Introduction
Primary testicular non-Hodgkin’s lymphoma (TNHL) was
first described as a clinical entity in 1866 (1,2). It is
a rare disease which accounts for 1% of all non-Hodgkin’s lymphoma
(NHL) cases, 2% of all extranodal lymphomas and 5% of all
testicular neoplasms (3). It is the
most common type of testicular tumor in males between 60 and 80
years of age. TNHL is unique in its high incidence of bilateral
involvement (8–38%), and it is also the most common bilateral
testicular tumor. TNHL has a predilection for spreading to
non-contiguous extranodal sites, particularly the central nervous
system (CNS) (1,2,4–7).
Hemophagocytic syndrome (HPS) also known as hemophagocytic
lymphohistiocytosis (HLH) is characterized by the impaired or
absent activity of natural killer (NK) cells and cytotoxic T-cells
leading to cytokine dysregulation with proliferation and activation
of histiocytes (8). HPS is
characterized by fever, pancytopenia, liver dysfunction and
hemophagocytosis in the bone marrow (9). HPS is divided into two types: primary
familial and secondary. Secondary type is often associated with
infection, autoimmune disease and malignancies.
In the present study, we report on a 47-year-old man
with primary TNHL who developed HPS 4 months after occurrence of
scrotal swelling. To the best of our knowledge, primary TNHL
associated with HPS has not been previously reported.
The search engine PubMed was used to search the
database Medline of the National Library of Medicine from 1966 to
the present. The search engine Science Direct was used to search
EMBASE from 1974 to November 2011. The search engine Web of Science
was used to search the Science Citation Index from 1980 to November
2011. Earlier sources were obtained by cross referencing. Subject
searches were conducted for hemophagocytosis, hemophagocytic
syndrome, hemophagocytic lymphohistiocytosis, primary testicular
non-Hodgkin’s lymphoma and testicular natural killer/T-cell
lymphoma. Studies considered to be of clinical importance and
additional references of key articles were included.
The study was approved by the ethics committee of
the university. Patient consent was also obtained.
Case report
A 47-year-old man presented with a 4-month history
of bilateral scrotal swelling, prolonged fever, weakness and night
sweats. The patient received antibiotics (trimethoprim and
sulfamethoxazole) for suspected orchitis and was admitted to
hospital due to pancytopenia and 15 kg weight loss.
Physical examination revealed a thin, chronically
ill-appearing Chinese male. Vital signs were as follows:
temperature, 37.7°C; pulse, 95 beats per min; respiratory rate, 20
breaths per min and blood pressure, 85/60 mmHg. No scleral icterus
was present. The patient’s abdomen and the upper right arm had an
approximately 2×1 cm red rash, respectively, which faded when
pressed. The oral cavity had good dentition without evidence of
thrush. No neck masses or lymphadenopathies were palpable. The
enlargement of the spleen was palpable under 6 cm of the left
costal margin.
Hepatomegaly was not noted. No lower extremity edema
was present. Neurological examination revealed normal cranial nerve
function, reflexes, speech, mental status and gait. No focal
weakness or sensory loss was present. Urological examination
revealed bilateral hard testicular masses. The patient was
otherwise in good health with no significant findings from medical
history. Family history was non-contributory.
Blood routine examination revealed severe
pancytopenia: hemoglobin was 93 g/dl, white blood cell count was
2.2×109/liter and platelet count was
28×109/liter. Routine urianlysis and stool routine
examination were normal. Serum albumin was decreased to 27.5 g/l.
Activated partial thromboplastin time was prolonged to 75.4 sec and
the concentration of fibrinogen was decreased to 0.55 g/l.
Laboratory examinations on admission revealed highly elevated
values of ferritin (957 ng/ml), lactate dehydrogenase (LDH; 1303
U/l), serum aspartate aminotransferase (AST; 207 U/l) and alanine
aminotransferase (ALT; 82 U/l), suggesting hematological
malignancies, malignant tumor of fibrous connective tissue and
severe infection. Therefore, an extensive rheumatologic and
infectious investigation was performed during hospitalization.
Rheumatologic work-up, including antinuclear antibodies,
anti-double-stranded DNA antibodies, complement C3 and C4 levels,
antimitochondrial antibodies, antiscleroderma-70 antibodies,
anti-Ro/SS-A and anti-La/SS-B antibodies, were all within normal
limits.
Infectious work-up included Mycoplasma
pneumoniae IgG and IgM levels, Herpes simplex virus IgG and IgM
levels, Toxoplasma gondii IgG and IgM levels, rubella IgG
and IgM levels, cytomegalovirus IgG and IgM levels, Epstein-Barr
virus antibodies and an hepatitis profile. Results from the
infectious disease laboratories were within normal limits. Other
laboratory tests performed, including multiple blood cultures,
international normalized ratio, erythrocyte sedimentation rate and
immunoglobulin levels (IgA, IgM, IgG and IgE), were also within
normal limits. Antibodies to HIV1/2 and antibody to Treponema
pallidum were negative. The β human chorionic gonadotropin
(HCG) and α-fetoprotein (AFP) levels were within normal limits.
Ultrasonography revealed bilateral testicular
enlargement (56 cc right testis, 36 cc left testis) and multifocal,
intensely hypoechoic areas, which demonstrated enhanced flow
velocity following Color Doppler sonography. A computed tomography
scan of the chest, abdomen and pelvis demonstrated lung infection
of the left inferior and right middle lobe, adiposis hepatica,
splenomegaly and bilateral testicular enlargement; however, no
lymph node enlargement was found (Fig.
1).
Contrast-enhanced MRI of the brain revealed no
abnormalities except for paranasal sinusitis. A bone marrow biopsy
and aspiration were performed. The biopsy results demonstrated the
following: bone marrow active proliferation; reduction in the
cellularity in all lineages; granulocytic series accounting for
50%, with relatively normal morphology; erythrocytic series
accounting for 30%, with intermediate erythroblasts and largely
acidophilic normoblasts, but no abnormal morphology; lymphocytic
series accounting for 17%; megakaryocyte with normal distribution,
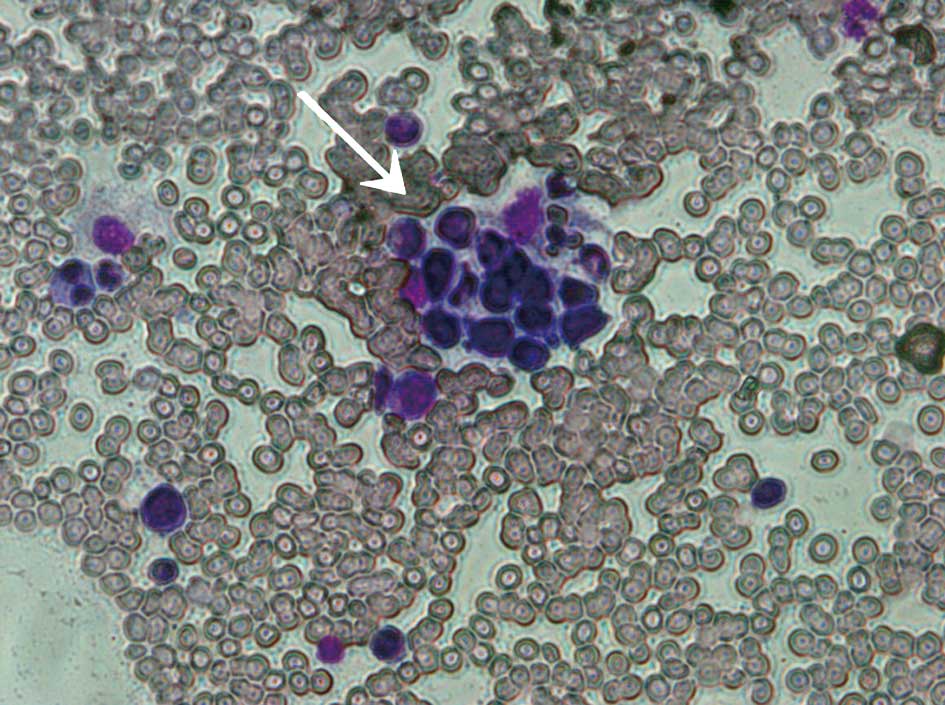
scattered piles of platelets; large platelets and hemophagocytosis
(Fig. 2).
According to the presented symptoms, laboratory
examinations and a bone marrow cell examination, the patient was
diagnosed with HPS. No other hereditary or acquired diseases
related to HPS were found. As the severe pancytopenia persisted,
regular substitution of erythrocytes, γ globulins, albumin and
platelets was required. Since the bilateral testicular tumor was
the only lesion potentially leading to HPS, a bilateral orchiectomy
was performed. Histological sections revealed small to medium-sized
neoplastic cells, typical heteromorphism and prominent necrosis
infiltrating and destroying seminiferous tubules (Fig. 3). The immunohistochemical staining
of paraffin-embedded sections demonstrated reactivity for LCA, Vim,
CD45RO, TIA-1, CD43, CD56 focal, IgG and Ki-67. The results of
staining for CD10, CD79a, CD3, CD5, CD1a, CD30, CD138, CD38, CD20,
TdT, MoPo, Mum-1, Ck, EMA and PLAP were negative. Staining for
CD45RB and IgA was positive (Fig.
4). The diagnosis was bilateral testicular non-Hodgkin’s
malignant lymphoma, as with NK/T-cell lymphoma.
Despite bilateral orchiectomy, the patient’s
clinical condition rapidly deteriorated with persistent fever and
severe pancytopenia. Given the extremely poor prognosis, the
patient refused chemotherapy and was discharged and transferred to
the local hospital, where the patient’s clinical condition
deteriorated with palliative supportive care. The patient succumbed
to acute multiple organ failure 2 weeks later.
Discussion
To the best of our knowledge, this is the first
report of a case in which HPS was caused by a primary testicular
non-Hodgkin’s malignant lymphoma, as with NK/T-cell lymphoma. TNHL
has a predilection for dissemination to non-contiguous extranodal
sites, including the CNS, Waldeyer’s ring, skin and lungs (1,5,10,11).
According to the Working Formulation of the United States National
Cancer Institute, approximately 68% of TNHL cases are classified as
intermediate grade, diffuse large B-cell subtype, followed by
high-grade, diffuse small non-cleaved subtype in approximately 30%
of patients (1,11). There is no prognostic advantage for
any pathological subtype (11).
Immunohistochemistry (IHC) studies confirm the majority of TNHL
cases to be of B-cell origin, with less occurrence of T-cell
lymphoma (1,12).
Extra-nasal NK/T-cell lymphomas, including the
testicular lymphoma detected in our patient, are rare. The most
common NK/T-cell lymphomas are nasal or nasal-type. Although
primary extranasal tumors occur, testicular lesions are
particularly uncommon, whether as primary tumors or as part of
multifocal disease presentation. NK/T-cell lymphomas with
testicular involvement share a number of clinicopathological
features with NK/T-cell lymphomas of other extranasal sites,
including a tendency to occur in individuals of Asian, Mexican and
South American origin and a notably poor clinical prognosis
(13,14). Almost all patients whose lesions
appeared to be limited to the testes relapsed within 6 months and
succumbed to widespread dissemination of the skin, CNS,
gastrointestinal (GI) tract or lung within 1 year, regardless of
treatment (15,16). Ornstein et al demonstrated
that in contrast to testicular B-cell lymphoma, primary testicular
NK/T-cell lymphoma occurred in younger males; the average age at
presentation for a solitary testicular lesion was 44 years, and 60%
of cases were diagnosed in patients younger than 40 years (17). In our case report, the patient also
had a rapidly progressive, fatal course, consistent with findings
from previous studies.
Concurrent occurrence or association with HPS may be
part of the reason for the fulminant course. Viral infection is the
most frequent trigger of secondary HPS. Malignancy is one of the
major causes of HPS, and lymphoproliferative disease is the main
type of malignancy associated with HPS (18). T-cell or B-cell lymphoma with HPS
can occur in immunocompromised patients, including individuals with
HIV infection and recipients of organ or bone marrow transplants,
as well as in otherwise immunocompetent populations (19). Notably, HPS is frequently reported
among patients who present with virus-induced lymphoproliferative
disorders, including EBV-driven B-cell lymphoma or human Herpes
virus-8-associated disease (20).
HPS is, therefore, able to complicate the course of T-cell
lymphoma, Hodgkin’s disease, NK-cell leukemia, myeloproliferative
disorders and acute leukemia (21).
HPS is a highly fatal disease if untreated. Since HPS is rare, no
randomized controlled clinical trials examining potential
treatments have been conducted. The immediate aim of therapy is
suppression of the increased inflammatory response and control of
cell proliferation using immunosuppressive or immunomodulatory
agents and cytotoxic drugs. Urologists require awareness of the
occurrence of HPS in testicular tumor patients with persistent
fever, organomegaly and cytopenia. Uropathologists should be aware
of this rare entity which may only be diagnosed after extensive
immunohistochemical studies.
To obtain a pathological diagnosis, bone marrow cell
examination and orchiectomy should be performed as soon as
possible. Management of HPS relies on early diagnosis,
identification of a triggering pathogen or an underlying disease,
and control of the lymphocyte/macrophage proliferation and
activation. Specific antimicrobial therapy can be beneficial in
selected cases. Severe cases are treated with chemotherapy,
generally an etoposide-containing regimen, and bone marrow
transplantation is the treatment for familial, severe and
persistent non-familial cases.
References
|
1
|
Shahab N and Doll DC: Testicular lymphoma.
Semin Oncol. 26:259–269. 1999.
|
|
2
|
Ballen KK and Hasserjian RP: Case records
of the Massachusetts General Hospital. Weekly clinicopathological
exercises Case 15-2004 A 31-year-old man with bilateral testicular
enlargement. N Engl J Med. 350:2081–2087. 2004.
|
|
3
|
Touroutoglou N, Dimopoulos MA, Younes A,
et al: Testicular lymphoma: late relapses and poor outcome despite
doxorubicin-based therapy. J Clin Oncol. 13:1361–1367.
1995.PubMed/NCBI
|
|
4
|
Eskey CJ, Whitman GJ and Chew FS:
Malignant lymphoma of the testis. AJR Am J Roentgenol. 169:8221997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buskirk SJ, Evans RG, Banks PM, et al:
Primary lymphoma of the testis. Int J Radiat Oncol Biol Phys.
8:1699–1703. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lobo FD, Bansal R, Naik R, et al: Primary
testicular lymphoma. J Indian Med Assoc. 96:193–194. 1998.
|
|
7
|
Sussman EB, Hajdu SI, Lieberman PH and
Whitmore WF: Malignant lymphoma of the testis: a clinicopathologic
study of 37 cases. J Urol. 118:1004–1007. 1977.PubMed/NCBI
|
|
8
|
Rouphael NG, Talati NJ, Vaughan C, et al:
Infections associated with haemophagocytic syndrome. Lancet Infect
Dis. 7:814–822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janka GE: Familial and acquired
hemophagocytic lymphohistocytosis. Eur J Pediatr. 166:95–109. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lagrange JL, Ramaioli A, Theodore CH, et
al: Non-Hodgkin’s lymphoma of the testis: a retrospective study of
84 patients treated in the French anticancer centres. Ann Oncol.
12:1313–1319. 2001.
|
|
11
|
Tepperman BS, Gospodarowicz MK, Bush RS
and Brown TC: Non-Hodgkin lymphoma of the testis. Radiology.
142:203–208. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moller MB, D’Amore F and Christensen BE:
Testicular lymphoma: a population-based study of incidence,
clinicopathological correlations and prognosis. The Danish Lymphoma
Study Group, LYFO. Eur J Cancer. 30A:1760–1764. 1994. View Article : Google Scholar
|
|
13
|
Ko Y, Cho E-Y, Kim J-E, et al: NK and
NK-like T-cell lymphoma in extranasal sites: a comparative
clinicopathological study according to site and EBV status.
Histopathology. 44:480–489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan J, Sin V, Wong K, et al: Nonnasal
lymphoma expressing the natural killer cell marker CD56: a
clinicopathologic study of 49 cases of an uncommon aggressive
neoplasm. Blood. 89:4501–4513. 1997.PubMed/NCBI
|
|
15
|
Cheung MM, Chan JK, Lau WH, et al: Primary
non-Hodgkin’s lymphoma of the nose and nasopharynx: clinical
features, tumor immunophenotype, and treatment outcome in 113
patients. J Clin Oncol. 16:70–77. 1998.
|
|
16
|
Falini B, Pileri S, De Solas I, et al:
Peripheral T-cell lymphoma associated with hemophagocytic syndrome.
Blood. 75:434–444. 1990.PubMed/NCBI
|
|
17
|
Ornstein DL, Bifulco CB, Braddock DT and
Howe JG: Histopathologic and molecular aspects of CD56+
natural killer/T-cell lymphoma of the testis. Int J Surg Pathol.
16:291–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Janka GE: Hemophagocytic syndromes. Blood
Rev. 21:245–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han AR, Lee HR, Park BB, et al:
Lymphoma-associated hemophagocytic syndrome: clinical features and
treatment outcome. Ann Hematol. 86:493–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fardet L, Blum L, Kerob D, et al: HHV-8
associated hemophagocytic lymphohistiocytosis in HIV-infected
patients. Clin Infect Dis. 37:285–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chuang HC, Lay JD, Chuang SE, et al: EBV
latent membrane protein-1 down-regulates TNF-[alpha] receptor-1 and
confers resistance to TNF-[alpha]-induced apoptosis in T cells:
implication for the progression to T-cell lymphoma in
EBV-associated hemophagocytic syndrome. Am J Pathol. 170:1607–1617.
2007.PubMed/NCBI
|