Introduction
Acute myeloid leukaemia is a neoplastic disease of
blood stem cells that is characterised by the continuous clonal
proliferation of myeloid precursors in the absence of
differentiation or maturation into terminal cells (1). In spite of advances in leukaemia
treatment, survival rates remain low, with 5-year survival rates of
60 and 10% in young and elderly patients, respectively (2). Thus, several new strategies are
currently being developed (3).
Casein is the principal protein in milk and a
significant component of the human diet that also regulates the
proliferation and activation of blood cells. For example, β-casein,
a component of bovine casein, has been found to activate free
radical production in granulocytes and to induce lymphocyte
proliferation (4). Casein also acts
as an inflammatory agent that induces the migration of myeloid and
lymphoid cells into the peritoneal cavity (5). In a previous study, we showed that
casein inhibits the proliferation of the normal mouse myeloid cell
line 32D by inducing its differentiation into the
monocyte-macrophage lineage (6).
Additionally, casein has been shown to inhibit the proliferation of
several leukaemia cell lines (7).
An ideal therapeutic agent is one that selectively
targets and kills cancer cells with minimal toxicity to normal
tissues (8) and there are naturally
occurring molecules that fulfill this requirement (9). As casein is able to induce the
differentiation of the 32D cell line and inhibit leukaemia cells,
the current study was performed to determine whether casein is
capable of inducing the apoptosis of WEHI-3 and mononuclear bone
marrow cells in vitro and inducing antileukaemic effects
in vivo. The results of this study indicate that casein
induces the apoptosis of leukaemia cells without exerting a
cytotoxic effect on normal haematopoietic cells, thereby prolonging
the survival of leukaemic mice.
Materials and methods
Experimental animals
BALB/c female mice between 8 and 12 weeks of age
were used and maintained in pathogen-free conditions. Experiments
were carried out in the animal facility of Facultad de Estudios
Superiores Zaragoza, Universidad Nacional Autónoma de México, in
accordance with institutional guidelines. Mice were provided with
autoclaved water and fed a standard powdered rodent diet ad
libitum. All experimental protocols were approved by the ethics
committee of our institution in accordance with national and
international regulations for the care and use of experimental
animals.
Cell culture
The WEHI-3 murine myelomonocytic leukaemia cell line
was obtained from ATCC (Rockville, MD, USA). The cells were
cultured in Iscove’s modified Dulbecco’s medium (IMDM) (Gibco-BRL,
Carlsbad, CA, USA) supplemented with 10% foetal bovine serum (FBS)
(Gibco-BRL), 100 U/ml penicillin and 100 μg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA). The cells were maintained in a
humidified atmosphere containing 5% CO2 at 37°C and the
culture medium was changed every 2 days.
Total bone marrow cells of the mice were obtained
from the femur and flushed with IMDM supplemented with 10% FBS.
Mononuclear cells (MNCs) were obtained from the total cells via
gradient separation with Ficoll-Paque (Amersham Biosciences AB,
Uppsala, Sweden) at a density of 1.077 g/ml and washed twice with
phosphate-buffered saline (PBS). MNCs were cultured for 120 h in
IMDM supplemented with 15% (v/v) FBS, 5% (v/v) horse serum
(Gibco-BRL) and 5 ng/ml recombinant mouse interleukin-3 (rmIL-3)
(R&D System, Minneapolis, MN, USA). The cells were maintained
in a humidified atmosphere containing 5% CO2 at 37°C and
were maintained in culture for a maximum period of 120 h.
Casein
Sodium caseinate, used as source of casein,
(Spectrum, New Brunswick, NJ, USA) was dissolved in PBS at a
concentration of 100 mg/ml. Autoclaved dilutions were made with PBS
to achieve concentrations of 0.5, 1 and 2 mg/ml.
Proliferation assay
To evaluate cell proliferation, 3×103 and
750 WEHI-3 cells and 1×105 MNCs/ml were cultured for
either 72 or 120 h with rmIL-3 and a range of casein concentrations
(0, 0.5, 1 or 2 mg/ml) in 24-well plates (Corning Costar, St.
Louis, MO, USA). The cultures were then fixed with 1.1%
glutaraldehyde and stained with crystal violet in 0.1% formic acid
(Sigma-Aldrich). The dye was solubilised in 10% acetic acid and the
optical density at 570 nm was determined using a plate reader
(Tecan Spectra, Grödig, Austria).
Cell viability
Trypan blue exclusion assays were used to determine
the number of viable cells in each culture. The cells were
incubated in the presence or absence of 2 mg/ml of casein for 120
h. Cell viability was determined by direct counting in a Neubauer
chamber; cells excluding the stain were counted as viable. The
results were shown as the mean percentage of cell viability ±
standard deviation (SD) of triplicate cultures.
Cell metabolic activity was quantified using the
CellTiter 96® aqueous non-radioactive cell proliferation
(MTT) assay (Promega, Madison, WI, USA) according to the
manufacturer’s instructions. Briefly, following 120 h in the
presence or absence of 2 mg/ml casein, 20 μl of CellTiter 96
Aqueous One solution reagent was added to the plate, which was
incubated for 4 h. The absorbance at 490 nm was determined using a
plate reader (Tecan Spectra).
Assessment of DNA fragmentation by
agarose gel electrophoresis
WEHI-3 or MNCs cells (1×106) were lysed
in DNA lysis buffer (1% NPO4, 20 nM EDTA, 50 mM Tris, pH
7.9) at 47°C for 15 min. The lysate was then centrifuged at 13,000
rpm for 10 min at 47°C. The supernatant containing the fragmented
DNA was collected and incubated with 1% sodium dodecyl sulphate
(SDS) and 2.5 μg/ml proteinase K, followed by RNaseA-mediated RNA
digestion at 37°C for 30 min. Following extraction with
phenol/chloroform/isoamyl alcohol (25:24:1), the DNA was
precipitated in 50% isopropanol at −20°C overnight. The
precipitated DNA was centrifuged at 14,000 rpm for 30 min, dried
and dissolved in TE (pH 8.0) buffer. Following electrophoresis in a
1% agarose gel containing ethidium bromide in TAE buffer, the DNA
bands were observed with UV light.
Establishment of the mouse leukaemia
model
WEHI-3 cells were washed twice with PBS, counted
with trypan blue to confirm >95% viability and adjusted to
2.5×104 cells/ml. BALB/c mice were injected
intraperitoneally (i.p.) with WEHI-3 cells and monitored for
survival. The mice and their spleens, livers and tumour samples
were obtained and weighed individually upon mortality or at the end
of the 40 day period; respective indices were determined as the
ratio of tumour or organ weight to body weight.
Electroporation procedure
The presence of leukaemia cells in the bone marrow
was determined by the fluorescence microscopy of WEHI-3 cells
electroporated with the pEGFP-C1 vector (Clontech, Mountain View,
CA, USA). For the electroporation experiments, growing cell
cultures at 60% confluence were collected and washed in room
temperature IMDM-GlutaMAX-I (Gibco-BRL). Pre-chilled hypoosmolar
electroporation medium (0.8 ml) was added to the cell pellets
(5×106 cells/ml) either with or without 5 μg of pEGFP-C1
vector and the cells were electroporated at 250 V following a 5 min
pre-incubation in the medium. Eight minutes after pulsing, the
cells were gently transferred to 4 ml of IMDM-GlutaMAX-I
supplemented with 10% FBS at 37°C and maintained at 5%
CO2. The total time of cell exposure to the
electroporation medium did not exceed 17 min. The centrifuged cells
were electroporated with a multiporator (Eppendorf AG, Hamburg,
Germany) in disposable sterile electroporation cuvettes (0.8 ml)
with a 4-mm gap between embedded aluminium electrodes (Eppendorf
AG).
Antileukaemic activity in BALB/c
mice
Four groups of 10 BALB/c mice were used. One group
of mice was injected i.p. with 1 ml of WEHI-3 cells. Another group
was inoculated with WEHI-3 cells, with 10% casein in 1 ml PBS added
after 48 h, and again every 48 h, for 35 days. The third and fourth
groups were inoculated with 1 ml of PBS or casein, maintained as
controls and observed for survival during the 40 day time course.
At 3 weeks from the start of treatment, the mice were sacrificed
and tissues were weighed individually.
Statistical analysis
Individual experiments were carried out in
triplicate. The experiments were repeated three times and values
were shown graphically as the mean ± SD. One-way ANOVA was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant result. Statistical software (SPSS, Inc.,
Chicago, IL, USA) was used to perform the analyses.
Results
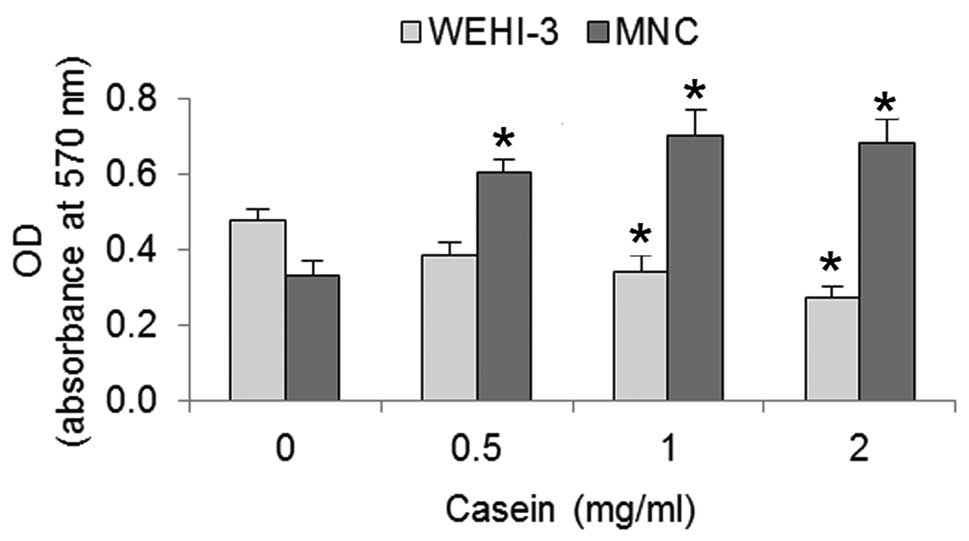
Casein inhibits the proliferation of
WEHI-3 cells and induces the proliferation of bone marrow MNCs
To homologise the culture conditions of the WEHI-3
cells and MNCs, we cultured 750 and 1×105 cells/ml,
respectively, with 5 ng/ml of rmIL-3 for 120 h in the presence or
absence of 0.5, 1 or 2 mg/ml casein. Using crystal violet, we
evaluated the number of cells present. The number of WEHI-3 cells
were decreased and the number of MNCs were increased as a function
of casein concentration (Fig. 1).
At 2 mg/ml casein, the inhibition of the WEHI-3 cells was >40%,
while the induced proliferation of the MNCs was >50% compared
with the control.
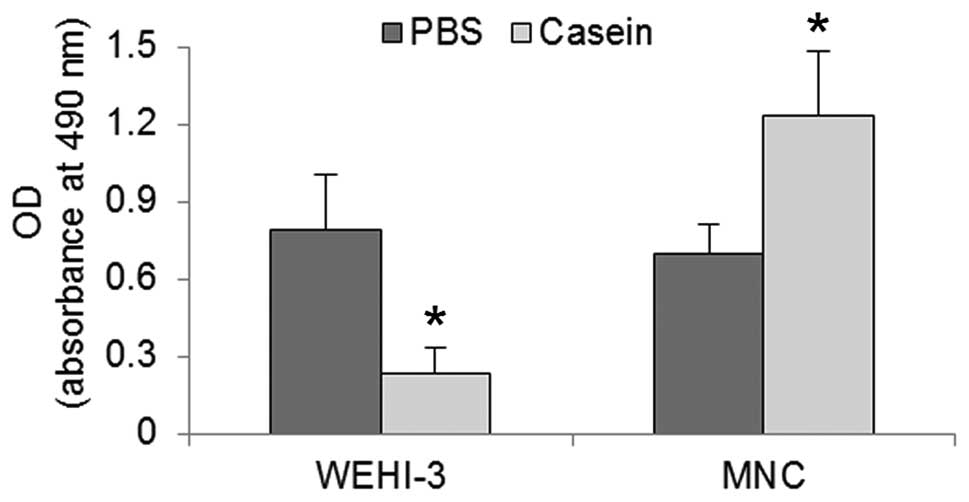
Casein reduced metabolic activity and
induced cell death in WEHI-3 cells, but did not impair the
viability of MNCs
Following the observation that casein inhibited the
proliferation of WEHI-3 cells and induced the proliferation of
MNCs, we evaluated the viability of those cells when cultured in
the presence of 2 mg/ml casein. Results of the trypan blue
exclusion assay revealed that the viability was <90% in the
WEHI-3 cells but was not reduced in the MNCs (Fig. 2). To evaluate the status of the two
cell types, we performed the MTT assay to determine their metabolic
activities. In response to the casein treatment, we observed that
WEHI-3 cells exhibited a 75% reduction in activity, while MNCs
showed an increase of their baseline rate by 50% relative to the
control (Fig. 3). The near-complete
loss of viability of WEHI-3 cells and the existence of significant
metabolic activity, indicated that cell death may have resulted
from an active cell mechanism.
Casein induced DNA fragmentation in
WEHI-3 cells but not in MNCs
To evaluate whether the casein-induced death in the
WEHI-3 cells resulted from an apoptotic mechanism, we examined the
fragmentation of DNA in those cells following a 120-h incubation
with 2 mg/ml casein. Our results showed that, for the WEHI-3 cells,
casein induced the typical apoptotic stair pattern in the agarose
gels; by contrast, this pattern was not observed for the MNCs
(Fig. 4). Therefore, our results
suggest that casein not only inhibits the proliferation, but also
induces the apoptosis, of WEHI-3 cells.
 | Figure 4DNA fragmentation, detected via
agarose gel electrophoresis, in WEHI-3 or MNC cultures in the
presence of 5 ng/ml rmIL-3. Lane 1, marker (bp); lane 2, WEHI-3
plus PBS; lane 3, WEHI-3 plus 2 mg/ml casein; lane 4, MNC plus PBS;
line 5, MNC plus 2 mg/ml casein. Results are representative of 2
separate experiments. bp, base pairs; MNC, mononuclear cell; PBS,
phosphate-buffered saline. |
Establishment of a BALB/c-WEHI-3
leukaemia model
Following the observation that casein was toxic to
leukaemia cells but promoted normal myeloid proliferation, we
developed a mouse leukaemia model to evaluate the antileukaemic
potential of casein. Our results showed that animals challenged
with WEHI-3 cells developed marked splenomegaly and hepatomegaly
and solid tumours (Table I),
resulting in the death of all experimental subjects within 30
days.
 | Table ITreatment of control or leukaemic mice
with and without casein treatment. |
Table I
Treatment of control or leukaemic mice
with and without casein treatment.
| PBS-1 | WEHI-3 |
|---|
|
|
|
|---|
| Index | PBS-2 | Casein | PBS-2 | Casein |
|---|
| Hepatic | 0.066±0.019 | 0.046±0.005 | 0.127±0.048a | 0.081±0.032a |
| Splenic | 0.004±0.0003 | 0.002±0.004 | 0.008±0.002a | 0.006±0.001a |
| Tumoural | ND | ND | 0.107±0.093a | 0.035±0.002a |
WEHI-3 cells infiltrated the bone marrow
of mice inoculated i.p. at 24 h
We determined whether WEHI-3 cells were able to
migrate out of the peritoneal cavity during the 48 h prior to
treatment with casein in our mouse leukaemia model. Therefore, we
evaluated the simultaneous injection of cells transiently
transfected with the pEGFP-1 vector to determine whether WEHI-3
cells were present in the bone marrow 24 h after inoculation. Our
results showed that WEHI-3 cells migrated to the bone marrow within
one day of i.p. inoculation (Fig.
5).
Casein reduced hepatomegaly and tumour
burden and increased the survival of mice inoculated with WEHI-3
cells
Leukaemia was induced by WEHI-3 cells as described
above and our results showed that treatment with casein markedly
reduced hepatomegaly and the presence of solid tumours (Table I). Conversely, we observed that
animals not treated with casein died by day 28, while those treated
with casein survived for longer, having a 40% survival rate after
40 days (Fig. 6). Together with the
increased survival of mice treated with casein, the decreased liver
and tumour indices indicate that casein inhibited the proliferation
and induced the apoptosis of WEHI-3 cells.
Discussion
Leukaemia cells undergo physiological alterations
that contribute to their self-sufficiency for survival, unlimited
growth capacity, apoptosis avoidance mechanisms and arrest of cell
differentiation, all of which collectively characterise their
malignant nature (10,11). Drugs including anthracyclines and
cytarabine are usually used to treat leukaemia, but survival rates
are low, with a 60% 5-year survival rate in young patients and a
10% survival rate for elderly patients (2). Given the side effects and poor
tolerance by elderly patients of these treatments (12), there is a pressing need for new
therapeutic alternatives (13).
We previously reported that casein inhibited the
proliferation of the myelomonocytic cell line WEHI-3 (7). In the present study, we have provided
evidence that casein also induces apoptosis in WEHI-3 cells. Casein
is known to induce the differentiation of the normal mouse myeloid
cell line 32D into a monocyte-macrophage lineage (6), thus we expected a similar response in
MNCs. However, casein induced a marked proliferation stimulus.
These data are significant since the usefulness of a potential
anticancer compound depends not only on its cytotoxicity towards
malignant cells, but also on a relative lack of toxicity in normal
tissues (8). There is evidence to
show that normal tissues may be less sensitive to the biological
effects of new molecules with potential antileukaemic properties.
It has previously been shown that betulinic acid reduces the
metabolic activity of WEHI-3 cells, but requires concentrations 10
times higher to achieve the same effect in normal human lymphocytes
(14). Curcumin induces apoptosis
in more than 80% of CD34+ cells from patients with AML,
but the same concentration is cytotoxic in only 20% of normal cells
(9).
Similar to a number of agents studied thus far
(15), casein inhibits
proliferation and induces the death of leukaemia cells. However, in
addition to exerting no cytotoxicity on non-leukaemia MNCs, casein
induces their proliferation, which is a rare property among the
majority of drugs tested for use in the treatment of acute myeloid
leukaemia. Therefore, we evaluated the possibility of using casein
as an antileukaemic agent in vivo.
The i.p. inoculation of leukaemia WEHI-3 cells in
BALB/c mice has been described as an ideal model for the study of
novel therapeutics (e.g., ATRA, aclacinomycin A, IL-6, G-CSF and
vitamin D3) (16). To determine
whether the leukaemia was restricted to the peritoneal cavity, we
evaluated whether i.p.-inoculated WEHI-3 cells were able to migrate
to the bone marrow. We observed that WEHI-3 cells were present in
the marrow of inoculated mice as early as 24 h post-inoculation,
demonstrating the ability of these cells to colonise secondary
sites. This observation demonstrates their aggressive nature, which
resulted in the mortality of all animals tested within 30 days.
When casein was injected i.p., it reduced the tumour
burden and suppressed hepatomegaly, which collectively increased
the survival of the leukaemic mice by a significant extent; this
was considered to be evidence of the inhibition of the growth of
WEHI-3 cells in vivo (17).
Therefore, casein exhibits antileukaemic properties in vitro
and in vivo, which is in contrast to the antibody gemtuzumab
ozogamicin, which efficiently eliminates leukaemia CD33+
cells in vitro but does not improve the survival of
leukaemic individuals (18).
Whether the antitumour properties exhibited in our
leukaemia mouse model resulted from the large induction of
inflammatory cell migration to the peritoneal cavity or from the
secretion of inflammatory differentiation or growth factors known
to be induced in vivo is unclear (19). Alternatively, it is possible that
these effects resulted from the bioactive components of casein,
products of the phagocyte-mediated digestion of casein (20) or a combination of these factors. Due
to its size, casein does not enter the bloodstream, but several of
its components do. Furthermore, biopeptide derivatives of casein
have been detected in blood plasma for several hours following the
ingestion of milk (21).
In conclusion, casein is not cytotoxic to normal
cells but has an inductive effect of haematopoiesis in
vitro. Conversely, under the same conditions, casein induces
apoptosis in leukaemia cells and prolongs the survival of leukaemic
mice, which is clear evidence of antileukaemic activity.
Acknowledgements
We would like to thank Mr. Ernesto J. Rivera Rosales
for the excellent technical assistance. We are indebted to CONACYT
for a Ph.D. scholarship to LME (48959); CGY (169059); a Master
scholarship to ASI (247169); and an undergraduate scholarship to
PCC (15750). This study was supported in part by Fondo SEP-CONACYT
(grant 104025) and PAPIIT (grant IN225610). Professor
Ledesma-Martínez acknowledges the Graduate Program in Biological
Sciences of the National Autonomous University of México (UNAM) for
the training received during the studies.
References
|
1
|
Gregory TK, Wald D, Chen Y, Vermaat JM,
Xiong Y and Tse W: Molecular prognostic markers for adult acute
myeloid leukemia with normal cytogenetics. J Hematol Oncol.
2:23–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robak T and Wierzbowska A: Current and
emerging therapies for acute myeloid leukemia. Clin Ther.
31:2349–2370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuendgen A and Germing U: Emerging
treatment strategies for acute myeloid leukemia (AML) in the
elderly. Cancer Treat Rev. 35:97–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong CW, Seow HF, Liu AH, Husband AJ,
Smithers GW and Watson DL: Modulation of immune responses by bovine
beta-casein. Immunol Cell Biol. 74:323–329. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Metcalf D, Robb L, Dunn AR, Mifsud S and
Di Rago L: Role of granulocyte-macrophage colony-stimulating factor
and granulocyte colony-stimulating factor in the development of an
acute neutrophil inflammatory response in mice. Blood.
88:3755–3764. 1996.
|
|
6
|
Ramos G, Santiago E, Martínez I, Zambrano
I, Manrique B and Weiss B: Sodium caseinate induces differentiation
of 32D pluripotential hematopoietic cells. Rev Invest Clin.
52:638–644. 2000.PubMed/NCBI
|
|
7
|
Ramos-Mandujano G, Weiss-Steider B, Melo
B, Cordova Y, Ledesma-Martinez E, Bustos S, Silvestre O, Aguiniga
I, Sosa N, Martinez I, et al: Alpha-, beta- and kappa caseins
inhibit the proliferation of the myeloid cell lines 32D cl3 and
WEHI-3 and exhibit different differentiation properties.
Immunobiology. 213:133–141. 2008. View Article : Google Scholar
|
|
8
|
Lickliter JD, Wood NJ, Johnson L, McHugh
G, Tan J, Wood F, Cox J and Wickham NW: HA14–1 selectively induces
apoptosis in Bcl-2-overexpressing leukemia/lymphoma cells, and
enhances cytarabine-induced cell death. Leukemia. 17:2074–2080.
2003.
|
|
9
|
Rao J, Xu DR, Zheng FM, Long ZJ, Huang SS,
Wu X, Zhou WH, Huang RW and Liu Q: Curcumin reduces expression of
Bcl-2, leading to apoptosis in daunorubicin-insensitive
CD34+ acute myeloid leukemia cell lines and primary
sorted CD34+ acute myeloid leukemia cells. J Trans Med.
9:71–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 7:57–70. 2000. View Article : Google Scholar
|
|
11
|
Montesinos JJ and Mayani H: New concepts
in the biology of acute myeloid leukemia. Gac Med Mex. 138:67–76.
2002.(In Spanish).
|
|
12
|
Tallman MS, Gilliland DG and Rowe JM: Drug
therapy for acute myeloid leukemia. Blood. 106:1154–1163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roboz GJ: Novel approaches to the
treatment of acute myeloid leukemia. Hematology Am Soc Hematol Educ
Program. 2011:43–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faujan NB, Alitheen SK, Yeap AM, Ali AH,
Muhajir FB and Ahmad H: Cytotoxic effect of betulinic acid and
betulinic acid acetate isolated from Melaleuca cajuput on
human myeloid leukemia (HL-60) cell line. Afr J Biotechnol.
9:6387–6396. 2010.
|
|
15
|
Shipley J and Butera J: Acute myelogenous
leukemia. Exp Hematol. 37:649–658. 2009. View Article : Google Scholar
|
|
16
|
He Q and Na X: The effects and mechanisms
of a novel 2 aminosteroid on murine WEHI3B leukemia cells in vitro
and in vivo. Leuk Res. 25:455–461. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon JS, Kim JY, Park HK, Kim ES, Ahn KS,
Yoon SS, Cho CG, Kim BK and Lee YY: Antileukemic effect of a
synthetic vitamin D3 analog, HY-11, with low potential to cause
hypercalcemia. Int J Oncol. 32:387–396. 2008.PubMed/NCBI
|
|
18
|
Majeti R: Monoclonal antibody therapy
directed against human acute myeloid leukemia stem cells. Oncogene.
30:1009–1019. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noursadeghi M, Bickerstaff MCM, Herbert J,
Moyes D, Cohen J and Pepys MB: Production of granulocyte
colony-stimulating factor in the nonspecific acute phase response
enhances host resistance to bacterial infection. J Immunol.
169:913–919. 2002. View Article : Google Scholar
|
|
20
|
Russell MW, Brooker BE and Reiter B:
Electron microscopic observations of the interaction of casein
micelles and milk fat globules with bovine polymorphonuclear
leucocytes during the phagocytosis of staphylococci in milk. J Comp
Pathol. 87:43–52. 1977. View Article : Google Scholar
|
|
21
|
Chabance B, Marteau P, Rambaud J, Migliore
D, Boynard M, Perrotin P, Jollès P and Fiat AM: Casein peptide
release and passage to the blood in humans during digestion of milk
or yogurt. Biochimie. 80:155–165. 1998. View Article : Google Scholar : PubMed/NCBI
|