Introduction
Angiogenesis is one of the eight hallmarks of cancer
which are gained during the multistep development of human tumors
(1). Similar to normal tissues,
tumors require sustenance in the form of nutrients and oxygen as
well as an ability to evacuate metabolic wastes and carbon dioxide.
The tumor-associated neovasculature, generated by the process of
angiogenesis, addresses these requirements. Tumors are known to
achieve vasculature by endothelial cell sprouting (2), cooption of preexisting vessels
(3–6), intussusceptive microvascular growth
(7,8), postnatal vasculogenesis (9), glomeruloid angiogenesis (10) or vasculogenic mimicry (11,12).
Vascular endothelial growth factor (VEGF) is well-established as a
key mediator in some or all of these processes. Furthermore, VEGF
is the only angiogenic factor known to be present throughout the
entire tumor lifecycle (13,14).
Based on this evidence, VEGF is considered as a rational target for
antiangiogenic drug development (14–16).
Since anti-VEGF approaches act by blocking tumor-associated
angiogenesis, which appears to be widely required by a number of
different types of tumor, these approaches have been shown to be
useful against a wide variety of solid tumors (16,17).
FP3 (KH902/KH903) is an engineered protein which
contains the extracellular domain 2 of VEGF receptor 1 (Flt-1) and
the extracellular domains 3 and 4 of VEGF receptor 2 (Flk-1, KDR),
fused to the Fc portion of human immunoglobulin G1 (16,18).
Recent studies have indicated that FP3 showed promise as a local
antiangiogenic treatment of human choroidal neovascularization
(CNV) caused by age-related macular degeneration (AMD) (16,19–21).
In subsequent studies, it was demonstrated that FP3 has an
inhibitory effect on VEGF-mediated proliferation and migration of
human umbilical vein endothelial cells, and on the VEGF-mediated
vessel sprouting of rat aortic rings in vitro (18). FP3 also exhibits an antitumor effect
in a non-small cell lung cancer cell line (A549) xenograft model in
nude mice (18). However, little is
known of the effects of FP3 on tumor vessels.
Measurement of microvascular density is one of the
most common microscopic methods used to quantify angiogenesis in
tumors, as performed in a previous study (18). However, it is not always an accurate
measure of efficacy as tumor mass may decrease in parallel with the
number of blood vessels (22).
Tumor burden, another standard endpoint, provides limited insight
into whether drugs act on blood vessels or tumor cells, and may not
reveal whether tumor growth is stabilized by angiogenesis
inhibition. Thus, new methods are required for evaluating the
vascular effects of FP3.
In the present study, we examined the cellular
effects of FP3 on blood vessels, mainly focusing on the endothelial
cells and pericytes of tumor vessels in a patient-derived tumor
tissue (PDTT) xenograft model of gastric carcinoma, using large
tumors with established vasculature. A fluorescent microscopic
approach was applied to reveal the cellular morphologic changes of
endothelial cells and pericytes of tumor vessels following
administration with FP3.
Materials and methods
Patients and tissue samples
Tumor samples were obtained at initial surgery from
a 57-year-old male patient with gastric carcinoma. Prior written
informed consent was obtained from the patient and the study
received ethics board approval from the First Affiliated Hospital,
College of Medicine, Zhejiang University, China. The patient had
not received chemotherapy or radiation therapy prior to surgery.
The histological type was determined according to WHO criteria. The
metastatic tumor was diagnosed as diffuse infiltrative signet ring
cell carcinoma. The tumor sample was placed into RPMI-1640 medium
immediately following surgical resection under sterile conditions,
and transported without delay to the animal facility.
Reagents and drugs
The antibody against platelet endothelial cell
adhesion molecule-1 (PECAM-1, CD31; rat monoclonal, clone MEC 13.3)
was purchased from BD Pharmingen (San Diego, CA, USA). The antibody
against α-smooth muscle actin (α-SMA, rabbit polyclonal) was
purchased from Abcam (Cambridge, UK). The fluorescent (Cy3- or
FITC-conjugated) secondary antibodies (goat anti-rat or goat
anti-rabbit) were purchased from Jackson ImmunoResearch (West
Grove, PA, USA). The RPMI-1640 medium, fetal bovine serum (FBS),
penicillin and streptomycin were purchased from Gibco (Grand
Island, NY, USA). Isofluorane, diethyl ether, ketamine, xylazine,
paraformaldehyde and bovine serum albumin (BSA) were purchased from
Sigma (St. Louis, MO, USA). Bevacizumab (Avastin) was purchased
from Roche, Inc. (Roche, South San Francisco, CA, USA). FP3 was
kindly provided as a gift from Kanghong Biotechnology Inc.
(Konghong, Chengdu, China).
Establishment of PDTT xenograft model of
gastric carcinoma
Four- to six-week-old female BALB/c nude mice were
purchased from Slaccas (Shanghai Laboratory Animal Center,
Shanghai, China). The mice were housed in a barrier facility and
acclimatized to 12-h light-dark cycles for at least three days
prior to use. The use of experimental animals adhered to the
‘Principles of Laboratory Animal Care’ (NIH publication no. 85–23,
revised in 1985). Experiments were approved by the Institutional
Animal Care and Use Committee of Zhejiang University [approval ID:
SYXK(ZHE)2005-0072]. A PDTT xenograft model of gastric carcinoma
was established for this study, as described in a previous study
(23–26).
Treatments
Xenografts from the second mouse-to-mouse passage
were allowed to grow to a size of 100–150 mm3, at which
time (10 days after xenograft) mice were randomized into 3 cohorts.
In cohort 1, 30 mice were divided into 3 groups of 10 mice; i)
control (100 μl saline, intravenously (i.v.), twice per week); ii)
bevacizumab (10 mg/kg, i.v., twice per week); iii) FP3 (15 mg/kg,
i.v., twice per week). Mice were treated during the following 25
days. Tumor size was evaluated every four days by caliper
measurements using the formula: tumor volume = (length ×
width2)/2. Experiments were terminated on day 34. In
cohort 2, 4 groups of female athymic nude mice (n=40) were
implanted with tumors for a further 3 weeks. One group (n=10) was
sacrificed for evaluation without treatment, the second group
(n=10) was treated with FP3 (15 mg/kg body weight) twice per week
for 3 weeks, and the third group (n=10) was treated with FP3 (15
mg/kg body weight) twice per week for 3 weeks. The treatment was
stopped for a 2 week period of recovery (total 5 weeks before
experiments were terminated). The fourth group (n=10) was treated
with FP3 (15 mg/kg body weight) twice per week for 3 weeks, then
stopped for a recovery of 2 weeks followed by 2 weeks of further
treatment (3 and 2 weeks of treatment, total 7 weeks of experiment
duration). In cohort 3, 3 groups of female athymic nude mice (n=30,
10 mice per group) were implanted with tumors for a further 3
weeks. One group (day 0) was sacrificed for vascular morphology
evaluation without treatment, whereas each of the other 2 groups
was treated with a single dose of FP3 (15 mg/kg), and sacrificed
for evaluation on the third day (day 2) and the fifth day (day 4)
following treatment.
Fixation by vascular perfusion
Selected mice were anesthetized with ketamine (87
mg/kg) plus xylazine (13 mg/kg), which was injected
intramuscularly. The chest was rapidly opened, and the vasculature
was perfused for 3 min at a pressure of 120 mmHg with fixative [4%
paraformaldehyde in 0.1 mol/l phosphate-buffered saline (PBS), pH
7.4] from an 18-gauge cannula, which was inserted into the aorta
via an incision in the left ventricle. Blood and fixative exited
through an opening in the right atrium. Following perfusion, the
implanted tumor was removed and placed into fixative for 2 h at
4°C. Samples were then rinsed several times with PBS, infiltrated
overnight with 30% sucrose, embedded in optimal cutting temperature
(OCT) medium, and frozen for cryostat sectioning (27).
Immunohistochemistry
Cryostat sections (8 to 10 μm) were brought to room
temperature, air dried overnight and fixed in acetone for 10 min.
Slides were allowed to air dry for 30 min, and were washed 3 times
for 5 min each in PBS. Samples were then incubated in 5% BSA in PBS
for 30 min at room temperature to block non-specific antibody
binding. The sections were then incubated overnight at room
temperature in humidified chambers in combinations of two primary
antibodies (CD31, 1:40; and α-SMA, 1:200) diluted in PBS. After
several rinses with PBS, specimens were incubated for 1 h at room
temperature with fluorescent (Cy3- or FITC-conjugated) secondary
antibodies (goat anti-rat or goat anti-rabbit) diluted (1:200) in
PBS. Specimens were rinsed again with PBS, and mounted in
Vectashield mounting medium (Vector Laboratories, Burlingame, CA,
USA) (28,29). Tissue sections were then examined
and images were digitally captured using a Zeiss Axiophot
fluorescence microscope (Carl Zeiss, Thornwood, NY, USA), equipped
with single, dual and triple fluorescence filters and a low-light,
externally cooled, three-chip charge-coupled device (CCD) camera
(480×640 pixel RGB-color images, CoolCam; SciMeasure Analytical
Systems, Atlanta, GA, USA), and saved as TIFF files.
Statistical analysis
Data were presented as the mean ± SEM and analyzed
by SPSS 16.0 software. Differences among the means of the groups
were determined using one-way ANOVA tests. P<0.05 is considered
to indicate a statistically significant difference.
Results
FP3 markedly blocks gastric carcinoma
growth in vivo
We examined the effect of FP3 on the growth of the
established PDTT xenograft model of gastric carcinoma. Following
implantation, tumors were allowed to grow for 10 days, forming
large retroperitoneal tumors of >100 mm3. Injections
of FP3 (15 mg/kg body weight), Avastin (10 mg/kg body weight) or
saline were then administered i.v. twice per week for 25 days,
after which the animals were sacrificed, and the tumors were
excised and measured. FP3 significantly inhibited the growth of
tumors (Fig. 1). The effects of
Avastin were evaluated for comparison.
FP3 results in regression of tumor
vasculature
To evaluate the effects of FP3 on tumor-associated
angiogenesis, the tumors from the abovementioned study on cohort 1
mice were sectioned and immunostained with antibodies to CD31 and
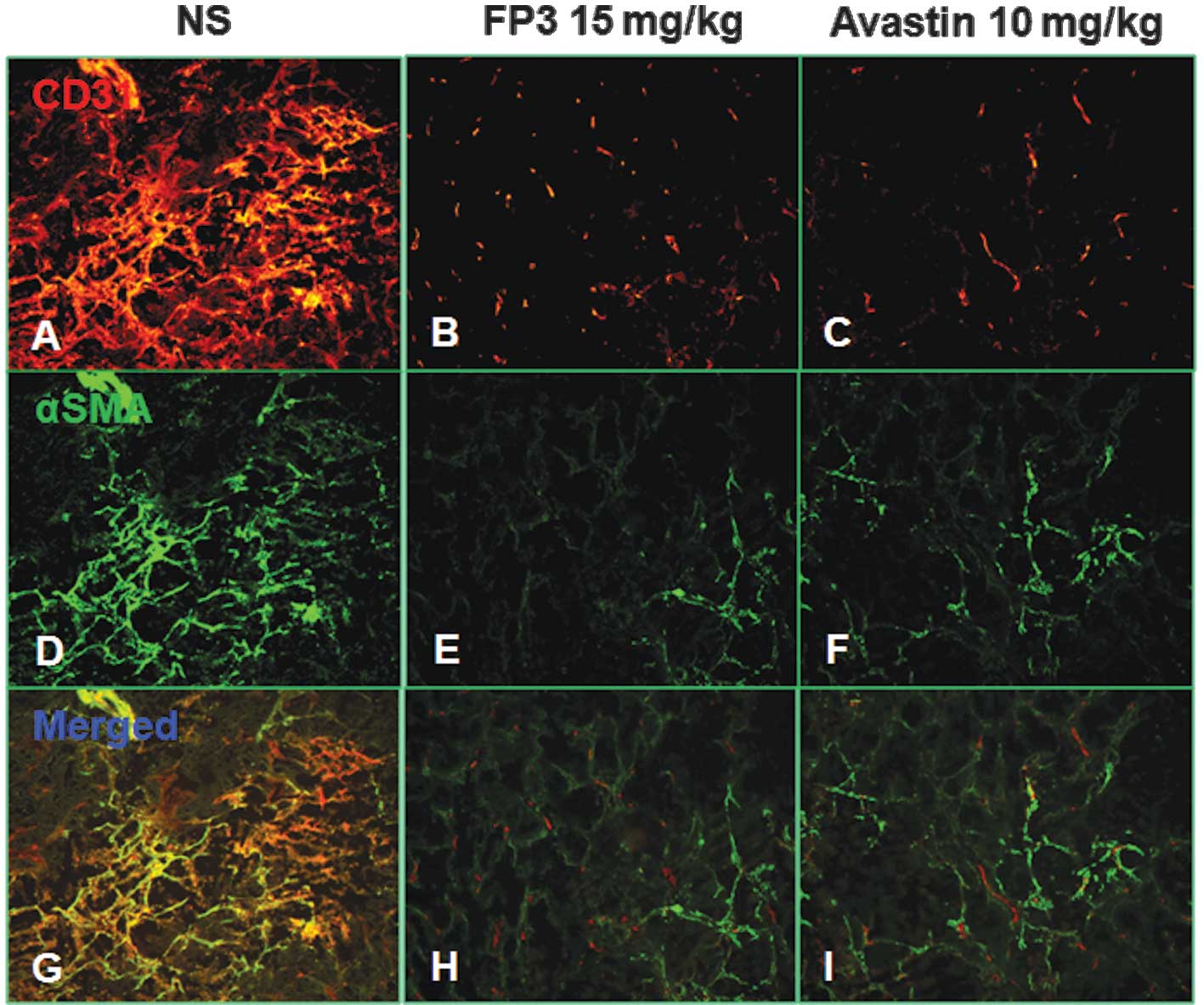
α-SMA, in order that the vasculature could be visualized (Fig. 2). This analysis revealed that
vasculature was almost absent in FP3-treated xenografts. FP3
(treatment for 25 days) almost completely blocked tumor-associated
angiogenesis, with the stunted tumors being largely avascular
(Fig. 2B,E and H). In contrast to
the FP3-treated tumors, control tumors in saline-treated mice were
not only much larger, but also had a high vascular density
(Fig. 2A,D and G). These results
indicated that FP3 administration reduces xenograft size and
concurrently causes decreased microvessel sprouting.
FP3 inhibits new and recurrent vessel
growth
Inhibition of VEGF signaling by FP3 blocks
angiogenesis and reduces tumor vascularity. However, little is
known regarding the events after treatment ends. We subsequently
evaluated the effects of FP3 on the growth of new and recurrent
tumor vessels (Fig. 3). Blood
vessels in untreated tumors were abundant, tortuous and variable in
diameter (Fig. 3A,E and I). The
vascularity of the tumors was conspicuously reduced following
treatment with FP3 for 3 weeks (Fig.
3B,F and J), but were as abundant as before the treatment after
withdrawal of FP3 for 2 weeks (Fig.
3C,G and K). However, after treatment with FP3 for another 2
weeks (3+2 weeks, treatment lasted 3 weeks, then stopped for a
recovery of 2 weeks followed by 2 weeks of further treatment),
tumor vessels were less tortuous, more uniform in caliber, and had
fewer branches and sprouts (Fig. 3D,H
and L).
Following treatments with FP3 for 3 weeks and 5
weeks (3+2 weeks, treatment lasted 3 weeks, then stopped for a
recovery of 2 weeks followed 2 weeks of treatment further), the
majority of surviving vessels in tumors did not have sprouts
(Fig. 3B,D,F,H,J and L).
Endothelial sprouts, appearing as cell protrusions tipped by
filopodia, were abundant on blood vessels in untreated tumors as
well as in tumors treated with FP3 for 3 weeks and stopped for a
recovery of 2 weeks (Fig. 3A,C,E,G,I
and K). These results showed that FP3 has a marked
antiangiogenic effect on preexisting or newly formed vessels.
FP3 normalizes existing tumor
vasculature
To determine whether the abovementioned results were
a direct effect of FP3 on tumor vessels, we examined its effect in
a short-term assay using large tumors with established vasculature.
The effect of VEGF inhibition on microvessel density, pericyte
coverage and tumor vessel phenotype on the third day (day 2) and
fifth days (day 4) following the administration of a single dose of
FP3 (15 mg/kg) to mice with established PDTT xenograft was
examined. Tumors from selected mice, which were sacrificed at each
of these times, demonstrated a significant, rapid and progressive
decrease in tumor microvessel density, as assessed by CD31
immunohistochemistry (Fig.
4A-C).
Serial tumor sections were also stained for α-SMA to
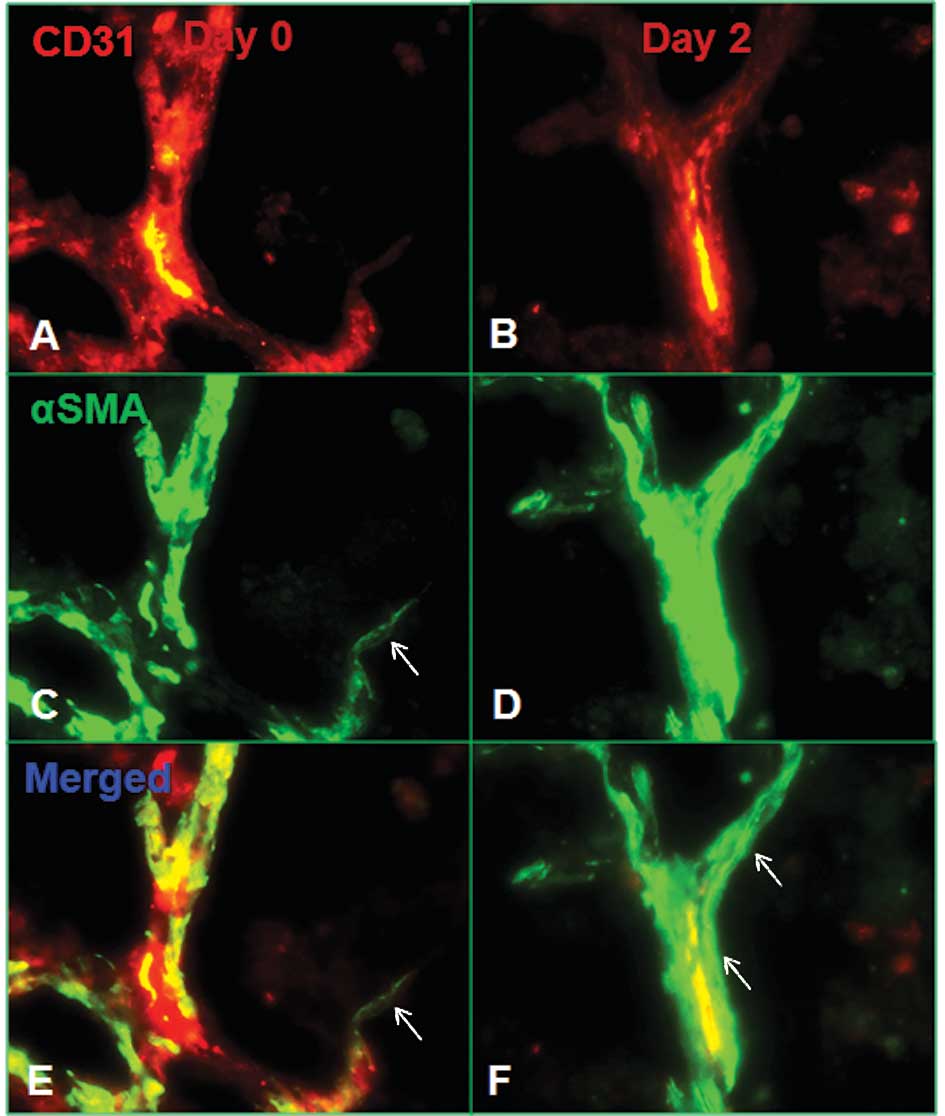
identify perivascular smooth muscle cells. Unlike the marked loss
of endothelial cells in treated tumors, the number of pericytes
remained stable after FP3 treatment (Fig. 4E and F). This observation suggests
that there was a selective loss of the more immature vessels that
lacked pericytes, whereas the more mature vessels remained. One
population of pericytes became closely associated with surviving
vessels (Fig. 5). These pericytes
were more tightly apposed to the endothelial cells compared to the
untreated tumors (Fig. 5). Unlike
the majority of pericytes in untreated tumors, some were oriented
circumferentially around the vessels (Fig. 5B,D and F), resembling smooth muscle
cells on arterioles. Another population of α-SMA-positive cells had
no apparent association with blood vessels. A number of of these
cells did not colocalize with the surviving CD31-positive blood
vessels (Fig. 5A,C and E). As
evidence of the greater impact of treatment on endothelial cells
compared to pericytes, the reduction in CD31 immunoreactivity was
greater than that in α-SMA immunoreactivity. These results indicate
that during the treatment period, tumor microvessel density
decreased and pericyte coverage of vessels increased, confirming
the hypothesis of vessel normalization. This ‘normalized’
vasculature is characterized by less leaky, less dilated and less
tortuous vessels, with a greater coverage by pericytes.
Discussion
FP3 is a humanized fusion protein that combines
ligand binding elements from the extracellular domains of VEGF
receptors 1 and 2 and the Fc portion of IgG1, and is able to bind
to all types of VEGF-A (16,18). A
previous study demonstrated that FP3 significantly blocked the
growth of a non-small cell lung cancer cell line (A549) tumor in a
subcutaneous xenograft model, and markedly decreased the vessel
density of the tumor (18). These
results were further confirmed in the present study in a PDTT
xenograft model of gastric carcinoma (Figs. 1 and 2). These preclinical observations have
indicated that FP3 is capable of causing regression of existing
tumor vasculature and significant reductions in tumor vascular
volume and density.
Although existing vasculature is essential for tumor
survival, tumors are unable to grow and spread without the
generation of new vasculature (14). Our findings have shown that FP3
resulted in almost complete inhibition of new vessel growth, which
is required for tumor growth and metastasis. We also found that
withdrawal of FP3 resulted in the regrowth of tumor vessels.
However, this regrowth was inhibited again by further treatment of
FP3, indicating that FP3 may result in the ongoing inhibition of
recurrent tumor vessel growth. In terms of clinical significance,
inhibition of recurrent tumor vessel growth by FP3 may provide
continued disease control.
The common hypothesis that antiangiogenic therapy
eradicates tumor vasculature, thus depriving the tumor of oxygen
and nutrients necessary for survival, has been challenged by
certain studies. These studies suggest that treatment with
antiangiogenic agents is able to transiently reverse some of the
abnormalities of tumor vessels or ‘normalize’ the tumor vasculature
for a short period of time, thereby providing a window of
opportunity for improving drug delivery and enhancing sensitivity
to conventional chemotherapy and radiation treatment (17,22,30–32).
Based on our previous results and those of the present study, it is
hypothesized that FP3 also has the potential to ‘normalize’ the
tumor vasculature, which is unique to this class of agents. To
determine this event, we assessed the direct cell effects of FP3 on
tumor vessels focusing on the endothelial cells and pericytes, by
examining the effect of FP3 on a short-term assay using large
tumors with established vasculature. A PDTT xenograft model of
gastric carcinoma was used for this purpose. We focused on changes
occurring during the first 5 days of treatment of established
tumors to identify primary vascular effects of FP3. Using a
fluorescent microscopic approach, we found that FP3 significantly
altered tumor vasculature, causing a rapid, quantitatively
significant decrease in the number of microvessels in as short a
time as 2 days. However, pericytes did not degenerate to the same
extent as endothelial cells, and those on surviving tumor vessels
achieved an increasingly normal phenotype. Pericytes have multiple
abnormalities, including a loose association with endothelial
cells. Treatment with FP3 normalized the phenotype of specific
pericytes, as manifested by a tighter association with endothelial
cells of surviving vessels. Following treatment with FP3, fewer
pericytes regressed compared to endothelial cells. Numerous
surviving α-SMA-positive cells were not associated with tumor
vessels, presumably due to the fact that their endothelial cells
had degenerated (Figs. 4 and
5). Our results indicate that
blockage of VEGF signaling with FP3 passively prunes the immature
and leaky growing blood vessels of transplanted tumors in mice,
causing an increased proportion of mature, functional vessels, and
actively remodeling the remaining vasculature to an increasingly
normal state.
The unregulated nature of tumor angiogenesis leads
to the production of structurally and functionally abnormal
vasculature, characterised by a number of different features,
including increased vessel density, diameter, length and
tortuosity, abnormally high interstitial fluid pressure, and
increased vascular permeability (33,34).
These abnormalities prevent the effective delivery of therapy to
the tumor. For example, the entry of large molecules, including
chemotherapeutic agents, into the tumor would be impeded and
hypoxia results from an inconsistent oxygen supply within the
tumor, producing regions that would be resistant to radiotherapy
and certain cytotoxic agents. The abovementioned vessel
normalization effect of FP3 may help make tumor cells increasingly
sensitive to cytotoxic chemotherapy and maximize the effectiveness
of the overall cancer treatment strategy. In terms of clinical
significance, normalization of tumor vasculature by FP3 may also
maximize the efficacy of concomitant therapy.
In conclusion, this study demonstrated that FP3 has
a direct and rapid antiangiogenic effect in solid tumors. The
antiangiogenic effects of FP3 were demonstrated via the regression
of tumor vasculature, inhibition of new and recurrent vessel
growth, and normalization of existing tumor vasculature. Whether
these morphological changes may be accompanied by functional
changes (including decreased interstitial fluid pressure, increased
tumor oxygenation, and improved penetration of drugs in solid
tumors), and functional consequences with regard to the
intratumoral delivery and antitumor activity of adjuvant anticancer
agents when administered together with FP3, are unknown and should
be ascertained in future studies.
Acknowledgements
This study was supported by the State Key Basic
Research and Development Program of China (973 Program, Grant No.
2009CB521704), the National High-Tech Research and Development
Program of China (863 Program, Grant No. 2006AA02A245), the
National Natural Science Foundation of China (Grant No. 81000894),
the Zhejiang Provincial Science and Technology Project (Grants No.
2009C13021, 2011C23087), the Science Research Fund of Shaoxing
(Grants No. 2011D10013) and the Science Research Fund of Zhuji
(Grants No. 2011CC7874). The funding groups had no role in the
study design, data collection and analysis, decision to publish, or
preparation of the manuscript.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paku S and Paweletz N: First steps of
tumor-related angiogenesis. Lab Invest. 65:334–346. 1991.PubMed/NCBI
|
|
3
|
Holash J, Maisonpierre PC, Compton D,
Boland P, Alexander CR, Zagzag D, Yancopoulos GD and Wiegand SJ:
Vessel cooption, regression, and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Döme B, Paku S, Somlai B and Tímár J:
Vascularization of cutaneous melanoma involves vessel co-option and
has clinical significance. J Pathol. 197:355–362. 2002.PubMed/NCBI
|
|
5
|
Vermeulen PB, Colpaert C, Salgado R,
Royers R, Hellemans H, Van Den Heuvel E, Goovaerts G, Dirix LY and
Van Marck E: Liver metastases from colorectal adenocarcinomas grow
in three patterns with different angiogenesis and desmoplasia. J
Pathol. 195:336–342. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paku S, Kopper L and Nagy P: Development
of the vasculature in ‘pushing-type’ liver metastases of an
experimental colorectal cancer. Int J Cancer. 115:893–902.
2005.
|
|
7
|
Kurz H, Burri PH and Djonov VG:
Angiogenesis and vascular remodeling by intussusception: from form
to function. News Physiol Sci. 18:65–70. 2003.PubMed/NCBI
|
|
8
|
Osawa M, Masuda M, Kusano K and Fujiwara
K: Evidence for a role of platelet endothelial cell adhesion
molecule-1 in endothelial cell mechanosignal transduction: is it a
mechanoresponsive molecule? J Cell Biol. 158:773–785. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sundberg C, Nagy JA, Brown LF, Feng D,
Eckelhoefer IA, Manseau EJ, Dvorak AM and Dvorak HF: Glomeruloid
microvascular proliferation follows adenoviral vascular
permeability factor/vascular endothelial growth factor-164 gene
delivery. Am J Pathol. 158:1145–1160. 2001. View Article : Google Scholar
|
|
10
|
Asahara T and Kawamoto A: Endothelial
progenitor cells for postnatal vasculogenesis. Am J Physiol Cell
Physiol. 287:572–579. 2004. View Article : Google Scholar
|
|
11
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in
vitro: vasculogenic mimicry. Am J Pathol. 155:739–752. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hendrix MJ, Seftor EA, Hess AR and Seftor
RE: Vasculogenic mimicry and tumour-cell plasticity: lessons from
melanoma. Nat Rev Cancer. 3:411–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrara N: Vascular endothelial growth
factor: basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Teng LS, Jin KT, He KF, Wang HH, Cao J and
Yu DC: Advances in combination of antiangiogenic agents targeting
VEGF-binding and conventional chemotherapy and radiation for cancer
treatment. J Chin Med Assoc. 73:281–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teng LS, Jin KT, He KF, Zhang J, Wang HH
and Cao J: Clinical applications of VEGF-trap (aflibercept) in
cancer treatment. J Chin Med Assoc. 73:449–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin K, He K, Teng F, Li G, Wang H, Han N,
Xu Z, Cao J, Wu J, Yu D and Teng L: FP3: a novel VEGF blocker with
antiangiogenic effects in vitro and antitumour effects in vivo.
Clin Transl Oncol. 13:878–884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang M, Zhang J, Yan M, Li H, Yang C and
Yu D: Recombinant anti-vascular endothelial growth factor fusion
protein efficiently suppresses choridal neovasularization in
monkeys. Mol Vis. 14:37–49. 2008.
|
|
20
|
Zhang M, Yu D, Yang C, Xia Q, Li W, Liu B
and Li H: The pharmacology study of a new recombinant human VEGF
receptor-fc fusion protein on experimental choroidal
neovascularization. Pharm Res. 26:204–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Zhang J, Yan M, Luo D, Zhu W,
Kaiser PK and Yu DC; KH902 Phase 1 Study Group. A phase 1 study of
KH902, a vascular endothelial growth factor receptor decoy, for
exudative age-related macular degeneration. Ophthalmology.
118:672–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inai T, Mancuso M, Hashizume H, Baffert F,
Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G,
Yancopoulos GD and McDonald DM: Inhibition of vascular endothelial
growth factor (VEGF) signaling in cancer causes loss of endothelial
fenestrations, regression of tumor vessels, and appearance of
basement membrane ghosts. Am J Pathol. 165:35–52. 2004. View Article : Google Scholar
|
|
23
|
Jin K, Teng L, Shen Y, He K, Xu Z and Li
G: Patient-derived human tumour tissue xenografts in
immunodeficient mice: a systematic review. Clin Transl Oncol.
12:473–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin K, He K, Li G and Teng L: Personalized
cancer therapy using a patient-derived tumor tissue xenograft
model: a translational field worthy of exploring further? Pers Med.
7:597–606. 2010. View Article : Google Scholar
|
|
25
|
Jin K, He K, Teng F, Han N, Li G, Xu Z and
Teng L: Heterogeneity in primary tumors and corresponding
metastases: could it provide us with any hints to personalize
cancer therapy? Pers Med. 8:175–182. 2011. View Article : Google Scholar
|
|
26
|
Jin K, He K, Han N, Li G, Wang H, Xu Z,
Jiang H, Zhang J and Teng L: Establishment of a PDTT xenograft
model of gastric carcinoma and its application in personalized
therapeutic regimen selection. Hepatogastroenterology.
58:1814–1822. 2011.PubMed/NCBI
|
|
27
|
Mancuso MR, Davis R, Norberg SM, O'Brien
S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B,
Shalinsky DR, Hu-Lowe DD and McDonald DM: Rapid vascular regrowth
in tumors after reversal of VEGF inhibition. J Clin Invest.
116:2610–2621. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morikawa S, Baluk P, Kaidoh T, Haskell A,
Jain RK and McDonald DM: Abnormalities in pericytes on blood
vessels and endothelial sprouts in tumors. Am J Pathol.
160:985–1000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baluk P, Morikawa S, Haskell A, Mancuso M
and McDonald DM: Abnormalities of basement membrane on blood
vessels and endothelial sprouts in tumors. Am J Pathol.
163:1801–1815. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Willett CG, Boucher Y, Di Tomaso E, Duda
DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et
al: Direct evidence that the VEGF-specific antibody bevacizumab has
antivascular effects in human rectal cancer. Nat Med. 10:145–147.
2004. View Article : Google Scholar
|
|
31
|
Baffert F, Le T, Sennino B, Thurston G,
Kuo CJ, Hu-Lowe D and McDonald DM: Cellular changes in normal blood
capillaries undergoing regression after inhibition of VEGF
signaling. Am J Physiol Heart Circ Physiol. 290:H547–559. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Byrne AT, Ross L, Holash J, Nakanishi M,
Hu L, Hofmann JI, Yancopoulos GD and Jaffe RB: Vascular endothelial
growth factor-trap decreases tumor burden, inhibits ascites, and
causes dramatic vascular remodeling in an ovarian cancer model.
Clin Cancer Res. 9:5721–5728. 2003.
|
|
33
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: a new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|