Introduction
Lung cancer was one of the most commonly diagnosed
cancers as well as the leading cause of cancer mortality in 2008
globally, particularly in males (1). Bronchial arterial infusion (BAI)
chemotherapy for lung cancer was introduced over 40 years ago
(2,3) and reductions in tumor size and
symptoms as well as in the incidence of adverse effects of
anticancer drugs were predicted due to the direct infusion of high
density chemotherapeutics into tumors. However, these outcomes have
not been confirmed and severe side-effects, including esophageal
ulceration and spinal cord damage, have been reported following
these therapies (4,5). Thus, BAI has not achieved wide
acceptance as a standard clinical therapy for lung cancer. Arterial
infusion chemotherapy for lung cancer utilizing only the bronchial
artery is not effective as feeding arteries for lung cancer other
than the bronchial artery are involved (6). The former studies indicated that
sufficient detection of feeding arteries may increase the
effectiveness of the local control of lung cancer by arterial
infusion chemotherapy (7,8). However, the disadvantage of this
method is the complexity of the extensive angiographic
examinations.
The efficacy of three-dimensional imaging using
multi-detector row helical computed tomography (MDCT) in the
preoperative assessment of the branching pattern of the pulmonary
artery (PA) prior to complete video-assisted thoracoscopic
lobectomy for lung cancer has been reported, particularly in the
superselective segmentectomy of deep and small pulmonary nodules
under the guidance of three-dimensional reconstructed computed
tomographic angiography (9–11). Several studies have evaluated
bronchial and non-bronchial systemic arteries using MDCT
angiography in patients with pulmonary disorders (12–15).
The purpose of this study was to evaluate the
effective use of chest MDCT angiography for detecting lung cancer
feeding arteries (bronchial and nonbronchial systemic arteries) and
tumor staining.
Materials and methods
Patients
The research protocol in our study was approved by
the Shanghai Chest Hospital Affiliated to Shanghai Jiaotong
University, Shanghai, China. Written consent was obtained from all
patients prior to commencement of the study.
This study involved 59 patients (44 males and 15
females; age range, 27–86 years; median age, 62 years) with 59
non-small cell lung cancers (NSCLCs; mean diameter, 4.2±1.3 cm;
diameter range, 1.5–7.0 cm), who underwent arterial infusion
chemotherapy. All patients were difficult to treat with standard
chemotherapy and thoracic radiotherapy due to poor performance
status (PS ≥2), advanced age (≥75 years old), severe hepatic
failure, severe respiratory failure or refusal of chemotherapy.
MDCT angiography examination
All patients were imaged using a 64-row MDCT scanner
(LightSpeed VCT, GE Medical Systems, Milwaukee, WI, USA) or a
dual-source CT scanner (Somatom Definition; Siemens AG, Medical
Solutions, Forchheim, Germany). The main imaging parameters were
0.625 mm collimation, 0.5 sec gantry rotation time and 250 mA with
a pitch of 0.938 in fast mode. The whole chest was scanned in ∼10
sec. The craniocaudal scan ranged from the mandible angle level to
the upper abdomen level. A power injector was used to administer
iopamidol (370 mg I/ml, Bracco, Milan, Italy) at a rate of 4 ml/sec
to provide a dose of 420 mg/kg body weight, followed by 20 ml
saline solution at the same rate. Imaging commenced after a 20–25
sec delay and lasted 6–12 sec. Axial images were reconstructed with
a thickness of 0.8 mm at 0.5 mm intervals.
From each data set, three series of images were
systematically reconstructed as follows: contiguous 1-mm thick
transverse CT scans viewed at mediastinal and lung window settings,
oblique coronal and sagittal maximum intensity projections (MIPs),
multi-planar reconstructions (MPRs) and three-dimensional
volume-rendered (VR) images of the thoracic vascular
structures.
Analysis of lung cancer feeding arteries
by MDCT angiography
CT angiograms were interpreted in consensus by two
faculty radiologists (X.Y. and X.X, with 8 and 25 years of
experience with CT, respectively), who were blinded to the
conventional angiography results but not to clinical information.
The CT images were reviewed as hard-copy images with the option of
a cine-mode display on the workstation, if needed. After several
weeks, the two readers reviewed the conventional angiographic
studies in consensus; subsequently, the degree of concordance
between the results of CT angiography and those of conventional
angiography was evaluated.
CT interpretation focused on the evaluation of
bronchial arteries ipsilateral to the side of the lesion by
recording the following parameters: a) the site of the ostium of
the bronchial artery (or arteries) and b) the total number of
bronchial arteries per patient. For each bronchial artery, the
images of its ostium and its mediastinal and hilar course were
analyzed on transverse CT scans and three-dimensional images to
determine the ability of CT angiography to depict the vessel.
Non-bronchial systemic arteries were defined as arteries that enter
the parenchyma through the inferior pulmonary ligament or through
the adherent pleura; their course is not parallel to that of the
bronchi (16). The lung cancer
feeding arteries detected by MDCT angiography were defined as those
which had an increased arterial diameter, entered the lesion, had
disorganized branches and varying degrees of angiogenesis. When
these criteria were met, CT angiography was considered to provide
an accurate identification of the feeding artery of interest. When
CT angiography was able to aid the depiction of the ostium of the
artery and recognize its course toward the adjacent lung
parenchymal zone, CT angiography was coded as suboptimal for the
depiction of this feeding artery. The numbers of lung cancer
feeding arteries detected by MDCT angiography were recorded.
Detection of lung cancer feeding arteries
by conventional angiography
Conventional angiography was performed with a
digital substraction technique within 1 week after CT. Angiography
was performed with a transfemoral approach and the Seldinger
technique. Various types of angiographic catheters were used to
selectively inject different systemic arteries. For the benefit of
each patient, angiographic procedures were performed with the
knowledge of the CT findings. We examined the bronchial arteries
based on the assumption that two bronchial arteries usually arise
from the thoracic aorta or that variants occasionally arise from
the internal thoracic, superior intercostal or thyro-cervical trunk
(17). When tumors had invaded the
pleura, thoracic wall or mediastinum, we examined the feeding
arteries according to tumor site and the extent of invasion into
the surrounding tissues as effectively as possible. We manually
applied angiography and infusion to avoid occluding the
corresponding feeding arteries with the catheter. To detect feeding
arteries, we observed tumor staining that was enhanced by filling
the tumor with contrast medium. Arteries with tumor staining
revealed by angiography were regarded as feeding arteries.
Multi-arterial infusion was based on gemcitabine and cisplatin as a
first-line therapy (8,18). We divided the total dose among the
detected feeding arteries according to the degree of tumor staining
in each artery. No complications were encountered following the
angiography and the infusion of bronchial and non-bronchial
systemic arteries in the studied population. The numbers of lung
cancer feeding arteries detected by conventional angiography were
recorded.
Evaluating tumor staining grades in all
detected feeding arteries by MDCT and conventional angiography
Tumor staining of lung cancers was arbitrarily
graded by the consensus of several physicians on a scale of I to
IV, which represented a percentage (%) of the tumor stained area to
the entire lesion on the chest X-ray or CT scan of: 0–25%, 26–50%,
51–75% and >75%, respectively (8). The tumor staining grades in all
feeding arteries detected by MDCT angiography and conventional
angiography were recorded and the results of conventional
angiography and MDCT angiography were compared.
Statistical analysis
The χ2 test or Fisher’s exact text were
used to investigate the statistical significance of the feeding
artery and tumor staining grades detected by MDCT angiography and
conventional angiography. P<0.05 was considered to indicate a
statistically significant result. All statistical analysis was
performed using SPSS software (version 10.0; SPSS, Inc., Chicago,
IL, USA).
Results
Comparison of lung cancer feeding
arteries detected by MDCT and conventional angiography
By conventional angiography and/or CT angiography,
we detected 80 feeding arteries (62 bronchial feeding arteries and
18 non-bronchial systemic arteries) for 59 lung cancers. The number
of feeding arteries detected in each lung cancer ranged from one to
four (mean±SD, 1.4±0.6), and we identified one bronchial feeding
artery for the lung tumor by conventional angiography/or CT
angiography in 56 of the 59 patients, 2 bronchial feeding arteries
in 3 patients, an intercostal feeding artery in 7 patients, 2
intercostal feeding arteries in one patient, an internal thoracic
feeding artery in 4 patients, as well as an inferior phrenic
feeding artery in 5 patients.
Among those 59 patients, CT angiography accurately
depicted 56 (56/80, 70%) feeding arteries (including 44 bronchial
feeding arteries, 3 intercostal feeding arteries, 4 internal
thoracic feeding arteries and 5 inferior phrenic feeding arteries)
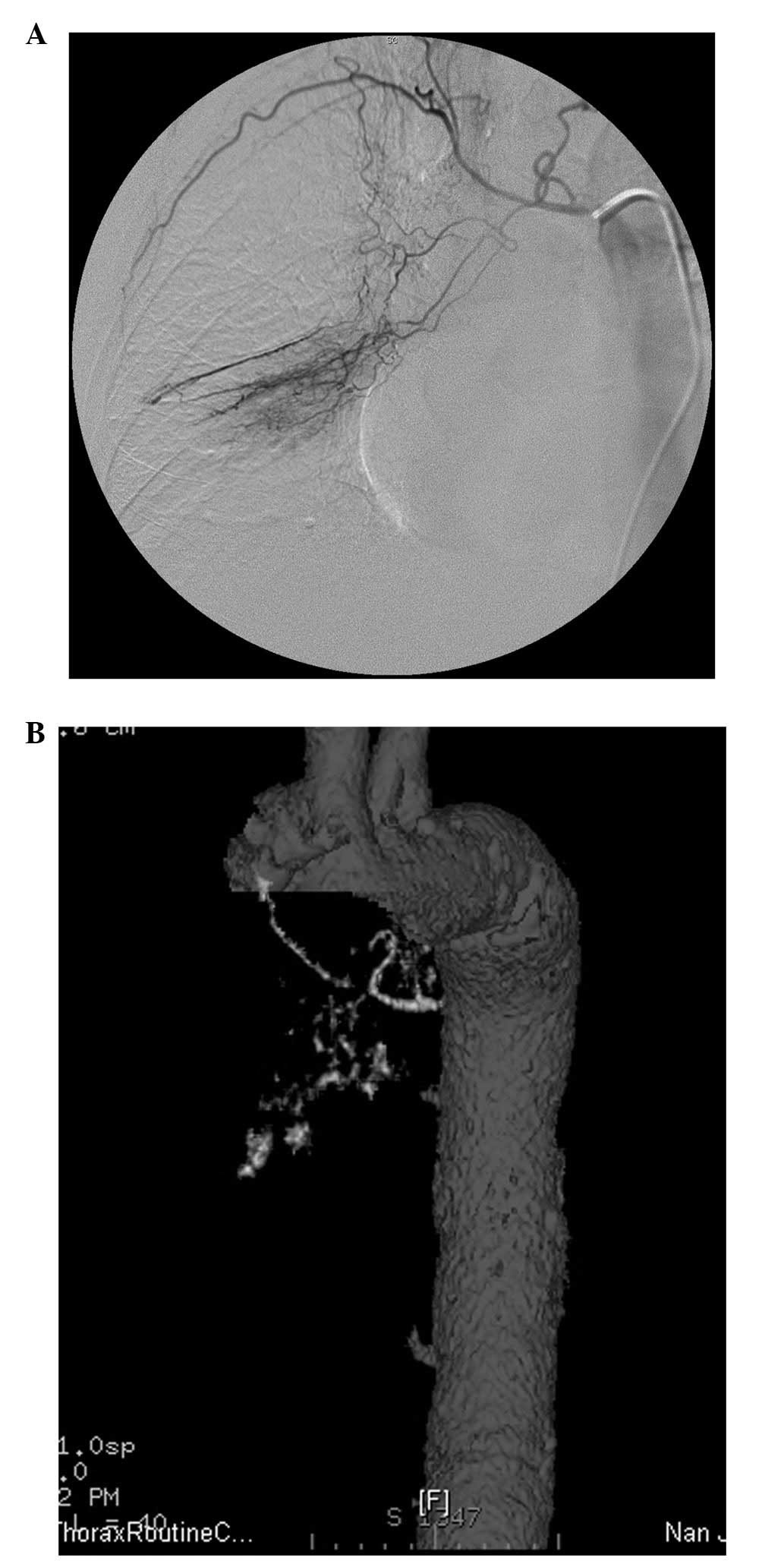
for the lung cancers (Fig. 1). A
total of 23 (23/80, 28.8%) feeding arteries defined by conventional
angiography also had clearly displayed origins and courses of the
major branches in the MDCT angiographic images, but no display of
their distal branches extending down to the lesions (Fig. 2). They were graded as suboptimal. In
one (1/80, 28.8%) case, the CT-defined feeding artery was not
selectively catheterized.
Table I compares the
results of MDCT angiography and conventional angiography in the
depiction of feeding arteries in the 59 patients who underwent
multi-arterial infusion chemotherapy.
 | Table IDepiction of feeding artery by MDCT
angiography and conventional angiography in 59 patients treated
with multi-arterial infusion chemotherapy. |
Table I
Depiction of feeding artery by MDCT
angiography and conventional angiography in 59 patients treated
with multi-arterial infusion chemotherapy.
| Conventional
angiography
|
|---|
| MDCT angiography | Selective
catheterization | Failure of
catheterization |
|---|
| Adequate
depiction | 55 | 1 |
| Suboptimal
depiction | 24 | 0 |
CT angiography accurately depicted the bronchial
artery as a feeding artery in all 21 patients with central lesions
(21/21, 100%). However, for the other 38 patients who had
peripheral lesions, CT angiography accurately depicted it in only
20 patients (20/38, 52.6%; P<0.05, χ2 test).
Evaluation of tumor staining grades in
all detected feeding arteries by MDCT and conventional
angiography
Table II summarizes
the tumor staining grades results evaluated by MDCT angiography and
conventional angiography. The results showed that tumor staining
grades were underestimated by CTA (P<0.001).
 | Table IIDepiction of the grade of tumor
staining by MDCT angiography and conventional angiography in 59
patients treated with multi-arterial infusion chemotherapy. |
Table II
Depiction of the grade of tumor
staining by MDCT angiography and conventional angiography in 59
patients treated with multi-arterial infusion chemotherapy.
| Tumor staining | Conventional
angiography (n) | MDCT angiography
(n) | P-valuea |
|---|
| I | 3 | 34 | <0.001 |
| II | 15 | 17 | |
| III | 19 | 5 | |
| IV | 22 | 3 | |
Discussion
Although BAI therapy is not a new therapy and has
not achieved wide acceptance as a standard clinical therapy for
lung cancer due to its unconfirmed outcomes and severe
side-effects, including esophageal ulceration and spinal cord
damage, several studies have indicated that it should be
reappraised as a preoperative adjuvant therapy for advanced lung
cancer as it has been observed histopathologically to have a high
response (7,8,18,19).
In the studies carried out by Nakanishi et al, many feeding
arteries (including bronchial and nonbronchial systemic arteries)
in patients with NSCLC were detected and the grade of total tumor
staining was significantly higher than that of tumor staining
supplied by the bronchial artery. Furthermore, the number of NSCLCs
that responded to multi-arterial infusion chemotherapy was
significantly increased among those with a higher grade of total
tumor staining (7,8). Therefore, we consider that the precise
definition of feeding arteries and sufficient tumor staining are
vital to ensure a successful outcome of arterial infusion
chemotherapy for patients with NSCLC. One point that should be
emphasized is the usefulness of their depiction with CT angiography
prior to a multi-artery infusion chemotherapy session. Of
particular importance are the subclavian artery and its branches
(most commonly, the internal mammary artery) for tumor-invaded
mediastinum, the inferior phrenic artery for lower lobe tumors, and
the intercostal artery for tumors which have invaded the pleura as
well as the thoracic wall. The time required to successfully
cannulate the appropriate vasculature may take hours or the
procedure may be ultimately unsuccessful for a number of technical
reasons. Therefore, the ability to concentrate on the relevant
vasculature reduces the procedure time and the potential iatrogenic
risks of a selective hunt for abnormal nonbronchial systemic
arteries. Based on this conclusion, we evaluated the feasibility of
detecting the feeding arteries and evaluating the grade of total
tumor staining by chest MDCT angiography. We detected 80 feeding
arteries (62 bronchial feeding arteries and 18 nonbronchial
systemic arteries) in 59 lesions by conventional angiography. These
80 feeding arteries detected by conventional angiography were also
clearly displayed with their origins, courses and main branches on
the MDCT angiographic images. However, only 56 of these arteries
were defined as lung cancer feeding arteries by MDCT angiography
(P<0.001). Similar results were also found when evaluating the
grade of total tumor staining by MDCT angiography (P<0.001). By
analysis of the patients who had no display of feeding arteries by
MDCT angiography, we estimated that the factors affecting the
detection rate of the lung cancer feeding arteries by MDCT
angiography are as follows. Firstly, the site of the tumor and the
extent of its invasion into the surrounding tissues may take
responsibility. In this study, the feeding arteries of centrally
located lung cancers were accurately depicted by MDCT angiography
according to conventional angiography (21/21, 100%). Conversely, of
the 38 patients with peripheral NSCLC, only 20 bronchial arteries
were accurately depicted as feeding arteries (20/38, 52.6%)
(P<0.05, χ2 test). The second factor is the diameter
of the tumor. In our study, we found that when the diameter of
tumor was smaller, the calibers of the feeding arteries were less
clearly enlarged. The size of the bronchial arteries greatly
affected their depiction on the CT image. This may be a limitation
of the spatial resolution of MDCT. The advent of high-spatial
resolution MDCT may allow radiologists to overcome the technical
limitation of displaying small feeding arteries such as bronchial
arteries.
From our results, we also identified that the grade
of total tumor staining was underestimated by MDCT angiography. In
this study, we started CT angiography examination 20–25 sec after
administration of the contrast medium to optimize the quality of
the CT angiography image. The transport of contrast medium through
the lung involves the intravascular interstitial spaces and the
washout phase from the interstitial spaces (20). The mean time to CT peak enhancement
for malignant pulmonary nodules is 3.2 min (ranging from 30 sec to
15 min) (21). At a 20–25 sec delay
after injecting the contrast medium, the transport of the contrast
medium only involves an intravascular phase. By allowing an
adequate delay for the contrast medium to penetrate into the
interstitial spaces, results closer to the tumor staining grade
evaluated by conventional angiography may be obtained. Therefore,
it could be explained that the grade of the tumor staining was
underestimated by MDCT angiography.
There were several limitations to our study.
Firstly, our analysis was based on small groups of patients and the
sensitivity of the imaging techniques might be under- or
overestimated. Therefore, our results are only preliminary results
and further studies with a larger number of patients would be
beneficial. Secondly, in this study, most of the MDCT angiography
was performed using a 64-row MDCT scanner which has a lower spatial
resolution than 256- or 320-row MDCT and so the ability to detect
feeding arteries by MDCT angiography may be underestimated.
Thirdly, we did not quantitatively measure the artery caliber. It
may be a predictive factor for the lung cancer feeding artery.
Finally, we did not evaluate the correlation between the CT peak
enhancement value to the grade of tumor staining by conventional
angiography.
In conclusion, although MDCT angiography is not able
to precisely or completely evaluate the lung cancer feeding
arteries and the grade of total tumor staining in this preliminary
study, it clearly displayed all feeding artery origins, courses and
main branches, which were defined by conventional angiography.
Therefore, chest MDCT angiography may provide an overview for
successful catheterization in multi-arterial infusion chemotherapy
for lung cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Viamonte M Jr: Selective bronchial
arteriography in man; preliminary report. Radiology. 83:830–839.
1964. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neyazaki T, Ikeda M, Seki Y, Egawa N and
Suzuki C: Bronchial artery infusion therapy for lung cancer.
Cancer. 24:912–922. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yiengpruksawa A, Watanabe G, Ono Y,
Tsurumaru M and Akiyama H: Tracheoesophageal fistula as a result of
bronchial artery infusion therapy. Int Surg. 69:351–355. 1984.
|
|
5
|
Munk PL, Morris DC and Nelems B: Left main
bronchial-esophageal fistula: a complication of bronchial artery
embolization. Cardiovasc Intervent Radiol. 13:95–97. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hellekant C and Jonsson K: Double blood
supply of bronchial carcinoma from multiple arteries. Acta Radiol
Diagn (Stockholm). 22:403–406. 1981.PubMed/NCBI
|
|
7
|
Nakanishi M, Umeda Y, Demura Y, Ameshima
S, Chiba Y, Miyamori I and Ishizaki T: Effective use of
multi-arterial infusion chemotherapy for advanced non-small cell
lung cancer patients: four clinical specified cases. Lung Cancer.
55:241–247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakanishi M, Demura Y, Umeda Y, Mizuno S,
Ameshima S, Chiba Y and Ishizaki T: Multi-arterial infusion
chemotherapy for non-small cell lung carcinoma-significance of
detecting feeding arteries and tumor staining. Lung Cancer.
61:227–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe S, Arai K, Watanabe T, Koda W and
Urayama H: Use of three-dimensional computed tomographic
angiography of pulmonary vessels for lung resections. Ann Thorac
Surg. 75:388–392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuhara K, Akashi A, Nakane S and Tomita
E: Preoperative assessment of the pulmonary artery by
three-dimensional computed tomography before video-assisted
thoracic surgery lobectomy. Eur J Cardiothorac Surg. 34:875–877.
2008. View Article : Google Scholar
|
|
11
|
Nakamoto K, Omori K and Nezu K; Lung
Cancer Project Group of West-Seto Inland Sea, Japan: Superselective
segmentectomy for deep and small pulmonary nodules under the
guidance of three-dimensional reconstructed computed tomographic
angiography. Ann Thorac Surg. 89:877–883. 2010. View Article : Google Scholar
|
|
12
|
Yu H, Liu SY, Li HM, Xiao XS and Dong WH:
Empirical description of bronchial and nonbronchial arteries with
MDCT. Eur J Radiol. 75:147–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murayama S, Hashiguchi N, Murakami J,
Sakai S, Matsumoto S, Mizushima A, Hasuo K and Masuda K: Helical CT
imaging of bronchial arteries with curved reformation technique in
comparison with selective bronchial arteriography: preliminary
report. J Comput Assist Tomogr. 20:749–755. 1996. View Article : Google Scholar
|
|
14
|
Remy-Jardin M, Bouaziz N, Dumont P,
Brillet PY, Bruzzi J and Remy J: Bronchial and nonbronchial
systemic arteries at multi-detector row CT angiography: comparison
with conventional angiography. Radiology. 233:741–749. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hartmann IJ, Remy-Jardin M, Menchini L,
Teisseire A, Khalil C and Remy J: Ectopic origin of bronchial
arteries: assessment with multidetector helical CT angiography. Eur
Radiol. 17:1943–1953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Remy J and Remy-Jardin M: Bronchial
bleeding. International Radiology. Dondelinger RF, Rossi P,
Kurdziel JC and Wallace S: Thieme; Stuttgart, Germany: pp. 325–341.
1990
|
|
17
|
Yoon W, Kim JK, Kim YH, Chung TW and Kang
HK: Bronchial and nonbronchial systemic artery embolization for
life-threatening hemoptysis: a comprehensive review. Radiographics.
22:1395–1409. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osaki T, Hanagiri T, Nakanishi R, Yoshino
I, Taga S and Yasumoto K: Bronchial arterial infusion is an
effective therapeutic modality for centrally located early-stage
lung cancer. results of a pilot study. Chest. 115:1424–1428. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe Y, Shimizu J, Murakami S, Yoshida
M, Tsubota M, Iwa T, Kitagawa M, Mizukami Y, Nonomura A and
Matsubara F: Reappraisal of bronchial arterial infusion therapy for
advanced lung cancer. Jpn J Surg. 20:27–35. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Littleton JT, Durizch ML, Moeller G and
Herbert DE: Pulmonary masses: contrast enhancement. Radiology.
177:861–871. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeong YJ, Lee KS, Jeong SY, Chung MJ, Shim
SS, Kim H, Kwon OJ and Kim S: Solitary pulmonary nodule:
characterization with combined wash-in and washout features at
dynamic multidetector row CT. Radiology. 237:675–683. 2005.
View Article : Google Scholar : PubMed/NCBI
|