1. Introduction
Malignant gliomas, the most common and lethal brain
tumors, exhibit a great deal of diversity in their location,
pathology, genetic status and response to therapy. Nevertheless,
survival for patients with glioblastoma, the most aggressive
glioma, although individually variable, has recently improved in
the last 5 years from an average of 10 to 14 months after diagnosis
due to improvements in the standard of care (1). A comprehensive understanding of the
genetic basis and pathology of gliomas has provided new information
regarding biologically based tumor classification and has
identified molecular prognostic biomarkers to improve the
management of patients with gliomas. Over the past decades the
application of sequencing, comparative genomic hybridization (CGH)
and single nucleotide polymorphism (SNP) array techniques have
revealed the molecular genetic background of neoplasms through the
identification of novel oncogenes and tumor suppressor genes.
2. IDH1/2 mutations in gliomas and
myeloid malignancies
One recently identified class of genes mutated in
cancer are those coding for isocitrate dehydrogenase (IDH), which
catalyzes the oxidative decarboxylation of isocitrate to
α-ketoglutarate (α-KG) leading to NADPH production (2,3). The
NADP+-dependent IDH1 (located in the cytoplasm and
peroxisomes) and its mitochondrial counterpart IDH2 are mutated in
up to 75% of grade II–III gliomas and secondary glioblastoma
multiforme (GBM) as well as myeloid malignancies including primary
and secondary acute myeloid leukemia (AML, 15–30%) and preleukemic
clonal malignancies, such as myelodysplasia and myeloproliferative
neoplasms (2–9). IDH1 mutation has rapidly
emerged as a novel prognostic and diagnostic marker with which to
identify low-grade gliomas and to distinguish secondary from
primary GBM (4,10,11).
IDH1/2 mutations are heterozygous, with tumors
retaining one wild-type copy of the relevant IDH1 or
IDH2 allele and producing single amino acid substitutions at
arginine 132 (R132) in IDH1 or corresponding arginine 172 (R172) in
IDH2 in glioma and leukemia, or at arginine 140 (R140) in IDH2 in
leukemia (8,12). Notably, the R132 mutation in IDH1
results not only in a marked decrease of normal catalytic activity,
but also in a gain of novel function catalyzing the
NADPH+-dependent reduction of α-KG to
R(-)-2-hydroxyglutarate (2-HG) (8,12).
Decreased α-KG and α-KG-dependent prolyl hydroxylase (PHD) activity
leads to an increase in hypoxia-inducible factor-1α (HIF-1α), since
PHDs use α-KG as a substrate for a reaction that normally targets
HIF-1α for degradation (13). α-KG
reduction and D-2-HG accumulation cooperatively contribute to
tumorigenesis (14), which may
resolve the continuing controversy over whether mutant IDH1
is a tumor suppressor gene or an oncogene (15,16).
3. TET2 mutations in gliomas and
myeloid malignancies
Other recently reported gene mutations are those of
the Ten-Eleven Translocation-2 gene (TET2), which have been
found in 20–26% of myelodysplastic syndromes or secondary AML
(17,18), 14% of myeloproliferative neoplasms
(17,19,20),
and 12% of de novo AML (21). TET2 is a putative tumor
suppressor gene located at chromosome 4q24, encoding a dioxygenase
that converts 5-methylcytosine to 5-hydroxymethylcytosine, leading
to DNA demethylation at selective loci. It has also been indicated
in the regulation of normal myelopoiesis (22–24),
and the disruption of TET2 enzymatic activity favours myeloid
tumorigenesis (24). Measurement of
5-hydroxymethylcytosine levels in myeloid malignancies may prove
valuable as a diagnostic and prognostic tool to tailor therapies
and assess responses to anticancer drugs (24). TET2 abnormalities are highly
heterogeneous and inactivating showing a relatively diverse pattern
of frame shift, nonsense, and missense mutations scattered across
several of its exons. Additionally, TET2 may occur as
hemizygous or heterozygous alterations, including loss of
heterozygosity, due to hemizygous deletion or uniparental disomy
(17,25). In contrast to the affirmatively
prognostic value of IDH1 mutation in gliomas (4,26), the
prognostic value of TET2 mutations in myeloid malignancies
remains unclear (27). Similarly,
survival in primary AML did not appear to be affected by the
presence of IDH mutations (2,8,28).
4. Correlation between TET2 and
IDH1/2 mutations
Mutations of TET2 gene have been shown to
coexist with other pathogenetically relevant mutations, including
the retinoic acid receptor α gene, thrombopoietin receptor gene
(myeloproliferative leukemia, MPL), janus kinase 2 gene (JAK2), KIT
gene, FMS-like tyrosine kinase 3 gene (FLT3), renin-angiotensin
system (RAS) gene, mixed lineage leukemia (MLL) gene, CCAAT
enhancer binding protein α gene (CEBA+) or nucleophosmin 1 gene
(NHMI) (18–21,29,30),
but occur in a manner mutually exclusive with that of IDH1
and IDH2 genes in AML (30).
Xu et al found that the expression of mutant IDH1/2 and
D-2-HG inhibited the activity of TET2 in catalyzing the 5mC-to-5hmC
conversion, which not only supported, but also provided a
biochemical basis for the mutual exclusivity of
IDH1/2 and TET2 gene mutations (14). The mechanisms of various gene
mutations involved in disease initiation and/or progression are not
isolated but associated, compatible or exclusive of each other.
Considering the ubiquitous nature of mutant TET2 in myeloid
malignancies, mutant IDH1/2 in gliomas and both
mutations in myeloid malignancies, it may be possible that
TET2 mutation is also present in malignant gliomas,
particularly those without IDH1/2 mutation.
5. Discussion
Mounting evidence has indicated that TET2
alterations are a common event in a spectrum of myeloid
malignancies, that IDH1/2 mutations are frequently
present in gliomas, and that they both exhibit similar frequencies
in myeloid malignancies. IDH1/2 mutations were
mutually exclusive with mutations in the α-KG-dependent enzyme
TET2, and TET2 loss-of-function mutations were associated with
similar epigenetic defects to those of IDH1/2 mutants in AML.
Expression of mutant IDH1/2 or TET2 depletion impaired
hematopoietic differentiation and increased stem/progenitor cell
marker expression, indicating a shared proleukemogenic effect
(30). Additionally, the two
mutations of IDH1 and TET2 have been reported to
occur at a relatively early stage during glioma and leukemia
development (17,18,31).
These findings suggest that TET2 mutations may be identified
as novel mutations in malignant gliomas without
IDH1/2 mutations, since no reports are currently
available regarding the association between TET2 mutations
and malignant gliomas.
IDH1/2 mutations are concurrent with
TP53 mutations in astrocytic tumors (9,31,32)
and 1p19q codeletion in oligodendroglial tumors (9,32,33),
but mutually exclusive with epidermal growth factor receptor (EGFR)
amplification (34) and the BRAF
fusion gene (10,35). IDH1/2 mutations are
inversely associated with numerous characteristic genetic changes
of primary glioblastomas, including EGFR amplification,
cyclin-dependent kinase inhibitor 2A or 2B deletion, and
phosphatase and tensin homolog mutations (9,32).
IDH mutations do not increase in frequency in the
progression to higher-grade gliomas and occur prior to other
genetic changes, indicating that these mutations arise at some
point in the transition from glial progenitor cells to a clinically
evident tumor (9,31,32,36,37).
Common genetic changes associated with various glioma subtypes are
shown in Fig. 1 in addition to the
hypothesis that TET2 mutations occur in gliomas. If the
above-mentioned hypothesis holds true, several questions may be
raised. The specific type of TET2 mutation in malignant
gliomas remains to be identified. It is also unknown whether
TET2 mutations are exclusive with IDH1/2 mutations in
gliomas, or perhaps even concurrent with them. In instances where
mutant TET2 coexists with IDH1/2 mutations or
other mutations, it is currently unclear as to whether it predates
or postdates their emergence. Within the context of a specific
disease, it is likely that the presence of mutant TET2
affects phenotype, prognosis or treatment response. Finally, it is
necessary to determine the pathogenetic contribution of mutant
TET2 in cancer, particularly in view of its occurrence
across different molecular profiles.
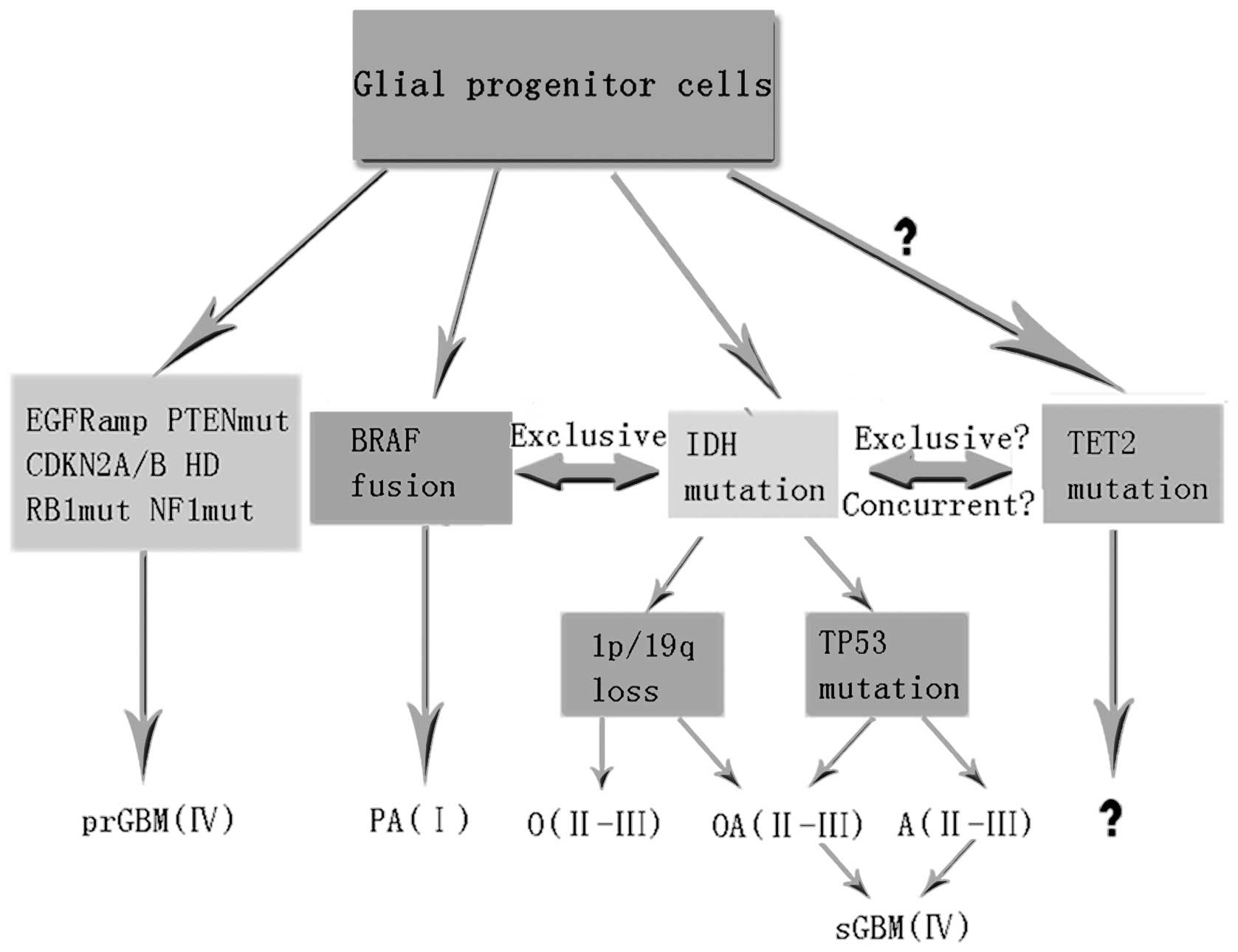 | Figure 1A model for tumorigenesis and
progression of gliomas based on genetic alterations. TET2
mutations are integrated into the model, but their role in gliomas
is unknown. PAI, pilocytic astrocytomas; OII, oligodendrogliomas;
OAII, oligoastrocytomas; AII, diffuse astrocytomas; OIII,
anaplastic oligodendrogliomas; OAIII, anaplastic oligoastrocytomas;
AIII, anaplastic astrocytomas; sGBMIV, secondary glioblastomas;
prGBM IV, primary glioblastomas occurring de novo; EGFR,
epidermal growth factor receptor; PTEN, phosphatase and tensin
homolog; CDKN2A and 2B, cyclin-dependent kinase inhibitor 2A and
2B; HD, homozygous deletion; RB1, retinoblastoma 1; mut, mutation;
NF1, neurofibromatosis type 1; BRAF fusion, v-raf murine sarcoma
viral oncogene homolog B1; IDH, isocitrate dehydrogenase; TP53,
tumor protein p53; 1p/19q loss, homozygous deletion of chromosome
arms 1p and 19q; TET2, Ten-Eleven Translocation-2 gene. |
Further clinical investigation from different
centers is required to confirm our hypothesis. Additional
laboratory studies are required to clarify the biological
consequence of these mutations prior to any clinical
applications.
References
|
1
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: the avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010.PubMed/NCBI
|
|
2
|
Mardis ER, Ding L, Dooling DJ, et al:
Recurring mutations found by sequencing an acute myeloid leukemia
genome. N Engl J Med. 361:1058–1066. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parsons DW, Jones S, Zhang X, et al: An
integrated genomic analysis of human glioblastoma multiforme.
Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kloosterhof NK, Bralten LB, Dubbink HJ,
French PJ and van den Bent MJ: Isocitrate dehydrogenase-1
mutations: a fundamentally new understanding of diffuse glioma?
Lancet Oncol. 12:83–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marcucci G, Maharry K, Wu YZ, et al: IDH1
and IDH2 gene mutations identify novel molecular subsets within de
novo cytogenetically normal acute myeloid leukemia: a Cancer and
Leukemia Group B study. J Clin Oncol. 28:2348–2355. 2010.
View Article : Google Scholar
|
|
6
|
Paschka P, Schlenk RF, Gaidzik VI, et al:
IDH1 and IDH2 mutations are frequent genetic alterations in acute
myeloid leukemia and confer adverse prognosis in cytogenetically
normal acute myeloid leukemia with NPM1 mutation without FLT3
internal tandem duplication. J Clin Oncol. 28:3636–3643. 2010.
View Article : Google Scholar
|
|
7
|
Tefferi A, Lasho TL, Abdel-Wahab O, et al:
IDH1 and IDH2 mutation studies in 1473 patients with chronic-,
fibrotic- or blast-phase essential thrombocythemia, polycythemia
vera or myelofibrosis. Leukemia. 24:1302–1309. 2010. View Article : Google Scholar
|
|
8
|
Ward PS, Patel J, Wise DR, et al: The
common feature of leukemia-associated IDH1 and IDH2 mutations is a
neomorphic enzyme activity converting alpha-ketoglutarate to
2-hydroxyglutarate. Cancer Cell. 17:225–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan H, Parsons DW, Jin G, et al: IDH1 and
IDH2 mutations in gliomas. N Engl J Med. 360:765–773. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ducray F, El-Hallani S and Idbaih A:
Diagnostic and prognostic markers in gliomas. Curr Opin Oncol.
21:537–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schiff D and Purow BW: Neuro-oncology:
isocitrate dehydrogenase mutations in low-grade gliomas. Nat Rev
Neurol. 5:303–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dang L, White DW, Gross S, et al:
Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 462:739–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao S, Lin Y, Xu W, et al: Glioma-derived
mutations in IDH1 dominantly inhibit IDH1 catalytic activity and
induce HIF-1alpha. Science. 324:261–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu W, Yang H, Liu Y, et al: Oncometabolite
2-hydroxyglutarate is a competitive inhibitor of
alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 19:17–30.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garber K: Oncometabolite? IDH1 discoveries
raise possibility of new metabolism targets in brain cancers and
leukemia. J Natl Cancer Inst. 102:926–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu J, Zuo J, Xu Q, Wang X, Wang Z and
Zhou D: Isocitrate dehydrogenase mutations may be a protective
mechanism in glioma patients. Med Hypotheses. 76:602–603. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Delhommeau F, Dupont S, Della VV, et al:
Mutation in TET2 in myeloid cancers. N Engl J Med. 360:2289–2301.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langemeijer SM, Kuiper RP, Berends M, et
al: Acquired mutations in TET2 are common in myelodysplastic
syndromes. Nat Genet. 41:838–842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tefferi A, Levine RL, Lim KH, et al:
Frequent TET2 mutations in systemic mastocytosis: clinical,
KITD816V and FIP1L1-PDGFRA correlates. Leukemia. 23:900–904. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tefferi A, Pardanani A, Lim KH, et al:
TET2 mutations and their clinical correlates in polycythemia vera,
essential thrombocythemia and myelofibrosis. Leukemia. 23:905–911.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdel-Wahab O, Mullally A, Hedvat C, et
al: Genetic characterization of TET1, TET2, and TET3 alterations in
myeloid malignancies. Blood. 114:144–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ito S, D’Alessio AC, Taranova OV, Hong K,
Sowers LC and Zhang Y: Role of Tet proteins in 5mC to 5hmC
conversion, ES-cell self-renewal and inner cell mass specification.
Nature. 466:1129–1133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tahiliani M, Koh KP, Shen Y, et al:
Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in
mammalian DNA by MLL partner TET1. Science. 324:930–905. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ko M, Huang Y, Jankowska AM, et al:
Impaired hydroxylation of 5-methylcytosine in myeloid cancers with
mutant TET2. Nature. 468:839–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jankowska AM, Szpurka H, Tiu RV, et al:
Loss of heterozygosity 4q24 and TET2 mutations associated with
myelodysplastic/myeloproliferative neoplasms. Blood. 113:6403–6410.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reitman ZJ and Yan H: Isocitrate
dehydrogenase 1 and 2 mutations in cancer: alterations at a
crossroads of cellular metabolism. J Natl Cancer Inst. 102:932–941.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tefferi A: Novel mutations and their
functional and clinical relevance in myeloproliferative neoplasms:
JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 24:1128–1138.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chou WC, Hou HA, Chen CY, et al: Distinct
clinical and biologic characteristics in adult acute myeloid
leukemia bearing the isocitrate dehydrogenase 1 mutation. Blood.
115:2749–2754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tefferi A, Lim KH, Abdel-Wahab O, et al:
Detection of mutant TET2 in myeloid malignancies other than
myeloproliferative neoplasms: CMML, MDS, MDS/MPN and AML. Leukemia.
23:1343–1345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Figueroa ME, Abdel-Wahab O, Lu C, et al:
Leukemic IDH1 and IDH2 mutations result in a hypermethylation
phenotype, disrupt TET2 function, and impair hematopoietic
differentiation. Cancer Cell. 18:553–567. 2010. View Article : Google Scholar
|
|
31
|
Watanabe T, Nobusawa S, Kleihues P and
Ohgaki H: IDH1 mutations are early events in the development of
astrocytomas and oligodendrogliomas. Am J Pathol. 174:1149–1153.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ichimura K, Pearson DM, Kocialkowski S, et
al: IDH1 mutations are present in the majority of common adult
gliomas but rare in primary glioblastomas. Neuro Oncol. 11:341–347.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Labussiere M, Idbaih A, Wang XW, et al:
All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2.
Neurology. 74:1886–1890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sanson M, Marie Y, Paris S, et al:
Isocitrate dehydrogenase 1 codon 132 mutation is an important
prognostic biomarker in gliomas. J Clin Oncol. 27:4150–4154. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Korshunov A, Meyer J, Capper D, et al:
Combined molecular analysis of BRAF and IDH1 distinguishes
pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol.
118:401–405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Balss J, Meyer J, Mueller W, Korshunov A,
Hartmann C and von Deimling A: Analysis of the IDH1 codon 132
mutation in brain tumors. Acta Neuropathol. 116:597–602. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ohgaki H and Kleihues P: Genetic
alterations and signaling pathways in the evolution of gliomas.
Cancer Sci. 100:2235–2241. 2009. View Article : Google Scholar : PubMed/NCBI
|















