Introduction
The FOLFOX regimen, which includes bolus/infusional
5-fluorouracil (5-FU) with folinic acid modulation and oxaliplatin,
has become one of the most common first-line treatments for
patients with metastatic colorectal cancer (mCRC) (1). Despite high response rates (RRs) to
the FOLFOX regimen, a pathological complete response (pCR) of CRC
liver metastases to systemic chemotherapy is rarely achieved.
Benoist et al reported that persistent macroscopic or
microscopic residual disease or early recurrence in situ was
observed in 83% of liver metastases downsized to a CR on imaging by
systemic chemotherapy (2).
Since treatment with infusional 5-FU has the
short-comings of increased inconvenience, cost and morbidity
related to the use of a portable infusion pump and a central venous
catheter, oral fluoropyrimidines were evaluated as alternatives to
infusional 5-FU. S-1 is an orally active prodrug of 5-FU that
contains tegafur, which is constantly metabolized to 5-FU, combined
with the modulators, gimeracil and potassium oxonate (3). In Japan, a phase I/II study of
oxaliplatin plus oral S-1 (SOX) showed promising efficacy with good
tolerability in patients with untreated mCRC (4). The efficacy of this combination was
superior to that reported for each drug as monotherapy (5,6), with
a RR of 50%, median progression-free survival (PFS) of 196 days,
and a 1-year survival rate of 78.6%. The results suggest that
tri-weekly treatment with the SOX regimen is an adequate substitute
for FOLFOX and can be administered more readily since it does not
require central vein access. A phase I study of SOX plus
bevacizumab (BV) showed that the maximum tolerated dose (MTD) of
S-1 is 25 mg/m2. Moreover, no increases in toxicities
were observed when BV and oxaliplatin were combined (7). However, the impact of SOX plus BV on
CRC liver metastasis remains unknown.
This is the first reported case of multiple CRC
liver metastases that had a marked response to SOX plus BV and
achieved a pCR following radical liver resection.
Case report
A 63-year-old man was referred to a prior hospital
due to bloody stool. Colonoscopic examination revealed a severely
stenotic lesion in the descending colon, although the patient did
not complain of any clinical symptoms. Subsequent abdominal
computed tomography (CT) scans showed a tumor occupying the
descending colon with possible invasion into the small intestine,
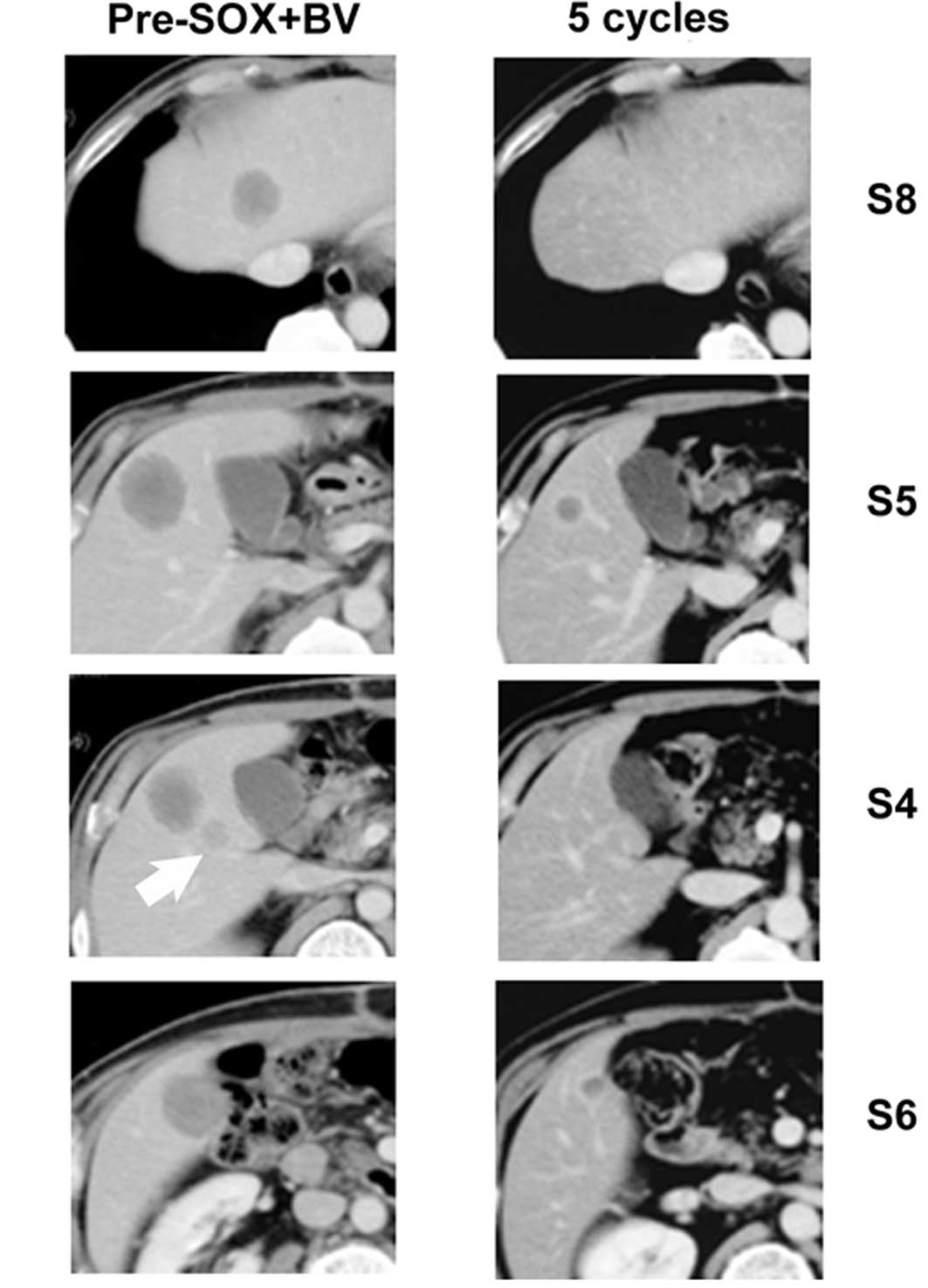
along with multiple liver tumors at segments (S) 4, S5, S6 and S8
(Fig. 1A-D) (8). All metastases with the exception of
that in S8 were >30 mm in diameter. Positron emission tomography
with 18-fluorodeoxyglucose (FDG-PET)/CT fusion imaging confirmed no
other distant metastatic lesions. To prevent ileus due to complete
obstruction in the descending colon, descending colectomy with
lymphadenectomy was performed. Since the tumor was not mobilized
due to direct invasion into the mesenterium and jejunum at surgery,
both the involved proximal jejunum and the mesenterium were
resected.
Pathological examination of the surgical specimens
revealed the presence of a poorly differentiated adenocarcinoma
(Fig. 2A) with direct invasion into
the serosa of the jejunum. Tumor cells metastasized to the regional
lymph nodes, including the paracolic (10/10) and left colic lymph
nodes (1/1). The patient was referred to our hospital at that point
for further treatment of the liver metastases.
Systemic chemotherapy with SOX plus BV was initially
administered in the neoadjuvant setting. Briefly, oxaliplatin (130
mg/m2) and BV (7.5 mg/kg) were administered
intravenously on day 1, and S-1 (80 mg/m2/day) was
administered orally twice daily for 14 days with a 1-week rest. The
regimen was repeated every 3 weeks. Although no serious adverse
events were observed, the patient developed grade 2 proteinuria
during the treatment, which required suspension of BV in cycles 5,
6 and 8. The patient did not receive BV during cycle 9 to allow
washout of BV prior to liver resection.
The patient was monitored regularly with CT of the
chest to pelvis, and by monthly measurement of carcinoembryonic
antigen (CEA) levels. Following the completion of two cycles of SOX
plus BV, each of the liver metastases was markedly decreased in
size on CT scans, considered to reflect a partial response to the
chemotherapy. The tumors in S4 and S8 were undetectable on the CT
scans after five cycles of SOX plus BV (Fig. 1E and G), and the tumors in S5 and S6
decreased to 65 and 59% of their original size, respectively
(Fig. 1F and H). No evidence of any
de novo detectable metastatic lesions was noted. The CEA
level was slightly elevated prior to chemotherapy (9.5 ng/ml) and
rapidly decreased to a normal level (2.7 ng/ml) at the completion
of the five cycles of SOX plus BV. Retention rate of ICG at 15 min
(ICGR15) was 23% after nine cycles of SOX plus BV.
On achievement of a markedly favorable response to
chemotherapy, the patient opted to undergo surgical treatment of
the liver metastasis after nine cycles of SOX plus BV. Partial
resection of S4, S5, S6 and S8 of the liver and cholecystectomy
were performed. No evidence of peritoneal dissemination was
observed on visual inspection. The liver displayed fatty change,
but not the dark features of ‘blue liver.’ All of the liver tumors
were successfully resected without any macroscopic residues.
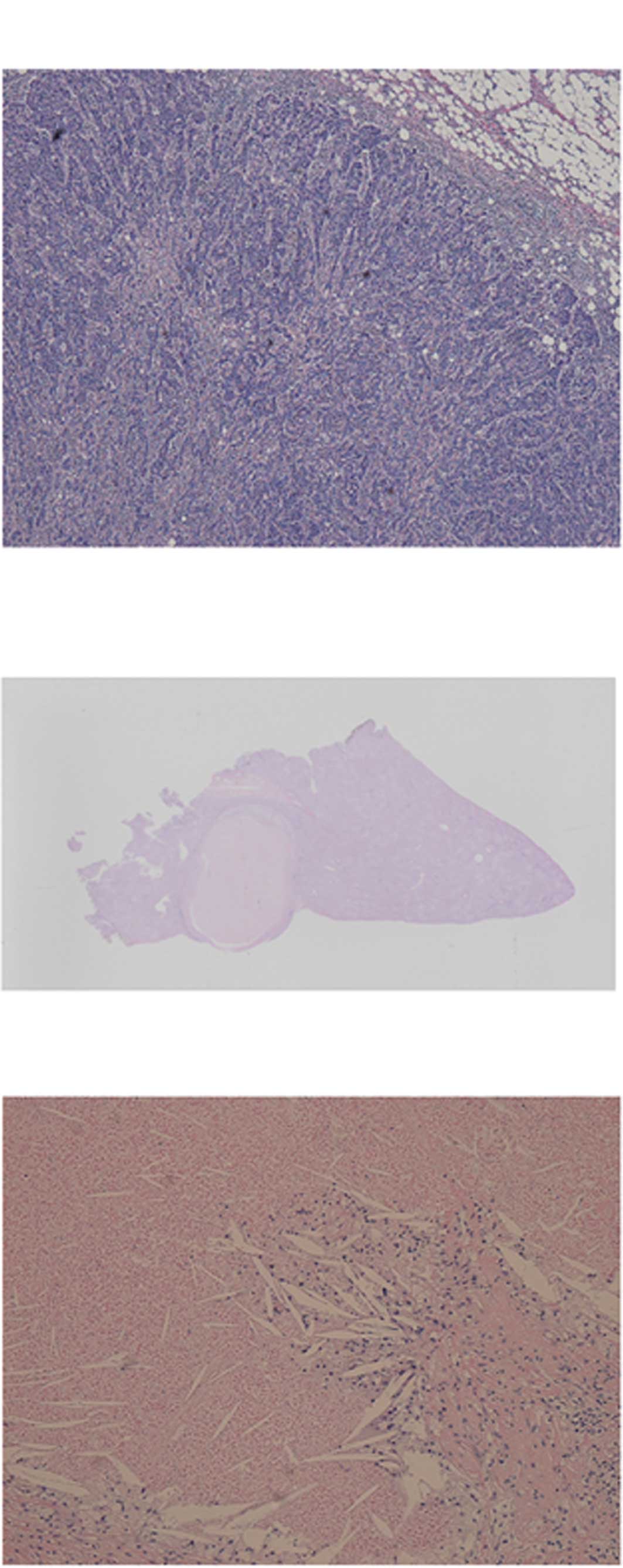
The resected tumors were yellowish nodules and
relatively large: 6×5 mm in S4, 15×10 mm in S5, and 13×9 mm in S6
(Fig. 3). No mass lesion was
identified in the S8 liver specimen, even after the tissue was
sectioned into 24 pieces at 5-mm intervals. Each nodule was sampled
and histologically analyzed. Microscopic examination of the tissue
revealed necrotic tissue without any viable tumor cells. The
necrotic nodule was surrounded by a collagen capsule and was mixed
with xanthogranuloma, comprising cholesterin cleft and foamy cells
with moderate infiltration of inflammatory cells (Fig. 2B and C). Sinusoidal obstruction was
not identified in the non-cancerous liver tissue. Thus, a pCR was
determined based on the absence of any viable tumor cells
irrespective of the proportions of necrosis and fibrosis in any
part of the resected liver tumor, corresponding to a grade 3
therapeutic effect.
Adjuvant chemotherapy with S-1 monotherapy is
ongoing following liver resection. No radiological recurrence was
observed 6 months following surgery.
Discussion
Patients with mCRC with synchronous liver metastases
are known to have a poorer prognosis than patients with
metachronous hepatic metastases (9–11). The
potential occurrence of occult hepatic metastases should be
considered in cases with synchronous liver metastases since primary
colorectal cancer possesses a stronger effect than in cases with
metachronous metastasis (12,13).
Survival rates of patients who do not undergo resection are also
poor and do not exceed 2% at 5 years (14,15).
Previously, the introduction of novel chemotherapeutic agents,
including oxaliplatin and irinotecan, increased the median survival
of these patients (1,16). However, outcomes of chemotherapy
remain inferior to those of curative hepatic resection, resulting
in 5-year survival rates of 40% overall (17) and even exceeding 50% in selected
patients (12,13,18,19).
The present case had a locally advanced colon tumor with direct
invasion into adjacent viscera and multiple metastatic regional
lymph nodes. Pathological examination of the primary lesion
revealed poorly differentiated adenocarcinoma. Multiple liver
metastases were observed in the two lobes and were found to be
synchronous. These findings suggested that the patient had a poor
prognosis. Thus, a cure was unlikely to be achieved. Consequently,
the Tumor Boards of our hospital recommended neoadjuvant
chemotherapy, although multiple liver metastases in the two lobes
were not technically unresectable.
Histopathological analysis of resected liver
specimens potentially allows for the evaluation of tumor response
following therapy. In particular, a pCR is defined as the complete
absence of residual viable tumor cells on examination by
pathologists as noted in the present case, and is associated with
relatively high survival rates (20,21). A
multivariate analysis identified three variables as independent
predictors of cure for patients with CRC liver metastases,
including a maximum size of metastases at diagnosis of less than 30
mm, three or fewer metastases at hepatectomy and a pCR (22). Thus, from an oncological
perspective, a pCR appears to have greater clinical relevance
compared to a radiologic CR.
No correlation between radiological imaging and
pathologic analysis was noted in this case. CT showed remnant liver
lesions in S5 and S6 following chemotherapy, although a pCR was
achieved. This suggests that the use of a single imaging modality,
i.e., CT, is insufficient for the prediction of a pathological
response in the evaluation of CRC liver lesions. Furthermore, a
complete disappearance of liver lesions on radiological imaging
does not always reflect a complete disappearance of viable tumor
cells on pathological examination. Data suggest that sites of liver
metastases with CR on imaging and without visible disease at
surgery indicate the presence of viable tumor cells on pathological
examination in 80% of patients (2).
Therefore, it is optimal to remove any lesions containing a tumor,
as performed in the present case. Furthermore, more accurate
assessments of tumor cell viability are required during neoadjuvant
chemotherapy. Adam et al reported that pCR was observed in
4% of patients with CRC liver metastases who received preoperative
chemotherapy (20). Various reports
proposed that the presence of metastases of 30 mm or less at
diagnosis is a key predictive factor for pCR (2,21).
Although the maximum size of the metastases at diagnosis was more
than 30 mm in the present case, a pCR was achieved.
In studies by Benoist et al (2) and Adam et al (20), the pathological response to
chemotherapy was analyzed in CRC patients treated with 5-FU and
leucovorin (LV5FU2), LV5FU2 plus oxaliplatin, and LV5FU2 plus
irinotecan, but not LV5FU2 plus BV. The majority of pCR patients
received FOLFOX without BV as the last line of systemic therapy
prior to hepatectomy. pCR was observed in 6% of patients treated
with FOLFOX and in 2% of patients who received FOLFIRI. No
significant differences in the chemotherapy characteristics were
noted between patients who did and did not experience a pCR
(20). However, the efficacy of BV
when combined with cytotoxic therapy in patients undergoing
preoperative chemotherapy followed by liver resection remains
unclear. For example, Ribero et al reported that the
addition of BV to 5FU/oxaliplatin did not appear to increase the
incidence of pCR (23). In
contrast, a recent study showed that the rates of radical surgery
are slightly higher in patients treated with an oxaliplatin-based
regimen plus BV versus oxaliplatin-based chemotherapy alone
(24). Furthermore, regimens
including BV have been shown to be associated with a significantly
higher frequency of complete or major responses compared to those
without BV (25).
Long-term exposure to oxaliplatin is associated with
an increased risk of postoperative liver insufficiency without
improvement of pathological response (25–27).
In the present case, a relatively high dose of total oxaliplatin
(1,930 mg) was administered, which may have been responsible for
the high ICGR15 prior to surgery. Therefore, hepatectomy
for CRC metastases should be performed as early as possible,
although there is no consensus regarding appropriate timing for
hepatic resection. However, a retrospective study showed that
oxaliplatin-related hepatic lesions occurred significantly less
frequently in patients treated with oxaliplatin plus BV compared to
those who received oxaliplatin alone (23,28).
This finding may support a model in which the preoperative use of
BV in combination with SOX may contribute, not only to improvement
of the pathological response, but also to a reduction of the risk
of hepatotoxicity.
In the present case, adjuvant chemotherapy with S-1
monotherapy is ongoing after surgery. The S-1 regimen was selected
for the reasons that: i) the patient was already treated with nine
cycles of oxaliplatin-based therapy prior to surgery (although no
persistent sensory neuropathy was observed), ii) no data support
the efficacy of the addition of BV to adjuvant chemotherapy, and
iii) no definite metastatic lesions were detected in other organs
on post-operative CT.
In conclusion, this is the first patient with
multiple CRC liver metastases who achieved a pCR following SOX plus
BV combination chemotherapy. The SOX regimen is a safe and
effective treatment for mCRC that does not require central vein
access. The addition of BV to the chemotherapy may also have played
a role in the pCR. Therefore, systemic chemotherapy with SOX plus
BV is promising as a conversion therapy for CRC liver metastasis
unlikely to be ‘cured’.
References
|
1
|
De Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F,
Wilson C, Morvan F and Bonetti A: Leucovorin and fluorouracil with
or without oxaliplatin as first-line treatment in advanced
colorectal cancer. J Clin Oncol. 18:2938–2947. 2000.
|
|
2
|
Benoist S, Brouquet A, Penna C, Julié C,
El Hajjam M, Chagnon S, Mitry E, Rougier P and Nordlinger B:
Complete response of colorectal liver metastases after
chemotherapy: does it mean cure? J Clin Oncol. 24:3939–3945. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato T, Shimamoto Y, Uchida J, Ohshimo H,
Abe M, Shirasaka T and Fukushima M: Possible regulation of
5-fluorouracil-induced neuro- and oral toxicities by two
biochemical modulators consisting of S-1, a new oral formulation of
5-fluorouracil. Anticancer Res. 21:1705–1712. 2001.PubMed/NCBI
|
|
4
|
Yamada Y, Tahara M, Miya T, Satoh T,
Shirao K, Shimada Y, Ohtsu A, Sasaki Y and Tanigawara Y: Phase I/II
study of oxaliplatin with oral S-1 as first-line therapy for
patients with metastatic colorectal cancer. Br J Cancer.
98:1034–1038. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohtsu A, Baba H, Sakata Y, Mitachi Y,
Horikoshi N, Sugimachi K and Taguchi T: Phase II study of S-1, a
novel oral fluoropyrimidine derivative, in patients with metastatic
colorectal carcinoma. Br J Cancer. 83:141–145. 2000.PubMed/NCBI
|
|
6
|
Shirao K, Ohtsu A, Takada H, Mitachi Y,
Hirakawa K, Horikoshi N, Okamura T, Hirata K, Saitoh S, Isomoto H
and Satoh A: Phase II study of oral S-1 for treatment of metastatic
colorectal carcinoma. Cancer. 100:2355–2361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Chung K, Zergebel C, Urrea P,
Quinones M and Saltz L: Phase I study of oral S-1 in combination
with oxaliplatin (oxali) and bevacizumab (bev) in patients with
advanced solid tumors. J Clin Oncol ASCO Annual Meeting Proceedings
Part I. 25(Suppl): 40912007.
|
|
8
|
Couinaud C: Le foie Etudes anatomiques et
chirurgicales. Masson; Paris: 1957
|
|
9
|
Beckurts KT, Holscher AH, Thorban S,
Bollschweiler E and Siewert JR: Significance of lymph node
involvement at the hepatic hilum in the resection of colorectal
liver metastases. Br J Surg. 84:1081–1084. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hananel N, Garzon J and Gordon PH: Hepatic
resection for colorectal liver metastases. Am Surg. 61:444–447.
1995.PubMed/NCBI
|
|
11
|
Scheele J, Stang R, Altendorf-Hofmann A
and Paul M: Resection of colorectal liver metastases. World J Surg.
19:59–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu Y, Yasui K, Sano T, Hirai T,
Kanemitsu Y, Komori K and Kato T: Treatment strategy for
synchronous metastases of colorectal cancer: is hepatic resection
after an observation interval appropriate? Langenbecks Arch Surg.
392:535–538. 2007. View Article : Google Scholar
|
|
13
|
Shimizu Y, Yasui K, Sano T, Hirai T,
Kanemitsu Y, Komori K and Kato T: Validity of observation interval
for synchronous hepatic metastases of colorectal cancer: changes in
hepatic and extrahepatic metastatic foci. Langenbecks Arch Surg.
393:181–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wood CB, Gillis CR and Blumgart LH: A
retrospective study of the natural history of patients with liver
metastases from colorectal cancer. Clin Oncol. 2:285–288.
1976.PubMed/NCBI
|
|
15
|
Wagner JS, Adson MA, Van Heerden JA, Adson
MH and Ilstrup DM: The natural history of hepatic metastases from
colorectal cancer: A comparison with respective treatment. Ann
Surg. 199:502–508. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saltz LB, Cox JV, Blanke C, Rosen LS,
Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta
N, Elfring GL and Miller LL: Irinotecan plus fluorouracil and
leucovorin for metastatic colorectal cancer: Irinotecan Study
Group. N Engl J Med. 343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
LiverMetSurvey. International registry of
liver metastases of colorectal cancer. http://www.livermetsurvey.org.
|
|
18
|
Choti MA, Sitzmann JV, Tiburi MF,
Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ and
Cameron JL: Trends in long-term survival following liver resection
for hepatic colorectal metastases. Ann Surg. 235:759–766. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdalla EK, Vauthey JN, Ellis LM, Ellis V,
Pollock R, Broglio KR, Hess K and Curley SA: Recurrence and
outcomes following hepatic resection, radiofrequency ablation, and
combined resection/ablation for colorectal liver metastases. Ann
Surg. 239:818–825. 2004. View Article : Google Scholar
|
|
20
|
Adam R, Wicherts DA, de Haas RJ, Aloia T,
Lévi F, Paule B, Guettier C, Kunstlinger F, Delvart V, Azoulay D
and Castaing D: Complete pathologic response after preoperative
chemotherapy for colorectal liver metastases: myth or reality? J
Clin Oncol. 26:1635–1641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blazer DG III, Kishi Y, Maru DM, Kopetz S,
Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, Zorzi D,
Ribero D, Ellis LM, Glover KY, Wolff RA, Curley SA, Abdalla EK and
Vauthey JN: Pathologic response to preoperative chemotherapy: a new
outcome end point after resection of hepatic colorectal metastases.
J Clin Oncol. 26:5344–5351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adam R, Wicherts DA, de Haas RJ, Ciacio O,
Lévi F, Paule B, Ducreux M, Azoulay D, Bismuth H and Castaing D:
Patients with initially unresectable colorectal liver metastases:
is there a possibility of cure? J Clin Oncol. 27:1829–1835. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ribero D, Wang H, Donadon M, Zorzi D,
Thomas MB, Eng C, Chang DZ, Curley SA, Abdalla EK, Ellis LM and
Vauthey JN: Bevacizumab improves pathologic response and protects
against hepatic injury in patients treated with oxaliplatin-based
chemotherapy for colorectal liver metastases. Cancer.
110:2761–2767. 2007. View Article : Google Scholar
|
|
24
|
Okines A, Puerto OD, Cunningham D, Chau I,
Van Cutsem E, Saltz L and Cassidy J: Surgery with curative-intent
in patients treated with first-line chemotherapy plus bevacizumab
for metastatic colorectal cancer First BEAT and the randomised
phase-III NO16966 trial. Br J Cancer. 101:1033–1038. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kishi Y, Zorzi D, Contreras CM, Maru DM,
Kopetz S, Ribero D, Motta M, Ravarino N, Risio M, Curley SA,
Abdalla EK, Capussotti L and Vauthey JN: Extended preoperative
chemotherapy does not improve pathologic response and increases
postoperative liver insufficiency after hepatic resection for
colorectal liver metastases. Ann Surg Oncol. June 22–2010.(Epub
ahead of print).
|
|
26
|
Karoui M, Penna C, Amin-Hashem M, Mitry E,
Benoist S, Franc B, Rougier P and Nordlinger B: Influence of
preoperative chemotherapy on the risk of major hepatectomy for
colorectal liver metastases. Ann Surg. 243:1–7. 2006. View Article : Google Scholar
|
|
27
|
Aloia T, Sebagh M, Plasse M, Karam V, Lévi
F, Giacchetti S, Azoulay D, Bismuth H, Castaing D and Adam R: Liver
histology and surgical outcomes after preoperative chemotherapy
with fluorouracil plus oxaliplatin in colorectal cancer liver
metastases. J Clin Oncol. 24:4983–4990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rubbia-Brandt L, Lauwers GY, Wang H, Majno
PE, Tanabe K, Zhu AX, Brezault C, Soubrane O, Abdalla EK, Vauthey
JN, Mentha G and Terris B: Sinusoidal obstruction syndrome and
nodular regenerative hyperplasia are frequent
oxaliplatin-associated liver lesions and partially prevented by
bevacizumab in patients with hepatic colorectal metastasis.
Histopathology. 56:430–439. 2010. View Article : Google Scholar
|