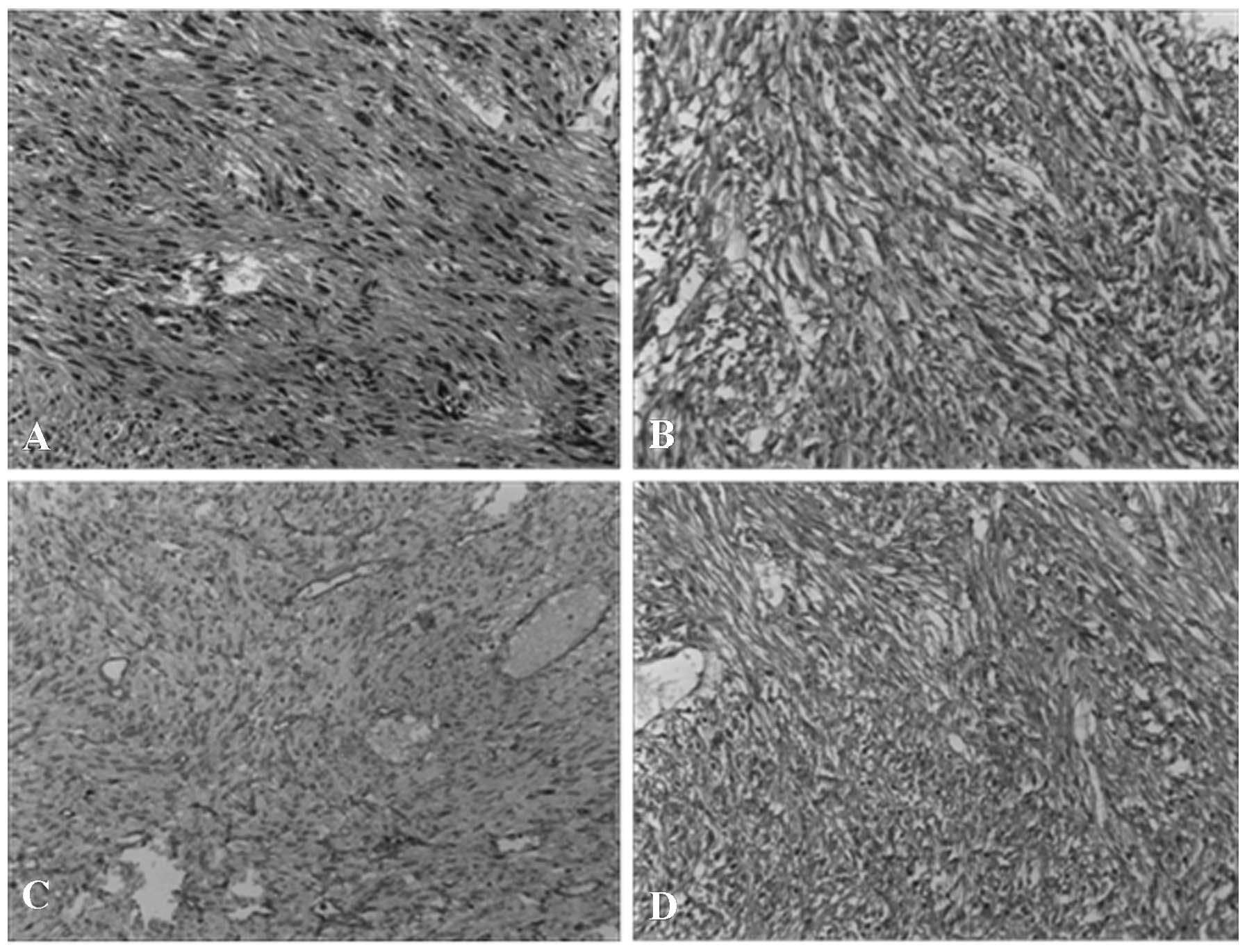
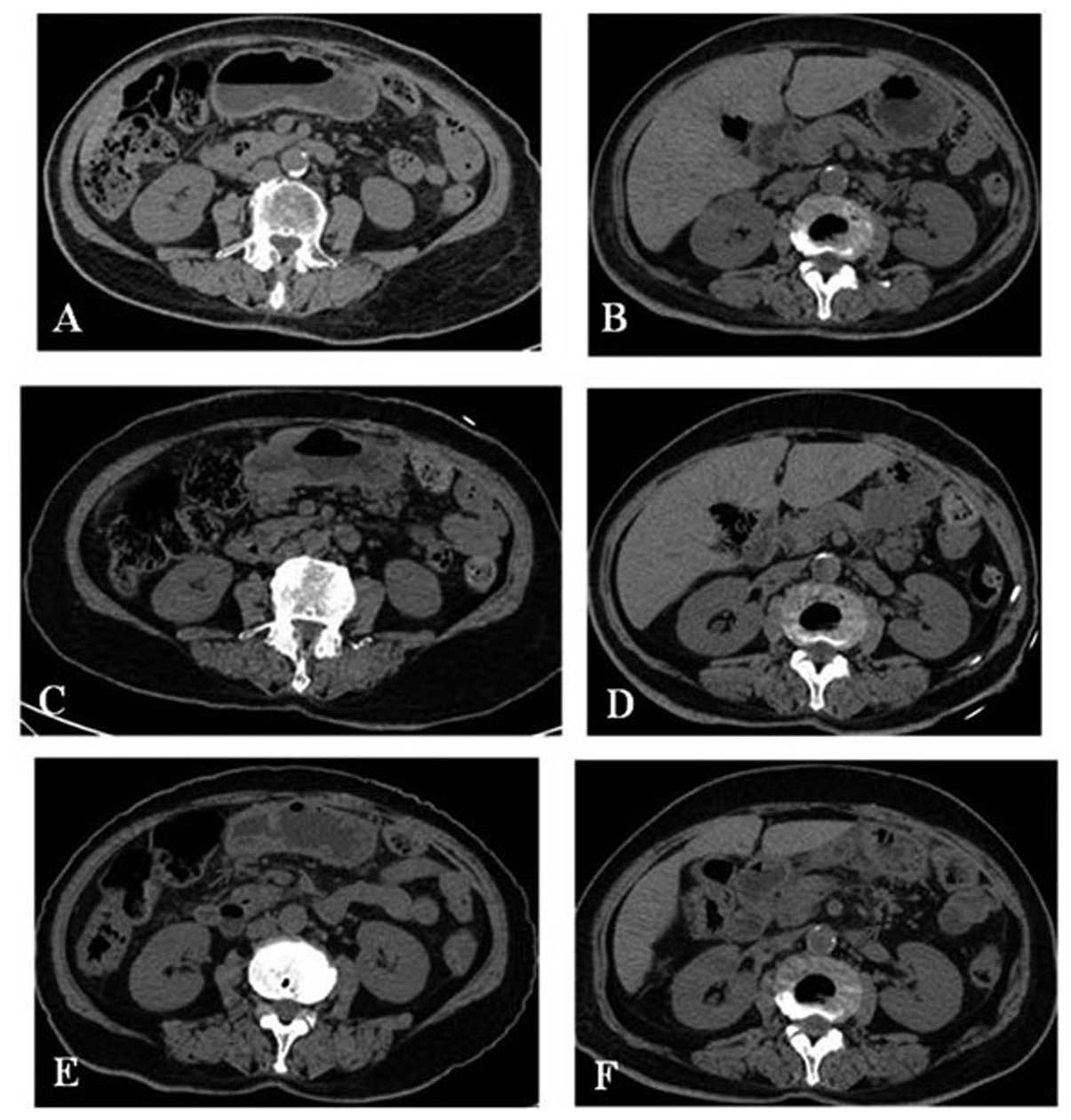
|
1.
|
Hauber AB, Gonzalez JM, Coombs J, et al:
Patient preferences for reducing toxicities of treatments for
gastrointestinal stromal tumor (GIST). Patient Prefer Adherence.
5:307–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Di Scioscio V, Greco L, Pallotti MC, et
al: Three cases of bone metastases in patients with
gastrointestinal stromal tumors. Rare Tumors. 3:e172011.
|
|
3.
|
Afuwape OO, Irabor DO and Ladipo JK:
Gastrointestinal stromal tumour in Ibadan, Nigeria: a case report
and review of current treatment. Afr Health Sci. 11:134–138.
2011.PubMed/NCBI
|
|
4.
|
Issar P, Dwivedi MK, Issar SK, Pal RK and
Dewanagan L: Malignant gastrointestinal stromal tumours. Indian J
Radiol Imaging. 16:65–67. 2006. View Article : Google Scholar
|
|
5.
|
Wingen CB, Pauwels PA, Debiec-Rychter M,
van Gemert WG and Vos MC: Uterine gastrointestinal stromal tumours
(GIST). Gynecol Oncol. 97:970–972. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
DeMatteo RP, Lewis JJ, Leung D, et al: Two
hundred gastrointestinal stromal tumors: recurrence patterns and
prognostic factors for survival. Ann Surg. 231:51–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chen YY, Yeh CN, Cheng CT, et al:
Sunitinib for Taiwanese patients with gastrointestinal stromal
tumor after imatinib treatment failure or intolerance. World J
Gastroenterol. 17:2113–2119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kindblom LG, Remotti HE, Aldenborg F, et
al: Gastrointestinal pacemaker cell tumor (GIPACT):
gastrointestinal stromal tumors show phenotypic characteristics of
the interstitial cells of Cajal. Am J Pathol. 152:1259–1269.
1998.
|
|
9.
|
Hirota S, Isozaki K, Moriyama Y, et al:
Gain-of-function mutations of c-kit in human gastrointestinal
stromal tumors. Science. 279:577–580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Demetri GD, von Mehren M, Blanke CD, et
al: Efficacy and safety of imatinib mesylate in advanced
gastronitestinal stromal tumors. N Engl J Med. 347:472–480. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Demetri GD, van Oosterom AT, Garrett CR,
et al: Efficacy and safety of sunitinib in patients with advanced
gastrointestinal stromal tumour after failure of imatinib: a
randomised controlled trial. Lancet. 368:1329–1338. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Choi H, Charnsangavej C, Faria SC, et al:
Correlation of computed tomography and positron emission tomography
in patients with metastatic gastrointestinal stromal tumor treated
at a single institution with imatinib mesylate: proposal of new
computed tomography response criteria. J Clin Oncol. 25:1753–1759.
2007. View Article : Google Scholar
|
|
14.
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: review on morphology, molecular pathology,
prognosis, and differential diagnosis. Arch Pathol Lab Med.
130:1466–1478. 2006.PubMed/NCBI
|
|
15.
|
Daniels M, Lurkin I, Pauli R, Erbstösser
E, et al: Spectrum of KIT/PDGFRA/BRAF mutations and
Phosphatidylinositol-3-Kinase pathway gene alterations in
gastrointestinal stromal tumors (GIST). Cancer Lett. 312:43–54.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: pathology and prognosis at different sites. Semin
Diagn Pathol. 23:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Corless CL, Fletcher JA, Heinrich MC, et
al: Biology of gastrointestinal stromal tumors. J Clin Oncol.
22:3813–3825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bucher P, Villiger P, Egger JF, et al:
Management of gastrointestinal stromal tumours: from diagnosis to
treatment. Swiss Med Wkly. 134:145–153. 2004.PubMed/NCBI
|
|
19.
|
Annaberdyev S, Gibbons J and Hardacre JM:
Dramatic response of a gastrointestinal stromal tumour to
neoadjuvant imatinib therapy. World J Surg Oncol. 7:302009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Dematteo RP, Ballman KV, Antonescu CR, et
al: Adjuvant imatinib mesylate after resection of localised,
primary gastrointestinal stromal tumour: a randomised,
double-blind, placebo-controlled trial. Lancet. 373:1097–104. 2009.
View Article : Google Scholar
|
|
21.
|
Ibrahim HH, Ahmad MS, Eskaf WA, et al:
Malignant gastrointestinal stromal tumor of the tongue: case report
and review of the literature. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 111:e24–e29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Nowain A, Bhakta H, Pais S, et al:
Gastrointestinal stromal tumors: clinical profile,
pathogenesis,treatment strategies and prognosis. J Gastroenterol
Hepatol. 20:818–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Arru JM and Richardson JD:
Gastrointestinal stromal tumors: pathogenesis and current
treatment. J Ky Med Assoc. 103:211–215. 2005.PubMed/NCBI
|
|
24.
|
Osusky KL, Hallahan DE, Fu A, et al: The
receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell
migration, tubule formation, and blood vessel formation in vivo,
but has little effect on existing tumor vessels. Angiogenesis.
7:225–233. 2004. View Article : Google Scholar
|
|
25.
|
Abrams TJ, Lee LB, Murray LJ, et al:
SU11248 inhibits KIT and platelet-derived growth factor receptor
beta in preclinical models of human small cell lung cancer. Mol
Cancer Ther. 2:471–478. 2003.PubMed/NCBI
|
|
26.
|
Mendel DB, Laird AD, Xin X, et al: In vivo
antitumor activity of SU11248, a novel tyrosine kinase inhibitor
targeting vascular endothelial growth factor and platelet-derived
growth factor receptors: determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.
|
|
27.
|
Murray LJ, Abrams TJ, Long KR, et al:
SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an
experimental breast cancer bone metastasis model. Clin Exp
Metastasis. 20:757–766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
O’Farrell AM, Abrams TJ, Yuen HA, et al:
SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent
activity in vitro and in vivo. Blood. 101:3597–3605.
2003.PubMed/NCBI
|
|
29.
|
Schueneman AJ, Himmelfarb E, Geng L, et
al: SU11248 maintenance therapy prevents tumor regrowth after
fractionated irradiation of murine tumor models. Cancer Res.
63:4009–4016. 2003.PubMed/NCBI
|
|
30.
|
Joensuu H, Trent JC and Reichardt P:
Practical management of tyrosine kinase inhibitor-associated side
effects in GIST. Cancer Treat Rev Feb. 37:75–88. 2011. View Article : Google Scholar : PubMed/NCBI
|