Introduction
Arachidonic acid (AA; 20:4n6) is a polyunsaturated
fatty acid (PUFA) present in the phospholipids of cell membranes,
and is particularly abundant in the retina and brain (1,2). AA
present in the human body originates from dietary sources including
egg yolk, or is synthesized from linoleic acid (3). AA is naturally occurring in human
breast milk. AA, together with docosahexaenoic acid (DHA), is
commonly added as a functional food ingredient to commercial infant
formula worldwide, in accordance with the international standard of
Codex Alimentarius (4). The
consensus recommendations by the World Association of Perinatal
Medicine and other such societies specify an intake of 0.2–0.5% DHA
of total fatty acids and a level of AA at least equal to that of
DHA (5). However, AA has been
demonstrated to affect carcinogenesis in certain organs. Skin tumor
models in mice, including the
12-O-tetradecanoylphorbol-13-acetate model, have
demonstrated an essential role for the AA cascade in skin tumor
promotion (6). AA has also promoted
the growth of orthotopically transplanted breast cancer cell line
tumors (KPL-1 cells) in female athymic BALB/c mice, and of urinary
bladder tumors in a medium-term multi-organ rat carcinogenesis
study (7).
Pancreatic cancer is the fourth leading cause of
cancer-related mortality in the United States, with an estimated
42,470 new cases and 35,240 mortalities in 2009. This is due to a
lack of early disease-specific symptoms and effective screening
diagnostics, and poor prognosis at the time of diagnosis (8). Principle risk factors for pancreatic
cancer include inherited germline mutations in genes and repeated
exposure to N-nitroso compounds (through tobacco smoke and
during the manufacturing of cured rubber products) or their
N-nitrosamines precursors (in protein-containing foods dried
at high temperatures) (9). Animal
models of exocrine pancreatic carcinogenesis have been established
in guinea pigs, hamsters and/or rodents exposed to several
chemicals, including N-nitrosamines and azaserine (10,11).
N-methyl-N-nitrosourea (MNU) is a
direct-acting alkylating agent that interacts with DNA. It is toxic
and carcinogenic to the immune, hematopoietic, reproductive,
mammary, dental, gastrointestinal, nervous and/or sensory systems
(12,13). MNU also possesses carcinogenic
potency in the pancreas of guinea pigs (14), hamsters (15) and rodents (16). MNU-treated rats develop acinar cell
hyperplasia (ACH), which may progress to cancer in certain cases
(16,17). These results suggest that increased
incidence of ACH is an early indicator of exocrine pancreatic
carcinogenesis (11). In the
present study, we aimed to elucidate the promoting effect of
prenatal and postnatal dietary AA on MNU-induced exocrine
pancreatic carcinogenesis in young Lewis rats.
Materials and methods
Animal procedures
The study protocol and all animal procedures were
approved by the Animal Care and Use Committee of Kansai Medical
University, and were in accordance with the guidelines for animal
experimentation at Kansai Medical University. Sixteen female
SPF/VAF rats [LEW/CrlCrlj], 10-weeks-old and one-week pregnant,
were purchased from Charles River Japan (Yokohama, Japan). Rats
were maintained in specific pathogen-free conditions and had access
to water and CE-2-modified diets containing different doses of AA
ad libitum. Rats were housed in plastic cages with
paper-chip bedding (Paper Clean, SLC, Hamamatsu, Japan) in an
air-conditioned room at 22±2°C and 60±10% relative humidity, with a
12-h light/dark cycle. The illumination intensity inside the cages
was less than 60 lux. Offspring were culled to a maximum of 10 per
dam and the dams were maintained on their respective diets during
the 21-day lactation period. During a post-weaning period of up to
60 days, the offspring were maintained on a CE-2 diet. A total of
116 male and female pups were used in this study. Five to ten rats
were sacrificed at each time point, and there was a similar number
of males and females in each dietary group.
Chemical and dose formulation
MNU was obtained from Sigma-Aldrich (St. Louis, MO,
USA) and was maintained at −80°C in the dark. MNU solution was
dissolved in physiological saline containing 0.05% acetic acid
immediately prior to use. MNU (35 mg/kg) or vehicle (physiological
saline containing 0.05% acetic acid) was administered once by
intraperitoneal (i.p.) injection. In our preliminary study,
pancreatic ACH developed in 11 of the 14 surviving rats that were
treated with 50 mg/kg MNU at birth (Fig. 1). However, almost 50% of these rats
did not survive and all surviving female rats developed mammary
cancers with severe hematotoxicity. Therefore, 35 mg/kg MNU was
selected as a non-lethal, lower dose that would not cause mammary
cancer to occur in the present short-term study.
AA-supplemented diet
AA (purity of 40.4% by our analysis) was purchased
from Cargill Alking Bioengineering (Wuhan) Co, Ltd. (Hubei, China)
and purified for use. The diet with 2.0 w/w% AA was semi-purified
based on the modified CE-2 formulation (CLEA Japan, Inc., Tokyo,
Japan). The basal diet consisted of modified CE-2. Gas
chromatographic analyses of the fatty acid composition of the diets
are displayed in Table I(18). The total fatty acid volumes were
47.20, 86.75 and 126.63 μg/mg of diet for the CE-2 (0.006%
w/w AA), basal (0.008% w/w AA) and 2.0% AA diets, respectively.
These diets were exposed to γ-ray (30 Gy) and formulated by CLEA
Japan. Food was stored at 4°C to prevent lipid oxidation before
use.
 | Table IFatty acid composition of diets given
to rats. |
Table I
Fatty acid composition of diets given
to rats.
| Fatty acid
component (wt%) | CE-2 | Basal | 2.0% AA |
|---|
| ∑Saturated | 19.53 | 23.06 | 24.45 |
|
∑Monounsaturated | 23.65 | 34.55 | 24.41 |
| ∑n-6 | 41.81 | 31.82 | 42.26 |
| ∑n-3 | 7.20 | 5.53 | 3.86 |
| n-3/n-6 | 0.17 | 0.17 | 0.09 |
| AA/DHA | 0.09 | 0.14 | 32.45 |
| n6 | | | |
| 18:2n6 | 41.56 | 31.58 | 23.73 |
| 18:3n6 | 0.00 | 0.00 | 0.85 |
| 20:2n6 | 0.11 | 0.15 | 0.27 |
| 20:3n6 | 0.00 | 0.00 | 1.40 |
| 20:4n6 | 0.14 | 0.10 | 15.90 |
| 22:2n6 | ND | ND | ND |
| 22:4n6 | ND | ND | 0.12 |
| 22:5n6 | ND | ND | ND |
| n3 | | | |
| 18:3n3 | 3.16 | 3.51 | 2.29 |
| 20:3n3 | 0.00 | 0.00 | 0.02 |
| 20:5n3 | 2.04 | 1.07 | 0.91 |
| 22:5n3 | 0.41 | 0.23 | 0.15 |
| 22:6n3 | 1.59 | 0.73 | 0.49 |
| Total fatty acid
(μg/mg) | 47.20 | 86.75 | 126.63 |
| AA% of total fatty
acid | 0.30 | 0.12 | 12.56 |
Experimental procedures
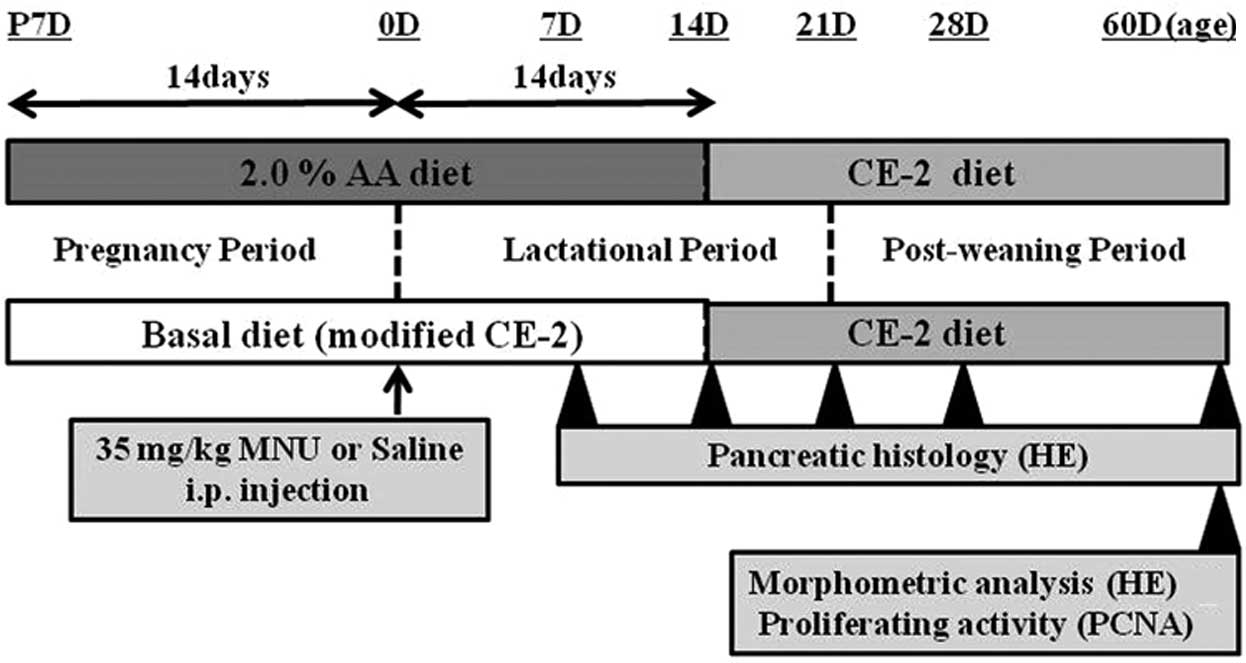
Male and female Lewis rats were exposed to either a
basal or an experimental diet (2.0% AA) from fertilization to
sacrifice. At birth (0 days of age), rats received an i.p.
injection of either vehicle (physiological saline) or 35 mg/kg MNU
(Fig. 2). At 7, 14, 21, 28 and 60
days after MNU or vehicle treatment, rats were anesthetized with
isoflurane (Forane; Abbot Japan, Tokyo, Japan) and sacrificed by
exsanguination from aortic transection. During the experiment, all
pups were observed daily for clinical signs of toxicity, and were
weighed at the time of MNU treatment and on the day of sacrifice.
The pancreas was immediately removed on sacrifice and complete
necropsies were conducted on all animals to check the systemic
toxicities induced by AA supplementation. Food consumption and body
weight of the dams were measured once per week to estimate the true
dosage of AA during the pregnancy and lactation periods.
Tissue fixation and processing
Pancreas tissues were fixed overnight in 10% neutral
buffered formalin, embedded in paraffin, sectioned at a thickness
of 4 μm and stained with hematoxylin and eosin (HE).
Histopathological and morphometrical evaluations were performed by
a toxicologic pathologist certified by the Japanese Society of
Toxicologic Pathology and/or the International Academy of
Toxicologic Pathology (K.Y. and A.T.). Previously defined
histopathological terminology and diagnostic criteria were used.
ACH is characterized by non-encapsulate glandular architecture with
zymogen granules, exhibiting larger size cytoplasm and nucleus,
basophilia, milder compression without higher mitotic index and
nuclear polymorphism (19,20). This lesion was distinguished from
regenerative hyperplasia that occurs in response to degeneration
and necrosis of acinar cells, as regeneration in response to a
single episode of severe toxic injury typically results in an
almost complete restoration of normal architecture, rather than a
focal nodular lesion (19).
However, multifocal ACH develops into frank neoplasia (20).
Morphometric analysis of pancreatic
ACH
HE-stained pancreas sections obtained from rats
sacrificed 60 days after MNU treatment were scanned with a
high-resolution digital slide scanner (NanoZoomer 2.0 Digital
Pathology; Hamamatsu Photonics, Hamamatsu, Japan) to prepare
digital images. The image files were opened with NDP.view software
(Hamamatsu Photonics). The total number of ACH lesions were counted
on each digital image. The areas of each ACH and whole pancreas
tissue were individually measured using NDP.view software. To
further evaluate the quantity and quality of ACH, the average area
and number of ACHs per 1 mm2 were calculated.
Proliferating cell nuclear antigen (PCNA)
staining and labeling index
Formalin-fixed pancreatic sections from rats (basal
and 2.0% AA diets) sacrificed 60 days after MNU or vehicle
treatment were used for proliferative activity analysis. Sequential
sections were immunohistochemically evaluated with anti-PCNA
monoclonal antibody (clone PC10, dilution 1:100; Leica Biosystems,
Newcastle-upon-Tyne, UK). A Labeled Streptavidin Biotin (LSAB)
staining kit (Dako, Carpinteria, CA, USA) and antigen retrieval by
pressure-cooker heating (Pascal, Dako) were used for immunostaining
(21). PCNA-stained sections were
scanned with a high-resolution digital slide scanner to prepare
digital images. The labeling index was calculated from digital
images by determining the number of PCNA-positive nuclei of acinar
cells per 1 mm2 in normal exocrine pancreatic tissue and
ACH, using NDP.view software.
Statistical analysis
All discrete values, expressed as the mean ±
standard error (SE), were analyzed using the two-tailed independent
Student's t-test for unpaired samples, after confirming the
homogeneity of variances. The incidence of ACH was analyzed using
the χ2 test. The Results include comparisons between
basal diet-fed rats and rats fed an AA-supplemented diet, in both
the MNU-treated and vehicle-treated groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
General remarks
No fatalities occurred, and no clinical signs or
symptoms related to treatment were evident in any of the pups or
dams during the experimental period. None of the pups in any of the
groups developed mammary tumors. The 2.0% AA diet did not influence
food consumption in dams during the experimental period. During the
pregnancy and lactation periods, the AA intake (mg/kg/day) of dams
was 6.3 and 8.5 in the basal diet group, and 1,477 and 1,876 in the
2.0% AA group, respectively. The 2.0% AA diet did not influence
body weight gain (the growth rate) in pups or cause weight changes
in dams with or without MNU treatment. However, the growth rate of
MNU-treated pups was typically lower than that of vehicle-treated
pups (data not shown).
Morphological and morphometric
analysis
The incidence of proliferative lesions of exocrine
pancreatic tissue is demonstrated in Table II. In vehicle-treated rats with or
without an AA-rich diet, no proliferative lesions were observed at
any time point. In contrast, multifocal ACHs occurred in the basal
diet-fed rats 60 days after MNU treatment (100% incidence). In rats
fed an AA-rich diet, multifocal ACH occurred 28 and 60 days after
MNU treatment (20 and 75% incidence, respectively). There was no
significant difference in incidence between MNU-treated rats fed a
basal diet and those fed an AA-rich diet. Regardless of diet, the
majority of MNU-induced ACH were characterized by
well-differentiated acidophilic acinar cells with cell polarity
(Fig. 3). However, among
MNU-treated rats, ACH were larger in rats fed an AA-rich diet,
compared with rats fed a basal diet (Fig. 3). In the area surrounding the ACH,
there was no evidence of capsulation. The neighboring pancreatic
tissue maintained a normal acinar structure with or without mild
compression. There was no significant difference in the incidence
of ACH between males and females (data not shown). Endocrine
pancreatic lesions and acinar cell tumors were not observed in any
group at any time point.
 | Table IIIncidence of ACH induced by MNU. |
Table II
Incidence of ACH induced by MNU.
| | Days after MNU
treatment
|
|---|
| Treatment | Diet | 7 | 14 | 21 | 28 | 60 |
|---|
| Vehicle | Basalb | 0 (0/6)d | 0 (0/6) | 0 (0/5) | 0 (0/5) | 0 (0/10) |
| AAc | 0 (0/6) | 0 (0/6) | 0 (0/5) | 0 (0/5) | 0 (0/5) |
| MNUa | Basal | 0 (0/6) | 0 (0/6) | 0 (0/5) | 0 (0/5) | 100 (5/5) |
| AA | 0 (0/6) | 0 (0/6) | 0 (0/5) | 20 (1/5)e | 75 (6/8)e |
The area and number of ACHs per 1 mm2 of
exocrine pancreas were determined (Table III). In MNU-treated rats, the area
of ACHs was 0.36 in rats fed a basal diet and 0.78 for rats fed an
AA-rich diet; thus, the area of ACH was significantly greater in
rats fed an AA-rich diet (P<0.01). The number of ACHs increased
significantly in MNU-treated rats fed an AA-rich diet (0.11),
compared with rats fed a basal diet (0.08) (P<0.05; Table III). Thus, the number and area of
ACH were highest in MNU-treated rats fed an AA-rich diet. This
result was consistent with the morphological characterization
(Fig. 3).
 | Table IIIMorphometrical analysis of acinar
cell hyperplasia induced by N-methyl-N-nitrosourea
(MNU). |
Table III
Morphometrical analysis of acinar
cell hyperplasia induced by N-methyl-N-nitrosourea
(MNU).
| Treatment | Diet | Area per
hyperplasia (mm2)a | Number per 1
mm2 |
|---|
| Vehicle | Basalc | 0 | 0 |
| AAd | 0 | 0 |
| MNUb | Basal | 0.36±0.18 | 0.08±0.02 |
| AA | 0.78e±0.19 | 0.11f±0.01 |
Macroscopic examination of other
organs
Atrophic changes in the spleen, thymus and testis
occurred 7–28 days after MNU treatment, regardless of diet. No
macroscopic lesions were detected in any organs, including the
pancreas, 60 days after MNU treatment.
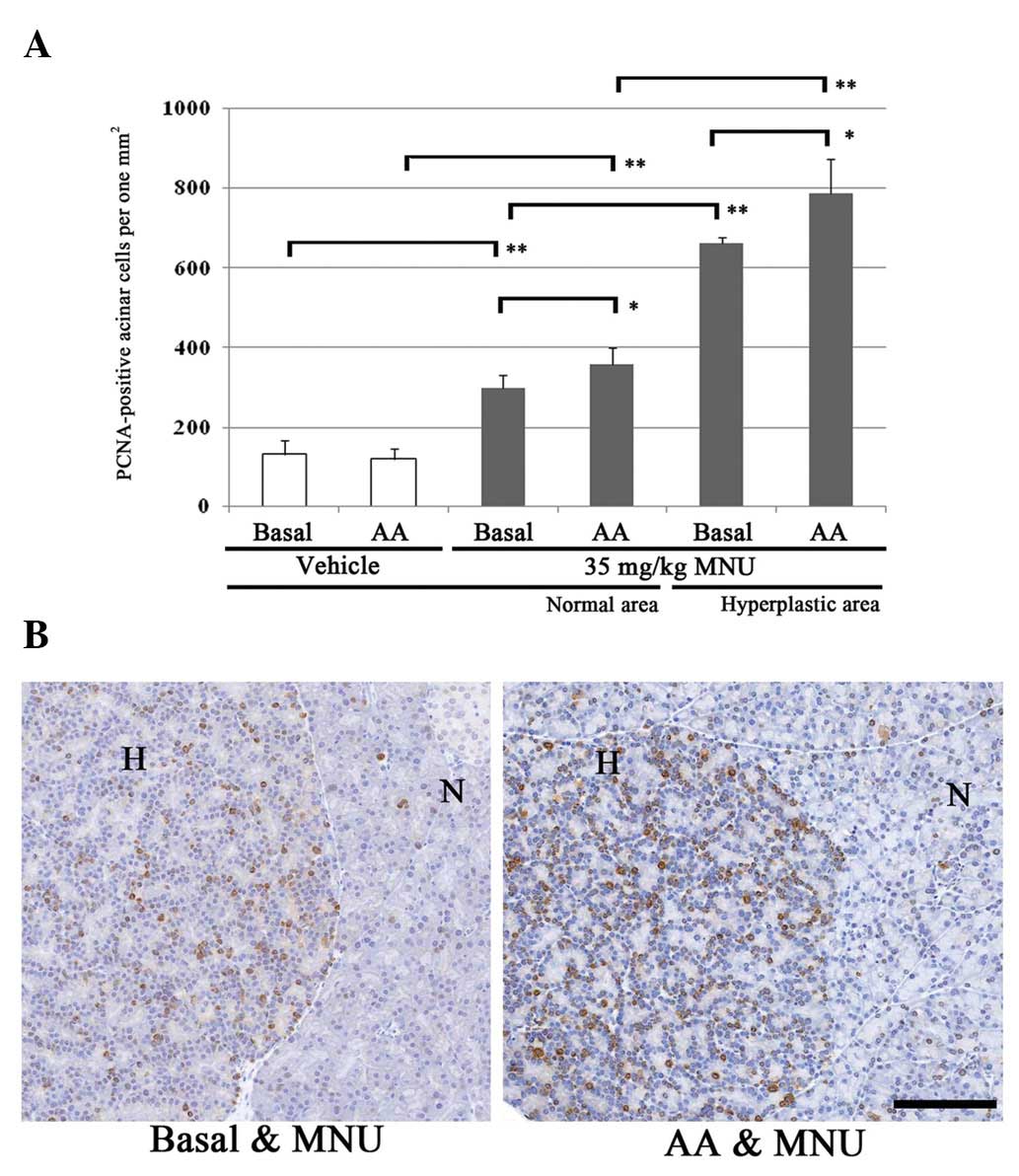
PCNA index
In order to compare the cell kinetics of normal
acinar areas and ACH areas among rats fed a basal or AA-rich diet,
the PCNA-positive cell number per 1 mm2 of exocrine
pancreas was determined. Changes in the number of PCNA-positive
acinar cells 60 days after MNU treatment are displayed in Fig. 4. In the basal and AA-rich diet
groups, PCNA-positive cells gradually and significantly increased
in the normal areas of vehicle- and MNU-treated rat exocrine
pancreas, and in the MNU-treated hyperplastic areas. In the normal
areas of vehicle-treated rats fed a basal diet or AA-rich diet, the
numbers of proliferative acinar cells were 133.2 and 121.0 (no
significant difference). In contrast, the number of PCNA-positive
cells in normal areas increased significantly in MNU-treated rats
fed an AA-rich diet (359.7), compared with rats fed a basal diet
(298.7) (Fig. 4A). The number of
PCNA-positive cells in ACHs was significantly increased in
MNU-treated rats fed an AA-rich diet (786.6), compared with rats
fed a basal diet (662.7) (Fig. 4A).
Thus, the proliferative activity of acinar cells in normal and ACH
areas was highest in MNU-treated rats fed an AA-rich diet (Fig. 4B).
Discussion
The main purpose of the present study was to
determine whether increased levels of AA during gestation and
lactation proportionally enhanced the development of preneoplastic
ACHs in the pancreas of MNU-treated rat pups. Pancreatic morphology
in rats treated with 35 mg/kg MNU exhibited small ACH in rats fed a
basal diet and large ACH in rats fed an AA-rich diet (2.0% AA). The
number and area of ACHs increased in MNU-treated rats fed an
AA-rich diet, which was consistent with the morphological
characterization. By PCNA immunohistochemistry, the proliferative
activity of acinar cells in both the normal and hyperplastic areas
was demonstrated to have significantly increased in MNU-treated
rats fed an AA-rich diet. These results demonstrated that 2.0% AA
had a strong promoting effect on ACHs, both morphologically and
morphometrically. In the MNU rat model of pancreatic carcinogenesis
with exposure to 50 mg/kg MNU at 3 days of age, mancozeb,
diethyldithiocarbamate, bayleton, phenobarbital and folithion
enhance preneoplastic lesions (acinar cell hyperplasia and
dysplasia), carcinoma in situ and/or adenocarcinoma at 24
weeks of age (16,17,22).
In our preliminary study, 50 mg/kg MNU induced mortality and
mammary cancers with severe hematotoxicity. Lewis rats are more
sensitive than other rat strains to chemical carcinogenesis in the
exocrine pancreas. Additionally, rats are most sensitive to the
induction of tumors if the chemical is administered during the
first several weeks after birth, when the rate of cell division in
the pancreas is highest (19).
Therefore, as with our experimental protocol, a short-term study
(60 days) with 35 mg/kg MNU as a non-lethal, lower dose that does
not cause mammary cancer occurrence, may be extremely useful for
testing the promoting, progressing or inhibitory effect of chemical
and physical agents on cell proliferation and transformation of rat
exocrine pancreas.
High levels of dietary PUFA promote tumor growth in
several animal models, including pancreatic cancer models (23). A higher incidence of proliferative
exocrine lesions in the pancreas have been observed in F344 rats
given corn oil in long-term studies (19,24).
The promoting effects of unsaturated fats have been attributed to
the development of these spontaneously initiated lesions (19,20).
In corn oil-treated models, males have a higher incidence and wider
distribution of ACH and tumors than female rats (25), and testosterone is considered to be
responsible for the higher incidence of these lesions in males. In
the present study, sex differences in the incidence of MNU-induced
ACH were not evident (data not shown), which is likely due to the
shorter study period (60 days).
Linoleic acid (LA; 18:2n6) is partly responsible for
the promoting effect of dietary polyunsaturated fats on pancreatic
carcinogenesis via accelerated prostaglandin synthesis caused by
the metabolism of linoleic-derived AA in preneoplastic tissue
(23,26). The strongest enhancing effect on the
growth of pancreatic (pre)neoplastic lesions in the azaserine rat
model and N-nitroso bis(2-oxopropyl)amine (BOP) hamster
model was obtained with LA-rich diets. In azaserine-induced ACH
(atypical acinar cell foci) in rats fed LA-rich diets, the
BrdU-labeling index was significantly increased, compared with rats
fed LA-low diets (26). In our
study, the proliferative activity of acinar cells in normal and ACH
areas was highest in the MNU-treated rats fed an AA-rich diet,
compared with those fed a basal diet. The LA concentration of the
AA-rich diet in our study was lower than that of the basal diet
(Table I); therefore, LA was not
responsible for the promoting effect on ACH in the present
study.
Cholecystokinin (CCK), which is produced and
secreted by highly specialized enteroendocrine cells located in the
duodenal and jejunal mucosa of the gut, strongly stimulates the
secretion of amylase from acinar cells (19,27).
Feeding raw soybean flour to rats produces hyperplasia and
neoplasia of the exocrine pancreas (28). Additionally, prolonged injection of
CCK accelerates the production of atypical acinar cell foci and
invasive cancers (11,19,20).
Pancreatic growth is considered to be stimulated by interference in
the feedback control of CCK by a heat-labile soybean trypsin
inhibitor. This effect appears to be independent of any promotion
by the high levels of unsaturated fats in the raw soybean flour
(11,20,29).
The composition of dietary fatty acids influences the CCK secretory
response. Short-term exposure of enteroendocrine STC-1 cells to AA
promotes CCK secretion (30).
Persistent stimulation of the pancreas by CCK induces acinar cell
hypersecretion of amylase, followed by hyperplasia, which is
capable of promoting chemically induced carcinogenesis of the
exocrine pancreas (27,29). Exogenous AA induces amylase
secretion in a concentration-dependent manner in the rat acinar
cell ex vivo model, suggesting a role for AA as a potential
intracellular mediator in the exocrine pancreas (31). In the present study, this
information collectively supports our speculation that CCK-related
amylase release is involved in the promoting effects of AA on
MNU-induced ACH.
Pancreatic cancer is the fourth leading cause of
cancer mortality in the United States (8). A previous large population-based,
case-control clinical study in San Francisco bay provided evidence
that the saturated fatty acids, monounsatu-rated palmitoleic and
oleic fatty acids, and polyunsaturated LA may increase the risk of
adenocarcinoma of the exocrine pancreas, whereas gadoleic acid
(monounsaturated) and ω-3 fatty acids (polyunsaturated) may
decrease this risk (8). However, no
association was observed between pancreatic cancer risk and a
dietary intake of 160 mg or more of AA. AA supplementation by
healthy adults appears to confer no toxicity or significant safety
risk; daily dosages of 1,500 mg for 50 days in the United States
and 838 mg for 14 days in Japan have been well-tolerated in
clinical studies with no significant side effects (32,33).
Previously, AA demonstrated no promoting effects on a rat
medium-term multi-organ carcinogenesis model using five carcinogens
including MNU (34).
The recommended intake of AA in Japan is 24
mg/kg/day in adult humans (http://www.suntory-kenko.com/supplement/main/433461;
in Japanese). The 2.0% AA diets used in the present study provide
an AA dose of 1,477 mg/kg during pregnancy and 1,876 mg/kg during
lactation, which are 61.6-and 78.2-fold higher than the recommended
human dose, respectively. Moreover, daily AA intake by Japanese
infants via breast milk is approximately 14.3 mg AA/kg/day
(34). Compared with the amounts of
AA tested in the present study, this is approximately 103- and
131-fold higher. Taken together, an AA-enriched diet in the
prenatal and postnatal periods is not likely to cause exocrine
pancreatic carcinogenesis in humans.
In conclusion, an AA-rich diet in dams during
gestation and lactation promotes MNU-induced pancreatic ACH in
young rats. An AA-rich diet induces increased proliferative
activity of acinar cells following MNU initiation, likely followed
by the development of exocrine pancreatic tumors. Several factors,
including AA itself, may affect the increased proliferative
activity of the exocrine pancreas. Further studies of the cascade
of proliferative action are necessary to understand the detailed
mechanisms of the promoting effects of AA on exocrine pancreatic
carcinogenesis.
Acknowledgements
This research was supported in part by
Health and Labour Sciences Research Grants
(H22-Shokuhin-Ippan-002). The authors thank Ms. T. Akamatsu for her
excellent technical assistance, Ms. A. Shudo for manuscript
preparation and Dr T. Sasaki (Maruho Co. Ltd.) for her scientific
advice.
References
|
1
|
Davis-Bruno K and Tassinari MS: Essential
fatty acid supplementation of DHA and ARA and effects on
neurodevelopment across animal species: a review of the literature.
Birth Defects Res B Dev Reprod Toxicol. 92:240–250. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uauy R, Hoffman DR, Peirano P, Birch DG
and Birch EE: Essential fatty acids in visual and brain
development. Lipids. 36:885–895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le HD, Meisel JA, de Meijer VE, Gura KM
and Puder M: The essentiality of arachidonic acid and
docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids.
81:165–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Codex Alimentarius Commission, Joint
FAO/WHO Food Standards Programme: Report of the 28th Session of the
Codex Committee on Nutrition and Foods for Special Dietary Uses,
2007.
|
|
5
|
Hoffman DR, Boettcher JA and
Diersen-Schade DA: Toward optimizing vision and cognition in term
infants by dietary docosahexaenoic and arachidonic acid
supplementation: a review of randomized controlled trials.
Prostaglandins Leukot Essent Fatty Acids. 81:151–158. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anonymous: Final report on the safety
assessment of arachidonic acid. Int J Toxicol. 12:481–559. 1993.
View Article : Google Scholar
|
|
7
|
Hamazaki T: Reports on “Research on the
toxicity of arachidonic acid supplementation”. The Health and Labor
Sciences Research Grants, Japan (H22-Shokuhin-Ippan-002). 2012.(In
Japanese).
|
|
8
|
Gong Z, Holly EA, Wang F, Chan JM and
Bracci PM: Intake of fatty acids and antioxidants and pancreatic
cancer in a large population-based case-control study in the San
Francisco bay area. Int J Cancer. 127:1893–1904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Risch HA: Etiology of pancreatic cancer,
with a hypothesis concerning the role of N-nitroso compounds and
excess gastric acidity. J Natl Cancer Inst. 95:948–960. 2003.
View Article : Google Scholar
|
|
10
|
Rao MS: Animal models of exocrine
pancreatic carcinogenesis. Cancer Metastasis Rev. 6:665–676. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woutersen RA, van Garderen-Hoetmer A,
Lamers CB and Scherer E: Early indicators of exocrine pancreas
carcinogenesis produced by non-genotoxic agents. Mutat Res.
248:291–302. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kimura A, Yoshizawa K, Sasaki T, Uehara N,
Kinoshita Y, Miki H, Yuri T, Uchida T and Tsubura A:
N-methyl-N-nitrosourea-induced changes in epithelial rests of
Malassez and the development of odontomas in rats. Exp Ther Med.
4:15–20. 2012.PubMed/NCBI
|
|
13
|
Tsubura A, Lai YC, Miki H, Sasaki T,
Uehara N, Yuri T and Yoshizawa K: Animal models of
N-methyl-N-nitrosourea-induced mammary cancer and retinal
degeneration with special emphasis on therapeutic trials. In Vivo.
25:11–22. 2011.PubMed/NCBI
|
|
14
|
Reddy JK and Rao MS: Pancreatic
adenocarcinoma in inbred guinea pigs induced by
N-methyl-N-nitrosourea. Cancer Res. 35:2269–2277. 1975.PubMed/NCBI
|
|
15
|
Furukawa F, Sato H, Imaida K, Toyoda K,
Imazawa T, Takahashi M and Hayashi Y: Induction of pancreatic
tumors in male Syrian golden hamsters by intraperitoneal
N-methyl-N-nitrosourea injection. Pancreas.
7:153–158. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monis B and Valentich MA: Promoting
effects of mancozeb on pancreas of nitrosomethylurea-treated rats.
Carcinogenesis. 14:929–933. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valentich MA, Eynard AR, Barotto NN, Diaz
MP and Bongiovanni GA: Effect of the co-administration of
pheno-barbital, quercetin and mancozeb on nitrosomethylurea-induced
pancreatic tumors in rats. Food Chem Toxicol. 44:2101–2105. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harauma A and Moriguchi T: Dietary n-3
fatty acid deficiency in mice enhances anxiety induced by chronic
mild stress. Lipids. 46:409–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eustis SL, Boorman GA and Hayashi Y:
Exocrine pancreas. Pathology of the Fischer Rat: Reference and
Atlas. Boorman GA, Montgomery CA Jr and MacKenzie WF: Academic
Press; San Diego: pp. 95–108. 1990
|
|
20
|
Longnecker DS and Millar PM: Tumors of the
pancreas. Pathology of Tumors in Laboratory Animals, Vol 1, Tumours
of the Rat. Turusov VS and Mohr U: IARC Scientific Publications No.
99, International Agency for Research in Cancer; Lyon: pp. 241–257.
1990
|
|
21
|
Yoshizawa K, Sasaki T, Kuro M, Miki H,
Kimura A, Uehara N, Yuri T and Tsubura A: Corneal damage induced in
adult mice by a single intraperitoneal injection of
N-ethyl-N-nitrosourea. In Vivo. 25:609–616. 2011.
|
|
22
|
Barotto NN, López CB, Eynard AR, Fernández
Zapico ME and Valentich MA: Quercetin enhances pretumorous lesions
in the NMU model of rat pancreatic carcinogenesis. Cancer Lett.
129:1–6. 1998.PubMed/NCBI
|
|
23
|
Appel MJ, van Garderen-Hoetmer A and
Woutersen RA: Effects of dietary linoleic acid on pancreatic
carcinogenesis in rats and hamsters. Cancer Res. 54:2113–2120.
1994.PubMed/NCBI
|
|
24
|
Boorman GA: Proliferative exocrine
pancreatic lesions in rats. The effect of sample size on the
incidence of lesions. Toxicol Pathol. 15:451–456. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dominick MA, Bobrowski WF and Metz AL:
Proliferative exocrine pancreatic lesions in aged Wistar rats.
Toxicol Pathol. 18:423–426. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Appel MJ and Woutersen RA: Modulation of
growth and cell turnover of preneoplastic lesions and of
prostaglandin levels in rat pancreas by dietary fish oil.
Carcinogenesis. 15:2107–2112. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshizawa K, Marsh T, Foley JF, Cai B,
Peddada S, Walker NJ and Nyska A: Mechanisms of exocrine pancreatic
toxicity induced by oral treatment with
2,3,7,8-tetrachlorodibenzo-p-dioxin in female Harlan
Sprague-Dawley rats. Toxicol Sci. 85:594–606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McGuiness EE, Morgan RGH and Wormsley KG:
Effects of soybean flour on the pancreas of rats. Environ Health
Perspect. 56:205–212. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haschek WM, Rousseaux CG and Wallig MA:
Pancreas, Section I, Exocrine pancreas. Fundamentals of Toxicologic
Pathology. 2nd edition. Academic Press; San Diego: pp. 237–251.
2010
|
|
30
|
Hand KV, Bruen CM, O'Halloran F, Giblin L
and Green BD: Acute and chronic effects of dietary fatty acids on
cholecystokinin expression, storage and secretion in
enteroendocrine STC-1 cells. Mol Nutr Food Res. 54:S93–S103. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hou W, Arita Y and Morisset J: Endogenous
arachidonic acid release and pancreatic amylase secretion.
Pancreas. 14:301–308. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kusumoto A, Ishikura Y, Kawashima H, Kiso
Y, Takai S and Miyazaki M: Effects of arachidonate-enriched
triacylglycerol supplementation on serum fatty acids and platelet
aggregation in healthy male subjects with a fish diet. Br J Nutr.
98:626–635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nelson GJ, Schmidt PC, Bartolini G, Kelley
DS and Kyle D: The effect of dietary arachidonic acid on platelet
function, platelet fatty acid composition, and blood coagulation in
humans. Lipids. 32:421–425. 1997. View Article : Google Scholar
|
|
34
|
Imai N, Kawabe M, Tamano S, Doi Y,
Nakashima H, Suguro M, Numano T, Hara T, Hagiwara A, Furukawa F,
Kaneda Y, et al: Arachidonate-enriched triglyceride oil does not
promote tumor development in a rat medium-term multi-organ
carcinogenesis model. Food Chem Toxicol. 50:2780–2791. 2012.
View Article : Google Scholar : PubMed/NCBI
|