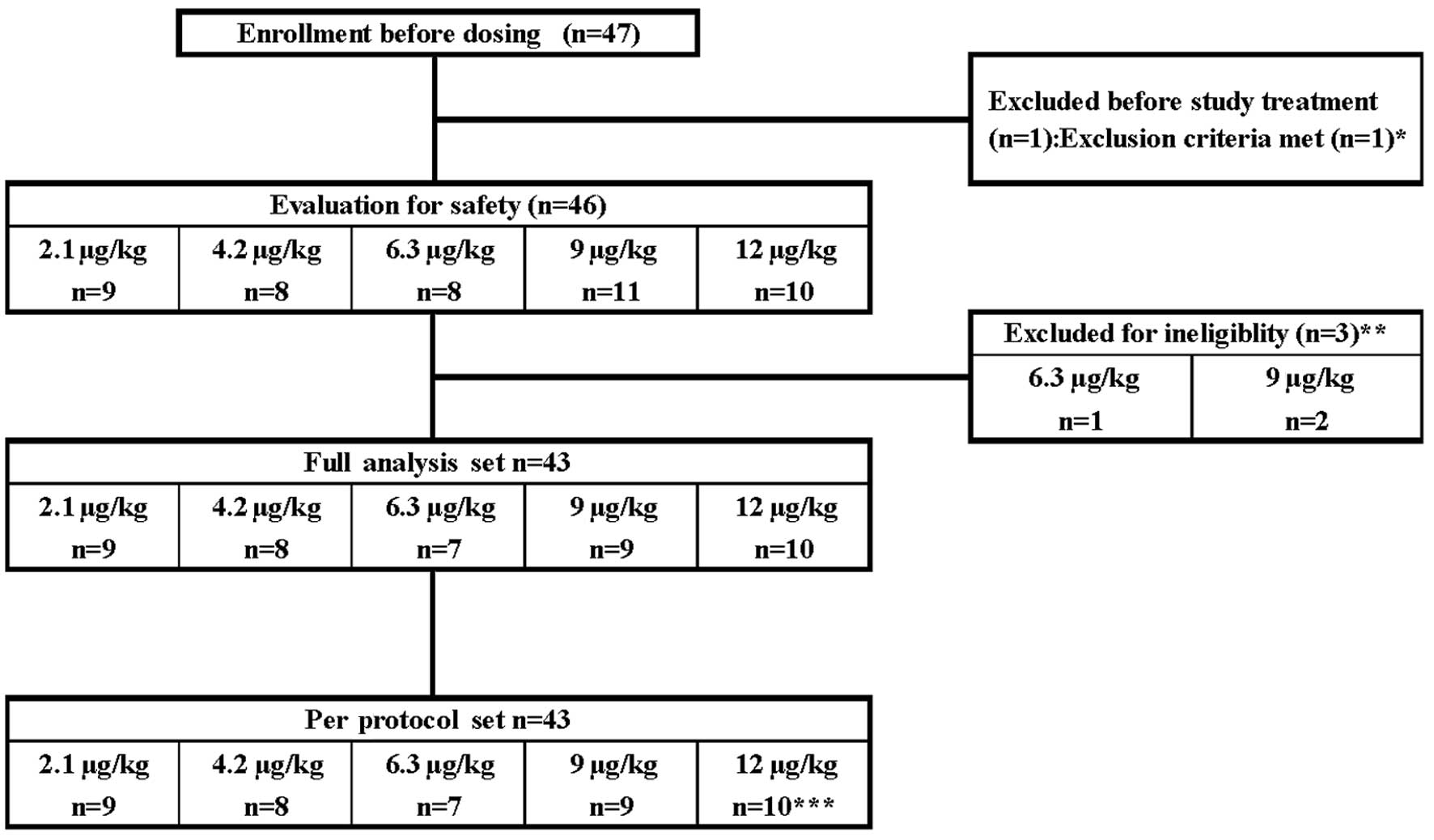
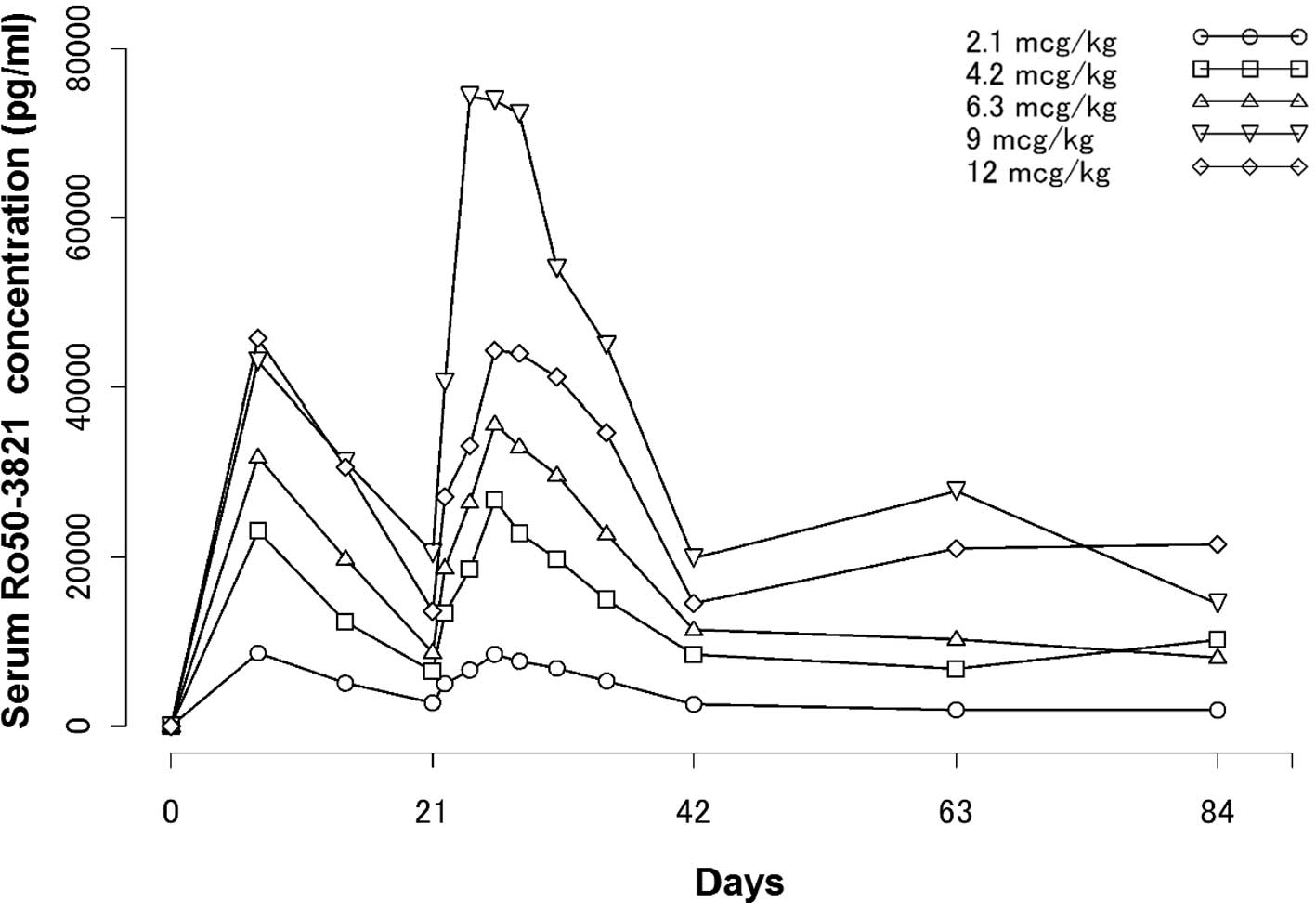
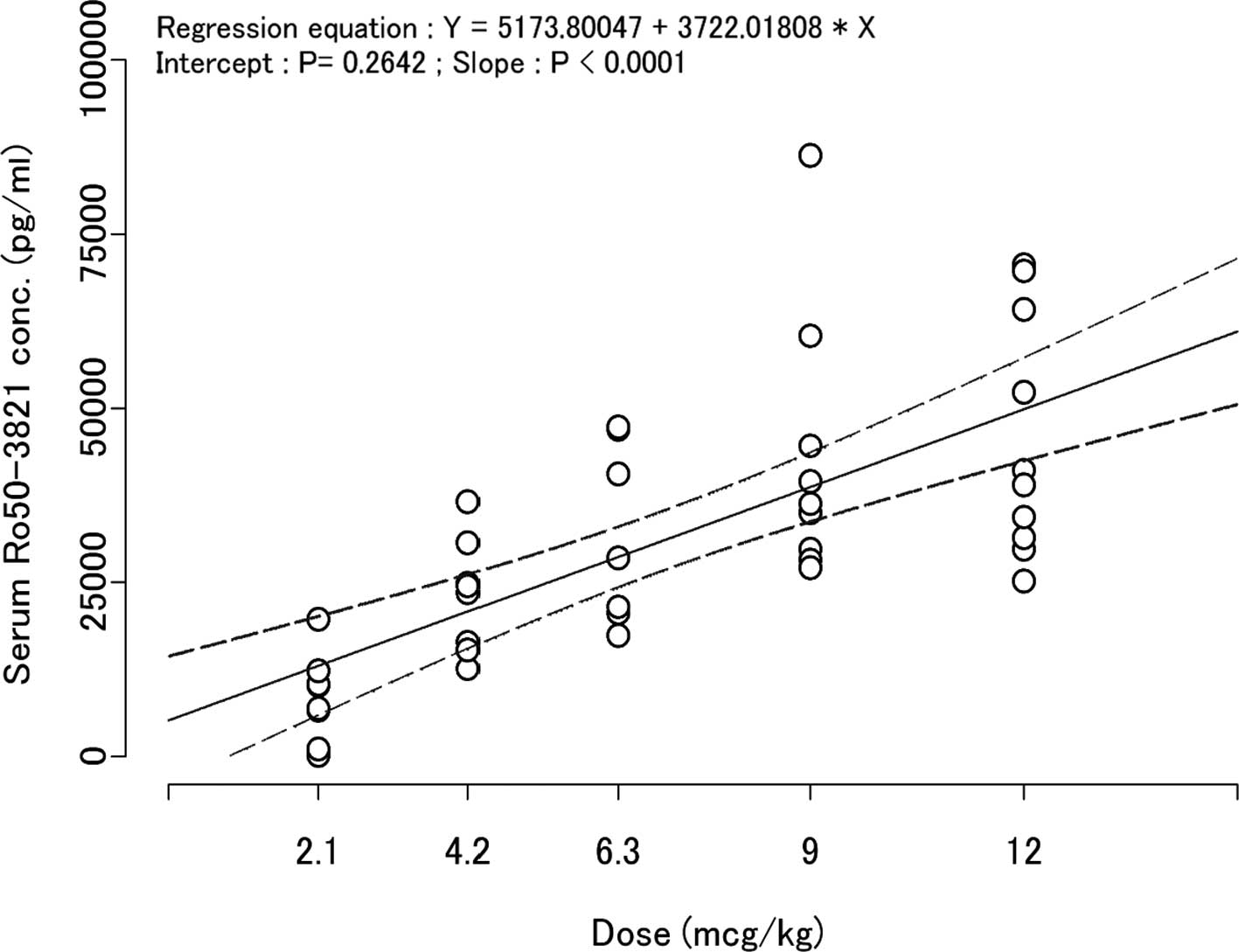
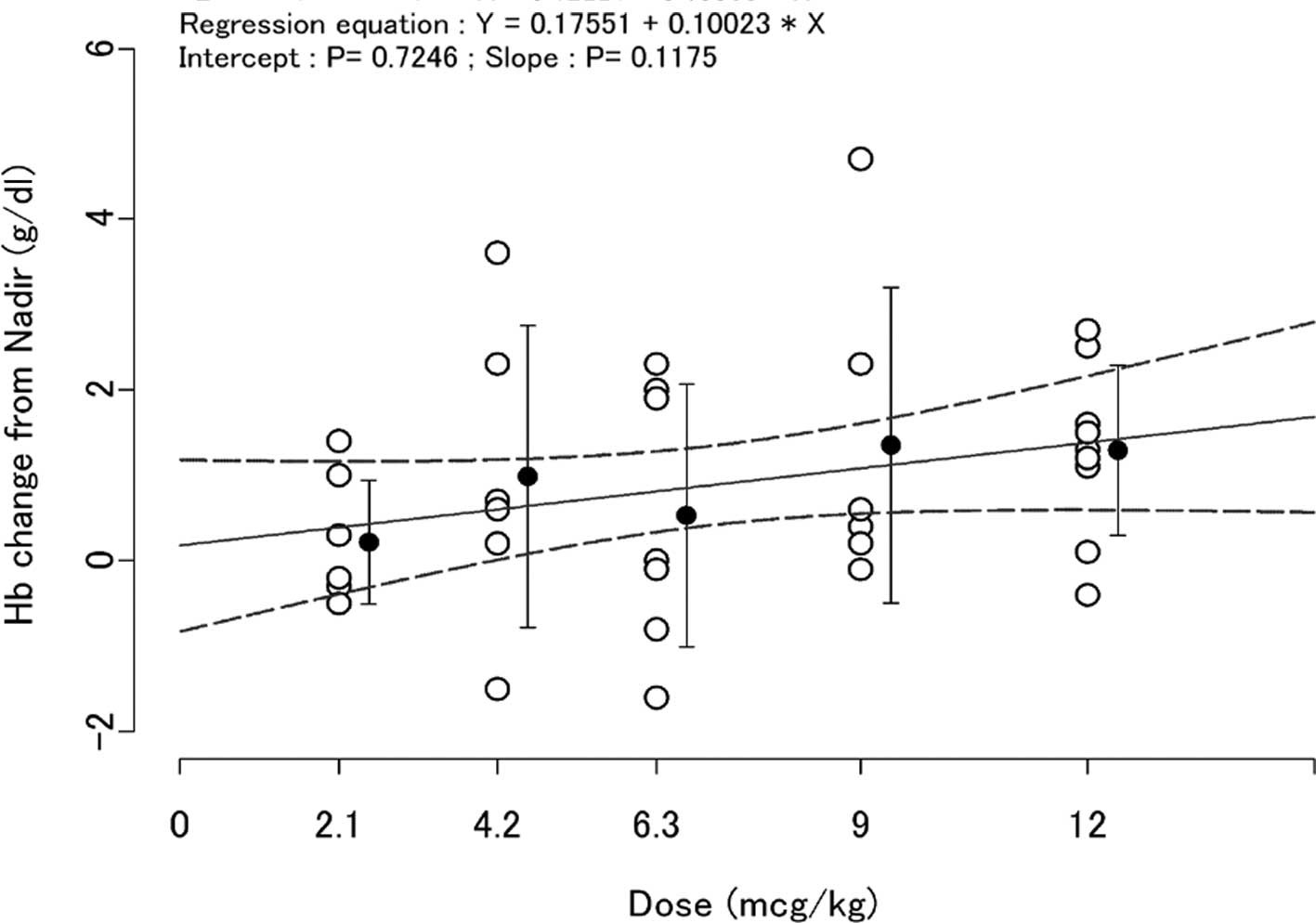
|
1
|
Oberhoff C, Neri B, Amadori D, Perty KU,
Gamucci T, Rebmann U, et al: Recombinant human erythropoietin in
the treatment of chemotherapy induced anemia and prevention of
transfusion requirement associated with solid tumors: a randomized,
controlled study. Ann Oncol. 9:239–241. 1998. View Article : Google Scholar
|
|
2
|
Littlewood TJ, Bajetta E, Nortier JW,
Vercammen E and Rapoport B; Epoetin Alfa Study Group. Effects of
epoetin alfa on hematologic parameters and quality of life in
cancer patients receiving nonplatinum chemotherapy; results of a
randomized, double-blind, placebo-controlled trial. J Clin Oncol.
19:2865–2874. 2001.
|
|
3
|
Vansteenkiste J, Pirker R, Massuti B,
Barata F, Font A, Fiegl M, Siena S, Gateley J, Tomita D, Colowick
AB and Musil J: Double-blind placebo-controlled, randomized phase
III trial of darbepoetin alfa in lung cancer patients receiving
chemotherapy. J Natl Cancer Inst. 94:1211–1220. 2002. View Article : Google Scholar
|
|
4
|
Ludwig H, Fritz E, Kotzmann H, Hocker P,
Gisslinger H and Barnus U: Erythropoietin treatment of anemia
associated with multiple myeloma. N Engl J Med. 322:1693–1699.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eshbach JW, Egrie JC, Dowing MR, Browne JK
and Adamson JW: Correction of the anemia of end-stage renal disease
with recombinant human erythropoietin. Results of a combined phases
I and II clinical trial. N Engl J Med. 316:73–78. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cazzola M, Beguin Y, Kloczko J, Spicka I
and Coiffier B: Once-weekly epoetin beta is highly effective in
treating anaemic patients with lymphoproliferative malignancy and
defective endogenous erythropoietin production. Br J Haematol.
122:386–393. 2003. View Article : Google Scholar
|
|
7
|
Waltzman R, Croot C, Justice GR, Fesen MR,
Charu V and Williams D: Randomized comparison of epoetin alfa
(40,000 U weekly) and darbepoetin alfa (200 microg every 2 weeks)
in anemic patients with cancer receiving chemotherapy. Oncologist.
10:642–650. 2005. View Article : Google Scholar
|
|
8
|
Canon JL, Vansteenkiste J, Bodoky G,
Mateos MV, Bastit L, Ferreira I, Rossi G and Amado RG: Randomized,
double-blind, active-controlled trial of every-3-week darbepoetin
alfa for the treatment of chemotherapy-induced anemia. J Natl
Cancer Inst. 98:273–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Locatelli F and Reigner B: C.E.R.A.
pharmacodynamics, pharmacokinetics and efficacy in patients with
chronic kidney disease. Expert Opin Investig Drugs. 16:1649–1661.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macdougall IC, Bailon P, Tare T and Pahlke
W: CERA (Continuous Erythropoiesis Receptor Activator) for the
treatment of renal anemia: an innovative agent with unique receptor
binding characteristics and prolonged serum half-life. J Am Soc
Nephrol. 14:769A(Abstract SU-PO 1063)2003.
|
|
11
|
Macdougall IC: CERA (Continuous
Erythropoietin Receptor Activator): a new
erythropoiesis-stimulating agent for the treatment of anemia. Curr
Hematol Rep. 4:436–440. 2005.PubMed/NCBI
|
|
12
|
Haselbeck A, Reigner B, Jordan B, Pannier
A and Glaspy J: Pre-clinical and Phase I pharmacokinetic and
mode-of-action studies of CERA (Continuous Erythropoiesis Receptor
Activator), an innovative erythropoietic agent with an extended
serum half-life. Proc Am Soc Clin Oncol. 22:748(Abstract
3006)2003.
|
|
13
|
Dougherty FC, Reigner B, Jordan P and
Pannier A: Continuous Erythropoiesis Receptor Activator (CERA)
provides dose-dependent erythropoietic activity with a prolonged
half-life in healthy volunteers. Ann Oncol. 15:iii157(Abstract
592)2004.
|
|
14
|
Dmoszynska A, Kloczko J, Rokicka M,
Hellmann A, Spicka I and Eid JE: A dose exploration, phase I/II
study of administration of continuous erythropoietin receptor
activator once every 3 weeks in anemic patients with multiple
myeloma receiving chemotherapy. Haematologica. 92:493–501. 2007.
View Article : Google Scholar
|
|
15
|
Osterborg A, Steegmann JL, Hellmann A,
Couban S, Mayer J and Eid JE: Phase II study of three dose levels
of continuous erythropoietin receptor activator (C.E.R.A.) in
anemic patients with aggressive non-Hodgkin’s lymphoma receiving
combination chemotherapy. Br J Haematol. 136:736–744.
2007.PubMed/NCBI
|
|
16
|
Hirsh V, Glaspy J, Mainwaring P, Manegold
C, Ramlau R and Eid JE: Phase II study of two dose schedules of
C.E.R.A. (Continuous Erythropoiesis Receptor Activator) in anemic
patients with advanced non-small cell lung cancer (NSCLC) receiving
chemotherapy. Trials. 8:82007. View Article : Google Scholar
|
|
17
|
Osterborg A, Brandberg Y, Molostova V,
Losava G, Abdalkadyrov K, Hedenus M and Messinger D: Randomized,
double-blind, placebo-controlled trial of recombinant human
erythropoietin, epoetin β, in hematologic malignancies. Journal of
Clinical Oncology. 20:2486–2494. 2002.
|
|
18
|
Crawford J: Anemia and lung cancer. Lung
Cancer. 38:S75–S78. 2002. View Article : Google Scholar
|
|
19
|
Langer CJ, Choy H, Glaspy JA and Colowick
A: Standards of care for anemia management in oncology; focus on
lung carcinoma. Cancer. 95:613–623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cella D: Factors influencing quality of
life in cancer patients: anemia and fatigue. Semin Oncol. 25:43–46.
1998.PubMed/NCBI
|
|
21
|
Pirker R, Wiesenberger K, Pohl G and Minar
W: Anemia in lung cancer: clinical impact and management. Clin Lung
Cancer. 5:90–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morère JF: Role of epoetin in the
management of anemia in patients with lung cancer. Lung Cancer.
46:149–156. 2004.PubMed/NCBI
|
|
23
|
Ludwig H, Van Belle S, Barett-Lee P,
Birgegard G, Bokemeyer C, Gascón P, Kosmidis P, Krzakowski M,
Nortier J, Olmi P, Schneider M and Schrijvers D: The European
Cancer Anemia Survey (ECAS): a large, multinational, prospective
survey defining the prevalence, incidence, and treatment of anemia
in cancer patients. Eur J Cancer. 40:2293–2306. 2004. View Article : Google Scholar
|
|
24
|
Fujisaka Y, Tamura T, Ohe Y, Kunitoh H,
Sekine I, Yamamoto N, Nokihara H, Horiike A, Kodama T and Saijo N:
Pharmacokinetics and Pharmacodynamics of Weekly Epoetin Beta in
Lung Cancer Patients. Jpn J Oncol. 36:477–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakagawa K, Ozaki T, Satoh T, Miyazaki M,
Akashi Y, Terashima M, Fujisaka Y, Tamura T, Fukuoka M and Saijo N:
Epoetin Beta Subcutaneous Administration to Lung Cancer Patients
with Anemia; Results of Pharmacokinetics/Pharmacodynamics Study.
Jpn J Lung Cancer. 47:313–322. 2007. View Article : Google Scholar
|
|
26
|
Tang L, Persky AM, Hochhaus G and Meibohm
B: Pharmacokinetic aspects of biotechnology products. J Pharm Sci.
93:2184–2204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Glaspy J, Henry D, Patel R, et al: Effects
of chemotherapy on endogenous erythropoietin levels and the
pharmacokinetics and erythropoietic response of darbepoetin alfa: a
randomized clinical trial of synchronous versus asynchronous dosing
of darbepoetin alfa. Eur J Cancer. 41:1140–1149, Epub 2005 Apr
8.
|
|
28
|
Agoram B, Heatherington AC and Gastonguay
MR: Development and evaluation of a population
pharmacokinetic-pharmacodynamic model of darbepoetin alfa in
patients with nonmyeloid malignancies undergoing multicycle
chemotherapy. AAPS J. 8:E552–E563. 2006. View Article : Google Scholar
|