weight of the target protein, denatured cellular proteins
(10–20 µg) were separated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis on 6–15% gels and were then transferred to
polyvinylidene fluoride membranes. The membranes were blocked with
5% non-fat dry milk in Tris-buffered saline-Tween-20 (0.5%) for 2 h
at room temperature. The membranes were incubated overnight at 4°C
with primary antibodies against GAPDH (1:5,000; cat. no.
10494-1-AP; Wuhan Sanying Biotechnology), p65 (1:1,000; cat. no.
ab32536; Abcam), phosphorylated (p)-p65 (1:1,000; cat. no.
ab239882; Abcam), acetylated p65 (cat. no. 3045s; Cell Signaling
Technology, Inc.), MCP-1 (1:1,000; cat. no. 26161-1-AP; Wuhan
Sanying Biotechnology), IL-6 (1:1,000; cat. no. 21865-1-AP; Wuhan
Sanying Biotechnology), VCAM-1 (1:1,000; cat. no. 11444-1-AP; Wuhan
Sanying Biotechnology), intercellular adhesion molecule 1 (ICAM-1;
1:1,000; cat. no. 16174-1-AP; Wuhan Sanying Biotechnology) and
SIRT1 (1:1,000; cat. no. ab110304; Abcam). The next day, the
membraned were incubated at room temperature for 1 h with the
corresponding HRP-conjugated Goat Anti-Rabbit IgG(H+L) (1:8,000;
cat. no. SA00001-2; Wuhan Sanying Biotechnology) and HRP-conjugated
Goat Anti-Mouse IgG(H+L) (1:8,000; cat. no. SA00001-1; Wuhan
Sanying Biotechnology) secondary antibodies. Chemiluminescence
(cat. no. STP262; Seyotin) was used for visualization, and ImageJ
(1.53K; National Institutes of Health) was used for analysis.
Co-immunoprecipitation (co-IP)
Cells were lysed in RIPA buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology), and the lysates were
collected. A total of 200 µg lysate was used per IP reaction. The
lysates were incubated with 1 µg anti-SIRT1 (1:1,000; cat. no.
ab189494; Abcam), anti-NF-κB p65 (1:1,000; cat. no. ab207297;
Abcam) or IgG (cat. no. ab18443; 1:10,000; Abcam), and mixed
thoroughly using a mixer at 4°C for 24 h. A total of 50 µl protein
A/G magnetic beads (cat. no. 10002D; Thermo Fisher Scientific,
Inc.) were then added and incubated at 4°C overnight. Beads were
collected by centrifugation at 16,000 × g at 4°C for 10 min. The
beads were washed with 1 ml cold PBS, and the bound proteins were
eluted by boiling. The samples were then eluted with SDS-PAGE
sample buffer to dissociate the protein complexes from the
antibodies. The eluted proteins were separated by SDS-PAGE,
transferred to a PVDF membrane and probed with anti-NF-κB p65 and
anti-SIRT1 antibodies for western blot analysis.
ELISA detection of MCP-1, IL-6, VCAM-1
and ICAM-1 levels in mouse serum
The mouse serum from each experimental group was
collected, and the levels of MCP-1, IL-6, VCAM-1 and ICAM-1 were
detected according to the instructions of the respective ELISA
kits: MCP-1 (cat. no. PC125; Beyotime Institute of Biotechnology),
IL-6 (cat. no. AF0201; Beyotime Institute of Biotechnology), VCAM-1
(cat. no. PV951; Beyotime Institute of Biotechnology) and ICAM-1
(cat. no. PI493; Beyotime Institute of Biotechnology).
Statistical analysis
GraphPad Prism (version 8.0; Dotmatics) was used for
statistical analysis. Data are presented as the mean ± SD of three
replicates. Significance was assessed using the unpaired Student's
t-test for two-group comparisons or one-way ANOVA with Tukey's
multiple comparisons test for comparisons among three or more
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
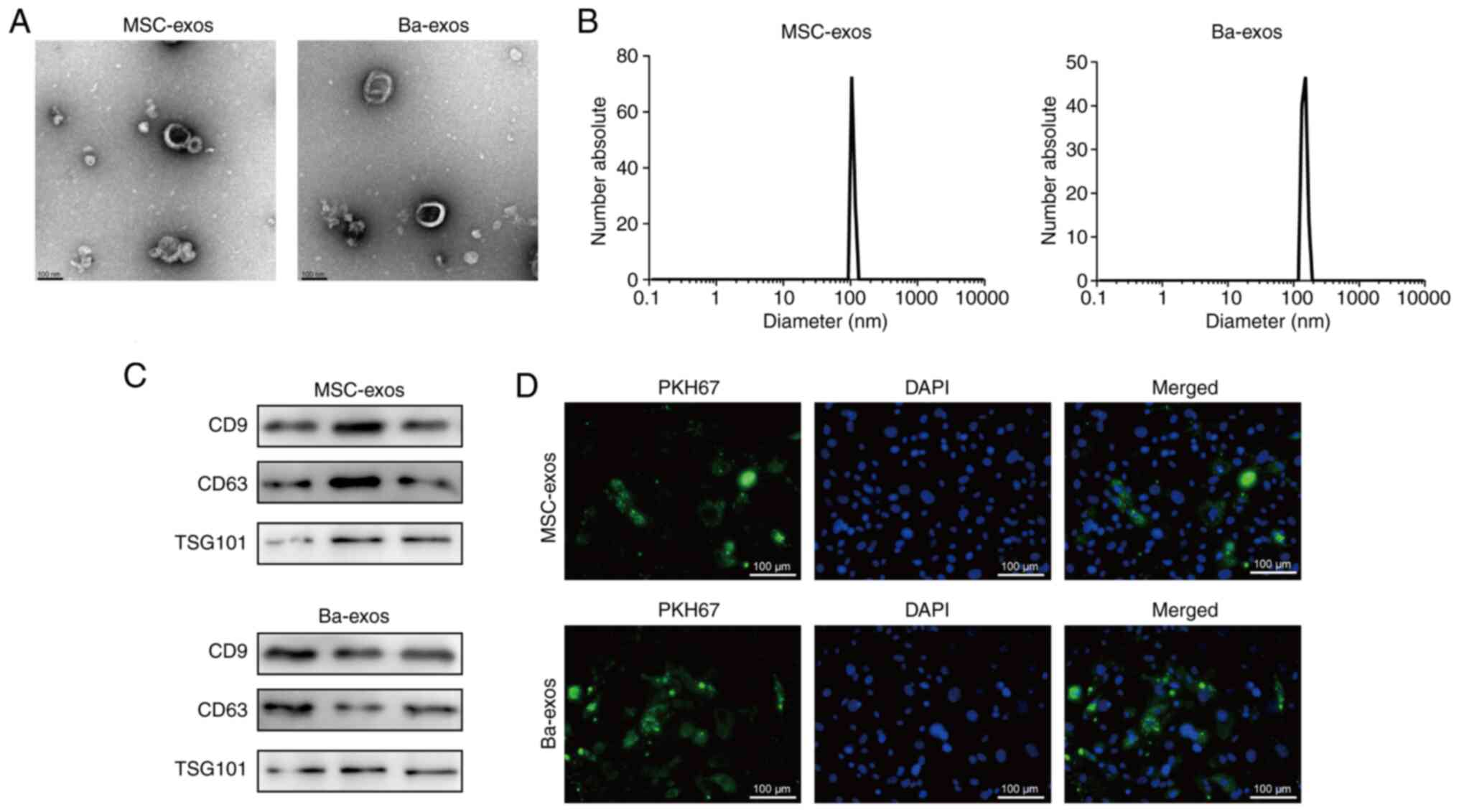
Isolation and identification of
exos
Exos were isolated from MSCs and MSCs treated with
Ba using differential ultracentrifugation. The morphology of
purified exos was observed under TEM (Fig. 1A) and characteristic disc-like
structures limited by a lipid bilayer were exhibited. The size
distribution of exos was determined by NTA, which showed a diameter
range of 70–200 nm (Fig. 1B).
Western blotting confirmed the expression of the exo-specific
markers CD9, CD63 and TSG101 (Fig.
1C). These results indicated the successful isolation of
MSC-exos and Ba-exos. Subsequently, MSC-exos and Ba-exos were
co-cultured with VSMCs, and immunofluorescence tracking results
demonstrated that both MSC-exos and Ba-exos were efficiently
internalized by VSMCs (Fig.
1D).
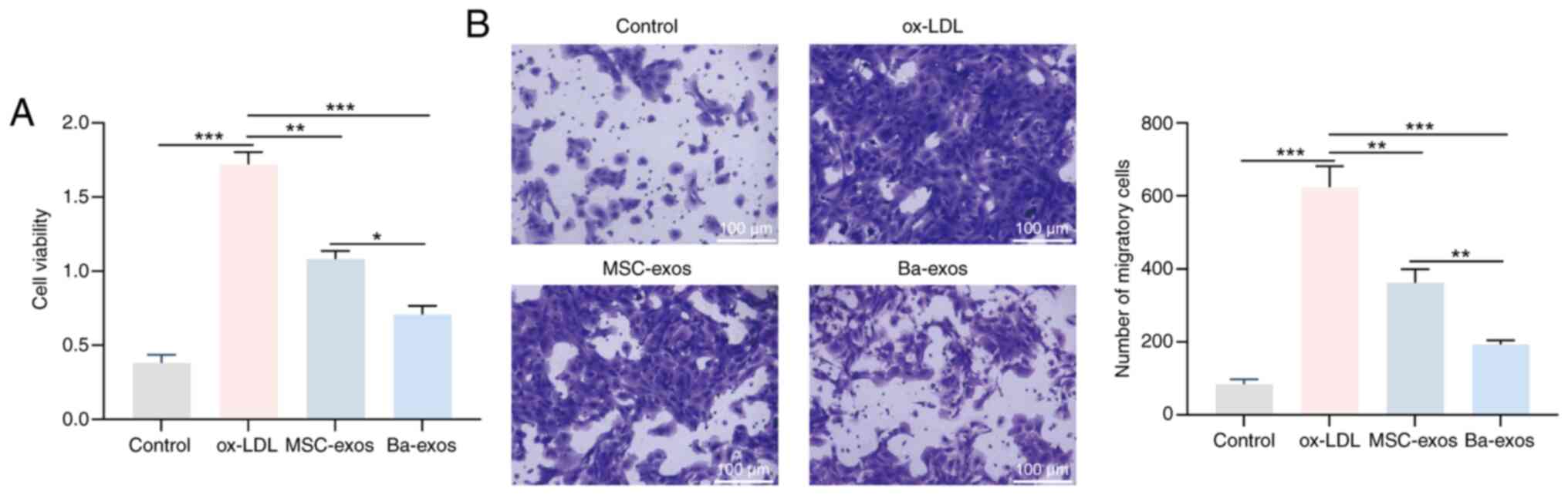
Ba-exos inhibit the viability and
migration of VSMCs
Abnormal proliferation and migration of VSMCs are
critical factors in accelerating the progression of AS (28). VSMCs were induced with ox-LDL to
investigate the effects of Ba-exos on viability and migration.
ox-LDL treatment significantly promoted the viability and migration
of VSMCs, whereas treatment with MSC-exos and Ba-exos inhibited
these abilities of VSMCs (Fig. 2A and
B). Notably, Ba-exos exhibited a better inhibitory effect on
VSMC viability and migration compared with MSC-exos treatment.
Ba-exos inhibit NF-κB and alleviate
inflammation in VSMCs
Inflammation is a driving force in the progression
of AS and late-stage AS plaque rupture (32); therefore, the effects of Ba-exos on
inflammation in AS were investigated. The results of RT-qPCR
demonstrated that the expression levels of the inflammatory genes
IL-6, MCP-1, VCAM-1 and ICAM-1 were upregulated in VSMCs induced
with ox-LDL, whereas treatment with MSC-exos reduced the expression
levels of these genes compared with the ox-LDL group (Fig. 3A). Furthermore, treatment with
Ba-exos further decreased the expression levels of IL-6, MCP-1,
VCAM-1 and ICAM-1 in VSMCs compared with MSC-exos; however, no
significant difference was observed in MCP-1 expression between the
MSC-exos and Ba-exos groups. Immunofluorescence results confirmed
these results; the expression levels of MCP-1 and VCAM-1 were
elevated in ox-LDL-induced VSMCs, and were reversed in the MSC-exos
and Ba-exos treatment groups (Fig.
3B). Furthermore, Ba-exos treatment significantly reduced the
levels of MCP-1 and VCAM-1 in VSMCs compared with those in the
MSC-exos group. These results revealed the inhibitory effects of
Ba-exos on inflammation in VSMCs induced by ox-LDL. Additionally, a
number of proinflammatory mediators, enzymes, chemokines and
cytokines involved in AS can be regulated by the NF-κB
transcription factor (33);
therefore, the role of Ba-exos in inflammation in AS through NF-κB
was investigated. Immunofluorescence results showed that treatment
with MSC-exos and Ba-exos markedly inhibited ox-LDL-mediated NF-κB
nuclear translocation in VSMCs, with a more pronounced effect
observed in the Ba-exos group (Fig.
3C). Western blotting showed reduced acetylation levels of the
NF-κB p65 subunit and decreased expression levels of p-p65 in the
MSC-exos and Ba-exos treatment groups compared with in the ox-LDL
group, with a more prominent trend observed in the Ba-exos group
(Fig. 3D and E). These results
suggested that Ba-exos may inhibit NF-κB and alleviate inflammation
in VSMCs.
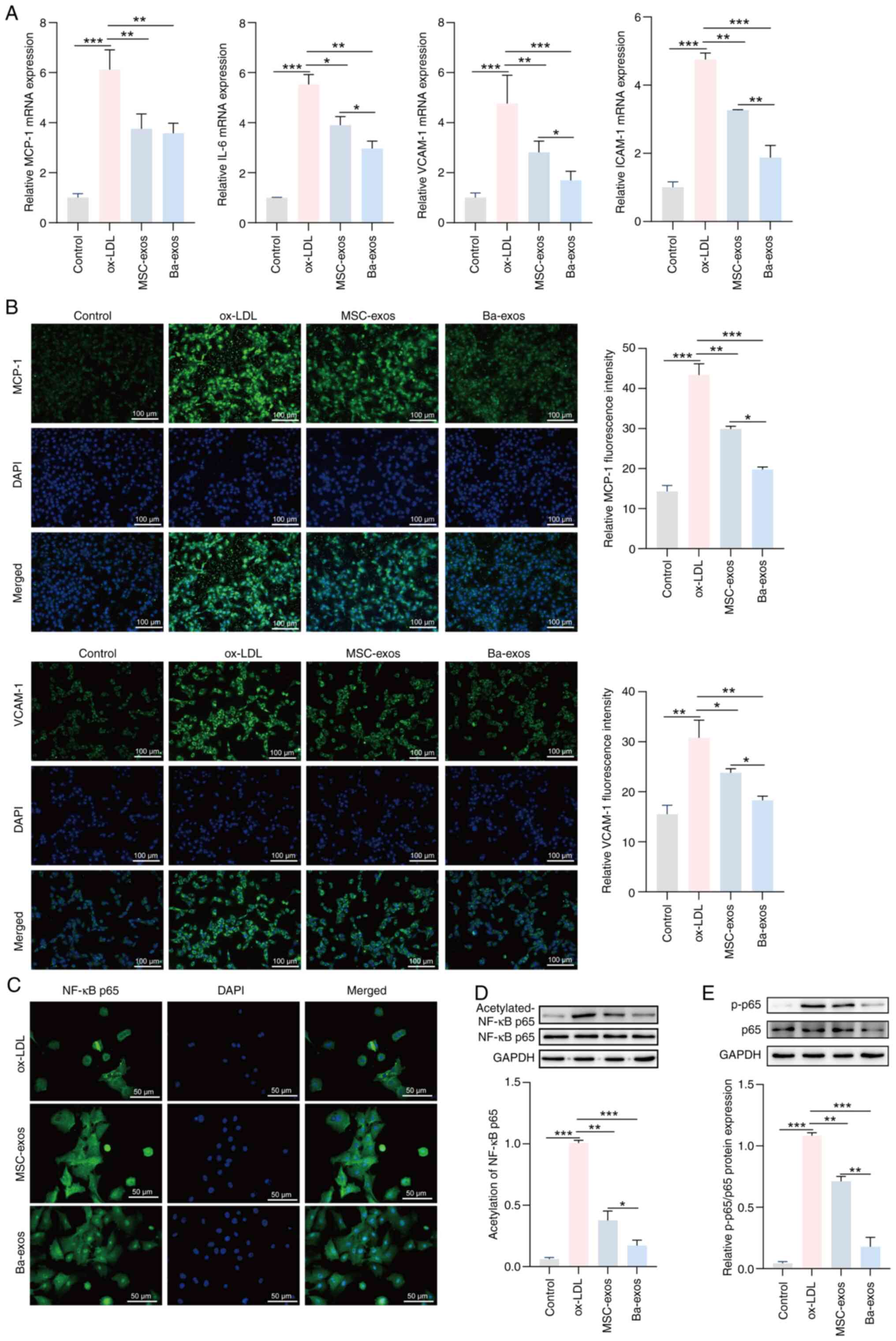 | Figure 3.Ba-exos inhibit NF-κB activation and
alleviate inflammation in VSMCs. (A) Reverse
transcription-quantitative PCR was performed to detect the
expression levels of the inflammatory genes MCP-1, IL-6, VCAM-1 and
ICAM-1 in VSMCs. Immunofluorescence was used to detect the (B)
levels of MCP-1 and VCAM-1, and (C) NF-κB nuclear translocation in
VSMCs. Western blotting was performed to detect the (D) acetylation
levels of NF-κB and the (E) protein expression levels of p65 and
p-p65 in VSMCs. n=3. *P<0.05, **P<0.01 and ***P<0.001.
Ba-exos, exos derived from baicalin-preconditioned MSCs; exos,
exosomes; ICAM-1, intercellular adhesion molecule 1; MCP-1,
monocyte chemoattractant protein 1; MSC-exos, MSC-derived exos;
MSC, mesenchymal stem cell; ox-LDL, oxidized low-density
lipoprotein; p-, phosphorylated; VCAM-1, vascular cell adhesion
molecule 1; VSMCs, vascular smooth muscle cells. |
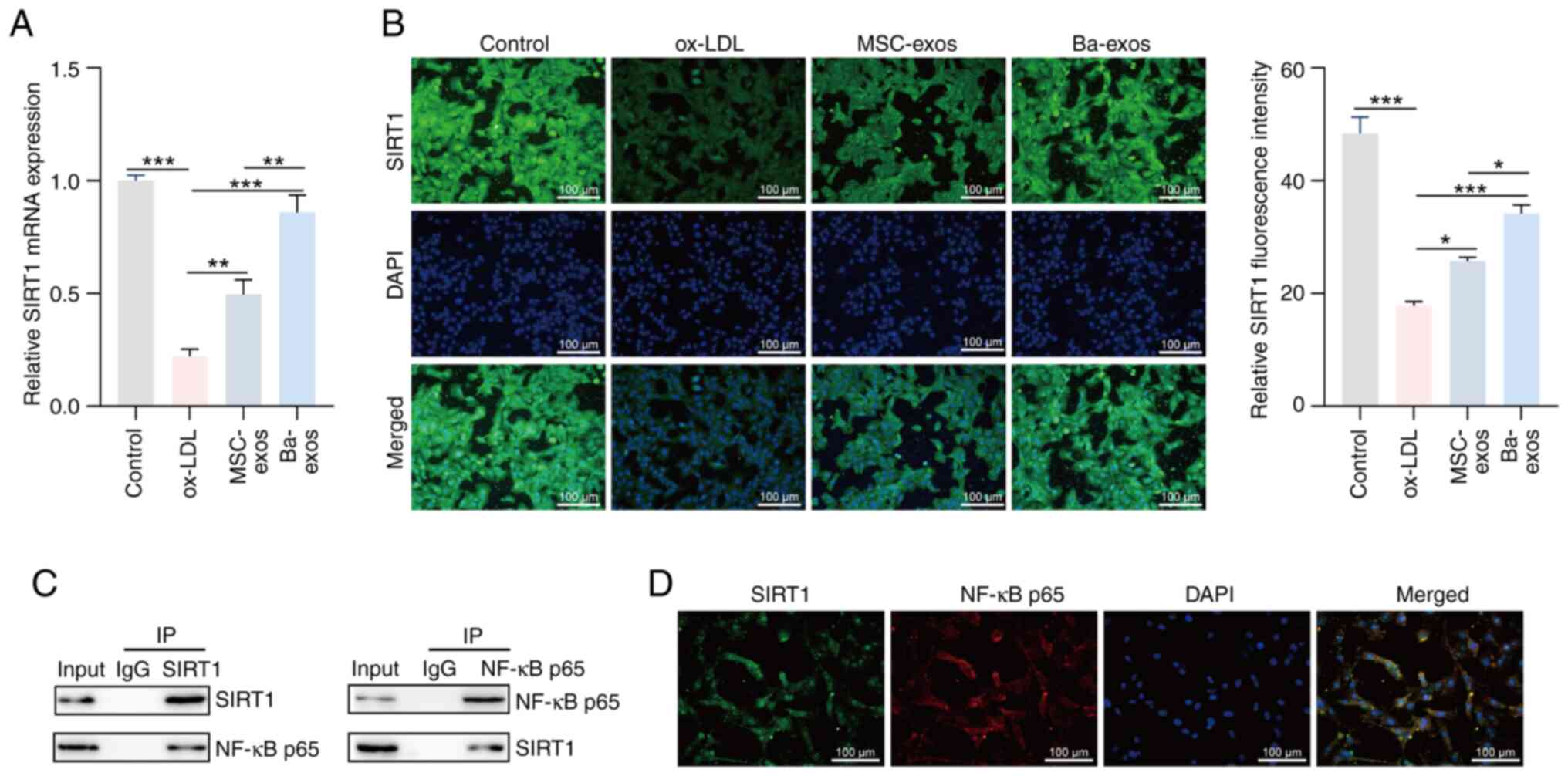
Ba-exos regulate the interaction
between SIRT1 and NF-κB by upregulating SIRT1
SIRT1 is a deacetylase that has been shown to
inhibit NF-κB signaling by deacetylating the p65 subunit of NF-κB,
thereby alleviating NF-κB-mediated inflammation (34). In the present study, the role of
Ba-exos in regulating the SIRT1/NF-κB signaling pathway was
investigated. The results of RT-qPCR revealed that the expression
levels of SIRT1 were low in ox-LDL-induced VSMCs, which was
reversed following treatment with MSC-exos and Ba-exos, with
Ba-exos showing a more significant effect (Fig. 4A). Immunofluorescence results also
validated this finding (Fig. 4B).
To elucidate the mechanism of interaction between SIRT1 and NF-κB,
the interaction between SIRT1 and NF-κB was confirmed through co-IP
(Fig. 4C), and the co-expression
of SIRT1 and NF-κB was further confirmed in VSMCs through
immunofluorescence (Fig. 4D).
These results indicated that Ba-exos may regulate the interaction
between SIRT1 and NF-κB by upregulating SIRT1.
Ba-exos alleviate inflammation, and
inhibit the viability and migration of VSMCs by regulating
SIRT1
si-NC and si-SIRT1 were transfected into
Ba-exos-treated VSMCs to further investigate the effect of Ba-exos
on AS progression through SIRT1. The results of RT-qPCR showed that
the mRNA expression levels of SIRT1 were reduced in the si-SIRT1
group compared with those in cells transfected with si-NC,
indicating successful transfection of si-SIRT1 (Fig. S1). As shown in Fig. S2A and B, knocking down SIRT1
reversed the inhibitory effects of Ba-exos on the viability and
migration of VSMCs, thus promoting their viability and migration.
The results of RT-qPCR showed that silencing SIRT1 reversed the
inhibitory effects of Ba-exos on the expression of inflammatory
factors in VSMCs, upregulating the mRNA expression levels of IL-6,
MCP-1, VCAM-1 and ICAM-1 in VSMCs (Fig. 5A). Immunofluorescence results also
indicated that MCP-1 and VCAM-1 expression levels were decreased in
the Ba-exos-treated group, whereas this trend was significantly
reversed following knockdown of SIRT1, although the difference was
not significant (Fig. 5B). In
addition, western blotting showed that, compared with those in the
Ba-exos-treated group, the protein expression levels of IL-6,
MCP-1, VCAM-1 and ICAM-1 were increased in VSMCs following SIRT1
knockdown, and the ratio of p-p65/p65 was also increased in VSMCs
(Fig. 5C). In summary, these
results suggested that Ba-exos may regulate SIRT1 to alleviate
inflammation, and inhibit the viability and migration of VSMCs.
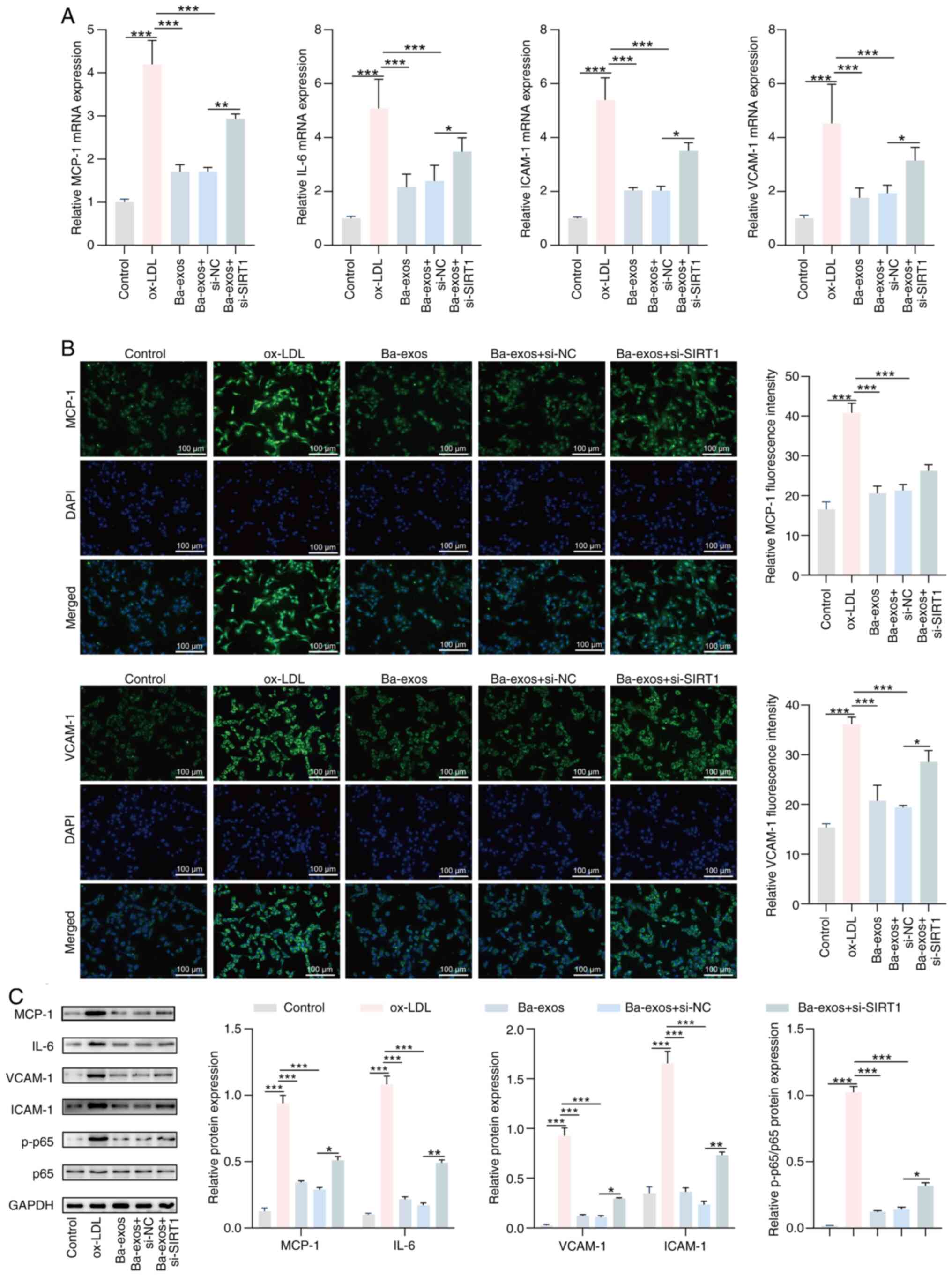 | Figure 5.Ba-exos attenuate inflammation of
VSMCs by regulating SIRT1. (A) Reverse transcription-quantitative
PCR was performed to detect the expression of inflammatory genes
MCP-1, IL-6, VCAM-1 and ICAM-1 in VSMCs. (B) Immunofluorescence was
used to detect the levels of MCP-1 and VCAM-1 in VSMCs. (C) Western
blotting was performed to detect the protein levels of MCP-1, IL-6,
VCAM-1, ICAM-1, p65 and p-p65 in VSMCs. n=3. *P<0.05,
**P<0.01 and ***P<0.001. Ba-exos, exos derived from
baicalin-preconditioned MSCs; exos, exosomes; ICAM-1, intercellular
adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1;
MSC-exos, MSC-derived exos; MSC, mesenchymal stem cell; NC,
negative control; ox-LDL, oxidized low-density lipoprotein; p-,
phosphorylated; si, small interfering RNA; SIRT1, sirtuin 1;
VCAM-1, vascular cell adhesion molecule 1; VSMCs, vascular smooth
muscle cells. |
Ba-exos alleviate AS progression in
vivo
Finally, the effect of Ba-exos on AS progression was
investigated in vivo. The results revealed that the levels
of blood glucose, total cholesterol, HDL-C, triglycerides and uric
acid were increased, whereas the levels of LDL-C were decreased in
the serum of the model group; by contrast, the levels of these
indicators were reversed following treatment with MSC-exos and
Ba-exos (Fig. 6A). Notably,
compared with in the MSC-exos group, the levels of these indicators
in the serum of mice were further reversed following Ba-exos
treatment, but the difference was not significant. Oil red O and
Masson staining results showed that the model group exhibited
markedly increased lipid-containing lesions and obvious AS plaques
in the aortic arch, which were reduced following treatment with
MSC-exos and Ba-exos, with a further reduction in lesion and plaque
area after Ba-exos treatment compared with MSC-exos (Fig. 6B and C). Furthermore, ELISA
detected increased levels of MCP-1, IL-6, VCAM-1 and ICAM-1 in the
serum of the model group, which were decreased following treatment
with MSC-exos and further decreased after Ba-exos treatment
compared with MSC-exos treatment, but the difference was not
significant (Fig. 6D). Notably,
western blotting results showed that MSC-exos and Ba-exos
upregulated the expression levels of SIRT1 in the model group and
lowered the ratio of p-p65/p65. However, no significant difference
in SIRT1 expression was detected between the MSC-exos group and the
model group. These trends were more pronounced in the Ba-exos
treatment group (Fig. 6E).
Concurrently, RT-qPCR and western blotting indicated that, compared
with those in the model group, the expression levels of TNF-α,
IL-1β, ICAM-1, MCP-1, VCAM-1 and IL-6 were significantly reduced in
both the MSC-exos and Ba-exos groups, with the Ba-exos group
showing a more pronounced effect (Fig. S3). In conclusion, Ba-exos may
alleviate AS progression in vivo by regulating
SIRT1/NF-κB.
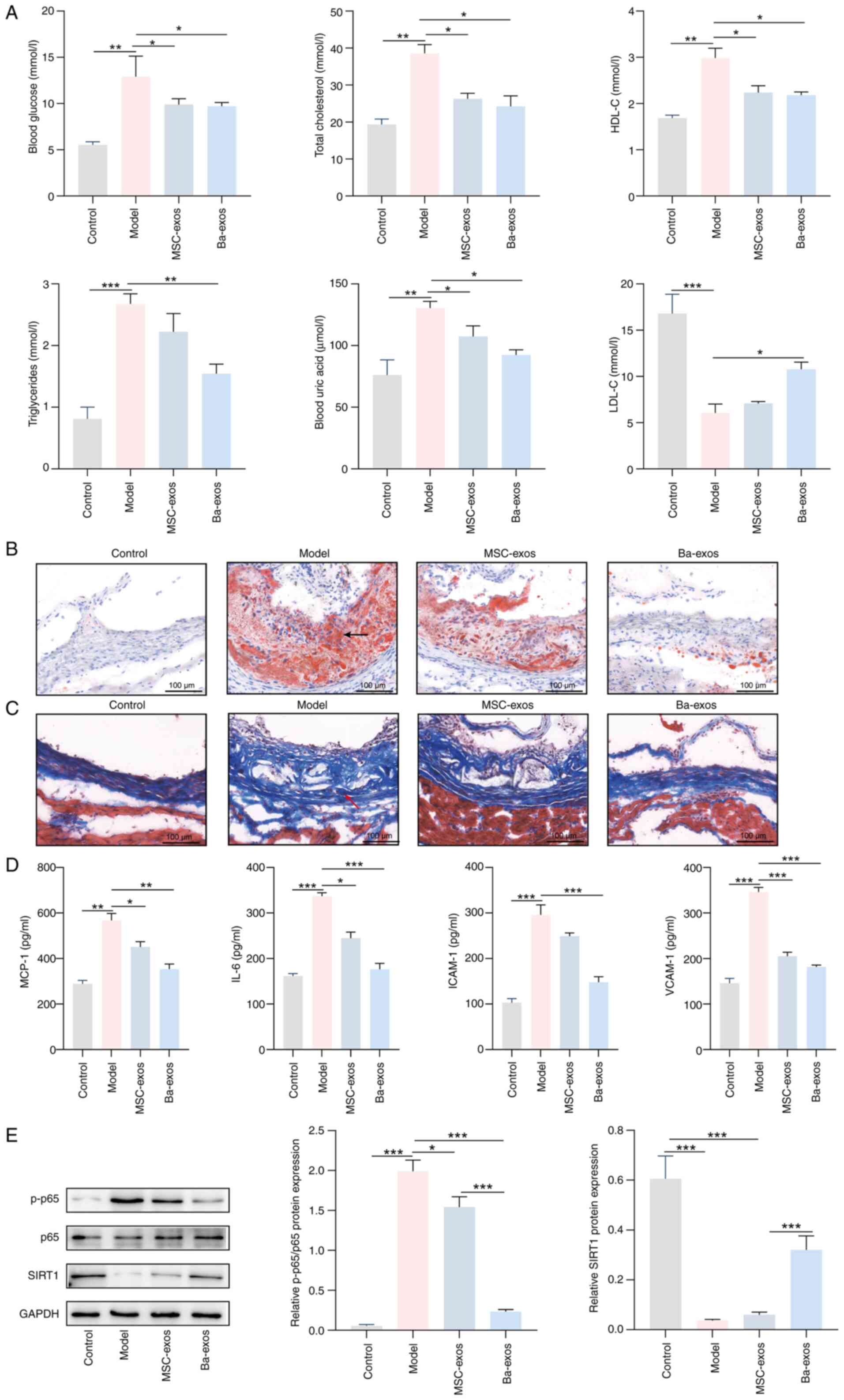 | Figure 6.Ba-exos may alleviate the progression
of atherosclerosis in vivo by regulating SIRT1/NF-κB. (A)
Automatic biochemical analyzer was used to detect blood glucose,
total cholesterol, LDL-C, HDL-C, triglycerides and uric acid levels
in mouse serum. (B) Oil Red O staining (arrows indicate the
locations of the lesions) and (C) Masson staining (arrows indicate
the locations of the lesions) were performed to assess tissue
pathology. (D) ELISA was used to detect the levels of MCP-1, IL-6,
VCAM-1 and ICAM-1 in mouse serum. (E) Western blotting was used to
detect the protein expression levels of SIRT1, p65 and p-p65 in
mouse tissues. n=5. *P<0.05, **P<0.01 and ***P<0.001.
Ba-exos, exos derived from baicalin-preconditioned MSCs; exos,
exosomes; HDL-C, high-density lipoprotein-cholesterol; ICAM-1,
intercellular adhesion molecule 1; MCP-1, monocyte chemoattractant
protein 1; LDL-C, low-density lipoprotein-cholesterol; MSC-exos,
MSC-derived exos; MSC, mesenchymal stem cell; p-, phosphorylated;
SIRT1, sirtuin 1; VCAM-1, vascular cell adhesion molecule 1. |
Discussion
In recent years, with changes in lifestyle, the
incidence of AS has increased, leading to a larger number of
patients with cardiovascular diseases, such as stroke, coronary
heart disease and myocardial infarction, which severely threaten
human life and health (35,36).
Although current clinical treatments for AS exhibit a certain
degree of effectiveness, there are toxic side effects and risks;
therefore, there is a need to identify new therapeutic methods that
are safe and effective with minimal side effects. MSCs are stem
cells with multi-differentiation potential and self-renewal
ability; due to their abundant sources and ease of culture and
expansion, they have garnered interest in various research fields
(37). In disease models, it has
been shown that stem cells can exert their unique anti-inflammatory
and immune-regulatory capabilities by secreting various bioactive
substances in conditions such as coronary heart disease,
Alzheimer's disease, fibrotic diseases and cancer (38–41).
As a long-term inflammatory disease of blood vessels, AS may be
markedly regulated by MSC-based therapies (42). In the present study, Ba-exos were
isolated from Ba-pretreated MSCs, and the mechanism by which
Ba-exos regulates the SIRT1/NF-κB signaling pathway to inhibit
inflammation and exert protective effects on AS was explored.
The advantages of exos in treating various diseases
have previously been confirmed (43,44).
MSC-exos contain a rich array of bioactive substances, including
miRNAs, cytokines and proteins, that can inhibit inflammation,
regulate immune cell activity and stimulate tissue regeneration
through targeted delivery of these bioactive molecules (45). Research has shown that the
substances carried by exos vary depending on the type of source
cells and their state, such as transformation, differentiation,
stimulation and stress (46).
Notably, pretreated MSC-exos have been reported to enhance
therapeutic and transplant efficacy. For example, exos derived from
atorvastatin-pretreated MSCs have been shown to accelerate diabetic
wound healing by enhancing angiogenesis through the AKT/eNOS
pathway (30). Additionally, exos
derived from TNF-α-pretreated gingival MSCs can enhance M2
macrophage polarization, inhibit inflammation and alleviate
periodontitis (47). Furthermore,
Ba has shown good therapeutic effects on cardiovascular diseases.
Previous studies have revealed that Ba exhibits potential in
treating AS and myocardial ischemia/reperfusion injury through
anti-inflammatory, antioxidant and lipid metabolism mechanisms
(48,49). Zhu et al (50) demonstrated that osteoclast-derived
factors and exosomes containing miRNAs can enhance or inhibit
osteoblast differentiation through paracrine and cell-contact
mechanisms, suggesting a central coupling role in bone formation.
Similarly, the present study explored the potential paracrine
effects of Ba-exos on VSMCs through the regulation of inflammatory
pathways, highlighting the therapeutic potential of exosome-based
interventions in AS. Additionally, Zhao et al (51) and Zhang et al (52) have reported that Ba-exos may
alleviate acute liver injury and improve ischemia/reperfusion
injury, highlighting their therapeutic potential in tissue repair
and regeneration. In the present study, it was hypothesized that
intervening with Ba in MSC-exos may endow them with
anti-inflammatory properties, thereby alleviating the progression
of AS. As anticipated, it was revealed that Ba-exos significantly
inhibited inflammation and alleviated AS progression compared with
MSC-exos. This result is consistent with the findings of a previous
study that reported Ba-exos can alleviate acute liver injury
(51). These findings indicated
that Ba pretreatment may enhance the therapeutic efficacy of
MSC-exos in diseases.
VSMCs are important components of the vascular wall,
which are responsible for regulating vascular contraction and
relaxation, while also secreting various vasoactive factors
(53,54). Abnormal proliferation and migration
of VSMCs are involved in plaque formation, and are key factors
driving the progression of AS (55,56).
An increasing number of studies have confirmed that exos serve a
critical role in AS by regulating the proliferation and migration
of VSMCs. For example, Guo et al (57) demonstrated that adipose
tissue-derived exos can exacerbate the progression of AS by
promoting the proliferation and migration of VSMCs within plaques.
Liu et al (58) also
reported that macrophage-derived exos induced by ox-LDL may promote
the progression of AS by regulating the
circ_100696/miR-503-5p/PAPPA axis, which mediates VSMC
proliferation and migration. Similarly, in the present study, it
was shown that Ba-exos significantly inhibited ox-LDL-induced
viability and migration of VSMCs. Notably, inflammatory factors
expressed in VSMCs, such as MCP-1, VCAM-1 and ICAM-1, accelerate
the progression of AS (59).
Therefore, effectively blocking these proteins may suppress the
development of AS. In the present study, it was revealed that
Ba-exos could inhibit the ox-LDL-induced upregulation of the
inflammatory genes IL-6, ICAM-1 and VCAM-1, and the chemokine MCP-1
in VSMCs. These results suggested that Ba-exos could alleviate the
progression of AS by inhibiting VSMC viability and migration, and
by downregulating cell inflammatory factors in VSMCs.
Inflammatory responses are closely related to AS,
and NF-κB is a key target in controlling inflammation. The
activation of NF-κB serves an important role in the occurrence and
development of AS by enhancing the transcription of various
proinflammatory cytokines (60).
However, to the best of our knowledge, the potential protective
effect of Ba-exos on AS related to the inhibition of NF-κB
activation has not yet been reported. The results of the current
study indicated that ox-LDL induced the phosphorylation of p65 in
VSMCs, promoting the nuclear translocation of NF-κB, whereas the
expression of NF-κB was significantly increased in AS tissues.
However, these trends were reversed following treatment with
Ba-exos. These findings suggested that Ba-exos may reduce the
production of proinflammatory factors by inhibiting the activation
of NF-κB in AS.
SIRT1 influences inflammation, apoptosis and other
processes through the deacetylation of transcription factors,
proteins and histones, acting as a regulator of inflammatory
processes related to the deacetylation of NF-κB (61). SIRT1 interacts with the RelA/p65
subunit of NF-κB and inhibits NF-κB transcription by deacetylating
the lysine 310 residue of this subunit (62). In the current study, an interaction
between SIRT1 and NF-κB was identified, and treatment with Ba-exos
significantly upregulated SIRT1 while reducing the acetylation
levels of NF-κB p65. These findings indicated that Ba-exos may
increase the deacetylation of NF-κB p65 by activating SIRT1,
thereby lowering the expression of NF-κB p65 and inhibiting
inflammation to alleviate AS. This is similar to the findings of
Wei et al (63), in which
platelet-derived exos were shown to regulate endothelial cell
inflammation in coronary thrombosis by promoting SIRT1 expression
and inhibiting NF-κB transcription. In summary, the aforementioned
results demonstrated that Ba-exos may alleviate AS by activating
SIRT1 and inhibiting NF-κB transcription, thereby suppressing
inflammation.
In conclusion, exos from MSCs and MSCs pretreated
with Ba were successfully isolated. Compared with MSC-exos, Ba-exos
markedly reduced the formation of AS plaques and decreased the
lesion area by reducing the secretion of inflammatory factors in
the mouse serum. Furthermore, Ba-exos significantly inhibited the
viability and migration of ox-LDL-induced VSMCs, and suppressed the
expression of the inflammatory factors MCP-1, IL-6, VCAM-1 and
ICAM-1 in VSMCs compared with MSC-exos. Mechanistically, Ba-exos
upregulated the expression of SIRT1, inhibited NF-κB activation and
thus suppressed inflammation to alleviate AS progression. In
summary, the results of the present study demonstrated that Ba-exos
exhibit notable ability to inhibit AS progression, providing novel
methods and perspectives for the clinical treatment of AS.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 62076103), the Guangdong Provincial
Bureau of Traditional Chinese Medicine Foundation (grant no.
20241038), the Guangdong Province Key Areas Research and
Development Plan (grant no. 2018B030339001) and the Guangdong
Province General University Youth Innovative Talent Project (grant
no. 2021KQNCX013).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XCY, YBH and WW conceived and designed the
experiments. WTH, JFF, YLC, XYC and XLL analyzed and interpreted
the data. YBH wrote the manuscript. All authors read and approved
the final version of the manuscript. XCY and YBH confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The animal experiments were approved by the
Experimental Animal Ethics Review Committee of the Guangdong Work
Injury Rehabilitation Hospital (approval no. GDWIRH2023043). The
use of primary cells was approved by the Experimental Animal Ethics
Review Committee of the Guangdong Work Injury Rehabilitation
Hospital (approval no. GDWIRH2023044).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fan J and Watanabe T: Atherosclerosis:
Known and unknown. Pathol Int. 72:151–160. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falk E: Pathogenesis of atherosclerosis. J
Am Coll Cardiol. 47 (8 Suppl):C7–C12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnett DK, Blumenthal RS, Albert MA,
Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A,
Lloyd-Jones D, McEvoy JW, et al: 2019 ACC/AHA guideline on the
primary prevention of cardiovascular disease: A report of the
american college of cardiology/American heart association task
force on clinical practice guidelines. Circulation. 140:e596–e646.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grundy SM, Stone NJ, Bailey AL, Beam C,
Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S,
Faiella-Tommasino J, Forman DE, et al: 2018
AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline
on the management of blood cholesterol: A report of the american
college of cardiology/American heart association task force on
clinical practice guidelines. Circulation. 139:e1082–e1143. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Collet JP, Thiele H, Barbato E, Barthélémy
O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T,
Folliguet T, et al: 2020 ESC guidelines for the management of acute
coronary syndromes in patients presenting without persistent
ST-segment elevation. Eur Heart J. 42:1289–1367. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aprotosoaie AC, Costache AD and Costache
II: Therapeutic strategies and chemoprevention of atherosclerosis:
What do we know and where do we go? Pharmaceutics. 14:7222022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Capodanno D, Alberts M and Angiolillo DJ:
Antithrombotic therapy for secondary prevention of atherothrombotic
events in cerebrovascular disease. Nat Rev Cardiol. 13:609–622.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lan W, Petznick A, Heryati S, Rifada M and
Tong L: Nuclear factor-κB: central regulator in ocular surface
inflammation and diseases. Ocul Surf. 10:137–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang D, Yu X, Gao K, Li F, Li X, Pu H,
Zhang P, Guo S and Wang W: Sweroside alleviates pressure
overload-induced heart failure through targeting CaMKIIδ to inhibit
ROS-mediated NF-κB/NLRP3 in cardiomyocytes. Redox Biol.
74:1032232024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hao T, Fang W, Xu D, Chen Q, Liu Q, Cui K,
Cao X, Li Y, Mai K and Ai Q: Phosphatidylethanolamine alleviates
OX-LDL-induced macrophage inflammation by upregulating autophagy
and inhibiting NLRP1 inflammasome activation. Free Radic Biol Med.
208:402–417. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang D, Gao W, Lu H, Qian JY and Ge JB:
Oxidized low-density lipoprotein stimulates dendritic cells
maturation via LOX-1-mediated MAPK/NF-κB pathway. Braz J Med Biol
Res. 54:e110622021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bian W, Jing X, Yang Z, Shi Z, Chen R, Xu
A, Wang N, Jiang J, Yang C, Zhang D, et al: Downregulation of
LncRNA NORAD promotes Ox-LDL-induced vascular endothelial cell
injury and atherosclerosis. Aging (Albany NY). 12:6385–6400. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang JW, Yao H, Caito S, Sundar IK and
Rahman I: Redox regulation of SIRT1 in inflammation and cellular
senescence. Free Radic Biol Med. 61:95–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stein S, Schäfer N, Breitenstein A, Besler
C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C,
Landmesser U, et al: SIRT1 reduces endothelial activation without
affecting vascular function in ApoE-/- mice. Aging (Albany NY).
2:353–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Z, Li T, Wang C, Meng M, Tan S and
Chen L: Dihydromyricetin inhibits M1 macrophage polarization in
atherosclerosis by modulating miR-9-mediated SIRT1/NF-κB signaling
pathway. Mediators Inflamm. 2023:25475882023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Golpanian S, Wolf A, Hatzistergos KE and
Hare JM: Rebuilding the damaged heart: Mesenchymal stem cells,
cell-based therapy, and engineered heart tissue. Physiol Rev.
96:1127–1168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ha DH, Kim HK, Lee J, Kwon HH, Park GH,
Yang SH, Jung JY, Choi H, Lee JH, Sung S, et al: Mesenchymal
stem/stromal cell-derived exosomes for immunomodulatory
therapeutics and skin regeneration. Cells. 9:11572020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang N, Luo Y, Zhang H, Zhang F, Gao X
and Shao J: Exosomes derived from mesenchymal stem cells ameliorate
the progression of atherosclerosis in ApoE-/- mice via
FENDRR. Cardiovasc Toxicol. 22:528–544. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma J, Chen L, Zhu X, Li Q, Hu L and Li H:
Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2
macrophage polarization and reduces macrophage infiltration to
attenuate atherosclerosis. Acta Biochim Biophys Sin (Shanghai).
53:1227–1236. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu C and Li L: Preconditioning influences
mesenchymal stem cell properties in vitro and in vivo. J Cell Mol
Med. 22:1428–1442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Wang J, Wang P, Zhong L, Wang S,
Feng Q, Wei X and Zhou L: Hypoxia-pretreated mesenchymal stem
cell-derived exosomes-loaded low-temperature extrusion 3D-printed
implants for neural regeneration after traumatic brain injury in
canines. Front Bioeng Biotechnol. 10:10251382022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li T, Zhao Y, Cao Z, Shen Y, Chen J, Huang
X, Shao Z, Zeng Y, Chen Q, Yan X, et al: Exosomes Derived from
apelin-pretreated mesenchymal stem cells ameliorate sepsis-induced
myocardial dysfunction by alleviating cardiomyocyte pyroptosis via
delivery of miR-34a-5p. Int J Nanomedicine. 20:687–703. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu J, Wang J, Sheng Y, Zou Y, Bo L, Wang
F, Lou J, Fan X, Bao R, Wu Y, et al: Baicalin improves survival in
a murine model of polymicrobial sepsis via suppressing inflammatory
response and lymphocyte apoptosis. PLoS One. 7:e355232012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Motoo Y and Sawabu N: Antitumor effects of
saikosaponins, baicalin and baicalein on human hepatoma cell lines.
Cancer Lett. 86:91–95. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu H, Chen B and Ren Q: Baicalin relieves
hypoxia-aroused H9c2 cell apoptosis by activating
Nrf2/HO-1-mediated HIF1α/BNIP3 pathway. Artif Cells Nanomed
Biotechnol. 47:3657–3663. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z, Pan X, Sheng Z, Yan G, Chen L and
Ma G: Baicalin suppresses the proliferation and migration of
Ox-LDL-VSMCs in atherosclerosis through upregulating miR-126-5p.
Biol Pharm Bull. 42:1517–1523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Y, Wang F, Fan L, Zhang W, Wang T, Du Y
and Bai X: Baicalin alleviates atherosclerosis by relieving
oxidative stress and inflammatory responses via inactivating the
NF-κB and p38 MAPK signaling pathways. Biomed Pharmacother.
97:1673–1679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu M, Liu W, Li J, Lu J, Lu H, Jia W and
Liu F: Exosomes derived from atorvastatin-pretreated MSC accelerate
diabetic wound repair by enhancing angiogenesis via AKT/eNOS
pathway. Stem Cell Res Ther. 11:3502020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ
and Han M: Inflammation and atherosclerosis: Signaling pathways and
therapeutic intervention. Signal Transduct Target Ther. 7:1312022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W, Yan C, Xiao Y, Sun Y, Lin Y, Li Q
and Cai W: Sulfasalazine induces autophagy inhibiting neointimal
hyperplasia following carotid artery injuries in mice. Front Bioeng
Biotechnol. 11:11997852023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng Z, Bian Y, Zhang Y, Ren G and Li G:
Metformin activates AMPK/SIRT1/NF-κB pathway and induces
mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer
cell pyroptosis. Cell Cycle. 19:1089–1104. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He B, Nie Q, Wang F, Han Y, Yang B, Sun M,
Fan X, Ye Z, Liu P and Wen J: Role of pyroptosis in atherosclerosis
and its therapeutic implications. J Cell Physiol. 236:7159–7175.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Groenen AG, Halmos B, Tall AR and
Westerterp M: Cholesterol efflux pathways, inflammation, and
atherosclerosis. Crit Rev Biochem Mol Biol. 56:426–439. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lotfy A, AboQuella NM and Wang H:
Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical
trials. Stem Cell Res Ther. 14:662023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiong J, Hu H, Guo R, Wang H and Jiang H:
Mesenchymal stem cell exosomes as a new strategy for the treatment
of diabetes complications. Front Endocrinol (Lausanne).
12:6462332021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo M, Yin Z, Chen F and Lei P:
Mesenchymal stem cell-derived exosome: A promising alternative in
the therapy of Alzheimer's disease. Alzheimers Res Ther.
12:1092020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen W, Lin F, Feng X, Yao Q, Yu Y, Gao F,
Zhou J, Pan Q, Wu J, Yang J, et al: MSC-derived exosomes attenuate
hepatic fibrosis in primary sclerosing cholangitis through
inhibition of Th17 differentiation. Asian J Pharm Sci.
19:1008892024.PubMed/NCBI
|
|
41
|
Liu J, Ren L, Li S, Li W, Zheng X, Yang Y,
Fu W, Yi J, Wang J and Du G: The biology, function, and
applications of exosomes in cancer. Acta Pharm Sin B. 11:2783–2797.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang Y, Yu M, Song ZF, Wei ZY, Huang J
and Qian HY: Targeted delivery of mesenchymal stem cell-derived
bioinspired exosome-mimetic nanovesicles with platelet membrane
fusion for atherosclerotic treatment. Int J Nanomedicine.
19:2553–2571. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia
QR and Ge JF: Exosomes derived from bone-marrow mesenchymal stem
cells alleviate cognitive decline in AD-like mice by improving
BDNF-related neuropathology. J Neuroinflammation. 19:352022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu S, Cheuk YC, Jia Y, Chen T, Chen J, Luo
Y, Cao Y, Guo J, Dong L, Zhang Y, et al: Bone marrow mesenchymal
stem cell-derived exosomal miR-21a-5p alleviates renal fibrosis by
attenuating glycolysis by targeting PFKM. Cell Death Dis.
13:8762022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao L, Qiu F, Cao H, Li H, Dai G, Ma T,
Gong Y, Luo W, Zhu D, Qiu Z, et al: Therapeutic delivery of
microRNA-125a-5p oligonucleotides improves recovery from myocardial
ischemia/reperfusion injury in mice and swine. Theranostics.
13:685–703. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li M, Li S, Du C, Zhang Y, Li Y, Chu L,
Han X, Galons H, Zhang Y, Sun H and Yu P: Exosomes from different
cells: Characteristics, modifications, and therapeutic
applications. Eur J Med Chem. 207:1127842020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nakao Y, Fukuda T, Zhang Q, Sanui T,
Shinjo T, Kou X, Chen C, Liu D, Watanabe Y, Hayashi C, et al:
Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2
macrophage polarization and inhibit periodontal bone loss. Acta
Biomater. 122:306–324. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu R, Cao H, Zhang S, Cai M, Zou T, Wang
G, Zhang D, Wang X, Xu J, Deng S, et al: ZBP1-mediated apoptosis
and inflammation exacerbate steatotic liver ischemia/reperfusion
injury. J Clin Invest. 134:e1804512024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu M, Li X and Song L: Baicalin regulates
macrophages polarization and alleviates myocardial
ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm
Biol. 58:655–663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu S, Yao F, Qiu H, Zhang G, Xu H and Xu
J: Coupling factors and exosomal packaging microRNAs involved in
the regulation of bone remodelling. Biol Rev Camb Philos Soc.
93:469–480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao S, Huang M, Yan L, Zhang H, Shi C,
Liu J, Zhao S, Liu H and Wang B: Exosomes derived from
baicalin-pretreated mesenchymal stem cells alleviate hepatocyte
ferroptosis after acute liver injury via the Keap1-NRF2 pathway.
Oxid Med Cell Longev. 2022:82872272022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang B, Su L, Chen Z, Wu M, Wei J and Lin
Y: Exosomes derived from baicalin-pretreated bone mesenchymal stem
cells improve Th17/Treg imbalance after hepatic
ischemia-reperfusion via FGF21 and the JAK2/STAT3 pathway. IUBMB
Life. 76:534–547. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pryma CS, Ortega C, Dubland JA and Francis
GA: Pathways of smooth muscle foam cell formation in
atherosclerosis. Curr Opin Lipidol. 30:117–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Grootaert MOJ, Finigan A, Figg NL, Uryga
AK and Bennett MR: SIRT6 protects smooth muscle cells from
senescence and reduces atherosclerosis. Circ Res. 128:474–491.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang
L and Wang Y: Exosomes from nicotine-stimulated macrophages
accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC
migration and proliferation. Theranostics. 9:6901–6919. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li H, Zhuang W, Xiong T, Park WS, Zhang S,
Zha Y, Yao J, Wang F, Yang Y, Chen Y, et al: Nrf2 deficiency
attenuates atherosclerosis by reducing LOX-1-mediated proliferation
and migration of vascular smooth muscle cells. Atherosclerosis.
347:1–16. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo B, Zhuang TT, Li CC, Li F, Shan SK,
Zheng MH, Xu QS, Wang Y, Lei LM, Tang KX, et al: MiRNA-132/212
encapsulated by adipose tissue-derived exosomes worsen
atherosclerosis progression. Cardiovasc Diabetol. 23:3312024.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu J, Zhang X, Yu Z and Zhang T: Exosomes
promote atherosclerosis progression by regulating
Circ_100696/miR-503-5p/PAPPA axis-mediated vascular smooth muscle
cells proliferation and migration. Int Heart J. 64:918–927. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park JY, Park HM, Kim S, Jeon KB, Lim CM,
Hong JT and Yoon DY: Human IL-32θA94V mutant attenuates
monocyte-endothelial adhesion by suppressing the expression of
ICAM-1 and VCAM-1 via binding to cell surface receptor integrin
αVβ3 and αVβ6 in TNF-α-stimulated HUVECs. Front Immunol.
14:11603012023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Feng X, Du M, Li S, Zhang Y, Ding J, Wang
J, Wang Y and Liu P: Hydroxysafflor yellow A regulates
lymphangiogenesis and inflammation via the inhibition of PI3K on
regulating AKT/mTOR and NF-κB pathway in macrophages to reduce
atherosclerosis in ApoE-/- mice. Phytomedicine. 112:1546842023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Morigi M, Perico L and Benigni A: Sirtuins
in renal health and disease. J Am Soc Nephrol. 29:1799–1809. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kauppinen A, Suuronen T, Ojala J,
Kaarniranta K and Salminen A: Antagonistic crosstalk between NF-κB
and SIRT1 in the regulation of inflammation and metabolic
disorders. Cell Signal. 25:1939–1948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wei K, Yu L, Li J, Gao J, Chen L, Liu M,
Zhao X, Li M, Shi D and Ma X: Platelet-derived exosomes regulate
endothelial cell inflammation and M1 macrophage polarization in
coronary artery thrombosis via modulating miR-34a-5p expression.
Sci Rep. 14:174292024. View Article : Google Scholar : PubMed/NCBI
|