Introduction
Ganoderma lucidum (G. lucidum), known
as ‘Lingzhi’ in China, is a lamella-less basidiomycetous fungus
that belongs to the Polyporaceae family. The medicinal
properties of this mushroom are well known in China and other parts
of Asia. Known as ‘miraculous Zhi’ or ‘auspicious herb’, Lingzhi is
considered to ‘symbolize happy augury, and to bespeak good fortune,
good health and longevity, even immortality’ (1). The fungus has been used as a
traditional Chinese medicine to treat a variety of diseases for
more than 4,000 years, and regular consumption of the mushroom
extracts is believed to preserve human vitality and promote
longevity (2,3).
A number of bioactive components have been
identified from its fruit bodies, mycelia, spores and culture
media. Polysaccharides and triterpenes are two major categories of
the bioactive ingredients. It was previously identified that
polysaccharides from G. lucidum exert their in vitro
and in vivo anticancer effect via an immune-modulatory
mechanism (4–6). Studies showed that triterpenes possess
the bioactivities of antioxidation (7), hepatoprotection (8), cholesterol stasis (9), anti-hypertension (10,11)
and inhibiting platelet aggregation (12) due to the inhibition of enzymes such
as h-galactosidase, cholesterol synthase and
angiotension-converting enzyme. Triterpenes isolated from G.
lucidum were reported to exhibit cytotoxic activity against
tumor cells (13–16). A triterpene from Ganoderma
tsugae was found to induce cell apoptosis and cell cycle arrest
in human hepatoma Hep3B cells, but its molecular mechanism was not
investigated (13).
Apoptosis is a form of cell death defined by a
characteristic set of morphological and biochemical changes.
Previous studies identified a significant role for caspases, a
family of cysteine-dependent aspartate-directed proteases, in
apoptotic death, especially in the context of cancer cells
(17). Individual members of the
caspase family mediate apoptosis in different cell types, and
different caspases have been found to mediate apoptosis even within
a given cell type depending on the apoptotic stimulus received by
the cells (18). Caspase-3 and -9
are reported to play key roles in caspase-mediated apoptosis, and
variations in their activity were correlated with apoptosis in a
variety of cancer cells (19,20).
In this study, we report that a triterpene-enriched
fraction from mycelia of G. lucidum inhibits the growth of
tumor cells and induces apoptosis in SW620 colorectal
adenocarcinoma cells. Consequently, the anticancer mechanism of
GLAI-induced apoptosis on SW620 human colorectal adenocarcinoma
cells was examined. Findings present evidence of the signaling
molecule involved in the anticancer activity of triterpene from
G. lucidum.
Materials and methods
Preparation of ganoderma extracts
G. lucidum fruiting bodies were extracted
with 95% (v/v) aqueous ethanol at room temperature. Combined
ethanolic extracts were evaporated to dryness, redissolved in water
and extracted with chloroform. After addition of a saturated
NaHCO3 solution, the chloroform layer containing
non-acidic triterpenoids was collected. The crude extracts were
purified using silica gel (200–300 mesh) column chromatography. The
column was eluted with petrol ether/acetone (v/v=1:0, 50:1, 30:1,
10:1, 3:1 sequentially) and eight fractions were collected. The
second fraction was separated further by MCI chromatography and the
elution gradient was 40–100% methanol in water. The fifth fraction,
obtained with 80% methanol elution, was termed GLAI. Triterpenes
were visualized as fluorescent spots under long wavelength UV
light.
Cell cultures
Human tumor cell lines, SW620 cells (colon), MCF-7
(breast), K562 (bone marrow), and mouse lymphocytic leukemia cell
line L1210 were obtained from the American Type Culture Collection
(ATCC) and maintained at 37°C in RPMI-1640 containing 10% fetal
calf serum (FCS) (Kraeber, Wedel, Germany), 100 U/ml penicillin and
100 μg/ml streptomycin.
Cell proliferation assay
Cells were adjusted to a concentration of
1×104 cells/ml. Cell suspension (180 μl) and different
test agents (20 μl) were added to each well of a 96-well microplate
reader. After incubation at 37°C in a 5% CO2 atmosphere
for a defined time, 20 μl Alamar Blue reagent (Biosource, Nivelles,
Belgium) were added to each well and incubation continued for
another 6 h. The extinction was measured using a micro ELISA
autoreader at 570 and 600 nm. The proliferation rate was calculated
according to the Biosource protocol.
Microscopic observation and nuclear
staining with Hoechst 33258
SW620 cells (1×104 cells/ml) were treated
for 24 h with a control and 10 μM GLAI. Morphological observations
of cultured cells were made by inverted, phase-contrast microscopy.
Samples treated with DMSO only served as controls. For nuclear
staining, SW620 cells (1×105 cells/ml) were treated for
24 h with DMSO and 10, 50 and 100 μM GLAI. After treatment, cells
were harvested and washed with ice-cold phosphate-buffered saline
(PBS). The cells were then incubated in nuclear fluorochrome
Hoechst 33258 at a final concentration of 10 μg/ml at room
temperature for 10 min in the dark. Nuclear morphology was then
examined with an Olympus fluorescent microscope.
Flow cytometric analysis of
apoptosis
To confirm the nature of the effects of GLAI on
SW620 cells, dual-staining [propidium iodide (PI) and annexin V
(AV)] flow cytometry was used to measure the externalization of
phosphatidylserine (PS). Aliquots (5×106) of SW620 cells
cultured as described above were treated with 20, 50 or 100 μmol/l
GLAI for 24 h. Controls were treated with DMSO only. After washing
and trypsinization, cell samples were collected by centrifugation
(400 g, 3 min, 4°C) and double-stained using the apoptosis
detection kit (BD Biosciences, San Jose, CA, USA) according to the
manufacturer’s instructions. Cells were incubated for 30 min at
25°C in 100 μl 1X buffer solution, 5 μl AV-FITC and 5 μl PI, and
then a further 400 μl of 1X solution was added. The green
fluorescence of AV-FITC-bound PS and the red fluorescence of
DNA-bound PI in individual cells were measured at 525 and 575 nm,
respectively, using a BD FACSCalibur. Cell populations were
classified as: AV−/PI−, viable cells;
AV+/PI−, early apoptotic cells;
AV+/PI+, apoptotic cells; and
AV−/PI+, residual damaged cells.
Caspase-3 activity assay
Caspase-3 activity in the lysates of SW620 cells was
measured using the Caspase-3 cellular activity assay (Calbiochem,
Darmstadt, Germany). SW620 cells were cultured as described above
and suspensions (2×107 cells) were treated with
different concentrations of GLAI (0, 10, 25 and 50 μmol/l) for 24
h. Controls were treated with DMSO only. After washing and
trypsinization, cell suspensions were centrifuged (400 g, 3 min,
4°C) and cell pellets were re-suspended in 1 ml ice-cold cell lysis
buffer for 5 min. Following centrifugation (400 g, 3 min, 4°C),
cytosol supernatants were collected and enzyme activity was
measured according to the manufacturer’s instructions. Reaction
mixtures (total volume 100 μl) were incubated at 37°C for 10 min
and the optical density value was measured for 15 h at 405 nm using
an ELISA reader (Bio-Tek, Atlanta, GA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
For RNA extraction, two experiments were performed.
Firstly, SW620 cells (3.6×106 cells/ml) were incubated
in RPMI-1640 containing 10% FCS and different concentrations of
GLAI (10, 50 and 100 μg/ml). The entry with 0 μg/ml GLAI, treated
with DMSO, was used as a negative control. The cells were cultured
for 24 h at 37°C, 5% CO2, in a humidified incubator.
Secondly, SW620 cells (2×106/ml) were incubated in
RPMI-1640 containing 10% FCS and GLAI (50 μg/ml). The cells were
cultured for 0, 6, 12, 18 and 24 h at 37°C, 5% CO2.
The cells were lysed in TRIzol reagent (Invitrogen
Life Technologies). Total RNA was extracted according to the
manufacturer’s instructions. The RNA pellet was dissolved in
diethyl pyrocarbonate (DEPC)-treated water prior to use for reverse
transcription, electrophoresed on a 1.5% agarose gel and visualized
by ethidium bromide (EB) staining under an ultraviolet light, and
two bands (28S and 18S) are evident.
RNA was primed with oligo(dT)15 and converted into
complementary DNA (cDNA) by Moloney murine leukemia virus (MMLV)
reverse transcriptase. The reaction mixture for reverse
transcription contained 5.0X buffer, 2.5 mM of each
deoxynucleotriphosphate (dNTP, i.e., dATP, dCTP, dGTP and dTTP), 40
U/μl RNase inhibitor, 200 U/μl reverse transcriptase, 10 μM
oligo(dT)15 and 2 μg total RNA (equal amounts of starting RNA were
used for each condition in the different experiments). The final
volume of the reaction was 30 μl. The program parameters were 70°C
for 5 min to heat, 37°C for 1.5 h for reverse transcription
reaction and 95°C for 5 min to inactivate the reverse
transcriptase. cDNA generated by reverse transcription was either
used immediately for PCR experiments for the cytokines of interest
or stored at −20°C.
Oligonucleotide primers for GAPDH, Bcl-2 and Bax
were purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA). Table I shows the sequence
primers and the sizes of the fragments generated by the PCR
reactions.
 | Table IList of polymerase chain reaction
primers. |
Table I
List of polymerase chain reaction
primers.
| Genes | Primer
sequences | Size (bp) |
|---|
| GAPDH |
| Sense | 5′ TGA AGG TCG GAG
TCA ACG GAT TTG GT 3′ | 566 |
| Antisense | 5′ CAT GTG GGC CAT
GAG GTC CAC CAC 3′ | |
| Bcl-2 |
| Sense | 5′ TGC ACC TGA CGC
CCT TCA C 3′ | 293 |
| Antisense | 5′ AGA CAG CCA GGA
GAA ATC AAA CAG 3′ | |
| Bax |
| Sense | 5′ ACC AAG AAG CTG
AGC GAG TGT C 3′ | 332 |
| Antisense | 5′ ACA AAG ATG GTC
ACG GTC TGC C 3′ | |
The components added to the sample to make up 20 μl
reaction mixture were: 1 μl cDNA, 2 μl 10X Taq DNA polymerase
buffer, 2 μl 25 mM Mg2+ (Promega, Madison, WI, USA), 0.5
μl 10 mM mixture of all four deoxynucleotide triphosphates
(Promega), 0.5 μl each of 5′ and 3′ primer (10 μM) and 1 unit Taq
DNA polymerase (Promega). PCR was performed for 35 cycles.
Temperature cycling was initiated with each cycle as follows: for
GAPDH, 95°C for 45 sec (denaturation), 58°C for 45 sec (annealing),
72°C for 30 sec (extension); for Bcl-2, 95°C for 45 sec, 56°C for
45 sec, 72°C for 30 sec; for Bax, 95°C for 45 sec, 58°C for 45 sec
and 72°C for 30 sec. The amplified products were detected in 1.5%
agarose gels stained with EB and visualized under UV light.
Western-blot analysis
For the analysis of p53 and XIAP, SW620 cells
(3.6×106/ml) were incubated in RPMI-1640 containing 10%
FCS and different concentrations of GLAI (10, 20, 50 and 100
μg/ml). The entry with 0 μg/ml GLAI, treated with DMSO, was used as
a negative control. The cells were cultured for 24 h at 37°C, 5%
CO2, in a humidified incubator. Cells were washed twice
with PBS and lysed in lysis buffer containing 50 mM Tris-HCl (pH
8.0), 150 mM NaCl, 1% Triton X-100 and 100 μg/ml PMSF at 4°C
overnight. The suspensions were then centrifuged at 12,000 rpm for
5 min; All lysates were subjected to BCA protein assay reagent
(Pierce, Rockford, IL, USA) for the quantification of protein
concentration, and then Western blot analysis was performed. Total
proteins (20–50 μg) were separated by sodium dodecyl
sulfate-polyacrylamide gelelectrophoresis using a 10%
polyacrylamide gel. The proteins in the gel were transferred to a
PVDF membrane. The membrane was blocked with 0.1% BSA in TBST for 1
h. Membranes were incubated with primary antibody (1:2,000) at 4°C
overnight and then with secondary antibody (1:2,000) for 1 h. The
membranes were washed three times in TBST for 10 min between each
step. The signal was detected using the Amersham ECL system
(Amersham-Pharmacia Biotech, Arlington Heights, IL, USA).
Statistical analysis
The data are shown as the means ± SD. The Student’s
t-test was used to determine the significance of differences
between population means with results considered as: significant,
p<0.05; very significant, p<0.01; extremely significant,
p<0.001.
Results
Cell proliferation assay
To determine the effect of GLAI on different tumor
cells, the proliferation assay was performed using the Alamar blue.
As shown in Fig. 1, GLAI at a
concentration of 2 μmol/l inhibited the growth of SW620, K562 and
L1210 cells to ~19, 12 and 60%, respectively. Exposure of the
SW620, K562 and L1210 cells to 10 μmol/l GLAI inhibited cell growth
by 35, 52 and 86%. respectively. However, no additional effect was
observed with MCF7 cells at these concentrations (Fig. 1). However, increased growth
inhibition of SW620, K562, MCF7 and L1210 cells (to 55, 90 and 98%,
respectively) was observed following exposure to 50 μmol/l GLAI.
Treatment with 100 μmol/l GLAI resulted in almost total inhibition
of cell growth and few viable cells (see below) in all cases.
Positive controls treated with 5-fluorouracil were inhibited 88–90%
under these conditions.
Microscopic observation
Normally adhesive SW620 cells were readily suspended
following treatment with 10 μmol/l GLAI for 24 h, and few viable
cells were observed following exposure to 50 μmol/l GLAI (data not
shown). Light microscopy showed that cells exposed to DMSO
(Fig. 2A) or 10 μmol/l GLAI
exhibited distinct morphological features, such as apoptotic
bodies, associated with programmed cell death (Fig. 2B).
To further determine the nuclear morphology of SW620
cells treated with GLAI, Hoechst 33258 staining was performed.
Following treatment of SW620 cells with different concentrations of
GLAI for 24 h, the chromatin stained with Hoechst 33258 had a
characteristic condensed and fragmented appearance (Fig. 2C-F).
Flow cytometry
The staining patterns of SW620 cells exposed to GLAI
for 24 h and treated with AV-FITC and PI are shown in Fig. 3. More than 94% of cells remained
viable following treatment for 24 h with DMSO alone (negative
control) and almost no apoptotic events were detected (Fig. 3, lower left quadrant). However, the
proportion of cells with externalized PS increased in cells
following treatment for 24 h with different concentrations of GLAI
(Fig. 3).
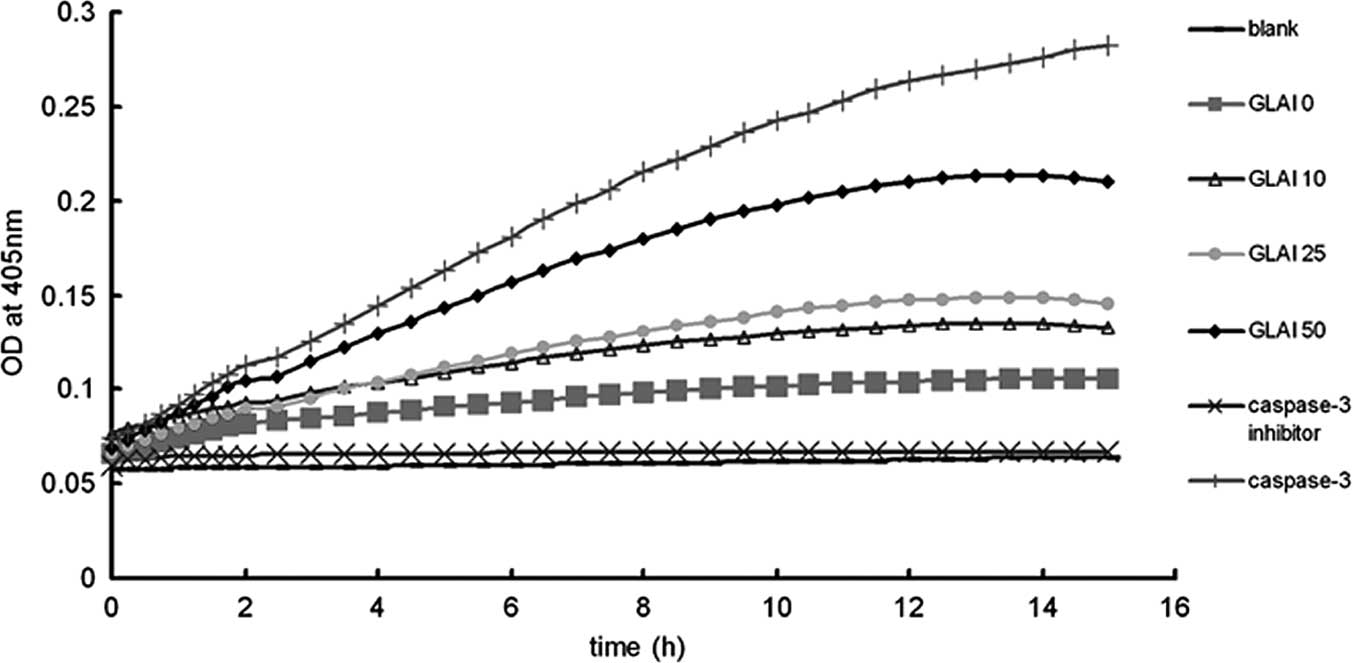
Activation of caspase-3 by treatment with
GLAI
Caspases are significant mediators of apoptosis in
mammalian cells (21), therefore,
we measured caspase-3 activities. When SW620 cells were treated
with different concentrations of GLAI, intracellular caspase-3
activity was analyzed (Fig. 4).
Caspase-3 activity was found to be up-regulated in SW620 cells in a
dose-dependent manner.
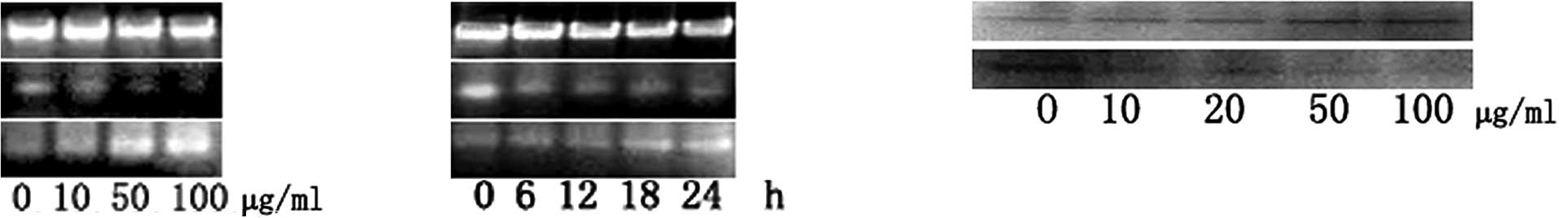
Molecular events responding to
GLAI-treatment
In order to understand the induction mechanism of
apoptosis by GLAI, we examined the expression levels of Bcl-2 and
Bax using RT-PCR, as well as p53 and XIAP using Western blotting.
The treatment of the SW620 cells with GLAI resulted in a marked
decrease of Bcl-2 at mRNA levels in a dose-dependent manner
(Fig. 5A). By contrast, the Bax
mRNA expression level was increased in a dose-dependent manner
(Fig. 5B). The results of Western
blotting showed that the p53 protein expression level was
up-regulated after SW620 cells were treated with different
concentrations of GLAI. By contrast, the XIAP protein expression
level was down-regulated (Fig.
5C).
Discussion
Colon cancer is a common cause of death among cancer
patients worldwide. Dysregulation of the normal colonic epithelium
is the causative factor of neoplastic transformation caused by
alterations in various parameters, including epithelial cell
proliferation and apoptosis. The latter two processes are highly
regulated in the constantly regenerating non-transformed colonic
epithelium and involve adhesion molecules, cytoskeletal proteins,
cell cycle regulators and apoptosis (22).
Annexin V is a protein that exhibits specific
affinity for PS. In non-apoptotic cells, most PS molecules are
localized on the inner leaflet of the plasma membrane, but shortly
after the onset of apoptosis, PS redistributes to the outer layer
of the membrane (23). Cells in the
early stages of apoptosis usually bind AV-FITC in the absence of PI
uptake (lower right quadrant), while those in the late stages of
apoptosis bind AV-FITC and in the presence of PI uptake (upper
quadrant).
Caspases are a family of intracellular cysteine
proteases with specificity for aspartic acid residues and play
important roles in drug-inducing apoptosis in a large variety of
cancer cells (24,25). Two members of this group of enzymes,
known as ‘initiator’ and ‘effector’ caspases, also play a
significant role in the apoptotic process (25,26).
Caspase-3 is the common effector for most apoptotic pathways
(19) and appears to play a special
role as a key ‘executioner’ in that its active form is responsible
for the cleavage and breakdown of several cellular components
related to DNA repair and regulation. Once activated, caspase-3 is
able to cleave a number of important cellular substrates and causes
membrane blebbing, disassembly of the cell structure and DNA
fragmentation, which eventually lead to cell death. Some initiator
caspases, such as caspase-9, activate pro-caspase-3, which then
cleaves the cellular substrates needed for the orchestration of
apoptosis and forms a ‘wheel of death’ (19,25–27).
Findings of studies have shown that apoptosis, especially
caspase-mediated cell death, plays an important role in the
etiology, pathogenesis and therapy of a variety of human
malignancies, such as human hepatocellular carcinoma. Additionally,
the cytotoxic effects of most anti-hepatocellular carcinoma drugs
are based on apoptosis induction (28). These studies indicate that induction
of apoptosis may be an index for new anti-tumor drug selection and
an important method of assessing the clinical efficacy of many
anti-carcinoma drugs (17).
Moreover, we found that the increase in caspase-3
activation is synchronized with the increase in Bax expression and
the decrease in Bcl-2, which is in agreement with other studies
(29–31). The Bcl-2 family of proteins plays a
crucial role in the regulation of apoptosis in many cellular
systems, by either inhibiting (Bcl-2, Bcl-XL, Bcl-W, Bfl-1 and
Mcl-1) or promoting apoptosis (Bax, Bak, Bad, Bcl-Xs, Bid and Hrk)
(32,33). Heterodimerization between pro- and
anti-apoptotic members of this family and relative levels of the
two types of proteins may determine the susceptibility to a given
apoptotic stimulus and the cell fate (34,35).
Moreover, these genes are known to be crucial regulators of
apoptosis in colon cancer cell lines (36,37).
In conclusion, our study has shown that GLAI
inhibits the growth of SW620 cells by inducing apoptosis via the
activation of caspase-3. These findings provide a basis for further
investigation of triterpenes from G. lucidum in the
treatment and prevention of colorectal adenocarcinoma.
Acknowledgements
The authors thank Mr. Hengbing Shi for the technical
assistance and Dr John Buswell for the linguistic revision of the
manuscript. This study was supported by the National Science
Foundation of China (30801031 to Z. Ji) and China Postdoctoral
Science Foundation (to Z. Ji).
References
|
1
|
Wasson RG: Soma-Divine Mushroom of
Immortality. Harcourt, Brace & World; New York: pp. 3811968
|
|
2
|
Chang ST and Buswell JA: Ganoderma
lucidum (Curt.:Fr) P. Karst. (Aphyllophoromycetideae) –
a mushrooming medicinal mushroom. International Journal of
Medicinal Mushrooms. 1:139–146. 1999. View Article : Google Scholar
|
|
3
|
Wachtel-Galor S, Benzie IFF, Tomlinson B
and Buswell JA: Lingzhi (Ganoderma lucidum): molecular
aspects of health effects. Herbal Medicines. Packer L, Halliwell B
and Ong CN: Marcel Dekker Inc; New York: pp. 179–228. 2004
|
|
4
|
Furusawa E, Chou SC, Furusawa S, Hirazumi
A and Dang Y: Antitumor activity of Ganoderma lucidum, an
edible mushroom, on intraperitoneal implanted Lewis lung carcinoma
in syngeneic mice. Phytotherapy Research. 6:300–304. 1992.
|
|
5
|
Lieu CW, Lee SS and Wang SY: The effect of
Ganoderma lucidum on induction of differentiation in
leukemic U937 cells. Anticancer Res. 12:1211–1215. 1992.
|
|
6
|
Wang SY, Hsu ML, Hsu HC, et al: The
anti-tumor effect of Ganoderma lucidum is mediated by
cytokines released from activated macrophages and T lymphocytes.
Int J Cancer. 70:699–705. 1997.
|
|
7
|
Zhu M, Chang Q, Wong LK, Chong FS and Li
RC: Triterpene antioxidants from ganoderma lucidum.
Phytother Res. 13:529–531. 1999. View Article : Google Scholar
|
|
8
|
Kim DH, Shim SB, Kim NJ and Jang IS:
Beta-glucuronidase-inhibitory activity and hepatoprotective effect
of Ganoderma lucidum. Biol Pharm Bull. 22:162–164. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komoda Y, Shimizu M, Sonoda Y and Sato Y:
Ganoderic acid and its derivatives as cholesterol synthesis
inhibitors. Chem Pharma Bull. 37:531–533. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kabir Y, Kimura S and Tamura T: Dietary
effect of Ganoderma lucidum mushroom on blood pressure and
lipid levels in spontaneously hypertensive rats (SHR). J Nutr Sci
Vitaminol. 34:433–438. 1988.
|
|
11
|
Lee SY and Rhee HM: Cardiovascular effects
of mycelium extract of Ganoderma lucidum: inhibition of
sympathetic outflow as a mechanism of its hypotensive action. Chem
Pharma Bull. 38:1359–1364. 1990.PubMed/NCBI
|
|
12
|
Su CY, Shiao MS and Wang CT: Differential
effects of ganodermic acid S on the thromboxane A2-signaling
pathways in human platelets. Biochem Pharmacol. 58:587–595. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gan KH, Fann YF, Hsu SH, Kuo KW and Lin
CN: Mediation of the cytotoxicity of lanostanoids and steroids of
Ganoderma tsugae through apoptosis and cell cycle. J Nat
Prod. 61:485–487. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Min BS, Gao JJ, Nakamura N and Hattori M:
Triterpenes from the spores of Ganoderma lucidum and their
cytotoxicity against meth-A and LLC tumor cells. Chem Pharma Bull.
48:1026–1033. 2000.PubMed/NCBI
|
|
15
|
Noda Y, Kaiya T, Kohda K and Kawazoe Y:
Enhanced cytotoxicity of some triterpenes toward leukemia L1210
cells cultured in low pH media: possibility of a new mode of cell
killing. Chem Pharma Bull. 45:1665–1670. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu TS, Shi LS and Kuo SC: Cytotoxicity of
Ganoderma lucidum triterpenes. J Nat Prod. 64:1121–1122.
2001.
|
|
17
|
Beauparlant P and Shore GC: Therapeutic
activation of caspases in cancer: a question of selectivity. Curr
Opin Drug Discov Devel. 6:179–187. 2003.PubMed/NCBI
|
|
18
|
Thorburn A: Death receptor-induced cell
killing. Cell Signal. 16:139–144. 2004. View Article : Google Scholar
|
|
19
|
Qi SN, Yoshida A, Wang ZR and Ueda T: GP7
can induce apoptotic DNA fragmentation of human leukemia cells
through caspase-3-dependent and -independent pathways. Int J Mol
Med. 13:163–167. 2004.PubMed/NCBI
|
|
20
|
Zhang JF, Liu JJ, Liu PQ, Lin DJ, Li XD
and Chen GH: Oridonin inhibits cell growth by induction of
apoptosis on human hepatocelluar carcinoma BEL-7402 cells. Hepatol
Res. 35:104–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thornberry NA and Lazebnik Y: Caspases:
enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subramaniam V, Vincent IR and Jothy S:
Upregulation and dephosphorylation of cofilin: modulation by CD44
variant isoform in human colon cancer cells. Exp Mol Pathol.
79:187–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin SJ, Reutelingsperger CP, McGahon
AJ, et al: Early redistribution of plasma membrane
phosphatidylserine is a general feature of apoptosis regardless of
the initiating stimulus: inhibition by overexpression of Bcl-2 and
Abl. J Exp Med. 182:1545–1556. 1995. View Article : Google Scholar
|
|
24
|
Donepudi M and Grutter MG: Structure and
zymogen activation of caspases. Biophys Chem. 101–102:145–153.
2002.PubMed/NCBI
|
|
25
|
Denault JB and Salvesen GS: Caspases: keys
in the ignition of cell death. Chem Rev. 102:4489–4500. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boatright KM and Salvesen GS: Caspase
activation. Biochem Soc Symp. 233–242. 2003.
|
|
27
|
Philchenkov AA: Caspases as regulators of
apoptosis and other cell functions. Biochemistry. 68:365–376.
2003.PubMed/NCBI
|
|
28
|
Huether A, Hopfner M, Sutter AP, Schuppan
D and Scherubl H: Erlotinib induces cell cycle arrest and apoptosis
in hepatocellular cancer cells and enhances chemosensitivity
towards cytostatics. J Hepatol. 43:661–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Srinivasan A, Roth KA, Sayers RO, et al:
In situ immunodetection of activated caspase-3 in apoptotic neurons
in the developing nervous system. Cell Death Differ. 5:1004–1016.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanabe H, Eguchi Y, Shimizu S, Martinou JC
and Tsujimoto Y: Death-signalling cascade in mouse cerebellar
granule neurons. Eur J Neurosci. 10:1403–1411. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhathena SJ and Velasquez MT: Beneficial
role of dietary phytoestrogens in obesity and diabetes. Am J Clin
Nutr. 76:1191–1201. 2002.PubMed/NCBI
|
|
33
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi YH, Kong KR, Kim YA, et al: Induction
of Bax and activation of caspases during β-sitosterol-mediated
apoptosis in human colon cancer cells. Int J Oncol. 23:1657–1662.
2003.
|
|
36
|
Levy P, Robin H, Bertrand F, Kornprobst M
and Capeau J: Butyrate-treated colonic Caco-2 cells exhibit
defective integrin-mediated signaling together with increased
apoptosis and differentiation. J Cell Physiol. 197:336–347. 2003.
View Article : Google Scholar
|
|
37
|
White E: Life, death, and the pursuit of
apoptosis. Genes Dev. 10:1–15. 1996. View Article : Google Scholar : PubMed/NCBI
|